Introduction
Breast cancer (BC) is the most common malignancy among women worldwide, with approximately 1.6 million new cases and 1.2 million BC-related deaths in China every year (1). Triple negative breast cancer (TNBC), an aggressive subtype of BC, accounts for about 15% of BC cases and carries a poor prognosis (2). The Chinese Society of Clinical Oncology (CSCO) BC guidelines have long been considered the gold standard reference for BC treatment in China. Chinese scholars have updated the guidelines annually since 2017, based on reliable clinical evidence and national conditions. Most significantly, risk stratification was introduced to provide precision medicine for BC and maximize the therapeutic benefits. This article aimed to summarize the most recent clinical studies and provide an in-depth insight into adjuvant therapy for TNBC after risk stratification.
Adjuvant treatment for TNBC patients
Surgery and chemotherapy are the mainstay for the treatment of TNBC, given that TNBC patients do not benefit from human epidermal growth factor receptor 2 (HER2)-targeted therapy or endocrine therapy (3). Randomized clinical trials have revealed that the sequential addition of taxane to anthracycline plus cyclophosphamide (AC) confers a prolonged disease-free survival (DFS) (4). According to the CSCO Guidelines for the Diagnosis and Treatment of Breast Cancer (2021 version), TNBC patients are recommended surgery followed by chemotherapy. The chemotherapy regimen differs based on risk stratification.
In the CALGB9344 study (5), the sequential addition of paclitaxel to AC chemotherapy was shown to prolong the DFS of patients with node-positive BC. Therefore, AC-T (anthracycline plus cyclophosphamide followed by taxane) is an effective and efficient scheme which is currently recommended for some patients with a higher risk of recurrence. However, no difference was observed in overall survival (OS) and DFS of node-positive BC between patients receiving TAC (docetaxel, anthracycline, and cyclophosphamide) and those treated with AC-T. Based on the CALGB 9741 study, which further revealed the effects of dose-dense (dd) chemotherapy in improving the DFS in BC, ddAC-ddT has been considered as an alternative treatment strategy for high-risk BC patients who are partially tolerable. As for those with a lower risk for recurrence, the US Oncology Research Trial 9735 showed that docetaxel and cyclophosphamide (TC) is superior to AC in improving the DFS of both younger and older patients (6).
The application of platinum agents has provided a new impetus due to DNA repair defects in TNBC, but the inclusion of platinum agents in adjuvant chemotherapy of TNBC is still controversial. The PATTERN study (7) showed that the paclitaxel-plus-carboplatin (PCb) regimen conferred significant improvements in DFS compared to cyclophosphamide, epirubicin, and fluorouracil followed by paclitaxel (CEF-T) regimen that was recommended in previous guidelines. Meanwhile, PCb was found to prolong the relapse-free survival (RFS) of patients with high homologous recombination defect (HRD) scores, highlighting the key role of carboplatin in adjuvant therapy. Recently published clinical research showed no difference in the DFS and OS between the subjects treated with taxanes combined with platinum (TP) and the counterparts with epirubicin, cyclophosphamide and taxanes (ECT) (8). Taken together, PCb could serve as an effective alternative adjuvant chemotherapy option for patients with operable TNBC, especially those with high HRD scores.
Capecitabine (X), an oral prodrug of fluorouracil (5FU), was approved in 1998 for the treatment of metastatic HER2-negative BC. A recent randomized clinical trial in China (CBCSG-010 study) (9) demonstrated that capecitabine concomitant with docetaxel, epirubicin, and cyclophosphamide conferred a more favorable DFS compared to a non-capecitabine regime, especially for patients with T2/T3, positive lymph nodes, histological grade III, and high expression of Ki-67. These findings demonstrated that capecitabine can be combined with anthracycline and taxol in early adjuvant therapy. Hence, three cycles of docetaxel and capecitabine followed by three cycles of capecitabine, epirubicin, and cyclophosphamide (TX-XEC) after surgery are strongly recommended for high-risk early TNBC patients.
In general, the addition of platinum or capecitabine to anthracycline + taxane regimen for TNBC patients may be applied.
Enhanced therapy after adjuvant therapy
Breast cancer susceptibility gene 1/2 (BRCA1/2) mutations are well documented and potent drivers for BC, as they are key molecules involved in DNA repair (10-12). Poly-ADP-ribose polymerase (PARP) is a nuclear enzyme that plays a pivotal role in DNA single-strand break repair (13). In vivo data demonstrates that functional deficiencies in BRCA1/2 enhance the sensitivity of cells to PARP inhibitor (PARPi) (14). In the OlympiA clinical trial (15), patients receiving olaparib, an oral PARPi, after completing local treatment and neoadjuvant or adjuvant chemotherapy, showed markedly longer survival free of invasiveness and distant disease compared to those in the placebo group. Therefore, we recommend olaparib for enhanced treatment after standard adjuvant chemotherapy in high-risk TNBC patients (i.e., those with positive axillary lymph nodes or tumor sizes <2 cm) with BRCA mutations (Figure 1). PARPis, unlike conventional chemotherapy, are an effective targeted therapy with a lower likelihood of acquired resistance. The TBCRC 048 study (16) uncovered that the patients with germline partner and localizer of BRCA2 (gPALB2) mutation or somatic BRCA1/2 mutation could achieve objective response rates (ORRs) of 80% and 50%, respectively, which is encouraging. With advances in high-throughput sequencing, genetic testing has shown promise for the maximal efficacy of targeted therapy.
Figure 1.
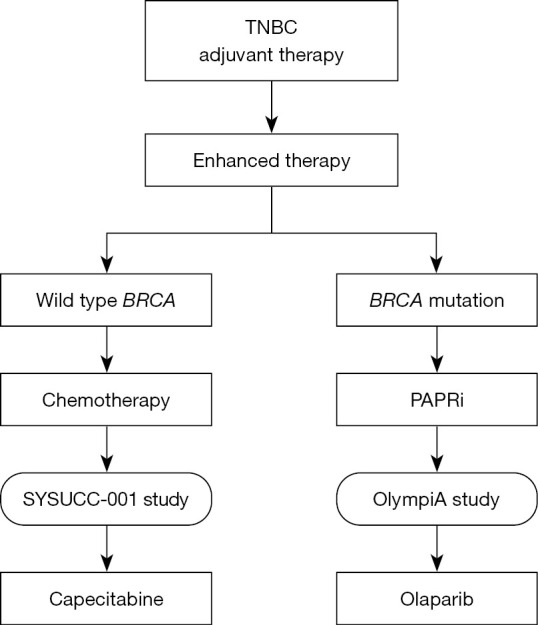
Enhanced therapy after adjuvant therapy of TNBC. TNBC, triple negative breast cancer; BRCA, breast cancer susceptibility gene; PARPi, poly-ADP-ribose polymerase inhibitor.
As for wild-type BRCA patients, capecitabine appears to be an effective strategy, which has been well illustrated in the SYSUCC-001 randomized clinical trial (17). This study, for the first time, introduced metronomic chemotherapy into the adjuvant treatment of TNBC. The dose for capecitabine metronomic chemotherapy, which has fewer safety concerns, was much lower than maximum tolerated dose. Furthermore, capecitabine effectively improved the prognosis of BC patients. Consequently, capecitabine is recommended to serve as maintenance therapy for low- and high-risk patients without BRCA mutations (Figure 1).
Immunotherapy for TNBC
Despite the high mutational load, programmed death ligand 1 (PD-L1) expression, and tumor-infiltrating lymphocytes, immunotherapy remains controversial in the treatment of TNBC. Keynote-522 (18), a prospective phase III study, was designed to explore the efficacy of pembrolizumab in neoadjuvant and adjuvant therapy of early TNBC. The promising results obtained in this trail lead to the National Comprehensive Cancer Network (NCCN) recommending the use of pembrolizumab combined with chemotherapy as neoadjuvant therapy. However, there was no direct evidence for the use of pembrolizumab in adjuvant treatment. Chinese scholars have voiced reservations about immunotherapy in TNBC, which is consistent with 2021 Chinese Anti-Cancer Association, Committee of Breast Cancer Society (CACA-CBCS) guidelines. As a result, immunotherapy in the adjuvant treatment of BC is not recommended. More research with larger sample sizes is required to identify effective immunotherapies in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
We acknowledged A. Kassem for the language editing of this article.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Breast Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-13/coif). The authors have no conflicts of interest to declare.
References
- 1.Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. 10.1016/S1470-2045(13)70567-9 [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Barlow WE, Godwin AK, et al. Validation of the DNA Damage Immune Response Signature in Patients With Triple-Negative Breast Cancer From the SWOG 9313c Trial. J Clin Oncol 2019;37:3484-92. 10.1200/JCO.19.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938-48. 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 4.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003;21:976-83. 10.1200/JCO.2003.02.063 [DOI] [PubMed] [Google Scholar]
- 5.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431-9. 10.1200/JCO.2003.09.081 [DOI] [PubMed] [Google Scholar]
- 6.Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol 2009;27:1177-83. 10.1200/JCO.2008.18.4028 [DOI] [PubMed] [Google Scholar]
- 7.Yu KD, Ye FG, He M, et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1390-6. 10.1001/jamaoncol.2020.2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng F, Du F, Wang W, et al. Updated efficacy of adjuvant epirubicin plus cyclophosphamide followed by taxanes versus carboplatin plus taxanes in early triple-negative breast cancer in phase 2 trial: 8.1-year median follow-up. Breast Cancer Res Treat 2022;191:97-105. 10.1007/s10549-021-06401-6 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yu K, Pang D, et al. Adjuvant Capecitabine With Docetaxel and Cyclophosphamide Plus Epirubicin for Triple-Negative Breast Cancer (CBCSG010): An Open-Label, Randomized, Multicenter, Phase III Trial. J Clin Oncol 2020;38:1774-84. 10.1200/JCO.19.02474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter C, Nilsson MP, Olsson E, et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann Oncol 2016;27:1532-8. 10.1093/annonc/mdw209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecarpentier J, Silvestri V, Kuchenbaecker KB, et al. Prediction of Breast and Prostate Cancer Risks in Male BRCA1 and BRCA2 Mutation Carriers Using Polygenic Risk Scores. J Clin Oncol 2017;35:2240-50. 10.1200/JCO.2016.69.4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drost R, Dhillon KK, van der Gulden H, et al. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J Clin Invest 2016;126:2903-18. 10.1172/JCI70196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolderson E, Richard DJ, Zhou BB, et al. Recent advances in cancer therapy targeting proteins involved in DNA double-strand break repair. Clin Cancer Res 2009;15:6314-20. 10.1158/1078-0432.CCR-09-0096 [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim YH, García-García C, Serra V, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov 2012;2:1036-47. 10.1158/2159-8290.CD-11-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021;384:2394-405. 10.1056/NEJMoa2105215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tung NM, Robson ME, Ventz S, et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J Clin Oncol 2020;38:4274-82. 10.1200/JCO.20.02151 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wang SS, Huang H, et al. Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA 2021;325:50-8. 10.1001/jama.2020.23370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cetin B, Gumusay O. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:e108. 10.1056/NEJMc2006684 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


