Abstract
Background:
Almost 10% of women in reproductive age are diagnosed with ovarian endometriomas and can experience symptoms and infertility disorders. Ovarian endometriomas can be treated with medical or surgical therapy.
Objective:
To assess whether long-term therapy with dienogest or oral cyclic estrogen-progestogens is effective in reducing the size of ovarian endometriomas, alleviating associated symptoms, and reducing the requirement for surgery.
Design:
Prospective non-interventional cohort study.
Methods:
We enrolled childbearing women diagnosed with ovarian endometriomas. We collected demographic, clinical, and surgical data, including the evaluation of ovarian endometrioma-associated symptoms and pain using the visual analog scale. We grouped the women according to treatment regimen into dienogest, estrogen-progestogens, and no-treatment. Patient’s assessment was performed at baseline and after 12 months evaluating the largest ovarian endometrioma diameter (in millimeters) and the associated symptoms. Furthermore, we analyzed the impact of hormonal treatment in a sub-group of women fulfilling at baseline the criteria for a first-line surgical approach (ovarian endometrioma > 30 mm with visual analog scale > 8 or ovarian endometrioma > 40 mm before assisted reproductive treatments or any ovarian endometrioma(s) > 60 mm).
Results:
We enrolled 142 patients: 62, 38, and 42 in dienogest, estrogen-progestogens, and no-treatment groups, respectively. No significant differences were found regarding baseline characteristics. After 12 months, the mean largest ovarian endometrioma diameter increased in the no-treatment group (31.1 versus 33.8; p < 0.01), while a significant reduction was registered in the dienogest (35.1 versus 25.8; p < 0.01) and estrogen-progestogens (28.4 versus 16.7; p < 0.01) groups; no significant difference in ovarian endometrioma diameter reduction between these two latter groups was noted (p = 0.18). Ovarian endometrioma-associated symptoms and pain improved in dienogest and estrogen-progestogens groups, with a significantly greater effect for dienogest than for estrogen-progestogens for dysmenorrhea (74% versus 59%; p < 0.01). In the sub-group of women eligible for first-line surgery at baseline, long-term treatment with dienogest and estrogen-progestogens reduced surgical eligibility by 30%.
Conclusions:
Decreased mean largest ovarian endometriomas’diameter after 12 months and reduction of the need for surgical treatment by 30% were observed in dienogest and estrogen-progestogens groups. Long-term treatment with dienogest had a greater effect in alleviating dysmenorrhea and pain.
Keywords: dienogest, endometrioma, estrogen-progestogens, minimally invasive surgery
Introduction
Endometriosis is a benign, chronic inflammatory condition characterized by endometrial tissue outside the uterus, and the etiology is probably multifactorial. 1 Endometriosis often causes pain, dysmenorrhea, dyspareunia, non-cyclical pelvic pain, and infertility disorders. 2 Almost 10% of women in reproductive age are diagnosed with ovarian endometriomas (OMAs), that is, localization of endometriosis in the ovaries. 3 OMAs diagnosis is based on ultrasound assessment, typically using a transvaginal approach, with a good mean specificity and sensitivity, both nearly 95%. 4
OMAs can be treated with medical or surgical therapy, and in-vitro fertilization (IVF) can be used in case of infertility.2,5,6 Medical treatment for symptomatic OMAs has been suggested to control pain, and to prevent recurrences, through the suppression of endogenous estrogen production.7,8 Surgical removal of OMAs can improve endometriosis-associated pain and accessibility of the follicles in the case of IVF procedures, even though there is a negative impact on ovarian reserve and has a recurrence rate near to 10% per year.9 –12 Of note, a second surgery for recurrent OMAs is associated with a greater loss of ovarian tissue and reserve. 13 The association of medical treatment and surgery can be used to further control symptoms and pain and to prevent recurrence. 14 The choice of medical treatment depends on the possibility of a long-term use with a good compliance and few side effects, 15 using usually progestogens or estrogens-progestogens. 2 Some authors reported a progressive decrease in the size of the OMAs in women taking dienogest 2 mg daily for at least 6 months,16 –18 while others analyzed the impact of estrogen-progestogens therapy. 19 Other relevant literature aims to compare the effect of a single progestin with that of estrogen-progestogens pills in the treatment of patients with OMAs. 20 In fact, the use of dienogest without estrogens can have a more beneficial effect on OMAs than estrogen-progestogens, 18 with a potential role in reducing the need of surgery. 21
The objective of this longitudinal observational study is to assess the impact of first-line medical therapy with dienogest or other cyclic estrogen-progestogens on the size of OMAs and related symptoms. Furthermore, we explored the eventual effects of the aforementioned medical treatments, in reducing the need for surgical approach.
Material and methods
Study design and setting
We conducted a prospective non-interventional cohort study in two tertiary academic hospitals (University of Brescia, Brescia, and University of Insubria, Varese), enrolling childbearing women, aged at least 18 years, diagnosed with OMA(s) who were referred to the endometriosis outpatient clinic at each participating center, from January 2022 to June 2022. The diagnosis of OMA was performed by transvaginal ultrasound (TV-US) scans based on pattern recognition carried out by GB, EBG, CC, DOR, EG, and JC. At least two TV-US assessments were conducted on each woman by a different operator, to guarantee an inter-operator agreement in the pattern recognition; furthermore, in each TV-US assessment, the measurements of the OMAs were recorded and finally used to calculate the means used in this study. In case of inter-operator disagreement, the woman was excluded from the study. OMA is represented by a regular margins mass, with ground glass echogenicity, one to four cyst locules, and no papillation with detectable blood flow on color or power Doppler.22 –24 We included women aged 18 years or older, with or without desire for pregnancy, regardless of the parity, with one or more OMAs measuring at least 20 mm. Exclusion criteria were premenarchal and menopausal status and a history of previous treatment with progestogens or estrogen-progestogens or surgery for endometriosis; we have also excluded patients that underwent surgery as first-line treatment.
We collected demographic (including age, body mass index (BMI), previous surgery, and nulligravida rates) and clinical (such as largest mean and median OMA diameter, bilaterality of the OMAs, type of hormonal treatment if used, the need for subsequent surgery) details, including the evaluation of OMA-associated symptoms and pain using the visual analog scale (VAS) at baseline, corresponding to the first approach to the patient. Investigated symptoms were dysmenorrhea, dyspareunia, and chronic pelvic pain (CPP).
Clinical management, using oral dienogest (2 mg/die), others using oral estrogen-progestogens or no-treatment, was chosen before and independently from the study recruitment, mainly depending on patient’s preference or choice. Dienogest was administered daily without any drug-free interval, and oral EPs were instead administrated cyclically. A TV-US assessment was performed at baseline (before starting hormonal treatment) and after 12 months to evaluate the largest OMA diameter (in millimeters); furthermore, we reassessed and analyzed the associated symptoms and pain using VAS, respectively, at baseline and after 12 months. In case of bilateral OMAs, we considered the largest diameter to assess the effect of the treatment. In case of refusal by the patient to take hormonal treatment, she was however enrolled in the study and undertook the same clinical assessment at baseline and after 12 months.
Based on treatment modalities, we grouped the population in three clinical management groups as follows: dienogest (D), estrogen-progestogens (EPs), and no-treatment (NoT) group.
The primary aim of the study was to demonstrate a reduction of the largest OMA diameter in each treatment group (D and EPs) after 12 months when compared to the women that did not receive any treatment (NoT group).
The secondary aim of the study is to investigate the magnitude of the OMA-related symptoms before and after treatment and their eventual reduction in the three groups.
We have also planned a post hoc analysis about the impact of hormonal treatment in a sub-group of women eligible at baseline for surgery fulfilling well-known criteria (OMA > 30 mm with VAS > 8 or OMA > 40 mm before assisted reproductive treatments or any OMAs > 60 mm 23 ), that refused to undergo surgery as first-line treatment.
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines to report our study as recommended for observational studies. 25
Sample size
In a sample of 25 consecutive historical women that attended our clinic, we found a mean and median OMA largest diameter of 35 mm (SD 9 mm), and hence, we estimated a decrease of 25% of the diameter as a comparison outcome of interest after 12 months, between each treatment groups (D and EPs) and the NoT group. The probability was 85% that the study will detect a difference at a two-sided 0.05 significance level, if the true difference between each treatment group was at least 25%, with a total of 85 women enrolled with a ratio 2:2:1, namely, at least 34, 34, and 17 women, respectively, for D, EPs, and NoT. We planned a different sample size for the last group, using the aforementioned ratio, because we presumed that most of the women would be keen to undertake the proposed hormonal treatment.
Statistical analysis
Descriptive statistics were reported as mean with standard deviation (SD) and median with interquartile range (IQR) for continuous variables, while categorical variables were presented as numbers and percentages. To compare data between baseline and the 12-month follow-up, appropriate statistical tests were utilized. Continuous variables were analyzed using either the independent samples t-test, one-way repeated-measures analysis of variance, or the Wilcoxon test, depending on the data distribution. Categorical variables were assessed using either the Pearson chi-square test or Fisher’s exact test. All statistical analyses were performed using GraphPad Prism (version 9.5.1 (528) for iMac). Statistical significance was considered for p < 0.05.
Results
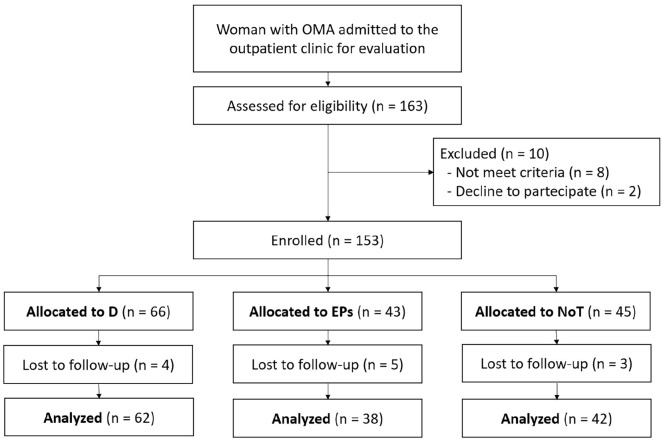
During the study period, we enrolled 153 women diagnosed with OMAs referred to our clinics, and we finally analyzed 142 of them (11 patients were lost to follow-up) during the follow-up (Figure 1). Mean age was 36.3 (SD 8.4) years with a mean BMI 22.6 (SD 1.9) and a mean OMA diameter equal to 30 mm (SD 11.6); bilateral OMAs was seen in 47 patients (33.3%). According to the study groups, we enrolled 62, 38, and 42 women into the D, EPs, and NoT groups, respectively. At baseline, the groups were homogeneous for medical history, BMI, mean and median largest OMA diameter, and previous non-gynecological surgeries (Table 1). Bilateral OMAs were seen in 28 (45.2%), 10 (27%), and 9 (21%) patients, respectively, in D, EPS, and NoT groups (p = 0.026). At baseline, we failed to notice any significant difference in the magnitude of referred symptoms (Table 2).
Figure 1.
Study flowchart.
OMA: ovarian endometrioma; D: dienogest; EPs: estro-progestogens; NoT: no treatment.
Table 1.
Characteristics at baseline for all patients and for dienogest (D), estrogen-progestogens (EPs), and no-treatment (NoT) groups.
| All patients (n = 142) | D (n = 62) | EPs (n = 38) | NoT (n = 42) | p-Value | |
|---|---|---|---|---|---|
| Age | 35.5 (9.8) | 35 (9.7) | 35.8 (7.8) | 37.9 (7.7) | 0.09 |
| BMI | 22.5 (2.1) | 22.3 (2.7) | 22.8 (1.3) | 22.6 (1.9) | 0.29 |
| Previous surgery | 59 (41%) | 24 (38%) | 15 (39%) | 20 (45%) | 0.13 |
| Mean OMA diameter | 33.1 (11.2) | 35.1 (12.5) | 28.4 (8.7) | 31.1 (13.7) | 0.24 |
| Median OMA diameter | 33.0 (22–43) | 35.0 (23–44) | 30.0 (22–37) | 30.0 (20–44) | 0.19 |
| Bilateral OMAs | 48 (33.8%) | 28 (45.2%) | 10 (27%) | 9 (21.4%) | 0.026 |
BMI: body mass index; OMA: ovarian endometrioma(s).
Data are expressed as mean (standard deviation), or median (interquartile range) or n (rate); mean and median OMA are expressed in millimeters.
Table 2.
Comparisons of OMA associated symptoms at baseline (TB) and after 12 months (T12), in dienogest (D), estrogen-progestogens (EPs), and no-treatment (NoT) groups.
| D (n = 62) | EPs (n = 38) | NoT (n = 42) | ||||
|---|---|---|---|---|---|---|
| TB | T12 | TB | T12 | TB | T12 | |
| Dysmenorrhea | 49 (79%) | 18 (29%) | 29 (76%) | 22 (42%) | 20 (47%) | 21 (50%) |
| VAS for dysmenorrhea | 5.59 (4.1) | 1.41 (2.7) | 5.44 (3.1) | 3.15 (3.3) | 5.19 (3.4) | 5.28 (3.5) |
| Dyspareunia | 32 (51%) | 17 (27%) | 22 (57%) | 17(44%) | 15 (35%) | 14(33%) |
| VAS for dyspareunia | 3.38 (3.8) | 1.87 (2.76) | 4.10 (3.5) | 2.55 (3.2) | 2.95 (3.2) | 3.2 (3.3) |
| CPP | 26 (41%) | 16 (25%) | 16 (42%) | 9 (21%) | 16 (38%) | 16 (38%) |
| VAS for CPP | 1.98 (2.7) | 1.09 (2.0) | 1.57 (2.5) | 1.02 (2.1) | 1.95 (2.9) | 1.85 (2.7) |
VAS: visual analog scale; CPP: chronic pelvic pain.
Data are reported as n (rate) and score (standard deviation).
At baseline, the largest mean and median OMA diameter in D were, respectively, 35.1 mm (SD 12.5) and 35 mm (IQR 23–44), and after 12 months, the mean and median diameter were 25.8 mm (SD 15.2) and 28 mm (IQR 14–39), respectively, with a statistically significant difference (p < 0.01), as can be seen in Table 3. The rate of bilateral OMAs dropped from 45.2% (28 patients) to 22.6% (14 patients) with a significant incidence rate difference (1:4; 95% CI 1:48–1:2; p = 0.03). The prevalence rates of dysmenorrhea decreased from 79% to 29%, dyspareunia from 51% to 29%, and CPP from 41% to 25%, with an improvement of VAS score for each symptom.
Table 3.
Comparisons of OMA diameters at baseline (TB) and after 12 months (T12), in dienogest (D), estrogen-progestogens (EPs), and no-treatment (NoT) groups.
| D (n = 62) | EPs (n = 38) | NoT (n = 42) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TB | T12 | p-Value | TB | T12 | p-Value | TB | T12 | p-Value | |
| Mean OMA diameter | 35.1 (12.5) | 25.8 (15.2) | <0.01 | 28.4 (8.7) | 16.7 (2.5) | <0.01 | 31.1 (13.7) | 33.8 (14.5) | <0.01 |
| Median OMA diameter | 35 (23–44) | 28 (14–39) | <0.01 | 30 (22–37) | 20 (13–30) | <0.01 | 31 (20–44) | 32.5 (21–44) | <0.01 |
| Bilateral OMAs | 28 (45.2%) | 14 (22.6%) | 0.03 | 10 (27%) | 4 (10.8%) | 0.30 | 9 (21.4%) | 9 (21.4%) | - |
TB: diameters at baseline; BMI: body mass index; OMA: ovarian endometrioma(s).
Data are expressed as mean (standard deviation), or median (interquartile range) or n (rate); mean and median OMA are expressed in millimeters.
In EPs, the largest mean and median OMA diameter were 28.4 (SD 8.7) and 30 (22–37) mm, respectively, while after 12 months, it was 16.7 (2.5) and 20 (13–30) with a significant difference (p < 0.01), as can be seen in Table 3. Dysmenorrhea decreased from 76% to 42%, dyspareunia from 51% to 44%, and CPP pain from 42% to 21%, with a significant improvement of VAS score for each symptom. The rate of bilateral OMAs dropped from 27% (10 patients) to 14% (4 patients) without a significant incidence rate difference (1:6; 95% CI −1:28 to 1:3; p = 0.30).
In NoT, the largest mean and median OMA diameters were 31.1 (SD 13.7) and 31 (20–44) mm, respectively, while after 12 months, we found increased mean and median largest diameters, respectively, 33.8 (SD 14.5) and 32.5 (21–44) mm, even with a significant difference (p = 0.01), as can be seen in Table 3. Prevalence of dysmenorrhea increased from 47% to 50%, that of dyspareunia decreased from 35% to 33%, and CPP was unmodified (38%). The rate of bilateral OMAs remained stable to 21% (9 patients).
Overall, an aggregate analysis of D and EPs versus group NoT confirmed a significant reduction of the largest OMA diameter (p = 0.001) and the prevalence of all symptoms (p = 0.001).
The were no significative difference between D and EPs in the reduction of the mean largest OMA diameter (p = 0.129). Regarding the control of the symptoms, there was a significant reduction of dysmenorrhea (p = 0.001) in D compared to that in EPs, while dyspareunia (p = 0.18) and CPP (p = 0.22) decreased without a significant difference compared to EPs.
The post hoc analysis was performed considering, at baseline, a sub-group of 20 women (14%) eligible for surgery (according to previously declared surgical criteria), who refused first-line surgical treatment. Half of them (10 patients) refused to undergo any hormonal treatment and hence were enrolled in NoT, while the remaining were enrolled in D (5 patients) and EPs (5 patients). After 12 months, 30% of the women (n = 3), enrolled in D and EPs, failed to meet surgical criteria and continued hormonal treatment, avoiding surgery. In the NoT group, 4 patients (40%) underwent urgent surgery due to severe pelvic pain, while the remaining 30%, after 12 months, maintained the surgical criteria and hence were counseled for surgical option.
Discussion
In our study, we enrolled 142 patients affected by OMAs, and we reported a significant reduction in the largest diameter of the ovarian lesion and prevalence of symptoms after 1 year of hormonal therapy, either with dienogest or estrogen-progestogens treatment. Of note, no difference was noted in OMA diameter decrement between these two treatment groups. Dienogest seems to be more effective in the control of associated OMA’s symptoms. Finally, hormonal therapy, either with dienogest or estrogen-progestogens, led to a 25% decrement in women’s eligibility for surgery after 1 year.
Dienogest can reduce OMA up to 75% in volume26,27 and 40% in diameter,13,17,26 and in literature, its safety, tolerability, and efficacy in the long-term treatment of symptomatic endometriosis are well demonstrated.17,20,28 A randomized controlled trial (comparing the efficacy of oral dienogest at a daily dose of 2 mg over a 12-week period with placebo) reported a significant reduction in the mean pain score in patients with endometriosis. 20 Our results are consistent with these data, scoring a reduction by 30% of the OMA’s largest diameter at 12 months, with a significant reduction of all the associated symptoms. Estrogen-progestogens are, according to guidelines, the first-line medical therapy in women with OMAs, compared to progestin-only pills. 26 Harada et al. 29 analyzed the efficacy of EPs showing a significant volume reduction of OMAs treated with monophasic EPs (ethinylestradiol and norethisterone), which was not observed in the placebo group. In line with our findings, in another randomized trial, an improved control of pain associated to OMAs was recorded using medical treatment with EPs (ethinylestradiol and drospirenone). 30
Angioni et al. 20 enrolled patients with OMA who started treatment with dienogest or dienogest plus ethinylestradiol and found that a significant reduction in the diameter of the lesion was observed only in the dienogest group. On the contrary, Xholli et al. 26 found that both dienogest and estrogen-progestogens were effective in reducing OMA volume and with a prominent effect during dienogest alone, similar to Vercellini et al. 31 who reported a better control of dysmenorrhea in patients treated with dienogest. Our results support that medical treatment could be a valid option, both using dienogest or EPs treatment with an improvement in the magnitude of symptoms and reduction of the largest diameter of the OMAs. Furthermore, it seems that the use of dienogest without the estrogens could have a more beneficial effect on OMAs symptoms but not on the size of the OMAs.
For many years, the most appropriate treatment of OMA was considered the surgical approach, 32 but this view changed 24 with the accumulated evidence of adverse effects induced by surgery on ovarian reserve33 –35 and the high rate of OMA recurrence, nearly 40%–50% at 5 years. 36 The improvement in ultrasonography and sonographers’skills led to an earlier detection of OMA recurrence, when they are smaller, less fibrotic, and more responsive to medical therapy, 37 which can help reduce the risk of subsequent surgery.26,38 Many authors have shown that OMA per se can impair the ovarian function because endometriotic cysts have been reported to contain high levels of reactive oxygen species (ROS), iron, inflammatory molecules, and cellular damaging factors. 39 Speculation of many authors reported that a reduction of the size of OMA can decrease the magnitude of the toxic substances that lead to oxidative stress and fibrogenetic effect. 18
The most recent guidelines suggest a tailor-made approach for the management of OMAs, based on the largest diameter and associated symptoms and taking into account the woman’s desire to conceive and personal preferences. 2 Control of pain and improvement of quality of life (QoL) are the primary goals of the treatment, and an effective, safe, and well-tolerated therapy, suitable for long-term use, is fundamental. 1
Considering the potential impairment of the ovarian reserve because of the presence of OMAs or following the ovarian surgery and the high risk of recurrence increasing with time, a long-term hormonal suppressive therapy should be advisable to decrease the first and second rate surgeries. 40 In literature, we did not find studies evaluating the rate of avoidable surgery in women with big symptomatic OMAs treated with medical therapy, even if there are some supporting reports. 18 In the post hoc analysis of our study, we reported a 30% reduction in the surgical eligibility after 1 year of treatment in a sub-group of women initially suitable for surgery; notably, we failed to find worsening outcomes in relation to an increment in OMAs’ diameter and prevalence of symptoms. These findings suggest the utility of hormonal treatment as a first-line approach even in big symptomatic OMAs. As a deductive conclusion, avoiding any hormonal treatment for women eligible to surgery can lead to urgent surgery, which could be potentially avoided in women who undertake D or EPs.
Our study presents some limits. First, it is a non-randomized study with a discrete sample size, and hence, we cannot exclude a selection bias. Again, the diagnosis of OMAs was not confirmed by histology, but it was based on ultrasound, even if, given its high accuracy, it can be considered a reliable method. 4 We did not explore the effect of the hormonal treatment on the ovarian reserve dosing serum anti-mullerian hormone at baseline and after 12 months, and unfortunately data on the side effects of the hormonal treatment (such as the bleeding pattern) were not collected in this study, in addition to other potentially relevant confounders such as education level, marital status, age at diagnosis, age of the first symptoms, and age at menarche. The strengths of our study are represented by the TV-US evaluation by at least two expert sonographers to guarantee interoperator agreement and hence reliable measurement, the low rate of lost women to follow-up, the rigorous inclusion criteria, the encouraging results in line with literature insights, and because these data derive from a real-world management of these women in two big referrals centers with a long-term follow-up protocol.
Conclusion
Hormal treatment in women with OMAs can be a worthwhile option, also for those women reluctant to undergo surgery at first instance. Of course, further randomized clinical trials are needed, but our results confirm that hormal treatment is effective either on the symptoms or on the OMA largest diameter. The reduction in the need for surgery is reached without worsening of the size of the lesion(s) and burden of symptoms, where dienogest seems to be the best approach for the latter effect. Finally, it has not yet been studied how long-lasting and progressive the effects of hormonal treatment are, and hence, further prospective multicenter studies are warranted.
Acknowledgments
Not applicable.
Footnotes
ORCID iDs: Federico Ferrari  https://orcid.org/0000-0001-7065-2432
https://orcid.org/0000-0001-7065-2432
Matteo Epis  https://orcid.org/0009-0001-8841-6961
https://orcid.org/0009-0001-8841-6961
Antonio Simone Laganà  https://orcid.org/0000-0003-1543-2802
https://orcid.org/0000-0003-1543-2802
Declarations
Ethics approval and consent to participate: The evaluation protocol was approved by the local institutional review board (Comitato Etico ASST Settelaghi) with number 35/2020. Consent to participate was obtained in written. During the study procedure, we were committed to meet and uphold the principles of the General Data Protection Regulation (UE 2016/679) and Italian laws concerning data protection; furthermore, we anonymized the data entered in the study database using a progressive numeration and avoiding relevant personal information (such as date of birth).
Consent for publication: The design, analysis, interpretation of data, drafting, and revisions conform to the Helsinki Declaration, the Committee on Publication Ethics guidelines (http://publicationethics.org/), and the Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement validated by the Enhancing the Quality and Transparency of Health Research Network (https://www.equator-network.org). The data collected were anonymized, considering the observational nature of the study, without personal data that could lead to formal identification of the patient.
Author contribution(s): Federico Ferrari: Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing—review and editing.
Matteo Epis: Conceptualization; Data curation; Formal analysis; Writing—original draft.
Jvan Casarin: Conceptualization; Writing—review and editing.
Giulia Bordi: Conceptualization; Writing—review and editing.
Emanuele Baldo Gisone: Conceptualization; Writing—review and editing.
Chiara Cattelan: Conceptualization; Writing—review and editing.
Diego Oreste Rossetti: Conceptualization; Writing—review and editing.
Giuseppe Ciravolo: Conceptualization; Writing—review and editing.
Elisa Gozzini: Conceptualization; Writing—review and editing.
Jacopo Conforti: Conceptualization; Writing—review and editing.
Antonella Cromi: Conceptualization; Writing—review and editing.
Antonio Simone Laganà: Conceptualization; Writing—review and editing.
Fabio Ghezzi: Conceptualization; Writing—review and editing.
Franco Odicino: Conceptualization; Writing—review and editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: All data were extracted retrospectively from prospectively collected electronic records in the context of evaluation for the endometriosis outpatient service and are available in our server.
References
- 1. Laganà AS, Ferrari F, Mangione D, et al. Molecular and cellular advances in endometriosis research: paving the way for future directions. Int J Mol Sci 2023; 24: 12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becker CM, Bokor A, Heikinheimo O, et al. ESHRE guideline: endometriosis. Hum Reprod Open 2022; 2022: hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell 2021; 184: 2807–2824. [DOI] [PubMed] [Google Scholar]
- 4. Orlov S, Jokubkiene L. Prevalence of endometriosis and adenomyosis at transvaginal ultrasound examination in symptomatic women. Acta Obstet Gynecol Scand 2022; 101(5): 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laganà AS, Vitagliano A, Chiantera V, et al. Diagnosis and treatment of endometriosis and endometriosis-associated infertility: novel approaches to an old problem. J Clin Med 2022; 11: 3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrari FA, Youssef Y, Naem A, et al. Robotic surgery for deep-infiltrating endometriosis: is it time to take a step forward? Front Med 2024; 11: 1387036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leonardi M, Uzuner C, Mestdagh W, et al. Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: prospective international pilot study. Ultrasound Obstet Gynecol 2022; 60(3): 404–413. [DOI] [PubMed] [Google Scholar]
- 8. Nie J, Zhao C, Laganà AS, et al. Identification of lesional attributes of dysmenorrhea severity and the serum antimüllerian hormone levels in women with ovarian endometriomas. Fertil Steril 2022; 118(1): 191–202. [DOI] [PubMed] [Google Scholar]
- 9. Busacca M, Marana R, Caruana P, et al. Recurrence of ovarian endometrioma after laparoscopic excision. Am J Obstet Gynecol 1999; 180: 519–523. [DOI] [PubMed] [Google Scholar]
- 10. Leone Roberti Maggiore U, Gupta JK, Ferrero S. Treatment of endometrioma for improving fertility. Eur J Obstet Gynecol Reprod Biol 2017; 209: 81–85. [DOI] [PubMed] [Google Scholar]
- 11. Naem A, Laganà AS. Editorial: minimally invasive surgery as a mean of improving fertility: what do we know so far? Front Surg 2023; 10: 1203816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donarini P, Ciravolo G, Rampinelli F, et al. Parametrial endometriosis with ureteral involvement: a case report of a conservative approach without ureteral resection. J Endometr Pelvic Pain Disord 2018; 10: 222–223. [Google Scholar]
- 13. Muzii L, Achilli C, Lecce F, et al. Second surgery for recurrent endometriomas is more harmful to healthy ovarian tissue and ovarian reserve than first surgery. Fertil Steril 2015; 103(3): 738–743. [DOI] [PubMed] [Google Scholar]
- 14. Chapron C, Marcellin L, Borghese B, et al. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol 2019; 15(11): 666–682. [DOI] [PubMed] [Google Scholar]
- 15. Vercellini P, Buggio L, Frattaruolo MP, et al. Medical treatment of endometriosis-related pain. Best Pract Res Clin Obstet Gynaecol 2018; 51: 68–91. [DOI] [PubMed] [Google Scholar]
- 16. Momoeda M, Harada T, Terakawa N, et al. Long-term use of dienogest for the treatment of endometriosis. J Obstet Gynaecol Res 2009; 35: 1069–1076. [DOI] [PubMed] [Google Scholar]
- 17. Sugimoto K, Nagata C, Hayashi H, et al. Use of dienogest over 53 weeks for the treatment of endometriosis: dienogest for endometriosis. J Obstet Gynaecol Res 2015; 41: 1921–1926. [DOI] [PubMed] [Google Scholar]
- 18. Muzii L, Galati G, Di Tucci C, et al. Medical treatment of ovarian endometriomas: a prospective evaluation of the effect of dienogest on ovarian reserve, cyst diameter, and associated pain. Gynecol Endocrinol 2020; 36(1): 81–83. [DOI] [PubMed] [Google Scholar]
- 19. Del Forno S, Mabrouk M, Arena A, et al. Dienogest or Norethindrone acetate for the treatment of ovarian endometriomas: can we avoid surgery? Eur J Obstet Gynecol Reprod Biol 2019; 238: 120–124. [DOI] [PubMed] [Google Scholar]
- 20. Angioni S, Pontis A, Malune ME, et al. Is dienogest the best medical treatment for ovarian endometriomas? Results of a multicentric case control study. Gynecol Endocrinol 2020; 36(1): 84–86. [DOI] [PubMed] [Google Scholar]
- 21. Vignali M, Belloni GM, Pietropaolo G, et al. Effect of Dienogest therapy on the size of the endometrioma. Gynecol Endocrinol 2020; 36(8): 723–727. [DOI] [PubMed] [Google Scholar]
- 22. Sasaran V, Alexa Bad CM, Muresan D, et al. Ultrasound pattern and diagnostic accuracy of primary ovarian endometrioma and its recurrence: a pictorial essay. Med Ultrason 2020; 22: 230–235. [DOI] [PubMed] [Google Scholar]
- 23. Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131: 557–571. [DOI] [PubMed] [Google Scholar]
- 24. Almog B, Sheizaf B, Shalom-Paz E, et al. Effects of excision of ovarian endometrioma on the antral follicle count and collected oocytes for in vitro fertilization. Fertil Steril 2010; 94(6): 2340–2342. [DOI] [PubMed] [Google Scholar]
- 25. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 26. Xholli A, Filip G, Previtera F, et al. Modification of endometrioma size during hormone therapy containing dienogest. Gynecol Endocrinol 2020; 36(6): 545–549. [DOI] [PubMed] [Google Scholar]
- 27. Uludag SZ, Demirtas E, Sahin Y, et al. Dienogest reduces endometrioma volume and endometriosis-related pain symptoms. J Obstet Gynaecol 2021; 41(8): 1246–1251. [DOI] [PubMed] [Google Scholar]
- 28. Strowitzki T, Faustmann T, Gerlinger C, et al. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol 2010; 151(2): 193–198. [DOI] [PubMed] [Google Scholar]
- 29. Harada T, Momoeda M, Terakawa N, et al. Evaluation of a low-dose oral contraceptive pill for primary dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertil Steril 2011; 95(6): 1928–1931. [DOI] [PubMed] [Google Scholar]
- 30. Harada T, Kosaka S, Elliesen J, et al. Ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: a randomized controlled trial. Fertil Steril 2017; 108(5): 798–805. [DOI] [PubMed] [Google Scholar]
- 31. Vercellini P, Buggio L, Berlanda N, et al. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril 2016; 106(7): 1552.e2–1571.e2. [DOI] [PubMed] [Google Scholar]
- 32. Rolla E. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000res 2019; 8: F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Somigliana E, Berlanda N, Benaglia L, et al. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertil Steril 2012; 98(6): 1531–1538. [DOI] [PubMed] [Google Scholar]
- 34. Cagnacci A, Bellafronte M, Xholli A, et al. Impact of laparoscopic cystectomy of endometriotic and non-endometriotic cysts on ovarian volume, antral follicle count (AFC) and ovarian doppler velocimetry. Gynecol Endocrinol 2016; 32(4): 298–301. [DOI] [PubMed] [Google Scholar]
- 35. Deckers P, Ribeiro SC, Simões RDS, et al. Systematic review and meta-analysis of the effect of bipolar electrocoagulation during laparoscopic ovarian endometrioma stripping on ovarian reserve. Int J Gynaecol Obstet 2018; 140(1): 11–17. [DOI] [PubMed] [Google Scholar]
- 36. Jiang D, Zhang X, Shi J, et al. Risk factors for ovarian endometrioma recurrence following surgical excision: a systematic review and meta‑analysis. Arch Gynecol Obstet 2021; 304(3): 589–598. [DOI] [PubMed] [Google Scholar]
- 37. Martone S, Troìa L, Marcolongo P, et al. Role of medical treatment of endometriosis. Minerva Obstet Gynecol 2021; 73: 304–316. [DOI] [PubMed] [Google Scholar]
- 38. Glasbey JC, Abbott TE, Ademuyiwa A, et al. Elective surgery system strengthening: development, measurement, and validation of the surgical preparedness index across 1632 hospitals in 119 countries. Lancet 2022; 400: 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanchez AM, Viganò P, Somigliana E, et al. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update 2014; 20(2): 217–230. [DOI] [PubMed] [Google Scholar]
- 40. Muzii L, Galati G, Mattei G, et al. Expectant, medical, and surgical management of ovarian endometriomas. J Clin Med 2023; 12: 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]