Abstract
The B-cell lymphoma-2 (Bcl-2) family of proteins plays a vital role in tumorigenesis. Cancer cells utilize the expression of Bcl-2 to evade therapy and develop resistance. Bcl-2 overexpression also causes cancer cells to be more invasive and metastatic. About 80% of cancer deaths are due to metastases, and yet targeted therapies for metastatic cancers are scarce. We discovered a small molecule, BFC1103, which changes the conformation of Bcl-2 to convert the antiapoptotic protein to a proapoptotic protein. BFC1103-induced apoptosis is dependent on the expression levels of Bcl-2, with higher levels causing more apoptosis. BFC1103 suppressed the growth of breast cancer lung metastasis. BFC1103 has the potential for further optimization and development for clinical testing in metastatic cancers that express Bcl-2. This study demonstrates a new approach to target Bcl-2 using a small molecule, BFC1103, to suppress metastatic disease.
Keywords: Bcl-2 functional conversion, small molecule therapeutics, BH3, TNBC, apoptosis, Bcl-2-selective killing, breast cancer lung metastasis, bioluminescent imaging, targeting Bcl-2 in solid cancers
The B-cell lymphoma-2 (Bcl-2) family proteins are attractive targets to treat a variety of cancers owing to their importance in regulating cell death.1−3 The Bcl-2 family of proteins is frequently overexpressed in many cancers.4−6 Targeting Bcl-2 is a promising approach, as it is independent of classical targets such as growth factor receptors and hormones.6 The Bcl-2 family of proteins consists of proapoptotic and antiapoptotic members and has conserved Bcl-2 homologous (BH) domains. While the antiapoptotic members have BH1–4 domains, the proapoptotic members have BH3-only sensitizers and BH1–3 domains containing effector members.4−6 The ratio of antiapoptotic to proapoptotic Bcl-2 members could be disrupted by various insults within the cell or from external stimuli, leading to tumorigenesis.4,6−9 The Bcl-2 family of proteins has been targeted by multiple therapeutic approaches. The overexpression of antiapoptotic Bcl-2 family members has been targeted by peptides derived from the BH3 domain and using small molecule inhibitors that mimic the BH3 α-helix.10−18 This approach inhibits the function of antiapoptotic proteins. Nur77, also referred to as NGFI-B and TR3, is an immediate-early member of the steroid/thyroid/retinoid nuclear receptor superfamily of transcription factors. Nur77 regulates both survival and proliferation of cancer cells.19,20 The subcellular localization of Nur77 is a determining factor in its biological function, influencing cancer cell fate. Nur77 translocates from the nucleus to the mitochondria upon stimulation with certain compounds and initiates apoptosis.19−21 Nur77 interacts with Bcl-2, resulting in mitochondrial localization of Nur77 and induction of apoptosis. We discovered a 9-amino acid Nur77-derived Bcl-2 converting peptide (NuBCP-9), which is capable of mimicking the mechanistic and functional activities of Nur77.21−35 NuBCP-9 binds to the loop domain of Bcl-2, altering its phenotype to a prodeath protein by exposing its BH3 domain.21−35 As with many anticancer compounds, resistance has been reported to small molecule inhibitors of the Bcl-2 family of proteins.36−38 While most therapies are targeted to effectively inhibit primary tumors, there are hardly any reliable therapies for metastatic cancers. Because of this, metastasis accounts for up to 80% of cancer mortality. Bcl-2 has been implicated in invasion and metastases of multiple types of cancer.39−42 Overexpression of Bcl-2 not only makes cancers resistant to chemotherapy but also increases their metastatic phenotype.39−42
In this study, we hypothesized that small molecule functional converters of Bcl-2 that function as peptido mimics of NuBCP-9 can be identified to alter the phenotype of Bcl-2 and effectively target and eliminate metastatic cancer cells. We used the highly metastatic triple-negative breast cancer cell line MDA-MB-231 with high and low Bcl-2 expression in a viability assay to screen for novel small molecules that selectively induce cell death in Bcl-2 high-expressing cells. The candidate molecule, BFC1103, identified from the Chembridge DIVERSet Compound Library, was used in a breast cancer lung metastatic model to test its therapeutic activity. BFC1103 has no known function or biological targets in the literature.
Results
Screening for Small Molecules Targeting Bcl-2
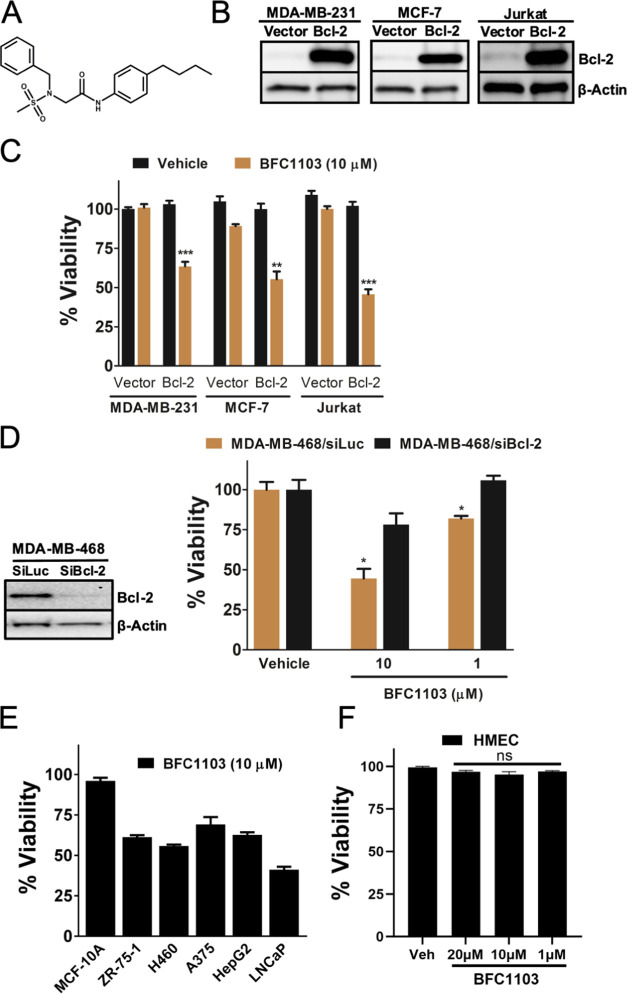
A library consisting of structurally diverse small molecules was used to screen and discover small molecules that induce death in cells with a higher Bcl-2 expression. A viability assay was employed to screen the library compounds in the triple-negative breast cancer cell line, MDA-MB-231, with either high or low expression levels of Bcl-2. The lead compound, designated as BFC1103 (Figure 1A), reduced the viability of cancer cells in a Bcl-2-dependent manner. MDA-MB-231 cells with high levels of Bcl-2 had reduced viability upon treatment with BFC1103 (Figure 1B,C). To demonstrate the Bcl-2-dependent effects of BFC1103 on other cancer cell types, we tested the effect of BFC1103 on MCF-7 breast cancer cells with high or low expression levels of Bcl-2. BFC1103 treatment reduced the viability of MCF-7 cells with high Bcl-2 levels, compared to Bcl-2 low-expressing control cells (Figure 1C). Similarly, BFC1103 reduced the viability of Jurkat human T cell leukemia cells with high Bcl-2 expression compared to control cells (Figure 1C). BFC1103 also reduced the viability of MDA-MB-468 triple-negative breast cancer cells. Suppression of the Bcl-2 expression reduced the effect of BFC1103 (Figure 1D), suggesting that BFC1103 requires Bcl-2 to induce cell death. We tested the effects of BFC1103 on both normal mammary epithelial cells and various types of cancer cells. BFC1103 had minimal effect on nontumorigenic mammary epithelial cells (MCF-10A) and human primary mammary epithelial cells (HMEC). However, BFC1103 reduced the viability of a host of cancer cell types including breast cancer ZR-75-1, nonsmall cell lung cancer H460, melanoma A375, liver cancer HepG2, and prostate cancer LNCaP (Figure 1E,F) cells.
Figure 1.
Bcl-2-dependent effects of BFC1103. (A) Structure of BFC1103 (N-2-benzyl-N-1-(4-butylphenyl)-N-2- (methylsulfonyl)glycinamide). (B) Western blots showing Bcl-2 protein expression in indicated cell lines. (C) Indicated cells were exposed to BFC1103 at 10 μM for 24 h in a medium containing 10% fetal bovine serum (FBS) and viability was assessed by CellTiter-Glo assay. (D) Left panel: Knockdown of Bcl-2 in MDA-MB-468 cells was determined by immunoblotting. Right panel: Cells were exposed to BFC1103 at the indicated concentrations for 48 h in a medium containing 10% FBS and viability was determined. (E) Comparison of viability of various cancer cells lines with MCF-10A nontransformed mammary epithelial cells upon treatment with BFC1103 for 24 h in 10% FBS containing medium. (F) Effect of BFC1103 on viability of primary human mammary epithelial cells (HMEC). ***P < 0.001, **P < 0.01, *P < 0.05, and ns—not significant.
We further tested if the Bcl-2-dependent induction of apoptosis by BFC1103 (Figure 2A) would occur under hypoxic conditions. We detected a significant reduction in the viability of MDA-MB-231 cells with high Bcl-2 expression compared to that of the control cells even under hypoxia (Figure 2B). The effect of BFC1103 on long-term clonogenic survival of MDA-MB-231 cells was also determined. Upon treatment with 10 μM BFC1103, MDA-MB-231 cells overexpressing Bcl-2 formed significantly fewer colonies compared to control cells (Figure 2C).
Figure 2.
Bcl-2-dependent antitumorigenic effects of BFC1103. (A) Apoptosis was determined by using nuclear fragmentation and condensation by using 4′,6-diamidino-2-phenylindole (DAPI) stain. Cells were treated with BFC1103 at 10 μM for 24 h in 10% FBS-containing media and the number of fragmented and condensed nuclei was counted under a fluorescent microscope. (B) Viability was assessed using CellTiter-Glo assay, while the cells were maintained under hypoxic (3% oxygen) conditions. (C) Effect of BFC1103 on the clonogenic survival of MDA-MB-231/Vector and MDA-MB-231/Bcl-2 cells was determined. Cells were treated with BFC1103 at 10 μM in medium containing 10% FBS for 48 h and colonies were counted after 2 weeks. **P < 0.01, *P < 0.05.
BFC1103 Interaction with Bcl-2
We investigated whether the Bcl-2-dependent apoptosis induced by BFC1103 correlated with a change in the conformation of Bcl-2, converting its function from an antiapoptotic to a proapoptotic protein.19−35 We used a Bcl-2-BH3 domain antibody that specifically recognizes Bcl-2 with a changed conformation. Following treatment with BFC1103, the altered conformation of Bcl-2 was detected in MDA-MB-231/Bcl-2 cells (Figure 3A). We previously demonstrated that the loop domain in Bcl-2 plays an important role in conformational changes, leading to the exposure of the BH3 domain in Bcl-2. To test if BFC1103 interacts with the loop domain of Bcl-2, we conducted a limited proteolysis of the loop domain of Bcl-2 with or without BFC1103. BFC1103 delayed the proteolysis of the loop domain of Bcl-2, suggesting its binding to the loop domain (Figure 3B).
Figure 3.

BFC1103 interacts with Bcl-2 and induces conformational change in Bcl-2. (A) MDA-MB-231/Bcl-2 cells were exposed to BFC1103 at 10 μM concentration for 48 h in a medium containing 10% FBS. Change in the conformation of Bcl-2 was determined by using Bcl-2-BH3 domain antibody followed by flow cytometric analysis (vehicle-treated cells in purple and BFC1108-treated cells in green). (B) Limited proteolysis of the Bcl-2 loop domain in the presence of BFC1103. Purified GST-tagged Bcl-2 loop domain (upper panel) or GST (lower panel) was incubated with 50 μM BFC1103 at the indicated times to determine the proteolysis pattern upon coincubation with trypsin.
BFC1103 Induces the Mitochondrial Intrinsic Death Pathway
To determine if BFC1103-induced cell death is dependent on the presence of Bax or Bak, we used mouse embryonic fibroblast cells (MEFs) with or without the expression of Bax or Bak. Upon exposure to BFC1103, wild-type MEF cells, Bax knockout MEF cells (Bax–/–), or Bak knockout MEF cells (Bak–/–) had significant reduction in their viability compared to the double-knockout (DKO, Bax–/– Bak–/–) MEF cells (Figure 4A). Thus, apoptosis induced by BFC1103 requires the expression of either Bax or Bak. BFC1103-treated H460 lung cancer cells showed a decrease in their mitochondrial outer membrane potential when stained with JC-1 dye, demonstrating collapse of the mitochondrial outer membrane (Figure 4B). Thus, BFC1103 induces a mitochondrial intrinsic apoptotic pathway.
Figure 4.
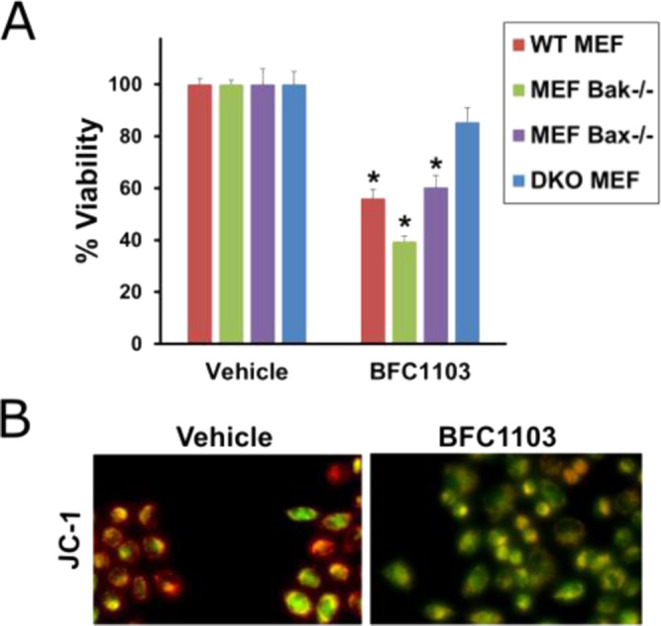
BFC1103 requires Bax/Bak for induction of apoptosis (A) WT MEF, Bax–/– MEF, Bak–/– MEF, and Bax–/– Bak–/– MEF cells were treated with 1 μM BFC1103 for 24 h in 10% FBS medium and viability was assessed using CellTiter-Glo assay. (B) JC-1 dye was used to stain live H460 cells treated with 10 μM BFC1103 for 16 h in 10% FBS-containing medium and images taken with fluorescein isothiocyanate (FITC) and rhodamine filters were overlaid. Cells stained orange have an intact mitochondrial outer membrane and the ones turning green have a compromised outer membrane, indicating loss of membrane potential. *P < 0.05.
Efficacy of BFC1103 in a Breast Cancer Lung Metastasis Model
We next determined the efficacy of BFC1103 in suppressing breast cancer lung metastasis. LMD231 cells are metastatic cells derived from the lung metastasis of MDA-MB-231 cells. LMD231 cells are preprogrammed to form lung metastasis when introduced in mice.43 LMD231 cells were engineered to stably express luciferase (LMD231-Luc) for easy detection in nude mice. Approximately 200,000 LMD231-Luc cells were injected into the tail vein of 6 weeks old nude mice, and lung metastasis was detected within 2 weeks. BFC1103 was administered at a dose of 50 mg/kg by the intraperitoneal route for 6 days per week. By the end of 7 weeks, BFC1103 significantly suppressed the growth of lung metastasis (Figure 5A). Immunohistochemical analysis of lung tissue by H&E staining revealed potent suppression of tumor growth by BFC1103 when compared with vehicle-treated mice (Figure 5B). Further, lung tissues from mice treated with BFC1103 had a markedly reduced number of proliferating cells, as measured by Ki-67 staining, compared to those treated with the vehicle (Figure 5C). Thus, BFC1103 was effective in reducing the breast cancer lung metastasis.
Figure 5.

BFC1103 suppresses lung metastasis in vivo (A) 200,000 LMD-231 cells stably expressing luciferase were injected into the tail vein of 6 weeks old nude mice. Lung metastasis was detectable in 2 weeks after injection of tumor cells. Mice were treated with BFC1103 at 50 mg/kg six times a week by intraperitoneal injections. Bioluminescent imaging was performed once a week and quantified. (B) H&E staining showed potent suppression of tumor cells in lung tissue of nude mice treated with BFC1103 compared to vehicle treatment. (C) Ki-67 staining detecting the presence of tumor cells with high proliferation in lung tissue from vehicle-treated mice, compared to BFC1103-treated mice. * P < 0.05.
Discussion
We found a new class of small molecule Bcl-2 functional converter, BFC1103, that suppresses breast cancer lung metastases. Current cancer therapies that target the Bcl-2 protein inhibit the antiapoptotic function of Bcl-2. In this study, we show a new way of targeting Bcl-2, using a small molecule that functionally converts the function of Bcl-2 from an antiapoptotic to a proapoptotic protein. We demonstrated that BFC1103 can successfully suppress triple-negative breast cancer lung metastasis in a mouse model.
We show that BFC1103 reduces viability of multiple cancer cell types expressing Bcl-2. The effect of BFC1103 increases with an increase in levels of Bcl-2 expression. This Bcl-2-dependent action of BFC1103 could be utilized for treating refractory and resistant cancers that express Bcl-2. BFC1103 induced a change in the conformation of Bcl-2, which correlated with the induction of apoptosis following the mitochondrial intrinsic death pathway. Our data suggest that BFC1103 has an affinity toward the loop domain of Bcl-2. Thus, BFC1103 mimics Nur77 or NuBCP-9 in interacting with the loop domain of Bcl-2, resulting in a change in its conformation and induction of apoptosis. Taken together, BFC1103 has the potential for treating highly metastatic cancers expressing Bcl-2.
This is a proof-of-concept study, demonstrating the ability of a small molecule to transform the phenotype of Bcl-2 into a proapoptotic protein. One limitation of this study is the absence of detailed optimization and characterization of BFC1103, particularly regarding its affinity and pharmacokinetic/pharmacodynamic (PK/PD) properties. These aspects are crucial for clinical translation and for understanding the nature of its molecular interaction with Bcl-2. These additional studies will pave the way for comprehensive preclinical testing and selection of a BFC1103 analogue for first-in-human trials. BFC1103 did not cause systemic toxicity or adverse effects in healthy mice during the maximum tolerated dose (MTD) studies. Development of small molecules such as BFC1103 will have significant impact in the clinic to tackle cancers that express Bcl-2 and those that have developed resistance to Bcl-2 inhibitors such as venetoclax. Bcl-2 inhibitors neutralize the survival function of Bcl-2 and are approved for treatment of some blood cancers. However, cancer cells become unresponsive to Bcl-2 inhibitors, as they become less dependent on Bcl-2 for survival. In contrast, BFC1103 will kill cancer cells, even if they do not require Bcl-2 for survival. BFC1103 can simply use Bcl-2 as a weapon to target and eliminate drug-resistant and other cancer cells expressing Bcl-2. Importantly, there are no Bcl-2-targeted Food and Drug Administration (FDA)-approved drugs for solid tumors. Additionally, the identification of combination treatments involving small molecules targeting other oncogenic pathways will help prime and sensitize resistant cancer cells for a more effective therapeutic outcome. We are currently pursuing a screening of known chemotherapeutics and other FDA-approved drugs to discover molecules similar to BFC1103, which induce the functional conversion of Bcl-2 and promote apoptosis in cancer cells for expedited clinical utility.
Materials and Methods
Cell Culture
Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) with l-glutamine (Corning, Cat #10-013-CV) supplemented with 10% fetal bovine serum (FBS) (tissue culture biologicals, Cat #101 HI), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Corning, Cat #30-002-Cl) in a humidified 5% CO2 incubator. Cells were cultured under 80% confluency (ATCC, cat. no. CRM-HTB-26, RRID:CVCL_0062), MCF-7 (ATCC, cat. no. HTB-22, RRID:CVCL_0031), HepG2 (ATCC, cat. no. HB-8065, RRID:CVCL_0027), ZR-75-1 (ATCC, cat. no. CRL-1500, RRID:CVCL_0588), A375 (ATCC, cat. no. CRL-1619, RRID:CVCL_0132), MEFs (ATCC, cat. no. CRL-2991, RRID:CVCL_U630), and H460 (ATCC, cat. no. HTB-177, RRID:CVCL_0459). LNCaP cells (ATCC, catalog no. CRL-1740, RRID:CVCL_0395) were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Corning, Cat #10-040-CM). MCF-10A cells (ATCC, catalog no. CRL-10317, RRID:CVCL_0598) were cultured in DMEM/F-12 (Gibco, catalog no. 11-320-033) with appropriate supplements. All cells were passaged once every 3 days. For hypoxic conditions, the cells were maintained under 1–3% O2 and 5% CO2 conditions.51
Generation of Bcl-2-Stable Cell Lines
Stable expression of Bcl-2 in MDA-MB-231 and MCF-7 breast cancer cell lines was generated by the electroporation technique and subsequent clonal expansion in G418-containing media. Stable expression of Bcl-2 was determined by Western blotting, and clones expressing high Bcl-2 were used for screening and characterization of lead molecules.
Chemicals
The ChemBridge library consisting of 50,000 active pharmacophores was purchased from ChemBridge (San Deigo, CA). Master library plates were diluted to 1 mM concentration for a final dimethyl sulfoxide (DMSO) concentration of 1% (v/v) in the screening assay. For validations, 10 mg of each hit compound was ordered from the respective supplier and 20 mM stocks in DMSO were made. All subsequent in vitro testing was done under 0.1% (v/v) DMSO concentration. BFC1103 (N-2-benzyl-N-1-(4-butylphenyl)-N-2-(methylsulfonyl)glycinamide) was custom-synthesized by ChemBridge Corporation, San Diego, CA, for in vivo testing.
Viability and Apoptotic Assays
Viability assays45,46 were performed using CellTiter-Glo assay (Promega, CA, Cat #G7573) as per the manufacturer’s instructions. DAPI stain was used to assess apoptosis.47−49 Colony-forming assays were performed as described previously.
Antibodies
Immunofluorescence and flow cytometry experiments to detect change in the conformation of Bcl-2 were performed using the Bcl-2 BH3 antibody (Cat #AP1303a) from Abgent, San Diego, CA. Western blotting experiments were performed using the Bcl-2 antibody (Cat #13-8800) from Invitrogen, CA. For tumor tissue staining, Ki-67 and hematoxylin and eosin (H&E) were obtained from Cell Signaling Technology. Mitochondrial membrane integrity experiments were performed using JC-1 dye (Cat #T3168) obtained from Roche, CA.
Bcl-2 Knockdown
To knock down Bcl-2, MDA-MB-468 cells were transfected with the Dharmafect reagent (catalog no. T-2001-03) with Bcl-2 siRNA (25 ng) (Dharmacon Research, Lafayette, CO) or 25 ng of control luciferase siRNA (Cat #D-002050-01, Dharmacon Research, Lafayette, CO). Briefly, cells were seeded at 4000 cells per well in a 96-well black plate and 72 h post-transfection, cells were treated with indicated compounds for 48 h, and viability was assayed with CellTiter Glo luminescent cell viability assay (Promega, CA, Cat #G7573). Bcl-2 knockdown was confirmed by Western blotting at the 72 h time point.
Limited Proteolysis Assay
To study the molecular interaction of BFC1103 with Bcl-2, a limited proteolysis assay was used. Briefly, Bcl-2 fused with GST and GST-only control fragments (60 μg) were incubated with 0.1% DMSO or BFC1103 (50 μM) for 1 h and proteolyzed by 3 μg/mL trypsin for 1, 2, 4, and 8 min. Laemmli buffer was used for quenching the reaction, protease cleavage fragments were resolved on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, and Coomassie blue stain was used for visualization.
Metastatic Model and Bioluminescence Analysis
For the lung metastasis model, LMD-231 cells derived from MDA-MB-231 that frequently colonized lungs were used.43 LMD-231 cells were transduced with lenti viral particles with a luciferase expression vector (GenTarget, San Diego, CA) to obtain a stable high-luciferase expression clone (LMD-Luc). The stable expression of luciferase was monitored for 7 weeks in vitro before using in animal experiments.
To establish lung metastasis in 5 weeks old female nude mice (Jackson Laboratories, Bar Harbor, ME), 200,000 LMD-Luc cells were injected into the lateral tail vein. Bioluminescence was measured once a week using an IVIS Lumina II (PerkinElmer, Waltham, MA) as per the manufacturer’s instructions, while the mice were under anesthesia. Animal experiments were executed as per approved protocol by the IACUC at Oregon State University. To comply with the animal ethics code of the IACUC (3Rs: replacement, refinement, and reduction), we utilized one control-treated group of mice to test two compounds at the same time.44
At the end of the study, mice were euthanized, and lung tissues were fixed for immunohistochemical analysis.44,50
Statistical Analysis
The statistical significance of difference between groups was analyzed by two-sided unpaired nonparametric Student’s t test with Mann–Whitney test using PRISM software version 9. Results were considered significant at p < 0.05.
Acknowledgments
The authors express their gratitude to Drs. Xiao-kun Zhang and Arnold Satterthwait for numerous discussions and support for this research. Additionally, Dr. Joseph G. Christison from Oregon State University Advantage is acknowledged for assistance with collaborative translational research agreements, and special thanks to Pranav J. Kolluri for editing. This work was supported by the U.S. Department of Agriculture’s (USDA) National Institute of Food and Agriculture (NIFA) Multistate project ORE00108E, National Institute of Environmental Health Sciences Core Center facilities and services (P30 ES030287), and grants to S.K. Kolluri from the US Army Medical Research and Material Command (W81XWH-08-1-0600 and W81XWH-12-1-0069), and the National Cancer Institute of the National Institutes of Health under the award number CA249627. The sponsors have no role in study design, conclusions, or submission of this article.
The authors declare no competing financial interest.
References
- Yip K. W.; Reed J. C. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- Kaloni D.; Diepstraten S. T.; Strasser A.; Kelly G. L. BCL-2 protein family: attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. 10.1007/s10495-022-01780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor I.; Bodo J.; Hill B. T.; His E. D.; Almasan A. Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death Dis. 2020, 11, 941 10.1038/s41419-020-03144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A. N.; Engelman J. A.; Faber A. C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discovery 2015, 5 (5), 475–487. 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.; Quinn B. A.; Das S. K.; Dash R.; Emdad L.; Dasgupta S.; Wang X.-Y.; Dent P.; Reed J. C.; Pellecchia M.; Sarkar D.; Fisher P. B. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 2013, 17, 61–75. 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M. S.; Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J. Clin. Oncol. 2012, 30, 3127–3135. 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. H.; Reynolds C. P. Bcl-2 Inhibitors: Targeting Mitochondrial Apoptotic Pathways in Cancer Therapy. Clin. Cancer Res. 2009, 15, 1126–1132. 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. E.; Davids M. S. BCL-2 Inhibitors, Present and Future. Cancer J. 2019, 25 (6), 401–409. 10.1097/PPO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- Montero J.; Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic?. Cell Death Differ. 2018, 25 (1), 56–64. 10.1038/cdd.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M. S.; Letai A. ABT-199: taking dead aim at BCL-2. Cancer Cell 2013, 23 (2), 139–141. 10.1016/j.ccr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros A. M.; Huth J. R.; Oost T.; Park C.-M.; Ding H.; Wang X.; Zhang H.; Nimmer P.; Mendoza R.; Sun C.; Mack J.; Walter K.; Dorwin S.; Gramling E.; Ladror U.; Rosenberg S. H.; Elmore S. W.; Fesik S. W.; Hajduk P. J. Discovery of a potent and selective Bcl-2 inhibitor using SAR by NMR. Bioorg. Med. Chem. Lett. 2010, 20, 6587–6591. 10.1016/j.bmcl.2010.09.033. [DOI] [PubMed] [Google Scholar]
- Moore V. D. G.; Letai A. Rational design of therapeutics targeting the BCL-2 family: are some cancer cells primed for death but waiting for a final push?. Adv. Exp. Med. Biol. 2008, 615, 159–175. 10.1007/978-1-4020-6554-5_8. [DOI] [PubMed] [Google Scholar]
- Oakes S. R.; Vaillant F.; Lim E.; Lee L.; Breslin K.; Feleppa F.; Deb S.; Ritchie M. E.; Takano E.; Ward T.; Fox S. B.; Generali D.; Smyth G. K.; Strasser A.; Huang D. C. S.; Visvader J. E.; Lindeman G. J. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc. Natl. Acad. Sci. U.S.A. 2012, 109 (8), 2766–2771. 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard C. BH3 mimetics: status of the field and new developments. Mol. Cancer Ther. 2013, 12 (9), 1691–1700. 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- Ploumaki I.; Triantafyllou E.; Koumprentziotis I. A.; Karampinos K.; Drougkas K.; Karavolias I.; Trontzas I.; Kotteas E. A. Bcl-2 pathway inhibition in solid tumors: a review of clinical trials. Clin. Transl. Oncol. 2023, 25 (6), 1554–1578. 10.1007/s12094-022-03070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos-Ortiz M.; Ryan J.; Mashaka T. N.; Opferman J. T.; Letai A. BH3 profiling discriminates on-target small molecule BH3 mimetics from putative mimetics. Cell Death Differ. 2020, 27 (3), 999–1007. 10.1038/s41418-019-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr S.; Vogler M. It’s time to die: BH3 mimetics in solid tumors. Biochim. Biophys. Acta, Mol. Cell Res. 2021, 1868 (5), 118987 10.1016/j.bbamcr.2021.118987. [DOI] [PubMed] [Google Scholar]
- Montero J.; Haq R. Adapted to Survive: Targeting Cancer Cells with BH3Mimetics. Cancer Discovery 2022, 12 (5), 1217–1232. 10.1158/2159-8290.CD-21-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Kolluri S. K.; Gu J.; Dawson M. I.; Cao X.; Hobbs P. D.; Lin B.; Chen G.; Lu J.; Lin F.; Xie Z.; Fontana J. A.; Reed J. C.; Zhang X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 2000, 289 (5482), 1159–1164. 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- Lin B.; Kolluri S. K.; Lin F.; Liu W.; Han Y. H.; Cao X.; Dawson M. I.; Reed J. C.; Zhang X.-k. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 2004, 116 (4), 527–540. 10.1016/S0092-8674(04)00162-X. [DOI] [PubMed] [Google Scholar]
- Kolluri S. K.; Zhu X.; Zhou X.; Lin B.; Chen Y.; Sun K.; Tian X.; Town J.; Cao X.; Lin F.; Zhai D.; Kitada S.; Luciano F.; O’Donnell E.; Cao Y.; He F.; Lin J.; Reed J. C.; Satterhwait A. C.; Zhang X.-K. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell 2008, 14 (4), 285–298. 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano F.; Krajewska M.; Ortiz-Rubio P.; Krajewski S.; Zhai D.; Faustin B.; Bruey J.-M.; Bailly-Maitre B.; Lichtenstein A.; Kolluri S. K.; Satterthwait A. C.; Zhang X.-K.; Reed J. C. Nur77 converts phenotype of Bcl-B, an antiapoptotic protein expressed in plasma cells and myeloma. Blood 2007, 109, 3849–3855. 10.1182/blood-2006-11-056879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi B.; Hardwick J. M. Bcl-2 turns deadly. Nat. Chem. Biol. 2008, 4 (12), 722–723. 10.1038/nchembio1208-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz L. BCL-2 double take. Science-Business eXchange 2008, 1 (39), 938 10.1038/scibx.2008.938. [DOI] [Google Scholar]
- Flemming A. New strategies to tip the BCL-2 balance. Nat. Rev. Drug Discovery 2008, 7, 977 10.1038/nrd2773. [DOI] [Google Scholar]
- Aschheim K.; DeFrancesco L.; Hare P. Flipping for apoptosis. Nat. Biotechnol. 2008, 26, 1250 10.1038/nbt1108-1250. [DOI] [Google Scholar]
- Kumar M.; Gupta D.; Singh G.; Sharma S.; Bhat M.; Prashant C. K.; Dinda A. K.; Kharbanda S.; Kufe D.; Singh H. Novel polymeric nanoparticles for intracellular delivery of peptide Cargos: antitumor efficacy of the BCL-2 conversion peptide NuBCP-9. Cancer Res. 2014, 74 (12), 3271–3281. 10.1158/0008-5472.CAN-13-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor S.; Gupta D.; Kumar M.; Sharma S.; Gupta A. K.; Misro M. M.; Singh H. Intracellular delivery of peptide cargos using polyhydroxybutyrate based biodegradable nanoparticles: Studies on antitumor efficacy of BCL-2 converting peptide, NuBCP-9. Int. J. Pharm. 2016, 511 (2), 876–889. 10.1016/j.ijpharm.2016.07.077. [DOI] [PubMed] [Google Scholar]
- Pearce M. C.; Gamble J. T.; Kopparapu P. R.; O’Donnell E. F.; Mueller M. J.; Jang H. S.; Greenwood J. A.; Satterthwait A. C.; Tanguay R. L.; Zhang X.-K.; Kolluri S. K. Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells. Oncotarget 2018, 9 (40), 26072–26085. 10.18632/oncotarget.25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D.; Kumar M.; Tyagi P.; Kapoor S.; Tyagi A.; Barman T. K.; Kharbanda S.; Kufe D.; Singh H. Concomitant Delivery of Paclitaxel and NuBCP-9 peptide for synergistic enhancement of cancer therapy. Nanomedicine 2018, 14 (4), 1301–1313. 10.1016/j.nano.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E.; Gamble J. T.; Pearce M. C.; Elson D. J.; Tanguay R. L.; Kolluri S. K.; Reich N. O. Improved in vivo targeting of BCL-2 phenotypic conversion through hollow gold nanoshell delivery. Apoptosis 2019, 24 (5–6), 529–537. 10.1007/s10495-019-01531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce M. C.; Satterthwait A. C.; Zhang X.-K.; Kolluri S. K. Cancer therapeutics based on BCL-2 functional conversion. Apoptosis 2019, 24 (1–2), 1–2. 10.1007/s10495-018-1504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Cao X.; Tu X.; Alitongbieke G.; Xia Z.; Li X.; Chen Z.; Yin M.; Xu D.; Guo S.; Li Z.; Chen L.; Zhang X.; Xu D.; Gao M.; Liu J.; Zeng Z.; Zhou H.; Su Y.; Zhang X.-k. BI1071, a Novel Nur77 Modulator, Induces Apoptosis of Cancer Cells by Activating the Nur77-Bcl-2 Apoptotic Pathway. Mol. Cancer Ther. 2019, 18 (5), 886–899. 10.1158/1535-7163.MCT-18-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Ge P.; Xu W.; Li M.; Kang Q.; Zhang X.; Xie J. Cancer-targeted and intracellular delivery of Bcl-2-converting peptide with functional macroporous silica nanoparticles for biosafe treatment. Mater. Sci. Eng., C 2020, 108, 110386 10.1016/j.msec.2019.110386. [DOI] [PubMed] [Google Scholar]
- Yang J.; Li Q.; Zhou R.; Zhou M.; Lin X.; Xiang Y.; Xie D.; Huang Y.; Zhou Z. Combination of mitochondria targeting doxorubicin with Bcl-2 function-converting peptide NuBCP-9 for synergistic breast cancer metastasis inhibition. J. Mater. Chem. B 2021, 9 (5), 1336–1350. 10.1039/D0TB02564J. [DOI] [PubMed] [Google Scholar]
- Yecies D.; Carlson N. E.; Deng J.; Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 2010, 115 (16), 3304–3313. 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan S.; Vogler M.; Butterworth M.; Dinsdale D.; Walnesky L. D.; Cohen G. M. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 2013, 20 (11), 1475–1484. 10.1038/cdd.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A. Hiding from ABT-737 in lymph nodes. Blood 2009, 113 (18), 4132–4133. 10.1182/blood-2008-12-192831. [DOI] [PubMed] [Google Scholar]
- Del Bufalo D.; Biroccio A.; Leonetti C.; Zupi G. Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J. 1997, 11 (12), 947–953. 10.1096/fasebj.11.12.9337147. [DOI] [PubMed] [Google Scholar]
- Zuo J.; Ishikawk T.; Boutros S.; Xiao Z.; Humtsoe J. O.; Kramer R. H. Bcl-2 overexpression induces a partial epithelial to mesenchymal transition and promotes squamous carcinoma cell invasion and metastasis. Mol. Cancer Res. 2010, 8 (2), 170–182. 10.1158/1541-7786.MCR-09-0354. [DOI] [PubMed] [Google Scholar]
- Choi J.; Choi K.; Benveniste E. N.; Rho S. B.; Hong Y.-S.; Lee J.-H.; Kim J.; Park K. Bcl-2 promotes invasion and lung metastasis by inducing matrix metalloproteinase-2. Cancer Res. 2005, 65 (13), 5554–5560. 10.1158/0008-5472.CAN-04-4570. [DOI] [PubMed] [Google Scholar]
- Real P. J.; Sierra A.; Juan A. D.; Segovia J. C.; Lopez-Vega J. M.; Fernandez-Luna J. L. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene 2002, 21 (50), 7611–7618. 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- Patel J. B.; Appaiah H. N.; Burnett R. M.; Bhat-Nakshatri P.; Wang G.; Mehta R.; Badve S.; Thomson M. J.; Hammond S.; Steeg P.; Liu Y.; Nakshatri H. Control of EVI-1 oncogene expression in metastatic breast cancer cells through microRNA miR-22. Oncogene 2011, 30 (11), 1290–1301. 10.1038/onc.2010.510. [DOI] [PubMed] [Google Scholar]
- Elson D. J.; Nguyen B. D.; Korjeff N. A.; Wilferd S. F.; Puig-Sanvicens V.; Sang Jang H.; Bernales S.; Chakravarty S.; Belmar S.; Ureta G.; Finlay D.; Plaisier C. L.; Kolluri S. K. Suppression of Ah Receptor (AhR) increases the aggressiveness of TNBC cells and 11-Cl-BBQ-activated AhR inhibits their growth. Biochem. Pharmacol. 2023, 215, 115706 10.1016/j.bcp.2023.115706. [DOI] [PubMed] [Google Scholar]
- Nguyen B. D.; Stevens B. L.; Elson D. J.; Finlay D.; Gamble J. T.; Kopparapu P. R.; Tanguay R. L.; Buermeyer A. B.; Kerkvliet N. I.; Kolluri S. K. 11-Cl-BBQ, a select modulator of AhR-regulated transcription, suppresses lung cancer cell growth via activation of p53 and p27Kip1. FEBS J. 2023, 290 (8), 2064–2084. 10.1111/febs.16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell E. F. 3rd; Jang H. S.; Liefwalker D. F.; Kerkvliet N. I.; Kolluri S. K. Discovery and Mechanistic Characterization of a Select Modulator of AhR-regulated Transcription (SMAhRT) with Anti-cancer Effects. Apoptosis 2021, 26 (5–6), 307–322. 10.1007/s10495-021-01666-0. [DOI] [PubMed] [Google Scholar]
- Elson D. J.; Nguyen B. D.; Bernales S.; Chakravarty S.; Jang H. S.; Korjeff N. A.; Zhang Y.; Wilferd S. F.; Castro D. J.; Plaisier C. L.; Finlay D.; Oshima R. G.; Kolluri S. K. Induction of Aryl Hydrocarbon Receptor-Mediated Cancer Cell-Selective Apoptosis in Triple-Negative Breast Cancer Cells by a High-Affinity Benzimidazoisoquinoline. ACS Pharmacol. Transl. Sci. 2023, 6 (7), 1028–1042. 10.1021/acsptsci.2c00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri S. K.; Bruey-Sedano N.; Cao X.; Lin B.; Lin F.; Han Y. H.; Dawson M. I.; Zhang X. K. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell. Biol. 2003, 23 (23), 8651–8667. 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopparapu P. R.; Pearce M. C.; Lohr C. V.; Duong C.; Jang H. S.; Tyavanagimatt S.; O’Donnell E. F.; Nakshatri H.; Kolluri S. K. Identification and characterization of small molecule Bcl-2 Functional Converter. Cancer Res. Commun. 2024, 4 (3), 634–644. 10.1158/2767-9764.CRC-22-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. L.; Löhr C. V.; Nguyen B. D.; Buermeyer A. B.; Kolluri S. K. Loss of the aryl hydrocarbon receptor increases tumorigenesis in p53-deficient mice. Toxicol. Appl. Pharmacol. 2022, 454, 116191 10.1016/j.taap.2022.116191. [DOI] [PubMed] [Google Scholar]
- Song Z.; Pearce M. C.; Jiang Y.; Yang L.; Goodall C.; Miranda C. L.; Milovancev M.; Bracha S.; Kolluri S. K.; Maier C. S. Delineation of hypoxia-induced proteome shifts in osteosarcoma cells with different metastatic propensities. Sci. Rep. 2020, 10, 727. 10.1038/s41598-019-56878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]