Abstract
Background
Autologous fat transfer (AFT) is gaining popularity in breast surgery, offering a natural-looking and minimally invasive approach for augmentation, reconstruction, and contouring. However, concerns about its impact on breast cancer necessitate an understanding of the interplay between transplanted adipose-derived stem cells (ADSCs) and the breast tissue microenvironment. Renowned for regeneration, ADSCs raise questions about their role in cancer promotion. This systematic review delves into the complex relationship between AFT and breast cancer, exploring how ADSCs may influence development, growth, and metastasis.
Methods
A systematic search of electronic databases, including PubMed, Embase, and BVS was conducted to identify relevant studies. The search strategy employed a combination of keywords, including “breast augmentation”, “fat grafting”, “breast enhancement”, “mammoplasty”, “cancer”, “neoplasm” and related terms. Two reviewers independently screened titles and abstracts. Full-text articles were then retrieved for further evaluation based on their potential contribution to the review objectives.
Results
Two hundred and forty records were identified. Among these, 104 duplicates were removed, resulting in 136 reports available for title and abstract screening. Subsequently, 54 papers were deemed potentially eligible for inclusion, and all reports were retrieved.
Conclusions
In vitro studies reveal ADSCs dual role in breast cancer, influencing proliferation, migration, and drug resistance through complex signaling pathways. Animal studies highlight distinct ADSC subpopulations impacting tumor growth via direct interactions and extracellular vesicle cargo. In vivo, ADSC-enriched fat grafting is generally safe, showing no increased cancer recurrence risk compared to other methods. Notably, cases of invasive breast carcinoma warrant special attention. ADSC-enriched fat grafts exhibit potential benefits in graft retention and survival rates. Despite promising evidence, further studies are needed to comprehensively understand the intricate relationship between ADSCs and breast cancer for optimized clinical applications and potential therapeutic innovations.
Keywords: Breast fat grafting (BFG), autologous fat transfer (AFT), adipose-derived stem cells (ADSCs), breast cancer
Highlight box.
Key findings
• This study confirms the safety and efficacy of autologous fat transfer in breast surgery.
• The study highlights adipose-derived stem cells (ADSCs) potential dual role in influencing breast cancer behaviors.
What is known and what is new?
• There are concerns about ADSCs regenerative properties and potential role in cancer promotion.
• We meticulously curate and synthesize the latest scientific advancements, offering a comprehensive and up-to-date resource for current knowledge.
What is the implication, and what should change now?
• The notable potential advantages of ADSCs enriched fat grafts lie in their ability to enhance graft retention and survival rates. This has the potential to revolutionize breast reconstruction options and introduce innovative therapeutic strategies aimed at improving overall patient outcomes.
Introduction
Breast fat grafting (BFG), or autologous fat transfer (AFT), is an increasingly popular technique in breast surgery. It involves harvesting adipose tissue from one body region and transplanting it into the breast. BFG is valued for its potential to provide a natural-looking and minimally invasive alternative for breast augmentation, reconstruction, and contouring. However, growing concerns have emerged regarding the implications of BFG on breast cancer, highlighting the need to understand the relationship between these two entities.
At the core of this issue lies the interplay between adipose-derived stem cells (ADSCs) in the transplanted fat and the breast tissue microenvironment. ADSCs have raised questions about their potential role in breast cancer promotion. Although renowned for their regenerative potential, ADSCs can also influence cellular behavior and even modulate tumor growth. The relationship between BFG and breast cancer is complex, encompassing both clinical and laboratory research. This systematic review explores the intricate mechanisms by which ADSCs could impact breast cancer development, growth, and metastasis, delving into the latest scientific findings and ongoing research in this domain. We present this article in accordance with the PRISMA reporting checklist (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-54/rc).
Methods
Study design
This article presents a systematic literature review of published studies and scientific literature on fat grafting, ADSCs, and their association with breast cancer. The review encompasses studies conducted across multiple geographic regions and various publication dates until the knowledge cutoff date of November 1, 2023.
Search strategy
A systematic search of electronic databases, including PubMed, Embase, and Biblioteca Virtual em Saúde (BVS) was conducted to identify relevant studies. The search strategy employed a combination of keywords, including “breast augmentation”, “fat grafting”, “breast enhancement”, “mammoplasty”, “cancer”, “neoplasm” and related terms.
Study selection
Two reviewers independently screened titles and abstracts. Full-text articles were then retrieved for further evaluation based on their potential contribution to the review objectives. Studies were included if they reported using fat grafting for breast augmentation, presented clinical outcomes, or provided relevant information on mechanisms, complications, and safety, regarding ADSCs and breast cancer. Literature reviews and case reports, as well as reports related to other malignancies, were excluded. Reviewers were unaware of each other’s decisions. Discordances were solved through discussion and further agreement between reviewers. Rayyan software was utilized to ensure blinding and to ease the inclusion process, especially for screening titles and abstracts.
Data extraction and synthesis
Data from the included studies was extracted using a standardized form for each reviewer. Information extracted included author, publication year, study design, sample size, patient demographics, outcomes assessed, and key findings. The extracted data was then narratively synthesized, with the focus on summarizing the key findings and common themes across the studies.
Data analysis
Given the nature of this systematic review as a literature synthesis, it is essential to note that no formal statistical analysis, such as a meta-analysis, was conducted. The suitability and feasibility of meta-analyses rely on the availability of data from studies with comparable methodologies and outcome measures. As the included studies exhibited heterogeneity, both in design and objectives, a qualitative analysis approach was adopted. This involved the qualitative synthesis of findings to offer a comprehensive overview of breast augmentation through fat grafting.
Results
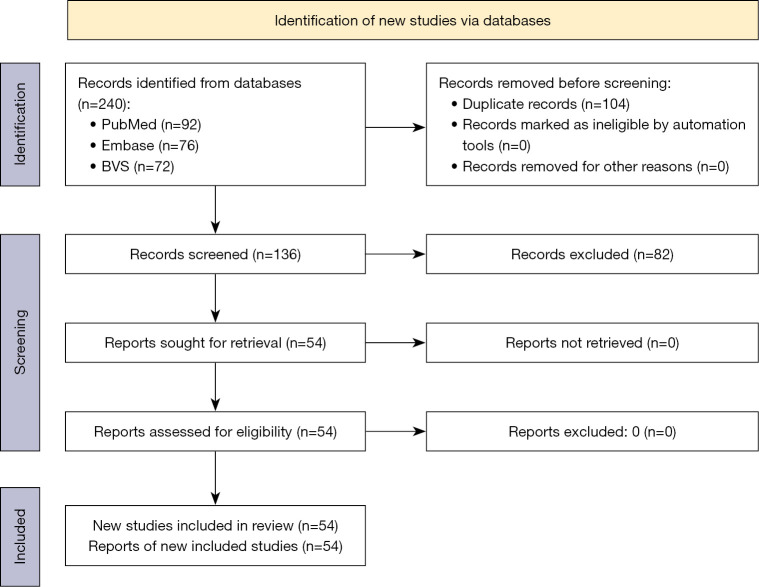
Following the search process, 240 records were identified. Among these, 104 duplicates were removed, resulting in 136 reports available for title and abstract screening. Subsequently, 54 papers were deemed potentially eligible for inclusion, and all reports were retrieved. The inclusion workflow is summarized in Figure 1.
Figure 1.
Study selection workflow. BVS, Biblioteca Virtual em Saúde.
Discussion
This section is delineated into three distinct sections, each addressing a specific facet of the research inquiry. The inaugural segment meticulously examines in vitro studies, scrutinizing outcomes derived from controlled laboratory settings to elucidate nuanced aspects of the investigated subject. Following this, the subsequent section encapsulates experimental studies, which entail controlled experiments designed to simulate real-world conditions and thereby contribute meaningful insights. The conclusive portion of this study concentrates on clinical studies, presenting empirical observations and outcomes garnered from research conducted in human subjects. This strategic organization ensures a systematic and comprehensive exploration of the research topic, spanning foundational laboratory investigations to the pragmatic applications witnessed in real-world clinical scenarios.
In vitro
In 26 studies, outcomes pertaining to the connection between ADSCs and breast cancer cell lines were examined. It is crucial to highlight the distinctions in adipocytes between the breast and other areas, given that the majority of studies gather fat from the abdomen. ADSCs collected from breast and abdomen have similar marker expression and differentiation potential. However, breast ADSCs presented higher self-renewal ability and a more unstable doubling compared with abdominal ADSCs (1).
The role of ADSCs in breast cancer progression in vitro is intricate and remains a subject of controversy in ongoing research. Co-culture of ADSCs and MCF-7 significantly increased MCF-7 migration rates, while the addition of a blocking anti-tenascin-C antibody in the medium didn’t affect the migration rate (2). Furthermore, when compared to a fat-only medium, the ADSC medium demonstrated an elevation in cancer cell proliferation, migration, and the transcription of arginase-1, transforming growth factor-β, and vascular endothelial growth factor (3). In a separate experiment involving MCF-7, the presence of ADSCs exosomes notably amplified breast cancer cell proliferation (4). MDA cell line viability increased to almost 100% in ADSCs presence in vitro (5). ADSCs also increased migration in MDA-MB-231 co-cultures (6). Notably, when breast cancer cells collected in vivo were co-cultured with ADSCs obtained from the same patient, migration was nearly ten times higher (7). Co-culture of cell line T47D (inflammatory breast carcinoma), also found higher levels of transcriptional genes for typical malignancy markers (8). BT-474 co-culture also increased proliferation, with TNF-alpha upregulation (9). Natural tropism of ADSCs towards breast cancer cells has been evaluated after independent cultures were done in silicon chambers, then chambers were removed and ADSCs tropism was confirmed, thus indicating attraction of ADSCs to malignant breast cells (10).
The presence of adipocyte exosomes was observed to reduce early apoptotic cells in 5-fluorouracil-treated MCF-7, suggesting that ADSCs play a protective role against chemotherapeutic drug-induced apoptosis (4). This is reinforced by the identification of an increased tumorigenesis pathway and transformation gene expression in co-cultures of ADSCs with both MCF-7 and BT-474 (HER-2+ model) after 48 hours, with BT-474 co-culture showing downregulation after 96 hours (11). When ex-vivo breast cancer cells were co-cultured with ADSCs-enriched medium and tamoxifen, tamoxifen treatment simultaneously reduced the differentiation of ADSCs into adipogenic and osteogenic cells, while also inhibiting their proliferation in a dose-dependent manner (12). While these findings contradict the results of a study by Boemi et al., which compared ADSCs from women with breast cancer who were taking tamoxifen to ADSCs from menopausal women, suggesting that tamoxifen (20 mg/day) had no effect on ADSC apoptosis compared to the control group (13). MDA-MB-231 and ADSCs underwent treatment with paclitaxel (1.0 µM) or low-dose paclitaxel (0.1 µM) in combination with a histone deacetylase inhibitor, providing additional insights into the interaction between ADSCs and chemotherapy. The combined therapy induced cytotoxicity in MDA-MB-231 while preserving the viability of ADSCs, suggesting that a synergistic approach may enhance treatment outcomes (14). Furthermore, in the presence of doxorubicin, ADSCs’ differentiation remained unaffected, highlighting the stronger affinity of doxorubicin and paclitaxel for BT-474 and MDA-MB-231 compared to ADSCs (15). These results emphasize the prospective application of chemotherapeutic fat grafts in the realm of breast reconstruction. Consistent with these observations, experimental data illustrated that ADSCs exhibit the capacity to uptake paclitaxel and subsequently release it, thereby furnishing supplementary substantiation for the aforementioned hypothesis (16).
In contrast, ADSCs were observed to stimulate both cell proliferation and cell apoptosis in MCF-7 and MDA-MB-231 models. This effect was attributed to the up-regulation of ATF4, a transcription factor known to induce cell apoptosis by disrupting translational processes (17). Further mechanisms were studied by Ejaz et al., in which ADSCs inhibited MCF-7 proliferation in contact co-culture, by cell cycle arrest mediated by the retinoblastoma protein, as well as MDA-MB-231 proliferation (18). The conditioned medium derived from ADSCs exhibited suppressive effects on the proliferation and viability of MCF-7, MDA-MB231, and MDA-MB468 breast cancer cell lines. This suppression was accompanied by the activation of ATM-Chk2 signaling cascades specifically in malignant cell lines, such as MCF-7 and MDA-MB231, but not in non-cancerous M10/H184B5F5 cells. These findings imply distinct responses to ADSC-derived conditioned medium between cancerous and non-cancerous breast cells (19). The triggering of MEK-ERK pathway in breast cancer cells co-culture with ADSCs was also indicated through nuclear CBP recruitment which later upregulates human telomerase reverse transcriptase transcription and telomerase activity (20). In another indirect co-culture of ADSCs and MDA-MB-231, and direct co-culture demonstrated no growth effect on breast cancer cell lines, but stimulated migration (21). In metastatic pleural effusion cell lines, ADSCs culture increased 5-fold its proliferation, suggesting metastatic behavior, later, an experiment with active and dormant cells was conducted, with ADSC enhancing growth of only active, not dormant cells (22). Additionally, it was observed that ADSCs did not possess the capability to induce the transformation of normal breast cells into a malignant phenotype. However, they demonstrated the ability to establish a susceptible microenvironment by inducing c-Met expression, a phenomenon validated through pharmacological inhibition (23). Therefore, AFT should be avoided until disease has been fully eliminated.
Animal models
Three papers exclusively focused on animal models, although certain in vitro studies also incorporated experimentation with these models. One study found ADSCs with low expression of CD90 (CD90low ADSCs) to significantly inhibit tumor progression compared to CD90 high ADSCs or fibroblasts (24). Tumor volume and mass were reduced with CD90low ADSC treatment versus other groups. Additional experiments explored the tumor-modulating effects of extracellular vesicles (EVs) derived from different ADSC subtypes. CD90low ADSC-derived EVs were significantly smaller in diameter than CD90 high ADSC-EVs. CD90low ADSC-EVs more potently suppressed breast cancer cell proliferation, migration, and induced apoptosis in vitro compared to CD90 high ADSC-EVs. The pro-apoptotic microRNA miR-16-5p was more abundant in CD90low ADSC-EVs and contributed to their enhanced anti-tumor activity.
Jin et al. and Millet et al. examined the impact of fat graft enrichment with ADSCs prior to transplantation in a mouse breast tumor model (25,26). Although the estimated tumor weight was similar between all groups, tumors in the highly enriched fat graft group exhibited significantly greater mass than those in other cohorts. In addition, the injection of ADSCs into the tumor site resulted in increased primary tumor growth and lung metastasis compared to the control group, as well as the fat graft and cell-assisted lipotransfer groups (3). Metastatic disease and tumor weight was also higher in an MDA-MB-231 and MCF-7 model in rats with ADSCs-high enhancement, with MDA-MB-231 being more aggressive (5). Higher vascular density was identified, as well as greater tumor growth, in tumors injected with ADSCs-enriched AFT, but not in AFT only models in Orbay et al. work (6). Proliferation marker Ki-67 staining indicated no difference in tumor cell proliferation between groups. Together these findings suggest ADSC enrichment of fat grafts may promote local tumor growth in animal models.
Paino et al. studied the inverse relationship between MCF-7 cancer cells and ADSCs, co-injecting MCF-7 cells and ADSCs in mice, with ADSCs integrating into the tumor, developing faster and bigger tumors than MCF-7 only cancer (27). Consistently, Rowan et al. did a similar experiment with MDA-MB-231 with human ADSCs that stimulated MDA-MB-231 multiple metastasis and increased angiogenesis (21). But when xenografts of CG5 tumor xenografts were injected with ADSCs enriched with paclitaxel (4 µM), tumor size was about 50% of controls, but lower doses (2 µM) rather increased xenograft growth (16). An interesting study involving the injection of ADSCs in a manner that encircled the tumor, leading to a reduction in growth and eventual disappearance within a span of 3 to 8 weeks. Remarkably, sustained complete recovery was observed and maintained for a minimum of 6 months throughout the study duration (28).
In summary, the animal studies collectively illustrate that various ADSCs subpopulations exert diverse regulatory influences on breast cancer progression. These effects are attributed to both direct interactions between tumors and ADSCs, as well as the secretion of EV cargo, particularly miR-16-5p, which exhibits antitumor functions. Further mechanistic investigations are essential to comprehensively understand the underlying pathways and mechanisms governing these observed effects.
In vivo
We’ve found 25 in-vivo studies in our search (detailed in Table 1). A retrospective case control study, elaborated on the recurrence risk in breast cancer after lipofilling, which included 412 women: 109 (26.5%) in the lipofilling group and 303 women in non-lipograft. In their study, lipofilling wasn’t a predictive factor for recurrence, for overall survival or for distance metastasis. Although women treated for invasive cancer presented an independent predictive factor for local recurrence [P<0.05, hazard ration (HR): 5.06 (1.97–10.6)] (29). A further retrospective cohort study, involving the examination of 93 women who had undergone autologous fat grafting (AFG) following breast cancer treatment, revealed no discernible difference in tumor recurrence or distant metastases. Notably, a high degree of patient satisfaction was maintained throughout the study (34). Similarly, patients who underwent mastectomy with immediate breast reconstruction involving AFT subsequent to an incidental diagnosis of invasive ductal carcinoma did not exhibit higher rates of recurrence (32).
Table 1. In vivo detailed studies assessment.
| Author, year | Design | Sample | Outcomes | Key findings |
|---|---|---|---|---|
| Berti, 2022, (29) | Retrospective, monocentric, case-control study | 412 women, with 109 (26.5%) in the lipofilling group and 303 in the non-lipofilling group | In the overall study population, lipofilling did not show predictive significance for recurrence (HR =1.39, P=0.41), overall survival (HR =0.84, P=0.79), or distant metastases (HR =1.10, P=0.84) | In women treated for invasive cancer, the multivariate analysis indicated that lipofilling was an independent predictive factor for local recurrence (HR =5.06, P=0.04) |
| Mazur, 2018, (30) | The study investigated tumor recurrence in patients with autologous ADSC breast reconstruction vs. those without reconstruction over a 3-year observation period | 56 reconstructed + SVF AFG vs. 252 without subsequent reconstruction | Cancer recurrence in patients treated with ASC-enriched fat for breast reconstruction was 3.7%, comparable to the control group (4.13%), with no observed adverse effects of therapy | Suggests safety of using ASC-enriched fat for breast reconstruction in terms of cancer recurrence |
| Toyserkani, 2017, (31) | Open-label, single-arm, single-center feasibility and safety study in patients with breast cancer-related lymphedema of the upper extremity | 11 patients | Arm heaviness reduced from a baseline median score of 5.5 to 2.5 after 6 months (P=0.0030). Arm tension decreased from a baseline median of 5.0 to 2.5 after 6 months (P=0.0097). DASH score improved from 21.3 at baseline to 12.9 after 6 months (P=0.0168) | Autologous ADRC with scar-releasing fat graft proved safe over 6 months, effectively alleviating breast cancer-related lymphedema symptoms and reducing the need for conservative treatment |
| Myckatyn. 2017, (32) | Mastectomy with immediate breast reconstruction between 2006 and 2011, age older than 21 years, female sex, and incident diagnosis of invasive ductal carcinoma (stage I, II, or III) | n=1,197, consisted of all recurrences during the study period (n=225) and a 30% random sample of the study population (n=972) | The HR for disease recurrence with fat transfer was 0.99 (95% CI: 0.56–1.7), and after adjustment, it remained at 0.97 (95% CI: 0.54–1.8) | In breast cancer patients who had mastectomy with immediate reconstruction, no association with a higher risk of cancer recurrence |
| Vester-Glowinski, 2022, (33) | Double-blind, randomized controlled trial of breast augmentation with ASC-enriched fat grafting | 20 women | After 1 year, ADSC-enriched fat grafts (54.0%, 95% CI: 30.4–77.6%) and placebo-enriched fat grafts (55.9%, 95% CI: 28.9–82.9%) demonstrated comparable volume retention in breasts (P=0.566, n=10 each) |
Both groups had similar volume retention |
| Kempa, 2022, (34) | Monocentric cohort from all consecutive patients who underwent AFG after breast cancer from 2008 to 2020 | 93 women | The study showed low rates of local recurrence (1.1%), distant metastases (2.2%), and tumor-related death (1.1%), with 67.12% of patients expressing satisfaction with autologous fat grafting | Study aligns with the literature, showing a consistent low incidence of tumor recurrence and metastasis following AFG use |
| Calabrese, 2018, (35) | Long-term cancer recurrence risk of SVF + AFT compared to AFT, after nipple sparing mastectomy | 41 women G1 (SVF + AFT), 64 G2 (AFT), and 64 G3 (control group) |
Loco-regional recurrence rates were 2.4%, 4.7%, and 1.6% for G1, G2, and G3, while systemic recurrence rates were 7.3%, 3.1%, and 3.1%, respectively | No significant risk factors for loco-regional or systemic recurrence were identified among the variables, including SVF + AFT (G1) which showed no increase in oncological recurrence |
| Ito, 2017, (36) | Female breast cancer patients who underwent breast-conserving surgery and adjuvant radiotherapy. They received an autologous fat graft enriched with ADRC during adipose tissue harvest | n=10 | Operation time: 188±30 min. Hospitalization: 1.2±0.4 days. No complications. No recurrence/metastasis in 7.8±1.5 years post-transplantation | ADRC-enriched autologous fat transplantation is safe without long-term recurrence and, a viable option for breast reconstruction, even following adjuvant radiotherapy |
| Kølle, 2013, (37) | Randomized, controlled, data assessor-blinded clinical trial. Participants undergoing breast augmentation | n=16, 8 each group | ASC-enriched fat grafts demonstrated significantly higher retention rates (mean =80.2%) compared to conventional fat grafts (mean =45.1%) | Potential use of ADSCs for both reconstructive and cosmetic volume restoration, presenting an attractive alternative to conventional fat grafting and implants |
| Intervention group: ASC-enriched fat grafts | Clinical photos showed statistically superior results in the intervention group, as assessed by independent clinical experts | |||
| Control group: conventional nonenriched fat grafts | ||||
| Gentile, 2013, (38) | Comparison of fat grafting with PRP to a control group using only centrifuged fat grafting | 50 patients treated with fat grafting + PRP and a control group of 50 patients treated with centrifuged fat grafting | Patients treated with PRP added to autologous fat grafts showed a 69% maintenance of contour restoration and 3-dimensional volume after 1 year | PRP + fat grafting leads to a significant improvement in maintaining breast volume in patients with breast soft-tissue defects compared to centrifuged fat grafting alone |
| The control group treated with centrifuged fat grafting showed a 39% maintenance | ||||
| Chiu, 2019, (39) | SVF-enriched fat grafting vs. conventional fat grafting | 105 patients controls; 101 SVF-AFG | Survival rate of transplanted fat at 12 months: 67.9% in group A, 68.7% in group B | Study does not support the use of SVF in autologous fat grafting for breast augmentation |
| Postoperative complication rate: 3.8% in group A, 5.9% in group B | ||||
| Statistically insignificant differences observed between the two groups | ||||
| Hu, 2022, (40) | Breast augmentation with autologous fat transplantation alone compared to cell-assisted autologous fat transplantation, randomized | n=34, 17 each | The observation group had higher CC, SN-N, and N-MF, a statistically significant higher uplift value after surgery and MRI revealed greater postoperative fat retention compared to the control group | Suggested superiority of cell-assisted AFG compared to AFG alone |
| Salgarello, 2011, (41) | 10% PRP + AFG vs. Coleman fat grafting | 17 patients (40%) received fat enriched with 10% PRP (group A), while 25 patients (60%) received only Coleman fat grafts (group B) | 10% PRP was not superior to Coleman fat grafting alone | The retrospective analysis does not show effect of 10% PRP on fat graft take compared to Coleman fat grafting |
| Kronowitz, 2016, (42) | Mastectomy for breast cancer or risk reduction and subsequent breast reconstruction with or without lipofilling | 719 breasts for lipofilling group, 305 risk reduction, and 670 no-lipofilling | Locoregional recurrence rates at 5 years were 1.6% for cases (lipofilling) and 4.1% for controls (no lipofilling) | Supports the oncologic safety of lipofilling in breast reconstruction, as there was no increase in locoregional recurrence, systemic recurrence, or second breast cancer |
| Systemic recurrence rates were 2.4% for cases and 3.6% for controls | ||||
| Tukiama, 2021, (43) | Retrospective matched cohort. 1:3 | 42 patients treated for breast cancer who underwent AFG vs. control: 126 patients without AFG | Locoregional recurrence (7.1% vs. 6.3%; P=0.856) | No evidence of increased risk in any of the survival outcomes, suggesting that lipofilling is a safe procedure for breast reconstruction after surgical treatment of breast cancer |
| Local recurrence (7.1% vs. 5.6%; P=0.705) | ||||
| Distant recurrence (14.3% vs. 7.9%; P=0.238) | ||||
| Disease-free survival (21.4% vs. 19.0%; P=0.837) | ||||
| Overall survival (14.3% vs. 7.1%; P=0.181) | ||||
| Juhl, 2018, (44) | Prospective, AFG reconstruction after breast conserving surgery | 42 patients undergoing 1–3 AFG procedures | Calcifications: 21%; oil cysts: 85%; increased scarring: 3% | Considerable radiologic breast imaging changes induced by AFG after breast conserving surgery |
| Serra-Mestre, 2017, (45) | Fat grafting to reduce asymmetry in breast reconstruction, mastopexy, and augmentation | 86 patients | Statistically significant reduction in mean intermammary distance. One capsular contracture in a breast reconstruction requiring capsulotomy and an oil cyst requiring aspiration | Safe remodeling of the medial cleavage of the breast |
| Kaoutzanis, 2016, (46) | Consecutive postmastectomy AFG | 108 women with a total of 167 breast reconstructions | AFG + breast reconstruction resulted in a biopsy rate of 4.8% | Suggests AFG is a relatively safe procedure for refinement of the reconstructed breast in postmastectomy patients |
| No cases of locoregional cancer recurrence | ||||
| Suspicious imaging findings requiring biopsy showed fat necrosis, scar, or oil cysts without evidence of malignancy | ||||
| Gale, 2015, (47) | Case-controlled AFG in women with breast cancer history | 211 participants (invasive carcinoma, n=184; ductal carcinoma in situ, n=27). Control subjects: matched 2:1 | Local recurrence: 0.95% vs. 1.90% (P=0.33) | No evidence of increased oncologic risk associated with fat grafting in women previously treated for breast cancer |
| Regional recurrence: 0.95% vs. 0% (P=0.16) | ||||
| Distant recurrence: 3.32% vs. 2.61% (P=0.65) | ||||
| Klinger, 2022, (48) | Differences in LRR and LRFS were assessed between patients who underwent AFG and those who did not | n=6,592 | LRR was 5.3% in the matched population, 3.9% in the AFG group, and 6.1% in the non-AFG group, suggesting non-inferiority of AFG (P=0.084) | Autologous fat grafting does not negatively interfere with cancer prognosis |
| Kaplan-Meier curves confirmed non-inferiority of the AFG procedure for LRFS (aHR =0.73, 95% CI: 0.41–1.30, P=0.291) | ||||
| Similar effects in terms of LRFS were observed among different biological subtypes (luminal-like group, HER-2 enriched-like, and TNBC) | ||||
| Yoshimura, 2008, (49) | SVF-AFG for breast augmentation | n=40 | Postoperative atrophy of injected fat was minimal and remained stable after 2 months. Cyst formation or microcalcification was detected in four patients | Effective and safe for soft tissue augmentation and may outperform conventional lipoinjection |
| Almost all patients reported satisfaction with the soft and natural-appearing augmentation | ||||
| Wang, 2015, (50) | SVF-AFG for breast augmentation | n=12 | Fat Resorption was 51.84% (16.74%) at 6 months postoperatively | Little complications using SVF + AFG for breast augmentation |
| Newly formed cysts and nodules in two cases; no calcification in MRI | ||||
| Shin, 2023, (51) | SVF-AFG for breast augmentation | n=384 | Higher SVF cell number associated with greater retention volume. Greater retention volume in patients with soft breasts | Limiting arm movement, increasing SVF cell count, and improving skin tension may enhance retention rates |
| Right breast retention rate (60.35%) lower than left breast (77.48%) at 18 months | ||||
| Jeon, 2021, (52) | SVF-AFG vs. AFG for correcting contour deformities of reconstructed breasts | n=20, 10 each group | Fat graft retention rate: Group 1 vs. Group 2 at 6 months: 73.8% vs. 62.2% (P=0.03); Group 1 vs. Group 2 at 12 months: 65.4% vs. 48.4% (P=0.03) | SVF is effective in increasing survival rates of autologous fat grafts for correcting volume deficit after breast reconstruction |
| Group 1 showed higher satisfaction | ||||
| Fat necrosis occurred in 1 patient in each group | ||||
| No locoregional recurrence during follow-up | ||||
| Pérez-Cano, 2012, (53) | ADSC-AFG for breast conservation therapy contour defects correction | n=71 | No serious adverse events associated with the ADRC-enriched fat graft injection procedure | Suggests safety and efficacy ADSC-AFG for breast conservative therapy contour defects |
| No reported local cancer recurrences. Injection site cysts reported in ten patients |
HR, hazard ratio; ADSC, adipose-derived stem cell; SVF, stromal vascular fraction; AFG, autologous fat grafting; ASC, adipose-derived stromal cell; DASH, Disabilities of the Arm, Shoulder and Hand; ADRC, adipose-derived regenerative cells; CI, confidence interval; AFT, autologous fat transfer; PRP, platelet-rich plasma; CC, chest circumference; SN-N, sternal notch-nipple distance; N-MF, distance between nipple and inframammary fold; MRI, magnetic resonance imaging; LRR, locoregional recurrence rate; LRFS, locoregional recurrence-free survival; aHR, adjusted hazard ratio; TNBC, triple-negative breast cancer.
Calabrese et al. evaluated risk factors in long-term cancer recurrence risk in stromal vascular fraction (SVF), which is mainly composed of ADSCs, + AFT cases, AFT-only and control after nipple sparing mastectomy. In their analysis, comparable rates of loco-regional and systemic recurrences were observed between groups, with no distinction based on factors such as technique, age over 50 years, lympho-vascular invasion, oncological stage, adjuvant or neoadjuvant chemotherapy, adjuvant radiotherapy, and adjuvant hormone therapy, which were not deemed independent risk factors (35). Thus, sustaining a medium enriched with ADSCs exhibited no discernible difference in oncological recurrence. Likewise, the occurrence of cancer recurrence in ADSCs-enriched fat utilized for breast reconstruction did not exhibit statistical variation compared to patients without breast reconstruction over a follow-up period of 3 years (30). This aligns with data obtained from ten patients who underwent an ADSCs-enriched graft following breast-conserving surgery and adjuvant radiotherapy, with a mean follow-up duration of 7 years (36). In this sense, AFG is known as not being an independent risk factor for breast cancer local recurrence as shown in matched research (42,43,47). In contrast, there may be a time-dependent association, which non-enriched AFG done before 12 postoperative months was linked to local recurrence, but AFG after 12 months was not (54). However, a great Italian multi-center study with more than 6,500 patients confirmed no association between loco-recurrence and AFG, consistently (48). In enriched-AFG, multiple studies reject the loco-regional recurrence association, therefore supporting oncological safety.
The main advantage of ADSC-enriched fat for AFT lies in its inherent properties that enhance the survivability and integration of transplanted fat. This results in a superior retention of the graft, surpassing the retention achieved with AFT alone (55). In the context of this review, many papers provided information on graft survival rates, revealing minimal differences between enhanced and conventional AFT methods (33). AFG volume retention on soft tissue seems to be influenced by many factors, such as menstrual cycle phase, operation stage, lactation history, and certainly, enhancement with ADSCs improves considerably the retention rate (56-58). Kølle et al. (37) conducted a randomized clinical trial, comparing ADSCs to non-enhanced AFG, with surprising results. In their research, enhanced AFG showed a substantially higher retention rate (80.2%) compared to conventional fat grafts (45.1%). Similarly, Xiong et al. presented that ADSCs and platelet-rich plasma (PRP)-enhanced AFG had higher retention rates compared to controls, even though there was no difference at 12 weeks in nude mice (59). Notably, Gentile et al. (38) showed that PRP-enhanced autologous fat grafts maintained 69% contour restoration and volume after 1 year, outperforming the control group with centrifuged fat grafts at 39% in breast reconstruction scenario. In contrast to this, its role in breast augmentation looks blurred, with contrasting results between ADSC enriched medium compared to control, from higher fat retention in cell-assisted AFG to dubious results. Despite the highly supportive evidence, low sample sizes research presenting no difference between AFG and enriched AFG exists (33,41). Therefore, results should be interpreted cautiously. In this context, Karam et al. conducted a meta-analysis comparing fat retention and necrosis, revealing a notable difference favoring ADSCs-enriched fat in terms of retention, however, no significant difference was observed in relation to necrosis (60).
Non-enhanced AFG after breast conserving surgery presented significant changes in radiological image, with 2 out of 10 developing calcifications, and 85% affected by oil cysts (44). Although highly present, these are benign changes, and when suspicious, had low biopsy rate and intervention needed (46). Similarly, SVF-AFG presented a small formation of 10% of oil cysts, while maintaining fat retention. Again, there was minimal formation of cysts, and no cases of fat necrosis. Jeon et al. (52) also found low rates of complications with SVF + AFG, with similar fat necrosis occurrence rates between SVF-AFG and AFG only, supporting procedure safety.
Enhancement fat grafting does not limit its use, as it can show prominent results even in scleroderma patients, with twice soft tissue after 6 months compared to control (61). Another successful trial used SVF in face lipotransfer, with significantly less loss of volume and higher grades by plastic surgeons. Notably, volume retention is only one of the benefits, with SVF-AFG better skin quality enhancement and volume compared to controls (62). Improvements in FACE-Q scores were present (63). Despite complete SVF enhancement working, platelet-derived growth factor (PDGF) alone does not improve results (64). These incremental improvements surely represent an incredible gain in patient satisfaction and surgical experience. SVF cell amount seems to influence fat retention, as higher cell amount in SVF associates with better volume retention (50,51).
Moreover, Toyserkani et al. investigated the application of ADSCs-enriched fat for breast cancer-related lymphedema of the upper extremity, yielding positive results in alleviating arm heaviness, tension, and dash scores. The study also reported a 6-month safety profile (31).
Purified AFG methods differ greatly, although mechanical and enzymatic digestion are the main ones. Gentile et al. (65) found out that enzymatic digestion was the best, with higher ADSCs amount and fat volume maintenance, while filtration was the best amongst mechanical methods. In concordance, Wu et al. (66) compared mechanical methods, also suggesting filtration superiority. Hence, it is suggested that the surgeon’s choice should consider its reality, but given preference to enzymatic digestion or filtration.
The external validity of this review is inherently constrained by the limited and variable number of included studies, posing challenges to the generalizability of findings to the broader population of women with breast cancer. The diverse quality of the studies further complicates the overall interpretation of results, necessitating caution in extrapolating conclusions to diverse clinical scenarios. The variations in study quality and heterogeneity across the included studies may impact the overall robustness of the evidence and its applicability to broader patient populations.
In conclusion, the in-vivo studies reviewed in this report offer valuable insights into the safety and efficacy of ADSC-enriched fat for breast cancer patients. The evidence suggests that ADSC-enriched fat grafting does not appear to increase the recurrence risk of cancer or distant metastases in breast cancer patients. Furthermore, it has been associated with high patient satisfaction, potential in breast reconstruction, and the capability to minimize cancer-related lymphedema of the upper extremity
The properties of ADSC-enriched fat, including its ability to enhance the survivability and integration of transplanted fat, appear to confer advantages over AFT-only retention. While this review has some limitations, further research is warranted to better comprehend the long-term effects and optimal applications of ADSC-enriched fat in breast cancer treatment, given the promising current findings. Continued investigation into its potential clinical benefits is imperative.
Conclusions
In-vitro investigations have elucidated the dual role of ADSCs in influencing breast cancer cell behaviors, manifesting both promotive and inhibitory effects on proliferation, migration, and drug resistance. This nuanced modulation is orchestrated through diverse secreted factors and signaling pathways. Expanding on these insights, animal studies have demonstrated that distinct subpopulations of ADSCs differentially impact tumor growth, employing mechanisms that involve both direct interactions and the effects of EV cargo.
In contrast to in vitro evidence, the limited yet noteworthy clinical reports from in-vivo studies suggest that ADSC-enriched fat grafting is generally considered a safe procedure. Comparative analyses with fat grafting alone or no reconstruction indicate no increased risk of breast cancer recurrence or metastasis. However, special attention is warranted for cases of invasive breast carcinoma, where a recurrence following AFT has been observed. ADSC-enriched fat grafts exhibit potential benefits in enhancing graft retention and survival rates.
Despite the promising current evidence, there remains a need for further studies to comprehensively unravel the intricate relationship between ADSCs and breast cancer. These endeavors aim to optimize the clinical applications of ADSC-enriched fat grafts, potentially revolutionizing breast reconstruction options and introducing novel therapeutic strategies to enhance overall patient outcomes.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This project has been funded by the Federal University of Health Sciences of Porto Alegre (UFCSPA).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-54/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-54/coif). The authors have no conflicts of interest to declare.
References
- 1.Guneta V, Tan NS, Sugii S, et al. Comparative Study of Adipose-Derived Stem Cells From Abdomen and Breast. Ann Plast Surg 2016;76:569-75. 10.1097/SAP.0000000000000797 [DOI] [PubMed] [Google Scholar]
- 2.Nyström M, Lauvrud AT, Pérez-Díaz S, et al. Interaction of adipose-derived stem cells with active and dormant breast cancer cells. J Plast Reconstr Aesthet Surg 2023;83:69-76. 10.1016/j.bjps.2023.05.006 [DOI] [PubMed] [Google Scholar]
- 3.Gebremeskel S, Gencarelli J, Gareau AJ, et al. Promotion of Primary Murine Breast Cancer Growth and Metastasis by Adipose-Derived Stem Cells Is Reduced in the Presence of Autologous Fat Graft. Plast Reconstr Surg 2019;143:137-47. 10.1097/PRS.0000000000005142 [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Su X, Xu M, et al. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther 2019;10:117. 10.1186/s13287-019-1220-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamat P, Schweizer R, Kaenel P, et al. Human Adipose-Derived Mesenchymal Stromal Cells May Promote Breast Cancer Progression and Metastatic Spread. Plast Reconstr Surg 2015;136:76-84. 10.1097/PRS.0000000000001321 [DOI] [PubMed] [Google Scholar]
- 6.Orbay H, Hinchcliff KM, Charvet HJ, et al. Fat Graft Safety after Oncologic Surgery: Addressing the Contradiction between In Vitro and Clinical Studies. Plast Reconstr Surg 2018;142:1489-99. 10.1097/PRS.0000000000004992 [DOI] [PubMed] [Google Scholar]
- 7.Charvet HJ, Orbay H, Harrison L, et al. In Vitro Effects of Adipose-Derived Stem Cells on Breast Cancer Cells Harvested From the Same Patient. Ann Plast Surg 2016;76 Suppl 3:S241-5. 10.1097/SAP.0000000000000802 [DOI] [PubMed] [Google Scholar]
- 8.Kuhbier JW, Bucan V, Reimers K, et al. Observed changes in the morphology and phenotype of breast cancer cells in direct co-culture with adipose-derived stem cells. Plast Reconstr Surg 2014;134:414-23. 10.1097/PRS.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 9.Tsuji W, Schweizer R, Plock JA, et al. Paracrine interaction between adipose-derived stem cells and breast cancer cells. Breast 2015;24:S27-8. 10.1016/S0960-9776(15)70057-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauter MA, Brett E, Müller CM, et al. Novel Assay Analyzing Tropism between Adipose-Derived Stem Cells and Breast Cancer Cells Reveals a Low Oncogenic Response. Breast Care (Basel) 2019;14:278-87. 10.1159/000503411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlottmann F, Bucan V, Strauß S, et al. Influence of Tamoxifen on Different Biological Pathways in Tumorigenesis and Transformation in Adipose-Derived Stem Cells, Mammary Cells and Mammary Carcinoma Cell Lines-An In Vitro Study. Cells 2022;11:2733. 10.3390/cells11172733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike S, Zhang P, Wei Z, et al. In vitro effects of tamoxifen on adipose-derived stem cells. Wound Repair Regen 2015;23:728-36. 10.1111/wrr.12322 [DOI] [PubMed] [Google Scholar]
- 13.Boemi I, Lisa AVE, Vitali E, et al. Evaluation of the ex vivo Effects of Tamoxifen on Adipose-Derived Stem Cells: A Pilot Study. Front Cell Dev Biol 2021;9:555248. 10.3389/fcell.2021.555248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koko KR, Chang S, Hagaman AL, et al. Histone Deacetylase Inhibitors Enhance Cytotoxicity Towards Breast Tumors While Preserving the Wound-Healing Function of Adipose-Derived Stem Cells. Ann Plast Surg 2017;78:728-35. 10.1097/SAP.0000000000001066 [DOI] [PubMed] [Google Scholar]
- 15.Tsuji W, Chung CW, McLaughlin MM, et al. Effect of doxorubicin and paclitaxel on adipose-derived stem cells and breast cancer cells: Can we incorporate chemotherapy into our reconstructive strategies? Cancer Res 2013;73:abstr P4-16-03.
- 16.Scioli MG, Artuso S, D'Angelo C, et al. Adipose-derived stem cell-mediated paclitaxel delivery inhibits breast cancer growth. PLoS One 2018;13:e0203426. 10.1371/journal.pone.0203426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren J, Kong W, Lu F, et al. Adipose-derived stem cells (ADSCs) inhibit the expression of anti-apoptosis proteins through up-regulation of ATF4 on breast cancer cells. Ann Transl Med 2021;9:1300. 10.21037/atm-21-3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejaz A, Yang KS, Venkatesh KP, et al. The Impact of Human Lipoaspirate and Adipose Tissue-Derived Stem Cells Contact Culture on Breast Cancer Cells: Implications in Breast Reconstruction. Int J Mol Sci 2020;21:9171. 10.3390/ijms21239171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu YC, Wang WT, Huang LJ, et al. Differential Response of Non-cancerous and Malignant Breast Cancer Cells to Conditioned Medium of Adipose tissue-derived Stromal Cells (ASCs). Int J Med Sci 2019;16:893-901. 10.7150/ijms.27125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Qian C, Ma F, et al. MAPK/ERK-CBP-RFPL-3 Mediates Adipose-Derived Stem Cell-Induced Tumor Growth in Breast Cancer Cells by Activating Telomerase Reverse Transcriptase Expression. Stem Cells Int 2022;2022:8540535. 10.1155/2022/8540535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowan BG, Gimble JM, Sheng M, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One 2014;9:e89595. 10.1371/journal.pone.0089595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerlin L, Donnenberg AD, Rubin JP, et al. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A 2011;17:93-106. 10.1089/ten.tea.2010.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eterno V, Zambelli A, Pavesi L, et al. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget 2014;5:613-33. 10.18632/oncotarget.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Zhou X, Wang J, et al. Adipose-derived mesenchymal stem cells and extracellular vesicles confer antitumor activity in preclinical treatment of breast cancer. Pharmacol Res 2020;157:104843. 10.1016/j.phrs.2020.104843 [DOI] [PubMed] [Google Scholar]
- 25.Jin X, Huang Y, Yoo HK, et al. Oncologic Safety and Efficacy of Cell-Assisted Lipotransfer for Breast Reconstruction in a Murine Model of Residual Breast Cancer. Aesthetic Plast Surg 2023;47:412-22. 10.1007/s00266-022-03021-3 [DOI] [PubMed] [Google Scholar]
- 26.Millet E, Haik J, Ofir E, et al. The Impact of Autologous Fat Grafting on Breast Cancer: An Experimental Model Using Magnetic Resonance Imaging. Isr Med Assoc J 2016;18:283-5. [PubMed] [Google Scholar]
- 27.Paino F, La Noce M, Di Nucci D, et al. Human adipose stem cell differentiation is highly affected by cancer cells both in vitro and in vivo: implication for autologous fat grafting. Cell Death Dis 2017;8:e2568. 10.1038/cddis.2016.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Illouz YG. Breast cancer treatment by adipose-derived stem cells: an experimental study. J Stem Cells 2014;9:211-7. [PubMed] [Google Scholar]
- 29.Berti M, Goupille C, Doucet M, et al. Oncological Safety of Autologous Fat Grafting in Breast Reconstruction after Mastectomy for cancer: A case-control study. J Gynecol Obstet Hum Reprod 2022;51:102257. 10.1016/j.jogoh.2021.102257 [DOI] [PubMed] [Google Scholar]
- 30.Mazur S, Zołocińska A, Siennicka K, et al. Safety of adipose-derived cell (stromal vascular fraction - SVF) augmentation for surgical breast reconstruction in cancer patients. Adv Clin Exp Med 2018;27:1085-90. 10.17219/acem/70798 [DOI] [PubMed] [Google Scholar]
- 31.Toyserkani NM, Jensen CH, Andersen DC, et al. Treatment of Breast Cancer-Related Lymphedema with Adipose-Derived Regenerative Cells and Fat Grafts: A Feasibility and Safety Study. Stem Cells Transl Med 2017;6:1666-72. 10.1002/sctm.17-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myckatyn TM, Wagner IJ, Mehrara BJ, et al. Cancer Risk after Fat Transfer: A Multicenter Case-Cohort Study. Plast Reconstr Surg 2017;139:11-8. 10.1097/PRS.0000000000002838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vester-Glowinski PV, Herly M, Ørholt M, et al. Fat Grafting With Expanded Adipose-Derived Stromal Cells for Breast Augmentation: A Randomized Controlled Trial. Aesthet Surg J 2022;42:1279-89. 10.1093/asj/sjac159 [DOI] [PubMed] [Google Scholar]
- 34.Kempa S, Brix E, Heine N, et al. Autologous fat grafting for breast reconstruction after breast cancer: a 12-year experience. Arch Gynecol Obstet 2022;305:921-7. 10.1007/s00404-021-06241-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrese C, Kothari A, Badylak S, et al. Oncological safety of stromal vascular fraction enriched fat grafting in two-stage breast reconstruction after nipple sparing mastectomy: long-term results of a prospective study. Eur Rev Med Pharmacol Sci 2018;22:4768-77. [DOI] [PubMed] [Google Scholar]
- 36.Ito S, Kai Y, Masuda T, et al. Long-term outcome of adipose-derived regenerative cell-enriched autologous fat transplantation for reconstruction after breast-conserving surgery for Japanese women with breast cancer. Surg Today 2017;47:1500-11. 10.1007/s00595-017-1544-4 [DOI] [PubMed] [Google Scholar]
- 37.Kølle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 2013;382:1113-20. 10.1016/S0140-6736(13)61410-5 [DOI] [PubMed] [Google Scholar]
- 38.Gentile P, Di Pasquali C, Bocchini I, et al. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg Innov 2013;20:370-6. 10.1177/1553350612458544 [DOI] [PubMed] [Google Scholar]
- 39.Chiu CH. Does Stromal Vascular Fraction Ensure a Higher Survival in Autologous Fat Grafting for Breast Augmentation? A Volumetric Study Using 3-Dimensional Laser Scanning. Aesthet Surg J 2019;39:41-52. 10.1093/asj/sjy030 [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Wang X, Jiang C. Clinical Efficacy Analysis of Augmentation Mammoplasty with Cell-Assisted Autologous Fat Transplantation. Aesthetic Plast Surg 2022;46:2218-27. 10.1007/s00266-022-02778-x [DOI] [PubMed] [Google Scholar]
- 41.Salgarello M, Visconti G, Rusciani A. Breast fat grafting with platelet-rich plasma: a comparative clinical study and current state of the art. Plast Reconstr Surg 2011;127:2176-85. 10.1097/PRS.0b013e3182139fe7 [DOI] [PubMed] [Google Scholar]
- 42.Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast Reconstr Surg 2016;137:385-93. 10.1097/01.prs.0000475741.32563.50 [DOI] [PubMed] [Google Scholar]
- 43.Tukiama R, Vieira RAC, Facina G, et al. Oncologic Safety of Autologous Fat Grafting after Breast Cancer Surgical Treatment: A Matched Cohort Study. Plast Reconstr Surg 2021;148:11-20. 10.1097/PRS.0000000000008037 [DOI] [PubMed] [Google Scholar]
- 44.Juhl AA, Redsted S, Engberg Damsgaard T. Autologous fat grafting after breast conserving surgery: Breast imaging changes and patient-reported outcome. J Plast Reconstr Aesthet Surg 2018;71:1570-6. 10.1016/j.bjps.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 45.Serra-Mestre JM, Fernandez Peñuela R, Foti V, et al. Breast Cleavage Remodeling with Fat Grafting: A Safe Way to Optimize Symmetry and to Reduce Intermammary Distance. Plast Reconstr Surg 2017;140:665e-72e. 10.1097/PRS.0000000000003788 [DOI] [PubMed] [Google Scholar]
- 46.Kaoutzanis C, Xin M, Ballard TN, et al. Autologous Fat Grafting After Breast Reconstruction in Postmastectomy Patients: Complications, Biopsy Rates, and Locoregional Cancer Recurrence Rates. Ann Plast Surg 2016;76:270-5. 10.1097/SAP.0000000000000561 [DOI] [PubMed] [Google Scholar]
- 47.Gale KL, Rakha EA, Ball G, et al. A case-controlled study of the oncologic safety of fat grafting. Plast Reconstr Surg 2015;135:1263-75. 10.1097/PRS.0000000000001151 [DOI] [PubMed] [Google Scholar]
- 48.Klinger M, Losurdo A, Lisa AVE, et al. Safety of autologous fat grafting in breast cancer: a multicenter Italian study among 17 senonetwork breast units autologous fat grafting safety: a multicenter Italian retrospective study. Breast Cancer Res Treat 2022;191:355-63. 10.1007/s10549-021-06444-9 [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 2008;32:48-55; discussion 56-7. 10.1007/s00266-007-9019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Luo X, Lu Y, et al. Is the Resorption of Grafted Fat Reduced in Cell-Assisted Lipotransfer for Breast Augmentation? Ann Plast Surg 2015;75:128-34. 10.1097/SAP.0000000000000068 [DOI] [PubMed] [Google Scholar]
- 51.Shin DJ. A Study on Breast Augmentation Using Fat Grafting With Stromal Vascular Fraction. Ann Plast Surg 2023;90:380-4. 10.1097/SAP.0000000000003506 [DOI] [PubMed] [Google Scholar]
- 52.Jeon HJ, Choi DH, Lee JH, et al. A Prospective Study of the Efficacy of Cell-Assisted Lipotransfer with Stromal Vascular Fraction to Correct Contour Deformities of the Autologous Reconstructed Breast. Aesthetic Plast Surg 2021;45:853-63. 10.1007/s00266-020-01981-y [DOI] [PubMed] [Google Scholar]
- 53.Pérez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol 2012;38:382-9. 10.1016/j.ejso.2012.02.178 [DOI] [PubMed] [Google Scholar]
- 54.Lee KT, Kim JH, Jeon BJ, et al. Association of Fat Graft with Breast Cancer Recurrence in Implant-Based Reconstruction: Does the Timing Matter? Ann Surg Oncol 2023;30:1087-97. 10.1245/s10434-022-12389-0 [DOI] [PubMed] [Google Scholar]
- 55.Moustaki M, Papadopoulos O, Verikokos C, et al. Application of adipose-derived stromal cells in fat grafting: Basic science and literature review. Exp Ther Med 2017;14:2415-23. 10.3892/etm.2017.4811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gentile P, Calabrese C, De Angelis B, et al. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. Int J Mol Sci 2019;20:5471. 10.3390/ijms20215471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin Y, Mu D, Zhang X, et al. Three-Dimensional Volumetric Analysis of the Effect of Interval Time in Autologous Fat Graft Breast Augmentation. Aesthetic Plast Surg 2023;47:1731-9. 10.1007/s00266-023-03367-2 [DOI] [PubMed] [Google Scholar]
- 58.Wang K, Mu D, Zhang X, et al. Lactation History Affects Postoperative Fat Volume Retention Rate in Autologous Fat Grafting Breast Augmentation. Aesthetic Plast Surg 2021;45:118-26. 10.1007/s00266-020-01683-5 [DOI] [PubMed] [Google Scholar]
- 59.Xiong BJ, Tan QW, Chen YJ, et al. The Effects of Platelet-Rich Plasma and Adipose-Derived Stem Cells on Neovascularization and Fat Graft Survival. Aesthetic Plast Surg 2018;42:1-8. 10.1007/s00266-017-1062-1 [DOI] [PubMed] [Google Scholar]
- 60.Karam M, Abul A, Rahman S. Stem Cell Enriched Fat Grafts versus Autologous Fat Grafts in Reconstructive Surgery: Systematic Review and Meta-Analysis. Aesthetic Plast Surg 2023;47:2754-68. 10.1007/s00266-023-03421-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, Long X, Si L, et al. A pilot study on ex vivo expanded autologous adipose-derived stem cells of improving fat retention in localized scleroderma patients. Stem Cells Transl Med 2021;10:1148-56. 10.1002/sctm.20-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin Y, Li J, Li Q, et al. Autologous fat graft assisted by stromal vascular fraction improves facial skin quality: A randomized controlled trial. J Plast Reconstr Aesthet Surg 2020;73:1166-73. 10.1016/j.bjps.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 63.Cao Z, Li H, Wang ZH, et al. High-Density Fat Grafting Assisted Stromal Vascular Fraction Gel in Facial Deformities. J Craniofac Surg 2022;33:108-11. 10.1097/SCS.0000000000008038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fontdevila J, Guisantes E, Martínez E, et al. Double-blind clinical trial to compare autologous fat grafts versus autologous fat grafts with PDGF: no effect of PDGF. Plast Reconstr Surg 2014;134:219e-230e. 10.1097/PRS.0000000000000409 [DOI] [PubMed] [Google Scholar]
- 65.Gentile P, Cervelli V, De Fazio D, et al. Mechanical and Enzymatic Digestion of Autologous Fat Grafting (A-FG): Fat Volume Maintenance and AD-SVFs Amount in Comparison. Aesthetic Plast Surg 2023;47:2051-62. 10.1007/s00266-023-03364-5 [DOI] [PubMed] [Google Scholar]
- 66.Wu R, Yang X, Jin X, et al. Three-dimensional Volumetric Analysis of 3 Fat-Processing Techniques for Facial Fat Grafting: A Randomized Clinical Trial. JAMA Facial Plast Surg 2018;20:222-9. 10.1001/jamafacial.2017.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]