Abstract
Pancreatic ductal adenocarcinoma (PDAC) patients have late presentation at the time of diagnosis and a poor prognosis. Metal dyshomeostasis is known to play a role in cancer progression. However, the blood and tissue metallome of PDAC patients has not been assessed. This study aimed to determine the levels of essential and toxic metals in the serum and pancreatic tissue from PDAC patients. Serum samples were obtained from PDAC patients before surgical resection. Tissue (tumor and adjacent normal pancreas) were obtained from the surgically resected specimen. Inductively coupled plasma–mass spectrometry (ICP‐MS) analysis was performed to quantify the levels of 10 essential and 3 toxic metals in these samples. Statistical analysis was performed to identify dysregulated metals in PDAC and their role as potential diagnostic and prognostic biomarkers. Significantly decreased serum levels of magnesium, potassium, calcium, iron, zinc, selenium, arsenic, and mercury and increased levels of molybdenum were shown to be associated with PDAC. There were significantly decreased levels of zinc, manganese and molybdenum, and increased levels of calcium and selenium in the pancreatic tumor tissue compared with the adjacent normal pancreas. Notably, lower serum levels of calcium, iron, and selenium, and higher levels of manganese, were significantly associated with a poor prognosis (i.e., overall survival) in PDAC patients. In conclusion, this is the first study to comprehensively assess the serum and tissue metallome of PDAC patients. It identified the association of metals with PDAC diagnosis and prognosis.
Keywords: diagnosis, essential metals, inductively coupled plasma–mass spectrometry, pancreatic ductal adenocarcinoma, prognosis, toxic metals
Metal dyshomeostasis is observed in cancer pathogenesis. However, there is very limited knowledge available on the metallome of pancreatic cancer. Serum and tissue levels of a number of biometals were significantly altered in pancreatic cancer patients. Furthermore, a panel of four essential metals demonstrated significant prognostic ability. Overall, these findings will aid in understanding the role of metals in pancreatic cancer pathogenesis, which could lead to the development of future therapies or prevention strategies.
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and has one of the worst prognostic outcomes (~9% 5‐year survival) among all major solid tumors. 1 In fact, it is projected to become the second leading cause of cancer‐related deaths by 2030. 2 The late‐stage diagnosis of PDAC is attributable to dismal survival outcomes, as a majority of patients have advanced, unresectable disease at diagnosis.
Metals play an essential role in the physiological functioning of the human body, and their levels are tightly regulated. 3 Metal dyshomeostasis is observed in a variety of pathological conditions, including cancer. 3 , 4 , 5 , 6 , 7 , 8 Abnormal metal levels could be the cause or effect of pathological conditions. Moreover, exposure to toxic metals can also initiate carcinogenesis. 9 Previous studies have assessed the levels of metallome in urine or toe‐nail specimens from pancreatic cancer patients. 10 , 11 However, current knowledge on the levels of essential and toxic metals in the serum and pancreatic tissue of PDAC patients is very limited. There are few studies that have assessed the serum levels of few metals in PDAC patients, 12 , 13 this is the first study to comprehensively determine the serum and tissue metallome of PDAC patients.
2. METHODOLOGY
2.1. Patient involvement statement
Patients across two tertiary centers, Royal North Shore Hospital and North Shore Private Hospital, who had surgically resected PDAC and serum and tissue collected at the time of surgery from 2004 to 2018, were included in this study. A retrospective cohort of serum and tissue (tumor and adjacent normal pancreas) specimens from PDAC patients was obtained from the Kolling Tumor Bank. Sera from age‐ and sex‐matched healthy controls were also obtained from the Kolling Tumor Bank. A control group of adjacent normal pancreas from patients with intraductal papillary mucinous neoplasm (IPMN) was obtained from the Pancreatic Tumor Bank maintained by the research team. Ethical approval was obtained from the Northern Sydney Local Health District HREC (Reference: 2019/ETH08639). Patients were not involved in the (1) development of the research question and outcome measure; (2) study design; and (3) dissemination of results.
2.2. Inductively coupled plasma–mass spectrometry (ICP‐MS)
Levels of 10 essential elements (i.e., magnesium (Mg), potassium (K), calcium (Ca), iron (Fe), copper (Cu), zinc (Zn), selenium (Se), manganese (Mn), cobalt (Co) and molybdenum (Mo)) metals and three toxic (arsenic (As), mercury (Hg) and lead (Pb)) elements were analyzed by ICP‐MS (Analytik‐Jena PlasmaQuant MS Elite, Jena, Germany). This instrument was maintained and operated to the manufacturer's specifications with plasma conditions optimized to ensure that Ba++ and CeO formation remained at <3%. Hydrogen was used as the collision reaction interface gas to minimize the presence of polyatomic interferences. Internal standards (Sc, Ga, Y, Ce, Lu) were added to the sample diluent to compensate for variations in sample delivery rate and element ionization. Measurements were performed in the NSW Health Pathology Trace Elements Laboratory, Royal North Shore Hospital, St Leonards, NSW, Australia.
Serum samples were prepared for analysis by thawing them to room temperature before being thoroughly mixed by vortexing. Calibrators, controls, blanks, and samples were diluted 26‐fold with 40 μL added to 1000 μL sample diluent. Some samples were available in <40 μL quantity and were diluted an additional two‐fold with a 20 μL sample and 20 μL reagent grade water added to the sample diluent to achieve the 40 μL volume. The sample diluent was prepared from reagent grade stocks to contain 1% (v/v) ammonia solution, 2% (v/v) ethanol, 0.01% (w/v) Tx100/EDTA, 2.73 g/L ammonium chloride and 10 μg/L internal standard. Reagent blanks of 40 μL reagent grade water and commercial trace element controls (SERO, Billingstad, Norway) were included throughout the run to maintain confidence in analytical accuracy. Solutions were vortex mixed and loaded onto the ICP‐MS autosampler for analysis.
Element concentrations were interpolated from standard curves generated by the instrument software. The calibration points were derived from aqueous solutions prepared from certified commercial standards. Calibration curves contained a blank and six calibration points at levels spanning the range of concentrations present in our samples. Results were corrected for signal drift using internal standards with interpolation applied as the method of correction where appropriate. Concentrations were reported in SI units with dilution factors and internal standard adjustments applied by the software. To ensure uniform sample uptake, internal standard percentage relative standard deviation (%RSD) was monitored across five replicate measurements taken per sample. Any sample with an internal standard %RSD >5% was rerun.
Tissue specimens were cut into sections of less than 1 mm diameter, then washed thoroughly in water and dried overnight at room temperature. Dried tissue samples were weighed on a five‐figure balance. A sample mass of up to 0.004 g was used for digestion. If a sample's mass exceeded 0.004 g, it was cut into portions weighing between 0.002 and 0.004 g. Standard Reference Material 1577b Bovine Liver was used as a control. Reagent blanks were also included with each run. Dried tissue samples were digested in 200 μL of freshly prepared reverse aqua regia (4:1 Suprapur nitric acid/hydrochloric acid) with loosely capped tubes placed in a 90°C heating block for approximately 30 min. When digested, 800 μL of water was added to bring the digestion solution to a final volume of 1 mL. A 40 μL aliquot of this solution was added to 1 mL of sample diluent for the subsequent analysis, as described for serum samples above.
2.3. Cell culture
Four pancreatic cancer cell lines (i.e., PANC1, MIAPaCa‐2, CFPAC1, and AsPC‐1) were used in this study. All cell lines were grown in DMEM supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin and grown at 37°C under 5% CO2. Mycoplasma testing was performed periodically on all cell lines using a commercial kit (Catalog#: rep‐mys‐10; InvivoGen).
2.4. Incucyte cell growth and proliferation assay
Cellular growth assay was performed using Incucyte Live Cell Imaging system using standard protocol. 14 Cells were seeded on 96‐well plates and allowed to attach for 24 h. Cells were then incubated with either control medium (10% FCS) or medium containing metal salts at different concentrations. Plates were then placed in the Incucyte Live Cell Imaging system to measure cellular growth and proliferation.
2.5. Incucyte wound healing assay
Cancer cell migration was assessed using the Incucyte wound healing assay using a standard protocol. 14 Cells were grown to 100% confluency in 96‐well plates, and a scratch was made using a wound maker tool. Cells were then washed twice with PBS and incubated with either control medium (5% FCS) or medium with metal salts at different concentrations. Plates were then placed in the Incucyte Live Cell Imaging system. Relative wound density was then calculated using Incucyte Scratch Wound Analysis software.
2.6. Data analysis
The distribution of data was assessed using the D'Agostino & Pearson normality test. The serum metal concentrations between PDAC and healthy controls were compared and analyzed using the Mann–Whitney U‐test or Kruskal–Wallis H‐test followed by Dunn's multiple comparison test. The diagnostic potential of metals was assessed using receiver operating characteristic (ROC) curves. Univariable survival analysis for biomarkers was performed using Kaplan–Meier curves, and statistical significance was assessed using the Log‐rank test with significance at a p‐value < 0.05. Multivariable survival analysis was performed using Cox proportional hazard analysis. The biomarker cut‐off values for the survival analysis were obtained from ROC analysis based on optimal sensitivity and specificity. All statistical analyses were performed using GraphPad Prism Software (Version 9).
3. RESULTS
3.1. Population demographics
In total, 138 PDAC patients and 88 sex‐ and age‐matched (±5 years) healthy controls were included in this study. Patient characteristics (age, sex, tumor stage, grade, lymph node involvement, margin status) are described in Table 1. Tissue specimens were available from 83 PDAC patients (Table 1).
TABLE 1.
Characteristics of PDAC patients and healthy controls.
| Serum | Tissue | ||
|---|---|---|---|
| PDAC | Healthy controls | PDAC | |
| Sex | |||
| M | 62 | 29 | 42 |
| F | 76 | 59 | 41 |
| Median age (years) | |||
| 66 | 62.5 | 66 | |
| Stage | |||
| IA | 7 | 4 | |
| IB | 17 | 8 | |
| IIA | 4 | 3 | |
| IIB | 51 | 34 | |
| III | 52 | 29 | |
| IV | 7 | 5 | |
| Grade | |||
| 1 | 4 | 2 | |
| 2 | 90 | 55 | |
| 3 | 44 | 26 | |
| Lymph node involved | |||
| Y | 108 | 67 | |
| N | 30 | 16 | |
| Margin | |||
| R0 | 67 | 41 | |
| R1 | 71 | 42 | |
| NAC | |||
| Y | 19 | 1 | |
| N | 119 | 82 | |
3.2. Serum and tissue metal levels
There was a significant (p < 0.01) decrease in the levels of Mg, K, Ca, Fe, Zn, Se, As, and Hg in the serum of PDAC patients compared with healthy controls (Table 2). In contrast, the serum levels of Mo and Pb were significantly (p < 0.05) increased in PDAC patients compared with healthy controls (Table 2). There was no significant change in the serum levels of Mn, Co, and Cu between PDAC patients and healthy controls (Table 2). Moreover, there was a significant (p < 0.001) increase in Cu:Zn and Cu:Fe ratios in the serum of PDAC patients compared with healthy controls (Table 2).
TABLE 2.
PDAC Metallome. (A) Levels of metals in serum from PDAC and healthy controls. (B) Levels of metals in tumor and adjacent normal pancreas tissue from PDAC patients.
| (A) Serum | |||||||
|---|---|---|---|---|---|---|---|
| PDAC | Healthy controls | p‐value* | |||||
| Mean | SD | Median | Mean | SD | Median | ||
| 24Mg (mM) | 0.715 | 0.126 | 0.720 | 0.815 | 0.129 | 0.820 | <0.001 |
| 39K (mM) | 4.167 | 2.134 | 4.000 | 4.317 | 0.664 | 4.350 | 0.001 |
| 44Ca (mM) | 2.106 | 0.324 | 2.135 | 2.334 | 0.324 | 2.300 | <0.001 |
| 57Fe (μM) | 14.790 | 9.393 | 12.750 | 21.540 | 7.062 | 20.300 | <0.001 |
| 63Cu (μM) | 16.770 | 4.779 | 16.200 | 16.310 | 3.486 | 16.000 | 0.690 |
| 68Zn (μM) | 9.875 | 2.279 | 9.800 | 10.980 | 2.626 | 10.900 | 0.003 |
| 78Se (μM) | 0.993 | 0.397 | 0.925 | 1.424 | 0.327 | 1.365 | <0.001 |
| 55Mn (nM) | 22.460 | 14.820 | 17.000 | 20.820 | 11.700 | 18.500 | 0.694 |
| 59Co (nM) | 4.754 | 7.786 | 2.000 | 4.080 | 8.595 | 3.000 | 0.443 |
| 95Mo (nM) | 14.990 | 7.609 | 13.500 | 12.250 | 7.730 | 11.000 | <0.001 |
| 63Cu:57Fe | 1.405 | 0.778 | 1.229 | 0.844 | 0.379 | 0.794 | <0.001 |
| 63Cu:68Zn | 1.759 | 0.608 | 1.691 | 1.533 | 0.367 | 1.497 | 0.002 |
| 75As (nM) | 25.320 | 70.120 | 8.500 | 19.030 | 22.550 | 11.000 | 0.022 |
| 202Hg (nM) | 3.681 | 3.052 | 3.000 | 5.318 | 3.168 | 5.000 | <0.001 |
| Pb (nM) | 0.812 | 1.359 | 0.000 | 0.421 | 0.656 | 0.000 | 0.035 |
| (B) Tissue | |||||||
|---|---|---|---|---|---|---|---|
| (μg/g) | Tumor tissue | Adjacent normal pancreas | p‐value* | ||||
| Mean | SD | Median | Mean | SD | Median | ||
| 24Mg | 417.200 | 147.900 | 424.100 | 410.900 | 231.700 | 452.500 | 0.6491 |
| 39K | 6962.000 | 3371.000 | 6743.000 | 6821.000 | 4238.000 | 7218.000 | 0.9154 |
| 44Ca | 545.900 | 208.000 | 523.200 | 432.400 | 257.500 | 370.700 | <0.0001 |
| 57Fe | 185.700 | 197.600 | 131.800 | 162.200 | 152.900 | 112.400 | 0.1550 |
| 63Cu | 5.728 | 4.852 | 4.870 | 6.471 | 10.510 | 4.510 | 0.5484 |
| 68Zn | 60.830 | 27.630 | 56.950 | 87.540 | 56.750 | 86.210 | 0.0026 |
| 78Se | 0.982 | 0.391 | 1.004 | 0.715 | 0.474 | 0.777 | <0.0001 |
| 55Mn | 1.242 | 1.234 | 0.890 | 2.929 | 2.262 | 2.510 | <0.0001 |
| 59Co | 0.034 | 0.029 | 0.027 | 0.034 | 0.024 | 0.031 | 0.4350 |
| 95Mo | 0.105 | 0.108 | 0.088 | 0.171 | 0.235 | 0.137 | 0.0005 |
| 63Cu:57Fe | 0.044 | 0.041 | 0.035 | 0.052 | 0.083 | 0.038 | 0.5251 |
| 63Cu:68Zn | 0.105 | 0.111 | 0.084 | 0.138 | 0.405 | 0.055 | 0.0182 |
| 75As | 0.075 | 0.078 | 0.048 | 0.060 | 0.067 | 0.037 | 0.1645 |
| 202Hg | 0.046 | 0.052 | 0.035 | 0.039 | 0.047 | 0.022 | 0.4870 |
| Pb | 0.065 | 0.094 | 0.042 | 0.255 | 0.684 | 0.104 | <0.0001 |
Mann–Whitney U‐test.
On the assessment of pancreas tissue, there was a significant (p < 0.01) decrease in the levels of Zn, Mn, Mo, and Pb in the tumor tissue compared with the adjacent normal pancreas (Table 2). Conversely, tumor levels of Ca and Se were significantly (p < 0.0001) increased compared to the adjacent normal pancreas (Table 2). There was no significant change in the tissue levels of Mg, K, Fe, Cu, Co, As, and Hg (Table 2). In order to confirm that adjacent normal pancreas from PDAC patients does not have aberrant metal levels, they were compared with adjacent normal pancreas from patients with IPMN, which are benign pancreatic cysts in the pancreatic ducts. Notably, none of the biometals showed any significant changes in adjacent normal pancreas obtained from PDAC and IPMN patients (Table S1). In contrast, a significant increase in the levels of toxic heavy metals (i.e., As, Hg, and Pb) was observed in adjacent normal pancreas from PDAC patients compared with IPMN patients (Table S1).
Some patients received neoadjuvant chemotherapy (NAC) treatment before serum collection, which could influence the levels of metals in the serum. Hence, analysis was also performed on patients who received upfront surgery. In comparison with their matched controls, patients who received upfront surgery demonstrated similar levels of metals in their serum (Table S2) as observed with the overall patient cohort (Table 2). These results indicated that the observed metal levels in serum were not influenced by the NAC treatment.
3.3. Diagnostic potential of serum and tissue metals
An area under the receiver operator characteristic (AUROC) curve was used to determine the diagnostic potential of serum metal concentrations. The following order of diagnostic potential was observed: Se (AUROC: 0.874) > Fe (AUROC: 0.789) > Mg (AUROC: 0.715) > Ca (AUROC: 0.702) > Hg (AUROC: 0.673) > Mo (AUROC: 0.659) > K (AUROC: 0.651) > Zn (AUROC: 0.615) > As (AUROC: 0.590) > Pb (AUROC: 0.573) > Co (AUROC: 0.529) > Cu or Mn (AUROC: 0.516) (Table 3). The AUROC for a panel of significantly different biometals (i.e., Mg, K, Ca, Fe, Zn, Se, and Mo) was 0.9012 (p < 0.0001).
TABLE 3.
Receiver operator curve analysis for: (A) serum levels of metals; and (B) tissue levels of metals.
| (A) Serum (PDAC vs. healthy controls) | |||||
|---|---|---|---|---|---|
| AUROC | p‐value | Optimal cut‐off | Sensitivity (%) | Specificity (%) | |
| 24Mg (mM) | 0.715 | <0.001 | <0.765 | 67.390 | 67.050 |
| 39K (mM) | 0.651 | 0.001 | <4.250 | 61.590 | 57.950 |
| 44Ca (mM) | 0.702 | <0.001 | <2.225 | 65.940 | 65.910 |
| 57Fe (μM) | 0.789 | <0.001 | <17.250 | 71.740 | 71.590 |
| 63Cu (μM) | 0.516 | 0.689 | >16.050 | 52.900 | 51.140 |
| 68Zn (μM) | 0.615 | 0.004 | <10.250 | 56.520 | 57.950 |
| 78Se (μM) | 0.874 | 0.001 | <1.215 | 85.510 | 78.410 |
| 55Mn (nM) | 0.516 | 0.693 | <17.500 | 53.620 | 56.820 |
| 59Co (nM) | 0.529 | 0.456 | <2.500 | 57.970 | 51.140 |
| 95Mo (nM) | 0.659 | 0.001 | >12.500 | 57.250 | 67.050 |
| 63Cu:57Fe | 0.778 | <0.001 | >0.927 | 73.190 | 73.860 |
| 63Cu:68Zn | 0.623 | 0.002 | >1.572 | 61.590 | 62.500 |
| 75As (nM) | 0.590 | 0.023 | <10.500 | 60.140 | 54.550 |
| 202Hg (nM) | 0.673 | <0.001 | <3.500 | 57.970 | 70.450 |
| Pb (nM) | 0.573 | 0.063 | >0.500 | 47.830 | 63.640 |
| (B) Tissue (tumor vs. adjacent normal pancreas) | |||||
|---|---|---|---|---|---|
| (μg/g) | AUROC | p‐value | Optimal cut‐off | Sensitivity (%) | Specificity (%) |
| 24Mg | 0.5205 | 0.6477 | <433.6 | 53.01 | 51.81 |
| 39K | 0.5049 | 0.9138 | >6299 | 57.83 | 43.37 |
| 44Ca | 0.6908 | <0.0001 | >462.9 | 68.66 | 63.86 |
| 57Fe | 0.5640 | 0.1544 | >123.9 | 53.63 | 53.63 |
| 63Cu | 0.5271 | 0.5470 | >4.515 | 54.22 | 50.60 |
| 68Zn | 0.6346 | 0.0027 | <64.21 | 62.65 | 62.65 |
| 78Se | 0.6978 | <0.0001 | >8970 | 65.06 | 62.65 |
| 55Mn | 0.7023 | <0.0001 | <1.320 | 73.49 | 69.88 |
| 59Co | 0.5352 | 0.4335 | <0.0295 | 59.04 | 57.83 |
| 95Mo | 0.6560 | 0.0005 | <0.1065 | 63.86 | 60.24 |
| 63Cu:57Fe | 0.5287 | 0.5236 | <0.03673 | 53.01 | 53.01 |
| 63Cu:68Zn | 0.6060 | 0.0184 | >0.07801 | 60.24 | 59.04 |
| 75As | 0.5625 | 0.1644 | >0.04125 | 59.04 | 54.22 |
| 202Hg | 0.5311 | 0.4885 | >0.02650 | 59.04 | 61.45 |
| Pb | 0.7309 | <0.0001 | <0.05873 | 68.67 | 68.67 |
The diagnostic ability of metals to identify tumor tissue compared with adjacent normal pancreas was also compared. The following order of diagnostic potential was observed: Pb (AUROC: 0.7309) > Mn (AUROC: 0.7023) > Se (AUROC: 0.6978) > Ca (AUROC: 0.6908) > Mo (AUROC: 0.6560) > Zn (AUROC: 0.6346) > Fe (AUROC: 0.5640) > As (AUROC: 0.5625) > Co (AUROC: 0.5352) > Hg (AUROC: 0.5311) > Cu (AUROC: 0.5271) > Mg (AUROC: 0.5205) > K (AUROC: 0.5049) (Table 3). The AUROC for a panel of significantly different biometals (i.e., Ca, Zn, Se, Mn and Mo) was 0.8596 (p < 0.0001).
3.4. Serum metal levels by PDAC stage
The serum levels of metals were further characterized against the disease stage. There was a significant (p < 0.05) decrease in Mg, K, Fe, and Se concentrations in the serum from all stages (I, II or III/IV) of PDAC patients compared with the healthy controls (Figure 1). There was a significant (p < 0.05) decrease in the levels of Ca and Hg from only Stage II or III/IV PDAC patients compared with healthy controls. Moreover, only Stage II patients demonstrated a significant decrease in Zn levels compared with healthy controls. In contrast, there was a significant (p < 0.05) increase in the serum levels of Mo in Stage II or III/IV patients, but not in Stage I patients, when compared with healthy controls. There was no significant change in the serum levels of Mn, Co, Cu, As and Pb between any stages of PDAC patients and healthy controls.
FIGURE 1.
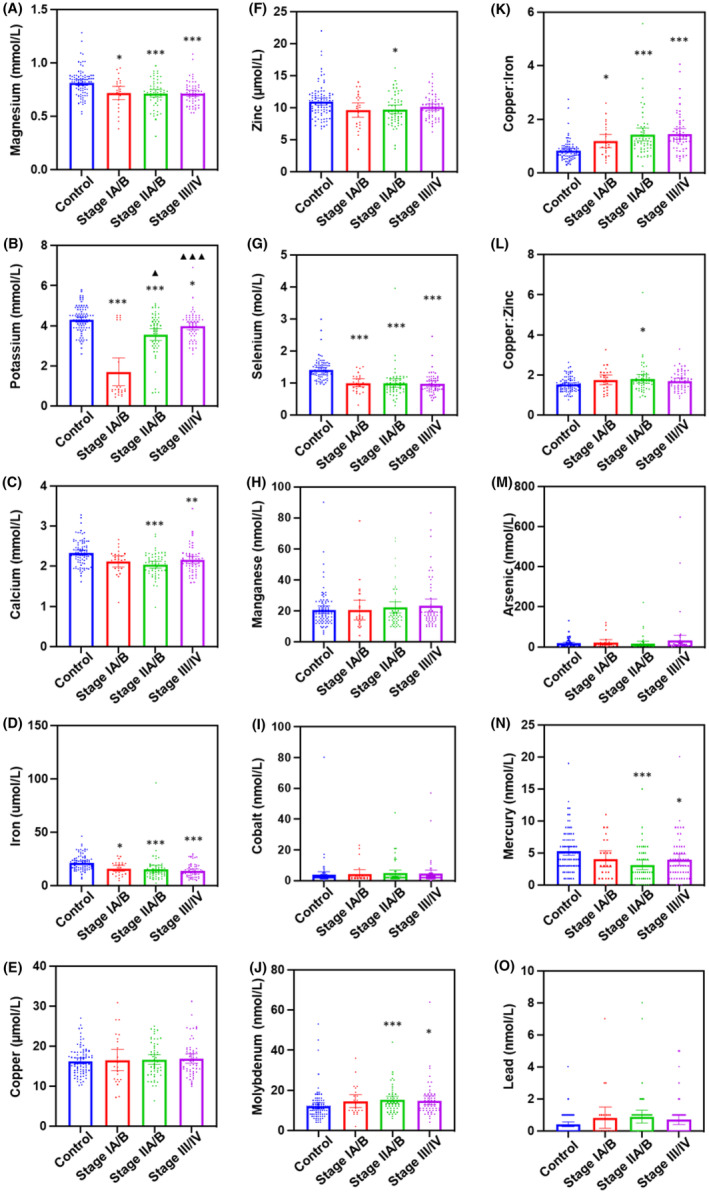
Serum metal level comparison between healthy controls and patients at different stages of PDAC. The levels of metals were compared between healthy controls, Stage IA/B, Stage IIA/B and Stage III/IV PDAC patients. (A) Magnesium (Mg); (B) potassium (K); (C) calcium (Ca); (D) iron (Fe); (E) copper (Cu); (F) zinc (Zn); (G) selenium (Se); (H) manganese (Mn); (I) cobalt (Co); (J) molybdenum (Mo); (K) Cu:Fe; (L) Cu:Zn; (M) arsenic (As); (N) mercury (Hg); and (O) lead (Pb). *p < 0.05; **p < 0.01; ***p < 0.001 compared with healthy controls. ▲ p < 0.05; ▲▲▲ p < 0.001 compared with Stage IA/B.
In comparison with the adjacent normal pancreas, the tumor tissue levels of Ca and Se were significantly increased in Stage II/III/IV patients. The tumor levels of Zn were significantly decreased in Stage III/IV patients only, while significantly decreased Mn, Mo, and Pb tumor levels were observed in Stage II/III/IV patients. No significant changes in tumor levels were observed for Mg, K, Fe, Cu, Co, As, and Hg at any tumor stage (Figure 2).
FIGURE 2.
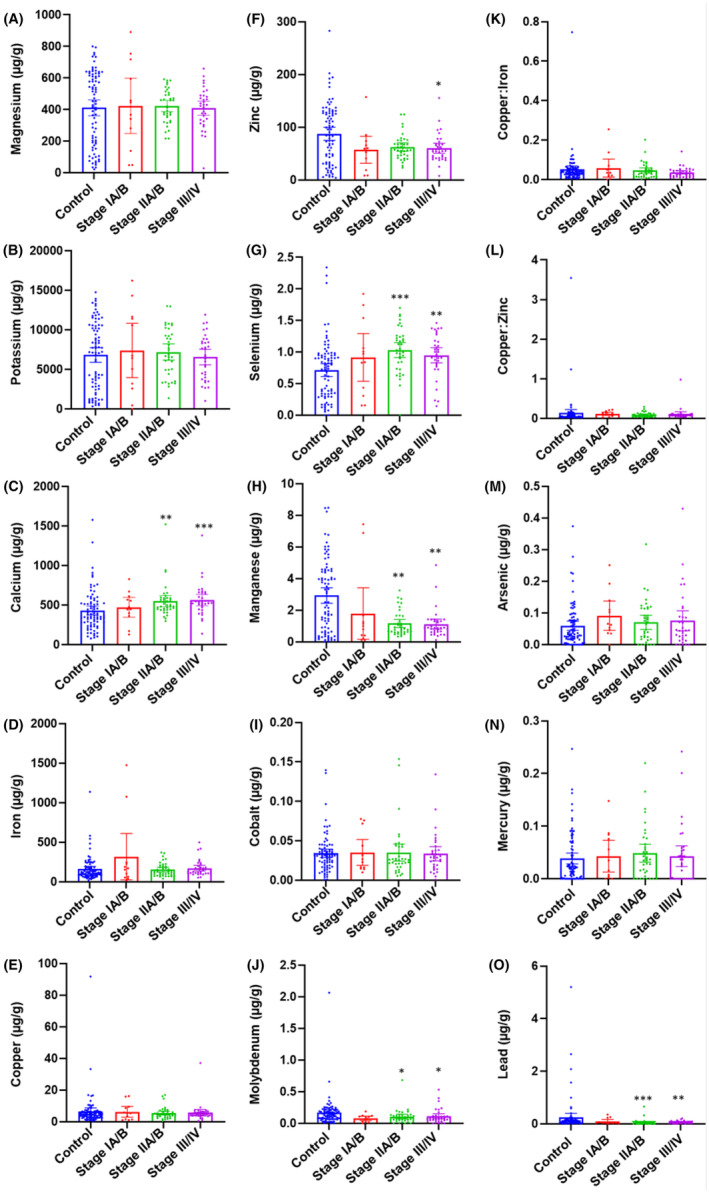
Tissue metal level comparison between adjacent normal pancreas and pancreatic tumor tissues at different stages of PDAC. The levels of metals were compared between adjacent normal pancreas and tumor tissue from PDAC patients at Stage IA/B, Stage IIA/B and Stage III/IV. (A) Magnesium (Mg); (B) potassium (K); (C) calcium (Ca); (D) iron (Fe); (E) copper (Cu); (F) zinc (Zn); (G) selenium (Se); (H) manganese (Mn); (I) cobalt (Co); (J) molybdenum (Mo); (K) Cu:Fe; (L) Cu:Zn; (M) arsenic (As); (N) mercury (Hg); and (O) lead (Pb). *p < 0.05; **p < 0.01; ***p < 0.001 compared with healthy controls. ▲ p < 0.05; ▲▲▲ p < 0.001 compared with Stage IA/B.
3.5. Prognostic potential of serum metals
The effect of metal serum levels on overall survival was assessed with patients divided into two groups based on the optimal cut‐offs obtained from the AUROC analysis (Table 3). There was a significant (p < 0.05) decrease in the overall survival of PDAC patients with lower levels of Ca, Fe, and Se or higher levels of Mn (Figure 3). No significant differences in survival were observed based on the serum levels of Mg, K, Cu, Zn, Co, Mo, As, Hg, and Pb or metal ratios Cu:Zn and Cu:Fe.
FIGURE 3.
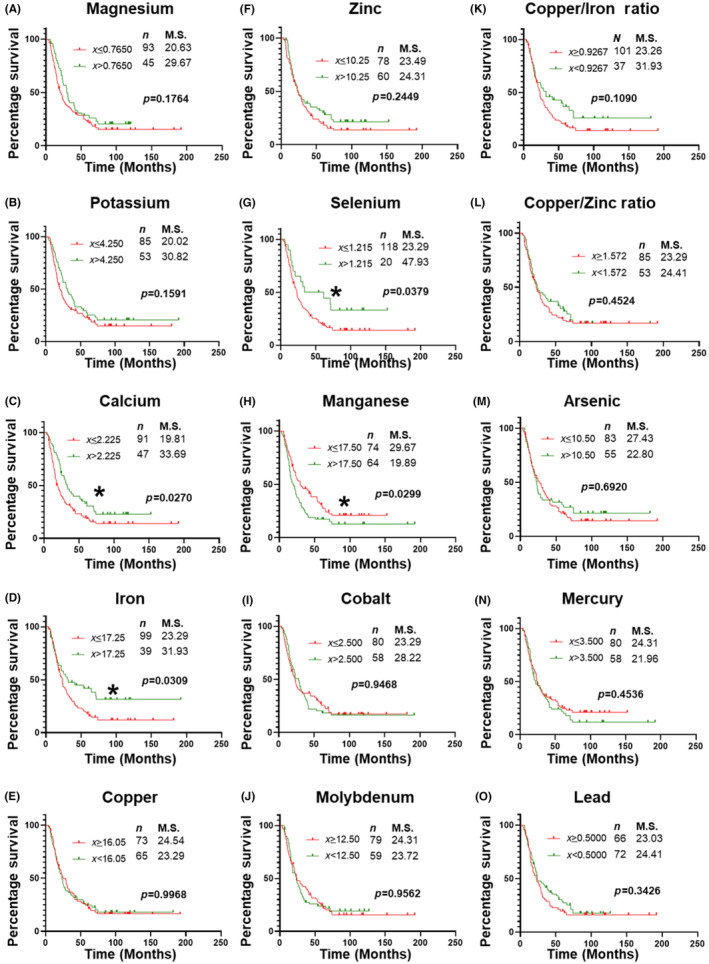
Univariable survival analysis of based on serum levels of individual metals. Kaplan–Meier survival curve analysis for PDAC patients based on cut‐offs for serum levels of different metals described in Table 3. Statistical significance between survival outcome was compared by Log‐rank test. (A) Magnesium (Mg); (B) potassium (K); (C) calcium (Ca); (D) iron (Fe); (E) copper (Cu); (F) zinc (Zn); (G) selenium (Se); (H) manganese (Mn); (I) cobalt (Co); (J) molybdenum (Mo); (K) Cu:Fe; (L) Cu:Zn; (M) arsenic (As); (N) mercury (Hg); and (O) lead (Pb). M.S., median survival in months. n = number of patients in the group. *, p < 0.05.
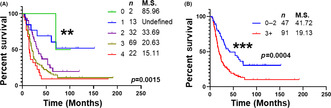
The levels of four metals with a significant prognostic ability (i.e., Ca, Fe, Se, and Mn) were assessed as a panel. Patients were categorized based on their metal levels, with decreased levels of Ca, Fe, and Se or increased levels of Mn being considered abnormal. The levels of four metals were able to significantly (p < 0.01) stratify patients based on their overall survival into five categories (Figure 4A). Furthermore, patients with abnormal levels of ≤2 metals showed a markedly significant (p < 0.001) difference in overall survival outcomes compared to patients with abnormal levels of >3 metals (median survival: 41.72 vs. 19.13 months; Figure 4B). Notably, the metal panel was an independent prognostic predictor of overall survival in a multivariable Cox proportional hazard model (p < 0.01; HR: 1.913; Table S3).
FIGURE 4.
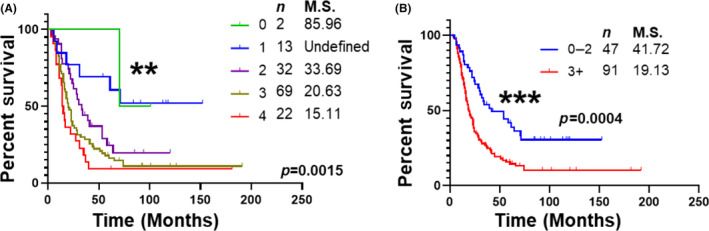
Univariable survival analysis of serum metals panel. Kaplan–Meier survival analysis of PDAC patients based on a selected metal panel of Ca, Fe, Se, and Mn. Statistical significance between survival outcome was compared by Log‐rank test. (A) Survival data of patients exhibiting abnormal levels of four, three, two, one or none of the metals in the panel. (B) Survival data of patients exhibiting abnormal levels of two or less of the metals was compared with those expressing abnormal levels of three or more metals. M.S., Median Survival in months. n = number of patients in the group. **, p < 0.01; ***, p < 0.001.
As margin status and NAC treatment are also known to affect the prognostic outcomes of PDAC patients, 15 , 16 the prognostic ability of a metal panel was also assessed in PDAC patients with (1) R0 margin; (2) R1 margin; (3) NAC treatment; and (4) No‐NAC treatment (i.e., upfront surgery). The metal panel was able to significantly (p < 0.01–0.05) stratify patients based on their overall survival in all four categories (Figure S1). These results further demonstrate the independent prognostic ability of the serum metal panel.
3.6. Effect of metals on cellular proliferation and migration
In order to determine whether metals exerted any direct effect on cellular proliferation and migration, four different PDAC cell lines (PANC1, MIAPaCa‐2, CFPAC1, and AsPC‐1) were incubated with different concentrations of each metal based on their serum levels. Iron showed a cell line‐specific effect with significantly decreased proliferation in PANC1 cells and increased proliferation in CFPAC1 cells, while having no significant effect in MIAPaCa‐2 and AsPC‐1 cell lines (Figure S2). Lower concentrations of Se led to a slight but significant decrease in proliferation of CFPAC1 cells, while increased proliferation of cells was observed after incubation of AsPC1 cells with Ca (Figure S2). In general, most metals did not have any significant effect on cellular growth/proliferation in the different PDAC cell lines examined.
Next, the effect of metals on cell migration was assessed. There was no significant effect of K, Fe, and Mo on cellular migration in all cell lines examined. Mg led to a significant increase in cell migration in all cell lines except PANC1, while a significant reduction in cell migration was observed after incubation with Ca in all cell lines except MIAPaCa‐2. Incubation with Cu led to a significant decrease in cell migration in PANC1 and AsPC‐1 cell lines, while it increased or had no effect in CFPAC1 and MIAPaCa‐2 cells, respectively (Figure S3). Zn, Se, and Mn demonstrated a significant increase in cell migration in some of the cell lines (Figure S3).
4. DISCUSSION
This study is the first that comprehensively determined the serum and tissue metallome of PDAC patients. Significant differences were observed between serum and tissue levels of essential trace elements as well as toxic heavy metals. Decreased serum metals/metalloids such as Se and Fe demonstrated a high potential to diagnose PDAC patients compared with healthy controls with AUROC values of 0.874 and 0.789. Conversely, increased levels of Se and Ca in tumor tissue were observed compared with adjacent normal pancreas. A panel of four metals (Ca, Fe, Se, and Mn) was able to significantly stratify patients based on overall survival and was shown to independently affect the prognosis in a multivariable survival model. These results indicated the potential utility of biometal levels as diagnostic and prognostic biomarkers for PDAC.
Mg, K, and Ca are macroelements that are known to play an important role in normal physiological functioning. 3 Interestingly, there was a significant decrease in the serum levels of these macroelements in PDAC patients. A prospective study has shown that Mg intake could reduce the risk of pancreatic cancer, 17 which is in direct alignment with reduced levels of Mg observed in the serum of PDAC patients in our study. A similar decrease in Ca levels was also observed in prostate cancer patients. 18 Interestingly, tumor levels of Ca were significantly increased compared with adjacent normal pancreas, indicating that decreased serum levels of Ca could be due to increased Ca uptake by the tumor. Ca was also shown to increase cellular growth in AsPC‐1 cells, indicating its role in the progression of primary tumors.
Fe, Cu, Zn, and Se are essential trace elements involved in a number of critical processes that play a role in oncogenesis. Iron is essential for key enzymes involved in cellular processes such as DNA synthesis (e.g., ribonucleotide reductase), cellular respiration (e.g., aconitase), etc. 19 , 20 The decrease in serum iron levels could be due to increased tumor demand for iron due to high cellular proliferation rates and altered energy metabolism, which are common characteristics of PDAC. 21 Although levels of Fe were increased in tumor tissue compared to adjacent normal pancreas, this effect did not achieve statistical significance. Copper‐containing enzymes are also involved in cellular respiration (e.g., cytochrome c oxidase) and angiogenesis (e.g., angiogenin). 22 , 23 A previous study has shown significantly increased serum Cu levels in pancreatic cancer patients. 13 Although we observed an increase in the Cu levels in serum and tumor tissue, these changes were not statistically significant. This discrepancy could be due to the small sample size (n = 29) of this previous study. 13
Free levels of both iron and copper redox cycles, which leads to the production of reactive oxygen species (ROS). 24 ROS production is one of the important initial steps for tumor initiation. 25 Conversely, Zn and Se have a cytoprotective role as cofactors for enzymes involved in antioxidant mechanisms such as metallothionines, superoxide dismutase, glutathione peroxidases, etc. 26 , 27 In this study, while serum levels of both Zn and Se were observed to be decreased in PDAC patients, contrasting results were obtained for tumor tissue where Se levels were significantly increased while Zn levels were significantly decreased. The decrease in serum levels of Zn and Se could be indicative of a weakened antioxidant response, which is a key feature of tumor progression. Previous studies have also shown a similar decrease in the Zn and Se levels in the serum of pancreatic cancer patients compared with healthy controls. 13 , 28 A recent study has shown increased urinary levels of Zn in PDAC patients, which might be responsible for the observed decrease in the levels of Zn in serum. 11 The decreased serum levels of Se could also be potentially due to increased tumor uptake of this metalloid. This could be due to an attempt by cancer cells to survive under harsh microenvironmental stress in PDAC tumors, which is associated with elevated redox stress.
There was a significant increase in the ratio of Cu:Zn in the serum and tumor tissue of PDAC patients. These results are in direct alignment with a previous study, 13 which demonstrated increased serum levels of Cu:Zn in PDAC patients compared with healthy controls or pancreatitis patients. Zn and Cu are both constituents of copper/zinc‐dependent superoxide dismutase (Cu/Zn‐SOD), which is an important antioxidant enzyme. 29 Adequate levels of Cu can support the activity of Cu/Zn‐SOD, but excess free Cu could lead to ROS production, resulting in an oxidative environment. 29 Conversely, Zn is inert to redox reactions and is mainly involved in antioxidant functions. 29 Thus, disruption of Cu and Zn homeostasis with a resultant increase in the Cu/Zn ratio indicates a pro‐oxidant environment, which could lead to an increased rate of carcinogenesis. 25
Mo was the only essential metal that was significantly increased in the serum of PDAC patients compared with healthy controls. In contrast, tumor tissue levels of Mo were significantly decreased. These interesting findings indicate a potential role of Mo in the pathogenesis of pancreatic cancer. There are four Mo‐containing enzymes found in humans, namely, xanthine oxidoreductase (XOR), aldehyde oxidase (AO), and the mitochondrial amidoxime‐reducing enzymes (mARC1 and 2). 30 The role of these enzymes in cancer pathogenesis is poorly understood, and future research in this area could shed more light on this interesting observation.
This is the first study assessing the levels of Mn in the serum and tissue from PDAC patients. Significantly, low levels of Mn were observed in tumor tissue compared with healthy normal pancreas. Mn is a cofactor for an important antioxidant protein, namely manganese superoxide dismutase (Mn‐SOD), and decreased levels of Mn in tumor tissue again re‐emphasize a weakened antioxidant response in tumors. No significant changes were observed in the serum levels of Mn in PDAC patients compared with healthy controls. However, patients with high serum levels of Mn had worse prognostic outcomes (i.e., lower overall survival). Previous studies have observed increased or decreased serum Mn levels in patients with other types of cancer (e.g., colorectal, renal cell, lung, breast cancers, etc.) compared with healthy controls, 31 , 32 , 33 , 34 , 35 , 36 , 37 but the effect on prognosis was not evaluated in any of these studies. Currently, there are only a few studies establishing the role of Mn in cancer pathogenesis. A recent study has shown that patients with high levels of Mn in tumors have poor overall survival. 38 Interestingly, increased Mn levels in drinking water are also shown to be linked with cancer incidence and mortality. 39 These results warrant future studies to examine the effect of Mn on PDAC pathogenesis and progression.
Notably, a panel of four elements (Ca, Fe, Se, and Mn) demonstrated a good prognostic ability and was able to independently affect the survival outcomes of PDAC patients. The measurement of metal levels is routinely performed in clinical pathology laboratories, 40 which are simple and inexpensive assays to perform. This metal panel has shown an excellent potential to be used as a surrogate biomarker panel for PDAC prognosis and, if verified independently, could be developed into a simple prognostic test for PDAC patients. This will be highly useful in stratifying patients to predict their prognostic outcomes, which could help in offering a personalized treatment plan to PDAC patients.
Using the multielement analytical technique of ICP‐MS, it was possible to determine a range of elements without compromising sample volume and analysis time. We were able to investigate several elements that would not normally be measured in serum (i.e., Mn, Hg, As, and Pb) as whole blood and/or urine samples provide superior information on exposure status. Significant changes between healthy controls and PDAC patients were observed in some of these elements that have prompted areas for further investigation. Serum levels of Hg and As showed significantly different ROC curves in diagnosing PDAC patients, which may reflect dietary changes with increased Hg and As concentrations pointing to a higher seafood intake. Significant differences in Pb concentrations may point to environmental variations resulting in higher lead exposure. Mn levels were able to stratify patients based on their overall survival, which may also indicate environmental factors, nutritional variations or undefined biochemical processes.
As an additional control for tissue metallome, levels of metals were also assessed on relatively more normal adjacent pancreas obtained from IPMN patients, who harbor benign pancreatic cysts instead of malignant pancreatic tumors. There were no changes in the levels of any biometals between adjacent normal pancreas obtained from PDAC or IPMN patients, indicating that potential pre‐cancerous aberrations in the adjacent normal pancreas of PDAC patients do not affect biometal levels. In contrast, levels of toxic heavy metals were increased in the adjacent normal pancreas of PDAC patients compared with IPMN patients. These results indicate the role of heavy metals in causing potential pre‐cancerous aberrations in pancreatic tumors and need to be assessed in more detail in future studies.
The main limitations of this study were a retrospective study design and a relatively small sample size. A future prospective study using a larger multi‐center cohort will be required to validate these results. Study strengths included similar age and sex distribution of PDAC patients and healthy controls and the application of a multielement technique enabling the simultaneous assessment of a large range of elements in a single laboratory, ensuring uniform result quality.
Overall, this was the first study to determine the serum and tissue metallome of PDAC patients. These results will provide a solid platform for further metallomics research in PDAC, which could potentially open new avenues for biomarker identification and therapeutic targeting of these belligerent tumors.
AUTHOR CONTRIBUTIONS
Sooin Byeon: Formal analysis; investigation; methodology; writing – review and editing. Taymin du Toit‐Thompson: Formal analysis; methodology. Luke Hipperson: Data curation; writing – review and editing. Sarah Maloney: Data curation; writing – review and editing. Ross Wenzel: Investigation; methodology; writing – review and editing. Anthony J. Gill: Data curation; resources; writing – review and editing. Jaswinder S. Samra: Conceptualization; supervision; writing – review and editing. Anubhav Mittal: Conceptualization; supervision; writing – review and editing. Sumit Sahni: Conceptualization; data curation; formal analysis; methodology; project administration; resources; supervision; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This project was supported by philanthropic funding received by Dr. Anubhav Mittal, Dr. Jaswinder Samra and Dr. Sumit Sahni.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Review Board: Ethical approval was obtained from the Northern Sydney Local Health District HREC (Reference: 2019/ETH08639).
Informed Consent: Specimens from PDAC patients and healthy controls were obtained from the Kolling Tumor Bank, which obtained these specimens after informed written consent.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Table S1
5. ACKNOWLEDGMENTS
A.M. would like to thank Sydney Vital for the Translational Centre for Excellence in Pancreatic Cancer Grant. S.S. would like to thank Mr. Guy Boncardo for the Boncardo Pancreatic Cancer Fellowship. S.S. would also like to thank the Pankind Foundation for a Collaboration Grant and the Ramsay Research Foundation for a Project Grant. T.d.T–T would like to acknowledge Team Lopez Foundation for a PhD scholarship. The authors would like to thank NSW Health Pathology for providing clinical data and ICP‐MS analysis. The authors would like to thank Kolling Tumor Bank for providing the specimens for this study. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Byeon S, du Toit‐Thompson T, Hipperson L, et al. Serum and tissue metallome of pancreatic ductal adenocarcinoma. Cancer Sci. 2024;115:1446‐1458. doi: 10.1111/cas.16124
Contributor Information
Anubhav Mittal, Email: anubhav.mittal@sydney.edu.au.
Sumit Sahni, Email: sumit.sahni@sydney.edu.au.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. [DOI] [PubMed] [Google Scholar]
- 3. Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM. The essential metals for humans: a brief overview. J Inorg Biochem. 2019;195:120‐129. [DOI] [PubMed] [Google Scholar]
- 4. Navarro Silvera SA, Rohan TE. Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control. 2007;18:7‐27. [DOI] [PubMed] [Google Scholar]
- 5. Greenough MA, Camakaris J, Bush AI. Metal dyshomeostasis and oxidative stress in Alzheimer's disease. Neurochem Int. 2013;62:540‐555. [DOI] [PubMed] [Google Scholar]
- 6. Lossow K, Schwarz M, Kipp AP. Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox Biol. 2021;42:101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callejón‐Leblic B, Arias‐Borrego A, Pereira‐Vega A, Gómez‐Ariza JL, García‐Barrera T. The metallome of lung cancer and its potential use as biomarker. Int J Mol Sci. 2019;20:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White AR, Aschner M, Costa LG, Bush AI. Biometals in Neurodegenerative Diseases: Mechanisms and Therapeutics. Academic Press; 2017. [Google Scholar]
- 9. Chen QY, DesMarais T, Costa M. Metals and mechanisms of carcinogenesis. Annu Rev Pharmacol Toxicol. 2019;59:537‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amaral AFS, Porta M, Silverman DT, et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012;61:1583‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schilling K, Larner F, Saad A, et al. Urine metallomics signature as an indicator of pancreatic cancer. Metallomics. 2020;12:752‐757. [DOI] [PubMed] [Google Scholar]
- 12. Lener MR, Scott RJ, Wiechowska‐Kozłowska A, et al. Serum concentrations of selenium and copper in patients diagnosed with pancreatic cancer. Cancer Res Treat. 2016;48:1056‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fabris C, Farini R, Del Favero G, et al. Copper, zinc and copper/zinc ratio in chronic pancreatitis and pancreatic cancer. Clin Biochem. 1985;18:373‐375. [DOI] [PubMed] [Google Scholar]
- 14. Abd El‐Aziz YS, McKay M, Molloy MP, et al. Inhibition of autophagy initiation: a novel strategy for oral squamous cell carcinomas. Biochim Biophys Acta, Mol Cell Res. 2024;1871:119627. [DOI] [PubMed] [Google Scholar]
- 15. Versteijne E, Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long‐term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022;40(11):1220‐1230. [DOI] [PubMed] [Google Scholar]
- 16. Birgin E, Rasbach E, Téoule P, Rückert F, Reissfelder C, Rahbari NN. Impact of intraoperative margin clearance on survival following pancreatoduodenectomy for pancreatic cancer: a systematic review and meta‐analysis. Sci Rep. 2020;10:22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dibaba D, Xun P, Yokota K, White E, He K. Magnesium intake and incidence of pancreatic cancer: the VITamins and lifestyle study. Br J Cancer. 2015;113:1615‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salem S, Hosseini M, Allameh F, Babakoohi S, Mehrsai A, Pourmand G. Serum calcium concentration and prostate cancer risk: a multicenter study. Nutr Cancer. 2013;65:961‐968. [DOI] [PubMed] [Google Scholar]
- 19. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164‐174. [PMC free article] [PubMed] [Google Scholar]
- 20. Stiban J, So M, Kaguni LS. Iron‐sulfur clusters in mitochondrial metabolism: multifaceted roles of a simple cofactor. Biochemistry (Mosc). 2016;81:1066‐1080. [DOI] [PubMed] [Google Scholar]
- 21. Reyes‐Castellanos G, Masoud R, Carrier A. Mitochondrial metabolism in PDAC: from better knowledge to new targeting strategies. Biomedicine. 2020;8:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uriu‐Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Asp Med. 2005;26:268‐298. [DOI] [PubMed] [Google Scholar]
- 23. Harris ED. A requirement for copper in angiogenesis. Nutr Rev. 2004;62:60‐64. [DOI] [PubMed] [Google Scholar]
- 24. Kalinowski DS, Stefani C, Toyokuni S, et al. Redox cycling metals: pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim Biophys Acta. 2016;1863:727‐748. [DOI] [PubMed] [Google Scholar]
- 25. Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)—induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167‐173. [DOI] [PubMed] [Google Scholar]
- 26. Chasapis CT, Ntoupa PA, Spiliopoulou CA, Stefanidou ME. Recent aspects of the effects of zinc on human health. Arch Toxicol. 2020;94:1443‐1460. [DOI] [PubMed] [Google Scholar]
- 27. Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94:739‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burney PG, Comstock GW, Morris JS. Serologic precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr. 1989;49:895‐900. [DOI] [PubMed] [Google Scholar]
- 29. Osredkar J, Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol S. 2011;S3:1. [Google Scholar]
- 30. Colette Daubner S, Lanzas RO. Cofactors and coenzymes|Pteridines. In: Jez J, ed. Encyclopedia of Biological Chemistry III. 3rd ed. Elsevier; 2018:395‐400. [Google Scholar]
- 31. Milde D, Novák O, Stuzka V, Vyslouzil K, Machácek J. Serum levels of selenium, manganese, copper, and iron in colorectal cancer patients. Biol Trace Elem Res. 2001;79:107‐114. [DOI] [PubMed] [Google Scholar]
- 32. Panaiyadiyan S, Quadri JA, Nayak B, et al. Association of heavy metals and trace elements in renal cell carcinoma: a case‐controlled study. Urol Oncol. 2022;40:111.e11‐111.e18. [DOI] [PubMed] [Google Scholar]
- 33. Wu HD, Chou SY, Chen DR, Kuo HW. Differentiation of serum levels of trace elements in normal and malignant breast patients. Biol Trace Elem Res. 2006;113:9‐18. [DOI] [PubMed] [Google Scholar]
- 34. Ding X, Jiang M, Jing H, et al. Analysis of serum levels of 15 trace elements in breast cancer patients in Shandong, China. Environ Sci Pollut Res Int. 2015;22:7930‐7935. [DOI] [PubMed] [Google Scholar]
- 35. Feng JF, Lu L, Zeng P, et al. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int J Clin Oncol. 2012;17:575‐583. [DOI] [PubMed] [Google Scholar]
- 36. Choi R, Kim M‐J, Sohn I, et al. Serum trace elements and their associations with breast cancer subgroups in Korean breast cancer patients. Nutrients. 2019;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saleh SAK, Adly HM, Abdelkhaliq AA, Nassir AM. Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr Urol. 2020;14:44‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doble PA, Miklos GLG. Distributions of manganese in diverse human cancers provide insights into tumour radioresistance. Metallomics. 2018;10:1191‐1210. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Q, Pan E, Liu L, et al. Study on the relationship between manganese concentrations in rural drinking water and incidence and mortality caused by cancer in Huai'an City. Biomed Res Int. 2014;2014:645056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang HS, LaFrance DR, Hao Y. Elemental testing using inductively coupled plasma mass spectrometry in clinical laboratories. Am J Clin Pathol. 2021;156:167‐175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.


