Abstract
Inflammatory myofibroblastic tumors (IMTs), which involve the proliferation of fibroblastic-myofibroblastic cells mixed with inflammatory infiltrates, are exceedingly rare in the extremities. There are no reported IMTs involving the sciatic nerve. This type of involvement may cause entrapment of the sciatic nerve, whose symptoms may mimic lumbar disc herniation (LDH), especially when it occurs in patients with lumbar degenerative disc disease. We describe the case of a 40-year-old male with lumbar degenerative disc disease accompanied by IMT involving the sciatic nerve whose symptoms mimicked LDH and posed a diagnostic challenge. We showed the course of the disease as well as the systematic imaging manifestations of IMTs involving the sciatic nerve and discussed their therapeutic management.
Keywords: Inflammatory myofibroblastic tumor, Sciatic nerve, Lumbar disc herniation
Introduction
Lumbar disc herniation (LDH) is a common cause of low back or radicular pain [1]. A majority of the underlying mechanisms, such as mechanical compression of the nerve root or inflammatory stimulation of the nerve root, have been proposed to explain the etiopathogenesis of pain [2, 3]. Among the components that contribute to pain, functional disorders involving immune cells, such as macrophages or T cells, can play vital roles in this process [4-7]. Studies have demonstrated that abnormal macrophage infiltration and activation, an increased proportion of Th17 cells and an increased concentration of interleukin-17 (IL-17) are causally related to pain in patients with intervertebral disc degeneration [4, 5]. However, mechanical compression derived from peripheral nerves, such as the sciatic nerve, can also cause radicular pain, which should be considered part of the full workup. We present an interesting case in which a man was found to have an inflammatory myofibroblastic tumor (IMT) entrapping his sciatic nerve. His symptoms mimicked LDH and posed a diagnostic challenge.
Case Report
Investigations
A 40-year-old man whose job was a bus driver was referred to our clinic for orthopedic evaluation. He complained of low back pain with the pain radiating to his right leg. His symptoms worsened during the past 2 months. He was diagnosed with LDH at another hospital. Many conservative treatments, including physical therapy and a root block at the outlet of the piriformis, were used, but these treatments were ineffective, and the patient’s symptoms continued to progress. On neurologic examination, his right big toe dorsiflexion weakness (motor power: grade 4) and sensory impairment were noted in the L5 dermatome, whose symptoms mimicked LDH. His Lasegue sign in the right leg was positive, but bilateral Babinski sign was negative. His laboratory studies on admission were unremarkable. Magnetic resonance imaging (MRI) revealed a mildly herniated lumbar disc at L4-L5 without significant compression of the nerve roots of L5 or deformation of the spinal cord (Fig. 1a, b). Until now, we found that the diagnosis of LDH could not completely explain his progressive symptoms.
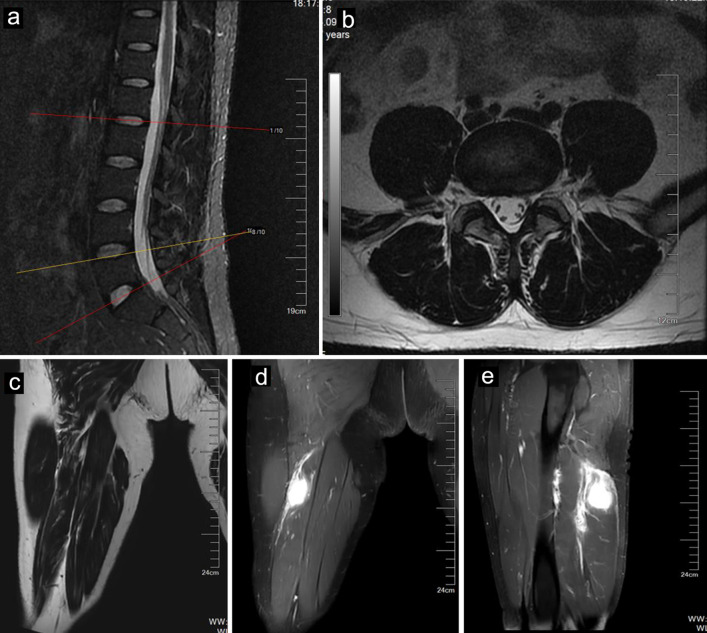
Figure 1.
MR images of a 40-year-old male with IMT. Sagittal (a) and axial (b) MR images of the lumbar spine showing a mildly herniated disc at the L4-L5 level. The tumor (arrows) showed mild hyperintensity on T1-weighted imaging (c) and hyperintensity on fat-suppressed T2-weighted imaging (d). The tumor (arrows) showed enhancement after contrast agent injection (e). IMT: inflammatory myofibroblastic tumor; MR: magnetic resonance.
Diagnosis and treatment
Therefore, his symptoms prompted us to conduct supplementary physical exams, which revealed a positive Tinel’s sign in the posterior right thigh. We thus conducted further evaluations, such as computed tomography (CT) and MRI of the right thigh, which revealed a mass along the right sciatic nerve (Fig. 1c, d). Finally, surgery was arranged for the patient to remove the mass. During surgery, the mass was found to originate from the sciatic nerve (Fig. 2a). A section of the mass was sent for pathology (Fig. 2b), and the tumor was negative for smooth muscle actin (SMA), S-100 and CD34 (Fig. 2d-f). The mass was histologically confirmed to be an IMT, which is rarely present in the peripheral nerves.
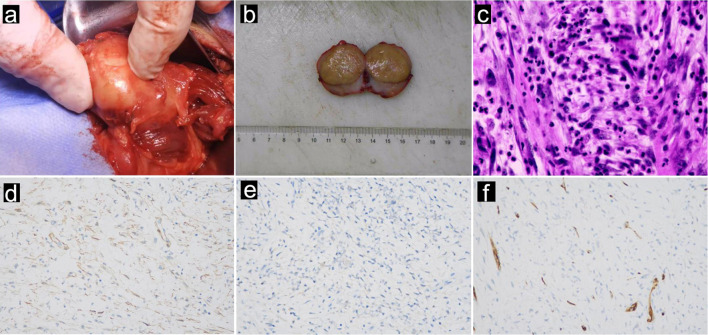
Figure 2.
Intraoperative and pathological images of a 40-year-old male with IMT derived from the sciatic nerve. (a) Intraoperative photograph, (b) macroscopic photograph, and (c) microscopic examination revealing IMTs. The tumor cells consisted of fusiform myofibroblasts, infiltrated plasma cells, lymphocytes, and eosinophils. The tumor cells did not express SMA (d), original magnification × 400; S100 (e), original magnification × 400, or CD34 (f), original magnification × 400, confirming the diagnosis of IMT. IMT: inflammatory myofibroblastic tumor.
Follow-up and outcomes
After surgery, the radiating pain in his right leg significantly improved. The patient remained in good condition and free of disease at the 2-year follow-up.
Discussion
LDH is one of the most common causes of low back pain, and its pathogenesis includes various factors, such as stress, nutritional status, strain, and trauma, as well as hereditary, developmental, and degenerative factors [8, 9]. In our case, the patient progressed to radiculopathy, and her symptoms and physical findings mimicked LDH. He had evident pathogenesis factors because he had to sit for a long time to drive, and his intervertebral disc may have suffered from continuous stress and strain. However, there were no obvious pathogenic findings in the imaging test of his lumbar spine, which is a diagnostic challenge. Our supplementary physical exams and imaging studies prompted us to determine the entrapment of the sciatic nerve by IMTs, which highlighted the importance of physical exams. However, IMTs derived from peripheral nerves have rarely been reported.
Proliferative fibroblastic-myofibroblastic cells admixed with inflammatory infiltrates make up IMTs [10]. In line with myofibroblastic differentiation, most IMTs exhibit SMA, desmin, and cytokeratin activity and negative immunostaining for CD34 and S-100 [11]. Studies have shown that approximately 86% of IMTs express SMA, while 70% of IMTs with negative SMA expression show a predominance of plasma cells in the stroma [12, 13]. In our patient, the tumor was composed of fascicularis and fusiform cells mixed with lymphocytes, neutrophils, and plasma cells (Fig. 2c). Usually, these tumors exhibit indolent behavior; however, they still have the potential for local aggressiveness, recurrence, metastasis, and malignant transformation [11]. Regarding the treatment of IMTs, studies have advocated extensive surgery with complete excision of the tumors, which can achieve an approximately 80% response rate. However, for IMTs that are not amenable to resection, such as intracranial IMTs or IMTs involving the central nervous system, steroids and radiotherapy should be applied [14]. Steroid administration can improve tumors, while radiotherapy (25 to 30 Gy) helps to induce remission and taper the use of steroids [14, 15].
Anaplastic lymphoma kinase (ALK) is a vital protein linked to cancer growth, and its activation can trigger a variety of signaling pathways, such as Janus kinase/signal transducers and activators of transcription (JAK/STAT), rat sarcoma/mitogen-activated protein kinase (RAS/MAPK), and phosphatidylinositol 3-kinase/Ak strain transforming (PI3K/AKT) pathways [16]. Approximately 56% of IMTs tend to have immunohistochemical reactivity with ALK [17]. Therefore, ALK inhibition has emerged as a powerful tool for the treatment of IMTs. According to a pediatric oncology group study on targeting ALK inhibition, 36% of patients with unresectable IMTs achieved a complete response, 50% of patients with unresectable IMTs achieved a partial response, and 14% of patients with unresectable IMTs remained stable [18]. Therefore, the outcome would be better for potentially malignant ALK-positive IMTs treated with ALK inhibition after surgical resection [19]. In our patient, we performed total resection of the tumor. However, our pathology department did not investigate the expression status of ALK. The patient remained free of disease during our last 2 years of follow-up.
IMTs, mesenchymal neoplasms with clonal proliferation of myofibroblasts, were originally described in the lung and tend to most commonly occur in children and young adults [10]. According to a retrospective case study, 64% of IMTs occur in the abdomen, retroperitoneum, or pelvis, 22% of IMTs are found in the lung, 8% of IMTs are found in the head and neck, and only 5% of IMTs occur in the extremities; the corresponding symptoms include fever, weight loss, malaise, and laboratory abnormalities (anemia, thrombocytosis, elevated erythrocyte sedimentation rate) [17]. However, another retrospective study of IMTs revealed that most IMTs were intracranial or orbital, and headache became the most common symptom, although proptosis and blurred vision can also occur [14]. Therefore, IMTs can occur in many parts of the body; however, the most common sites involved in IMTs are still unclear. Our case was unique in that the tumor was located in the sciatic nerve of a patient with symptoms of LDH. Furthermore, understanding the imaging features of the disease may facilitate quick diagnosis [20, 21]. Therefore, our case was also unique in that we showed the systematic imaging manifestations of IMTs involving the sciatic nerve. Therefore, IMTs involving the sciatic nerve may become an unexpected cause of radiating pain, which may complicate the diagnosis of LDH. As such, IMTs should be included in the differential diagnosis of LDH.
Acknowledgments
None to declare.
Funding Statement
This work was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2023QH517; ZR2022QH252); Clinical Medical Science and Technology Innovation Program of Jinan Science and Technology Bureau (grant no. 202328059); Foundation of Research Hospital Association of Shandong Province (grant no. 2022016); Foundation of Scientific Research Incubation of Shandong First Medical University and Shandong Academy of Medical Sciences (grant no. 202201-086); Taishan Scholar Program of Shandong Province (No.tsqn202312357); National Science Foundation of China (No.81902188).
Conflict of Interest
The authors declared no conflict of interest.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
CJC and JFY participated in the drafting, writing, and revising of the manuscript. XZ, JWZ, HXZ, MC and DYP participated in the data selection and analysis. CJC and DYP contributed to the study concept and acquired and analyzed the data. All authors contributed to the drafting of the manuscript and figure preparation.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Chen C, Ma Q, Qi Y. et al. Osteolytic schwannoma in an older patient with lumbar degenerative disk disease: a case report. HSS J. 2023 doi: 10.1177/15563316231200862. [DOI] [Google Scholar]
- 2.Li XC, Luo SJ, Fan W, Jiang C, Wang W, Chen JH, Chen YL. et al. Influence of macrophage polarization in herniated nucleus pulposus tissue on clinical efficacy after lumbar discectomy. JOR Spine. 2023;6(2):e1249. doi: 10.1002/jsp2.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Wang S, Chen J, Liu X, Zhang M, Wang X, Xu W. et al. Escin suppresses HMGB1-induced overexpression of aquaporin-1 and increased permeability in endothelial cells. FEBS Open Bio. 2019;9(5):891–900. doi: 10.1002/2211-5463.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu X, Chen L, Jiang C, Cao K, Gao Z, Wang Y. Microglia and macrophages contribute to the development and maintenance of sciatica in lumbar disc herniation. Pain. 2023;164(2):362–374. doi: 10.1097/j.pain.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Nie L, Guo YJ, Han LX, Wang X, Zhao H, Han YG. et al. Th17 cell frequency and IL-17 concentration correlate with pre- and postoperative pain sensation in patients with intervertebral disk degeneration. Orthopedics. 2014;37(7):e685–e691. doi: 10.3928/01477447-20140626-62. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Zhao X, Luo Y, Li B, Li Q, Zhao C, Huang Y. et al. Imbalanced T-cell subsets may facilitate the occurrence of osteonecrosis of the femoral head. J Inflamm Res. 2022;15:4159–4169. doi: 10.2147/JIR.S367214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Fu L, Luo Y, Zeng W, Qi X, Wei Y, Chen L. et al. Engineered exosome-functionalized extracellular matrix-mimicking hydrogel for promoting bone repair in glucocorticoid-induced osteonecrosis of the femoral head. ACS Appl Mater Interfaces. 2023;15(24):28891–28906. doi: 10.1021/acsami.3c01539. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Gao X, Li H. et al. Probable lumbar acute noncompressive nucleus pulposus extrusion: a case report of acute pain caused by a hydrated nucleus pulposus and review of the literature. Emerg Crit Care Med. 2022;2(2):94–100. doi: 10.1097/ec9.0000000000000008. [DOI] [Google Scholar]
- 9.Chen C, Gao X, Li H, Pan X, Wang S. Intravertebral insertion of interbody fusion cage via transpedicular approach for the treatment of stage III Kummell disease: a technical note and case presentation. Br J Neurosurg. 2023;37(6):1909–1914. doi: 10.1080/02688697.2021.1892590. [DOI] [PubMed] [Google Scholar]
- 10.Gurzu S, Bara T, Jung I. Inflammatory myofibroblastic tumor of the colon. J Clin Oncol. 2013;31(10):e155–e158. doi: 10.1200/JCO.2012.42.8961. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Zhao X, Zhao Z, Lu P, Jin F, Li G. Malignant inflammatory myofibroblastic tumor of the prostate. J Clin Oncol. 2013;31(10):e144–e147. doi: 10.1200/JCO.2012.44.4851. [DOI] [PubMed] [Google Scholar]
- 12.Makhlouf HR, Sobin LH. Inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the gastrointestinal tract: how closely are they related to inflammatory fibroid polyps? Hum Pathol. 2002;33(3):307–315. doi: 10.1053/hupa.2002.32213. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim MMA. Role of myofibroblasts in oral plasma cell granuloma: immunohistochemical evaluation of alpha-SMA and ALK in a retrospective study of 30 cases. J Stomatol Oral Maxillofac Surg. 2023;124(6S):101595. doi: 10.1016/j.jormas.2023.101595. [DOI] [PubMed] [Google Scholar]
- 14.Nishadham V, Rao S, Saravanan A, Kulanthaivelu K, Vengalil S, Venkatappa HA, Valasani RK. et al. Inflammatory myofibroblastic tumors: a short series with an emphasis on the diagnostic and therapeutic challenges. Clin Neuropathol. 2023;42(3):100–111. doi: 10.5414/NP301540. [DOI] [PubMed] [Google Scholar]
- 15.Mahadev DS, Praveen NVS, Suryadevara A, Naga Kishore MG. Inflammatory myofibroblastic disease of right petrous apex: a rare case with review of literature. J Cancer Res Ther. 2024 doi: 10.4103/jcrt.JCRT_1451_20. [DOI] [PubMed] [Google Scholar]
- 16.Mousa DV, Mavrovounis G, Argyropoulos D, Stranjalis G, Kalamatianos T. Anaplastic lymphoma kinase (ALK) in posterior cranial fossa tumors: a scoping review of diagnostic, prognostic, and therapeutic perspectives. Cancers (Basel) 2024;16(3):650. doi: 10.3390/cancers16030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31(4):509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 18.Mosse YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, Adamson P. et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A Children’ Oncology Group Study. J Clin Oncol. 2017;35(28):3215–3221. doi: 10.1200/JCO.2017.73.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig E, Wiltsie LM, Beaupin LK, Baig A, Kozielski R, Rothstein DH, Li V. et al. Anaplastic lymphoma kinase inhibitor therapy in the treatment of inflammatory myofibroblastic tumors in pediatric patients: case reports and literature review. J Pediatr Surg. 2021;56(12):2364–2371. doi: 10.1016/j.jpedsurg.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen CJ, Zhao X, Zhao JW, Ma XJ, Xu WH, Qi YB, Li JK. et al. Osteoblastic bone reaction developing during treatment with sintilimab and bevacizumab in a patient with KRAS(G12V)-mutant lung adenocarcinoma. World J Oncol. 2023;14(6):580–583. doi: 10.14740/wjon1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C, Ren Y, Qi Y, Zhang G, Hu H, Zhang K, Wu Y. et al. Congenital lumbosacral malformations with low-grade neuroepithelial tumor causing progressive postpoliomyelitis paralytic limb. Ann Neurol. 2023;94(1):160–162. doi: 10.1002/ana.26667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.