Abstract
Propranolol reduces experimental murine cerebral cavernous malformations (CCMs) and prevents embryonic caudal venous plexus (CVP) lesions in zebrafish that follow mosaic inactivation of ccm2. Because morpholino silencing of the β1 adrenergic receptor (adrb1) prevents the embryonic CVP lesion, we proposed that adrb1 plays a role in CCM pathogenesis. Here we report that adrb1−/− zebrafish exhibited 86% fewer CVP lesions and 87% reduction of CCM lesion volume relative to wild type brood mates at 2dpf and 8–10 weeks stage, respectively. Treatment with metoprolol, a β1 selective antagonist, yielded a similar reduction in CCM lesion volume. Adrb1−/− zebrafish embryos exhibited reduced heart rate and contractility and reduced CVP blood flow. Similarly, slowing the heart and eliminating the blood flow in CVP by administration of 2,3-BDM suppressed the CVP lesion. In sum, our findings provide genetic and pharmacological evidence that the therapeutic effect of propranolol on CCM is achieved through β1 receptor antagonism.
Introduction
Cerebral cavernous malformations (CCMs), accounting for 5–15% of cerebrovascular abnormalities, are characterized by blood-filled endothelial-lined cavities, and can cause seizures, headaches, neurological deficits, and recurrent stroke risk1. Familial CCMs are due to heterozygous loss of function mutations in the KRIT1, CCM2, or PDCD10 genes with a second mutation inactivating the normal allele in random brain endothelial cells2. We previously developed a zebrafish CCM model using CRISPR-Cas9 to inactivate ccm2 in a manner that replicates the mosaic genetic background of the human disease3. This zebrafish model exhibits two phenotypic phases: lethal embryonic caudal venous plexus (CVP) cavernomas at 2 days post-fertilization (dpf) in ~30% of embryos and histologically-typical CNS CCMs in ~100% of surviving 8 week old fish3. Both phases of this model, like their murine counterpart, depend on Krüppel-like factor 2 (KLF2), confirming shared transcriptional pathways. Furthermore, both phases of the zebrafish model exhibit similar pharmacological sensitivities (Supplementary Table 1) to murine and human lesions. Thus, this zebrafish model offers a powerful tool for genetic and pharmacological analysis of the mechanisms of CCM formation.
Anecdotal case reports4–6 and a recent phase 2 clinical trial have suggested that propranolol a non-selective β adrenergic receptor antagonist benefit patients with symptomatic CCMs7. In previous studies, we observed this effect of propranolol in both zebrafish and murine models of CCM8. Importantly, propranolol is a racemic mixture of R and S enantiomers and elegant work from the Bischoff lab has implicated the R enantiomer, which lacks β adrenergic antagonism, as the component that inhibits SOX18 thereby suppressing infantile hemangiomas9. We previously found that the anti-adrenergic S enantiomer rather than the R enantiomer inhibited development of embryonic CVP cavernomas in ccm2 CRISPR zebrafish8. Furthermore, morpholino silencing of the gene that encodes the β1 (adrb1) but not β2 (adrb2) receptor also prevents CVP cavernomas8, suggesting that the β1 adrenergic receptor (β1AR), which primarily impacts hemodynamics10, contributes to the pathogenesis of CCM. Here we have inactivated the adrb1 gene to eliminate the β1AR and observed the expected reduction of heart rate and contractility which resulted in reduced blood flow through the CVP. These adrb1−/− zebrafish were protected from embryonic CVP cavernomas and, importantly, also exhibited much reduced number and volume of adult brain CCM. Furthermore, a β1-selective antagonist, metoprolol, also inhibited adult CCM suggesting that β1AR-specific antagonists may be useful for CCM treatment and will carry less potential for β2 AR-related side effects such as bronchospasm11.
Results
β1AR is important in for development of CVP cavernomas
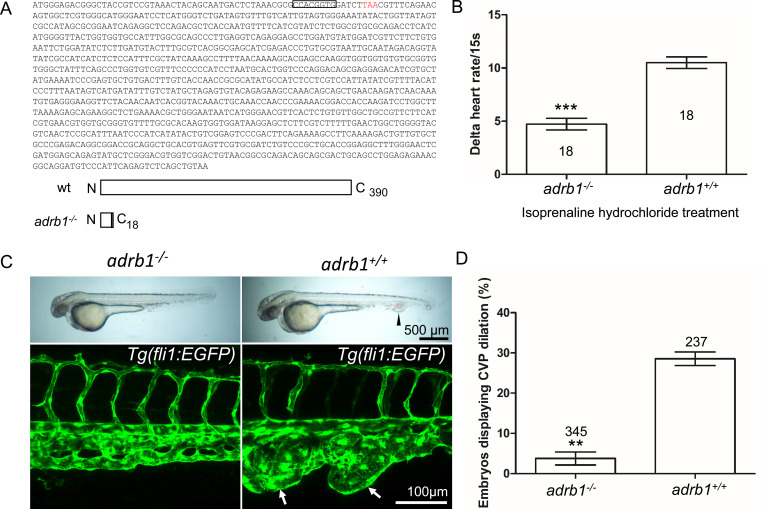
We previously reported that morpholino silencing of adrb1 rescued CVP cavernomas in zebrafish embryos8. Because of morpholinos potential for off target effects12, 13, we sought to confirm the β1AR’s potential involvement in CCM pathogenesis by inactivating adrb1. We used CRISPR-Cas9 to generate an 8-bp deletion resulting in a premature stop codon at 57 bp (Figure 1A). To exclude potential off-target effects, the top 20 potential off-target sites predicted by Cas-OFFinder (www.rgenome.net/cas-offinder) (Supplementary Table 2) were sequenced and were not mutated (data not shown). We intercrossed adrb1+/− F1 offspring resulting in adrb1−/− zebrafish embryos that displayed reduced cardiac contractility (Supplementary Video 1) and decreased heart rate (Supplementary Figure 1A). Similar to Adrb1−/− mice14, adrb1−/− zebrafish exhibited a blunted chronotropic response to a β1AR agonist, isoprenaline hydrochloride (Figure 1B). Adrb1−/− embryos had no obvious defects in vascular development and they survived to adulthood and were fertile.
Figure 1.
Adrb1 signaling is essential for CVP dilation. (A) The targeted adrb1 allele shows an 8-nucleotide deletion producing a pre-stop codon. Adrb1 null cDNA is predicted to encode truncated adrb1 protein. The wild type adrb1 protein contains 390 amino acids, while the predicted adrb1 null protein would contain 2 missense amino acids (gray bar) and would terminate after amino acid 18. (B) Isoprenaline hydrochloride (50 μM) treatment at 72hpf lead to a heart rate increase in zebrafish, while the delta heart rate in adrb1−/− is significantly smaller than that of wild type. Heartbeat was counted in 18 embryos of each group before and immediately after adding the chemical. Paired two-tailed t test, P<0.0001. (C) After ccm2 CRISPR injection, representative bright field and confocal images of 2dpf Tg(fli1:EGFP) embryos show that wild type embryos display CVP dilation, while adrb1−/− embryos were resistant to this defect. Arrowhead and arrows indicate the dilation in CVP. Scale bar: 500 μm (bright field), 100μm (confocal). (D) Paired two-tailed t test shows that percentage of embryos displaying CVP dilation is significantly smaller on adrb1−/− background than that of control embryos. P=0.0012. 345 adrb1−/− embryos and 237 control embryos from 4 experiments were examined for CVP cavernoma.
We intercrossed adrb+/− individuals and injected one-cell stage embryos with ccm2 CRISPR and blindly scored the presence of CVP cavernomas at 48hpf. As expected we observed CVP cavernomas in 28% of adrb1+/+ embryos. In sharp contrast only 3% of adrb1−/− embryos exhibited cavernomas (Fig. 1C and D) indicating that loss of β1AR prevents CVP cavernomas. these observations demonstrate that the β1AR is required for embryonic CVP cavernoma formation.
β1AR mediates formation of adult CCM in the brain
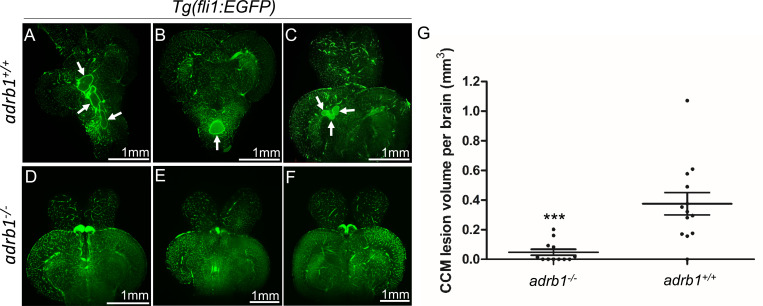
Adult ccm2 CRISPR zebrafish display highly penetrant CCMs throughout the central nervous system3. Brains from adult ccm2 CRISPR fish on adrb1−/− (12 brains) or wild type (13 brains) background were treated with CUBIC (clear, unobstructed brain/body imaging cocktails and computational analysis)15, and these transparent brains were then scanned with light-sheet microscopy and lesions were enumerated and volumes were estimated with NIH ImageJ. While the typical multi-cavern CCMs were observed in brains on wild type background appearing as blood filled dilated vessels (Figure 2A through C), ccm2 CRISPR adrb1−/− fish exhibited an 87% reduction in lesion volume (Figure 2D through G). Thus, genetic inactivation of β1AR prevented CCMs.
Figure 2.
Genetic inhibition of adrb1 signaling could rescue CCM in ccm2 CRISPR zebrafish. (A through F) Representative light sheet microscopy scanning pictures of brains from ccm2 CRISPR adult zebrafish of adrb1+/+ (A through C) and of adrb1−/− (D through F) on Tg(fli1:EGFP) background. Brains from ccm2 CRISPR on wild type background show lesions indicated by arrows (A through C), while brains from ccm2 CRISPR on adrb1−/− do not show lesions (D through F). Scale bar: 1mm. (G) Statistical analysis of total lesion volume by unpaired two-tailed t test. P=0.0005. 12 adrb1−/− brains and 13 adrb1+/+ brains were analyzed.
A selective β1AR antagonist prevents CCMs
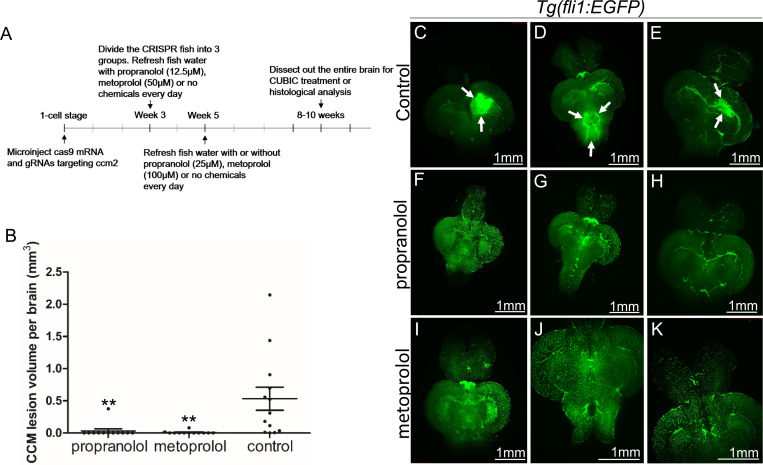
The non-selective β blocker propranolol reduces lesion volume in murine CCM models8, 16. To ascertain whether propranolol had a similar effect in the zebrafish model, the chemical treatment was started from larval stage at concentration which allows the fish to develop to two months for CCM inspection. We added 12.5μM propranolol to or vehicle control to fish water of ccm2 CRISPR zebrafish starting at 3 weeks of age. The water was refreshed daily with drug or vehicle until fish were sacrificed and brains were examined as described (Figure 3A). Quantification based on light-sheet scanning of zebrafish brains (Figure 3B) showed that compared to vehicle-treated controls (Figure 3C, D, and E), propranolol-treated groups displayed a 94% reduction in CCM lesion volume (Figure 3F, G, and H). Similarly, administration of 50μM racemic metoprolol a β1-selctive antagonist produced a similar (98%) reduction in lesion volume (Figure 3B, I, J, and K). Importantly, neither drug at the doses administered reduced the growth of the fish or the volume of their brains. In sum, both genetic and pharmacological loss of β1 adrenergic receptor signaling markedly reduces the lesion burden in the zebrafish ccm2 CRISPR model of CCM.
Figure 3.
Both propranolol and metoprolol could rescue CCM in ccm2 CRISPR zebrafish. (A) A diagram outlines the drug treatment experiment, CUBIC treatment and following recording of CCMs in adult zebrafish brain. The chemical treatment was started from week 3 with 12.5 μM propranolol or 50 μM metoprolol, and increased to 25 μM propranolol and 100 μM metoprolol, respectively from week 5. The fish water with chemicals or vehicle control are refreshed on a daily basis. (B) Statistical analysis of lesion volume by one-way ANOVA followed by Tukey’s multiple comparison test. P<0.01. 12 propranolol treated, 12 metoprolol treated, and 13 vehicle brains were analyzed. (C through K) Representative light sheet microscopy scanning pictures of brains from ccm2 CRISPR adult zebrafish on Tg(fli1:EGFP) background. In controls without chemical treatment (C, D, and E) there were vascular anomalies indicated by arrows. Neither propranolol (F, G, and H) nor metoprolol (I, J, and K) treated fish showed vascular lesions in the brain. Scale bar: 1mm.
Loss of β1AR does not prevent increased klf2a expression in ccm2 null embryos
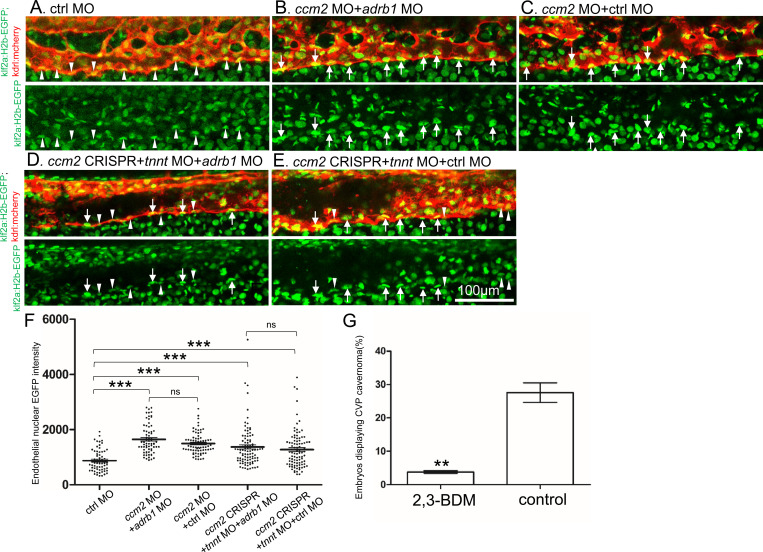
Inactivation of CCM genes leads to increased endothelial KLF2 expression17, 18, a transcription factor important in cavernoma formation3, 19. Nevertheless, silencing of adrb1 did not prevent the expected increased endothelial klf2a17 expression in ccm2 morphant Tg(klf2a:H2b-EGFP) fish in which the nuclear EGFP expression is driven by the klf2a promoter (Figure 4A, B and C). We previously reported that mosaic expression of KLF2a occurs in tnnt morphant 2 dpf ccm2 CRISPR embryos, as judged by a widely variable in endothelial klf2a reporter expression3. Nevertheless, combination of tnnt2a morphant with the adrb1 morphant (Figure 4D) or control morphant (Figure 4E) ccm2 CRISPR Tg(klf2a:H2b-EGFP) embryos both displayed a similar widely variable klf2a reporter intensity (Figure 4F) indicative of similar mosaicism. Similarly, merely slowing the heart and reducing contractility with 2,3-butanedione monoxime (BDM)20 also prevented CVP cavernomas (Fig 4G, and Supplementary Figure 2). Thus, silencing β1AR does not prevent the generalized increase in endothelial KLF2a in ccm2 morphants nor does it prevent the mosaic KLF2a increase in ccm2 CRISPR zebrafish.
Figure 4.
Adrb1 signaling does not alter klf2a expression in ccm2 CRISPR embryos. Tg(klf2a:H2b-EGFP; kdrl:mcherry) embryos were injected and nuclear EGFP signal in mcherry labeled vascular endothelial cells is recorded by confocal. Representative images from each group are shown. (A) Control MO alone injected embryos were used as control. (B and C) Ccm2 morphant embryos co-injected with adrb1 MO (B) or control MO (C) both displayed significant increase of endothelial nuclear EGFP intensity (P<0.0001) compared to that of control (A), and there is no significant difference between them. (D and E) All the ccm2 CRISPR embryos were co-injected with tnnt MO, which are absent of blood flow. Compared to that of control (A), ccm2 CRISPR embryos co-injected with adrb1 MO (D) or control MO (E) both displayed a mosaic increase of nuclear EGFP intensity of vascular endothelial cells compared to control (A) (P<0.0001), and there is no significant difference between them. Arrows indicated the endothelial nuclei with significant higher EGFP intensity than those indicated by arrowheads. Scale bar:100μm. (F) EGFP intensity of endothelial nuclei were quantified with Image J. The number of analyzed nuclei were: 63 from 10 embryos (control MO), 70 from 10 embryos (ccm2 MO+adrb1 MO), 77 from 10 embryos (ccm2 MO+control MO), 93 from 13 embryos (ccm2 CRISPR+adrb1 MO), and 94 from 13 embryos (ccm2 CRISPR+control MO). Statistical analysis is performed by one-way ANOVA followed by Tukey’s multiple comparison test. (G) At 2dpf, 2,3-BDM prevented the CVP cavernoma dramatically. 164 embryos in 2,3-BDM treated group and 177 in control group were used for Two-tailed paired t-test. P=0.0013.
Discussion
In this study, we provide genetic and pharmacological evidence to implicate the β1 adrenergic receptor (β1AR) in the pathogenesis of CCM. Our data suggest that inactivating β1AR via gene inactivation or pharmacological inhibition can significantly reduce the volume of CCM lesions in a zebrafish model.
Case reports4–6, 21 and our previous study8 revealed the potential benefit of the non-selective β-adrenergic receptor blocker propranolol in reducing CCMs in patients and mouse models, respectively. However, propranolol is a racemic mixture, and its R enantiomer which lacks β-adrenergic antagonism was reported to show therapeutic effect for infantile hemangiomas in an animal study9. Notably, our study demonstrates that adrb1−/− zebrafish are significantly protected against the formation of CCMs, suggesting that propranolol’s therapeutic effect on CCM is through β1AR antagonism. Together with the observed significant reduction of CCM lesion volume upon metoprolol treatment, a selective β1AR antagonist, supports the therapeutic potential of β1AR antagonism in CCMs. β1 selective blockers offer the advantage of causing fewer mechanism-based side effects compared to propranolol, such as bronchospasm22, and are already in clinical use (including agents like atenolol, metoprolol, nebivolol, and bisoprolol). Thus, further studies are warranted to evaluate the potential value of β1 selective antagonists in this disease.
Consistent with the Adrb1KO mice14, the adrb1−/− zebrafish embryos displayed the decreased chronotropic response to a beta-adrenergic agonist, isoprenaline, and decreased basal heart rate compared to that of adrb1+/+ brood mates; however, CVP morphology of adrb1−/− embryo was not perturbed (Supplementary Figure 3). adrb1−/− embryo also displayed a marked decrease of blood flow in CVP (Supplementary Video 2, and Supplementary Figure 1B), A similar reduction of heart rate and contractility by 2,3-BDM, a cardiac myosin ATPase inhibitor, prevented CVP dilation (Figure.4F). We previously found that arresting blood flow prevents aberrant intussusceptive angiogenesis and the resulted CVP cavernomas in ccm2 CRISPR embryos3. Taken together, these data suggest that reduced blood flow secondary to reduced cardiac function accounts for the protective effect of loss of β1AR signaling on CVP lesions and on CCM. To further confirm that reduced blood flow underlies the role of β1AR antagonism in rescuing these vascular defects, chemicals such as cardiac glycosides or phosphodiesterase inhibitors could be employed to restore cardiac pumping function in adrb1−/− embryos. Importantly, although β1AR are highly expressed in cardiomyocytes and contribute to increased cardiac output23, β1ARs are also expressed in other tissues24, 25 including endothelial cells of vascular anomalies in patients26. Thus, future studies will be required to delineate the tissue-specific contributions of β1AR signaling to CCM pathogenesis.
Experimental procedures
Zebrafish lines and handling
Zebrafish were maintained and with approval of Institutional Animal Care and Use Committee of the University of California, San Diego. The following mutant and transgenic lines were maintained under standard conditions: Tg(fli1:EGFP)y1 27, Tg(klf2a:H2b-EGFP) 28, and Tg(kdrl:mcherry)is5 29. adrb1−/− zebrafish was obtained by coninjection of Cas9 protein (EnGen Spy Cas9 NLS, M0646, NEB) with gRNA targeting adrb1. Genotyping of adrb1−/− was performed with forward primer (5’-AGAGCAGAGCGCGGATGGAA-3’) and reverse primer (5’-GATCCATACATCCAGGCT-3’).
Plasmids and morpholino
pCS2-nls-zCas9-nls (47929) and pT7-gRNA (46759) were bought from Addgene. The CRISPR RNA (crRNA) sequences used in this study are as follow: ccm2-1 5’-GGTGTTTCTGAAAGGGGAGA-3’, ccm2-2 5’- GGAGAAGGGTAGGGATAAGA-3’, ccm2-3 5’-GGGTAGGGATAAGAAGGCTC-3’, ccm2-4 5’-GGACAGCTGACCTCAGTTCC-3’, adrb1 5’-GACTCTAAACGCGCCACGG-3’. Target gRNA constructs were generated as described before30. Morpholino sequence used in this study are: adrb1 (5’-ACGGTAGCCCGTCTCCCATGATTTG-3’)31, ccm2 (5’-GAAGCTGAGTAATACCTTAA CTTCC-3’)32, tnnt2a (5’-CATGTTTGCTCTGATCTGACACGCA-3’)33, control (5’-CCTCTTACCTCAGTTACAATTTATA-3’).
RNA synthesis
The pCS2-nls-zCas9-nls plasmid containing Cas9 mRNA was digested with NotI enzyme, followed by purification using a Macherey-Nagel column, serving as the template. The capped nls-zCas9-nls RNA was synthesized using the mMESSAGE mMACHINE SP6 Transcription Kit from ThermoFisher Scientific. The resulting RNA was purified through lithium chloride precipitation, as per the instructions provided in the kit. For gRNA synthesis, the gRNA constructs were linearized using BamHI enzyme and purified using a Macherey-Nagel column. The gRNA was synthesized via in vitro transcription using the MEGAshortscript T7 Transcription Kit from ThermoFisher Scientific. After synthesis, the gRNA was purified by alcohol precipitation, as instructed in the same kit. The concentration of the nls-zCas9-nls RNA and gRNA was measured using a NanoDrop 1000 Spectrophotometer from ThermoFisher Scientific, and their quality was confirmed through electrophoresis on a 1% (wt/vol) agarose gel.
Microinjection
All injections were performed at 1-cell stage with a volume of 0.5nl. The final injection concentrations are as follow: Cas9 protein (10μM), Cas9 mRNA (750ng/μl), gRNA 120ng/μl, adrb1 MO (4ng/μl), ccm2 MO (4ng/μl), tnnt2a MO (5.3 ng/μl), control MO (4ng/μl).
Chemical treatment
Propranolol (P0995, TCI) (12.5μM) and metoprolol (M1174, Spectrum) (50μM) were used to treat the zebrafish larva from Day 21. Beginning from Day 35, adjusted concentration of propranolol (25μM) or metoprolol (100μM) were used to treat the juvenile fish. The chemicals were dissolved in fish water, and fish water containing chemicals were refreshed daily. Egg water without above chemicals was refreshed daily for fish used as negative control. 2,3- butanedione monoxime (BDM) (6mM) was added to egg water of the ccm2 CRISPR embryos at 25hpf, and CVP cavernoma was observed at 2dpf.
Airyscan imaging and fluorescence intensity analysis
To prepare the embryos for imaging, they were first anesthetized using egg water containing 0.016% tricaine (3-amino benzoic acid ethyl ester) from Sigma-Aldrich. Subsequently, the anesthetized embryos were embedded in 1% low melting point agarose obtained from Invitrogen (product number 16520050). The imaging process was carried out using a Zeiss 880 Airyscan confocal microscope, utilizing the standard Airyscan stack mode, with a Plan-Apochromat 20x/0.8 M27 objective. The scanning setup is as follow: Lasers (Green 488nm: 26.0%, Red 561nm: 15.0%), Master Gain (800), Digital Gain (1.00), Scaling X (0.415μm), Scaling Y (0.415μm), and Scaling Z (0.800μm). The intensity of nuclear EGFP (enhanced green fluorescent protein) was quantified using ImageJ software. The selected background area signal was measured by running Analyze>Measure. Then the background value was subtracted by running Process>Math>Subtract. “Freehand selections” button was used for outlining the endothelial nucleus stack by stack along Z-axis. By running Analyze>Measure, the information of “Area” and “IntDen (Integrated Density)” of the selected endothelial nucleus was obtained. The average EGFP intensity of a nucleus equals to the summation of “IntDen” divided by summation of “Area”.
Heartbeat and blood flow recording
Heartbeat and blood flow were recorded using Olympus MVX10. The embryos were treated with 0.004% tricaine which does not have effect on heartbeat34, 35.
Zebrafish brain dissection, CUBIC treatment and lightsheet imaging
The dissection of zebrafish brains followed the methodology described in a previous study by Gupta et al.36. The CUBIC method was optimized based on the findings from a previous report by Susaki et al.15. The brains were fixed in 4% paraformaldehyde (PFA) with a pH of 7.5 for 24 hours and subsequently washed with PBS (phosphate-buffered saline) for an additional 24 hours. Following the PBS wash, the brains underwent CUBICR1 treatment at 37°C in a water bath for 42 hours. For imaging, the samples were placed in CUBICR2 as the imaging medium and imaged using a ZEISS Lightsheet Z.1 microscope. Scanning was carried out utilizing 5X dual illumination optics in combination with a 5X objective.
Statistical analysis
The statistical analysis was conducted using GraphPad Prism software. P-values were calculated using an unpaired two-tailed Student’s t-test, unless otherwise specified. The bar graphs display the mean values along with their corresponding standard deviations (SD).
Supplementary Material
Acknowledgements
We gratefully acknowledge Douglas A Marchuk, and Issam A. Awad for their invaluable advice on both this study and the manuscript. This work was supported by NIH grants P01 NS092521 and P01 HL151433. We also acknowledge Jennifer Santini and Marcy Erb for microscopy technical assistance and resources provided by the UCSD School of Medicine Microscopy Core (NINDS P30 NS047101).
Literature Cited
- 1.Leblanc GG, Golanov E, Awad IA, Young WL and Biology of Vascular Malformations of the Brain NWC. Biology of vascular malformations of the brain. Stroke. 2009;40:e694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labauge P, Denier C, Bergametti F and Tournier-Lasserve E. Genetics of cavernous angiomas. Lancet Neurol. 2007;6:237–44. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Tran V, Shaked I, Xue B, Moore T, Lightle R, Kleinfeld D, Awad IA and Ginsberg MH. Abortive intussusceptive angiogenesis causes multi-cavernous vascular malformations. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg J, Jaeggi C, Schoeni D, Mordasini P, Raabe A and Bervini D. Bleeding risk of cerebral cavernous malformations in patients on beta-blocker medication: a cohort study. J Neurosurg. 2018:1–6. [DOI] [PubMed] [Google Scholar]
- 5.Moschovi M, Alexiou GA, Stefanaki K, Tourkantoni N and Prodromou N. Propranolol treatment for a giant infantile brain cavernoma. J Child Neurol. 2010;25:653–5. [DOI] [PubMed] [Google Scholar]
- 6.Reinhard M, Schuchardt F, Meckel S, Heinz J, Felbor U, Sure U and Geisen U. Propranolol stops progressive multiple cerebral cavernoma in an adult patient. J Neurol Sci. 2016;367:15–7. [DOI] [PubMed] [Google Scholar]
- 7.Lanfranconi S, Scola E, Meessen J, Pallini R, Bertani GA, Al-Shahi Salman R, Dejana E, Latini R and Treat CCMI. Safety and efficacy of propranolol for treatment of familial cerebral cavernous malformations (Treat_CCM): a randomised, open-label, blinded-endpoint, phase 2 pilot trial. Lancet Neurol. 2022. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Shenkar R, Detter MR, Moore T, Benavides C, Lightle R, Girard R, Hobson N, Cao Y, Li Y, Griffin E, Gallione C, Zabramski JM, Ginsberg MH, Marchuk DA and Awad IA. Propranolol inhibits cavernous vascular malformations by beta1 adrenergic receptor antagonism in animal models. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overman J, Fontaine F, Wylie-Sears J, Moustaqil M, Huang L, Meurer M, Chiang IK, Lesieur E, Patel J, Zuegg J, Pasquier E, Sierecki E, Gambin Y, Hamdan M, Khosrotehrani K, Andelfinger G, Bischoff J and Francois M. R-propranolol is a small molecule inhibitor of the SOX18 transcription factor in a rare vascular syndrome and hemangioma. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Meiracker AH, Man in’t Veld AJ, Boomsma F, Fischberg DJ, Molinoff PB and Schalekamp MA. Hemodynamic and beta-adrenergic receptor adaptations during long-term beta-adrenoceptor blockade. Studies with acebutolol, atenolol, pindolol, and propranolol in hypertensive patients. Circulation. 1989;80:903–14. [DOI] [PubMed] [Google Scholar]
- 11.Maclagan J and Ney UM. Investigation of the mechanism of propranolol-induced bronchoconstriction. Br J Pharmacol. 1979;66:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA and Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen JS and Smith JC. Controlling morpholino experiments: don’t stop making antisense. Development. 2008;135:1735–43. [DOI] [PubMed] [Google Scholar]
- 14.Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP Jr., Barsh GS, Bernstein D and Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci U S A. 1996;93:7375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A and Ueda HR. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc. 2015;10:1709–27. [DOI] [PubMed] [Google Scholar]
- 16.Oldenburg J, Malinverno M, Globisch MA, Maderna C, Corada M, Orsenigo F, Conze LL, Rorsman C, Sundell V, Arce M, Smith RO, Yau ACY, Billstrom GH, Magi CO, Beznoussenko GV, Mironov AA, Fernando D, Daniel G, Olivari D, Fumagalli F, Lampugnani MG, Dejana E and Magnusson PU. Propranolol Reduces the Development of Lesions and Rescues Barrier Function in Cerebral Cavernous Malformations: A Preclinical Study. Stroke. 2021;52:1418–1427. [DOI] [PubMed] [Google Scholar]
- 17.Renz M, Otten C, Faurobert E, Rudolph F, Zhu Y, Boulday G, Duchene J, Mickoleit M, Dietrich AC, Ramspacher C, Steed E, Manet-Dupe S, Benz A, Hassel D, Vermot J, Huisken J, Tournier-Lasserve E, Felbor U, Sure U, Albiges-Rizo C and Abdelilah-Seyfried S. Regulation of beta1 integrin-Klf2-mediated angiogenesis by CCM proteins. Dev Cell. 2015;32:181–90. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Rawnsley DR, Goddard LM, Pan W, Cao XJ, Jakus Z, Zheng H, Yang J, Arthur JS, Whitehead KJ, Li D, Zhou B, Garcia BA, Zheng X and Kahn ML. The cerebral cavernous malformation pathway controls cardiac development via regulation of endocardial MEKK3 signaling and KLF expression. Dev Cell. 2015;32:168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z, Tang AT, Wong WY, Bamezai S, Goddard LM, Shenkar R, Zhou S, Yang J, Wright AC, Foley M, Arthur JS, Whitehead KJ, Awad IA, Li DY, Zheng X and Kahn ML. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature. 2016;532:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartman T, Walsh EC, Wen KK, McKane M, Ren J, Alexander J, Rubenstein PA and Stainier DY. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2:E129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miquel J, Bruneau B and Dupuy A. Successful treatment of multifocal intracerebral and spinal hemangiomas with propranolol. J Am Acad Dermatol. 2014;70:e83–e84. [DOI] [PubMed] [Google Scholar]
- 22.Ji Y, Chen S, Wang Q, Xiang B, Xu Z, Zhong L, Yang K, Lu G and Qiu L. Intolerable side effects during propranolol therapy for infantile hemangioma: frequency, risk factors and management. Sci Rep. 2018;8:4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohrer DK, Chruscinski A, Schauble EH, Bernstein D and Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem. 1999;274:16701–8. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes S and Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–56. [PubMed] [Google Scholar]
- 25.Osswald W and Guimaraes S. Adrenergic mechanisms in blood vessels: morphological and pharmacological aspects. Rev Physiol Biochem Pharmacol. 1983;96:53–122. [DOI] [PubMed] [Google Scholar]
- 26.Stanciulescu MC, Popoiu MC, Cimpean AM, David VL, Heredea R, Cerbu S and Boia ES. Expression of beta1 adrenergic receptor in vascular anomalies in children. J Int Med Res. 2021;49:3000605211047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson ND and Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–18. [DOI] [PubMed] [Google Scholar]
- 28.Heckel E, Boselli F, Roth S, Krudewig A, Belting HG, Charvin G and Vermot J. Oscillatory Flow Modulates Mechanosensitive klf2a Expression through trpv4 and trpp2 during Heart Valve Development. Curr Biol. 2015;25:1354–61. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Kaiser MS, Larson JD, Nasevicius A, Clark KJ, Wadman SA, Roberg-Perez SE, Ekker SC, Hackett PB, McGrail M and Essner JJ. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. 2010;137:3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jao LE, Wente SR and Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A. 2013;110:13904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steele SL, Yang X, Debiais-Thibaud M, Schwerte T, Pelster B, Ekker M, Tiberi M and Perry SF. In vivo and in vitro assessment of cardiac beta-adrenergic receptors in larval zebrafish (Danio rerio). J Exp Biol. 2011;214:1445–57. [DOI] [PubMed] [Google Scholar]
- 32.Mably JD, Chuang LP, Serluca FC, Mohideen MA, Chen JN and Fishman MC. santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development. 2006;133:3139–46. [DOI] [PubMed] [Google Scholar]
- 33.Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M and Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–10. [DOI] [PubMed] [Google Scholar]
- 34.Schwerte T, Uberbacher D and Pelster B. Non-invasive imaging of blood cell concentration and blood distribution in zebrafish Danio rerio incubated in hypoxic conditions in vivo. J Exp Biol. 2003;206:1299–307. [DOI] [PubMed] [Google Scholar]
- 35.Langheinrich U, Vacun G and Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol. 2003;193:370–82. [DOI] [PubMed] [Google Scholar]
- 36.Gupta T and Mullins MC. Dissection of organs from the adult zebrafish. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.