Abstract
Objective
According to a seminal hypothesis stated by Crick and Koch in 1995, one is not aware of neural activity in primary visual cortex (V1) because this region lacks reciprocal connections with prefrontal cortex (PFC).
Methods
We provide here a neuropsychological illustration of this hypothesis in a patient with a very rare form of cortical blindness: ventral and dorsal cortical pathways were lesioned bilaterally while V1 areas were partially preserved.
Results
Visual stimuli escaped conscious perception but still activated V1 regions that were functionally disconnected from PFC.
Interpretation
These results are consistent with the hypothesis of a causal role of PFC in visual awareness.
Keywords: prefrontal cortex, vision, consciousness, cortical blindness, primary visual cortex
In a seminal paper published more than a quarter century ago, Crick and Koch hypothesized that one is not aware of neural activity that occurs in primary visual cortex (V1). 1 They proposed that the lack of direct reciprocal neuronal connections between prefrontal cortex (PFC) and V1 2 should prevent awareness of V1 activity. We would only be aware of visual representations coded within ventral (occipito‐temporal) or dorsal (occipito‐parietal) cortical pathways that are fed by V1 and connected to PFC. 3 This hypothesis is also supported by a study suggesting that extrastriate lesion involving V2/V3 while preserving V1 may be sufficient to create a visual field defect. 4 This prediction is difficult to test in healthy volunteers in whom V1 is connected indirectly to PFC through ventral and dorsal cortical pathways. Checking its validity is still crucial today because it is compatible with only one group of current theories of consciousness, 5 , 6 , 7 while incompatible with another group. 8 , 9 , 10 We provide here a case report compatible with this prediction through the exploration of a neurological patient with cortical blindness caused by an extremely rare pattern of posterior brain lesions: While ventral and dorsal cortical pathways were severely lesioned in both hemispheres, V1 areas were partially preserved. Using a set of behavioral, electrophysiological, and fMRI data, we show visual stimuli were still activating V1 areas in this patient, but that bilateral lesions of ventral and dorsal pathways abolished the indirect functional connectivity between V1 and PFC present in healthy sighted volunteers.
A 59 years‐old male patient free of any prior neurological or psychiatric history was hospitalized in Intensive Care Unit for an acute respiratory distress syndrome secondary to SARS‐CoV‐2 infection. He presented several complications and notably a severe posterior encephalopathy syndrome with refractory status epilepticus. We examined and tested this patient between February–August 2021 (see Supplementary Material for Ethical agreement and consent, and for detailed clinical report), and his condition was stable across all sessions: When awake, he was conscious and aware of his blindness. He often complained about it (e.g., “I am so so sad, I am blind, I can't see!”), with emotionally congruent facial and prosodic expressions of sadness. He also showed the following cognitive deficits: (i) impaired executive functions including working memory and cognitive control deficits, as well as perseverations, (ii) short‐term memory deficit, (iii) space and time disorientation, and (iv) language deficits combining impaired lexical access, impaired repetition with a phrase‐length effect, and impaired understanding of complex syntactic structures (see Table S1) and (iv) parietal symptoms including astereognosia. Examination of motor and primary somatosensory systems was normal. Finally, the patient did not present evidence of unilateral neglect in auditory and tactile sensory modalities.
Neurovisual examination showed preserved direct and consensual photomotor reflexes, confirming functionality of retinal and brainstem visual pathways. However, behavioral examination could not reveal any evidence of visual cortical processing: blink to visual threat, optokinetic reflex, visual fixation, and pursuit were absent. 11 When presented with visual stimuli, he invariably denied any subjective conscious perception. He could not read any word, letter or number. When engaged on color naming and object naming tasks on visual input, he remained silent and could not produce any correct response. The patient could not perform any of the classical visual tasks related to ventral (word reading, letter reading, digit reading, color naming, object naming, object discrimination) or to dorsal (fixation, saccade orienting, pointing, grasping) visual cortical pathways (see Table S2).
Engaged in a forced‐choice semantic discrimination task (artifact versus natural object), he performed at chance‐level in the visual modality (22/48 correct responses =45.8%; Z‐test = −0.58; p‐value = 0.56), contrasting with an almost perfect performance with auditory input (47/48 correct responses =97.9% vs 50%; Z‐test = 6.64; p‐value <0.0001). A chi‐square test confirmed the massive difference between these two modalities (χ 2 = 26.6; p‐value <0.00001). The semantic knowledge required to perform this task correctly was therefore fully preserved, confirming the perceptual origin of his chance‐level performance with visual input.
Structural brain MRI revealed severe bilateral and atrophic lesions of ventral and dorsal pathways in each hemisphere, contrasting with an anatomical preservation of more anterior structures including frontal lobes. Crucially, V1 cortices were partially preserved (see Fig. 1a).
Figure 1.
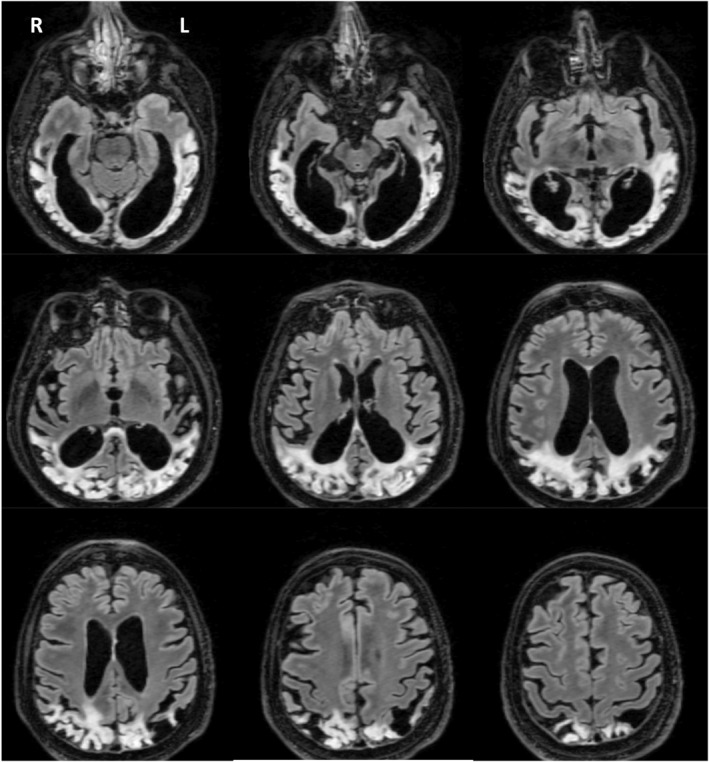
Bilateral severe structural lesions of ventral and dorsal cortical pathways with partial structural preservation of V1. Brain axial sections of FLAIR MRI reveal bilateral hyperintensities and severe brain atrophy of occipito‐temporal (ventral) and occipito‐parietal (dorsal) cortical pathways. Crucially, V1 area of each hemisphere appeared partially preserved.
This unique lesion pattern causing cortical blindness invited us to test the two seminal predictions stemming from Crick and Koch’ hypothesis: (Prediction 1) Visual stimuli should still activate V1 in this patient lacking conscious vision, and (Prediction 2) functional connectivity between V1 and PFC should be massively impaired, as compared to healthy sighted volunteers.
Electroretinographic recordings first confirmed the normality of retinal processing: P50 and N95 components evoked by visual stimulation of each eye showed normal latencies and amplitudes. 12 Recordings of visual evoked potentials (VEP) using the standardized checkerboard procedure revealed the preservation N75 and P100 VEP components that originate from V1 areas 13 , 14 , 15 (see Fig. S1). P100 latencies showed a moderate but significant slowdown: 124 and 122 ms, respectively, after right‐eye and left‐eye stimulation, versus 107 ± 3.61 ms in healthy male participants of comparable age (Z‐scores = 4.24 and 4.79 respectively; both p‐value <10−5). N75/P100 peak‐to‐peak responses showed a non‐significant decrease of amplitudes: 3.6 μV and 5.8 μV, respectively, after right‐eye and left‐eye stimulation, versus 8.75 ± 3.13 μV for healthy participants (Z‐scores = −1.64 and −0.94 respectively; both p‐value >0.1).
We then scanned the patient with fMRI in order to confirm V1 functionality with a space‐resolved technic. He was presented with images from different categories (i.e., printed words, numbers, faces, houses, and tools; see Supplementary Material for details). When contrasting all visual categories versus rest, we found an activation in the left primary visual cortex (MNI −3 −63 −22, Z = 3.78, n = 17 voxels), significant only after small volume correction with the primary visual cortex smoothed mask (p = 0.034 FWE‐correction, see Fig. 2). The reverse contrast (rest >all categories) did not reveal any significant result at the whole brain level, or restricted to the abovementioned inclusive mask. VEP and fMRI therefore confirmed Prediction 1, by demonstrating unconscious neural activity elicited by visual stimuli in patient's residual V1. Interestingly, the mismatch between normal amplitudes of N75‐P100 VEP components on the one hand (that reflect early cortical processing), and (ii) reduced amplitudes of V1 fMRI signal on the other hand, is highly suggestive of a lack of recurrent processing between V1 and extrastriate areas—that were massively lesioned in this patient—required for V1 sustained activity. 10
Figure 2.
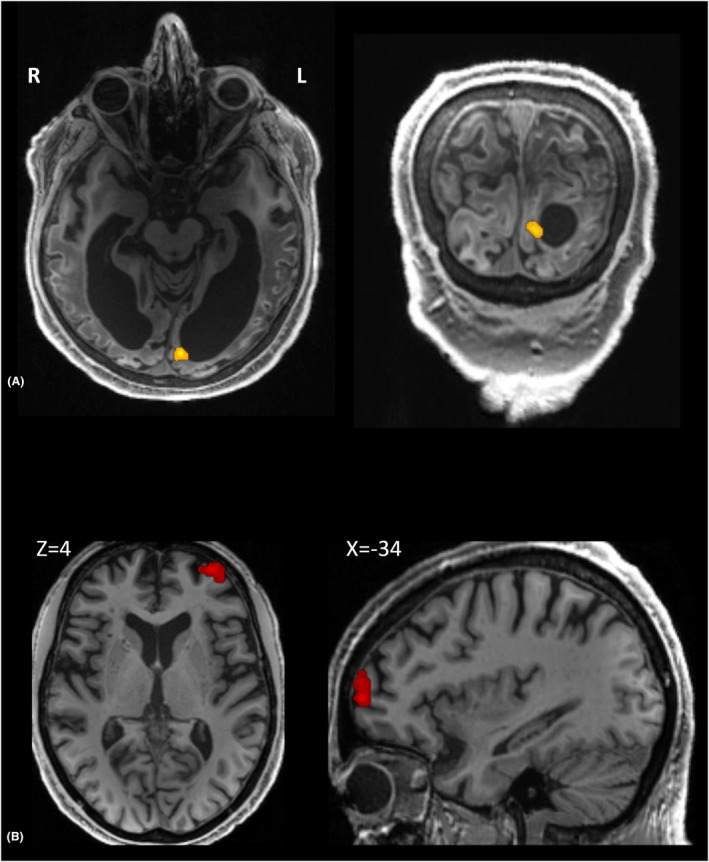
Loss of conscious vision associated with V1 activation and impaired functional connectivity with PFC. (A) Patient's left primary visual cortex (shown in native space) was activated by visual stimuli (voxel p ≤ 10−3; p = 0.034 FWE‐correction within V1 inclusive mask). (B) As compared to healthy sighted volunteers, patient's V1 area (overlaid on healthy anatomy in MNI space) showed a significant decrease of functional connectivity with a left PFC region previously identified as playing a causal role in conscious vision. 15
In order to test the V1‐PFC disconnection prediction, we then recorded resting‐state fMRI. We used as a seed fMRI time‐series averaged within V1 inclusive mask defined above and found a higher connectivity in healthy volunteers as compared to the patient in two regions: right temporal pole (MNI: 16 14 −40) and left anterior prefrontal cortex (MNI: −32 58 4, Z = 4.31; see Table 1).
Table 1.
Regions more connected to V1 in controls than in the patient during resting state.
| Region | Contrast | Peak coordinates (MNI) | Z value | Cluster size | p‐value (cluster‐level, FWE corrected) |
|---|---|---|---|---|---|
| Right temporal pole | Controls > MG | 16 4–40 | 4.84 | 425 | <0.001* |
| Left anterior prefrontal cortex | Controls > MG | −32 58 4 | 4.31 | 190 | <0.014* |
Significant cluster‐level p, with voxelwise p < 0.001 and clusterwise p < 0.05 corrected for multiple comparisons across the whole brain.
Crucially, this last PFC region has previously been reported in a lesion study as playing a putative causal role in conscious access to visual stimuli 16 (MNI −32 54 −6; peak‐distance between two studies = 11 mm). Importantly, the reverse contrast did not reveal any area that would have been more connected to V1 in the patient than in healthy volunteers.
One may wonder why this contrast did not also capture additional areas, in particular those contributing to visual ventral pathway downstream V1 (e.g., V2, V4, …) all the way to the right infero‐temporal cortex area that was found significant. In order to better explore this question, we first ran restricted analyses for controls and for the patient separately. However, both in controls and in the patient, we did not find any significant correlation between V1 seed and ventral pathway areas. We then replicated these restricted analyses using a less stringent approach: We removed from our statistical model the age covariate—which is already partially accounted for by the group regressor (the two controls groups differed in age and were acquired on two distinct 3 T MRI magnets, so this group regressor is absolutely necessary). Using this less stringent approach, we could observe the two expected results: (i) no correlation between V1 seed and other cortical areas in the patient, and (ii) in controls only an extensive ventral pathway cluster of functional connectivity with V1: This cluster was correlated with the visual cortex using the as a seed the mask defined in the patient (voxelwise p < 0.001 and clusterwise p < 0.05 corrected for multiple comparisons across the whole brain, see below a transversal view at Z = 0; see Fig. S2). Therefore, the absence of significant difference in V1 functional connectivity in controls as compared to the patient seems to stem from a lack of statistical power that could originate at different levels. The massive atrophy of these posterior ventral pathway areas in the patient may contribute to this lack of power: CSF and motion related signals probably out‐weighted neuronal signal in these areas. Indeed, the right temporal cortex region (included within right temporal pole cluster) was much less atrophied to posterior areas of ventral pathways.
To conclude, we first found that bilateral structural lesions of visual pathways preserving substantial part of V1 cortices induced a state of cortical blindness comparable to bilateral lesions of V1. We then demonstrated that visual stimuli he was not aware of were still processed in patient's V1 areas. Finally, we showed that in healthy volunteers V1 is functionally connected to PFC indirectly through ventral pathways relays, and that this indirect V1‐PFC connectivity was lost in this patient. These findings are consistent with Crick & Koch's hypothesis and suggest that we are not aware of V1 neural activity.
Author Contributions
LN conceived the study. FH and LN designed the MRI and fMRI study and analyzed MRI results. AS, EMM, NDD, SC, FB and LN contributed to behavioral and cognitive data acquisition and analyses. CM, AS and LN contributed to VEP data acquisition and analysis. LN and FH drafted and revised the manuscript.
Funding Information
This study was funded by the UNIM, ICM, Dumont Family donation, the “TOPLEX” ANR program to Lionel Naccache, and the “Fondation pour la Recherche Médicale” to Fabien Hauw.
Conflict of Interest
Fabien Hauw, Claire Meyniel, Aude Sangaré, Esteban Munoz‐Musat, Nina Di Donato, Sylvie Chokron, Lionel Naccache, and Frédérique Bozon declared no disclosures.
Checklist Statement
We used the CARE checklist when writing our report. 17
Supporting information
Appendix S1.
Acknowledgments
We thank the patient and his wife for their active cooperation to this study.
Funding Statement
This work was funded by Agence Nationale de la Recheche ; UNIM; Fondation pour la Recherche Médicale ; Institut du Cerveau et de la Moelle Epinière .
References
- 1. Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121‐123. [DOI] [PubMed] [Google Scholar]
- 2. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1‐47. [DOI] [PubMed] [Google Scholar]
- 3. Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJ, eds. Analysis of Visual Behavior. MIT Press; 1982:549‐586. [Google Scholar]
- 4. Horton JC, Hoyt WF. Quadrantic visual field defects. A hallmark of lesions in extrastriate (V2/V3) cortex. Brain. 1991;114(Pt 4):1703‐1718. [DOI] [PubMed] [Google Scholar]
- 5. Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1‐37. [DOI] [PubMed] [Google Scholar]
- 6. Lau H, Rosenthal D. Empirical support for higher‐order theories of conscious awareness. Trends Cogn Sci. 2011;15:365‐373. [DOI] [PubMed] [Google Scholar]
- 7. Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn Sci. 2004;8:457‐464. [DOI] [PubMed] [Google Scholar]
- 8. Tononi G. Integrated information theory of consciousness: an updated account. Arch Ital Biol. 2012;150:56‐90. [DOI] [PubMed] [Google Scholar]
- 9. Tononi G, Boly M, Massimini M, Koch C. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci. 2016;17:450‐461. [DOI] [PubMed] [Google Scholar]
- 10. Lamme VA. Towards a true neural stance on consciousness. Trends Cogn Sci. 2006;10:494‐501. [DOI] [PubMed] [Google Scholar]
- 11. Naccache L. Minimally conscious state or cortically mediated state? Brain. 2018;141:949‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birch DG, Anderson JL. Standardized full‐field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571‐1576. [DOI] [PubMed] [Google Scholar]
- 13. Arroyo S, Lesser RP, Poon WT, Webber WR, Gordon B. Neuronal generators of visual evoked potentials in humans: visual processing in the human cortex. Epilepsia. 1997;38:600‐610. [DOI] [PubMed] [Google Scholar]
- 14. Shigeto H, Tobimatsu S, Yamamoto T, Kobayashi T, Kato M. Visual evoked cortical magnetic responses to checkerboard pattern reversal stimulation: a study on the neural generators of N75, P100 and N145. J Neurol Sci. 1998;156:186‐194. [DOI] [PubMed] [Google Scholar]
- 15. Di Russo F, Pitzalis S, Spitoni G, et al. Identification of the neural sources of the pattern‐reversal VEP. NeuroImage. 2005;24:874‐886. [DOI] [PubMed] [Google Scholar]
- 16. Del Cul A, Dehaene S, Reyes P, Bravo E, Slachevsky A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain. 2009;132:2531‐2540. [DOI] [PubMed] [Google Scholar]
- 17. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. J. Diet. Suppl. 2013;10:381‐390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


