Abstract
Pleural effusion is a common problem in our country, and most of these patients need invasive tests as they can’t be evaluated by blood tests alone. The simplest of them is diagnostic pleural aspiration, and diagnostic techniques such as medical thoracoscopy are being performed more frequently than ever before. However, most physicians in India treat pleural effusion empirically, leading to delays in diagnosis, misdiagnosis and complications from wrong treatments. This situation must change, and the adoption of evidence-based protocols is urgently needed. Furthermore, the spectrum of pleural disease in India is different from that in the West, and yet Western guidelines and algorithms are used by Indian physicians. Therefore, India-specific consensus guidelines are needed. To fulfil this need, the Indian Chest Society and the National College of Chest Physicians; the premier societies for pulmonary physicians came together to create this National guideline. This document aims to provide evidence based recommendations on basic principles, initial assessment, diagnostic modalities and management of pleural effusions.
KEY WORDS: Chest tube, guidelines, pleural biopsy, pleural disease, pleural effusion, thoracentesis, thoracoscopy
1 BACKGROUND AND METHODS
The National pleural effusion guidelines
Indian Chest Society and the National College of Chest Physicians are the premier National societies in India, and they come together to create the National guidelines for various respiratory diseases. In the past, guidelines have been created for Asthma, COPD, Interstitial Lung diseases, etc., The task of chairing this initiative was entrusted to Dr D J Christopher, and the face-to-face meeting was organised by the Pulmonary and Respiratory Medicine departments, Christian Medical College, Vellore.
Background
Pleural effusions are common in our country, and the majority of the patients need invasive evaluation as they cannot be evaluated by blood tests alone. The simplest of them is diagnostic pleural aspiration, and diagnostic techniques such as medical thoracoscopy are being performed more frequently than ever before. However, in reality, most physicians in India treat pleural effusions empirically, leading to delays in diagnosis, misdiagnosis and complications from wrong treatments. This situation must change, and the adoption of evidence-based protocols is urgently needed. Furthermore, the spectrum of pleural disease in India is different from that in the West, and yet we use Western guidelines and algorithms. Therefore, India-specific consensus guidelines are needed.
Aim of the guideline
This guideline aims to provide evidence-based recommendations on initial assessment, diagnostic modalities and management of pleural effusion caused by varied aetiology. Pleural effusion is a relatively common condition, but no guidelines have been published so far for India. The problems faced in India are unique, and thus, there is a compelling case for setting out to develop a comprehensive, evidence-based guideline.
Intended users of the guideline
The guideline will be of interest to clinicians in India caring for adults with pleural effusion, who include respiratory physicians, general physicians, emergency physicians and postgraduate trainees in these specialities.
Scope of the guideline
The guideline particularly delves into different aspects of the evaluation and management of pleural effusion. The topics covered have been endorsed by the expert group that was set up to prepare the guidelines. This guideline is not exhaustive and is not intended to be a textbook on pleural effusion but a guidance document for the care of patients. The guidelines have been updated to the extent feasible up to the time of its publication, however, those who use the recommendations should ensure that more recent information has not become available on the topic, rendering the recommendations on this document outdated.
Areas not covered by the guideline
Primary pleural tumours, pneumothorax and pleural effusion in children have not been covered in this guideline.
Limitations of the guideline
Physicians are expected to use sound clinical judgement and discretion when applying the recommendations in this guideline. When appropriate, referral to those with more expertise and experience or transfer to centres with higher facilities should be carried out. All decisions should be made after providing adequate information and obtaining the consent of the concerned patients.
Members of the guideline development group
The process of developing guidelines was undertaken as a joint exercise by the two major societies covering the practice of respiratory diseases, namely, the Indian Chest Society and the National College of Chest Physicians. After the completion of the approval processes, the task of coordinating this exercise was taken up by the Department of Pulmonary Medicine and Department of Respiratory Medicine, Christian Medical College, Vellore. The committee constituted for this purpose included experts nominated by the two associations.
Methods
The faculty and postgraduate trainees of the departments of Pulmonary and Respiratory Medicine prepared extensively to collate available evidence over several months. This was shared with the members of the committee. A face-to-face meeting was required to derive a consensus between experts on various aspects of this guideline. This occurred over 2 days on 11 and 12 October 2019. A working group of more than 30 National experts, comprising pulmonologists and thoracic surgeons, came down to Vellore for this meeting. The core group prepared the search questions to address the clinically relevant situations. A systematic search had been performed on the PubMed, Embase databases, and the Cochrane Library before the meeting. Data related to each question was presented by the faculty and postgraduates of the Christian Medical College, Vellore and was reviewed during the meeting in discussions across subgroups and the whole group.
A consensus was sought for all questions. When high-quality evidence was available, it was invariably unanimous. Greater than 80% agreement was used as a threshold to determine consensus for those with a lesser quality of evidence. Therefore, the evidence statements and the recommendations reflect the consensus opinion of the working group. A modified GRADE approach was used to grade the evidence. [see Table 1]
Table 1.
Grading based on level of evidence and strength of recommendations
| Grade of evidence | Criterion | |
|---|---|---|
| Level 1 | Evidence from ≥1 good quality and well-conducted randomised control trial (s) or meta-analysis of RCTs | |
| Level 2 | Evidence from at least 1 RCT of moderate quality, or well-designed clinical trial without randomisation, or from cohort or case-controlled studies | |
| Level 3 | Evidence from descriptive studies. Not backed by sufficient evidence; |
|
| GPP | A recommendation for best practice based on the experience of guideline development group. | |
| Strength of recommendation | ||
| A | Strong recommendation: to do (or not to do) where the benefits clearly outweigh the risk (or vice versa) for most, if not all, patients. E.g.,1A, 2A | |
| B | Weak recommendation: where benefits and risks are more closely balanced or are more uncertain. E.g., 1B, 2B, 3B |
RCT: Randomised Controlled Trial, GPP: Good Practice Point
Manuscript writing and publication
After gruelling sessions, the working group concluded its deliberations, and the CMC team was entrusted with the task of writing the final manuscript. This is expected to take six months. When the preparation was in full swing, the COVID-19 pandemic struck the globe. The work on the manuscript had to be halted, and all hands were called on deck to combat the ravaging pandemic. In 2022, after the pandemic abated, work was recommenced. Since more than two years had elapsed, the writing team had the job of reviewing the evidence that had accumulated over this period and updating the manuscript. When the manuscript was ready later that year, the release was put on hold pending the impending publication of some other reputed societal guidelines. These guidelines have also been reviewed, and the manuscript has now been revised to ensure that this guideline is as up-to-date as possible.
This guideline will be simultaneously published in the ‘Lung India’ and ‘Indian Journal of Chest Diseases and Allied Sciences’ and is likely to be widely circulated to medical practitioners through various media. The guidelines will also be submitted to the Ministry of Health and the Indian Council of Medical Research for official endorsement.
Acknowledgements
Special mention should be made of the efforts taken by the postgraduate trainees at the Christian Medical College, Vellore, in performing literature searches and collating the material needed for discussions.
2 BASIC SCIENCE RELEVANT TO PLEURAL PROCEDURES
The pleura
The pleura is a serous membranous structure that covers the parenchyma of the lung, the mediastinum, the diaphragm, and the rib cage. It is divided into the visceral and the parietal pleura.[1] The lung parenchyma is covered by the visceral pleura at its contact sites with the chest wall, diaphragm, mediastinum and interlobar fissures. The parietal pleura covers the inner chest wall and is divided into costal, mediastinal and diaphragmatic pleura in accordance with the intrathoracic surface it lines. The potential space between the two layers of the pleura is the pleural space, which usually has a thin layer of fluid (pleural fluid) that enables the visceral pleura to slide along the parietal pleura. The pleura consists of loose, uneven connective tissue enclosed by a single mesothelial cell layer.
What are the anatomical variations in the course of the intercostal artery, and what is its clinical significance?
The intercostal vessels and nerves are arranged as a neurovascular bundle, which lies within the intercostal groove located in the inferior margin of the ribs.[2] Hence, pleural interventions performed close to the superior margin of the lower (caudal) rib avoid the intercostal artery (ICA) and are considered to be safe.[2] However, studies in cadavers have reported variation in the course of the intercostal arteries, which is not always protected posteriorly as commonly believed.[3] This potentially exposed intercostal artery can be injured during pleural interventions. Although less frequent, injury to the intercostal artery can be a life-threatening complication to all pleural interventions.[4] The course of ICA was studied by Helm et al.[2] using CT pulmonary angiography. In the first 6 centimetres lateral to the vertebral spine, the ICA appeared to be within the intercostal space and unprotected by the rib above. This variability is more remarkable in older patients and in more cephalad rib spaces. Therefore, if the procedure is carried out in regions closer to the spine or in more cephalad intercostal spaces or if the patient is elderly, there is a higher chance of suffering damage to an intercostal artery.[2]
According to recent research, vascular ultrasonography combined with colour flow may be used to identify the intercostal artery.[5,6] However, this is limited by the absence of prospective studies to assess the usefulness of thoracic ultrasound in identifying the ICA in the presence of pleural disease when an intervention is required. There is no conclusive evidence for the use of thoracic ultrasound for the visualisation of the intercostal artery.[7] Thus, further studies are required to address this question.
Evidence statement
The course of the intercostal arteries is not consistently deeper to the inferior margin of the upper (cranial) rib and may be exposed during part of its course. This potentially renders the ICA vulnerable to injury during pleural interventions. There is an increased risk of injury to the ICA up to 6 cm lateral to the spine of the vertebra, and the risk is higher in older patients and in more cephalad rib spaces.
Recommendation
Pleural interventions in the intercostal space should be performed close to the superior margin of the lower (caudal) rib and at least 6 cm lateral to the spine to avoid injuring the intercostal artery. (Grade 2A)
What is the safe triangle?
This is an anatomical region in the axilla that is safe for the placement of the intercostal drainage tube. It is triangular with the apex in the axilla, and it is bordered anteriorly by the lateral border of the pectoralis major, posteriorly by the anterior border of the latissimus dorsi, and inferiorly by a horizontal line from the nipple (often corresponds to the fifth intercostal space).[8,9] [See Figure 1][10]
Figure 1.
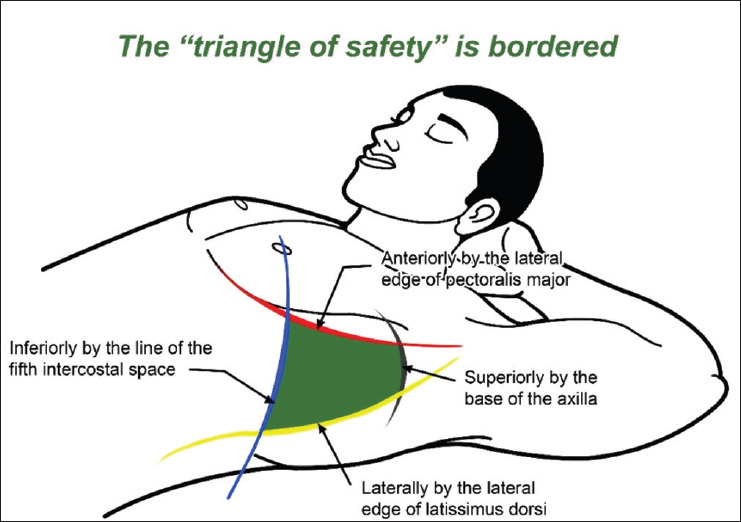
Safe triangle
What are the mechanisms of pleural fluid formation?
The current consensus is that the pleural fluid is derived from the systemic vessels of the pleural membranes (both parietal and visceral) and not from the pulmonary vessels.[11] In other words, pleural fluid is the interstitial fluid of systemic pleural micro vessels. The parietal vessels (intercostal micro vessels) are thought to be of primary importance because they are closer to the pleural space and have a higher filtration pressure than the bronchial micro vessels of the visceral pleura. The formation of pleural fluid is dependent upon a balance of hydrostatic pressures (microvascular minus pleural) opposed by the counterbalancing osmotic pressures (microvascular minus pleural). These pressures can be quantified by the application of Starling’s equation.[11] Pleural fluid is initially partially reabsorbed by the micro vessels, and the remaining fluid exits the pleural space via the lymphatic stomata in the parietal pleura.[11]
Tuberculous pleural effusion
When tuberculous pleural effusion occurs in the absence of radiologically apparent tuberculosis (TB), it can be a recurrence of tuberculosis (TB) or an extension of a primary infection that occurred 6–12 weeks ago.[11,12] It is believed that the rupture of a subpleural necrotic focus in the lung into the pleural space causes tuberculous pleural effusion.[13] Delayed hypersensitivity occurs when tuberculous proteins gain access to the pleural space, which increases the permeability of the pleural capillaries to protein.[14] The rate at which pleural fluid forms is significantly higher due to the elevated protein levels in the pleural fluid, which accordingly results in the accumulation of pleural fluid.[15,16] The intense inflammatory reaction in the parietal pleura also impedes the lymphatic drainage from the pleural space and leads to pleural fluid accumulation.[16] There is also evidence to suggest that tuberculous pleurisy may occur at any time in the natural course of tuberculosis, following the primary infection.[17]
Malignant pleural effusion
The mechanisms that cause the accumulation of pleural fluid directly are pleural metastasis with increased permeability, obstruction of lymphatic vessels, mediastinal lymph node involvement with impaired drainage, thoracic duct interruption (chylothorax), bronchial obstruction and pericardial involvement.[11] Causes of malignant pleural effusion through indirect mechanisms include hypoproteinemia, post-obstructive pneumonitis, pulmonary embolism, and post-radiation therapy.[11]
A combination of increased fluid production due to fluid extravasation from hyper-permeable parietal or visceral pleural and/or tumour vessels and impaired lymphatic outflow underlie the development of malignant pleural effusion.[11,16,18,19] Direct causes of pleural effusion formation include malignancy interfering with the integrity of the lymphatics system or direct tumour involvement of the pleura.[20] Tumour cells metastasise to the pleura mainly through the bloodstream and initially invade the visceral pleura. Most lung carcinomas translocate to the ipsilateral visceral pleura via the pulmonary vessels.[20,21] Secondary dissemination to the parietal pleura occurs by tumour seeding along adhesions or by exfoliated tumour cells floating in the effusion. The pleura may also be invaded through lymphangitic spread or even through direct extension of tumours infiltrating adjacent structures.[4] Higher levels of vascular endothelial growth factor (VEGF) are seen in malignant than in pleural effusions due to other inflammatory diseases.[22,23] Protein ratios and LDH, two indicators of enhanced vascular permeability and pleural inflammation, correlated with VEGF levels.[24]
Increased capillary permeability is the likely way that pulmonary emboli cause pleural effusion, which is probably due to the release of inflammatory mediators released by the platelet-rich thrombi.[11,25] VEGF and ischaemia of the pulmonary capillaries may also contribute to increased permeability.[23]
No specific mechanism is described for pleural effusion developing post-radiation therapy, but every patient has had concomitant pneumonitis.[26] Thoracoscopy of certain case reports has shown enlarged lymphatic vessels in the visceral pleura and diffuse thickening of the pleura.[27,28]
Hepatic hydrothorax
The direct passage of peritoneal fluid via diaphragmatic defects appears most plausible in explaining most cases of hepatic hydrothorax, which has been demonstrated by various methods.[11,29,30,31] Large effusions are probably because the fluid is permitted to flow from the peritoneal into the pleural cavity until the pleural pressure approaches the peritoneal pressure through the diaphragmatic defect.[23]
Pancreatic diseases
The following non-malignant conditions of the pancreas may present with pleural effusion: acute pancreatitis, pancreatic abscess, chronic pancreatitis with pseudocyst, and pancreatic ascites.[11] The pleural effusion in acute pancreatitis results from the trans-diaphragmatic transfer of the exudative fluid formed due to acute pancreatic and diaphragmatic inflammation. The high levels of pancreatic enzymes result in increased permeability of the pleural lymphatics. The diaphragm may be inflamed by the adjacent inflammatory process, and this may increase the permeability of the capillaries in the diaphragmatic pleura.[32] The mechanism responsible for pleural effusion in patients with a chronic pseudocyst is the development of pancreatic-pleural fistula (PPF). The fistula is usually formed due to a leak from an incompletely formed or ruptured pseudocyst or a direct pancreatic duct leakage.[33]
Chylothorax
Chylothorax is caused by the disruption of the lymphatic duct, which can occur via the following mechanisms:[34,35,36]
Compressive obstruction (e.g., malignancy)
Direct involvement (e.g., malignant or infectious lymphadenitis, tear or rupture from trauma or surgery)
Congenital anomalies (e.g., Gorham syndrome)
Dysfunction (e.g., reverse flow of chyle toward the lung), stimulation of excess amounts of lymphatic fluid (typically from lymphatic masses or malformations) resulting in rupture or seepage of chyle from lymphatic ducts.
Transfer of chyle across the diaphragm from abdominal or retroperitoneal chyle accumulations.
3 CHARACTERISTICS OF PLEURAL FLUID
What is the volume of pleural fluid in the pleural space?
A small amount of fluid is present in the pleural space in healthy individuals.[37] In a study conducted by Noppen et al.[37] on subjects undergoing thoracoscopy for the treatment of idiopathic hyperhidrosis by using a novel pleural lavage technique, the volume of pleural fluid was determined to be 8.4 ± 4.3 ml or 0.26 ± 0.1 mL/kg.
What is the cellular composition of normal pleural fluid?
Noppen et al.[37] reported that the total white blood cell count of the normal pleural fluid was 1,716 × 103 cells/mL. Differential cell count showed that macrophages constituted the predominant cell type. [See Table 2]
Table 2.
Cellular composition of normal pleural fluid
| Cells | Median (%) | Interquartile range | ||
|---|---|---|---|---|
| Macrophages | 75 | 64-80% | ||
| Lymphocytes | 23 | 18-36% | ||
| Mesothelial cells | 1 | 0-2% | ||
| Neutrophils | 0 | 0-1% | ||
| Eosinophils | 0 | 0% |
What are the biochemical characteristics of normal pleural fluid?
Animal studies have shown that the concentration of protein in the normal pleural fluid is about 1.33 g/dL.[38,39] In the normal physiologic state, pleural fluid and blood glucose concentrations were found to be identical in an animal study by Rolf et al.[40] In the only human study by Yamada et al., the pleural fluid pH in a healthy individual was found to be 7.64, which was 0.23 pH units greater than the simultaneously measured blood pH.[41,42] An animal study by Rolf et al.[40] showed that the pleural fluid bicarbonate concentration was 20% to 25% more than that of plasma.
4 COAGULATION SCREENING BEFORE PLEURAL PROCEDURES
Are coagulation parameters essential before thoracentesis?
British Thoracic Society (BTS) pleural disease guidelines 2010 recommends avoiding non-urgent pleural aspirations or chest drain insertions in anticoagulated patients with INR >1.5.[43] The review article by DeBiasi et al.[44] has described the complications associated with thoracentesis with different bleeding risk factors, namely clopidogrel use, deranged coagulation parameters and thrombocytopenia. There was no increased rate of haemorrhagic complications in patients with bleeding risks. After ultrasonography-guided thoracentesis, haemorrhagic complications were rare, and trying to treat a deficient platelet count or INR before the procedure is unlikely to be beneficial.
Evidence statement
Avoid non-urgent pleural aspirations or chest drain insertions in anticoagulated patients with INR >1.5
No significant increase or serious bleeding complications were seen with thoracentesis in patients with deranged coagulation parameters or with patients continuing antiplatelet drugs like Clopidogrel.
Recommendations
Routine evaluation of coagulation parameters is not necessary for thoracentesis. However, a good clinical history of bleeding complications in the past and other high-risk conditions like chronic kidney disease, chronic liver disease, and bleeding diatheses should be obtained before attempting thoracentesis. In these patients risk–benefit assessment would determine proceeding with thoracentesis (GPP).
In patients on antiplatelet drugs (clopidogrel) requiring non-urgent thoracentesis, it may be performed after assessing the risk–benefit ratio. (Grade 2 A)
Do we need to assess coagulation parameters before pleural biopsy?
The American College of Chest Physicians guidelines (2003) cite uncorrectable coagulopathy as a contraindication to pleural biopsy.[45] However, there are no studies that compare pleural biopsy with and without assessment of coagulation workup. However, all patients should be assessed for their previous history of bleeding. A pleural biopsy should not be performed on a patient with a coagulation defect that includes:
Patients with haemophilia or other factor deficiencies.
Patients with low platelets or platelet function abnormalities.
Patients with abnormal PT and PTT.
Anticoagulants and antiplatelet drugs may need to be stopped, as recommended in Table 3.
Table 3.
Recommendations for stopping anticoagulants and antiplatelet drugs before pleural biopsy
| Drugs | Recommendations | |
|---|---|---|
| Warfarin | It should be discontinued 5 days before the procedure. INR should be <1.5 on the day of the procedure. | |
| Lowmolecular weight heparin | Stop at least 24 h before the procedure. Resume 24 h after the procedure. | |
| Unfractionated heparin (intravenous) | Should be discontinued 6 h before the procedure. | |
| Dabigatran | Discontinue 4 days before the procedure. Resume 24 h after the procedure. | |
| Rivaroxaban, edoxaban, apixaban | It should be discontinued 2 days before the procedure. Resume 24 h after the procedure. | |
| Tirofiban | Discontinue 8 h before the procedure. | |
| Eptifibatide | Discontinue 8 h before the procedure. | |
| Abciximab | Discontinue 24 h before the procedure. | |
| Aspirin (lowdose) | Discontinue 3 days before the procedure. | |
| Clopidogrel | Discontinue 7 days before the procedure. | |
| Prasugrel | Discontinue 7 days before the procedure. |
Evidence statement
Coagulation parameters and a history of antiplatelet drug intake have been routinely done before attempting pleural biopsy.
Recommendations
Coagulation workup (PT, INR, and APTT with haemoglobin and platelet counts) should be performed before attempting pleural biopsy. (GPP)
All patients should be assessed for any risk factor for high risk of bleeding, and alternative means of diagnosis should be discussed with patients. (GPP)
Antiplatelet and anticoagulant medications should be stopped as per the standard guidelines. (GPP)
5 PLEURAL FLUID ANALYSIS
How should pleural fluid be collected and stored?
Pleural fluid should be collected in a glass or plastic tube with an anticoagulant (EDTA) – as fluid may clot or the cells may clump, providing inaccurate cell counts and differentials. Conner et al.[46] showed that cell counts were not affected by refrigerated storage for up to 24 hours.
Evidence statement
Cell counts in pleural fluid collected in a glass or plastic tube with anticoagulant (EDTA) were not affected by refrigeration for up to 24 hours.
Recommendation
Pleural fluid collected in an EDTA container could be stored for up to 24 hours in the refrigerator before performing cell count. (Grade 3A)
How is pleural fluid processed for measuring pH?
Exposure to local anaesthetic (lidocaine) and heparin decreases pleural fluid pH. Delay after sample collection does not seem to substantially alter measured pH up to 1 hour. Pleural fluid pH level also increases if the sample is exposed to air (due to the escape of carbon dioxide) or if there is a delay in testing for over 4 hours. 0.5-1 ml of pleural fluid drawn into an ABG syringe is considered adequate for testing. The presence of purulent pleural fluid is generally considered a contraindication for analysis in blood gas machines.[47,48,49]
Evidence statement
Exposures to local anaesthetic, heparin, air and delay of more than 1 hour for measuring pH affect the pleural fluid pH. The presence of purulent pleural fluid is generally not considered for analysis in blood gas machines.
Recommendation
At least 0.5-1 ml of pleural fluid should be sent for pH measurement in an ABG syringe at room temperature after clearing preloaded heparin and residual air. It should be processed in an ABG machine within 1 hour. (Grade 3A)
Frank pus should not be analysed in a blood gas machine. (Grade 3A)
6 DIFFERENTIAL DIAGNOSIS
What are the causes of transudative pleural effusion?
The commonest causes of transudative pleural effusion are congestive cardiac failure and hepatic hydrothorax. Congestive cardiac failure as a cause of pleural effusion occurs in 5-10% of the pleural effusions in Indian studies.[50,51,52] The list of causes for transudative pleural effusion is enumerated in Table 4.
Table 4.
| Congestive cardiac failure Hepatic hydrothorax Renal failure Constrictive pericarditis Nephrotic syndrome Peritoneal dialysis Pulmonary embolism Hypoalbuminemia Hypothyroidism Mitral stenosis Urinothorax Meig’s syndrome Superior Vena Caval obstruction |
What are the causes of exudative pleural effusion?
The causes of exudative pleural effusion are enumerated in Table 5.
Table 5.
Causes of exudative pleural effusion
| Infections | Connective tissue disease-related | |
|---|---|---|
| Bacterial pneumonia Tuberculosis Parasitic or fungal infections Viral pneumonia Nocardia Other sources of infections like liver abscesses, subphrenic abscesses |
Systemic lupus erythematosus Rheumatoid pleural effusion Vasculitis – GPA * and EGPA ** |
|
|
| ||
| Malignancy related | Endocrine related | |
|
| ||
| Carcinoma Lymphoma Mesothelioma Paramalignant effusion |
Hypothyroidism Ovarian hyperstimulation |
|
|
| ||
| Trauma or instrumentation | Gastrointestinal causes | |
|
| ||
| Central venous access misplaced in pleura Oesophageal perforation Hemothorax Chylothorax |
Ascites due to any cause Pancreatitis Abdominal source of sepsis |
|
|
| ||
| Miscellaneous | ||
|
| ||
| Pulmonary embolism Radiation Meigs’s syndrome Uremic pleural effusion Post cardiac injury (Dressler’s) syndrome Asbestos-related pleural effusion IgG4-related pleural effusion |
||
|
| ||
| *GAA Granulomatosis with Polyangiitis *EGPA Eosinophilic granulomatosis with polyangiitis | ||
How are exudative and transudative pleural effusions differentiated?
In the original study published by Dr Richard Light, which included 47 transudates and 103 exudates, Light’s criteria [See Table 6] yielded 99% sensitivity and 98% specificity for identifying exudates.[58]
Table 6.
Light’s criteria
| Pleural fluid is an exudate if one or more of the following criteria are met: 1. Pleural fluid protein divided by serum protein is >0.5 2. Pleural fluid lactate dehydrogenase (LDH) divided by serum LDH is >0.6 3. Pleural fluid LDH >2/3 the upper limits of laboratory normal value for serum LDH. |
However, in congestive cardiac failure (CCF), diuretic therapy can lead to a rise in the pleural fluid protein, lactate dehydrogenase and lipids.[59] Light’s criteria tend to misclassify a significant proportion of these transudative effusions as exudates.[59] The pleural effusion from approximately 1/3rd of patients with CCF may be classified as exudates by Light’s criteria.[54,60]
The pleural fluid cholesterol level > 38–65 mg/dl or pleural fluid/serum cholesterol ratio ≥ 0.3 and pleural fluid to serum bilirubin ratio > 0.6 suggest an exudative pleural effusion. However, studies have shown that they are not superior to Light’s criteria, and there is no consensus on the best cutoff value for pleural fluid cholesterol.[55,56,61]
Evidence statement
Light’s criteria have a sensitivity of 99% and specificity of 98% in identifying an exudative pleural effusion. The pleural effusion from approximately one-third of patients with CCF may be classified as exudative by Light’s criteria. The pleural fluid cholesterol, pleural fluid to serum cholesterol ratio, and pleural fluid to serum bilirubin ratio are not superior to Light’s criteria.
Recommendations
Light’s criteria should be considered the initial choice for differentiating exudates from transudates. (Grade 2A)
We do not recommend the use of pleural fluid cholesterol, pleural fluid to serum cholesterol ratio, and pleural fluid to serum bilirubin ratio as a replacement for Light’s criteria. (Grade 3A)
How can a transudative effusion misclassified as exudative by Light’s criteria be identified?
The serum-effusion protein gradient (>3.1 g/dL) and serum-effusion albumin gradient (>1.2 g/dL) can be used as cut off to identify transudative effusions, which were wrongly classified as exudative by the Light’s criteria. The serum-effusion albumin gradient has been found to fare better than the serum-effusion protein gradient in this regard and was shown to correctly identify transudative effusions due to CCF and hepatic hydrothorax, misclassified by Light’s criteria in around 80% and 60%, respectively[53] [see Figure 2].
Figure 2.
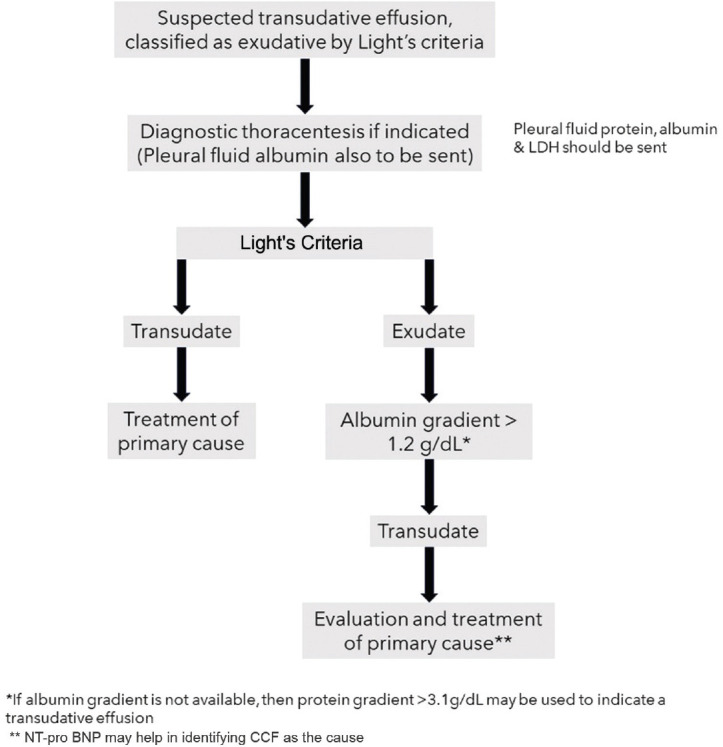
Diagnostic algorithm for correct identification of a misclassified transudative effusion by Light’s criteria
NT pro-BNP has been shown to correctly diagnose transudative effusions due to CCF that have been misclassified as exudates by Light’s criteria. The pleural fluid and blood NT pro-BNP levels are comparable, and either of them can be used. A cutoff value of 1500 pg/ml has been found to have the best sensitivity and specificity.[62,63] However, it may also be elevated in other causes of fluid overload.
Evidence statement
The serum-effusion protein gradient (>3.1 g/dL) and serum-effusion albumin gradient (>1.2 g/dL) can be used as cut off to identify transudative effusions, which were wrongly classified as exudative by Light’s criteria. The later is better in this regard.
Serum or pleural fluid NT pro-BNP of more than or equal to 1500 pg/ml has been shown to accurately diagnose transudative effusions due to CCF that have been misclassified as exudative by Light’s criteria.
Recommendations
When a transudative effusion is suspected clinically but is classified as exudative according to Light’s criteria, the serum-effusion albumin gradient should be used to discriminate. (Grade 2A)
If albumin values are not available, then protein gradient may be used instead. (Grade 2B)
Serum or pleural fluid NT pro-BNP may help in identifying transudative effusion in CCF. (Grade 2A)
What are the exudative effusions that can present as bilateral pleural effusion?
A bilateral effusion is likely to be transudate. It is most commonly caused by congestive heart failure.[44,64,65]
Malignancy, autoimmune diseases, para-pneumonic effusions, tuberculosis and heart failure on diuretics are exudative effusions that can present as bilateral effusion.[44,64,65,66,67]
Can bilateral pleural effusion present with two different aetiologies?
Contarini’s syndrome is characterised by bilateral pleural fluid collection, each side of which has a unique cause. A common combination is pneumonia, causing exudative pleural effusion on one side and triggering a contralateral transudate by decompensating a pre-existing cardiac failure.
In the study published by Porcel et al.,[65] out of 2605 patients from the author’s database, 546 (21%) had bilateral pleural effusion. The most common cause was heart failure in 286 patients, followed by malignancy in 102 patients. Only 5 (0.9%) patients had bilateral effusions of different causes.
What are the commonly used drugs that could cause pleural effusion?
A detailed list of drugs causing pleural effusion can be found on the Pneumotox website: (https://www.pneumotox.com/drug/index/).[68] The commonly used drugs are listed in Table 7.
Table 7.
Commonly used drugs causing pleural effusion
| 1. Tyrosine kinase inhibitor 2. Amiodarone 3. Gonadotrophin stimulating hormones 4. Methotrexate 5. Beta-blockers 6. Nitrofurantoin 7. Ergot alkaloids 8. Phenytoin 9. L tryptophan |
7 IMAGING FOR PLEURAL EFFUSION
What is the role of chest X-ray in the evaluation of pleural effusion?
Evidence statement
The most basic and widely available radiological investigation is chest X-ray standard postero-anterior and lateral chest radiographs. The amount of fluid to be evident on a posteroanterior film is 200 mL. On a lateral film, costophrenic angle blunting is visible after around 50 mL of fluid has collected.[69]
Recommendations
In all suspected cases of pleural effusion, a chest X-ray PA view should be performed. (GPP)
Lateral and lateral decubitus views of the affected side are useful for detecting minimal pleural effusion if USG is not available or feasible. (GPP)
Should thoracic ultrasound (TUS) be used for the diagnosis of pleural effusion?
Evidence statement
TUS is a simple, non-invasive, portable method to diagnose pleural effusion. TUS is superior to chest radiography, with better sensitivity and specificity. It is a useful aid in diagnostic aspiration, insertion of chest drain, and it also helps to assess the underlying lung, which may be difficult with other radiological investigations.[69,70,71]
Recommendation
TUS should be used for diagnosing and quantifying pleural effusion, when available. (Grade 2A)
Can TUS characterise the underlying pleural effusion?
Evidence statements
Recommendation
We recommend TUS in the diagnosis and evaluation of pleural effusion. (Grade 2A)
What are the indications for CT thorax in pleural effusion?
Evidence statement
Contrast-enhanced CT allows assessment of the entire thorax and specific features like enhancement of thickened pleura, known as split pleura sign, helps in diagnosing empyema. CT helps to distinguish parenchymal abscess from pleural collections.
In suspected cases of malignancy, CT demonstrates pleural thickening, nodularity and underlying lung mass within the effusion.[74,75,76]
Recommendation
When pleural fluid evaluation is inconclusive, we recommend CECT thorax before pleural biopsy (whenever malignancy is suspected) and before full pleural drainage. (GPP)
What are the indications for other imaging modalities like Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) in pleural effusion?
Evidence statements
PET-CT imaging: It has been proven that malignant pleural effusions have a higher uptake of 18 FDG. Patients with pleural inflammation, including pleural infection and those who had talc pleurodesis can have false positive results.[77,78]
MRI: According to some studies, the morphological features of pleural malignancy identified by MRI are equivalent to those identified by CT. The MRI assessment of diaphragmatic and chest wall involvement is superior.[79]
Recommendation
PET-CT and MRI may have a role but are not routinely recommended for the evaluation of pleural effusion. (GPP)
8 DIAGNOSTIC THORACENTESIS
What are the indications for diagnostic thoracentesis?
A variety of diseases can cause pleural effusion, which can be exudative or transudative in nature. Diagnostic thoracentesis is an outpatient procedure performed to identify the cause of effusion [see Table 8].
Table 8.
| 1. Undiagnosed pleural effusion 2. Suspected parapneumonic effusion – for diagnosis and decision on drainage of pleural effusion 3. Suspected hemothorax – for pleural fluid haematocrit 4. Suspected transudative pleural effusion with atypical features or not responding to primary therapy. |
What are the contraindications for attempting thoracentesis?
There are no absolute contraindications; however, it is not advisable to do thoracentesis in certain situations [see Table 9].
Table 9.
| 1. Insufficient pleural fluid (<10 mm in USG/lateral decubitus X-ray) 2. Skin infection at the site of puncture 3. Severe hemodynamic or respiratory compromise 4. Severe bleeding diathesis 5. Uncooperative patient. |
While attempting thoracentesis in a mechanically ventilated patient, one should be cautious as there is a theoretical risk of pneumothorax due to positive pressure ventilation.[82] In case of hemodynamic or respiratory compromise, the procedure should be withheld until the patient is stable. Risk versus benefit is to be assessed on an individual basis before performing thoracentesis.
Is diagnostic aspiration mandatory in all cases of pleural effusion?
In all cases of unilateral pleural effusion, diagnostic thoracocentesis should be performed when an appropriate amount of pleural fluid is present to perform the procedure safely. In case of bilateral effusion, suspected to be due to cardiac failure, liver disease or renal disease, diagnostic thoracocentesis should not be performed, unless atypical features are present.[83,84]
Treating the underlying cause will resolve the effusion. However, the presence of atypical features or failure to respond to treatment of underlying cause warrants diagnostic thoracentesis. A few atypical features of cardiac failure include:
Unexplained fever
Pleural effusions that are greatly disparate in size (effusion on the left is larger than on the right)
Unilateral pleural effusion
Pleuritic chest pain
No evidence of cardiomegaly in the chest X-ray
Echocardiogram that is inconsistent with heart failure
Effusions that do not disappear within a few days after initiating therapy.
Atypical features that suggest alternative aetiology in liver disease include
Fever
Pleural effusion without ascites
Unilateral left-sided pleural effusion
Evidence statement
In case of suspected transudative pleural effusion, if the clinical assessment is suggestive of cardiac, hepatic or renal disease – diagnostic pleural fluid aspiration is not routinely indicated.
Atypical features or failure to respond to adequate therapy for at least three days may indicate an alternative diagnosis.
Recommendation
Diagnostic thoracentesis is not routinely recommended in suspected transudative pleural effusion unless there are atypical features or failure to respond to primary treatment with adequate therapy for at least three days. (Grade 1A)
When should thoracentesis be considered on both sides in a patient with bilateral pleural effusion?
Bilateral thoracentesis may be considered only under certain circumstances. The indications are enumerated in Table 10. The diagnostic algorithm for the evaluation of bilateral pleural effusion is enumerated in Figure 3.
Table 10.
Indications for bilateral thoracentesis[30]
| 1. Unilateral parenchymal lung involvement 2. Significantly disparate-sized effusions 3. Markedly different attenuation values (Hounsfield units) or appearance (e.g. unilateral pleural loculations or enhancement) on CT 4. Atypical clinical findings (fever or pleuritic chest pain in the context of decompensated heart failure) 5. Resolution of pleural effusion only on one side 6. Evaluation of pleural diseases usually associated with unilateral effusion (e.g. pneumonia) |
Figure 3.
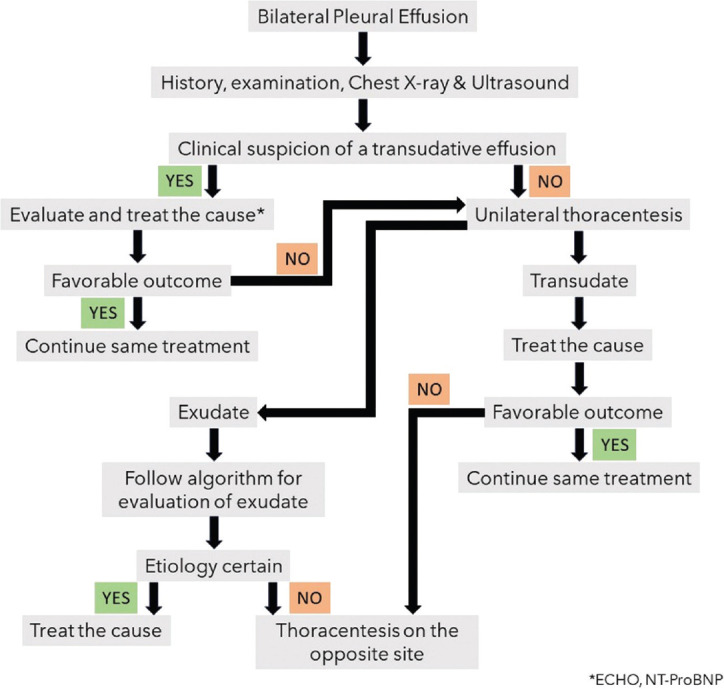
Diagnostic algorithm for bilateral pleural effusions
How to choose a safe site for thoracentesis?
The triangle of safety has been the preferred site by most (Figure 1). As per British Thoracic Society guidelines, ultrasound guidance is mandatory before performing thoracentesis.[7] Ultrasound enables the identification of the depth, safe site, characteristics and extent of pleural effusion. It also helps to identify the distance of visceral organs (Lung, Heart, Liver and Spleen) and diaphragm from the proposed pleural aspiration site. A study by Helm et al.[2] in which CT was performed to assess the intercostal artery position showed that at 3 cm lateral to the spine, only 17% of intercostal vessels were protected by the superior border of the rib. However, at 6 cm lateral to the spine in the infrascapular area, 97% of arteries were under the superior border of the upper rib. Hence, the needle should always be introduced along the upper border of the chosen rib at appropriate intercostal space to prevent injury to the intercostal vessels.
Evidence statement
There are no studies comparing the triangle of safety and the interscapular area to identify which is safer for thoracentesis.
The risk of intercostal vessel trauma increases with thoracentesis attempts made near the midline or spine.
Ultrasound guidance decreases risk of injury to intercostal vessels and visceral organs.
Recommendations
In usual circumstances, thoracentesis can be safely performed under ultrasound guidance in the infrascapular area. (GPP)
It is recommended to stay 6 cm or more lateral to midline if possible, and the aspiration needle should be introduced along the superior border of the lower rib at the appropriate intercostal space to avoid interrupting the neurovascular bundle. (GPP)
Triangle of safety may be the preferred site for thoracentesis, provided that adequate fluid is demonstrated there on ultrasound. (GPP)
What are the instruments needed for diagnostic thoracentesis?
Numerous commercial prepackaged sterile kits are also available, which can be used based on institution protocol [see Table 11].
Table 11.
Instruments for diagnostic thoracentesis
| 1. Sterile gloves 2. Sterile drape 3. Skin sterilising fluid like povidone iodine or chlorhexidine 4. Needle – 21G 5. Syringes – 5,10, 20 or 50 ml 6. For administering local anaesthesia – 26 g needle, ten cc syringes, 1% or 2% lignocaine 7. For therapeutic thoracentesis – 18-gauge over-the-needle catheter, a three-way stopcock, drainage tubing, and one or two large containers. |
What are the complications of diagnostic thoracentesis?
Thoracentesis is a relatively safe procedure in the hands of a trained person. Common complications are pain, cough, and shortness of breath. Serious complications include hemothorax, or hemoperitoneum (0.01%), pneumothorax (6%), spleen or liver puncture (0.01%), and re-expansion pulmonary oedema (<1%). Pneumothorax is seldom large and rarely requires intercostal drainage tube placement.
Other complications are vasovagal events, empyema, soft tissue infection, tumour seeding of the tract caused by the needle, dry tap and drug-related adverse reactions, secondary to anaesthetics or antiseptic solutions.[7,87]
Post-thoracentesis, how much time does a patient need to be kept under observation?
Once the procedure is over, the patient should be observed for complications. A routine chest X-ray is not recommended. Vital signs, including heart rate, blood pressure, respiratory rate and oxygen saturation, should be documented before discharging the patient.
Evidence statement
There is no literature addressing this particular question.
Recommendation
Post-procedure patients should be observed for at least 1 hour and can be discharged after this, provided there are no new-onset symptoms. (GPP)
Is chest radiography routinely needed after thoracentesis?
Recommendation
Post-thoracentesis chest radiography is not routinely indicated for asymptomatic patients.
Chest Radiography may be considered when post-procedure complications such as pneumothorax are suspected when the procedure requires multiple attempts, if the volume of fluid aspirated is large (>1500 ml), and in patients on mechanical ventilation.[88]
9 PLEURAL BIOPSY
What are available methods of pleural biopsy?
Establishing the aetiology of exudative pleural effusion in the setting of an inconclusive pleural fluid analysis often requires biopsy from the parietal pleura.[89] Pleural biopsy can be performed either by closed or image-guidance methods. However, more and more biopsies are obtained by medical thoracoscopy (MT), which has caused a paradigm shift in the evaluation of pleural effusion.[90,91,92] MT is a minimally invasive endoscopic technique performed under local anaesthesia (LA) and conscious sedation, which offers direct visualisation of the pleural surfaces and the option to perform diagnostic and therapeutic procedures. Thoracoscopic pleural biopsy is compared with closed pleural biopsy in Table 12. The most common indication for performing MT is for the evaluation of undiagnosed exudative pleural effusion. The diagnostic yield of MT reaches nearly 100% for tubercular aetiology when combining histopathology and mycobacterial cultures/XPERT on thoracoscopic samples.[49,93] It offers a high diagnostic yield for malignancy and has the added advantage of performing pleurodesis in the same sitting.
Table 12.
Comparing thoracoscopic and closed pleural biopsies
| Medical thoracoscopic pleural biopsy | Closed pleural biopsy (percutaneous) | |
|---|---|---|
| It has a very high diagnostic yield. 91 to 95% in malignant pleural effusion 98 to 100% in tuberculous pleural effusion |
The diagnostic yield is good. Up to 60% in malignant pleural effusions (without image guidance) Up to 80 to 87% in tuberculous pleural effusions (at least six biopsy samples should be obtained) |
|
| Advantages 1. In malignancy, Talc poudrage could be performed if required. 2. Clearing adhesions 3. It is possible to ensure complete drainage of pleural fluid. |
Advantages 1. Readily accessible 2. Minimally invasive 3. Rapid and low-cost 4. Can be performed in patients unfit for Thoracoscopy. 5. Can be performed in patients with pleural thickening but without pleural effusion. |
It is an excellent minimally invasive diagnostic and therapeutic modality in the management of undiagnosed pleural effusion. However, appropriate training is required.
What are the indications for pleural biopsy?
The biochemical, microbiological, and cytological analysis of pleural effusion often provides information about the etiology. However, up to 40% of the effusions are not diagnosed after the initial pleural fluid evaluation.[49,89,93,94]
Pleural fluid microbiological and cytological analysis fails to provide a diagnosis in
40% cases of malignancy
60% cases of mesothelioma
70% cases of tuberculosis
In cases of tuberculosis pleural effusion, Adenosine D Aminase (ADA)-based diagnosis fails to guide treatment in the case of drug-resistant tuberculosis.[94] Pleural biopsy can further provide an etiological diagnosis in these situations.[45] The indications for pleural biopsy are enumerated in Table 13.
Table 13.
Indications for pleural biopsy
| 1. Undiagnosed cases of exudative pleural effusion (Non-diagnostic pleural fluid evaluation) 2. Pleural thickening without pleural effusion 3. Pleural mass 4. Sample for additional tests (such as mutation analysis in Malignancy and culture and drug sensitivity tests in tuberculosis) |
Recommendation
In cases of pleural effusion, after doing a complete pleural fluid analysis, if the aetiology of pleural effusion remains unknown, pleural biopsy should be performed to obtain a definitive diagnosis. (Grade 2A)
What is the role of medical thoracoscopy (MT) in the evaluation and management of pleural effusion?
Determining the cause of exudative pleural effusion when pleural fluid analysis yields inconclusive results often necessitates obtaining biopsies from the parietal pleura. Pleural biopsy can be performed either closed or image-guided. The advent of Medical thoracoscopy has caused a paradigm shift, and it has overshadowed other modes of obtaining pleural biopsy. MT is a single-port minimally invasive endoscopic technique, which offers the opportunity for direct visualisation of the pleural surfaces and provides the option of obtaining biopsy samples for diagnosis under direct vision. It is performed under local anaesthesia and conscious sedation and is usually performed in the bronchoscopy suites or in dedicated thoracoscopy suites, but less frequently in the operating theatre. It affords the opportunity to obtain ample quantities of tissue, which is important for cancer diagnostics, as well as microbiologic tests for the diagnosis of TB. In addition, perform minor therapeutic procedures such as pleurodesis by talc poudrage and management of complex septated pleural effusion and early empyema stages 1 and 2.
The most common indication for performing MT is for the evaluation of undiagnosed exudative pleural effusion. The diagnostic yield of MT reaches close to 100% for tubercular aetiology when histopathology and mycobacterial cultures and GeneXpert are performed on thoracoscopic samples.[95,96] MT also offers a high diagnostic yield for malignancy, providing adequate tissue samples for immunohistochemistry (IHC) studies in addition to histopathology. It also has the added advantage of being able to perform pleurodesis in the same sitting.
MT can be performed with a rigid or semi-rigid instrument with almost the same yield and safety profile, except that the rigid instrument fares a little better in the presence of multiple septations and thick fibrous pleura. With the advent of newer diagnostic tools such as the cryoprobe, there is the possibility of obtaining larger, good quality specimens with well-preserved cellular architecture and tissue integrity for pathologic and IHC evaluation.[94]
Thus, it is an excellent minimally invasive diagnostic and therapeutic modality in the management of effusion. However, appropriate training is required to ensure that the procedure is safe and obtains optimal outcomes. The ICS-NCCP MT guidelines has recently been published in Lung India.
What is the diagnostic yield of closed pleural biopsy in comparison to thoracoscopic and image-guided Abrams needle pleural biopsy in malignant and tuberculous pleural effusion?
Malignant pleural effusion
Diagnostic yield of pleural fluid cytology ranges from 40 to 87% in malignant pleural effusion.[1] Loddenkemper et al.[97] reported a diagnostic yield of 44% for closed pleural biopsy and 62% for pleural fluid cytology in cases of malignant pleural effusion.
Percutaneous needle biopsy of the costal pleura is usually carried out using Abrams, Copes or Tru-cut biopsy needle. Tomlinson et al.[90] reviewed more than 2,500 pleural biopsies cases performed with the Abrams needle. They reported a diagnostic yield of 57% for pleural biopsy in the case of malignant pleural effusion. Abrams needle biopsy added only 12% more diagnostic yield to pleural fluid cytology.[90] In an Indian study by Christopher et al.,[98] the diagnostic yield of pleural biopsy with a Tru-Cut biopsy needle without image guidance was 71% in cases of malignant pleural effusion. In a randomised controlled trial on 58 patients with exudative pleural effusion from Calicut, Kerala, Haridas N, et al.[99] reported lower diagnostic yield (62%) and increased complication rates (17%) with non-image-guided costal pleural biopsy with Abrams biopsy needle in comparison to thoracoscopic pleural biopsy (Diagnostic yield = 86.2%, Complication rate = 10.3%). In a prospective comparative study from Lucknow, India, on 46 patients of exudative pleural effusion, the diagnostic yield of non-image-guided costal pleural biopsy with Copes needle was only 22% in comparison to a diagnostic yield of 78% with rigid thoracoscopic pleural biopsy.[100] One reason for low diagnostic yield with Abrams or Cope’s biopsy needle is the irregular deposition of malignant nodules in the pleura. These nodules often cluster around the diaphragm and midline – areas dangerous to access with Abrams or copes needle biopsy.[90] Image-guided biopsy performed under ultrasound or CT guidance allows accurate and safe biopsies of pleural thickening or nodules.[101] Also, inferior biopsy sites closer to the diaphragm have been shown to be more likely to give positive biopsy samples as metastases are most likely to be found here.[102] Maturu et al.,[103] in their study, demonstrated a diagnostic yield of 93.2% with diagnostic thoracoscopy in comparison to 84.5% with closed blind pleural biopsy. Irrespective of the type of needles used, CT-guided biopsy has a marginally better diagnostic yield (75 – 82.4%) compared to USG-guided techniques (66.7 – 71.4%).[104,105] The choice between CT and USG-guided techniques depends on the availability, cost and expertise. The use of CT allows areas inaccessible to the USG to be biopsied (e.g., behind ribs). The size of the target lesions dictates the ease of the procedure; however, pleural thickening as little as 5 mm has been effectively biopsied.[106]
Tuberculous pleural effusion
In comparison to malignant pleural effusion, the diagnostic yield of non-image-guided percutaneous biopsy of pleura was high and ranged from 67 to 92% in most of the studies.[90,98,107,108,109,110,111] The reason for this increased diagnostic yield compared to malignancy is the diffuse involvement of pleura in tuberculosis. Bibby AC et al.,[111] in their review of various methods of pleural biopsy in undiagnosed pleural effusion, concluded that non-image-guided pleural biopsies are often used as a first-line diagnostic tool in resource-poor settings where the prevalence of tuberculosis is high. However, outside of this scenario, alternative methods are advocated.
Metintas M et al.,[112] in a randomised controlled trial on 124 patients with exudative pleural effusion in Turkey, compared CT-guided Abrams Needle biopsy of costal pleura with thoracoscopic pleural biopsy. CT guidance was obtained from a hard copy of CT images; thus, real-time image guidance was not used. The diagnostic yield of CT-guided Abrams needle pleural biopsy was found to be high (87% in malignant pleural effusion and 89% in tuberculous pleural effusion) and comparable to thoracoscopic pleural biopsy; the differences between the yields were not statistically significant. However, in cases of Malignant Mesothelioma, the diagnostic yield of CT-guided Abrams needle biopsy was low (80% vs 94%) compared to thoracoscopic pleural biospy.[22]
Evidence statements
Closed pleural biopsy provides a modest diagnostic yield in both malignancy and tuberculosis, with a higher yield in the latter due to the diffuse pleural involvement.
The yield of MT and CT-guided Abrams needle biopsy are generally comparable and higher than closed pleural biopsy in both malignancy and tuberculosis.
MT provides excellent yield for pleural biopsy, especially in cases with no pleural thickening or nodules demonstrated on CT or USG.
In cases of suspected malignant mesothelioma, the diagnostic yield of thoracoscopic pleural biopsy was superior to CT-guided Abrams needle biopsy.
Recommendations
Closed pleural biopsies may be used as a first-line diagnostic tool in resource-poor settings, where prevalence of tuberculosis is high and facilities for medical thoracoscopy or image-guided biopsy are not available. When these modalities are available, they should be used. (Grade 1A)
When there is no resource constraint, medical thoracoscopy should be the preferred method for pleural biopsy, especially in cases with no pleural thickening or nodules on CT or USG. CT-guided Abrams needle biopsy is the next best option. (Grade 1A)
When malignant mesothelioma is suspected, thoracoscopic pleural biopsy is preferred to in comparison with CT-guided Abrams needle biopsy. (Grade 2A)
Does image guided pleural biopsy have higher yield compared to closed pleural biopsy?
Chang et al.[102] compared the outcomes of closed pleural biopsy with Abrams needle and USG-guided pleural biopsy with a Tru-Cut needle. The closed pleural biopsy diagnosed 20% of tuberculosis and 44% of malignancy correctly. In contrast, the USG-guided diagnosed 86% of tuberculosis and 70% of malignancies correctly and proved superior.
Evidence statement
The yield of image-guided pleural biopsy is higher than closed pleural biopsy.
Recommendation
Image-guided pleural biopsy should be the preferred over closed pleural biopsy. (Grade 1A)
What are the complications reported with pleural biopsy?
The complications of pleural biopsy are enumerated in Table 14.
Table 14.
| Biopsy site pain (up to 15%) Pneumothorax (up to 15%) Vasovagal symptoms (approximately 5%) Hemothorax (2%) Transient fever (1%) Death due to massive haemorrhage has been reported, but this is rare. |
10 THERAPEUTIC THORACENTESIS
What are the indications for therapeutic thoracentesis?
The main indication for performing therapeutic thoracentesis is symptom relief.[7] In case of symptomatic malignant pleural effusion, if the cause of dyspnoea is uncertain or if lung expansion is uncertain, then therapeutic thoracentesis can be performed to decide on intercostal drainage or intra pleural catheter placement.[113]
10.2 How much pleural fluid can be drained safely in a single Sitting?
The main concerns while performing large-volume thoracentesis are re-expansion pulmonary oedema and pneumothorax. In the study by Ault MJ et al., where 9320 inpatient thoracentesis were performed, the incidence of re-expansion pulmonary oedema, pneumothorax or any complication was significantly higher in those with > 1500 mL pleural fluid removed than in the group with < 1500 mL pleural fluid removed (P < 0.0001).[10] In another study by Josephson et al.[114] where 735 thoracentesis were performed, the incidence of pneumothorax was 34 (4.6%). When compared to removal of 0.8-1.2 L pleural fluid, removal of 1.8–2.2 L pleural fluid showed three times more pneumothorax and ≥ 2.3 L pleural fluid removal showed six times more pneumothorax. Nine out of 11 tube thoracostomies occurred after thoracentesis of 1.8 L pleural fluid or more.
In another study by Jones et al.,[115] where 941 thoracentesis were performed, when > 1,100 mL of fluid were removed, the incidence of pneumothorax requiring tube thoracostomy and pain were increased significantly (P < 0.05). Re-expansion pulmonary oedema complicated 2 of 373 thoracenteses (0.5%) in which more than 1,000 mL of pleural fluid were removed. British Thoracic Society recommends stopping pleural fluid aspiration when a patient develops cough/chest discomfort or when no more fluid can be aspirated or when 1500 ml of fluid has been aspirated.[7]
Evidence statement
Removal of more than 1500 ml of pleural fluid in a single sitting is associated with an increase in the rate of complications like pneumothorax, re-expansion pulmonary oedema and need for chest tube drainage.
Recommendation
It is recommended that in a single sitting, pleural aspiration should be stopped once 1500 ml of pleural fluid has been aspirated or earlier if the patient develops symptoms. (Grade 2A)
11 TUBE THORACOSTOMY
What are the indications of chest tube insertion in pleural effusion?
Table 15.
Indications of chest tube insertion
| 1 | Empyema thoracis | |
| 2 | Complicated Parapneumonic effusion | |
| 3 | Hemothorax | |
| 4 | Hydropneumothorax/pyopneumothorax | |
| 5 | Symptomatic Malignant pleural effusion ± pleurodesis | |
| 6 | For symptom relief in transudative effusion – requiring repeated therapeutic thoracentesis, i.e., refractory to medical management |
Does chest tube size matter while draining pleural effusion?
The two important questions with regard to the size of the chest tube are as follows:
Are the large-size chest tubes associated with better clinical outcomes in those with pleural infections?
Are small-size chest tubes associated with lesser procedure-related adverse events, including less procedure-related pain?.
Multicenter Intrapleural Sepsis Trial (MIST1) compared different chest tube sizes in patients with pleural infection.[118] The important clinically relevant characteristic was that predominantly patients had visibly purulent pleural fluid, i.e. empyema thoracis. The primary outcomes assessed were death and surgical intervention combined. Small-size chest tubes (<15F tubes) performed as well as large-size chest tubes (>15F tubes) in terms of chest X-ray clearance, improvement in FVC and FEV1, hospital stay and surgical intervention and death combined. Chest tube insertion methods (Seldinger vs blunt dissection) showed similar clinical outcomes and adverse events. Small-size chest tubes were associated with less procedure-related chest pain. Small chest tubes were placed with image guidance, which could have led to better outcomes in terms of success in this study.
BTS pleural diseases guideline 2010 recommends the use of small-bore catheters for most pleural infection patients, and flushing of the catheter is also recommended.[43] Large-bore catheters can be used in case the small-bore catheter fails to drain the effusion. The American Association for Thoracic Surgery (2017) recommends the usage of image-guided small tube placement in minimally septated empyema. Additional drain placement needs to be planned in case of undrained effusion.[119]
Evidence statement
Small-bore chest tubes (12 -14 F) performed as good as large-size chest tubes in terms of clinical outcome.
Small-bore chest tubes were associated with less pain.
Tube blockage and dislodgement may be more with small-bore chest tubes.
Recommendations
Small-bore drains (12 to 14) may be used as first-line therapy. Clinicians should be vigilant for tube dislodgement while using small-bore chest drains. (Grade 1A)
Whenever feasible – USG image guidance should be used to improve treatment outcomes with small-bore chest drains. (Grade 2A)
Daily flushing of small-bore drains is recommended to avoid tube blockage (GPP)
Large-bore drains may be helpful in case small-bore drainage fails (Grade 2A)
What is the best position for chest tube insertion?
The positions used for chest tube insertion include semi-recumbent, upright and lateral decubitus [for description see Table 16].
Table 16.
Positions for chest tube insertion
| Semi-recumbent | Upright | Lateral decubitus | ||
|---|---|---|---|---|
| Commonly used | Commonly used | During Thoracoscopy | ||
| With arms behind the head | With a table for leaning | With arms over the side of the head |
There are no trials addressing the most optimal positioning of patients for the insertion of the chest tube. It is usually the clinician’s preference and the patient’s comfort that dictates the positioning.
How should we select the appropriate site for chest tube insertion?
There are no studies comparing the different sites of chest tube insertion. However, the triangle of safety should be preferred in the case of free-flowing effusion. In the case of loculated pleural effusions, ultrasound or CT thorax should be used to identify the largest locule for insertion of chest drains.
Does image guidance decrease chest tube insertion-related complications and improve outcomes?
A systemic review by Menegozzo et al. evaluated the use of ultrasound for chest tube insertion. Ultrasound can correctly identify a site for safe insertion and accurately find a vulnerable intercostal artery.[120]
Evidence statement
During chest tube insertion, thoracic ultrasound guidance prevents injury to vascular structures and visceral organs.
Thoracic ultrasound guidance helps in inserting the chest tube in the largest locule to improve drainage.
Recommendations
Point of care ultrasound should be guide chest tube insertion to decrease adverse events. (Grade 2A)
Which are the pleural fluid drainage systems used?
Four types of drainage systems used for pleural effusion are:[116]
Three-compartment drainage system
Digital or Electronic chest drain system
Simple vacuum bottles for IPC drainage
Three-compartment drainage system
The chest drainage system includes a collection chamber, a water-seal chamber and a suction control chamber [Figure 4 shows the one, two & three bottle system].
Figure 4.
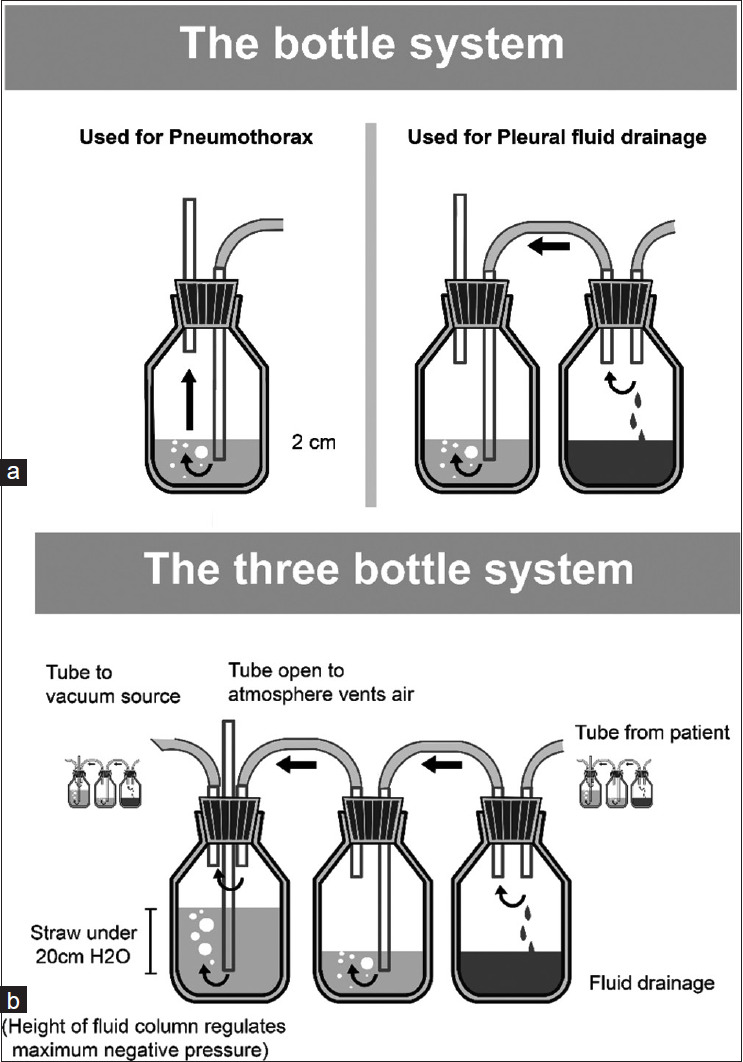
a) The one & two bottle systems for chest drainage b) The three bottle system
1st Bottle: Fluid or air drain into the Collection chamber.
2nd Bottle: The water-seal chamber holds a column of water (2 cm), which prevents air from being sucked into the pleural space with inspiration.
3rd Bottle: Suction chamber – may use a wet (water column), which allows a maximum of − 25 cm suction pressure [See Figure 4] or a dry (valve regulator) suction mechanism that allows the suction pressures up to − 40 cm H2O
Wet suction control
The level of the water column in the suction bottle/container dictates the degree of suction exerted on the typical chest drainage system. Sterile fluid is used to fill the suction control chamber to the preferred height, and the short suction tubing is linked to a suction source, with adjustments made to generate mild bubbling within the suction control chamber.
Dry suction control
The advantages of dry suction control over wet suction control are:
Higher suction pressure levels can be achieved.
Set up is easy.
There is no reduction in suction pressure on account of fluid evaporation.
The disadvantage of dry suction control is that it does not provide the same level of patient assessment information as a conventional water seal (i.e. the clinician cannot see changes in the water level reflecting pressure changes in the chest).
Digital or electronic chest drain system
These devices are used for pneumothorax treatment and post-thoracic surgery. It facilitates early discharge from the hospital. They provide a continuous digital recording of air leak, fluid drainage and intrapleural pressure, thus reducing inter-observer variability in air leak assessment. They maintain a preset intrathoracic pressure (usually − 8 cm H2O), and the system ensures that this pressure is maintained.
Vacuum bottles for IPC drainage
The drainage of pleural fluid via an IPC involves linking the external one-way valve to the vacuum bottle provided by the IPC manufacturer.
What is the role of applying suction to the chest drain tube?
There are no studies addressing the role of suction in patients with pleural effusion. BTS pleural diseases guideline 2010 recommends the usage of suction in selected cases after understanding the risks involved.[43]
Evidence statement
There is no direct evidence of the advantage of using suction in non-surgical patients with pleural effusion.
Recommendations
Suction should not be applied routinely to an underwater seal system in patients with pleural effusion. (GPP)
In selected patients with unexpanded lungs with no BPF, suction to underwater seal system may be applied with proper precautions. (GPP)
What are the complications of chest tube insertion?
The complications of chest tube insertion are enumerated in Table 17.[116]
Table 17.
Complications of chest tube insertion
| Insertion-related complication | Malposition of tube Hemothorax Lung injury Diaphragm injury Injury to mediastinal structures Injury to abdominal organs |
|
| Infectious complications | Chest tube site infection Empyema Necrotising chest wall infection |
|
| Mechanical complications | Tube dislodgement/kinking/occlusion Arrhythmias Phrenic nerve palsy Horner’s syndrome |
|
| Miscellaneous | Pain Subcutaneous emphysema Re-expansion pulmonary oedema Chest wall arterio-venous fistula |
The most common complication is tube malposition. Complications were less common with chest tube size 16F, compared to larger-sized tubes.[121]
As described earlier, use of thoracic ultrasound aids safe & more precise placement, especially in loculated effusion.
Recommendations
Small-sized chest tubes (16 F or less) should be used to minimise chest tube-related complications. (Grade 2A)
USG-guided chest tube placement in pleural effusion can minimise tube position-related complications. (Grade 2A)
What are the precautions while applying suction to ICD drainage systems?
The underwater seal bottle should not be connected to wall-mounted suction directly. (GPP)
When suction is being applied to the underwater seal bottle system, the pressure should not exceed -10 to -20 cm H2O, and the patient should be monitored for new-onset chest pain or BPF. (GPP)
The 24-hour ICD drain, air leak and column movement should be monitored. (GPP)
The ICD drainage bottle should always be kept upright and below the level of the patient’s chest while transporting the patient. (GPP)
The Chest tube should never be clamped during the transportation of patients. (GPP)
How is a non-functioning or radiologically confirmed malpositioned chest drain managed?
Recommendations
Cessation of swinging is evidence of a tube blockage. This could be rectified by saline flushing by adopting a sterile technique. (GPP)
A functional chest tube with all side holes within the chest cavity does not require a change of position or replacement, regardless of where the tip lies, unless it is too far in and produces symptoms of pleural irritation. (GPP)
CT is a good tool for assessing malpositioned tubes. (GPP)
Tubes that are not placed deep enough should not be pushed in due to the risk of introducing infection. In such a situation, and when the tube is dislodged, if the need for drainage remains, a fresh tube should be inserted in another site.[122] (GPP)
Financial support and sponsorship
Indian Chest Society and the National College of Chest Physicians provided financial support towards this exercise.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Light RW. Pleural Diseases. Lippincott Williams and Wilkins. 2007:456. [Google Scholar]
- 2.Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143:634–9. doi: 10.1378/chest.12-1285. [DOI] [PubMed] [Google Scholar]
- 3.Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: A cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18:346–9. doi: 10.1002/ca.20133. [DOI] [PubMed] [Google Scholar]
- 4.Psallidas I, Helm EJ, Maskell NA, Yarmus L, Feller-Kopman DJ, Gleeson FV, et al. Iatrogenic injury to the intercostal artery: Aetiology, diagnosis and therapeutic intervention. Thora×. 2015;70:802–4. doi: 10.1136/thoraxjnl-2014-206658. [DOI] [PubMed] [Google Scholar]
- 5.Salamonsen M, Dobeli K, McGrath D, Readdy C, Ware R, Steinke K, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18:942–7. doi: 10.1111/resp.12088. [DOI] [PubMed] [Google Scholar]
- 6.Kanai M, Sekiguchi H. Avoiding vessel laceration in thoracentesis: A role of vascular ultrasound with color Doppler. Chest. 2015;147:e5–7. doi: 10.1378/chest.14-0814. [DOI] [PubMed] [Google Scholar]
- 7.Havelock T, Teoh R, Laws D, Gleeson F BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thora×. 2010;65(Suppl 2):ii61–76. doi: 10.1136/thx.2010.137026. [DOI] [PubMed] [Google Scholar]
- 8.Gareeboo S, Singh S. Tube thoracostomy: How to insert a chest drain. Br J Hosp Med (Lond) 2006;67:M16–8. doi: 10.12968/hmed.2006.67.Sup1.20339. [DOI] [PubMed] [Google Scholar]
- 9.Laws D, Neville E, Duffy J Pleural Diseases Group; Standards of Care Committee; British Thoracic Society. BTS guidelines for the insertion of a chest drain. Thora×. 2003;58(Suppl 2):ii53–9. doi: 10.1136/thorax.58.suppl_2.ii53. Suppl 2. doi: 10.1136/thorax. 58.suppl_2.ii53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxford Medical Education. Oxford Medical Education-Free Online Medical Education. Available from: https://oxfordmedicaleducation.com/ [Last accessed on 2023 Nov 13]
- 11.Staub N, Wiener-Kronish J, Albertine K. New York: Marcel Dekker; 1985. Transport through the pleura: Physiology of normal liquid and solute exchange in the pleural space. [Google Scholar]
- 12.Moudgil H, Sridhar G, Leitch AG. Reactivation disease: The commonest form of tuberculous pleural effusion in Edinburgh, 1980-1991. Respir Med. 1994;88:301–4. doi: 10.1016/0954-6111(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 13.Berger HW, Mejia E. Tuberculous pleurisy. Chest. 1973;63:88–92. doi: 10.1378/chest.63.1.88. [DOI] [PubMed] [Google Scholar]
- 14.Allen JC, Apicella MA. Experimental pleural effusion as a manifestation of delayed hypersensitivity to tuberculin PPD. J Immunol. 1968;101:481–7. [PubMed] [Google Scholar]
- 15.Mohammed KA, Nasreen N, Hardwick J, Van Horn RD, Sanders KL, Antony VB. Mycobacteria induces pleural mesothelial permeability by down-regulating beta-catenin expression. Lung. 2003;181:57–66. doi: 10.1007/s00408-003-1006-1. [DOI] [PubMed] [Google Scholar]
- 16.Leckie WJ, Tothill P. Albumin turnover in pleural effusions. Clin Sci. 1965;29:339–52. [PubMed] [Google Scholar]
- 17.Wallgren A. The time-table of tuberculosis. Tubercle. 1948;29:245–51. doi: 10.1016/s0041-3879(48)80033-4. [DOI] [PubMed] [Google Scholar]
- 18.Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: Tumor-host interactions unleashed. Am J Respir Crit Care Med. 2012;186:487–92. doi: 10.1164/rccm.201203-0465PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Light RW, Hamm H. Malignant pleural effusion: Would the real cause please stand up? Eur Respir J. 1997;10:1701–2. doi: 10.1183/09031936.97.10081701. [DOI] [PubMed] [Google Scholar]
- 20.Meyer PC. Metastatic carcinoma of the pleura. Thora×. 1966;21:437–43. doi: 10.1136/thx.21.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J. 1989;2:366–9. [PubMed] [Google Scholar]
- 22.Ishimoto O, Saijo Y, Narumi K, Kimura Y, Ebina M, Matsubara N, et al. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology. 2002;63:70–5. doi: 10.1159/000065723. [DOI] [PubMed] [Google Scholar]
- 23.Cheng D, Rodriguez RM, Perkett EA, Rogers J, Bienvenu G, Lappalainen U, et al. Vascular endothelial growth factor in pleural fluid. Chest. 1999;116:760–5. doi: 10.1378/chest.116.3.760. [DOI] [PubMed] [Google Scholar]
- 24.Economidou F, Antoniou KM, Tzanakis N, Sfiridaki K, Siafakas NM, Schiza SE. Angiogenic molecule Tie-2 and VEGF in the pathogenesis of pleural effusions. Respir Med. 2008;102:774–9. doi: 10.1016/j.rmed.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Wiener-Kronish JP, Broaddus VC, Albertine KH, Gropper MA, Matthay MA, Staub NC. Relationship of pleural effusions to increased permeability pulmonary edema in anesthetized sheep. J Clin Invest. 1988;82:1422–9. doi: 10.1172/JCI113747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachman AL, Macken K. Pleural effusions following supervoltage radiation for breast carcinoma. Radiology. 1959;72:699–709. doi: 10.1148/72.5.699. [DOI] [PubMed] [Google Scholar]
- 27.Morrone N, Gama e Silva Volpe VL, Dourado AM, Mitre F, Coletta EN. Bilateral pleural effusion due to mediastinal fibrosis induced by radiotherapy. Chest. 1993;104:1276–8. doi: 10.1378/chest.104.4.1276. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-García JL, Fraile G, Moreno MA, Sánchez-Corral JA, Peñalver R. Recurrent massive pleural effusion as a late complication of radiotherapy in Hodgkin’s disease. Chest. 1991;100:1165–6. doi: 10.1378/chest.100.4.1165. [DOI] [PubMed] [Google Scholar]
- 29.Kiafar C, Gilani N. Hepatic hydrothorax: Current concepts of pathophysiology and treatment options. Ann Hepatol. 2008;7:313–20. [PubMed] [Google Scholar]
- 30.Huang PM, Chang YL, Yang CY, Lee YC. The morphology of diaphragmatic defects in hepatic hydrothorax: Thoracoscopic finding. J Thorac Cardiovasc Surg. 2005;130:141–5. doi: 10.1016/j.jtcvs.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 31.Mittal BR, Maini A, Das BK. Peritoneopleural communication associated with cirrhotic ascites: Scintigraphic demonstration. Abdom Imaging. 1996;21:69–70. doi: 10.1007/s002619900014. [DOI] [PubMed] [Google Scholar]
- 32.Gumaste V, Singh V, Dave P. Significance of pleural effusion in patients with acute pancreatitis. Am J Gastroenterol. 1992;87:871–4. [PubMed] [Google Scholar]
- 33.Ali T, Srinivasan N, Le V, Chimpiri AR, Tierney WM. Pancreaticopleural fistula. Pancreas. 2009;38:e26–31. doi: 10.1097/MPA.0b013e3181870ad5. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez FG, Denlinger CE, Patterson GA, Kreisel D, Krupnick AS. Massive bilateral chylothoraces complicating mediastinal granulomatous disease. Ann Thorac Surg. 2009;88:1012–3. doi: 10.1016/j.athoracsur.2009.01.053. [DOI] [PubMed] [Google Scholar]
- 35.Karapolat S, Sanli A, Onen A. Chylothorax due to tuberculosis lymphadenopathy: Report of a case. Surg Today. 2008;38:938–41. doi: 10.1007/s00595-007-3739-6. [DOI] [PubMed] [Google Scholar]
- 36.Nadolski G. Nontraumatic chylothorax: Diagnostic algorithm and treatment options. Tech Vasc Interv Radiol. 2016;19:286–90. doi: 10.1053/j.tvir.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Noppen M, De Waele M, Li R, Gucht KV, D’Haese J, Gerlo E, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med. 2000;162:1023–6. doi: 10.1164/ajrccm.162.3.9910050. [DOI] [PubMed] [Google Scholar]
- 38.Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219–25. doi: 10.1183/09031936.97.10010219. [DOI] [PubMed] [Google Scholar]
- 39.Miserocchi G, Agostoni E. Contents of the pleural space. J Appl Physiol. 1971;30:208–13. doi: 10.1152/jappl.1971.30.2.208. [DOI] [PubMed] [Google Scholar]
- 40.Rolf LL, Travis DM. Pleural fluid-plasma bicarbonate gradients in oxygen-toxic and normal rats. Am J Physiol. 1973;224:857–61. doi: 10.1152/ajplegacy.1973.224.4.857. [DOI] [PubMed] [Google Scholar]
- 41.Yamada S, Mitarbeitern Über die seröse Flüssigkeit in der Pleurahöhle der gesunden Menschen. Z Ges Exp Med. 1933;90:342–8. [Google Scholar]
- 42.Sahn SA. The value of pleural fluid analysis. Am J Med Sci. 2008;335:7–15. doi: 10.1097/MAJ.0b013e31815d25e6. [DOI] [PubMed] [Google Scholar]
- 43.Du Rand I, Maskell N. Introduction and methods: British Thoracic Society Pleural Disease Guideline 2010. Thora×. 2010;65(Suppl 2):ii1–3. doi: 10.1136/thx.2010.137042. [DOI] [PubMed] [Google Scholar]
- 44.DeBiasi EM, Puchalski J. Thoracentesis: State-of-the-Art in procedural safety, patient outcomes, and physiologic impact. PLEURA. 2016;3:2373997516646554. [Google Scholar]
- 45.Ernst A, Silvestri GA, Johnstone D American College of Chest Physicians. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123:1693–717. doi: 10.1378/chest.123.5.1693. [DOI] [PubMed] [Google Scholar]
- 46.Conner BD, Lee YCG, Branca P, Rogers JT, Rodriguez RM, Light RW. Variations in pleural fluid WBC count and differential counts with different sample containers and different methods. Chest. 2003;123:1181–7. doi: 10.1378/chest.123.4.1181. [DOI] [PubMed] [Google Scholar]
- 47.Rahman NM, Mishra EK, Davies HE, Davies RJO, Lee YCG. Clinically important factors influencing the diagnostic measurement of pleural fluid pH and glucose. Am J Respir Crit Care Med. 2008;178:483–90. doi: 10.1164/rccm.200801-062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishra EK, Rahman NM. Factors influencing the measurement of pleural fluid pH. Curr Opin Pulm Med. 2009;15:353–7. doi: 10.1097/MCP.0b013e32832b98d4. [DOI] [PubMed] [Google Scholar]
- 49.Hooper C, Lee YCG, Maskell N BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thora×. 2010;65(Suppl 2):ii4–17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 50.Maikap MK, Dhua A, Maitra MK. Etiology and clinical profile of pleural effusion. Int J Med Sci Public Health. 2018;7:316–21. [Google Scholar]
- 51.Villena V, López Encuentra A, Echave-Sustaeta J, Alvarez Martínez C, Martín Escribano P. [Prospective study of 1,000 consecutive patients with pleural effusion. Etiology of the effusion and characteristics of the patients] Arch Bronconeumol. 2002;38:21–6. doi: 10.1016/s0300-2896(02)75142-9. [DOI] [PubMed] [Google Scholar]
- 52.Chinchkar NJ, Talwar D, Jain SK. A stepwise approach to the etiologic diagnosis of pleural effusion in respiratory intensive care unit and short-term evaluation of treatment. Lung India. 2015;32:107–15. doi: 10.4103/0970-2113.152615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porcel JM, Light RW. Pleural effusions. Dis Mon. 2013;59:29–57. doi: 10.1016/j.disamonth.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Bielsa S, Porcel JM, Castellote J, Mas E, Esquerda A, Light RW. Solving the Light’s criteria misclassification rate of cardiac and hepatic transudates. Respirology. 2012;17:721–6. doi: 10.1111/j.1440-1843.2012.02155.x. [DOI] [PubMed] [Google Scholar]
- 55.Shen Y, Zhu H, Wan C, Chen L, Wang T, Yang T, et al. Can cholesterol be used to distinguish pleural exudates from transudates? Evidence from a bivariate meta-analysis. BMC Pulm Med. 2014;14:61. doi: 10.1186/1471-2466-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lépine PA, Thomas R, Nguyen S, Lacasse Y, Cheah HM, Creaney J, et al. Simplified criteria using pleural fluid cholesterol and lactate dehydrogenase to distinguish between exudative and transudative pleural effusions. Respiration. 2019;98:48–54. doi: 10.1159/000496396. [DOI] [PubMed] [Google Scholar]
- 57.Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician. 2006;73:1211–20. [PubMed] [Google Scholar]
- 58.Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: The diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 59.Romero-Candeira S, Fernández C, Martín C, Sánchez-Paya J, Hernández L. Influence of diuretics on the concentration of proteins and other components of pleural transudates in patients with heart failure. Am J Med. 2001;110:681–6. doi: 10.1016/s0002-9343(01)00726-4. [DOI] [PubMed] [Google Scholar]
- 60.Gotsman I, Fridlender Z, Meirovitz A, Dratva D, Muszkat M. The evaluation of pleural effusions in patients with heart failure. Am J Med. 2001;111:375–8. doi: 10.1016/s0002-9343(01)00881-6. [DOI] [PubMed] [Google Scholar]
- 61.Meisel S, Shamiss A, Thaler M, Nussinovitch N, Rosenthal T. Pleural fluid to serum bilirubin concentration ratio for the separation of transudates from exudates. Chest. 1990;98:141–4. doi: 10.1378/chest.98.1.141. [DOI] [PubMed] [Google Scholar]
- 62.Porcel JM, Vives M, Cao G, Esquerda A, Rubio M, Rivas MC. Measurement of pro-brain natriuretic peptide in pleural fluid for the diagnosis of pleural effusions due to heart failure. Am J Med. 2004;116:417–20. doi: 10.1016/j.amjmed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Porcel JM, Chorda J, Cao G, Esquerda A, Ruiz-González A, Vives M. Comparing serum and pleural fluid pro-brain natriuretic peptide (NT-proBNP) levels with pleural-to-serum albumin gradient for the identification of cardiac effusions misclassified by Light’s criteria. Respirology. 2007;12:654–9. doi: 10.1111/j.1440-1843.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 64.Ferreiro L, San José ME, Gude F, Lama A, Suárez-Antelo J, Golpe A, et al. Unilateral or bilateral thoracocentesis for bilateral pleural effusion. A prospective study. Arch Bronconeumol. 2016;52:189–95. doi: 10.1016/j.arbres.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 65.Porcel JM, Civit MC, Bielsa S, Light RW. Contarini’s syndrome: Bilateral pleural effusion, each side from different causes. J Hosp Med. 2012;7:164–5. doi: 10.1002/jhm.981. [DOI] [PubMed] [Google Scholar]
- 66.Pankaj B, Puja B. Tuberculous pleural effusion: A study on 250 patients. J Med Sci Research. 2010;1:46–9. [Google Scholar]
- 67.Bouros D, Pneumatikos I, Tzouvelekis A. Pleural involvement in systemic autoimmune disorders. Respiration. 2008;75:361–71. doi: 10.1159/000119051. [DOI] [PubMed] [Google Scholar]
- 68.Krenke R, Light RW. Drug-induced eosinophilic pleural effusion. Eur Respir Rev. 2011;20:300–1. doi: 10.1183/09059180.00005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brockelsby C, Ahmed M, Gautam M. Pleural effusion size estimation: US, CXR or CT? Thora×. 2016;71(Suppl 3):A83. [Google Scholar]
- 70.Kalokairinou-Motogna M, Maratou K, Paianid I, Soldatos T, Antipa E, Tsikkini A, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12–6. [PubMed] [Google Scholar]
- 71.Roch A, Bojan M, Michelet P, Romain F, Bregeon F, Papazian L, et al. Usefulness of ultrasonography in predicting pleural effusions>500 mL in patients receiving mechanical ventilation. Chest. 2005;127:224–32. doi: 10.1378/chest.127.1.224. [DOI] [PubMed] [Google Scholar]
- 72.Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: Analysis of 320 cases. AJR Am J Roentgenol. 1992;159:29–33. doi: 10.2214/ajr.159.1.1609716. [DOI] [PubMed] [Google Scholar]
- 73.Ibitoye BO, Idowu BM, Ogunrombi AB, Afolabi BI. Ultrasonographic quantification of pleural effusion: Comparison of four formulae. Ultrasonography. 2018;37:254–60. doi: 10.14366/usg.17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abramowitz Y, Simanovsky N, Goldstein MS, Hiller N. Pleural effusion: Characterization with CT attenuation values and CT appearance. AJR Am J Roentgenol. 2009;192:618–23. doi: 10.2214/AJR.08.1286. [DOI] [PubMed] [Google Scholar]
- 75.Arenas-Jiménez J, Alonso-Charterina S, Sánchez-Payá J, Fernández-Latorre F, Gil-Sánchez S, Lloret-Llorens M. Evaluation of CT findings for diagnosis of pleural effusions. Eur Radiol. 2000;10:681–90. doi: 10.1007/s003300050984. [DOI] [PubMed] [Google Scholar]
- 76.Traill ZC, Davies RJ, Gleeson FV. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin Radiol. 2001;56:193–6. doi: 10.1053/crad.2000.0573. [DOI] [PubMed] [Google Scholar]
- 77.Bury T, Paulus P, Dowlati A, Corhay JL, Rigo P, Radermecker MF. Evaluation of pleural diseases with FDG-PET imaging: Preliminary report. Thora×. 1997;52:187–9. doi: 10.1136/thx.52.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akkurt B, Torun Parmaksız E, Doğan C, Beyhan Sagmen S, Kıral N, Fidan A, et al. The role of PET-CT in the differential diagnosis of malignant-paramalignant pleural effusion. South Clin Ist Euras. 2019;30:315–9. [Google Scholar]
- 79.Usuda K, Iwai S, Funasaki A, Sekimura A, Motono N, Matoba M, et al. Diffusion-weighted imaging can differentiate between malignant and benign pleural diseases. Cancers (Basel) 2019;11:811. doi: 10.3390/cancers11060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346:1971–7. doi: 10.1056/NEJMcp010731. [DOI] [PubMed] [Google Scholar]
- 81.Sokolowski JW, Burgher LW, Jones FL, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140:257–8. doi: 10.1164/ajrccm/140.1.257. [DOI] [PubMed] [Google Scholar]
- 82.Thomsen TW, DeLaPena J, Setnik GS. Thoracentesis. N Engl J Med. 2006;355:e16. doi: 10.1056/NEJMvcm053812. [DOI] [PubMed] [Google Scholar]
- 83.Karkhanis VS, Joshi JM. Pleural effusion: Diagnosis, treatment, and management. Open Access Emerg Med. 2012;4:31–52. doi: 10.2147/OAEM.S29942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitazono MT, Lau CT, Parada AN, Renjen P, Miller WT. Differentiation of pleural effusions from parenchymal opacities: Accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407–12. doi: 10.2214/AJR.09.2950. [DOI] [PubMed] [Google Scholar]
- 85.Froudarakis ME. Diagnostic work-up of pleural effusions. Respiration. 2008;75:4–13. doi: 10.1159/000112221. [DOI] [PubMed] [Google Scholar]
- 86.McGrath EE, Anderson PB. Diagnosis of pleural effusion: A systematic approach. Am J Crit Care. 2011;20:119–27. doi: 10.4037/ajcc2011685. quiz 128. [DOI] [PubMed] [Google Scholar]
- 87.Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: A 12-year experience. Thora×. 2015;70:127–32. doi: 10.1136/thoraxjnl-2014-206114. [DOI] [PubMed] [Google Scholar]
- 88.Capizzi SA, Prakash UBS. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc. 1998;73:948–50. doi: 10.4065/73.10.948. [DOI] [PubMed] [Google Scholar]
- 89.Murthy V, Bessich JL. Medical thoracoscopy and its evolving role in the diagnosis and treatment of pleural disease. J Thorac Dis. 2017;9(Suppl 10):S1011–21. doi: 10.21037/jtd.2017.06.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomlinson JR, Sahn SA. Invasive procedures in the diagnosis of pleural disease. Semin Respir Med. 1987;9:30–6. [Google Scholar]
- 91.Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991;4:320–4. [PubMed] [Google Scholar]
- 92.Kirsch CM, Kroe DM, Azzi RL, Jensen WA, Kagawa FT, Wehner JH. The optimal number of pleural biopsy specimens for a diagnosis of tuberculous pleurisy. Chest. 1997;112:702–6. doi: 10.1378/chest.112.3.702. [DOI] [PubMed] [Google Scholar]
- 93.Diacon AH, Van de Wal BW, Wyser C, Smedema JP, Bezuidenhout J, Bolliger CT, et al. Diagnostic tools in tuberculous pleurisy: A direct comparative study. Eur Respir J. 2003;22:589–91. doi: 10.1183/09031936.03.00017103a. [DOI] [PubMed] [Google Scholar]
- 94.Muhammad RSE, Hussein SAM, Mohammad MF, Ahmed MM, Ali GA. Thoracoscopic pleural cryobiopsy versus conventional forceps biopsy in diagnosis of exudative pleural effusion of unknown etiology. Egypt J Bronchol. 2019;13:162–9. [Google Scholar]
- 95.Gao S, Wang C, Yu X, Teng T, Shang Y, Jia J, et al. Xpert MTB/RIF Ultra enhanced tuberculous pleurisy diagnosis for patients with unexplained exudative pleural effusion who underwent a pleural biopsy via thoracoscopy: A prospective cohort study. Int J Infect Dis. 2021;106:370–5. doi: 10.1016/j.ijid.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 96.Christopher DJ, Coelho V, Ebby GS, Shankar D, Gupta R, Thangakunam B. Incremental yield of Xpert® MTB/RIF Ultra over Xpert® MTB/RIF in the diagnosis of extrapulmonary TB. Int J Tuberc Lung Dis. 2021;25:939–44. doi: 10.5588/ijtld.21.0280. [DOI] [PubMed] [Google Scholar]
- 97.Loddenkemper R, Grosser H, Gabler A, Mai J, Presseuler H, Brandt H. Prospective evaluation of biopsy methods in the diagnosis of malignant pleural effusions. Inpatient comparison between pleural fluid cytology, blind needle biopsy and thoracoscopy. Am Rev Respir Dis. 1983;127:114–1983. [Google Scholar]
- 98.Christopher DJ, Peter JV, Cherian AM. Blind pleural biopsy using a Tru-cut needle in moderate to large pleural effusion--an experience. Singapore Med J. 1998;39:196–9. [PubMed] [Google Scholar]
- 99.Haridas N K. P. S, T.P. R, P.T. J, Chetambath R. Medical thoracoscopy vs closed pleural biopsy in pleural effusions: A randomized controlled study. J Clin Diagn Res. 2014;8:MC01–4. doi: 10.7860/JCDR/2014/7476.4310. doi: 10.7860/JCDR/2014/7476.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mishra AK, Verma SK, Kant S, Kushwaha RA, Garg R, Kumar S, et al. A study to compare the diagnostic efficacy of closed pleural biopsy with that of the thoracoscopic guided pleural biopsy in patients of pleural effusion. South Asian J Cancer. 2016;5:27–8. doi: 10.4103/2278-330X.179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dixon G, de Fonseka D, Maskell N. Pleural controversies: Image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis. 2015;7:1041–51. doi: 10.3978/j.issn.2072-1439.2015.01.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang DB, Yang PC, Luh KT, Kuo SH, Yu CJ. Ultrasound-guided pleural biopsy with Tru-Cut needle. Chest. 1991;100:1328–33. doi: 10.1378/chest.100.5.1328. [DOI] [PubMed] [Google Scholar]
- 103.Maturu VN, Dhooria S, Bal A, Singh N, Aggarwal AN, Gupta D, et al. Role of medical thoracoscopy and closed-blind pleural biopsy in undiagnosed exudative pleural effusions: A single-center experience of 348 patients. J Bronchology Interv Pulmonol. 2015;22:121–9. doi: 10.1097/LBR.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 104.Metintas M, Yildirim H, Kaya T, Ak G, Dundar E, Ozkan R, et al. CT scan-guided Abrams’ needle pleural biopsy versus ultrasound-assisted cutting needle pleural biopsy for diagnosis in patients with pleural effusion: A randomized, controlled trial. Respiration. 2016;91:156–63. doi: 10.1159/000443483. [DOI] [PubMed] [Google Scholar]
- 105.Sivakumar P, Jayaram D, Rao D, Dhileepan V, Ahmed I, Ahmed L. Ultrasound-guided abrams pleural biopsy vs CT-guided tru-cut pleural biopsy in malignant pleural disease, a 3-year follow-up study. Lung. 2016;194:911–6. doi: 10.1007/s00408-016-9933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology. 2001;219:510–4. doi: 10.1148/radiology.219.2.r01ma07510. [DOI] [PubMed] [Google Scholar]
- 107.James P, Gupta R, Christopher DJ, Balamugesh T. Evaluation of the diagnostic yield and safety of closed pleural biopsy in the diagnosis of pleural effusion. Indian J Tuberc. 2010;57:19–24. [PubMed] [Google Scholar]
- 108.Poe RH, Israel RH, Utell MJ, Hall WJ, Greenblatt DW, Kallay MC. Sensitivity, specificity, and predictive values of closed pleural biopsy. Arch Intern Med. 1984;144:325–8. [PubMed] [Google Scholar]
- 109.Von Hoff DD, LiVolsi V. Diagnostic reliability of needle biopsy of the parietal pleura. A review of 272 biopsies. Am J Clin Pathol. 1975;64:200–3. doi: 10.1093/ajcp/64.2.200. [DOI] [PubMed] [Google Scholar]
- 110.Mestitz P, Purves MJ, Pollard AC. Pleural biopsy in the diagnosis of pleural effusion; a report of 200 cases. Lancet. 1958;2:1349–53. doi: 10.1016/s0140-6736(58)91439-9. [DOI] [PubMed] [Google Scholar]
- 111.Bibby AC, Maskell NA. Pleural biopsies in undiagnosed pleural effusions; Abrams vs image-guided vs thoracoscopic biopsies. Curr Opin Pulm Med. 2016;22:392–8. doi: 10.1097/MCP.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 112.Metintas M, Ak G, Dundar E, Yildirim H, Ozkan R, Kurt E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: A randomized, controlled trial. Chest. 2010;137:1362–8. doi: 10.1378/chest.09-0884. [DOI] [PubMed] [Google Scholar]
- 113.Feller-Kopman DJ, Reddy CB, DeCamp MM, Diekemper RL, Gould MK, Henry T, et al. Management of malignant pleural effusions. An Official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839–49. doi: 10.1164/rccm.201807-1415ST. [DOI] [PubMed] [Google Scholar]
- 114.Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50:42–7. doi: 10.1080/02841850802590460. [DOI] [PubMed] [Google Scholar]
- 115.Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YCG, Light RW. Ultrasound-guided thoracentesis: Is it a safer method? Chest. 2003;123:418–23. doi: 10.1378/chest.123.2.418. [DOI] [PubMed] [Google Scholar]
- 116.Porcel JM. Chest tube drainage of the pleural space: A concise review for pulmonologists. Tuberc Respir Dis (Seoul) 2018;81:106–15. doi: 10.4046/trd.2017.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ritchie M, Brown C, Bowling M. Chest tubes: Indications, sizing, placement, and management. Clin Pulm Med. 2017;24:37. [Google Scholar]
- 118.Rahman NM, Maskell NA, Davies CWH, Hedley EL, Nunn AJ, Gleeson FV, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137:536–43. doi: 10.1378/chest.09-1044. [DOI] [PubMed] [Google Scholar]
- 119.Shen KR, Bribriesco A, Crabtree T, Denlinger C, Eby J, Eiken P, et al. The American association for thoracic surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg. 2017;153:e129–46. doi: 10.1016/j.jtcvs.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 120.Menegozzo CAM, Artifon ELA, Meyer-Pflug AR, Rocha MC, Utiyama EM. Can ultrasound be used as an adjunct for tube thoracostomy? A systematic review of potential application to reduce procedure-related complications. Int J Surg. 2019;68:85–90. doi: 10.1016/j.ijsu.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 121.Vilkki VA, Gunn JM. Complications related to tube thoracostomy in Southwest Finland hospital district between 2004 and 2014. Scand J Surg. 2020;109:314–9. doi: 10.1177/1457496919857262. [DOI] [PubMed] [Google Scholar]
- 122.Kwiatt M, Tarbox A, Seamon MJ, Swaroop M, Cipolla J, Allen C, et al. Thoracostomy tubes: A comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143–55. doi: 10.4103/2229-5151.134182. [DOI] [PMC free article] [PubMed] [Google Scholar]


