Abstract
The neutrophil to lymphocyte ratio (N:L) is an emergent transdiagnostic biomarker shown to predict peripheral inflammation as well as neuropsychiatric impairment. The afferent signaling of inflammation to the central nervous system has been implicated in the pathophysiology of sickness behavior and depression. Here, the N:L was compared to structural and functional limbic alterations found concomitant with depression within a geriatric cohort. Venous blood was collected for a complete blood count, and magnetic resonance imaging as well as phenotypic data were collected from the 66 community-dwelling older adults (aged 65–86 years). The N:L was regressed on gray matter volume and resting-state functional connectivity (rsFC) of the subgenual anterior cingulate (sgACC). Thresholded parameter estimates were extracted from structural and functional brain scans and bivariate associations tested with scores on the geriatric depression scale. Greater N:L predicted lower volume of hypothalamus and rsFC of sgACC with ventromedial prefrontal cortex. Both parameters were correlated (p < 0.05) with greater symptomology in those reporting moderate to severe levels of depression. These findings support the N:L as a transdiagnostic biomarker of limbic alteration underpinning mood disturbance in non-treated older adults.
Keywords: Neutrophilia, Lymphocytopenia, Geriatric depression, Resting state functional connectivity, Medial frontal cortex, Perigenual anterior cingulate
1. Introduction
The chronicity of low-grade inflammation has of recent garnered significant attention in the etiology of depression, particularly amongst older adults (Krishnadas and Cavanagh, 2012). Concomitant with immunosenescent processes and age-related multi-morbidity, persons aged over 65 years are susceptible to chronically elevated levels of peripheral inflammation (Orimo et al., 2006). Elevated levels of pro-inflammatory cytokines may subjugate the brain altering dopaminergic and glutamatergic neurotransmission within the neural circuits underpinning mood disturbance (Miller and Raison, 2016). As the scope of pro-inflammatory biomarker involvement in the manifestation of depression broadens it becomes important to investigate upstream changes in adaptive and innate immune function from which these age-related inflammatory-immune mechanisms progress.
The relative state of peripheral neutrophilia to lymphocytopenia has emerged as a potential transdiagnostic biomarker of neuropsychiatric disturbance. The largest pool of innate immune cells, i.e., neutrophils, are driven by cortisol levels and contribute to nonspecific inflammation via phagocytosis, chemotaxis, release of reactive oxygen species, degranulation, and the production and liberation of cytokines which affect BBB function (Aubé et al., 2014). Although neutrophil recruitment is key for host defense, the overexuberance of these cells brought on by trauma or physiologic stress appears to be amplified in older adults and lead to a paradoxical increase in systemic damage to bodily tissues(Liew and Kubes, 2019). In juxtaposition, through the anti-inflammatory effects of regulatory T-cells on the activated state of microglia and the tryptophan-metabolizing enzyme indoleamine-2,3-dioxygenase enzyme peripheral has been implicated in mitigation of depressive symptoms (Laumet et al., 2018). Moreover, the age-related changes in mitochondrial stress and telomerase activity within peripheral lymphocytes resulting in leukocytosis and lymphocytopenia are further implicated in age-related mood disturbance (Lindqvist et al., 2015). Hence, driven by disease and immunosenescent processes in addition to elevated presence of endogenous neuroendocrine response to acute physiologic stress, the neutrophil to lymphocyte ratio (NLR) has emerged as a highly reproducible index of the severity of stress and systemic inflammation amongst critically ill patients following trauma, major surgery or sepsis (Zahorec, 2001; Rainer et al., 1999). This relatively low-cost marker of neutrophil expansion is also used to indicate life-threatening inflammation and organ failure in older adults (Demir and YÜCEL M., 2020; Song et al., 2020). Yet, amongst older adults, the prognostic capacity for the NLR in neuropsychiatric and neurodegenerative disease remains unclear.
Afferent signaling of an inflammatory peripheral state is a central tenet of the geriatric manifestation of depression (Alexopoulos and Morimoto, 2011). Meta-analyses on the correlates of depression implicate chronically and experimentally elevated levels of the acute-phase reactant C-reactive protein (CRP), Interleukins (IL)-6, IL-1, and tumour necrosis factor alpha (TNF-α) in the severity of mood disturbance (Valkanova et al., 2013; Haapakoski et al., 2015; Howren et al., 2009). Levels of CRP are most germane to geriatric populations as they are found elevated amongst depresses older adults living with(Lu et al., 2013; Viscogliosi et al., 2013) or without (van den Biggelaar et al., 2007) chronic disease. Neutrophilia in combination with lymphocytopenia are closely intertwined with systemic inflammation in older adult cohorts. Most recently, literature has emerged correlating NLR with depression severity in middle- to older-aged adults diagnosed with major depressive disorder (MDD) (Sunbul et al., 2016; Kayhan et al., 2017; Arabska et al., 2018). Furthermore, NLR is higher in persons experiencing manic bipolar disorder episodes comparison with depressive bipolar and MDD suggesting the increased likelihood for severe neuropsychiatric disease with increasing NLR (Mazza et al., 2019). Although compelling, it remains unclear whether a neutrophilic environment predicts greater severity of depressive symptomology amongst individuals not currently treated for MDD and what are the neural regions implicated in this process.
In recent years, attention has turned to elucidating the role of peripheral inflammatory-immune processes in the structural integrity and functional connectivity of limbic regions implicated in depression (Alexopoulos and Morimoto, 2011). In a meta-analysis of functional Magnetic Resonance Imaging (fMRI) and positron emission tomography studies, peripheral markers of inflammation correlated with greater co-activation throughout limbic regions of interest (ROI) including the ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC), temporal cortices, amygdala, hippocampus, hypothalamus, striatum, insula as well as other subcortical regions extending into the midbrain and brainstem (Kraynak et al., 2018). Notably, the subgenual region of the ACC (sgACC) was amongst the ROIs surviving the more stringent statistical correction. In the context of depression and sickness behavior, the sgACC is primarily implicated in behavioral withdrawal, resource conservation, and the promotion of safety behaviors (Marsland et al., 2017). This neurobehavioral adaptation of what may be collectively described as sickness behaviors further involves interactions between neuroendocrine and inflammatory-cytokine signaling response (Critchley et al., 2003). Not only do acute inflammatory changes predict enhanced sgACC activity during limbic processing, but increased connectivity of the sgACC with the amygdala, vmPFC, and nucleus accumbens (Harrison et al., 2009a). In addition to these experimentally induced changes in limbic activity neuroimaging the functional connectivity of these regions at rest has also garnered interest. Unlike task-related blood oxygen level dependent (BOLD) activity, resting state functional connectivity (rsFC) reflects the intrinsic energy demands of neuronal assemblies of brain regions involved in a common behavior (Lewis et al., 2009). Meta-analytic findings also implicate increased limbic connectivity with the sgACC in the presentation of depressive rumination (Hamilton et al., 2015). Although brain structure does not always implicate function deficits volumetric reductions of the sgACC are reported in depressed individuals (Kaltenboeck and Harmer, 2018; Schmaal et al., 2017; Campbell et al., 2004). Furthermore, in a large sample of non-demented older adults, serum levels of TNF-α and IL-1β were associated with smaller volume in the sgACC and inferior parietal lobule after controlling for age-related factors including APOE ε4 status, cardiovascular risk and geriatric depression (Corlier et al., 2018). Although inflammatory signaling in the periphery may contribute to the structural and functional alterations implicated in mood disturbance it remains unclear whether the upstream markers of immune dysfunction, such as the NLR, can be related to the neurobehavioral processes involved in the manifestation of geriatric depression.
The NLR has emerged as a potential transdiagnostic marker for psychiatric disease (Brinn and Stone, 2020). The sgACC is a region of interest implicated in the inflammatory signaling of mood disturbance, however, it is unclear whether the neural integrity and connectivity of this and other limbic regions relates to the NLR in older adults with subclinical depressive symptoms is unclear. Hence, the current study aimed to determine whether the neural correlates of the NLR, indexed by whole-brain volume and rsFC of the sgACC, predict the severity of geriatric depression in a cohort of older adults not diagnosed or treated for MDD.
2. Methods
2.1. Participants
The fMRI and physiological data were acquired from the Enhanced NKI Rockland Sample (Nooner et al., 2012a). Data collection for this study was approved by the Nathan Kline Institute and Montclair State University institutional review boards and all participants provided informed consent. Data collection involved a semi-structured diagnostic psychiatric interview, battery of psychiatric, cognitive, and behavioral assessments as well as a multimodal brain imaging session (Nooner et al., 2012a). The initial sample of individuals with structural and functional brain scans was n = 801. Individuals with a history of treatment or diagnosis for schizophrenia, Alzheimer’s disease, dementia, Parkinson’s Disease, Huntington’s Disease, Multiple Sclerosis, and loss of consciousness were excluded from the study. Seventy-eight older adults, (age of 65 years or older), provided a blood sample NLR assay. Of these, 66 individuals passed quality control for resting state fMRI. Of those individuals four did not have quantifiable T1-weighted scans.
2.2. Measures
2.2.1. Self-report
The Geriatric Depression Scale (GDS) is a 30-item “yes/no” self-report measure of depressive symptoms designed to distinguish depressed elders with neurovegetative symptoms from nondepressed elders with symptoms that overlap with depression yet are concomitant with normal aging or comorbid medical disorder (Yesavage et al., 1982). During administration, the individual is asked to report whether they have experienced several symptoms over the past week. The GDS has a maximum score of 30, with higher scores representing more severe symptoms. The GDS emphasizes cognitive symptoms of depression rather than somatic symptoms. The instrument shows good validity amongst healthy, medically ill, or mild to moderately cognitively impaired older adults(Jung et al., 1997).
2.2.2. Biomarker
The blood sample was obtained on day 1 and the R-fMRI scans on day 2 (~1–2 weeks apart) (Nooner et al., 2012a). A 5 ml venous blood sample was collected at study entry during the first visit and tested between 2 and 6 h for complete blood count including absolute number and percentage of neutrophils, lymphocytes, and monocytes. CBCs are performed in house on a Beckman Coulter LH780 using samples collected in K3EDTA. C-Reactive protein was sent out for reference at Bioreference Laboratories™, using serum samples and would have to meet Bioreferences specimen requirements.
2.2.3. Neuroimaging
Acquisition.
A 3.0-T Siemens MAGNETOM Trio-Tim scanner was used to collect resting state scans with using the following imaging parameters: TR = 1400 ms, TE = 30 ms, slice thickness = 3.0 mm, flip angle = 65°, field of view = 224 mm, slices = 64, and voxel size = 2.0 × 2.0 × 2.0 mm. The acquisition time was 10-min for each participant using a multi-band imaging sequence. The subjects were instructed to lay still inside the scanner with their eyes open thinking of nothing and not to fall asleep. High-resolution anatomical images (MPRAGE) were acquired using the following scanning parameters: TR = 1900 ms, TE = 2.52 ms, slice thickness = 1.0 mm, flip angle = 9°, field of view = 256 mm, and voxel size = 1.0 × 1.0 × 1.0 mm. All fMRI data used in the analysis are part of the NKI Enhanced dataset made publicly available by the international neuroimaging data sharing initiative (Nooner et al., 2012b).
Structural Image Preprocessing.
Voxel Based Morphometry (VBM) (Ashburner, 2010) analysis was conducted using the following steps implemented in SPM12 software: 1) origin manually set to the anterior commissure; (2) images reoriented to fit a 2 mm Montreal Neurological Institute (MNI) template; (3) images segmented into gray matter, white matter, and cerebrospinal fluid-based probability maps using DARTEL templates; (4) deformations estimated; (5) Jacobian modulation performed to avoid volumetric changes during the normalization to MNI space; and (6) Jacobian-scaled warped gray matter tissue images were produced for statistical analysis.
fMRI pre-processing.
Resting state scans were preprocessed using DPARSF-A in DPABI (http://rfmri.org/DPARSF) (Chao-Gan and Yu-Feng, 2010; Yan et al., 2016). The pipeline was implemented in MATLAB R2017a (MathWorks, Natick, MA, USA) using the following steps: 1) first five images were removed; 2) nuisance covariates (Friston 24 motion parameters, white matter, & cerebrospinal fluid) and linear trends were regressed out; 3) band-pass filtering at 0.01 to 0.1 Hz (Damoiseaux et al., 2006); 4) data were despiked through AFNI 3dDespike, realigned and normalized with DPARSF-A, and smoothed to 6 mm with AFNI 3dBlur; and 5) independent component analysis (ICA-FIX) was applied through FSL MELODIC to identify signal and noise components, which were extracted and transformed into 3 mm MNI space (i.e., subject space). Frramewise displacement (FD) was calculated (Power et al., 2012) and a total of 12 individuals were removed from the analysis due to excessive head motion (FD > 0.50).
Seed-to-whole-brain connectivity analysis.
Whole-brain rsFC was examined using a 6-mm spherical seed centered on the sgACC (MNI: x = 1, y = 22, z = −10). This region was defined from coordinates demonstrating maximal increase in response to administration of an endotoxin (Harrison et al., 2009a). Individual preprocessed data were used to extract fMRI time series data from the sgACC seed in the filtered data, and then Pearson’s correlation coefficients were calculated between the sgACC time series and the time series from all other voxels in the brain. The correlation coefficient at each voxel was transformed to a z-value using Fisher’s r-to-z transformation to enhance normality. The resultant sgACC functional connectivity map for each participant was entered into the group level analysis.
To identify significant associations between the inflammation index and functional connectivity of the sgACC, a voxel-wise multiple regression analysis was conducted using the group-level sgACC connectivity maps as the dependent variable, the inflammation index as the independent variable, gender, and age as covariates of no interest. Mean framewise displacement (FD), averaged across the entire time series, was added as a nuisance covariate to minimize the impact of head motion-related variance on group inference (Satterthwaite et al., 2013). The relationship between inflammation index and sgACC functional connectivity was tested using t-statistics and reported as a z-score after the t-value was transformed into the standard normal distribution. Clusters were considered statistically significant if they reached the extent threshold of p <0.001, uncorrected with a with an FWE-corrected cluster-forming threshold of p < 0.001.
Extraction of regions of interest (ROI).
The contrast parameter estimates were extracted from the seed-based connectivity or VBM regression analyses for voxels defining clusters within the ROIs that exhibited significant effects for high or low levels of inflammation at k ≥ 25 using the program MarsBaR (http://marsbar.sourceforge.net). Extracted parameter estimates were averaged within each ROI and imported into SPSS before being submitted to a partial correlation with GDS scores controlling for head motion and/or age.
3. Results
Sociodemographic and cardiometabolic data is provided in Table 1. The bivariate correlation between GDS scores and NLR was not significant (r = 0.158, p = 0.10). However, compared with individuals with an elevated NLR (>3) (mean = 5.53 ± 5.55) those with lower neutrophil ratios (mean = 2.86 ± 2.54) reported significantly lower levels of geriatric depression (t(64) = −2.65, p = 0.01).
Table 1.
Sample characteristics.
| Characteristics | n = 66 |
|---|---|
| Sociodemographic | |
| Age | 71.9 ± 5.6 |
| Gender (Female %)) | 57.6 |
| Ethnicity (%) | |
| Caucassian | 90.9 |
| African-American | 6.1 |
| Hispanic | 3.0 |
| Primary language (%) | |
| English | 92.8 |
| Socioeconomic Status | 52.7 ± 8.1 |
| Parents’ Socioeconomic Status | 34.3 ± 14.3 |
| Full Scale Sum T-score | 217.7 ± 29.6 |
| Medical History (%) | |
| Cancer | 25.8 |
| Myocardial Infarction | 3.0 |
| Coronary Artery Disease | 4.5 |
| Hyperlipidemia | 43.3 |
| Hypertension | 40.9 |
| Irritable Bowel Syndrome | 14.4 |
| Crohn’s Disease | 1.0 |
| Ulcerative Colitis | 2.1 |
| Hepatitis | 1.5 |
| Type II Diabetes Mellitus | 10.6 |
| Arthritis | 45.5 |
| Psychiatric History (%) | |
| Major Depression | 4.5 |
| Bipolar Disorder | 1.0 |
| Social Anxiety | 4.5 |
| Delusional Disorder | 1.1 |
| Specific Phobia | 2.2 |
| Posttraumatic Stress Disorder | 2.2 |
| Bereavement | 1.1 |
| Substance Abuse | |
| Alcohol Abuse | 4.3 |
| Alcohol Dependence | 2.2 |
| Biomarkers | |
| Body mass index | 27.0 ± 5.1 |
| Systolic blood pressure | 128.6 ± 12.3 |
| Diastolic blood pressure | 75.8 ± 8.0 |
| Neutrophil % | 60.7 |
| Lymphocyte % | 27.9 |
| Monocyte % | 7.9 |
| Hemoglobin A1c1 | 0.4 ± 1.4 |
To identify gray matter areas that were associated with a high or low peripheral inflammation index, voxel-wise multiple regression analysis was tested in SPM12 controlling for the effects of age gender, and total gray matter volume. The results indicate that three clusters surviving an uncorrected primary threshold of p < 0.001 and a secondary cluster-level FDR-corrected threshold of p < 0.01 showed greater gray matter volume that corresponded with a lower index of peripheral inflammation (see Fig. 1). These clusters included the hypothalamus (k = 137, T = 4.68, FWE < 0.001, FDR < 0.001), the left mid-posterior insula (k = 82, T = 4.45, FWE = 0.048, FDR < 0.01), and the left parahippocampal gyrus (k = 108, T = 4.29, FWE = 0.014, FDR < 0.001). The trending correlation for parameter estimates extracted from the hypothalamus with the GDS scores emerged for the entire cohort (r = −0.181, p = 0.073, n = 66), however, upon restricting the sample to those with moderate to severe depressive symptomology, a significant negative correlation emerged (r = −0.452, p = 0.023, n = 20).
Fig. 1.
Three volumetric regions in the hypothalamus (MNI x = −6, y = −2, z = −12), left mid-posterior insula (x = −36, y = −8, z = −6) and the left parahippocampal gyrus (x = −24, y = − 8, z = −36) corresponding to lower neutrophil-to-lymphocyte ratios (uncorrected primary p < 0.001 and secondary cluster-level FDR p < 0.01).
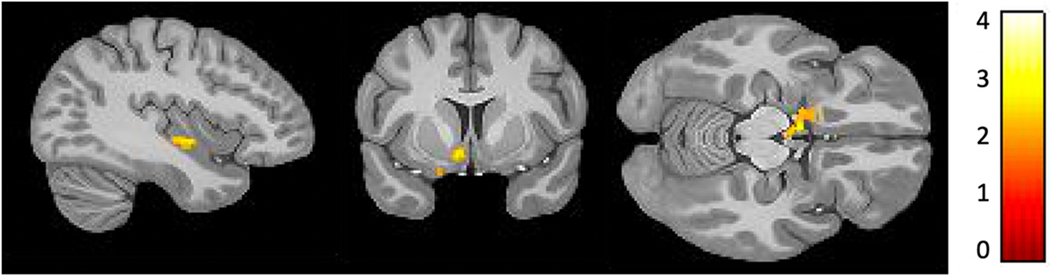
Based on the hypothesis put forth regarding the sgACC as an anterior cortical structure involved in the interoceptive signaling of inflammation, this structure was used as the seed ROI for a whole brain functional connectivity analysis with the inflammatory index as a covariate of interest. The results showed one large cluster emerging from an area in the left ventromedial prefrontal cortex (x = −30, y = −57, z = −3), (Brodmann Area 10), wherein increased rsFC with the sgACC was associated with lower NLR (k = 132, T = 4.79, FWE < 0.001, FDR < 0.01) (see Fig. 2). Parameter estimates extracted from left BA10 did not correlate with GDS scores when controlling for age and head-motion for the entire cohort (r = −0.137, p > 0.05, n = 66). However, upon restricting the sample to individuals with moderate to severe depression, parameter estimates from BA10 negatively predicted GDS scores (r = −0.383, p < 0.05, n = 20).
Fig. 2.
Greater resting state functional connectivity of the subgenual cingulate with an area in the left ventromedial prefrontal cortex (BA10) (x = −30, y = −57, z = −3), that corresponds with lower ratio of neutrophils to lymphocytes (k = 132, T = 4.79, FWE < 0.001, FDR < 0.01).
4. Discussion
The aim of the current study was to assess the relationship of the NLR, an emergent transdiagnostic biomarker of neuropsychiatric dysfunction, to geriatric depression and its structural and functional neural underpinnings. In this sample a NLR ratio of more than 3:1 was associated with greater endorsement of depressive symptomology on a scale validated in geriatric populations. Previous studies examining systemic markers of critical illness commonly implicate inflammatory biomarkers and acute-phase reactants in mood disturbance amongst older adults (Kushner, 2001; Michaud et al., 2013; Puzianowska-Kuźnicka et al., 2016; Smith et al., 2018). The sgACC was selected as the a priori region of interest because its activity reflects acute changes in peripheral levels of inflammation (Harrison et al., 2009b). This region exhibited greater rsFC with the left vmPFC in conjunction with a lower NLR. This sgACC connectivity to the vmPFC was inversely associated with severity of geriatric depressive symptoms, albeit amongst those endorsing moderate to severe symptoms. In the full sample, greater NLR was associated with volumetric deficits in the hypothalamus, left mid-posterior insula, and parahippocampal cortex. Parameter estimates of hypothalamus volume were inversely associated with moderate to severe depression symptoms with a similar trend found across the entire spectrum of symptom severity.
The correspondence between neutrophilia and hypothalamus atrophy in relation to depressive symptomology is a novel finding amongst older adults. In addition to breakdown of the blood brain barrier the accumulation of neutrophils in the brain parenchyma can perpetuate local microglial activation yielding neurogenerative effects (Cernackova et al., 2020; Zhang et al., 2016). Hypothalamic inflammation is commonly observed in conjunction with atrophy in this region. Chronic interoceptive signaling of an inflammatory peripheral state has been linked to subsequent reductions in insula volume over time (Craig and Craig, 2009; Nusslock and Miller, 2016; Jiang et al., 2015). Specifically, induction of systemic inflammation following endotoxin administration is shown to induce abnormal glucose metabolism in both anterior and posterior insula (Lekander et al., 2016). Meta-analytic findings highlight a strong association between markers of systemic inflammation and resting BOLD activity in left posterior and right anterior insula (Kraynak et al., 2018). Although the causal effect for systemic inflammation on volumetric brain reduction has been mainly inferred amongst older adults (Taki et al., 2013), some mechanistic evidence has been levied. A longitudinal study genotyping sequences for IL-1β C-511 T and CRP polymorphisms measured 2 years apart found these pro-inflammatory biomarkers accurately predicted shrinkage within the entorhinal and ambient gyri of the parahippocampal cortex (Persson et al., 2014; Ladenvall et al., 2006; Reitz et al., 2007; Meisenzahl et al., 2001). Notably, the hypothalamus was the only correlate of greater NLR and geriatric depressive symptom severity. The hypothalamus has long been implicated in major depression by virtue of its role in the adaptive control of energy homeostasis (Cernackova et al., 2020; Pimentel et al., 2014; Valdearcos et al., 2015; Mravec et al., 2019). For example, obesity-mediated inflammation has not only been linked to atrophy of hypothalamic nuclei (Cazettes et al., 2011; Puig et al., 2015), but also M1-activation, HPA-axis reactivity, and total mood disturbance in older adults (Hryhorczuk et al., 2013; Soczynska et al., 2011; Schachter et al., 2018; Martinac et al., 2014). Thus, the pattern of volumetric deficits observed in relation to the NLR supports this measure as a transdiagnostic marker of the limbic atrophy concomitant with depressive disorder.
Coinciding with extant literature implicating inflammation with changes in mood and coactivation patterns with the sgACC (Kraynak et al., 2018; Harrison et al., 2009a; O’Connor et al., 2009), lower rsFC was observed between sgACC and vmPFC amongst individuals with greater NLR. Despite a growing number of studies implicating rsFC between the vmPFC and sgACC in inflammation-based mood disturbance the precise neurobiological mechanism remains unclear (Beckmann et al., 2009; Torta and Cauda, 2011; Yu et al., 2011). Neuroanatomical tracing studies show the murine homologue of the vmPFC is laden with cytokine receptors that mediate activity with an extensive network of subcortical regions including hypothalamus as well as parabrachial and solitary nuclei of the brainstem (Vertes, 2004; Gabbott et al., 2005). Furthermore, brainstem and hypothalamus expression of cytokines, chemokines, and pro-inflammatory transcription factors are observed following systemic inflammation induced by changes in diet(Dalvi et al., 2017) or stress (Sirivelu et al., 2012; Kanemitsu, 2000). Indeed, the highest density of IL-1 receptors are found in the preoptic, supraoptic, and paraventricular areas of the hypothalamus (Konsman et al., 2004). It should be noted that IL-1 and CRP share common gene variation (Eklund et al., 2003; Berger et al., 2002), proliferate in tandem (Pue et al., 1996), and are both linked to major depression (Howren et al., 2009). Intriguingly, thrombocytosis, in association with elevated production of chemotactic proteins for neutrophil proliferation is shown to coincide with elevated levels of TNF-α and the IL-1 family (Strieter et al., 1989; Clancy et al., 2017). Although altered interaction of activated microglia with neurons and axons are an established downstream mechanism of inflammatory signaling to the brain (Fujita and Yamashita, 2021), research has recently emerged implicating neutrophils in cerebrovascular function. In a study examining a mouse model for Alzheimer’s disease found that an the administration of a neutrophil-specific signaling marker that increases migration of neutrophils toward sites of inflammation by modulation of β2-integrin-dependent adhesion resulted in the stalling of blood flow in cerebral capillary segments (Hernandez et al., 2019). According to the neurovascular coupling hypothesis, increased cerebral blood flow may be coupled with higher degree of functional brain connectivity (Zhu et al., 2017). Nevertheless, if a neutrophilic environment is conducive to neuroinflammatory signaling and cerebrovascular dysfunction, then the volumetric reductions of the hypothalamus in conjunction with mitigated vmPFC connectivity with sgACC may reflect independent pathways for inflammatory-immune dysfunction to subjugate the limbic regions that signal sickness behavior (Jin et al., 2016; Dantzer, 2009; Hennessy et al., 2017).
5. Limitations
Amongst the inherent limitations of this cross-sectional design is the inference of causality of NLR on volumetric atrophy and aberrant functional connectivity. Nor can we determine, with any degree of specificity, the chronicity of neutrophilia or inflamation in the current sample. However, supplementary analyses suggest individuals with NLR ratio greater than 3:1 were more likely to have detectable levels of CRP. Furthermore, literature does support neutrophilia is concomitant with immunosenescence and propensity for chronic inflammation amongst older adults (Uhl et al., 2016; Ward et al., 2011; Drew et al., 2018). As a function of hypothalamic atrophy and vmPFC dysconnectivity this study supports NLR as a transdiagnostic index of inflammatory-immune and mood disruption (Kushner, 2001; Michaud et al., 2013; Puzianowska-Kuźnicka et al., 2016). However, other mechanisms involving HPA-axis disruption have been implicated in the etiology of depression (Cernackova et al., 2020). Given the specificity of our volumetric findings and the role this structure plays in mediating glucocorticoid and inflammatory receptor signaling collection of basal cortisol measures would have helped to further support and elucidate the biobehavioral mechanism for hypothalamic inflammation in the geriatric presentation of depressive symptoms. Future studies should include a protocol for cortisol measurement, particularly given that cortisol levels sampled from awakening to pre-scan predicts aberrant rsFC of the medial frontal gyrus with limbic and non-limbic regions (Veer et al., 2012; Wu et al., 2015; Wang et al., 2018). Clinical implications for our findings are limited by the self-reported measure of depression. Although older adults are at greater risk for major depression they are less likely to endorse depressive symptomology (Gallo et al., 1994). Diagnostic interview may provide a more reliable index of depression, however, compared to other frequently used self-report indices the GDS demonstrates superior sensitivity in detecting depression in adults over the age of 65 (Lyness et al., 1997).
6. Clinical implications
Neural tracing studies show dense connection between sgACC and VMPFC lead a large field cortical network that actively responds to metabolic demands (Joyce and Barbas, 2018). Although the current study did not implicate hypothalamus connectivity with the sgACC in the presentation of the NLR or depression, activation of these subcortical nuclei may trigger an extended network that regulates internal homeostasis (Chiba et al., 2001; Freedman et al., 2000). Our neurobiological findings relating to the hypothalamus supports a mechanism whereby inflammation predicts mood disturbance amongst individuals exposed to chronic metabolic or inflammatory-immune dysfunction (Cernackova et al., 2020). It should be noted that selective serotonin-reuptake inhibitors show an inverse effect on inflammation, suggesting bidirectional relationships might be at play (Gałecki et al., 2018; Adzic et al., 2018). Moreover, preliminary yet compelling evidence suggests cumulative exposure to stress and major depression can induce reductions in leukocyte telomere length and activity (Kinser and Lyon, 2013). Concomitantly, waning NLR is observed in MDD following 3 months of selective serotonin-reuptake inhibitor treatment. Hence, future longitudinal studies should elucidate the bidirectional effects of im munosenescent processes and the neuropathophysiology processes underpinning the etiology of geriatric manifestations of depression, particularly in persons living with chronic disease.
Funding
This work was supported by 5K01HL139722 and T32 DA031098 to JDL.
Footnotes
Availability of data and material
Raw and pre-processed resting state fMRI data available.
Code availability
Not applicable.
Ethics approval
The study was approved by the appropriate institutional and/or national research ethics committee (Nathan Kline Institute and Montclair State University) and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was attained from all subjects participating in this study.
Consent for publication
Not applicable.
Declaration of Competing Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Adzic M, Brkic Z, Mitic M, et al. , 2018. Therapeutic strategies for treatment of inflammation-related depression. Curr. Neuropharmacol. 16 (2), 176–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Morimoto SS, 2011. The inflammation hypothesis in geriatric depression. Int. J. Geriatric Psychiatry 26 (11), 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabska J, Łucka A, Magierski R, Sobow T, Wysokiński A, 2018. Neutrophil-lymphocyte ratio is increased in elderly patients with first episode depression, but not in recurrent depression. Psychiatry Res. 263, 35–40. [DOI] [PubMed] [Google Scholar]
- Ashburner J, 2010. VBM tutorial. Tech repWellcome Trust Centre for Neuroimaging, London, UK. [Google Scholar]
- Aubé B, Lévesque SA, Paré A, et al. , 2014. Neutrophils mediate blood–spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J. Immunol. 193 (5), 2438–2454. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF, 2009. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 29 (4), 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger P, McConnell JP, Nunn M, et al. , 2002. C-reactive protein levels are influenced by common IL-1 gene variations. Cytokine. 17 (4), 171–174. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, et al. , 2007. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp. Gerontol. 42 (7), 693–701. [DOI] [PubMed] [Google Scholar]
- Brinn A, Stone J, 2020. Neutrophil-lymphocyte ratio across psychiatric diagnoses: an electronic health record investigation. BMJ Open 10 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM, 2004. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry 161 (4), 598–607. [DOI] [PubMed] [Google Scholar]
- Cazettes F, Cohen JI, Yau PL, Talbot H, Convit A, 2011. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res. 1373, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernackova A, Durackova Z, Trebaticka J, Mravec B, 2020. Neuroinflammation and depressive disorder: the role of the hypothalamus. J. Clin. Neurosci. 75, 5–10. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z, 2010. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K, 2001. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 888 (1), 83–101. [DOI] [PubMed] [Google Scholar]
- Clancy DM, Henry CM, Sullivan GP, Martin SJ, 2017. Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines. FEBS J. 284 (11), 1712–1725. [DOI] [PubMed] [Google Scholar]
- Corlier F, Hafzalla G, Faskowitz J, et al. , 2018. Systemic inflammation as a predictor of brain aging: contributions of physical activity, metabolic risk, and genetic risk. Neuroimage. 172, 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Craig A, 2009. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10 (1). [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, et al. , 2003. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 126 (10), 2139–2152. [DOI] [PubMed] [Google Scholar]
- Dalvi P, Chalmers J, Luo V, et al. , 2017. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int. J. Obes. 41 (1), 149–158. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S, Barkhof F, et al. , 2006. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. 103 (37), 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, 2009. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. 29 (2), 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir I, YÜCEL M, 2020. Investigation of relation between mortality of geriatric patients with sepsis and C-Reactive Protein, Procalcitonin and Neutrophil/Lymphocyte ratio in admission to intensive care unit. Family Practice Palliative Care 5 (1), 12–17. [Google Scholar]
- Drew W, Wilson DV, Sapey E, 2018. Inflammation and neutrophil immunosenescence in health and disease: targeted treatments to improve clinical outcomes in the elderly. Exp. Gerontol. 105, 70–77. [DOI] [PubMed] [Google Scholar]
- Eklund C, Jahan F, Pessi T, Lehtimäki T, Hurme M, 2003. Interleukin 1B gene polymorphism is associated with baseline C-reactive protein levels in healthy individuals. Eur. Cytokine Netw. 14 (3), 168–171. [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y, 2000. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J. Comp. Neurol. 421 (2), 172–188. [PubMed] [Google Scholar]
- Fujita Y, Yamashita T, 2021. Mechanisms and significance of microglia–axon interactions in physiological and pathophysiological conditions. Cell. Mol. Life Sci. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 492 (2), 145–177. [DOI] [PubMed] [Google Scholar]
- Gałecki P, Mossakowska-Wójcik J, Talarowska M, 2018. The anti-inflammatory mechanism of antidepressants–SSRIs, SNRIs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 80, 291–294. [DOI] [PubMed] [Google Scholar]
- Gallo JJ, Anthony JC, Muthén BO, 1994. Age differences in the symptoms of depression: a latent trait analysis. J. Gerontol. 49 (6), P251–P264. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M, 2015. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH, 2015. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry 78 (4), 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD, 2009a. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66 (5), 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD, 2009b. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66 (5), 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy E, Gormley S, Lopez-Rodriguez AB, Murray C, Murray C, Cunningham C, 2017. Systemic TNF-α produces acute cognitive dysfunction and exaggerated sickness behavior when superimposed upon progressive neurodegeneration. Brain Behav. Immun. 59, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JCC, Bracko O, Kersbergen CJ, et al. , 2019. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 22 (3), 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J, 2009. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71 (2), 171–186. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C, Sharma S, Fulton SE, 2013. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 7, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Wen W, Brown DA, et al. , 2015. The relationship of serum macrophage inhibitory cytokine–1 levels with Gray matter volumes in community-dwelling older individuals. PLoS One 10 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Kim JG, Park JW, Koch M, Horvath TL, Lee BJ, 2016. Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis. Sci. Rep. 6 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MKP, Barbas H, 2018. Cortical connections position primate area 25 as a keystone for interoception, emotion, and memory. J. Neurosci. 38 (7), 1677–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung IK, Kwak DI, Shin DK, Lee MS, Lee HS, Kim JY, 1997. A reliability and validity study of geriatric depression scale. J. Korean Neurol. Assoc. 36 (1), 103–112. [Google Scholar]
- Kaltenboeck A, Harmer C, 2018. The neuroscience of depressive disorders: a brief review of the past and some considerations about the future. Brain Neurosci Adv. 2, 2398212818799269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu Y, 2000. Quantitation of mRNA in micro-samples of the brain by competitive PCR: analysis of expression of interleukin-1 beta mRNA in rat hypothalamus and hippocampus after inflammatory or non-inflammatory stress. Fukuoka igaku zasshi= Hukuoka acta medica 91 (7), 170–183. [PubMed] [Google Scholar]
- Kayhan F, Gündüz S¸., Ersoy SA, Kandeger A, Annagür BB, 2017. Relationships of neutrophil–lymphocyte and platelet–lymphocyte ratios with the severity of major depression. Psychiatry Res. 247, 332–335. [DOI] [PubMed] [Google Scholar]
- Kinser PA, Lyon DE, 2013. Major depressive disorder and measures of cellular aging: an integrative review. Nurs. Res. Pract. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A, 2004. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J. Comp. Neurol. 472 (1), 113–129. [DOI] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, Wager TD, Gianaros PJ, 2018. Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neurosci. Biobehav. Rev. 94, 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, Cavanagh J, 2012. Depression: an inflammatory illness? J. Neurol. Neurosurg. Psychiatry 83 (5), 495–502. [DOI] [PubMed] [Google Scholar]
- Kushner I, 2001. C-reactive protein elevation can be caused by conditions other than inflammation and may reflect biologic aging. Cleve. Clin. J. Med. 68 (6), 535–537. [DOI] [PubMed] [Google Scholar]
- Ladenvall C, Jood K, Blomstrand C, Nilsson S, Jern C, Ladenvall P, 2006. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke. 37 (8), 2018–2023. [DOI] [PubMed] [Google Scholar]
- Laumet G, Edralin JD, Chiang AC-A, Dantzer R, Heijnen CJ, Kavelaars A, 2018. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology. 43 (13), 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander M, Karshikoff B, Johansson E, et al. , 2016. Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain Behav. Immun. 56, 34–41. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M, 2009. Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. U. S. A. 106 (41), 17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew PX, Kubes P, 2019. The neutrophil’s role during health and disease. Physiol. Rev. 99 (2), 1223–1248. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Epel ES, Mellon SH, et al. , 2015. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 55, 333–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP, 2013. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir. Res. 14 (1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED, 1997. Screening for depression in elderly primary care patients: a comparison of the Center for Epidemiologic Studies—Depression Scale and the Geriatric Depression Scale. Arch. Intern. Med. 157 (4), 449–454. [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun. 64, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac M, Pehar D, Karlović D, Babić D, Marčinko D, Jakovljević M, 2014. Metabolic syndrome, activity of the hypothalamic-pituitary-adrenal axis and inflammatory mediators in depressive disorder. Acta Clinica Croatica. 53 (1), 55–70. [PubMed] [Google Scholar]
- Mazza MG, Tringali AGM, Rossetti A, Botti RE, Clerici M, 2019. Cross-sectional study of neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratios in mood disorders. Gen. Hosp. Psychiatry 58, 7–12. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Rujescu D, Kirner A, et al. , 2001. Association of an interleukin-1β genetic polymorphism with altered brain structure in patients with schizophrenia. Am. J. Psychiatr. 158 (8), 1316–1319. [DOI] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, et al. , 2013. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 14 (12), 877–882. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16 (1), 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec B, Horvathova L, Cernackova A, 2019. Hypothalamic inflammation at a crossroad of somatic diseases. Cell. Mol. Neurobiol. 39 (1), 11–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe S, Tobe R, et al. , 2012a. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front. Neurosci. 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, et al. , 2012b. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front. Neurosci. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Miller GE, 2016. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol. Psychiatry 80 (1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Irwin MR, Wellisch DK, 2009. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 47 (3), 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M, 2006. Reviewing the definition of “elderly”. Geriatr Gerontol Int 6 (3), 149–158. [Google Scholar]
- Persson N, Ghisletta P, Dahle CL, et al. , 2014. Regional brain shrinkage over two years: individual differences and effects of pro-inflammatory genetic polymorphisms. Neuroimage. 103, 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel GD, Ganeshan K, Carvalheira JB, 2014. Hypothalamic inflammation and the central nervous system control of energy homeostasis. Mol. Cell. Endocrinol. 397 (1–2), 15–22. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59 (3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pue CA, Mortensen RF, Marsh CB, Pope HA, Wewers MD, 1996. Acute phase levels of C-reactive protein enhance IL-1 beta and IL-1ra production by human blood monocytes but inhibit IL-1 beta and IL-1ra production by alveolar macrophages. J. Immunol. 156 (4), 1594–1600. [PubMed] [Google Scholar]
- Puig J, Blasco G, Daunis-i-Estadella J, et al. , 2015. Hypothalamic damage is associated with inflammatory markers and worse cognitive performance in obese subjects. J. Clin. Endocrinol. Metabolism 100 (2), E276–E281. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuźnicka M, Owczarz M, Wieczorowska-Tobis K, et al. , 2016. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun. Ageing 13 (1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer T, Chan T, Cocks R, 1999. Do peripheral blood counts have any prognostic value following trauma? Injury. 30 (3), 179–185. [DOI] [PubMed] [Google Scholar]
- Reitz C, Berger K, de Maat MP, et al. , 2007. CRP gene haplotypes, serum CRP, and cerebral small-vessel disease: the Rotterdam Scan Study and the MEMO Study. Stroke. 38 (8), 2356–2359. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, et al. , 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J, Martel J, Lin C-S, et al. , 2018. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav. Immun. 69, 1–8. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Samann PG, et al. , 2017. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22 (6), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirivelu MP, MohanKumar P, MohanKumar SM, 2012. Differential effects of systemic interleukin-1β on gene expression in brainstem noradrenergic nuclei. Life Sci. 90 (1–2), 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Au B, Ollis L, Schmitz N, 2018. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: a systematic review and meta-analysis. Exp. Gerontol. 102, 109–132. [DOI] [PubMed] [Google Scholar]
- Soczynska JK, Kennedy SH, Woldeyohannes HO, et al. , 2011. Mood disorders and obesity: understanding inflammation as a pathophysiological nexus. NeuroMolecular Med. 13 (2), 93–116. [DOI] [PubMed] [Google Scholar]
- Song H, Kim HJ, Park KN, Kim SH, Oh SH, Youn CS, 2020. Neutrophil to lymphocyte ratio is associated with in-hospital mortality in older adults admitted to the emergency department. Am. J. Emerg. Med. 40, 133–137. [DOI] [PubMed] [Google Scholar]
- Strieter R, Kunkel S, Showell H, et al. , 1989. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 243 (4897), 1467–1469. [DOI] [PubMed] [Google Scholar]
- Sunbul EA, Sunbul M, Yanartas O, et al. , 2016. Increased neutrophil/lymphocyte ratio in patients with depression is correlated with the severity of depression and cardiovascular risk factors. Psychiatry Investig. 13 (1), 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, et al. , 2013. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum. Brain Mapp. 34 (10), 2418–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Cauda F, 2011. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 56 (4), 2157–2172. [DOI] [PubMed] [Google Scholar]
- Uhl B, Vadlau Y, Zuchtriegel G, et al. , 2016. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood 128 (19), 2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Xu AW, Koliwad SK, 2015. Hypothalamic inflammation in the control of metabolic function. Annu. Rev. Physiol. 77, 131–160. [DOI] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 150 (3), 736–744. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA, 2012. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 37 (7), 1039–1047. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 51 (1), 32–58. [DOI] [PubMed] [Google Scholar]
- Viscogliosi G, Andreozzi P, Chiriac IM, et al. , 2013. Depressive symptoms in older people with metabolic syndrome: is there a relationship with inflammation? Int. J. Geriatric Psychiatry 28 (3), 242–247. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen G, Zhong S, et al. , 2018. Association between resting-state brain functional connectivity and cortisol levels in unmedicated major depressive disorder. J. Psychiatr. Res. 105, 55–62. [DOI] [PubMed] [Google Scholar]
- Ward JR, Heath PR, Catto JW, Whyte MK, Milo M, Renshaw SA, 2011. Regulation of neutrophil senescence by microRNAs. PLoS One 6 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhang S, Li W, et al. , 2015. Cortisol awakening response predicts intrinsic functional connectivity of the medial prefrontal cortex in the afternoon of the same day. Neuroimage. 122, 158–165. [DOI] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F, 2016. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 14 (3), 339–351. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. , 1982. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 17 (1), 37–49. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhou Y, Liu Y, et al. , 2011. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage. 54 (4), 2571–2581. [DOI] [PubMed] [Google Scholar]
- Zahorec R, 2001. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 102 (1), 5–14. [PubMed] [Google Scholar]
- Zhang H, Sachdev PS, Wen W, et al. , 2016. The relationship between inflammatory markers and voxel-based gray matter volumes in nondemented older adults. Neurobiol. Aging 37, 138–146. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhuo C, Xu L, Liu F, Qin W, Yu C, 2017. Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr. Bull. 43 (6), 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]