Abstract
Introduction
Subacute adult‐acquired hemichorea is a striking presentation with a broad differential, including ischemic, metabolic, and inflammatory causes.
Case
We encountered a 74‐year‐old woman with rapid onset of hemichorea and associated encephalopathy. Following a thorough workup without identification of clear imaging or laboratory abnormalities, we empirically treated with IVIg. Her hemichorea dramatically improved. Due to relapses of hemichorea, she required repeat immunotherapy with IVIg or high dose steroids followed by maintenance mycophenolate.
Discussion
This case of seronegative autoimmune hemichorea highlights the importance of a high index of suspicion for an inflammatory etiology of chorea when other causes are ruled out and performing an immunotherapy trial.
Introduction
Whereas insidious onset of chorea in adults is most often attributed to genetic disease, the differential for acute‐subacute chorea is unique. Cerebrovascular, metabolic, and endocrine causes of acute‐ and subacute‐onset chorea have been well established. A growing body of evidence now also highlights inflammatory causes of quickly progressive adult chorea, including paraneoplastic, para‐infectious, and idiopathic etiologies. 1 , 2 While causative autoantibodies are identified in select cases, much remains unknown about immune‐mediated movement disorders, and as a result some cases may go unrecognized and untreated. Here we present a patient with subacute relapsing hemichorea responsive to immunomodulatory therapies, with laboratory testing and imaging unrevealing of a specific diagnosis, suggesting a seronegative autoimmune hemichorea.
Case Description
A 74‐year‐old woman with a history of anxiety, depression, tobacco use, and several years of mild cognitive decline presented to our hospital with subacute onset of left‐sided chorea involving the face, arm, and leg. One day prior to symptom onset she had developed a painless and non‐pruritic confluent erythematous rash on her left arm. Six weeks prior to symptom onset she had received her second dose of the Pfizer‐BioNTech COVID‐19 vaccine. She had no other recent fevers, infections, or vaccinations. She had no exposure to antidopaminergic or estrogenic agents. She had no family history of cognitive, psychiatric, autoimmune, or movement disorders. Within several days of first appearance, her choreiform movements progressed to a nearly constant state, only ceasing during sleep. She received a course of doxycycline and clindamycin which improved her rash but did not affect her chorea. Over the subsequent days she developed a progressive change in mental status manifesting as disorientation, tangential speech, grandiose hallucinations, and euphoric affect. On hospital presentation she was unable to describe the reason for her admission. Her exam was notable for nearly continuous left‐sided hemichorea without any focal abnormalities in other domains of the neurologic exam.
Non‐contrast head CT and 1.5T brain MRI with gadolinium revealed symmetric frontal‐predominant atrophy but no focal lesions, with normal basal ganglia structure and volume. A comprehensive metabolic panel, complete blood count, blood glucose, and serum inflammatory markers were normal. Serum antinuclear antibody was mildly positive at a 1:40 titer but extensive rheumatologic labs were otherwise normal. Antiphospholipid antibody testing was negative. PET‐CT from skull‐base to thighs revealed no evidence of malignancy or infection. CSF studies were not completed during the initial presentation due to the patient's continuous movements, though a serum Mayo autoimmune encephalopathy panel was unremarkable. She was empirically treated with IVIg 0.4 g/kg of ideal body weight ×5 days and had marked improvement in her hemichorea and mental status even after the first dose. Within days of completing IVIg she achieved complete resolution of chorea and significant though not full improvement in encephalopathy.
After resolution of her choreiform movements she underwent a lumbar puncture, which revealed a normal CSF profile. CSF Mayo autoimmune encephalopathy panel was negative, and a repeat serum autoimmune encephalopathy panel after IVIg was only notable for trace positivity of anti‐GAD65. Brain FDG‐PET, which was also obtained after IVIg, revealed nonspecific patchy hypometabolism in the right anterior temporal lobe of unclear clinical significance. Genetic testing was not performed given that neurodegenerative causes of hemichorea would not be expected to have such rapid onset.
Approximately 5 months later she relapsed with identical symptoms of left‐sided hemichorea and encephalopathy, again progressive over several days (Video S1). Repeat brain MRI and CSF studies were unremarkable. Routine EEG was normal. Extensive laboratory testing was again negative with the exception of an elevated anticardiolipin IgM at 70.7 (normal range 0–15 MPL), but this normalized on repeat testing 4 months later. She received an identical dose of IVIg, which decreased but did not resolve her hemichorea during the hospitalization.
In the outpatient setting IVIg was replaced with IV methylprednisolone 1.3 mg/kg weekly. Her hemichorea began to improve after approximately 4 doses, and by Week 10 she had complete remission of hemichorea and methylprednisolone was discontinued (Video S2). Her encephalopathy did not improve and she continued to display irritability and deficits in short‐term memory. After 4 months her left‐sided hemichorea again returned. IV methylprednisolone was restarted at 1.3 mg/kg weekly for 6 weeks then transitioned to mycophenolate and monthly 2 g/kg IVIg for maintenance treatment. She has achieved sustained remission of her hemichorea, enduring for over 1 year at the time of publication of this case study.
Although her chorea has not returned while on maintenance immunotherapy, she continues to have worsening cognitive decline with a MoCA score of 16 out of 30 revealing deficits in language, executive function, and memory. Further cognitive workup included a low CSF A‐beta 42 to total tau index with normal CSF phosphorylated tau, inconsistent with Alzheimer's disease, and a repeat brain MRI and brain FDG‐PET 2 years after initial scans now showing prominent bilateral frontotemporal hypometabolism and atrophy most suggestive of frontotemporal dementia. Her underlying frontotemporal dementia has progressed on a timeline independent from her subacute immunotherapy‐responsive hemichorea. The case is summarized in Figure 1 and components of the workup are listed in Table 1.
Figure 1.
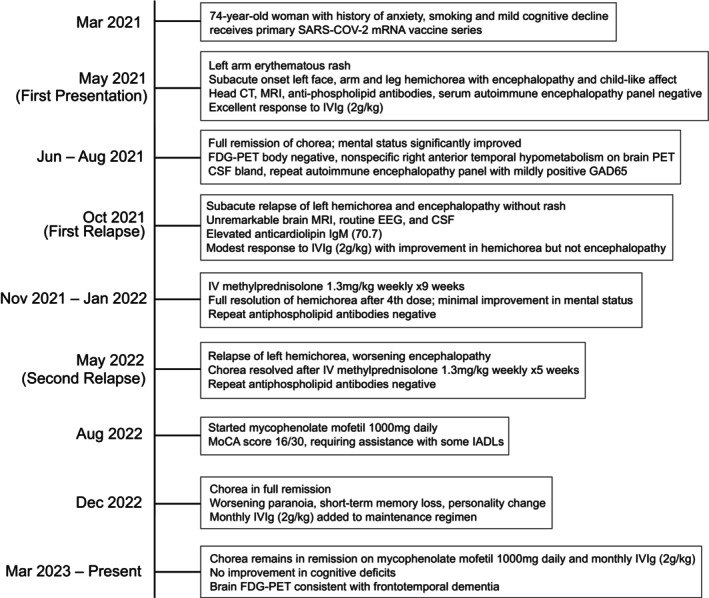
Case summary.
Table 1.
Workup completed for cognitive decline and hemichorea including laboratory, imaging, and EEG testing.
| Test category | Components | Result |
|---|---|---|
| Basic serum labs | CBC, BMP, LFTs, electrolytes | Normal |
| CSF labs (2×) | Protein, glucose, cell counts, IgG synthesis rate, IgG index, oligoclonal bands | Normal |
| Antiphospholipid antibodies (4× serum) | B2‐glycoprotein‐1 IgG/IgM, cardiolipin IgG/IgM | Positive cardiolipin IgM (70.7 MPL) once during relapse, negative on repeat twice |
| Autoimmune encephalopathy antibodies (2× serum, 3× CSF) | AGNA‐1, AMPAR, Amphiphysin, ANNA‐1, ANNA‐2, ANNA‐3, CASPR2, CRMP‐5, DPPX, GABA‐B, GAD65, GFAP, GlyR, IgLON5, KLHL11, LGI1, mGluR1, NIF, NMDA, Ma, PCA‐1, PCA‐2, PCA‐Tr, Ta. Serum Thyroglobulin, TPO | Serum anti‐GAD65 mildly elevated (0.03 nmol/L) once during remission, negative on repeat. All other tests normal |
| Rheumatologic labs | ANA, C3, C4, CRP, ESR, dsDNA, anti‐La, anti‐Ro, RHF, anti‐RNP, anti‐Scl‐70, anti‐Sm | ANA 1:40; All other tests normal |
| Dementia lab evaluation | B12, ceruloplasmin, HIV, syphilis, TSH, CSF A‐beta 42, CSF T‐tau, CSF P‐tau | Indeterminate Alzheimer's profile with reduced CSF A‐beta 42 to T‐Tau index and normal CSF P‐Tau; All other tests normal |
| Imaging | Brain MRI (2×), brain FDG‐PET (2×) | No infarct or mass; scattered hypometabolism in right anterior temporal lobe on initial PET; development of progressive frontotemporal atrophy with frontotemporal hypometabolism on 2‐year interval scans |
| EEG (2×) | Routine EEG | Normal |
Discussion
The most common causes of acute‐subacute chorea and hemichorea include infarct or structural lesions of the basal ganglia, followed by metabolic perturbations including non‐ketotic hyperglycemic hemichorea/hemiballismus. 3 , 4 , 5 Additionally, autoimmunity accounts for an unknown fraction of cases and should be considered when a workup for common causes is negative. Accompanying features that may strengthen suspicion for an autoimmune etiology include history of a systemic autoimmune disease, recent infection or recent vaccination. Notably, the predictive value of these elements is unknown, so their absence should not eliminate autoimmunity from consideration. Our patient's subacute change in personality likely stemmed from autoimmunity, though this also occurred on a background of slowly progressive cognitive decline, which would ultimately be revealed as a separate neurodegenerative process. Her rash during initial presentation was likely unrelated to her neurologic symptoms.
Aside from paraneoplastic cases, the trigger for autoimmune chorea is often unknown. There have been a small number of case reports of chorea and hemichorea occurring after COVID‐19 vaccination and infection, 6 including at least two corticosteroid‐responsive cases associated with the same Pfizer‐BioNTech COVID‐19 vaccine that our patient received. 7 , 8 Many of these cases were associated with encephalopathy. However, all other reported cases had sudden symptom onset within days or up to 2 weeks after vaccination, whereas our patient's symptoms were delayed by 6 weeks. Due to this disparity in timeline from other cases, it is unclear what role our patient's vaccine played.
Workup for autoimmune chorea should include CSF sampling, which can show markers of inflammation or can be bland as in this case. 1 The presence of antiphospholipid antibodies is the most well‐known cause of autoinflammatory chorea and hemichorea in adults. 9 , 10 , 11 Among antibody‐mediated encephalitides anti‐CRMP‐5 is best known for causing generalized or unilateral chorea, 12 though chorea has also been described in cases of anti‐NMDA, anti‐CASPR2, anti‐LGI1, anti‐PDE10A, anti‐IgLON5, anti‐Yo, and anti‐MOG. 2 , 13 , 14 , 15 , 16 , 17 Comprehensive neuronal autoantibody testing should be performed from serum and CSF in patients for whom an autoimmune mechanism is under consideration, and may be repeated if initial testing is negative. Our patient's transiently positive serum anti‐GAD65 and anticardiolipin IgM support an autoimmune milieu, but do not fit into an established diagnostic entity.
In the absence of a positive autoantibody there is no other definitive test to identify cases of hemichorea that may respond to immune modulation. Brain MRI is often normal or nondiagnostic in autoimmune hemichorea. 1 Contralateral striatal hypermetabolism can be seen on PET, though this is not a reliable finding across cases. 18 We argue that the index of suspicion for an autoimmune process should be high even when these tests are normal, assuming that other structural and metabolic causes have been ruled out. An empiric immunotherapy trial should be offered unless there is a strong contraindication.
There is no established standard of care for treatment of autoimmune chorea. In paraneoplastic cases, treatment of the underlying malignancy is paramount, and all patients with a presumed autoimmune movement disorder should undergo a malignancy workup. Corticosteroids are generally preferred in Sydenham's chorea, 19 though some cases are treated with plasma exchange or IVIg. Other antibody‐mediated chorea disorders are often responsive to corticosteroids, though have also been treated with steroid‐sparing agents including rituximab, cyclophosphamide, IVIg, and plasma exchange. 1 , 2 , 20 In small cohorts, the rate of relapse with steroid treatment alone is high, but improved control is seen with prolonged use of steroid‐sparing agents. 1 Long‐term maintenance immunotherapy should be initiated in relapsing cases such as the one highlighted here.
In summary, this case emphasizes the importance of considering autoimmune etiologies of acute‐subacute chorea presenting in adults, especially after common structural, vascular, and metabolic causes have been exonerated. Even in the absence of specific inflammatory laboratory or imaging findings, if infection has been reasonably excluded then it may be appropriate to empirically treat with immunomodulatory therapy. Choice of immunosuppressive agent and duration of therapy remain open questions that may vary among patients, and both natural history of the disease and standard treatment courses will become clearer as more cases are reported.
Author Contributions
Rachel E. Rodin and Denis T. Balaban designed the case report, collected data, and consented the patient for participation. Rachel Rodin wrote the manuscript. Denis T. Balaban and Nagagopal Venna edited the manuscript. All authors participated in the clinical care of the patient.
Funding Information
No funding information provided.
Conflict of Interest Statement
Denis T. Balaban is a member of a Massachusetts General Hospital translational neuroscience fellowship that receives financial support from Biogen Inc.
Supporting information
Video S1. Left‐sided hemichorea during admission for first relapse.
Video S2. Neurologic exam after completion of IVIg following first relapse of hemichorea.
Acknowledgments
We thank the patient and her family for their generous participation in this report and for sharing their video and clinical information.
Contributor Information
Rachel E. Rodin, Email: rrodin@bwh.harvard.edu.
Denis T. Balaban, Email: dbalaban@mgb.org.
References
- 1. O'Toole O, Lennon VA, Ahlskog JE, et al. Autoimmune chorea in adults. Neurology. 2013;80(12):1133‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kyle K, Bordelon Y, Venna N, Linnoila J. Autoimmune and paraneoplastic chorea: a review of the literature. Front Neurol. 2022;13:829076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cincotta M, Walker RH. One side of the story; clues to etiology in patients with asymmetric chorea. Tremor Other Hyperkinet Mov (N Y). 2022;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cosentino C, Torres L, Nuñez Y, et al. Hemichorea/hemiballism associated with hyperglycemia: report of 20 cases. Tremor Other Hyperkinet Mov (N Y). 2016;6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryan C, Ahlskog JE, Savica R. Hyperglycemic chorea/ballism ascertained over 15 years at a referral medical center. Parkinsonism Relat Disord. 2018;48:97‐100. [DOI] [PubMed] [Google Scholar]
- 6. Rosca EC, Bilavu R, Cornea A, Simu M. Chorea following SARS‐CoV‐2 infection and vaccination: a systematic review of reported cases. Int J Infect Dis. 2023;134:256‐260. [DOI] [PubMed] [Google Scholar]
- 7. Batot C, Chea M, Zeidan S, et al. Clinical and radiological follow‐up of a Pfizer‐BioNTech COVID‐19 vaccine‐induced hemichorea‐hemiballismus. Tremor Other Hyperkinet Mov (N Y). 2022;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryu D‐W, Lim E‐Y, Cho A‐H. A case of hemichorea following administration of the Pfizer‐BioNTech COVID‐19 vaccine. Neurol Sci. 2022;43(2):771‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sastre‐Garriga J, Montalban X. APS and the brain. Lupus. 2003;12(12):877‐882. [DOI] [PubMed] [Google Scholar]
- 10. Safarpour D, Buckingham S, Jabbari B. Chorea associated with high titers of antiphospholipid antibodies in the absence of antiphospholipid antibody syndrome. Tremor Other Hyperkinetic Mov (N Y). 2015;5:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cervera R, Asherson RA, Font J, et al. Chorea in the antiphospholipid syndrome. Clinical, radiologic, and immunologic characteristics of 50 patients from our clinics and the recent literature. Medicine (Baltimore). 1997;76(3):203‐212. [DOI] [PubMed] [Google Scholar]
- 12. Vernino S, Tuite P, Adler CH, et al. Paraneoplastic chorea associated with CRMP‐5 neuronal antibody and lung carcinoma. Ann Neurol. 2002;51(5):625‐630. [DOI] [PubMed] [Google Scholar]
- 13. Hacohen Y, Dlamini N, Hedderly T, et al. N‐methyl‐d‐aspartate receptor antibody‐associated movement disorder without encephalopathy. Dev Med Child Neurol. 2014;56(2):190‐193. [DOI] [PubMed] [Google Scholar]
- 14. Tofaris GK, Irani SR, Cheeran BJ, Baker IWS, Cader ZM, Vincent A. Immunotherapy‐responsive chorea as the presenting feature of LGI1‐antibody encephalitis. Neurology. 2012;79(2):195‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vynogradova I, Savitski V, Heckmann JG. Hemichorea associated with CASPR2 antibody. Tremor Other Hyperkinetic Mov (N Y). 2014;4:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren M, Zhou Q. Stroke‐like presentation of autoimmune chorea with positive anti‐Yo and anti‐MOG antibodies: a case report. Neurol Sci. 2023;44(1):347‐349. [DOI] [PubMed] [Google Scholar]
- 17. Méneret A, Garcin B, Frismand S, Lannuzel A, Mariani LL, Roze E. Treatable hyperkinetic movement disorders not to be missed. Front Neurol. 2021;12:659805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu SW, Graham B, Gelfand MJ, Gruppo RE, Dinopolous A, Gilbert DL. Clinical and positron emission tomography findings of chorea associated with primary antiphospholipid antibody syndrome. Mov Disord. 2007;22(12):1813‐1815. [DOI] [PubMed] [Google Scholar]
- 19. Paz JA, Silva CAA, Marques‐Dias MJ. Randomized double‐blind study with prednisone in Sydenham's chorea. Pediatr Neurol. 2006;34(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 20. Feinstein E, Walker R. Treatment of secondary chorea: a review of the current literature. Tremor Other Hyperkinetic Mov (N Y). 2020;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Left‐sided hemichorea during admission for first relapse.
Video S2. Neurologic exam after completion of IVIg following first relapse of hemichorea.


