Abstract
Background
Many recent studies suggest the existence of a relationship between oral health and the occurrence of depressive symptoms. The aim of this study was to assess the relationship between the number of lost teeth and the occurrence of depressive symptoms in middle-aged adults.
Methods
An analysis was performed on the data obtained from the PONS project (POlish-Norwegian Study), conducted in the Świętokrzyskie Province in Poland in 2010–2011. The research material included the cross-sectional data of 11,901 individuals aged 40–64 years (7967 women). Depressive symptoms, used as outcome variables, were assessed with a questionnaire. The participants provided the responses to questions concerning the occurrence of eight symptoms over the last 12 months. The answers were scored as 1 point or 0 points. The participants were divided into three tercile groups based on their total scores: no or mild (0–2 points), moderate (3–5 points), and severe depressive symptoms (6–8 points). The self-reported number of lost teeth was analysed according to the following categories: 0–4, 5–8, 9–27, and a complete lack of natural teeth. Multivariable logistic regression analysis for depressive symptoms was used in relation to the number of lost teeth. The following covariates were included in the adjusted model: age, sex, place of residence, education, marital status, BMI, diabetes status, stressful life events in the last year, use of antidepressants, smoking, and sugar and sweet consumption.
Results
The likelihood of both moderate (OR = 1.189; 95%CI: 1.028–1.376; p < .020) and severe (OR = 1.846; 95%CI: 1.488–2.290; p < .001) depressive symptoms showed the strongest relationship with a total lack of natural teeth. A loss of more than 8 natural teeth was also significantly associated (OR = 1.315; 95%CI: 1.075–1.609; p < .008) with the occurrence of severe depressive symptoms.
Conclusions
The loss of natural teeth was positively related to the occurrence of depressive symptoms in middle-aged adults. Thus, there is an urgent need to intensify stomatological prophylaxis, education and treatment for middle-aged individuals.
Keywords: Depressive symptoms, Tooth loss, Middle-aged adults
Introduction
Depression is one of the most common mental health disorders worldwide and can originate from genetic, biological, and environmental factors [1, 2]. Depressive symptoms, such as lowered mood (sadness, despondency, reliving of negative events, anhedonia, and indifference); decreased psychomotor drive (psychomotor retardation or inhibition, loss of energy, and persistent fatigue); abnormal circadian rhythm; and somatic symptoms (hyposomnia, hypersomnia, dry mucous membranes in the mouth, and weight changes), may also occur in people who do not meet the clinical diagnostic criteria for depression and who experience subthreshold depression. Many recent studies suggest the existence of a relationship between oral health and the occurrence of depressive symptoms [3, 4]. One of the most severe oral conditions is tooth loss [5, 6]. Its direct causes are usually untreated caries and periodontal diseases [7–9]. The prevalence of tooth loss and the factors that directly determine it depend largely on economic development, access to dental care, social factors, and lifestyle, which is why they may differ between countries and regions [8–15]. Loss of teeth not only reduces the effectiveness of mastication but also contributes to a limitation, or even complete elimination, of hard products from the diet (e.g., nuts and some raw fruits and vegetables), significantly reducing the nutritional value of food [16]. Consequently, such changes may impact the nutritional status and health of individuals. Loss of teeth results in a gradual decrease in bone tissue and may lead to malocclusion and disorders of the temporomandibular joints. It also causes speech impediments, especially lisping, since without proper support in the form of teeth, the tongue cannot be positioned to correctly articulate some sounds [17]. Furthermore, visibly missing teeth constitute an aesthetic blemish, while poorly fitted dentures and incorrect articulation reduce self-confidence, make it difficult for individuals to be active in the job market, and cause individuals to withdraw from social life, which in turn may reduce mental well-being [17, 18]. These relationships have, to date, been analysed predominantly among elderly individuals, with very few publications addressing the topic among middle-aged adults. The aim of this study was to assess the relationship between the number of lost teeth and the occurrence of depressive symptoms among individuals aged 40–64 years. According to our hypothesis, the number of lost natural teeth shows a positive relationship with the occurrence of depressive symptoms among middle-aged adults.
Materials and methods
Study design
An analysis was performed on the cross-sectional data obtained as part of the PONS project (POlish-Norwegian Study), which was conducted in the Świętokrzyskie Province in Poland in 2010–2011. The goal of the project was to observe the health and prevalence of chronic noncommunicable diseases among the residents of southeastern Poland [19]. This was a facility-based survey. All participants of the project were volunteers. The study in its entirety (both the measurements and the interviews) was conducted in the medical institutions that took part on the project. The Ethics Committee at the Cancer Center and Institute of Oncology in Warsaw (No. 69/2009/1/2011) approved the project and method of data collection. Further data analysis was also approved by the Committee on Bioethics at the Faculty of Health Sciences, Jan Kochanowski University in Kielce (No. 45/2016).
Participants
Participants were recruited by invitations to participate in the project to all men and women aged 45–64 years living in the Kielce District (Świętokrzyskie Province, Poland). The participants were invited to take part in the study through advertisements and promotional articles in local newspapers, radio and TV programmes, informative materials (leaflets, billboard advertising, information charts and a website), co-operation with family doctors and medical institutions participating in the project, which sent the invitations via mail. Therefore, all participants of the project were volunteers. The sole inclusion criterion for the whole project was age 45–64 years and being a resident of the city of Kielce and the surrounding rural area (the Kielce County). We did not directly calculate the sample size. We assumed a response rate of at least 10% among the target group and the actual response rate was 12%. Many more women than men from the target age group volunteered to participate. A small number of participants who were younger (37–44 years) and older (65–66 years) than the target age group also volunteered for the study. Consequently, the collected data encompassed the results of 13,172 individuals aged 37–66 years. For the purposes of the analyses conducted in this study, individuals aged ≥ 65 years and < 40 years, as well as participants whose data were incomplete, were excluded (1271 individuals). Ultimately, the research material for the study included the data of 11,901 individuals aged 40–64 years (3934 men).
Measurements
Sociodemographic data and data related to depressive symptoms, the declared number of lost teeth, and lifestyle factors were collected via face-to-face interviews with structured questionnaires. The sociodemographic variables included sex (male or female), age (years), place of residence (the city of Kielce (200 thousand inhabitants) and the rural county of Kielecki (village), education level (higher, secondary, primary, or vocational), and marital status (married or in a stable relationship, single, or widowed/widowered). Measurements of depressive symptoms, used as outcome variables, were adapted from the Prospective Urban and Rural Epidemiological (PURE) study [20]. The participants were assessed based on the responses provided to eight questions concerning the occurrence of the following symptoms in the last 12 months: fatigue (loss of energy), weight gain or loss, problems falling asleep, loss of concentration, loss of interest and pleasure, feeling of helplessness (low self-esteem), sadness (worry), and thoughts about death. The respondents answered “yes” or “no” to each question. The answers were scored as 1 point or 0 points. The participants were then divided into three tercile groups based on their total scores (from 0 to 8 points): no or mild depressive symptoms (0–2 points), moderate depressive symptoms (3–5 points), and severe depressive symptoms (6–8 points) [21]. The respondents were also asked about the use of antidepressants and whether they had experienced any stressful life events in the last year, such as a serious disease or injury or death or serious disease of a close family member. The categories of the variable “number of lost teeth” were distinguished based on the quartile distribution of this characteristic among the analysed population. Thus, the self-reported number of lost teeth was analysed according to the following categories: 0–4, 5–8, 9–27, and a complete lack of natural teeth. Body height and mass were measured and subsequently used to calculate BMI (kg/m2). The assessment of diabetes status included a diagnosis of diabetes, diabetes treatment, or current abnormal fasting glucose in the blood serum (≥ 100 mg/dL/5.5 mmol/L) measured using the enzyme method with hexokinase. Participants who did not meet the above criteria were classified as nondiabetic. Total consumption of sugar, sweets, and sweetened beverages was assessed with a validated, semiquantitative food frequency questionnaire from the PURE study, which was used in the PONS project [22]. The questionnaire was administrated in Polish. The method of FFQ development and validation was carefully standardised for all PURE study participating countries [22]. One hundred and forty-six study participants in the Polish arm of the PURE study completed the 134-item FFQs as well as four 24-h dietary recalls during a 12-month period. The FFQ has good validity and reproducibility in relations to the reference method. The study participants were asked about how frequently they consumed certain portions of each product containing sugar over the last year. The frequencies of consumption were classified as follows: 6 times a day or more, 4–5 times a day, 2–3 times a day, once a day, 5–6 times a week, 2–4 times a week, once a week, 1–3 times a month, less frequently than once a month or not at all. The reported frequencies of consumption and sizes of portions of the analysed food products were converted into mean daily doses (min–max = 0.00–27.64 times/day). Smoking status was determined by dividing the respondents into current smokers (smoking every day), former smokers (those who had not smoked for more than six months at the time of the study), and never smokers (the remaining respondents).
Statistical analysis
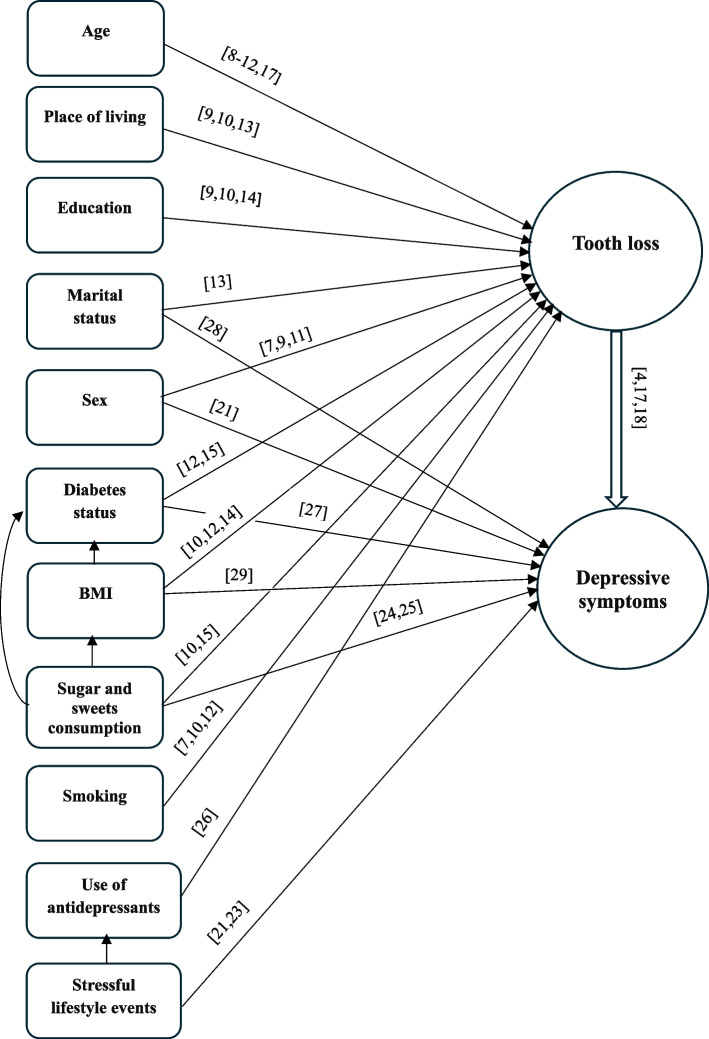
The data were analysed using STATISTICA 13.3 software (STATSOFT, PL). The results were considered significant at p ≤ 0.05. Counts and percentages were calculated for each categorical (qualitative) variable. The arithmetic mean (X) and standard deviation (SD) were calculated for the continuous variables, i.e., age, BMI, and sugar and sweets consumption. The internal consistency and reliability of the eight-question test used to assess depressive symptoms were calculated based on Cronbach’s alpha, which equaled 0.81 in this sample. Simple and multivariable logistic regression analyses were conducted to calculate odds ratios (ORs) and 95% confidence intervals (CIs). The number of lost teeth was an independent variable (ref. 0–4). Depressive symptoms were analysed according to three categories, with moderate (3–5 points) and severe (6–8 points) symptoms forming the model categories and no or mild (0–2 points) symptoms forming the reference group (ref.). Multivariable models were calculated adjusted for the following covariates: age and consumption of sugar and sweets (continuous variables) and categorical variables: sex (ref. women), place of residence (ref. city), education (ref. higher), marital status (ref. in a relationship), BMI (ref. 18.5–24.9 kg/m2), diabetes status (ref. no), stressful life events in the last year (ref. no), use of antidepressants (ref. no), and smoking (ref. never smokers). The goodness-of-fit model was assessed using the Akaike Information Criterion (AIC). The covariates were selected based on a review of the subject literature [7–15, 17, 18, 23–29] and a previous study [21] (Fig. 1).
Fig. 1.
Relationship between dependent and independent variables and confounders [7–15, 17, 18, 21, 23–29]
Results
Table 1 presents the sociodemographic and clinical data of the participants. The majority of the participants were women (63.89%), lived in a city, had secondary education, and were in a stable relationship. The mean BMI was 28.14 ± 4.65 kg/m2, and diabetes or elevated glucose was observed in more than 1/3 of the participants. In terms of the number of lost teeth, the largest group included individuals who lost 9 or more teeth (28.56%). The mean intensity of depressive symptoms was 2.22 ± 2.29 points. Only 2.91% of the participants used antidepressants regularly. Over 22% declared experiencing stressful life events in the last year. The mean total consumption of sugar, sweets, and sweetened beverages was 4.32 ± 3.60 times/day. Almost 1/5 of the participants were current smokers.
Table 1.
Sociodemographic and clinical characteristics of the respondents (N = 11,901)
| Variables | N (%) | ||
|---|---|---|---|
| Sociodemographic | Sex | Men | 3934 (33.1) |
| Women | 7967 (63.9) | ||
| Age (years) | X ± SD | 55.64 ± 5.3 | |
| Place of residence | City | 7414 (62.3) | |
| Village | 4487 (37.7) | ||
| Education | Higher | 3127 (26.3) | |
| Secondary | 5310 (44.6) | ||
| Primary or vocational | 3464 (29.1) | ||
| Marital status | In a relationship | 9441 (79.3) | |
| Single | 2460 (20.7) | ||
| Clinical and health | BMI (kg/m2) | X ± SD | 28.14 ± 4.65 |
| < 18.5 | 51 (0.5) | ||
| 18.5–24.9 | 3068 (28.2) | ||
| 25.0–29.9 | 5198 (43.2) | ||
| ≥ 30.0 | 3584 (28.2) | ||
| Diabetes status | No | 7941 (66.7) | |
| Yes | 3960 (33.3) | ||
| Number of lost teeth | 0–4 | 2908 (24.5) | |
| 5–8 | 2932 (24.6) | ||
| 9–27 | 3399 (28.6) | ||
| No natural teeth | 2662 (22.4) | ||
| Depressive symptoms | X ± SD (points) | 2.22 ± 2.29 | |
| None or mild (0–2 points) | 7469 (62.8) | ||
| Moderate (3–5 points) | 3038 (25.5) | ||
| Severe (6–8 points) | 1394 (11.7) | ||
| Use of antidepressants | No | 11555 (97.1) | |
| Yes | 346 (2.9) | ||
| Stressful life events in the last year | No | 9255 (77.8) | |
| Yes | 2646 (22.2) | ||
| Lifestyle | Sugar and sweets consumption (times/day) | X ± SD | 4.32 ± 3.60 |
| Smoking | Never | 5588 (47.0) | |
| Former | 3996 (33.6) | ||
| Current | 2317 (19.5) | ||
According to the unadjusted model, a greater number of lost teeth was associated with a greater likelihood of both moderate and severe depressive symptoms (Table 2). After adjusting for the confounding variables, the relationships between the number of lost teeth and depressive symptoms were weaker, but a total lack of natural teeth was still the factor that was most strongly connected with the likelihood of both moderate (p < 0.020) and severe (p < 0.001) symptoms (Table 3). A loss of more than 8 natural teeth was also significantly associated (p < 0.008) with the occurrence of severe depressive symptoms.
Table 2.
Simple multinomial logistic regression analysis of moderate and severe depressive symptoms in relation to the number of lost teeth
| Depressive symptoms | Number of lost teeth | OR (95% CI) | p |
|---|---|---|---|
| Moderate vs. none or mild | 0–4 | Ref | |
| 5–8 | 1.129 (0.999—1.276) | 0.051 | |
| 9–27 | 1.346 (1.197—1.513) | < 0.001 | |
| No natural teeth | 1.474 (1.301—1.669) | < 0.001 | |
| Severe vs. none or mild | 0–4 | Ref | |
| 5–8 | 1.342 (1.116—1.613) | 0.002 | |
| 9–27 | 1.854 (1.562—2.202) | < 0.001 | |
| No natural teeth | 2.624 (2.207—3.120) | < 0.001 |
Akaike Information Criterion – AIC = 16,201.74
OR (95% CI) Odds Ratio and 95% Confidence Interval
Table 3.
Multivariable multinomial logistic regression analysis of depressive symptoms in relation to the number of lost teetha
| Depressive symptoms | Number of lost teeth | OR (95% CI) | p |
|---|---|---|---|
| Moderate vs. none or mild | 0–4 | Ref | |
| 5–8 | 1.052 (0.926—1.196) | 0.437 | |
| 9–27 | 1.132 (0.993—1.291) | 0.064 | |
| No natural teeth | 1.189 (1.028—1.376) | 0.020 | |
| Severe vs. none or mild | 0–4 | Ref | |
| 5–8 | 1.165 (0.951—1.426) | 0.141 | |
| 9–27 | 1.315 (1.075—1.609) | 0.008 | |
| No natural teeth | 1.846 (1.488—2.290) | < 0.001 |
Akaike Information Criterion – AIC = 15076.62
OR (95% CI) Odds Ratio and 95% Confidence Interval
aadjusted for sex, age, place of residence, education, marital status, BMI, diabetes status, use of antidepressants, stressful life events in the last year, sugar and sweets consumption, and smoking
Discussion
The obtained results confirmed the hypothesis about the existence of a positive relationship between the number of lost teeth and the occurrence of depressive symptoms among middle-aged adults. Furthermore, the results indicated that individuals with severe edentulism in particular should be assessed for depressive symptoms.
Most long-term studies have demonstrated that oral health problems and a lack of natural teeth may play a role in the development or worsening of depressive symptoms [30, 31]. In a study conducted in Brazil, individuals who experienced tooth loss over a six-year follow-up were at a greater risk of exhibiting depressive symptoms (adjusted prevalence ratio = 1.86; 95% CI: 1.01–3.53) [32]. In the Chilean population, individuals aged 38–74 years with fewer than 20 natural teeth were shown to have higher odds of incident depression at two- and four-year follow-ups [33]. The existence of positive relationships between tooth loss and the occurrence of depressive symptoms was also substantiated by the results of meta-analyses [34] and cross-sectional studies [35, 36]. Matsuyama et al. [36] reported that the effect of tooth loss on depression seemed to be greater in young adults. This phenomenon can be explained by the fact that individuals usually associate the loss of teeth with old age, and consequently, if the problem occurs at a young age, it may exacerbate depressive symptoms. Elderly individuals may treat tooth loss as a natural consequence of old age and adapt their daily life to it more easily than young individuals. The oral health of Poles has remained unsatisfactory for many years compared to that of other European countries, despite a recent trend toward improvement [12, 37, 38]. A total lack of natural teeth, reported by 22.37% of the participants in this study aged 40–64 years, was similar in prevalence to the global prevalence among individuals aged ≥ 45 years, which amounts to 22% [39]. As a result, all negative health outcomes of tooth loss can be expected to appear much earlier in the Polish population.
The mechanism of the relationship between the loss of natural teeth and depressive symptoms has not been investigated or explained in depth thus far. In their long-term study, Yamamoto et al. [30] observed that problems with smiling, laughing, and exposing teeth without embarrassment may cause individuals to isolate themselves and eat alone, which in turn exacerbates their depressive symptoms. Kusama et al. [18] also reported that the deterioration of oral function and orofacial appearance are the main factors contributing to the development of depression due to the loss of natural teeth. Sun et al. [40], based on research conducted among the Chinese population, concluded that dietary dissatisfaction was a contributing factor to the development of depressive symptoms following the loss of teeth. This occurs because food plays an important role in meetings with family and friends and is a medium for maintaining social contact, expressing friendships, and caring for family members. In middle-aged individuals, embarrassment and dissatisfaction with one’s own appearance due to loss of teeth also led to frustration and problems with satisfying activity on the job market, even when dentures were used [17], because removable, unstable dentures did not eliminate certain problems related to aesthetics and oral health. Researchers suggest that depressive symptoms may also be further exacerbated by inflammation of the nervous system resulting from a history of periodontitis or autonomic nerve imbalance caused by oral pain and discomfort [34]. Moreover, Wingfield et al. [41] noted that depression may be related to the state of the oral cavity microbiome. The authors described explicit changes in the composition and amount of specific bacterial taxa in the salivary microbiome in young adults with depression compared to a reference group of individuals without depression.
Limitations
The limitations of this study include, first and foremost, its cross-sectional design. Moreover, the persons who agreed to participate were volunteers. Thus, they may not be representative of the entire target population. This may result in a sampling bias and limit the generalisability of the results. It should be noted that the relationship between tooth loss and the occurrence of depressive symptoms may be bidirectional [34]. Analysis of depressive symptoms suggested that self-neglect, lowered mood, and lack of energy may cause individuals to neglect oral hygiene and proper eating habits, leading to health problems in the form of oral diseases and tooth loss [35]. Aldosari et al. [31] demonstrated that oral cavity disorders, including tooth loss, were more prevalent among individuals with severe internalization problems. Another limitation that should be mentioned is the potential effect of other factors that have not been included in this study on loss of teeth and the occurrence of depressive symptoms, such as oral hygiene habits [10, 12], number of visits to a dentist [10, 12], well-being [42] or overuse of alcohol [43]. Furthermore, the research tool did not allow for a clinical diagnosis of depression. However, the questionnaire used in this paper has been successfully applied for the assessment of depressive symptoms in other international studies [20]. Conversely, the strengths of this study were the large sample size, uniformity in terms of age, and large number of confounders included in the analysis.
As has been mentioned in the introduction, the prevalence of missing teeth and factors that determine it depend on economic development, access to dental care, social factors and lifestyle, which is why they may differ between countries and regions [8–15]. The obtained results indicated a need for improvement in dental care for middle-aged patients. However, the results may also be used in other European countries with similar living conditions, in particular, in East-Central European countries.
Conclusions
The results indicated that loss of natural teeth was positively related to the occurrence of depressive symptoms in middle-aged adults. Edentulism showed the strongest relationship with the likelihood of both moderate and severe depressive symptoms. The results of this study have indicated an urgent need to intensify stomatological prophylaxis, education and treatment for middle-aged individuals. Preventing the loss of teeth may potentially reduce the risk of depressive symptoms in this group. This will require the involvement of not only psychologists but also the entire medical community, including dentists, as well as the introduction of appropriate changes in the health care system in Poland aimed at increasing the real availability of dental services.
Better integration of mental and oral health prevention and treatment are recommended. Further longitudinal studies are required to establish the causal and temporal relationship between depressive symptoms and oral health status.
Acknowledgements
The data collection was supported by the Maria Sklodowska-Curie Institute of Oncology in Warsaw (Poland) and the Polish-Norwegian Foundation Research Fund.
Abbreviations
- PONS
POlish-Norwegian Study
- PURE
Prospective Urban and Rural Epidemiological Study
- X
Arithmetic mean
- SD
Standard deviation
- BMI
Body mass index
- OR
Odds ratio
- CI
Confidence interval
Authors’ contributions
MG-O contributed to the study conception and interpretation of the data and wrote the manuscript. EC performed the statistical analysis and revised the paper. ES contributed to the study conception and interpretation of the data and revised it critically for important intellectual content. All authors approved the final version of the manuscript prior to submission.
Funding
This study was supported by Jan Kochanowski University in Kielce, Poland (No 2024). The founders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee at the Cancer Center and Institute of Oncology in Warsaw (No. 69/2009/1/2011). Written informed consent was obtained from participants before the survey. The material analyses were approved by the Committee on Bioethics at the Faculty of Health Sciences, Jan Kochanowski University in Kielce (No. 45/2016).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2019;2022(9):137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 3.Joury E, Kisely S, Watt RG, Ahmed N, Morris AJ, Fortune F, Bhui K. Mental disorders and oral diseases: future research directions. J Dent Res. 2023;102:5–12. doi: 10.1177/00220345221120510. [DOI] [PubMed] [Google Scholar]
- 4.Skallevold HE, Rokaya N, Wongsirichat N, Rokaya D. Importance of oral health in mental health disorders: an updated review. J Oral Biol Craniofac Res. 2023;13:544–552. doi: 10.1016/j.jobcr.2023.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu C, Wang G, Hu Z, Wang S, Yan Q, Liu X. Burden of oral disorders, 1990–2019: estimates from the global burden of disease study 2019. Arch Med Sci. 2023;19:930–940. doi: 10.5114/aoms/165962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño CC, Kearns C, Benzian H, Allison P, Watt RG. Oral diseases: a global public health challenge. Lancet. 2019;394:249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 7.Costa FO, Lages EJ, Cota LO, Lorentz TC, Soares RV, Cortelli JR. Tooth loss in individuals under periodontal maintenance therapy: 5-year prospective study. J Periodontal Res. 2014;49:121–128. doi: 10.1111/jre.12087. [DOI] [PubMed] [Google Scholar]
- 8.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe tooth loss: a systematic review and meta-analysis. J Dent Res. 2014;93(7 Suppl):20S–S28. doi: 10.1177/0022034514537828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang L, Li J, Yang Z, Huang X, Zhong X, Huang Y, Liu B, Wu L, Huang S, Fan W. Analysis of epidemiological trends of and associated factors for tooth loss among 35- to 44-year-old adults in Guangdong, Southern China, 1995–2015: a population-based cross-sectional survey. BMC Oral Health. 2023;23:74. doi: 10.1186/s12903-023-02776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskiliene A, Kriaucioniene V, Siudikiene J, Petkeviciene J. Self-reported oral health, oral hygiene and associated factors in lithuanian adult population, 1994–2014. Int J Environ Res Public Health. 2020;17:5331. doi: 10.3390/ijerph17155331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooray U, Watt RG, Tsakos G, Heilmann A, Hariyama M, Yamamoto T, Kuruppuarachchige I, Kondo K, Osaka K, Aida J. Importance of socioeconomic factors in predicting tooth loss among older adults in Japan: evidence from a machine learning analysis. Soc Sci Med. 2021;291:114486. doi: 10.1016/j.socscimed.2021.114486. [DOI] [PubMed] [Google Scholar]
- 12.Gabiec K, Bagińska J, Łaguna W, Rodakowska E, Kamińska I, Stachurska Z, Dubatówka M, Kondraciuk M, Kamiński KA. Factors associated with tooth loss in general population of Bialystok, Poland. Int J Environ Res Public Health. 2022;19:2369. doi: 10.3390/ijerph19042369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tôrres LHDN, Hilgert JB, Hugo FN, Sousa MDLR, De Marchi RJ. Predictors of tooth loss in Brazilian older adults: an 8-year follow-up. Gerodontology. 2023;40:207–212. doi: 10.1111/ger.12634. [DOI] [PubMed] [Google Scholar]
- 14.Österberg T, Dey DK, Sundh V, Carlsson GE, Jansson JO, Mellstrom D. Edentulism associated with obesity: a study of four national surveys of 16416 Swedes aged 55–84 years. Acta Odontol Scand. 2010;68:360–367. doi: 10.3109/00016357.2010.514721. [DOI] [PubMed] [Google Scholar]
- 15.Wiener RC, Shen C, Findley PA, Sambamoorthi U, Tan X. The association between diabetes mellitus, sugar-sweetened beverages, and tooth loss in adults: evidence from 18 states. J Am Dent Assoc. 2017;148:500–9.e4. doi: 10.1016/j.adaj.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu KJ, Lee HE, Wu YM, Lan SJ, Huang ST, Yen YY. Masticatory factors as predictors of oral health-related quality of life among elderly people in Kaohsiung City. Taiwan Qual Life Res. 2014;23:1395–1405. doi: 10.1007/s11136-013-0574-7. [DOI] [PubMed] [Google Scholar]
- 17.Cortez GFP, Barbosa GZ, Tôrres LHDN, Unfer B. Reasons for and consequences of tooth loss in adults and elderly people in Brazil: a qualitative metasynthesis. Cien Saude Colet. 2023;28:1413–1424. doi: 10.1590/1413-81232023285.01632022. [DOI] [PubMed] [Google Scholar]
- 18.Kusama T, Kiuchi S, Umehara N, Kondo K, Osaka K, Aida J. The deterioration of oral function and orofacial appearance mediated the relationship between tooth loss and depression among community-dwelling older adults: a JAGES cohort study using causal mediation analysis. J Affect Disord. 2021;286:174–179. doi: 10.1016/j.jad.2021.02.071. [DOI] [PubMed] [Google Scholar]
- 19.Zatonski WA, Manczuk M. Kielce PONS Team: POlish-Norwegian Study (PONS): Research on chronic noncommunicable diseases in European high risk countries – study design. Ann Agric Environ Med. 2011;18:203–206. [PubMed] [Google Scholar]
- 20.Koon T, Chow CK, Vaz M, Rangarajan S, Yusuf S. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal infl uences on chronic noncommunicable diseases in low-, middle-, and high income countries. Am Heart J. 2009;158:1–7. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Głuszek-Osuch M, Cieśla E, Suliga E. Relationship between multimorbidity and sociodemographic factors, depressive symptoms, and lifestyle in middle-aged adults. Medical Studies/Studia Medyczne. 2023;39:182–191. doi: 10.5114/ms.2023.129047. [DOI] [Google Scholar]
- 22.Dehghan M, Ilow R, Zatonska K, Szuba A, Zhang X, Mente A, Regulska-Ilow B. Development, reproducibility and validity of the food frequency questionnaire in the Poland arm of the Prospective Urban and Rural Epidemiological (PURE) study. J Hum Nutr Diet. 2012;25:225–232. doi: 10.1111/j.1365-277X.2012.01240.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson LR, Monden CWS, Bukodi E. Stressful life events, differential vulnerability, and depressive symptoms: critique and new evidence. J Health Soc Behav. 2022;63:283–300. doi: 10.1177/00221465211055993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werneck AO, Schuch FB, Stubbs B, Oyeyemi AL, Szwarcwald CL, Vancampfort D, Silva DR. Independent and combined associations of sugar-sweetened beverage consumption, TV viewing, and physical activity with severe depressive symptoms among 59,402 adults. Braz J Psychiatry. 2021;43:574–583. doi: 10.1590/1516-4446-2020-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Ansari W, Adetunji H, Oskrochi R. Food and mental health: relationship between food and perceived stress and depressive symptoms among university students in the United Kingdom. Cent Eur J Public Health. 2014;22:90–97. doi: 10.21101/cejph.a3941. [DOI] [PubMed] [Google Scholar]
- 26.Kisely S, Sawyer E, Siskind D, Lalloo R. The oral health of people with anxiety and depressive disorders - a systematic review and meta-analysis. J Affect Disord. 2016;200:119–132. doi: 10.1016/j.jad.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Maina JG, Balkhiyarova Z, Nouwen A, Pupko I, Ulrich A, Boissel M, Bonnefond A, Froguel P, Khamis A, Prokopenko I, Kaakinen M. Bidirectional mendelian randomization and multiphenotype gwas show causality and shared pathophysiology between depression and type 2 diabetes. Diabetes Care. 2023;46:1707–1714. doi: 10.2337/dc22-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recksiedler C, Stawski RS. Marital transitions and depressive symptoms among older adults: examining educational differences. Gerontology. 2019;65:407–418. doi: 10.1159/000493681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulugeta A, Zhou A, Power C, Hyppönen E. Obesity and depressive symptoms in mid-life: a population-based cohort study. BMC Psychiatry. 2018;18:297. doi: 10.1186/s12888-018-1877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Aida J, Kondo K, Fuchida S, Tani Y, Saito M, Sasaki Y. Oral health and incident depressive symptoms: JAGES project longitudinal study in older Japanese. J Am Geriatr Soc. 2017;65:1079–1084. doi: 10.1111/jgs.14777. [DOI] [PubMed] [Google Scholar]
- 31.Aldosari M, Helmi M, Kennedy EN, Badamia R, Odani S, Agaku I, Vardavas C. Depression, periodontitis, caries and missing teeth in the USA, NHANES 2009–2014. Fam Med Community Health. 2020;8:e000583. doi: 10.1136/fmch-2020-000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunrath I, Silva AER. Oral health and depressive symptoms among older adults: longitudinal study. Aging Ment Health. 2021;25:2265–2271. doi: 10.1080/13607863.2020.1855104. [DOI] [PubMed] [Google Scholar]
- 33.Ortuño D, Martínez C, Caneo C. Association between number of remaining teeth and incident depression in a rural Chilean cohort. BMC Oral Health. 2023;23:633. doi: 10.1186/s12903-023-03374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cademartori MG, Gastal MT, Nascimento GG, Demarco FF, Corrêa MB. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta-analysis. Clin Oral Investig. 2018;22:2685–2702. doi: 10.1007/s00784-018-2611-y. [DOI] [PubMed] [Google Scholar]
- 35.Skośkiewicz-Malinowska K, Malicka B, Ziętek M, Kaczmarek U. Oral health condition and occurrence of depression in elderly individuals. Medicine (Baltimore) 2018;97:e12490. doi: 10.1097/MD.0000000000012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuyama Y, Jürges H, Dewey M, Listl S. Causal effect of tooth loss on depression: evidence from a population-wide natural experiment in the USA. Epidemiol Psychiatr Sci. 2021;30:e38. doi: 10.1017/S2045796021000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehr K, Olszanecka-Glinianowicz M, Chudek J, Szybalska A, Mossakowska M, Zejda J, Wieczorowska-Tobis K, Grodzicki T, Piotrowski P. Dental status in the Polish senior population and its correlates—Results of the national survey PolSenior. Gerodontology. 2018;35:398–406. doi: 10.1111/ger.12364. [DOI] [PubMed] [Google Scholar]
- 38.Raport 2021; Olczak-Kowalczyk, D. Choroba Próchnicowa i Stan Tkanek Przyzebia Populacji Polskiej. Podsumowanie Wyników Badan z Lat 2016–2019; Ministerstwo Zdrowia: Warszawa, Polska, 2021 [Caries Disease and the condition of periodontal tissues in the Polish population. Summary of research results from 2016–2019; Ministry of Health: Warsaw, Poland, 2021.]. Available online: https://www.gov.pl/attachment/e837445b-41ef-4b49-b261-d21d869e0018 (accessed on 4 September 2023) ISBN 978–83–7637–555–7. (In Polish)
- 39.Borg-Bartolo R, Roccuzzo A, Molinero-Mourelle P, Schimmel M, Gambetta-Tessini K, Chaurasia A, Koca-Ünsal RB, Tennert C, Giacaman R, Campus G. Global prevalence of edentulism and dental caries in middle-aged and elderly persons: a systematic review and meta-analysis. J Dent. 2022;127:104335. doi: 10.1016/j.jdent.2022.104335. [DOI] [PubMed] [Google Scholar]
- 40.Sun Q, Wang Y, Chang Q. Oral health and depressive symptoms among older adults in urban China: a moderated mediation model analysis. BMC Geriatr. 2022;22:829. doi: 10.1186/s12877-022-03542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wingfield B, Lapsley C, McDowell A, Miliotis G, McLafferty M, O'Neill SM, Coleman S, McGinnity TM, Bjourson AJ, Murray EK. Variations in the oral microbiome are associated with depression in young adults. Sci Rep. 2021;11:15009. doi: 10.1038/s41598-021-94498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhammad T, Srivastava S. Tooth loss and associated self-rated health and psychological and subjective wellbeing among community-dwelling older adults: a cross-sectional study in India. BMC Public Health. 2022;22:7. doi: 10.1186/s12889-021-12457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grocock R. The relevance of alcohol to dental practice. Br Dent J. 2018;223:895–899. doi: 10.1038/sj.bdj.2017.997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.