Abstract
The clinical manifestations observed in human immunodeficiency virus type 1 (HIV-1)-infected patients are primarily due to the capacity of the virus and its components to inactivate the immune system. HIV-1 Tat protein could participate in this immune system disorder. This protein is secreted by infected cells of HIV-infected patients and is free in the plasma, where it can interact and be taken up by both infected and noninfected cells. In asymptomatic patients infected by HIV-1, production of interleukin-10 (IL-10), a highly immunosuppressive cytokine, is associated with disease progression to AIDS. In the present work, we tested the capacity of Tat to induce IL-10 production by peripheral blood monocytes of healthy donors. The results show that Tat causes the production of IL-10 in a dose- and stimulation time-dependent manner. Investigations of the mechanisms involved in signal transduction show that (i) the calcium pathway is not or only slightly involved in Tat-induced IL-10 production, (ii) the protein kinase C pathway plays an essential role, and (iii) monocyte stimulation by Tat results in the intranuclear translocation of transcription factor NF-κB and in the induction of phosphorylation of the mitogen-activated protein kinases ERK1 and ERK2; activation of these two potential substrates of protein kinase C is required for the production of IL-10. Finally, our results suggest that the effect of Tat is exerted at the membrane level and that the active domain is located within N-terminal residues 1 to 45. This production of IL-10 induced by Tat could participate in the progression of HIV infection to AIDS.
The clinical manifestations observed in patients infected by human immunodeficiency virus (HIV) are primarily due to the capacity of the virus to inactivate the immune system. Before the decrease in the number of CD4 T lymphocytes, a disorder in the immune response is observed (17, 18, 38). The cellular and molecular mechanisms of this deficiency of the immune system cannot be explained solely by the direct lytic effect of the virus on infected CD4 T lymphocytes. HIV-1 can infect target cells and remain in the form of a latent provirus. In addition to this mechanism to escape the defenses of the immune system, the virus uses other strategies involving viral and cellular factors.
One of the potential candidates is HIV Tat gene product, a 14-kDa protein known for its transactivating activity on the viral genome (34). Tat binds to the secondary-structure sequence TAR (Tat activation region) 5′ of viral RNA during transcription and thereby enables the recruitment of cellular factors forming the complex of cyclin T1 and cdk9, called TAK (Tat-associated kinase), that phosphorylates the C-terminal domain on RNA polymerase 2, thereby activating transcription elongation (28). Tat also participates in the pathogenesis of HIV-1 infection by its capacity to interact with different cell types. Tat is found in the serum of HIV-infected patients (26, 54). It is secreted by infected cells (26) and can act on other cells, whether or not they are infected (10, 21, 22, 39, 57). Tat activates quiescent CD4+ T cells, rendering them permissive for HIV-1 infection (37). This effect is accentuated by the capacity of Tat to increase the rate of expression of coreceptors for the chemokines CXCR4 and CCR5 (29). Tat also contributes to immune system disorders by inducing apoptosis of T lymphocytes (36). Tat interferes with the cell-mediated immune response by inhibiting major histocompatibility complex class I molecule expression, as reported for Jurkat cells (30), NK cell activity (59), and interleukin-12 (IL-12) production by dendritic cells (45) and by monocytes (31).
It is now established that deregulation of cytokine production contributes to the attenuated functioning of the immune system in the course of HIV-1 infection. HIV-1-infected patients thus develop a progressive decrease in the TH1-type cellular immune response that results in an increase in the TH2-type humoral immune response mediated by IL-4, IL-6, and IL-10 (16, 17, 38).
The infection of T-cell (H9) or promonocytic (U937) lines by HIV in vitro stimulates the secretion of IL-10 (40). In line with these reports, Shearer's group, in a study including more than 1,000 patients (18), identified four patient classes depending on the capacity of their CD4 T lymphocytes to respond to different stimuli (mitogen, alloantigen, influenza virus, and HIV-1 antigens). The progressive loss of the response of the immune system to these stimuli was found to be associated with a course leading to AIDS. Considerable production of IL-10 by peripheral blood mononuclear cells (PBMC) was observed in these patients and paralleled the alteration in CD4+ T-cell proliferative function (18). In addition, the immunosuppressive effect of IL-10 also correlated with the capacity of isolated mononuclear cells of patients infected by HIV and immunodepressed to proliferate in vitro after stimulation by peptide antigens of the HIV envelope glycoproteins in the presence of a neutralizing anti-IL-10 antibody (18).
The production of cytokines involves primarily two signaling pathways, the calcium pathway and activation of protein kinase C (PKC) (13, 3). The simultaneous use of a calcium ionophore such as ionomycin and a PKC activator such as phorbol myristate acetate (PMA) would thus lead to the stimulation of production of most cytokines (in particular IL-2 and IL-4). These pathways are activated following the binding of a ligand to its receptor. Activated phospholipase C (PLC) cleaves phosphatidylinositol biphosphate to inositol 1,4,5-triphosphate, responsible for the mobilization of intracellular calcium, and to diacylglycerol, which initiates PKC activation. These two pathways lead to the phosphorylation and activation of cellular proteins (mitogen-activated protein [MAP] kinases) and of transcription factors (NF-AT, NF-κB, AP-1, and CREB) responsible for the induction of cytokine genes (25).
The HIV-mediated production of IL-10, a cytokine with immunosuppressive properties (41), seems to be a crucial event during HIV infection.
The aims of the present work were to determine if Tat could have a direct effect on human monocytes, a prime target of HIV but also a key cell in the immune system, by inducing the production of IL-10 and to elucidate the intracellular mechanisms responsible for this production of IL-10.
Our results show that Tat from HIV-1 induces the production of IL-10 by human peripheral blood monocytes. This IL-10 production is highly dependent on the activation of PKC. Transcription factor NF-κB and MAP kinases ERK1 and ERK2 (ERK1/2), potential substrates of PKC, are active and are apparently involved in the production of IL-10 induced by Tat.
MATERIALS AND METHODS
Monocyte isolation.
PBMC were isolated from blood or the buffy coat from healthy HIV-negative donors in a Ficoll density gradient (Pharmacia). The PBMC were resuspended in 60/30 complete medium (60% AIM V and 30% Iscove [Gibco]) containing penicillin (100 IU/ml), streptomycin (100 μg/ml), and 10% fetal calf serum (FCS). PBMC were then plated at a density of 106 cells/well in 24-well Primaria (Becton Dickinson) tissue culture plates. After 24 h of culture at 37°C in 5% CO2, nonadherent cells were removed, and the remaining cells were washed twice and then incubated with the different compounds tested.
Recombinant HIV-1 Tat protein. (i) Native recombinant Tat.
Recombinant HIV-1 Tat protein was obtained from Agence Nationale de la Recherche sur le SIDA (Paris, France). The level of endotoxin contamination in purified HIV-1 Tat was assessed by using the Limulus amebocyte lysate assay (BioSepra, Villeneuve la Garenne, France). HIV-1 Tat protein contained less than 0.3 EU/μg. This Tat preparation has been shown to be biologically active (7, 53).
(ii) Chemically oxidized Tat.
Native recombinant Tat was oxidized with 3% H2O2 in phosphate-buffered saline (PBS) for 1 h at 25°C as previously described (19). In contrast to unmodified native Tat, no transactivation activity was found with this oxidized Tat (data not shown), in accordance with data reported by Cohen et al. (19).
(iii) Tat mutants.
HIV Tat mutants were produced as glutathione-S-transferase (GST) fusion proteins in Escherichia coli. The wild-type GST-Tat 1-101 and Tat-deleted mutants GST-Tat 1-72, GST-Tat 1-55, GST-Tat 1-45, GST-Tat 20-72, and GST-Tat 30-72 were purified as previously described (8). As a control, GST was purified in the same conditions and used in the same experiments. All these constructions are lipopolysaccharide (LPS) free (less than 0.3 EU/μg) and biologically active, as previously described (8).
Signal transduction experiments.
Isolated monocytes were cultured in 60/30 complete medium in the absence or presence of HIV-1 Tat protein or LPS. HIV-1 Tat (3.6 × 10−5 M) and LPS (500 μg/ml) were prepared as stock solutions in PBS and water, respectively. Further dilutions were done in FCS-free medium.
Monocytes were incubated for 30 min with various signal transduction pathway inhibitors, and HIV-1 Tat (10 nM) was added for an additional 24 h. The following inhibitors were used: U73122 (1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione) (Calbiochem, La Jolla, Calif.) as an inhibitor of PLC; cyclosporin A (Calbiochem) as an inhibitor of calcineurin; BAPTA/AM (Calbiochem) as an intracellular calcium chelator; RO31-8220 (3-[1-[3-(amidinothio)propyl-1H-indol-3-yl]-3-(1-methyl-1H-indol-3-yl) maleimide methane sulfonate) (Calbiochem) as a specific inhibitor of PKC that competes with ATP (29); H89 (N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide HCl) (Calbiochem) as a selective inhibitor of PKA; TLCK HCl (Nα-tosyl-lysine chloromethyl ketone hydrochloride) (Calbiochem) as an inhibitor of trypsin-like serine proteinases; and PD98059 (2′-amino-3′-methoxyflavone) (Calbiochem) as a selective inhibitor of MAP kinase kinase (MEK) that acts by inhibiting the activation of MAP kinase (ERK) and subsequent phosphorylation of MAP kinase substrates.
Cyclosporin A (25 or 10 mg/ml) was dissolved in ethanol; BAPTA/AM (10−2 M), RO31-8220 (1.81 mM), H89 (10−2 M), U73122 (10−2 M), TLCK (1.35 10−1 M), and PD98059 (1.87 10−2 M) were initially dissolved in dimethyl sulfoxide (DMSO). Further dilutions were done in FCS-free Iscove modified Dulbecco's medium.
PMA (phorbol-12-myristate-13-acetate) (Calbiochem) was used as an activator of PKC. It was prepared at 1.62 × 10−3 M in DMSO, and further dilutions were done in FCS-free medium.
Rolipram (4-[3-(cyclopentyloxy)-4-methoxy-phenyl]-2-pyrolidinone) (Sigma), a cyclic AMP (cAMP)-specific phosphodiesterase inhibitor, was used as a PKA activator. It was initially dissolved in ethanol at 9 mM, and further dilutions were done in FCS-free medium.
A putative cytotoxic effect of the different inhibitors was tested by a trypan blue dye exclusion assay, and none was found to be cytotoxic (viability was >90%) at the concentrations used.
Immobilized HIV Tat protein.
Tat was immobilized in wells by incubation for 2 h at 37°C. After two washes to eliminate nonfixed Tat, monocytes (106) were added and cultured for 24 h with different concentrations of Tat (5, 50, or 500 nM). To control that in these conditions Tat protein could not enter cells, an intracellular Tat-dependent transactivation assay was performed. HeLa P4 cells stably transfected with an HIV-1 long terminal repeat (LTR)-lacZ construct as previously described (47) were added to Tat-coated wells. LacZ expression, which reflects Tat penetration into cells and LTR transactivation, was analyzed by monitoring blue staining of the cells in the presence of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (53). Briefly, cells were cultured overnight at 37°C in 5% CO2. They were washed twice with PBS (0.5 mM MgCl2, 1 mM CaCl2), fixed with 0.5% glutaraldehyde for 10 min, and washed twice with the same buffer. Cells were then incubated for 3 h in a mixture containing 1 mg of β-galactosidase substrate (X-Gal) per ml in PBS containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM magnesium chloride. X-Gal had been previously dissolved in DMSO at 40 mg/ml. The enzymatic reaction was stopped by removing the X-Gal reaction mixture. Stained cells were stored in PBS buffer.
IL-10 detection by ELISA.
IL-10 production was quantified by using a two-site sandwich enzyme-linked immunosorbent assay (ELISA). MAB217 (R & D Systems, Oxon, U.K.) monoclonal antibody (MAb) (4 μg/ml) was used for capture overnight at room temperature. After three washes with PBS containing 0.05% Tween 20 (wash buffer), plates were blocked by adding 300 μl of PBS containing 1% bovine serum albumin and 5% sucrose to each well for a minimum of 1 h. After three washes, culture supernatants (100 μl/well) were incubated for 2 h at room temperature. Plates were then washed three times and incubated with biotinylated anti-human IL-10 polyclonal antibody (BAF217), obtained from R & D Systems, for 2 h at room temperature. After washing, the bound biotinylated polyclonal antibody was visualized by an additional 20 min of incubation with streptavidin-peroxidase (Sigma, Saint Quentin Fallavier, France) diluted 1:16,000 in PBS-Tween-bovine serum albumin. After washing, the plates were incubated with the substrate O-phenylendiamine dihydrochloride plus H2O2 (Sigma). The reaction was stopped by adding 50 μl of H2SO4 (4 N) to each well. Absorbance was read at 490 nm, with a wavelength correction of 600 nm. Cytokines were quantified from a standard curve generated by using various concentrations of recombinant human IL-10 (R & D Systems). The limit of detection was 15 pg/ml.
Intracellular Ca2+ concentrations.
Intracellular Ca2+ concentrations were determined by emission microspectrofluorimetry as previously described (3, 41). Cells were incubated with 5 μM 3-fluo-acetoxymethylester (AM; Molecular Probes, Leiden, The Netherlands) for 30 min at 37°C. Intracellular Ca2+ concentrations were measured in cells stimulated by Tat (10 or 100 nM) or 1 μM ionomycin (Sigma). Ionomycin was initially dissolved in DMSO at a concentration of 2 mM. Cell preparations were placed on the stage of an inverted Diaphot microscope (Nikon) and observed with a 40× objective. The excitation wavelength was 490 nm, with a 525-nm barrier filter. Fluorescence was detected by an intensified charge coupled device camera (C2400-80; Hamamatsu, Photonics, Hamamatsu, Japan). With the magnification used (40×) a field of 200 by 200 μm was recorded by the camera. Three to five fields were observed for each type of experiment, and in each field 12 windows (9 μm) were distributed on different cells and analyzed for fluorescence. Images were captured at intervals of 5 s and processed with the Argus 50 image processing system (Hamamatsu Photonics). Time courses of Ca2+ signals in cells were analyzed with Argus 50 software. Data are presented as the ratio of fluorescence (F) in stimulated cells to fluorescence (F0) at the baseline level. Cells were scored as positive if the variation in fluorescence intensity was 5% above the baseline level.
EMSA.
For the electrophoretic mobility shift assay (EMSA), nuclear extracts were prepared as previously described (50). Briefly, cold Tris-buffered saline (TBS, pH 7.8) was added to monocytes (2 × 106 cells), which were scraped and harvested after 16 h of incubation. Monocytes, whole PBMC, or monocyte-depleted PBMC (2 × 106 cells) were washed and collected. Cells were transferred to 1.5-ml Eppendorf tubes and microcentrifuged at 4°C for 15 s. The pellet was resuspended in 400 μl of lysis buffer (10 mM HEPES [pH 7.9], 0.1 mM EGTA, 0.1 mM EDTA, 10 mM KCl, 1 mM dithiothreitol [DTT], and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). After 15 min on ice, 25 μl of 10% Nonidet P-40 (Sigma) solution was added to the samples, and cells were homogenized with a Vortex and microcentrifuged at 4°C for 30 s. The pellets were resuspended in 50 μl of B lysis buffer (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF), and the cells were agitated vigorously for 15 min at 4°C on a shaking platform. The nuclear extracts were microcentrifuged for 5 min at 4°C, and aliquots of supernatants were frozen at −80°C. Total protein levels were determined with the Bradford assay, using a commercial protein assay reagent (Bio-Rad, Ivry Sur Sein Cedex, France). The NF-κB mobility shift assays were performed using 6 μg of protein of nuclear extract, 104 cpm of radiolabeled double-stranded NF-κB probe in C buffer (100 mM KCl, 1 mM DTT, 1 μM ZnSO4, 20% glycerol, 0.01% Nonidet P-40, 50 mM HEPES [pH 7.9]) supplemented with bovine serum albumin, tRNA, and poly(dI:dC) in a final volume of 20 μl. After 20 min at room temperature, electrophoresis of the mixture was carried out at 120 V in a 5% polyacrylamide gel.
Two oligonucleotide sequences were used. The first was the HIV-1 LTR NF-κB sequence 5′-GCTGGGGACTTTCCAGGGAG-3′, and in order to determine specificity of binding, the second was the NF-κB mutated sequence 5′-GCTGTTTACTGGCCCAGGGAG-3′.
SDS-PAGE and Western blot.
After incubating cells (106) in the presence or absence of Tat or PMA for 15 or 30 min, cold TBS (pH 7.8) was added, and the cells were transferred to 1.5-ml Eppendorf tubes and microcentrifuged at 4°C for 15 s. The pellet was resuspended in 200 μl of lysis buffer (10 mM HEPES [pH 7.9], 0.1 mM EGTA, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT, 0.5 mM PMSF). After 15 min on ice, 7 μl of 10% Nonidet P-40 solution was added to the samples and the cells were homogenized with a Vortex. The cytoplasmic extracts were microcentrifuged at 4°C for 30 s, and the supernatants were stored at −80°C until used.
Generated extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and separated proteins were transferred to nitrocellulose membranes. Immunoblotting was conducted using either rabbit polyclonal anti-phospho-p44/42 MAP kinase (Thr-202/Tyr-204) antibody (1:1,000) (New England Biolabs, Hertfordshire, England) or rabbit anti-p44/42 MAP kinase antibody (1:1,000) (New England Biolabs). Membranes were incubated with the primary antibody (2 h at room temperature). Immunoreactive bands were then detected by incubation for 2 h at room temperature with swine anti-rabbit immunoglobulins conjugated with horseradish peroxidase (1:1,000) (Dako A/S, Roskilde, Denmark). The membranes were then visualized using a chemiluminescent substrate (Pierce, Rockford, Ill.).
Statistical analysis.
The Mann-Whitney nonparametric test was used in this study to compare data for stimulated cells in the absence and presence of inhibitors.
RESULTS
Tat induces the production of IL-10 by human monocytes.
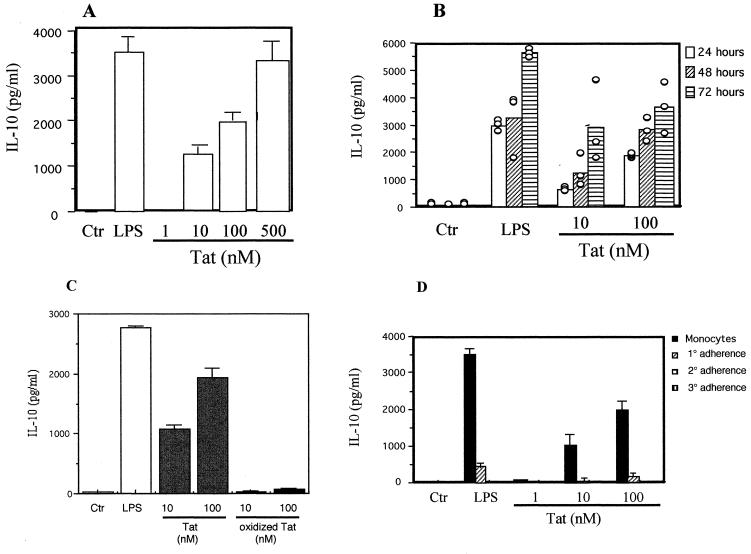
Monocytes from healthy donors were purified from the buffy coat and cultured for 24 h in the presence of Tat at 1, 10, 100, or 500 nM Tat or LPS at 100 ng/ml. No IL-10 production was detected in the supernatant of monocytes cultured in the absence of Tat or LPS (Fig. 1). In contrast, addition of various concentrations of Tat induced strong and dose-dependent IL-10 production by monocytes from 1,234 ± 186 pg/ml with 10 nM Tat up to 3,280 ± 427 pg/ml by cells treated with Tat at 500 nM (Fig. 1A). Production that was observed at 24 h increased when Tat and monocytes were incubated for 48 and 72 h (Fig. 1B). The quantity of IL-10 produced by monocytes in response to Tat is thus dose and stimulation time dependent. To verify the specificity of the Tat effect, a chemically inactivated Tat mutant was used as a control. When tested in the same conditions, oxidized Tat was unable to induce IL-10 production by human monocytes (Fig. 1C). This specificity was further characterized using a mixture of three monoclonal antibodies directed against Tat. Preincubation of Tat (10 nM) with these antibodies totally inhibits IL-10 production (Fig. 2B).
FIG. 1.
Production of IL-10 by monocytes treated with Tat. (A) Monocytes (106) were incubated in the absence or presence of Tat (1, 10, 100, or 500 nM) or LPS (100 ng/ml) for 24 h. (B) Monocytes were identically treated with LPS (100 ng/ml) or Tat (10 or 100 nM) but for 24, 48, or 72 h. (C) Specificity of Tat. Monocytes (106) were incubated in the absence or presence of native Tat (10 or 100 nM), oxidized Tat (1 h at 25°C in PBS plus 3% H2O2 at 10 and 100 nM), or LPS (100 ng/ml) for 24 h. (D) PBMC were depleted of monocytes by three successive adherence steps (1°, 2°, and 3°) in 24-well plates. After each adherence step, cells remaining in suspension (106) were incubated in the absence or presence of LPS at 100 ng/ml or Tat at 1, 10, or 100 nM. Culture supernatants were recovered, and the presence of IL-10 was determined by ELISA. For A, B, and D, the values are the means ± standard deviation (SD) of three experiments with cells from one donor. Similar results were obtained with cells from three different donors. For C, the values are from results obtained with three different donors. Ctr, control.
FIG. 2.
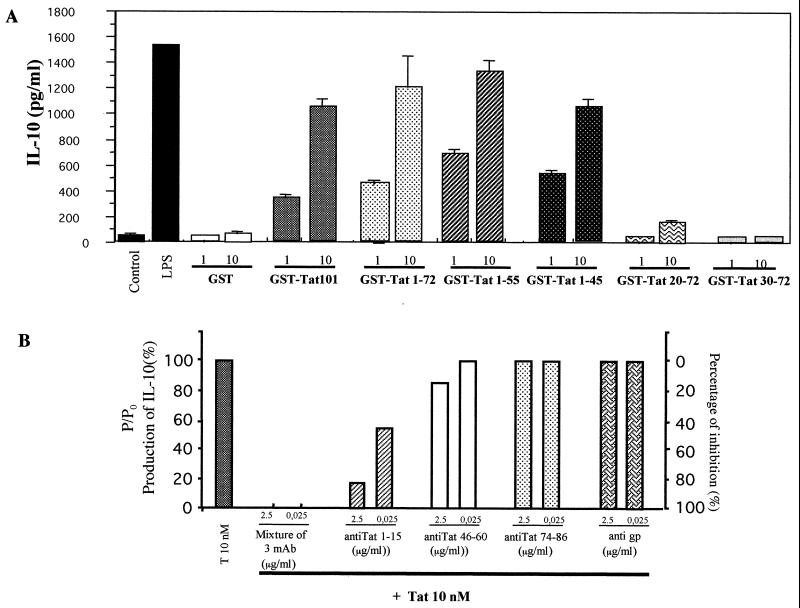
Specificity of Tat-induced IL-10 production and mapping of the active domain. (A) Monocytes (106) were incubated with wild-type GST-Tat 1-101 (1 or 10 nM) or with recombinant mutants GST-Tat 1-72, GST-Tat 1-55, GST-Tat 1-45, GST-Tat 20-72, or GST-Tat 30-72 (1 or 10 nM) or with GST as a negative control for 24 h. Culture supernatants were recovered, and the presence of IL-10 was determined by ELISA. The values are the means ± SD of three experiments with cells from one donor. Similar results were obtained with cells from three different donors. (B) Tat (10 nM) was incubated for 1 h or not with MAbs directed against Tat epitopes 1 to 15, 46 to 60, or 74 to 86 (2.5 or 0.025 μg/ml) or the mixture of the three MAbs. After 24 h, culture supernatants were recovered, and the presence of IL-10 was determined by ELISA. As control, an MAb directed against gp140 of simian immunodeficiency virus was used as a control in the same conditions. Results represent the ratio of production of IL-10 by monocytes stimulated by Tat (10 nM) incubated with MAb and production of IL-10 produced after stimulation by Tat (10 nM) alone (P/P0). On the right, the percent inhibition of IL-10 production induced by Tat (10 nM) is represented. Mouse MAbs were obtained from the Agence Nationale de la Recherche sur le SIDA (Paris, France).
As expected, LPS used at 100 ng/ml also induced high IL-10 production (Fig. 1A). Thus, the recombinant protein Tat was tested to detect possible contamination by this component. The Tat preparation used in this work contained no LPS within the limit of sensitivity of the test. Furthermore, LPS at the limit of detection in this test, 50 pg/ml, does not cause the production of IL-10 by monocytes in our system (data not shown). The production of IL-10 by monocytes is thus due to HIV-1 Tat.
Since our experiments were run with monocytes obtained from the buffy coat, the induction of IL-10 production was also confirmed with monocytes isolated from the fresh whole blood of healthy donors (data not shown). In order to determine if cells other than monocytes from peripheral blood can produce IL-10 following stimulation by Tat, monocytes in the PBMC population were depleted by several adherence steps and treated with Tat in the same conditions. The monocyte-free nonadherent cells did not produce IL-10 after treatment with 1, 10, and 100 nM Tat (Fig. 1D), indicating the direct implication of monocytes in the production of IL-10.
To determine the Tat region implicated in IL-10 production, we used first exon-deleted mutants GST-Tat 1-72 (RGD domain deleted), GST-Tat 1-55 (RGD and glutamic domains deleted), GST-Tat 1-45 (RGD, glutamic, and basic domains deleted), GST-Tat 20-72 (N-terminal domain deleted), and GST-Tat 30-72 (cysteine-rich region deleted). Results show that the C-terminally deleted mutants GST-Tat 1-72, GST-Tat 1-55, and GST-Tat 1-45 induced the same amount of IL-10 as the wild-type GST-Tat 1-101. Weak stimulation was observed with GST-Tat 20-72 (10 nM), while no stimulation was observed with GST-Tat 30-72 or with GST alone (Fig. 2A). These results indicate that the critical region responsible for the stimulation was located within residues 1 to 45. In a second approach, we used three anti-Tat MAbs recognizing epitopes located within regions 1 to 15, 46 to 60, and 74 to 86. Preincubation of Tat with MAb 1-15 greatly inhibited (80.7%) the capacity of Tat to induce IL-10 production (Fig. 2B). Only a weak inhibition was obtained with Tat MAb 46-60, and no inhibition was observed with MAb 74-86. Thus, in agreement with the results with Tat recombinant mutants, the N-terminal region of Tat, amino acids 1 to 45, seems to be crucial for IL-10 stimulation.
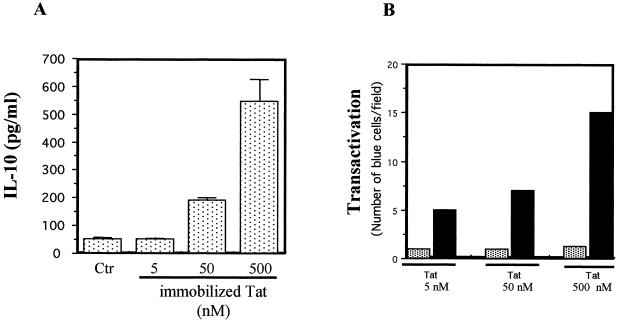
Tat contains a nuclear localization sequence, and so there are two possible levels of action, the membrane and the nucleus. Tat was immobilized in wells in order to test whether it must penetrate monocytes to induce IL-10 production. In these conditions, monocyte stimulation by increasing concentrations of immobilized Tat led to dose-dependent production of IL-10 (Fig. 3A). In order to rule out the possibility that under these conditions some Tat entered the monocyte and induced IL-10 by an intracellular mechanism, Tat transactivation activity was evaluated in a comparative assay, depending on the intracellular localization of Tat, using HeLa P4 cells cultured with immobilized or soluble Tat. Tat immobilized in these conditions was unable to transactivate the HIV LTR, contrary to soluble Tat added at the same concentrations (Fig. 3B). Thus, Tat coated on the wells did not enter the cells, at least at the limit of sensitivity of the test. This suggests that Tat probably mediates its effect by direct interaction with the cell membrane. On the other hand, using flow cytometry analysis, we have shown, in agreement with previous reports (2, 21, 27, 58, 49), that fluorescein isothiocyanate (FITC)-labeled Tat was able to bind to the cell membrane in a dose-dependent manner (data not shown).
FIG. 3.
Production of IL-10 by monocytes stimulated by Tat immobilized in wells. (A) Tat was immobilized in wells by incubation for 2 h at 37°C. After two washes to eliminate nonfixed Tat, monocytes (106) were added and cultured for 24 h with different concentrations of Tat (5, 50, or 500 nM). Culture supernatants were recovered, and the presence of IL-10 was determined by ELISA. (B) HeLa P4 cells were incubated with soluble (solid bars) or immobilized (shaded bars) Tat, and a Tat-dependent-transactivating test was done as described in Materials and Methods. The values are the means ± SD of three experiments. For A, similar results were obtained with cells from two different donors.
Calcium pathway seems not to be involved in the production of IL-10 induced by Tat.
We then sought to determine the signal transduction pathways involved in this production of IL-10. Two predominant signaling pathways known to induce the expression of cytokine genes were studied, the calcium pathway and the PKC pathway. These pathways are activated after stimulation of a membrane receptor that activates PLC, an enzyme that hydrolyzes phosphatidylinositol biphosphate to inositol 1,4,5-triphosphate, which initiates the calcium pathway, and to diacylglycerol, which activates PKC.
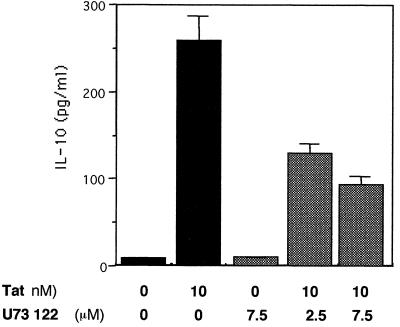
We initially used U73122, an inhibitor of PLC, to determine if this enzyme, the starting point for these signaling pathways, was involved in the activation by Tat. Significant inhibition (36 and 61.2%) of Tat-induced IL-10 production was observed when U73122 was used at 2.5 and 7.5 μM, respectively (Fig. 4). This suggests that the PLC pathway is involved in Tat-induced IL-10 production.
FIG. 4.
Effect of PLC inhibitor U73122 on Tat-induced production of IL-10. Monocytes (106) were treated or not with U73122 (2.5 or 7.5 μM) for 30 min. Tat (10 nM) was then added for 24 h. Culture supernatants were recovered, and the presence of IL-10 was determined by ELISA. The values are the means ± SD of three experiments. Similar results were obtained with cells from three different donors.
The calcium pathway is initiated by the presence of IP3, responsible for mobilizing intracellular calcium stores and thus the increased concentration of intracellular calcium. This pathway leads to the activation of calcineurin, a phosphatase that dephosphorylates transcription factor NF-AT. This enables the factor to undergo translocation to the nucleus, where it binds to specific sites on gene promoters, especially those coding for cytokines (47).
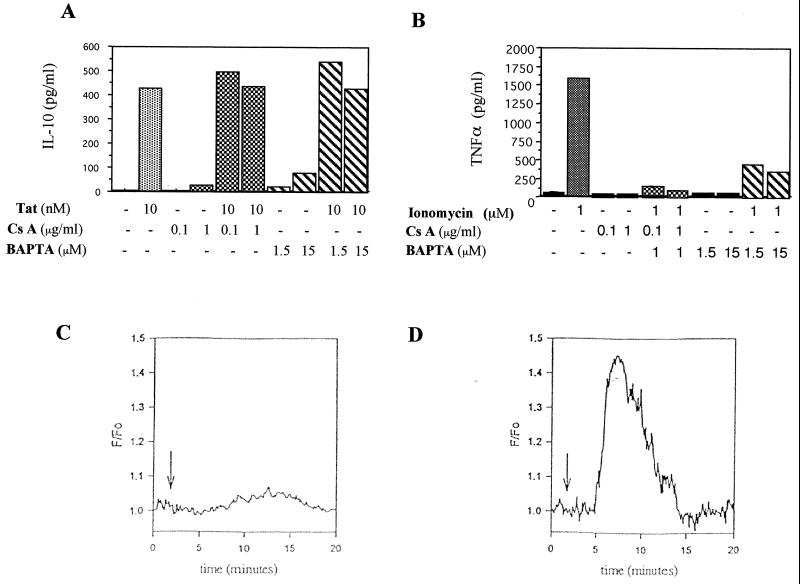
Two complementary approaches were used to determine the involvement of the calcium pathway in IL-10 induction by Tat, the first of which involved inhibitors. The compounds used were cyclosporin A (0.1 and 1 μg/ml), which inhibits calcineurin and acts by sequestering phosphorylated NF-AT in the cytoplasm, and BAPTA/AM (1.5 or 15 μM), a chelator of intracellular calcium. These two compounds did not significantly modify the Tat-mediated production of IL-10 by monocytes (Fig. 5A). As controls, we verified that the concentrations of cyclosporin A and BAPTA/AM used are biologically active (Fig. 5B). To this end, monocytes were stimulated with the calcium ionophore ionomycin, and production of tumor necrosis factor-alpha (TNF-α) was measured in the presence of these inhibitors. The results show that TNF-α induced by ionomycin was totally inhibited by cyclosporin A and markedly reduced (81%) by BAPTA/AM (Fig. 5B).
FIG. 5.
Involvement of the calcium pathway. (A) Effect of calcineurin inhibitor cyclosporin A (Cs A) and of the chelator of intracellular calcium BAPTA/AM on the Tat-induced production of IL-10. Monocytes were treated or not for 30 min as indicated and then treated or not with Tat at 10 nM. Culture supernatants were recovered, and the presence of IL-10 was determined by ELISA. (B) Positive control of inhibition by cyclosporin A and BAPTA/AM on the ionomycin-induced production of TNF-α. Monocytes were treated or not for 30 min as indicated and then treated or not with ionomycin (1 μM) for 24 h. Culture supernatants were then collected, and the presence of TNF-α was determined by ELISA. These results are representative of three independent experiments done on cells from two donors. (C and D) Variations in intracellular calcium concentrations determined by microspectrofluorimetry using fluo-3AM as the probe. Cells were incubated for 30 min in the presence of fluo-3 (5 μM) and observed microscopically after two washes. Monocytes were stimulated by 100 nM Tat (C) or 1 μM ionomycin (D). The curves are the results obtained from the means for 11 different cells from a single donor. Similar results were obtained with cells from two different donors.
In the second approach, the role of calcium in the production of IL-10 was investigated by following the variations in cytoplasmic free Ca2+ concentrations ([Ca2+]i) at the cellular level by microspectrofluorimetry with the fluorescent probe fluo3-AM. Monocyte stimulation by Tat (100 nM) led to a very slight increase in the intracellular calcium concentration (Fig. 5C) that was at the limit of significance. These results were the mean for 11 cells analyzed. The calcium ionophore ionomycin was used as a positive control in the same experimental conditions. The addition of 1 μM ionomycin caused a transient increase in [Ca2+]i that returned to the baseline after 15 min of stimulation (Fig. 5D).
These results show that the calcium pathway does not play an essential role in the induction of IL-10 production by Tat.
PKC is indispensable for Tat-induced production of IL-10.
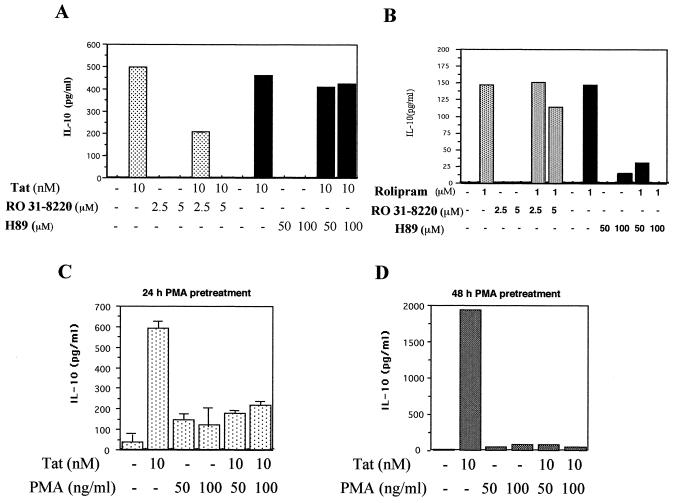
Monocytes treated with RO31-8220 (2.5 or 5 μM), a specific inhibitor of PKC that competes with ATP, could no longer produce IL-10 after treatment with Tat. Inhibition by the PKC inhibitor used was dose dependent, being partial (58%) at 2.5 μM and total at 5 μM (Fig. 6A). This result suggests that PKC plays an important role in the mechanism of induction of IL-10 by Tat. In order to rule out a possible interference with PKA sometimes observed with PKC inhibitors, a PKA inhibitor, H89, was used at 50 or 100 μM. In these conditions, H89 had no significant effect on Tat-induced production of IL-10 (Fig. 6A). The specific inhibitory effects of RO31-8220 and H89 were tested in an alternative IL-10 production assay in which the stimulation of IL-10 was mediated by the PKA pathway. To this end, monocytes were treated with rolipram, a phosphodiesterase inhibitor known to induce IL-10 production via the PKA pathway (20). H89 inhibits in a dose-dependent manner the IL-10 production mediated by rolipram, and this inhibition became total at 100 μM. In contrast, no significant inhibition was observed with the PKC inhibitor RO318220 (Fig. 6B). Together these results argued for the specific effect of protein kinase inhibitors used in this study and underlined the major role of PKC in Tat-induced IL-10 production.
FIG. 6.
Involvement of PKC in Tat-induced production of IL-10. (A) Monocytes (106) were treated or not with the PKC inhibitor RO318220 (RO) at the concentrations indicated (2.5 and 5 μM) or by the PKA inhibitor H89 (50 and 100 μM) for 30 min. Tat was then added for 24 h. These results are representative of three independent experiments done on cells from three donors. (B) Control of the specificity of the kinase inhibitors H89 and RO318220. Monocytes (106) were treated or not with the PKC inhibitor RO318220 (2.5 and 5 μM) or by the PKA inhibitor H89 (50 and 100 μM) for 30 min. Rolipram was then added for 24 h. Culture supernatants were then collected, and the presence of IL-10 was determined by ELISA. (C and D) Monocytes were treated or not with PMA (50 or 100 ng/ml), a PKC activator, for 24 h (C) or 48 h (D), and Tat (10 nM) was then added. After 24 h, culture supernatants were recovered, and the presence of IL-10 was determined by ELISA. The values in C are the means ± SD of three experiments. The results in C and D are representative of three independent experiments done on cells from three donors.
The involvement of the PKC pathway was further characterized. Monocytes were depleted of PKC by a long treatment (24 or 48 h) with PMA (a PKC activator) at 50 or 100 ng/ml. Treated monocytes were then incubated with Tat (10 nM) for 24 h, and the concentration of IL-10 was measured. The results showed a strong inhibition of IL-10 production, up to 70% (Fig. 6C), with monocytes treated with PMA for 24 h. Inhibition became total after 48 h of treatment with PMA (Fig. 6D). Residual production of IL-10 after 24 h of treatment would be due to activation by PMA and not to activation by Tat, since it was also detected in cells treated with PMA alone (Fig. 6C). In this procedure, PKC depletion was checked in experiments involving restimulation of monocytes treated with PMA for 24 or 48 h. In these conditions of PMA restimulation, the cells became unable to produce TNF-α (data not shown). We can note that there was no cell toxicity in these experimental conditions, as shown by the trypan blue dye exclusion test. These results strongly suggest that PKC plays an essential role in the production of IL-10 by human monocytes after stimulation by Tat.
We then attempted to investigate pathways activated downstream from the PKC by studying the known PKC substrate transcription factor NF-κB and the PKC-activated MAP kinase ERK1/2 pathway.
Involvement of transcription factor NF-κB.
Transcription factor NF-κB is a likely candidate for the transactivation of the IL-10 gene, since the organization of the IL-10 promoter shows the presence of nine potential NF-κB sites (23).
In inactivated cells, NF-κB is sequestered in the cytoplasm by the inhibitor protein IκB, which masks its nuclear localization sequence. In order to be active, NF-κB must be translocated into the nucleus, and this translocation required the degradation of IκB, which, once phosphorylated, is degraded in the proteasome pathway (4).
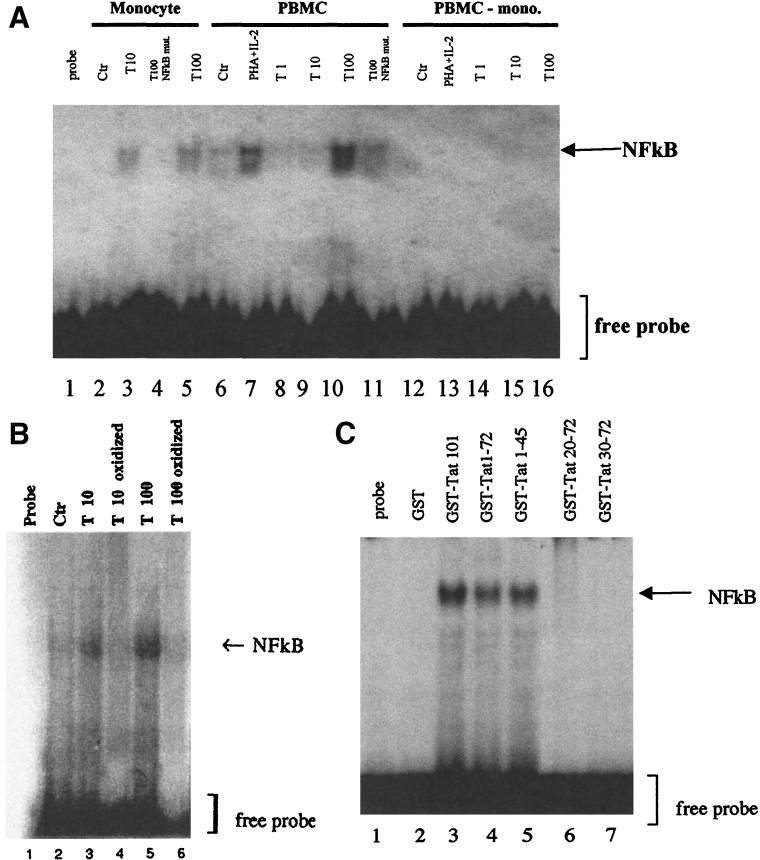
We examined the involvement of NF-κB by first testing the capacity of Tat to activate the nuclear translocation of this factor with the electrophoretic mobility shift technique. These experiments were done with an NF-κB site and showed the formation of a complex with nuclear extracts of monocytes stimulated with 10 and 100 nM Tat (Fig. 7A, lanes 3 and 5). The observed interaction between factor NF-κB and the probe seems to be specific, since no complex was observed when the protein extract was incubated in the same conditions with the mutated NF-κB site (Fig. 7A, lane 4). Similar results were obtained with nuclear proteins obtained from whole PBMC (Fig. 7A, lanes 6 to 11) using phytohemagglutinin (PHA, 3 μg/ml) plus IL-2 (10 U/ml) as a positive control. On the other hand, no complex was detected when monocyte-depleted PBMC were treated with PHA plus IL-2 or with different concentrations of Tat (1, 10, and 100 nM) (Fig. 7A, lanes 12 to 16). To verify that NF-κB activation was specifically mediated by Tat, monocytes were treated with a chemically mutated Tat (oxidized Tat, used at 10 and 100 nM), and nuclear extracts were analyzed by EMSA. In agreement with the inability of this mutant Tat to stimulate the production of IL-10, no complex was detected in these conditions (Fig. 7B). These results indicate that Tat induces NF-κB activation specifically in monocytes. We then investigated if the region involved in IL-10 production was able to induce NF-κB activation. EMSA results show that only the C-terminally deleted mutants tested (GST-Tat 1-72 and GST-Tat 1-45) activate NF-κB as the wild-type GST-Tat 1-101 does (Fig. 7C, lanes 3 to 5). In contrast, no activation was observed with GST-Tat 20-72, GST-Tat 30-72 (Fig. 7C, lanes 6 and 7), or GST (Fig. 7C, lane 2). This result shows that NF-κB activation is correlated with the ability of Tat and Tat mutants to mediate IL-10 production.
FIG. 7.
Activation of NF-κB by Tat in human monocytes. (A) Activation of NF-κB determined by EMSA. Nuclear protein extracts of monocytes, total PBMC, or monocyte-depleted PBMC (PBMC-mono.) were treated with Tat at 1 nM (lanes 8 and 14), 10 nM (lanes 3, 9, and 15), or 100 nM (lanes 5, 10, and 16) or with PHA (3 mg/ml) plus IL-2 (10 U/ml) (lanes 7 and 13) for 16 h and then incubated with a sequence containing the wild-type NF-κB site. The specificity of interaction was tested by using a 32P-labeled mutated NF-κB probe (NF-κB mut.) incubated with extracts from cells treated with 100 nM Tat (lanes 4 and 11). These results are representative of two independent experiments done on cells from two donors. Ctr, control. (B) Specificity of Tat-mediated NF-κB activation. Nuclear protein extracts of monocytes treated with native Tat (10 and 100 nM) (lanes 3 and 5, respectively) or with oxidized Tat (10 and 100 nM) (lanes 4 and 6, respectively) for 16 h were incubated with the 32P-labeled wild-type NF-κB probe as described above. (C) Localization of the domain of Tat involved in NF-κB activation. Nuclear protein extracts of monocytes treated with wild-type GST-Tat 1-101 (lane 3) or Tat-deleted mutants (GST-Tat 1-72, GST-Tat 1-45, GST-Tat 20-72, and GST-Tat 30-72) at 10 nM (lanes 4 to 7) or as a negative control with GST (10 nM) for 16 h were incubated with 32P-labeled NF-κB probe.
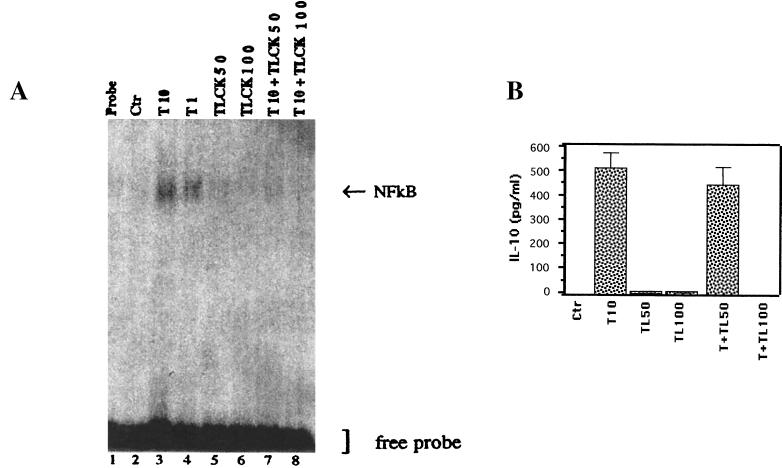
We next tested the role of NF-κB activation in the production of IL-10. Monocytes were treated with nontoxic doses (50 and 100 μM) of TLCK and stimulated by Tat at 10 nM. NF-κB activation and IL-10 production were analyzed by EMSA and ELISA, respectively. The results depicted in Fig. 8A and 6B clearly showed that TLCK prevents both NF-κB translocation (Fig. 8A, lanes 7 and 8) and IL-10 production (Fig. 8B). These results indicate that the Tat-induced IL-10 production is correlated with Tat-induced NF-κB activation in monocytes. Tat thus activated transcription factor NF-κB, one of the substrates of PKC, thereby causing induction of the IL-10 gene.
FIG. 8.
Role of NF-κB in Tat-induced IL-10 production. (A) Effect of the serine protease inhibitor TLCK (50 and 100 μM) on NF-κB nuclear translocation induced by Tat (10 nM) (lanes 7 and 8). Lanes 3 and 4 correspond to the activation of NF-κB with Tat (10 and 1 nM, respectively). Ctr, control. (B) Effect of treatment with TLCK (TL) at 50 and 100 μM on IL-10 production by monocytes (106) treated with 10 nM Tat (T). The values are the means ± SD of three experiments. Similar results were obtained with cells from three different donors.
Involvement of MAP kinases ERK1/2.
MAP kinases p42 and p44, also called ERK1 and ERK2, can be activated by PKC (11, 12, 14, 51). This activation occurs after their phosphorylation by a cascade of kinases (Raf, MEK, and ERK) initiated by PKC. Once ERK1/2 are phosphorylated, they can activate transcription factors that bind to the promoters of cytokine genes.
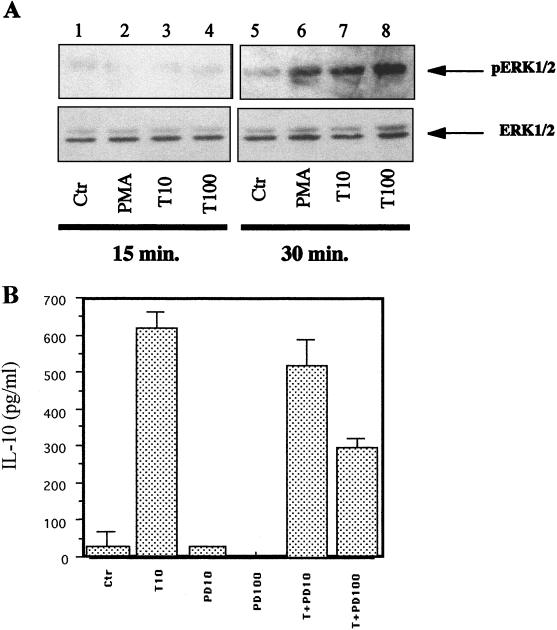
In order for MAP kinases p42 and p44 to be activated, they must be phosphorylated on their tyrosine and threonine residues. The extent of activation of p42 and p44 was determined by treating monocytes with Tat (10 or 100 nM) for 15 or 30 min. Immunoblotting of the cytoplasmic extracts was then carried out, initially with an antibody against total p42 and p44 and then with an antibody against phosphorylated tyrosine and threonine residues. Western blots immunolabeled by specific antibodies against the phosphorylated residues showed a nonsignificant activation of ERK1/2 in cells treated with Tat (10 and 100 nM) after 15 min of stimulation (Fig. 9A). In contrast, treatment of monocytes with Tat for 30 min (10 and 100 nM) allowed a dose-dependent activation of MAP kinases ERK1/2. The amounts of total MAP kinases analyzed were equivalent in all the lanes (Fig. 9A). Tat thus activates MAP kinases ERK1/2 in human monocytes.
FIG. 9.
Involvement of MAP kinases ERK1/2 in the Tat-induced production of IL-10. (A) Western blot analysis of the activation of MAP kinases ERK1/2 by Tat. Cytoplasmic protein extracts were prepared from monocytes (2 × 106) treated with PMA at 50 ng/ml (lanes 2 and 6) or 10 nM (lanes 3 and 7) or 100 nM (lanes 4 and 8) Tat for 15 or 30 min. Visualization was done with an antibody recognizing total ERK1/2 or only phosphorylated ERK1/2 (pERK1/2). These results are representative of two independent experiments done on cells from two different donors. Ctr, control. (B) Effect of PD 98 059 (PD) at 10 or 100 μM, an inhibitor of MAP kinases ERK1/2, on monocytes (106) treated with 10 nM Tat (T). Similar results were obtained with cells from three different donors.
The involvement of MAP kinases ERK1/2 in the Tat-induced production of IL-10 was tested by using a specific inhibitor of these MAP kinases PD98 059. When monocytes were treated with PD98 059 (10 and 100 μM), IL-10 production was partially inhibited. Inhibition was significant and was 52% with 100 μM inhibitor (Fig. 9B). These results suggest that Tat also activates MAP kinases ERK1/2 and thereby contributes to the production of IL-10.
DISCUSSION
There are now a large number of arguments in favor of a role of Tat protein in immune disorders occurring during HIV infection. Tat has a direct involvement in the stimulation of viral replication (34), activation of the provirus, and overexpression of virus coreceptors CXCR4 and CCR5 (30). It also disturbs the equilibrium of the immune system, e.g., by inducing the apoptosis of T cells (26), by inhibiting the activity of superoxide dismutase (55), and by acting on the expression of genes of numerous cytokines, both those that it activates, such as IL-2 (44), IL-4, IL-8, IL-6, IL-1β, transforming growth factor beta, TNF-α, and TNF-β (26, 48) and those that it inhibits, such as IL-12 (45). IL-12 is a key cytokine that causes the differentiation of precursor T cells to TH1 cells (43).
In the present work, we have shown that HIV-1 Tat induces the production of IL-10 by human peripheral blood monocytes. The analysis of the signal transduction pathways showed that the calcium pathway is not or is only slightly involved; the PKC pathway apparently plays an essential role in the production of IL-10; transcription factor NF-κB, one of the main targets of PKC, is activated and is involved in this Tat-induced production of IL-10; and in parallel to the activation of NF-κB, MAP kinases ERK1/2 are also partially involved in the production of IL-10.
Tat also induces the production of TNF-α by human monocytes (15 and data not shown). It has been reported that TNF-α can potentiate the production of IL-10 (24). It is important to note, however, that TNF-α alone cannot induce the production of IL-10 by human monocytes (24). In agreement with these results, the addition of a neutralizing anti-TNF-α antibody in our work did not block the production of IL-10 (not shown).
Prior immobilization of Tat in the culture wells to prevent its intracellular penetration also allowed the stimulation of IL-10 production. This indicates that the interaction of Tat with a membrane receptor suffices to induce the production of IL-10 by human monocytes. Different regions of Tat have been implicated in interactions with membrane proteins: the N-terminal region with receptor CD26 (27), the tripeptide RGD (arginine-glycine-aspartate) with integrins αvβ3 and α5β1 of dendritic cells (58), and the basic region with membrane lipids (49) or with the vegetative epidermal growth factor receptor of endothelial cells (2). Among this panoply of potential Tat receptors, it would be interesting to determine the receptor(s) that participates in the transduction of the signal leading to the production of IL-10 after stimulation by Tat. However, the observation that oxidized Tat is unable to mediate the production of IL-10 by monocytes suggests the importance of the cysteine-rich domain in the activity of Tat to stimulate IL-10 induction. To map the Tat domain implicated in this activity, the use of both recombinant Tat mutants and MAbs directed against the N-terminal, central, or C-terminal region of Tat showed the involvement of N-terminal residues 1 to 45 of Tat in this activity. It is interesting to note that the implication of the N-terminal region, which contains the cysteine-rich domain but not the basic domain (responsible for penetration and nuclear localization of Tat), is in agreement with (i) the absence of activity of the cysteine-oxidized Tat and (ii) the fact that the effect of Tat is exerted at the membrane level.
It has recently been reported that Nef protein (9) and the transmembrane glycoprotein gp41 (5) can also induce the production of IL-10. Comparison of these results with ours suggests that Tat operates via a signaling pathway different from those used by Nef and gp41. Nef apparently uses the calcium/calmodulin phosphodiesterase pathway. In the presence of W7, an inhibitor of this phophodiesterase, or in the presence of EGTA, a calcium chelator, the induction of IL-10 is inhibited (9). In the case of Tat, on the contrary, the calcium pathway is not or is only very slightly involved, as shown by the absence of inhibition by cyclosporin A, an inhibitor of calcineurin, and BAPTA/AM, a chelator of intracellular calcium. These results obtained with calcium pathway inhibitors are consistent with microspectrofluorimetry determinations, at the cellular level, of variations in intracellular calcium concentrations. In addition, it has been reported that Tat blocks L-type calcium channels in dendritic cells (45) and NK cells (59). In contrast to these studies, it has been reported that synthetic Tat can induce a calcium signal in monocytes at concentrations from 6.6 to 33 nM (1). This effect was also observed with a peptide (CysL24–51) containing the cysteine-rich region and the core region (1). Confocal microscopy will lead to a finer determination of the existence of possible variations in calcium concentrations in different cell compartments.
The induction of IL-10 by gp41 of HIV-1 rather seems to involve adenylate cyclase and cAMP (6). Sequences of the promoter of the IL-10 gene contain a cAMP-responsive element for transcription factors activated by cAMP. This pathway involves PKA and apparently is not involved in the mechanism of induction of IL-10 by Tat, since H89, a PKA inhibitor, has no effect on this production. In contrast, PKC activation is essential because the PKC inhibitor RO318220 inhibits the Tat-induced production of IL-10 in a dose-dependent manner; PKC depletion of monocytes by treatment with PMA abolishes the Tat-induced production of IL-10; and PMA, a direct activator of PKC, induces the production of IL-10. Thus, in contrast to the mechanisms used by Nef and gp41, the induction of IL-10 by Tat does involve the PKC pathway. This difference in signal transduction between Nef, gp41, and Tat can be partially explained by the nature of the membrane receptors involved, upstream, in the ligand-receptor interaction.
In the activation cascade involving PKC, we have thus demonstrated the activation of NF-κB, which is translocated into the nucleus as shown by gel mobility shift. We have also demonstrated the activation of the MAP kinases ERK1/2 pathway, leading to the activation of transcription factors, including AP-1, known for its involvement in the induction of several genes, including those of cytokines (25). It has also been reported that Tat produced endogenously in the U937 promonocyte line can induce c-Jun N-terminal kinase (33), another member of the family of MAP kinases. It nevertheless remains to be determined if activation is accompanied by the modulation of expression of certain cellular genes.
The induction of inflammatory and immunomodulating cytokine genes (26, 44, 48), as well as the chemotaxis that can be exerted by Tat (1), suggests an activating role in the immune response. In spite of this, the overall effect of Tat in patients infected by HIV-1 appears rather suppressive. This can be explained by the inhibition of the proliferative T response (52, 56), the induction of T-lymphocyte apoptosis (26), the inhibition of phagocytosis of apoptotic bodies by dendritic cells (58), the cytotoxic activity of NK cells (59), and finally the suppression of IL-12 production by dendritic cells (45) and monocytes (31). The effect of Tat on IL-10 production reported here agrees with all of these reports concerning the potential immunosuppressive role of Tat during infection by HIV.
IL-10 is a cytokine produced by monocytes/macrophages, B cells, and T lymphocytes that suppresses cell-mediated immunity (41). IL-10 acts at different levels and is able to inhibit macrophage activity and to suppress the production of cytokines such as IL-1, TNF-α, and granulocyte-macrophage colony-stimulating factor (41). IL-10 also inhibits the proliferation of T lymphocytes by reducing major histocompatibility complex class II molecule expression on the surface of monocytes (41).
In summary, we have identified a signal transduction pathway used by Tat that leads to the production of IL-10 by human monocytes. Tat acts on membranes to initiate the activation of PKC, a key protein that can mobilize and activate NF-κB and also the members ERK1/2 of the MAP kinase family. NF-κB and other transcription factors activated by ERK1/2 are likely implicated in IL-10 gene induction. The multiple effects of IL-10 could contribute to the course of HIV infection to AIDS. The understanding of the molecular and cellular mechanisms involved in the production of this cytokine, by the identification of new specific targets, may suggest possible targeted therapeutic approaches to neutralize these effects.
ACKNOWLEDGMENTS
Abdallah Badou was supported by SIDACTION (ensemble contre le SIDA). This work was supported by Agence Nationale de Recherche sur le SIDA, Conseil Régional Midi-Pyrénées, and SIDACTION.
We acknowledge P. Druet and L. Pelletier for helpful discussions.
REFERENCES
- 1.Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi M G, Proudfoot A E I, Alouani S, Wells T N C, Mariani G, Rabin R L, Farber J M, Noonan D M. HIV-1 Tat mimicry of chemokines. Proc Natl Acad Sci USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 3.Badou A, Savignac M, Moreau M, Leclerc C, Pasquier R, Druet P, Pelletier L. HgCl2-induced interleukin-4 gene expression in T cells involves a protein kinase C-dependent calcium influx through L-type calcium channels. J Biol Chem. 1997;272:32411–32418. doi: 10.1074/jbc.272.51.32411. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Baltimore D. NF-kB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 5.Barcova M, Kacani L, Speth C, Dierich M P. gp41 envelope protein of human immunodeficiency virus induces interleukin (IL)-10 in monocytes, but not in B, T, or NK cells, leading to reduced IL-2 and interferon-γ production. J Infect Dis. 1998;177:905–913. doi: 10.1086/515230. [DOI] [PubMed] [Google Scholar]
- 6.Barcova M, Speth C, Kacani L, Überall F, Stoiber H, Dierich M P. Involvement of adenylate cyclase and p70s6-kinase activation in IL-10 up-regulation in human monocytes by gp41 envelope protein of human immunodeficiency virus type 1. Pflügers Arch. 1999;437:538–546. doi: 10.1007/s004240050815. [DOI] [PubMed] [Google Scholar]
- 7.Barthelemy S, Vergnes L, Moynier M, Guyot D, Labidalle S, Bahraoui E. Curcumin and curcumin derivatives inhibit Tat-mediated transactivation of type 1 human immunodeficiency virus long terminal repeat. Res Virol. 1998;149:43–52. doi: 10.1016/s0923-2516(97)86899-9. [DOI] [PubMed] [Google Scholar]
- 8.Benkirane M, Chun R F, Xiao H, Ogryzko V V, Howard B H, Nakatani Y, Jeang K T. Activation of integrated provirus requires histone acetyltransferase: p300 and P/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 9.Brigino E, Haraguchi S, Koutsonikolis A, Cianciolo G J, Owens U, Good R A, Day N K. Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3178–3182. doi: 10.1073/pnas.94.7.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonaguro L, Barillari G, Chang H K, Bohan C A, Kao V, Morgan R, Gallo R C, Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992;66:7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cacace A, Ueffing M, Philipp A, Han E K, Kolch W, Weinstein I B. PKC epsilon functions as an oncogene by enhancing activation of the Raf kinase. Oncogene. 1996;13:2517–2526. [PubMed] [Google Scholar]
- 12.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco M T, Moscat J, Rapp U, Cooper G M. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–265. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 14.Caroll M P, May W S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994;269:1249–1256. [PubMed] [Google Scholar]
- 15.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-α production. J Biol Chem. 1997;272:22385–22388. doi: 10.1074/jbc.272.36.22385. [DOI] [PubMed] [Google Scholar]
- 16.Clerici M, Hakim F T, Venzon D J, Blatt S, Hendrix C W, Wynn T A, Shearer G M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Investig. 1993;91:759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clerici M, Shearer G M. A TH1/TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 18.Clerici M, Wynn T A, Berzofsky J A, Blatt S P, Hendrix C W, Sher A, Coffman R L, Shearer G M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Investig. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S S, Li C, Cao Y, Pardee A B, Shevach E M, Cohen D I. Pronounced acute immunosuppression in vivo mediated by HIV Tat challenge. Proc Natl Acad Sci USA. 1999;96:10842–10847. doi: 10.1073/pnas.96.19.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eigler A, Siegmund B, Emmerich U, Baumann K H, Hartmann G, Endres S. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J Leukocyte Biol. 1998;63:101–107. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- 21.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R A, Wingfield P, Gallo R C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang H K, Brady J N, Gallo R C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature. 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 23.Eskdale J, Kube D, Tesch H, Gallagher G. Mapping of the human IL10 gene and further characterization of the 5′ flanking sequence. Immunogenetics. 1997;46:120–128. doi: 10.1007/s002510050250. [DOI] [PubMed] [Google Scholar]
- 24.Foey A D, Parry S L, Williams L M, Feldmann M, Foxwell B J, Brennan F M. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-α: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- 25.Fraser J D, Straus D, Weiss A. Signal transduction events leading to T-cell lymphokine gene expression. Immunol Today. 1993;14:357–362. doi: 10.1016/0167-5699(93)90236-E. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein G. HIV-Tat protein as a potential AIDS vaccine. Nat Med. 1996;2:960. doi: 10.1038/nm0996-960. [DOI] [PubMed] [Google Scholar]
- 27.Gutheil W G, Subramanyam M, Flenpke G R, Sanford D G, Munoz E, Huber B T, Bachovchin W W. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): A possible mechanism for Tat's immunosuppressive activity. Proc Natl Acad Sci USA. 1994;91:6594–6598. doi: 10.1073/pnas.91.14.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howcroft T K, Strebel K, Martin M A, Singer D S. Repression of MHC class I gene promoter activity by two-exon Tat of HIV. Science. 1993;260:1320–1322. doi: 10.1126/science.8493575. [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Bosch I, Hofmann W, Sodroski J, Pardee A. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J Virol. 1998;72:8952–8960. doi: 10.1128/jvi.72.11.8952-8960.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito M, Ishida T, He L, Tanabe F, Rongge Y, Miyakawa Y, Terunuma H. HIV type 1 Tat protein inhibits interleukin 12 production by human peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 1998;14:845–849. doi: 10.1089/aid.1998.14.845. [DOI] [PubMed] [Google Scholar]
- 32.Keller H U, Niggli V. The PKC inhibitor RO 31-8220 selectively suppresses PMA- and diacylglycerol-induced fluid pinocytosis and actin polymerisation in PMNs. Biochem Biophys Res Commun. 1993;194:1111–1116. doi: 10.1006/bbrc.1993.1936. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Manna S, Dhawan S, Aggarwal B B. HIV-1 protein activates c-Jun N-terminal kinase and activator protein-1. J Immunol. 1998;161:776–781. [PubMed] [Google Scholar]
- 34.Laspia M F, Rice A P, Mathews M B. Tat protein increases transcription initiation and stabilizes elongation. Cell. 1989;59:283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann M H, Berg H. Interleukin-10 expression is induced by increase of intracellular calcium levels in the monocytic cell line U937. Pflugers Arch. 1998;435:868–870. doi: 10.1007/s004240050596. [DOI] [PubMed] [Google Scholar]
- 36.Li C J, Friedman D J, Wang C, Metelev V, Pardee A. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 37.Li C J, Ueda Y, Shi B, Borodyansky L, Huang L, Li Y, Pardee A. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc Natl Acad Sci USA. 1997;94:8116–8120. doi: 10.1073/pnas.94.15.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maggi E, Mazetti M, Ravina A, Annunziato F, De Carli M, Piccini M P, Manetti R, Carbonari M, Pesce A M, Del Prete G, Romagnani S. Ability of HIV to promote a TH1 to TH2 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265:244–246. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 39.Mann D A, Frankel A D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masood R, Lunardi-Iskandar Y, Moudgil T, Zhang Y, Law R E, Huang C L, Puri R K, Levine A M, Gill P S. IL-10 inhibits HIV-1 replication and is induced by tat. Biochem Biophys Res Commun. 1994;15:374–383. doi: 10.1006/bbrc.1994.1938. [DOI] [PubMed] [Google Scholar]
- 41.Moore K W, O'Garra A, De Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 42.Moreau M, Leclerc C, Gualandris-Parisot L, Duprat A M. Increase in internal Ca2+ mediates neural induction in the amphibian embryo. Proc Natl Acad Sci USA. 1994;91:12639–12646. doi: 10.1073/pnas.91.26.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 44.Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science. 1997;275:1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 45.Poggi A, Rubartelli A, Zocchi M R. Involvement of dihydropyridine-sensitive calcium channels in human dendritic cell function. J Biol Chem. 1998;273:7205–7209. doi: 10.1074/jbc.273.13.7205. [DOI] [PubMed] [Google Scholar]
- 46.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15:274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 47.Rocancourt D, Bonneret C, Jouin H, Emerman M, Nicolas J F. Activation of a β-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J Virol. 1990;64:2660–2668. doi: 10.1128/jvi.64.6.2660-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubartelli A, Poggi A, Sitia R, Zocchi M R. HIV-1 Tat: a polypeptide for all seasons. Immunol Today. 1998;19:543–545. doi: 10.1016/s0167-5699(98)01351-6. [DOI] [PubMed] [Google Scholar]
- 49.Sabatier J, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. Evidence for neurotoxic activity of Tat from human immunodeficiency virus type 1. J Virol. 1991;65:961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schreiber E, Matthias P, Müller M M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 52.Viscidi R P, Mayur K, Lederman H M, Frankel A D. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science. 1989;246:1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- 53.Vives E, Charneau P, Van Rietschoten J, Rochat H, Bahraoui E. Effects of the Tat basic domain on human immunodeficiency virus type 1 transactivation, using chemically synthesized Tat protein and Tat peptides. J Virol. 1994;68:3343–3353. doi: 10.1128/jvi.68.5.3343-3353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Waiczak H, Deabtin K, Krammer P. Sensitization of T cell to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 55.Westendorp M O, Shatrov V L, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer P H, Dröge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-kB activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wrenger S, Hoffmann T, Faust J, Mrestani-Klaus C, Brandt W, Neubert K, Kraft M, Olek S, Frank R, Ansorge S, Reinhold D. The N-terminal structure of HIV-1 Tat is required for suppression of CD26-dependent T cell growth. J Biol Chem. 1997;272:30283–30288. doi: 10.1074/jbc.272.48.30283. [DOI] [PubMed] [Google Scholar]
- 57.Zauli G, Gibellini D, Celeghini C, Mischiati C, Bassini A, Laplaca M, Capitani S. Pleiotropic effects of immobilized versus soluble recombinant HIV-1 Tat protein on CD3-mediated activation, induction of apoptosis, and HIV-1 long terminal repeat transactivation in purified CD4+ T lymphocytes. J Immunol. 1996;157:2216–2224. [PubMed] [Google Scholar]
- 58.Zocchi M R, Poggi A, Rubartelli A. The RGD-containing domain of exogenous HIV-1 Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS. 1997;11:1227–1235. doi: 10.1097/00002030-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Zocchi M R, Rubartelli A, Morgavi P, Poggi A. HIV-1 Tat inhibits human natural killer cell function by blocking L-type calcium channels. J Immunol. 1998;161:2938–2943. [PubMed] [Google Scholar]