Abstract
OBJECTIVES:
Consensus regarding biomarkers for detection of infection-related organ dysfunction in the emergency department is lacking. We aimed to identify and validate biomarkers that could improve risk prediction for overt or incipient organ dysfunction when added to quick Sepsis-related Organ Failure Assessment (qSOFA) as a screening tool.
DESIGN:
In a large prospective multicenter cohort of adult patients presenting to the emergency department with a qSOFA score greater than or equal to 1, admission plasma levels of C-reactive protein, procalcitonin, adrenomedullin (either bioavailable adrenomedullin or midregional fragment of proadrenomedullin), proenkephalin, and dipeptidyl peptidase 3 were assessed. Least absolute shrinkage and selection operator regression was applied to assess the impact of these biomarkers alone or in combination to detect the primary endpoint of prediction of sepsis within 96 hours of admission.
SETTING:
Three tertiary emergency departments at German University Hospitals (Jena University Hospital and two sites of the Charité University Hospital, Berlin).
PATIENTS:
One thousand four hundred seventy-seven adult patients presenting with suspected organ dysfunction based on qSOFA score greater than or equal to 1.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The cohort was of moderate severity with 81% presenting with qSOFA = 1; 29.2% of these patients developed sepsis. Procalcitonin outperformed all other biomarkers regarding the primary endpoint (area under the curve for receiver operating characteristic [AUC-ROC], 0.86 [0.79–0.93]). Adding other biomarkers failed to further improve the AUC-ROC for the primary endpoint; however, they improved the model regarding several secondary endpoints, such as mortality, need for vasopressors, or dialysis. Addition of procalcitonin with a cutoff level of 0.25 ng/mL improved net (re)classification by 35.2% compared with qSOFA alone, with positive and negative predictive values of 60.7% and 88.7%, respectively.
CONCLUSIONS:
Biomarkers of infection and organ dysfunction, most notably procalcitonin, substantially improve early prediction of sepsis with added value to qSOFA alone as a simple screening tool on emergency department admission.
Keywords: adrenomedullin, biomarker, C-reactive protein, procalcitonin, proenkephalin, sepsis
KEY POINTS.
Question: Can the combined use of biomarkers and clinical scores in the emergency department (ED) improve the early identification of patients at risk for sepsis?
Findings: Biomarkers of infection and organ dysfunction, most notably procalcitonin, substantially improved early prediction of sepsis over and above quick Sepsis-related Organ Failure Assessment (qSOFA) alone.
Meanings: A combination of qSOFA and procalcitonin provides a simple screening tool to improve prediction of sepsis on ED admission. This may potentially impact upon patient management though this needs to be tested in prospective studies.
Sepsis is a potential life-threatening complication of an infectious disease. Development of acute organ dysfunction as a result of an inappropriate host response to the underlying infection is the hallmark of this syndrome (1). This remains the most frequent driver of death on ICUs worldwide (2). While infection is, in general, a commonplace and frequently self-limiting event, progression to organ dysfunction is relatively rare (3). As such, a broad range of patients with suspected or proven infection, most of which are benign, present to the emergency department (ED). Delayed identification of patients with overt or incipient organ dysfunction is a well-documented problem in the ED (4–6) and may have a significant impact on morbidity and mortality (7).
In this context, biomarkers providing objective and measurable indicators of sepsis could offer considerable utility for diagnostic, prognostic, and theranostic enrichment. This is particularly relevant in view of the large numbers of patients presenting with fever or other nonspecific signs of infection to the ED, as well as those with a noninfectious mimic that can simulate sepsis (8). Many articles have addressed various biomarkers reflecting the host response in sepsis (9), early recognition of organ dysfunction (10–12), risk stratification (13, 14), and patient management (15) including biomarker-guided antibiotic stewardship (16). However, clear recommendations regarding use of biomarkers in guidelines such as the “Surviving Sepsis Campaign” are scarce. Only the incorporation of plasma lactate levels to diagnose septic shock (1) and the use of procalcitonin to terminate anti-infective therapy in the context of antibiotic stewardship are supported by an evidence base and subsequent guideline recommendations (17). This paucity reflects the absence of adequately powered studies to assess any potential patient-centered benefit through measuring biomarkers, such as diagnosing sepsis in unselected patients presenting to an ED (18).
We thus conducted a prospective multicenter trial to prospectively assess the performance of both established (procalcitonin, C-reactive protein [CRP]) and novel (adrenomedullin—either bioavailable adrenomedullin [bio-ADM] or midregional fragment of proadrenomedullin [MR-proADM], proenkephalin, dipeptidyl peptidase 3 [DPP3]) biomarkers to improve diagnosis of incipient or overt organ dysfunction (sepsis) in patients presenting to the ED with clinical signs of suspected life-threatening infection as reflected by a quick Sepsis-related Organ Failure Assessment (qSOFA) score greater than or equal to 1 with its inherent limitation regarding sensitivity (19).
MATERIALS AND METHODS
Study Design
From December 19, 2016, to June 7, 2019, we conducted a multicenter, prospective, observational study recruiting patients presenting with suspected organ dysfunction based on a qSOFA score greater than or equal to 1 to three large tertiary care EDs in Germany with approximately 125,000 inpatients and 740,000 outpatients for Charité and 54,000 inpatients and 475,000 outpatients for Jena University Hospital, respectively. (More details regarding spectrum and features for the recruiting hospitals can be found at https://www.charite.de/en/charite/about_us/facts_figures/ and https://www.uniklinikum-jena.de/Uniklinikum+Jena/Wir+%C3%BCber+uns/Portrait.html). All locations have comparable equipment with 5–10% high dependency beds. No changes in staffing levels, organization, or number of beds occurred during the inclusion period. Protocols for managing patients with suspected sepsis were in place at all sites.
The study conducted in accordance with the Helsinki Declaration of 1975, as most recently amended (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/) and was approved by the Ethics Committee of Jena University Hospital on September 27, 2016 (approval number 4912-08/16; study title: Lebensbedrohliche Infektionen Früh Erkennen!—Schnelle Differentialdiagnose durch multivalentes innovatives Biomarkerpanel auf mobile Point-of-Care (POC) Plattform. Biomarker for Early Sepsis Diagnosis “BEdSide”). The Ethics Committee of Charité University Hospital Berlin agreed with this approval. The study was registered in the German Registry of Clinical Trials (No: DRKS00011188).
All adult patients (> 18 yr old) who visited the ED with a qSOFA score greater than or equal to 1 were eligible for potential recruitment. The qSOFA score was assessed at triage and confirmed at the time of enrollment by the study personnel. The qSOFA score was calculated based on the following three variables: systolic blood pressure less than or equal to 100 mm Hg, respiratory rate greater than or equal to 22 breaths/min, and altered mental status defined as a Glasgow Coma Scale less than 15. Exclusion criteria were pregnancy, acute trauma, stroke, myocardial infarction, and limited life expectancy (< 28 d) due to concomitant disease. In Berlin patients were recruited on alternating shifts, whereas in Jena in a daily basis from 09:00 to 17:00 hours. All participants or their legal representatives gave informed consent to the study.
Biomarker Measurements
After informed consent was obtained either from the patient or from a legal representative, 18 mL of whole blood was drawn from a peripheral vein catheter or by aseptic venipuncture. The blood was collected into EDTA, lithium heparin, and serum bottles within 12 hours of ED arrival. In Berlin, following centrifugation blood samples were aliquoted and stored initially at –20°C and, within 72 hours, at –80°C. Aliquots were transported at weekly intervals on dry ice to the Integrated Biobank at Jena. In Jena, blood samples were immediately transferred to this biobank where they were centrifuged, aliquoted, and stored at –80°C.
Procalcitonin and MR-proADM were measured in EDTA plasma using commercially available automated immunofluorescent assays (procalcitonin sensitive KRYPTOR and MR-proADM KRYPTOR [BRAHMS GmbH, Hennigsdorf, Germany]). The 95th percentile reported in healthy individuals is 0.064 µg/L for procalcitonin, and the 97.5th percentile is 0.55 nmol/L for MR-proADM.
Proenkephalin A 119–159 was measured in EDTA plasma samples using the immunoluminometric Sphingotest assay (SphingoTec GmbH, Hennigsdorf, Germany) (20). The 97.5th percentile in healthy adult subjects is 89 pmol/L. The upper normal range (89 pmol/L) is also the clinical cutoff for diagnosis of acute kidney injury.
Bio-ADM 1–52 was measured in EDTA plasma samples using the immunoluminometric Sphingotest assay (SphingoTec GmbH) (21). The 97.5th percentile in healthy adult subjects is 29 pg/mL (90% CI, 27–38 pg/mL) while the clinical cutoff for patients with sepsis and septic shock is 70 pg/mL (22, 23).
Circulating DPP3 was measured in EDTA plasma samples by 4TEEN4 Pharmaceuticals (Hennigsdorf, Germany) using the immunoluminometric Sphingotest DPP3 assay (SphingoTec GmbH) (24). The 97.5th percentile for Sphingotest DPP3 in healthy adult subjects is 22 ng/mL (90% CI, 18–34 ng/mL) while the clinical cutoff for critically ill patients is 40 ng/mL (25, 26).
For biomarker measurements, samples were blinded regarding clinical and demographic patient data.
Data Collection
For each enrolled patient all relevant secondary data were extracted, reviewed, and recorded from the hospital database for 4 calendar days starting from the time of ED presentation. These included demographic data, comorbidities summarized as Charlson Comorbidity Index (27), laboratory findings, diagnostic tests, ventilation settings, administration of sepsis-relevant medication (e.g. antibiotics, catecholamines), fluid management, dialysis parameters/urine output, and time of admission or transfer. Death or survival was assessed up to 28 days. In case of earlier hospital discharge, patients received a follow-up call from study personnel as agreed on enrollment.
Definitions and Endpoints
The primary endpoint was sepsis onset within 96 hours. Sepsis was defined as an acute infection-related change in the Sepsis-related Organ Failure Assessment (SOFA) score greater than or equal to 2 points in accordance with the Sepsis-3 criteria (1). The SOFA score was calculated daily and compared against the baseline SOFA score of the patient. Where no prior values were provided, the entry SOFA score of that patient was considered “0.” Regarding the respiratory SOFA, imputation with oxygen saturation/Fio2 ratios was performed according to a conversion table (eTable 1, http://links.lww.com/CCM/H496). After finalizing data input, a diagnosis of sepsis was verified by a panel of experts blinded to the laboratory data. The panel consisted of four board-certified ED physicians. In case of disagreements, two further experts in emergency medicine and intensive care medicine were called in. Overall, only few cases needed the backup panel, reflecting a team well experienced in the conduct of this type of studies.
Secondary objectives were septic shock within 96 hours, hospital and 28-day all-cause mortality, ICU admission and length of stay, length of hospital stay, and organ dysfunction, that is, need for vasopressors, mechanical ventilation, or acute kidney failure, as reflected by increased creatinine, oliguria, and need for renal replacement therapy. Septic shock was defined as persisting hypotension requiring catecholamines to maintain a mean arterial pressure greater than or equal to 65 mm Hg plus hyperlactatemia greater than 20 mg/dL (2.2 mmol/L) despite adequate fluid administration.
Statistical Analysis
We expected to analyze 5–12 biomarkers to discriminate 10–30% septic patients from nonseptic patients. To detect small effects with 80% power a sample size of 1500 patients was deemed sufficient (28). Cohen’s d effect size varied between 0.21 (five biomarkers, 30% septic patients) and 0.34 (12 biomarkers, 10% septic patients) depending on the number of biomarkers and sepsis prevalence. The significance level was set at 5% taking the Bonferroni correction into account.
Receiver operating characteristic (ROC) analysis was performed with five different biomarkers to predict the primary endpoint (sepsis within 96 hours). The area under the curve (AUC) was generated for each biomarker with 95% CIs. AUC of the biomarkers was compared pairwise by the DeLong test; a significance level was set at 0.005 for each test to account for multiple comparisons (Bonferroni correction).
Least absolute shrinkage and selection operator (LASSO) regression for variable selection was used to find the combination of biomarkers with the highest discrimination of septic and nonseptic patients (29) (Methods glossary, http://links.lww.com/CCM/H496). The LASSO regression model with logit link function was fitted for the primary endpoint including all biomarkers as independent variables. Logarithmic values of the biomarkers were used since the data were not normally distributed. Ten-fold cross validation was applied to optimize the shrinkage parameter λ in the LASSO regression. AUCs with 95% CIs are reported for the model after cross-validation. Sensitivity, specificity, and positive and negative predictive values (PPV and NPV) are provided with 95% CI for the cutoff corresponding to the highest Youden index using the bootstrap method. Model calibration was assessed by plotting deciles of the predicted probabilities in comparison to the observed probabilities. Goodness-of-fit was evaluated by the Brier score (BS) with values ranging between 0 (perfect accuracy) and 1 (noninformative).
The biomarkers selected by the LASSO regression were analyzed together with additional predictors (including comorbid conditions reflected in the Charlson Comorbidity Index, body temperature, respiratory rate, heart rate, arterial blood pressure, Pao2/Fio2 ratio, cardiac insufficiency, peripheral artery disease, cerebrovascular disease, chronic lung disease, connective tissue disease, liver disease, renal disease, and diabetes mellitus) in a binary logistic regression model to predict the primary endpoint. The AUC with 95% CIs are reported for the model after cross-validation with diagnostic measures (sensitivity, specificity, PPV, NPV) are provided with 95% CIs.
The same analyses (DeLong test, LASSO regression with all biomarkers, logistic regression with additional predictors) were performed for all binary secondary endpoints (e.g., septic shock, 28-d mortality). A linear LASSO regression model was applied for the continuous secondary endpoints (length of ICU stay, length of hospital stay), with the coefficient of determination reported to assess the model fit.
Continuous baseline parameters were summarized by median and 25th–75th percentiles. Absolute and relative frequencies are provided for categorical baseline variables. Statistical analysis was performed with SAS 9.4 (SAS Institute, Cary, NC) and R 4.1.0 (The R Foundation, Vienna, Austria).
RESULTS
Demographics and Frequency of Organ Dysfunctions
A total of 1477 patients were enrolled with a near-equal split between Jena (n = 736) and Berlin (n = 741). Sepsis status over 96 hours could be determined for 1426 patients. The trial flow chart is summarized in Figure 1 and demographics displayed in Table 1. Average age was 68 ± 16 years with 56% male. The cohort was of overall moderate disease severity with 81% presenting with qSOFA 1 and 19% with qSOFA 2–3. Hospital admission was required in 83.1% of patients, of whom 27.8% were admitted to intensive care.
Figure 1.
Patient flow chart.
TABLE 1.
Patient Characteristics at Admission
| Patient Characteristics | Patients With Sepsis (n = 417) | Patients With No Sepsis (n = 1009) | Total (n = 1426) |
|---|---|---|---|
| Age (yr) | 74 (63–80) | 71 (59–80) | 72 (61–80) |
| Female (%) | 175 (42.0) | 452 (44.8) | 627 (44.0) |
| Quick SOFA | |||
| 1 | 278 (66.7) | 876 (86.8) | 1154 (80.9) |
| 2 | 124 (29.7) | 129 (12.8) | 253 (17.7) |
| 3 points | 13 (3.1) | 3 (0.3) | 16 (1.1) |
| SOFA | 7 (6–8) | 5 (4–6) | 6 (5–7) |
| Charlson Comorbidity Index | 2 (1–3) | 1 (0–2) | 1 (0–3) |
| Body temperature (°C) | 37.9 (37.1–38.5) | 37.1 (36.7–37.7) | 37.3 (36.8–38) |
| Heart rate (beats/min) | 96 (82–110) | 89 (76–104) | 90 (77–107) |
| Respiratory rate (breaths/min) | 25 (22–28) | 23 (22–26) | 24 (22–27) |
| Mean arterial blood pressure (mm Hg) | 77 (65–93) | 88 (74–103) | 86 (71–100) |
| Pao2/Fio2 ratio | 310 (247–409) | 376 (307–457) | 348 (286–457) |
| Glasgow Coma Scale | 15 (15–15) | 15 (15–15) | 15 (15–15) |
| pH | 7.4 (7.35–7.45) | 7.4 (7.36–7.43) | 7.4 (7.36–7.44) |
| Standard base excess (mmol/L) | –0.1 (–3.1 to 2.5) | 1.1 (–1.1 to 3.2) | 0.8 (–1.7 to 3.0) |
| Creatinine (mg/dL) | 1.3 (1.0–2.1) | 0.9 (0.8–1.3) | 1.0 (0.8–1.5) |
| Bilirubin (µmol/L) | 0.8 (0.5–1.3) | 0.5 (0.4–0.8) | 0.6 (0.4–1.1) |
| Lactate (mmol/L) | 2.0 (1.4–2.9) | 1.6 (1.2–2.2) | 1.7 (1.2–2.4) |
| C-reactive protein (mg/L) | 128.5 (57–237) | 15.9 (3.8–57.5) | 34.5 (7.2–106.4) |
| Leukocytes (Gpt/L) | 12.5 (8.5–17.2) | 9.5 (7.3–13.0) | 10.3 (7.5–14.0) |
| Thrombocytes (Gpt/L) | 211 (148–286) | 242 (193–309) | 231 (182–303) |
| Biomarker | |||
| Mid-regional fragment of proadrenomedullin (nmol/L) | 2.1 (1.4–3.8) | 1.0 (0.7–1.6) | 1.2 (0.8–2.1) |
| Procalcitonin (µg/L) | 1.2 (0.3–6.0) | 0.1 (0.1–0.2) | 0.1 (0.1–0.6) |
| Bioavailable adrenomedullin (pg/mL) | 75.3 (43.2–131.6) | 37.4 (24.9–61.9) | 44.0 (28.4–79.4) |
| Proenkephalin (pmol/L) | 80.3 (48.7–136.4) | 60.9 (42.5–88.4) | 64.3 (44.4–99.4) |
| Dipeptidyl peptidase 3 (ng/mL) | 20.4 (13.2–38.9) | 17.4 (12.1–28.0) | 18.3 (12.3–31.2) |
SOFA = Sepsis-related Organ Failure Assessment.
For continuous variables, median (25th–75th percentiles) and for categorical variables absolute and relative frequencies are reported.
Of the 1426 patients, 417 (29.2%) developed organ dysfunction due to infection, thus fulfilling the criteria for a sepsis diagnosis (the primary endpoint), of whom 14.6% progressed to septic shock. Requirement for mechanical ventilation (9.2%) and renal replacement therapy (2.9%) was low, thus reflecting a cohort with a low pre-test probability as would be expected in allcomers presenting to an ED.
Performance of Biomarkers to Predict the Primary Endpoint
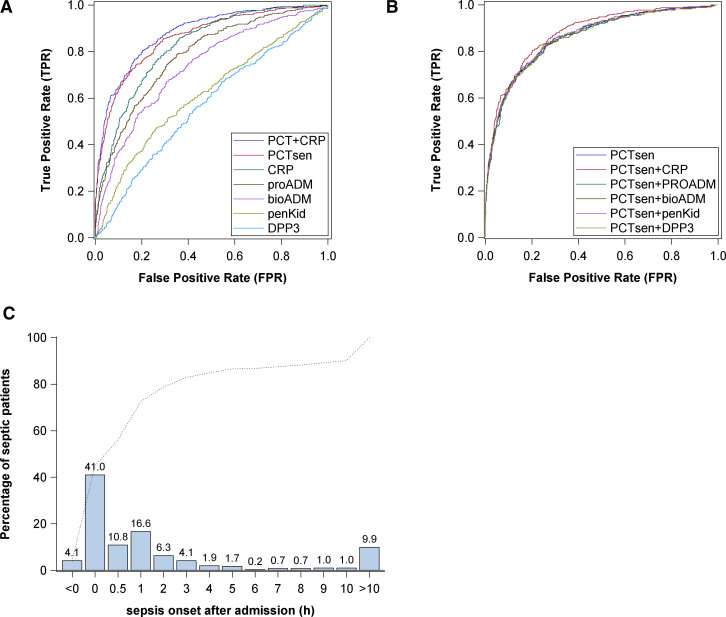
The area under the curve for receiver operating characteristic (AUC-ROC) for achieving the primary endpoint—sepsis within 96 hours of admission—was best for procalcitonin (AUC, 0.86 [95% CI, 0.79–0.93]), followed by CRP (Fig. 2A). Discrimination of the model with procalcitonin as predictor was also significantly higher than the model with qSOFA (AUCqSOFA = 0.60 [95% CI, 0.57–0.63]; p < 0.001). The AUC-ROC for procalcitonin to predict infection without organ dysfunction was comparable (0.843; 95% CI, 0.812–0.874), suggesting procalcitonin as a biomarker of infection. The low BS of the model (BS = 0.13) indicates that the model fitted the data well. eFigure 1 (http://links.lww.com/CCM/H496) shows only small deviations of predicted and observed probabilities, thus confirming good calibration of the model. Additional analyses considering only patients admitted to ICU (AUC, 0.85 [95% CI, 0.81–0.90]) and patients not admitted to hospital (AUC, 0.95 [95% CI, 0.92–0.99]) confirms the result of the primary analysis.
Figure 2.
Performance of biomarkers to predict the primary endpoint and time of sepsis onset after admission. A, Receiver operating characteristic (ROC) analysis—area under the curve (AUC) of biomarkers for the primary endpoint sepsis within 96 hr. B, ROC analysis—AUC of biomarkers combined with procalcitonin (PCT) for the primary endpoint sepsis within 96 hr. C, Time to onset of sepsis within the observation period of 96 hr. bioADM = bioavailable adrenomedullin, CRP = C-reactive protein, DPP3 = dipeptidyl peptidase 3, PCTsen = procalcitonin as measured using the sensitive Kryptor method, penKid = proenkephalin, proADM = proadrenomedullin.
The added value of a composite biomarker, combining several of the biomarkers, did not, in general, improve the classification compared with procalcitonin. Only a slight improvement could be achieved by combining procalcitonin with CRP (Fig. 2B).
A procalcitonin value with a cutoff of 0.25 µg/L substantially improved net reclassification compared with qSOFA alone (Table 2). The Net Reclassification Improvement (NRI) describes the improvement in model performance by using procalcitonin compared with qSOFA as a predictor. The NRI indicates how much more frequently appropriate reclassification (e.g., 197 cases of sepsis with procalcitonin ≥ 0.25 and qSOFA = 1) occurs than inappropriate reclassification (e.g., 24 cases of sepsis with procalcitonin < 0.25 and qSOFA ≥ 2) when using procalcitonin instead of qSOFA. This resulted in a sensitivity of 75.3% (95% CI, 70.8–79.4%), specificity of 80.0% (95% CI, 77.3–82.4%), a PPV of 60.7% (95% CI, 56.3–65.0%), and a NPV of 88.7% (95% CI, 86.4–90.8%).
TABLE 2.
Net Reclassification Improvement by Adding Procalcitonin
| Model Without Procalcitonin | Model With Procalcitonin at a Cutoff Value of 0.25 µg/L | Sum | |
|---|---|---|---|
| Risk Category 1, Procalcitonin < 0.25 | Risk Category 2, Procalcitonin ≥ 0.25 | ||
| Patients with sepsis | |||
| Risk category 1, qSOFA = 1 | 78 | 197 | 275 |
| Risk category 2, qSOFA ≥ 2 | 24 | 112 | 136 |
| Sum | 102 | 309 | 411 |
| Patients without sepsis | |||
| Risk category 1, qSOFA = 1 | 708 | 163 | 871 |
| Risk category 2, qSOFA ≥ 2 | 94 | 37 | 131 |
| Sum | 802 | 200 | 1002 |
| Net reclassification improvement | 35.2% | ||
qSOFA = quick Sepsis-related Organ Failure Assessment.
Applying a procalcitonin cutoff of 0.5 µg/L gave a lower sensitivity (64.2%; 95% CI, 59.3–68.8%) and lower NPV (85.9%; 95% CI, 83.6–88.0%), but higher specificity (89.6%; 95% CI, 87.6–91.5%) and higher PPV (71.8%; 66.9–76.4%).
Regarding the timepoint for development of sepsis, 45% fulfilled the Sepsis-3 criteria at admission to the ED. Only 10% developed organ dysfunction 10–96 hours after admission (Fig. 2C).
There were 196 patients with infection and no organ dysfunction, and procalcitonin discriminated well between septic and nonseptic patients in the population of patients without organ dysfunction (AUC, 0.84; 95% CI, 0.81–0.88; p < 0.001). On the other hand, there were 120 patients with organ dysfunction and without infection, and the discrimination with procalcitonin as a predictor of organ dysfunction was only moderate in the population of nonseptic patients (AUC, 0.66; 95% CI, 0.61–0.71; p < 0.001).
Performance of Biomarkers to Predict Secondary Endpoints
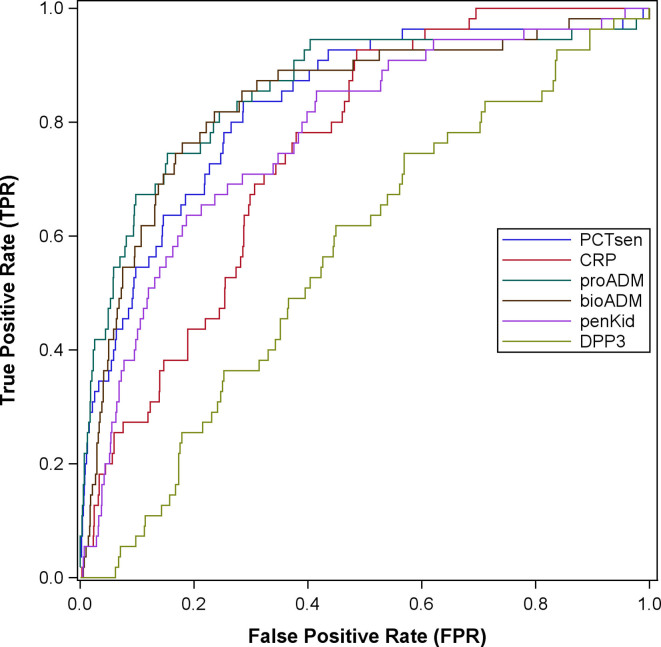
Regarding the secondary endpoint of septic shock, MR pro-ADM and procalcitonin were able to identify cardiovascular insufficiency associated with sepsis (Fig. 3). The AUC for predicting septic shock using the combination of these two biomarkers was 0.86 (95% CI, 0.67–0.93), that is, similar to the results obtained for the primary endpoint (sepsis within 96 hr of admission). Model calibration is shown in eFigure 2 (http://links.lww.com/CCM/H496); prediction accuracy was high (BS = 0.03). Additional analyses confirmed good discrimination between septic and nonseptic patients in the subgroup of patients admitted to ICU (AUC, 0.77 [95% CI, 0.70–0.84]) and the subgroup of patients not admitted to hospital (AUC, 0.95 [95% CI, 0.92–0.98]). An additional analysis for immunocompromised patients revealed similar results (eFig. 3, http://links.lww.com/CCM/H496). The only biomarker remaining in the LASSO regression model for hospital mortality was MR pro-ADM with an AUC of 0.75 (95% CI, 0.56–0.93) and accurate predictions (BS = 0.05); calibration of the model is shown in eFigure 4 (http://links.lww.com/CCM/H496). eFigure 5 (http://links.lww.com/CCM/H496) illustrates the AUC of procalcitonin and/or lactate to qSOFA for the primary endpoint of sepsis within 96 hours. Table 3 summarizes all analyses of secondary endpoints by LASSO regression regarding the various biomarkers.
Figure 3.
Receiver operating characteristic analysis— area under the curve of biomarkers for the secondary endpoint septic shock within 96 hr. bioADM = bioavailable adrenomedullin, CRP = C-reactive protein, DPP3 = dipeptidyl peptidase 3, PCTsen = procalcitonin as measured using the sensitive Kryptor method, penKid = proenkephalin, proADM = proadrenomedullin.
TABLE 3.
Area Under the Curve of Biomarker Combinations Selected by Least Absolute Shrinkage and Selection Operator Regression for Primary and Secondary Endpoints
| Primary Endpoint | Biomarkersa | Area Under the Curve (95% CI) |
|---|---|---|
| Sepsis within 96 hr | Procalcitonin | 0.86 (0.79–0.93) |
| Secondary Endpoints | Biomarkersa | Area Under the Curve (95% CI) |
|---|---|---|
| Septic shock within 96 hr | Procalcitonin, proADM | 0.86 (0.67–0.93) |
| 28-d mortality | proADM, bioADM, dipeptidyl peptidase 3 | 0.74 (0.57–0.91) |
| Hospital mortality | proADM | 0.75 (0.56–0.93) |
| ICU admission | proADM, bioADM | 0.68 (0.57–0.79) |
| Vasopressors | Procalcitonin, proADM | 0.77 (0.62–0.93) |
| Ventilation | Procalcitonin, proADM, bioADM | 0.66 (0.50–0.82) |
| Dialysis | penKid | 0.82 (0.59–1.00) |
| Acute kidney failure | proADM, penKid | 0.88 (0.80–0.95) |
| Oliguria | proADM | 0.84 (0.68–1.00) |
| Length of hospital stay | proADM, bioADM | 0.02b |
| Length of ICU stay | Procalcitonin, proADM, bioADM | 0.07b |
bioADM = bioavailable adrenomedullin, penKid = proenkephalin, proADM = proadrenomedullin.
Predictive biomarkers after variable selection in least absolute shrinkage and selection operator (LASSO) regression.
Coefficient of determination in the linear LASSO regression model.
DISCUSSION
In this prospective, multicenter, observational study enrolling 1477 patients, biomarkers of inflammation, most notably procalcitonin, were able to substantially improve early identification of incipient organ dysfunction within the first 96 hours after presentation to the ED. These added value to qSOFA as a simple screening tool for early identification of life-threatening complications of infection.
The studied biomarkers were selected as they complementarily reflect injury to typically affected organs, that is, kidneys (proenkephalin A) and cardiovascular system (adrenomedullin), or pathophysiologic mechanisms such as endothelial dysfunction (adrenomedullin), or cell death (DPP3). While the qSOFA score is no longer recommended by the Surviving Sepsis Campaign as a screening tool for sepsis (17), our data lend support to the notion that addition of procalcitonin with a cutoff of 0.25 µg/L can substantially improve diagnostic accuracy. The combined metric of procalcitonin-supported qSOFA outperformed the lactate-enhanced qSOFA metric proposed by Liu et al (30) (eFig. 5, http://links.lww.com/CCM/H496). This combination of qSOFA and procalcitonin could potentially support decision making in the ED, assuming that delayed identification of organ dysfunction in infected patients may negatively affect outcomes. It should be acknowledged that PPV was moderate at 60.7% (95% CI, 56.3–65.0%) though NPV was high at 88.7% (95% CI, 86.4–90.8%).
Of note, addition of other biomarkers to procalcitonin-supported qSOFA, that were selected to reflect complementary facets of injury or organ involvement, did not further improve sepsis prediction using Sepsis-3 criteria as the primary endpoint. These biomarkers included adrenomedullin (either bio-ADM or MR-proADM), proenkephalin, and DPP3. Adding CRP slightly improved the classification over the use of procalcitonin alone.
Adrenomedullin (a protein with 52 amino acids) its precursor pre-proadrenomedullin (consisting of 185 amino acids), and its cleavage products are found in a broad range of tissues (31). As adrenomedullin and its variants have vasodilatory properties, they have been suggested as biomarkers for severity, for example, development of shock and outcome of sepsis (32–35). Consistent with these findings, LASSO regression confirmed an added value for both adrenomedullin variants on secondary endpoints associated with disease severity, that is, length of stay, development of shock, and 28-day mortality. Both cleavage products performed similarly while simultaneous use of both adrenomedullin variants in a combined biomarker was not intended.
Kidney injury has a strong association with mortality in sepsis, with an approximate doubling of mortality rate if dialysis is required (36). Proenkephalin reflects levels of the hormone enkephalin, and thus glomerular filtration rate, that is, it acts a surrogate for kidney dysfunction in the dysregulated host response defining sepsis. LASSO regression identified this biomarker alone could predict eventual severe kidney dysfunction but not development of sepsis in general.
DPP3 is a member of the S9B family of the serine proteases. On release into the bloodstream it can inactivate peptide hormones such as angiotensin II with ensuing hemodynamic instability (37). DPP3 levels were only associated with mortality but neither with development of shock nor sepsis within 96 hours of admission.
These data regarding our secondary endpoints might explain why—against the frequently assumed superiority of multiplexed biomarker combinations over single markers (38)—in our group of ED patients with moderate pre-test probability for sepsis, the ROC for procalcitonin alone was not improved by adding further markers of disease severity (such as adrenomedullin and DPP3) or specific organ dysfunctions (such as proenkephalin). Procalcitonin apparently has a favorable profile to indicate (incipient) sepsis out of the chosen biomarkers of infection, albeit there is a broad range of more than 200 further suggested biomarkers to indicate the host response to infection (39). Noteworthy, a combined metric of qSOFA and procalcitonin with a cutoff of 0.25 µg/L performed superior to the “lactate-enhanced qSOFA” (eFig. 5, http://links.lww.com/CCM/H496) as a screening tool (30, 40), albeit still with a moderate PPV of 60.7% (95% CI, 56.3–65.0%) and a NPV of 88.7% (95% CI, 86.4–90.8%).
The role of procalcitonin to rule out life-threatening infection and either not start or de-escalate antibiotic treatment has been explored in several important studies and meta-analyses (e.g., [41, 42]). By contrast, our current study was designed to address the role of various biomarkers to rule in sepsis in allcomers presenting to the ED with suspected infection where clinical symptoms of organ dysfunction, as reflected by the qSOFA score, were used to identify a subgroup of patients at increased risk of sepsis. Among the chosen biomarkers, procalcitonin was the best biomarker in enhancing the qSOFA score as a triage tool, albeit with relatively low PPV but high NPV. Adding further biomarkers in a combined metric, however, failed to improve performance further regarding the primary endpoint.
Some limitations of our study have to be addressed. Our study design failed to address performance of the bedside clinician in their estimation of the primary outcome. As biomarkers are increasingly available as point-of-care (POC) assays, prospective assessment of the added value of procalcitonin-enhanced qSOFA over clinical assessment alone appears warranted in light of the urgency of triage decisions that are often required in these patients and before formal laboratory test results are obtained. An additional limitation may relate to center effects resulting from inclusion of patients who presented during the daytime or during alternate shifts, as well as the evaluation occurring in two large urban teaching hospitals. Finally, new nosocomial infections ± sepsis may have developed between the time of presentation and the 96-hour timepoint used for the primary endpoint.
CONCLUSIONS
Biomarkers of inflammation and organ dysfunction taken at ED presentation, most notably procalcitonin, improve the early prediction of sepsis and provide added value over qSOFA alone. Their performance to inform triage decision warrants further evaluation, preferentially by applying POC testing.
ACKNOWLEDGMENTS
We thank the staff of the Emergency Department of the Charité University Hospital in Berlin at both sites and of Jena University Hospital. Also, we thank to study nurses Sandra Signert and Carine Werk-Wenzel, to Thomas Fricke, Margarita Suitchmezian, Cora Richert, and Kay Stötzer for their technical support, and to all students who contributed to recruitment and data acquisition in Berlin.
Supplementary Material
Footnotes
Drs. Bauer, Winning, Möckel, Slagman, Reinhart, Kiehntopf, and Stacke were responsible for the conception and the design of the study. Drs. Bauer und Bolanaki drafted the article. Dr. Bolanaki was responsible for data acquisition in Berlin, and mainly done in Jena by Drs. Kiehntopf, Stacke, and Neumann. Dr. Lehmann analyzed and interpreted the data. Dr. Kiehntopf was responsible for sample biobanking and distribution of samples to cooperation partners. All authors read and approved the final article for publication.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
This study was funded by the Federal Ministry of Education and Research (Grant No. 03ZZ0810B) within the INFECTCONTROL framework.
Drs. Bolanaki’s, Winning’s, Slagman’s, Kiehntopf’s, Stacke’s, and Möckel’s institutions received funding from the Federal Ministry of Education and Research (Germany) (Bundesministerium für Bildung und Forschung [BMBF]). Dr. Slagman reports research funds from the German Research Council (Deutsche Forschungsgemeinschaft), the Innovation Fonds (Gemeinsamer Bundesausschuss), Roche Diagnostics, and ThermoFisher BRAHMS. Dr. Bolanaki reports funds from the Roche Diagnostics and ThermoFisher BRAHMS. Dr. Reinhart reported research grants from the Innovation Fonds (Gemeinsamer Bundesausschuss) and holds shares of the InflaRx NV, Jena, Germany. Dr. Bauer participated in advisory boards of BRAHMS/ThermoFisher and Roche Diagnostics regarding the use of biomarkers to diagnose sepsis. Dr. Winning disclosed he is an employee of the Jena University Hospital (JUH) and at the Ernst-Abbe-Hochschule in Jena. Drs. Kiehntopf’s and Bauer’s institutions received funding from the BMBF (Grant No. 03ZZ0810B). Dr. Kiehntopf disclosed they are inventor of a patent covering a method for quantification of C-terminal peptides of α1-antitrypsin (applicant: JUH; EP22154836.5; status: application), and the inventor of other patents covering C-terminal AAT peptides in inflammation (applicant: JUH: Method for determining the origin of an infection; EP17719610.2 [application]; EP3239712 [granted]) and Diagnosis of Sepsis and Systemic Inflammatory Response Syndrome (CN104204808B, EP2592421, EP2780719, US10712350B2, JP6308946B2 [all granted]). Dr. Möckel’s institution received funding from Roche Molecular Diagnostics German Innovation Funds; he received funding from ThermoFisher BRAHMS GmbH, AstraZeneca, Abbott, Medtronic, Radiometer, and Sanofi; and he disclosed he is Editor-in-Chief of Biomarkers. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Bolanaki, Winning, Möckel, and Bauer shared authorships.
The study was performed in accordance with the Declaration of Helsinki and has been approved by ethical committees of both participating clinics (Jena University Hospital and Charité Hospital Berlin) and was registered under DRKS00011188 accordingly to the German Registry of clinical trials. All patients participating in the study or their legal representatives have given informed consent to participate in the study.
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
The trial was registered in the German Registry for Clinical Trials (DRKS00011188) on October 20, 2016; the first patient was enrolled on December 19, 2016.
*See also p. 979.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer M, Gerlach H, Vogelmann T, et al. : Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—results from a systematic review and meta-analysis. Crit Care. 2020; 24:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer M, Inada-Kim M, Shankar-Hari M: Sepsis hysteria: Excess hype and unrealistic expectations. Lancet. 2019; 394:1513–1514 [DOI] [PubMed] [Google Scholar]
- 4.Morr M, Lukasz A, Rubig E, et al. : Sepsis recognition in the emergency department—impact on quality of care and outcome? BMC Emerg Med. 2017; 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husabo G, Nilsen RM, Flaatten H, et al. : Early diagnosis of sepsis in emergency departments, time to treatment, and association with mortality: An observational study. PLoS One. 2020; 15:e0227652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Conte P, Thibergien S, Obellianne JB, et al. : Recognition and treatment of severe sepsis in the emergency department: Retrospective study in two French teaching hospitals. BMC Emerg Med. 2017; 17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy MM, Dellinger RP, Townsend SR, et al. ; Surviving Sepsis Campaign: The surviving sepsis campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010; 38:367–374 [DOI] [PubMed] [Google Scholar]
- 8.Barichello T, Generoso JS, Singer M, et al. : Biomarkers for sepsis: More than just fever and leukocytosis—a narrative review. Crit Care. 2022; 26:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parlato M, Cavaillon JM: Host response biomarkers in the diagnosis of sepsis: A general overview. Methods Mol Biol. 2015; 1237:149–211 [DOI] [PubMed] [Google Scholar]
- 10.Marino R, Struck J, Hartmann O, et al. : Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol. 2015; 28:717–724 [DOI] [PubMed] [Google Scholar]
- 11.Schaalan M, Mohamed W: Predictive ability of circulating osteoprotegerin as a novel biomarker for early detection of acute kidney injury induced by sepsis. Eur Cytokine Netw. 2017; 28:52–62 [DOI] [PubMed] [Google Scholar]
- 12.Liu XW, Ma T, Cai Q, et al. : Elevation of serum PARK7 and IL-8 levels is associated with acute lung injury in patients with severe sepsis/septic shock. J Intensive Care Med. 2019; 34:662–668 [DOI] [PubMed] [Google Scholar]
- 13.Ploder M, Kurz K, Spittler A, et al. : Early increase of plasma homocysteine in sepsis patients with poor outcome. Mol Med. 2010; 16:498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Liang S, Geng J, et al. : Development of a nomogram to predict 30-day mortality of patients with sepsis-associated encephalopathy: A retrospective cohort study. J Intensive Care. 2020; 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C, Murphy MV, Li L, et al. ; Centers for Disease Control and Prevention Prevention Epicenters Program: Lactate testing in suspected sepsis: Trends and predictors of failure to measure levels. Crit Care Med. 2015; 43:1669–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyriazopoulou E, Giamarellos-Bourboulis EJ: Antimicrobial stewardship using biomarkers: Accumulating evidence for the critically ill. Antibiotics (Basel). 2022; 11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021; 47:1181–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierrakos C, Velissaris D, Bisdorff M, et al. : Biomarkers of sepsis: Time for a reappraisal. Crit Care. 2020; 24:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand V, Zhang Z, Kadri SS, et al. ; CDC Prevention Epicenters Program: Epidemiology of quick sequential organ failure assessment criteria in undifferentiated patients and association with suspected infection and sepsis. Chest. 2019; 156:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donato LJ, Meeusen JW, Lieske JC, et al. : Analytical performance of an immunoassay to measure proenkephalin. Clin Biochem. 2018; 58:72–77 [DOI] [PubMed] [Google Scholar]
- 21.Weber J, Sachse J, Bergmann S, et al. : Sandwich immunoassay for bioactive plasma adrenomedullin. J Appl Lab Med. 2017; 2:222–233 [DOI] [PubMed] [Google Scholar]
- 22.Marino R, Struck J, Maisel AS, et al. : Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit Care. 2014; 18:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mebazaa A, Geven C, Hollinger A, et al. ; AdrenOSS-1 study investigators: Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: The prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) study. Crit Care. 2018; 22:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehfeld L, Funk E, Jha S, et al. : Novel methods for the quantification of dipeptidyl peptidase 3 (DPP3) concentration and activity in human blood samples. J Appl Lab Med. 2019; 3:943–953 [DOI] [PubMed] [Google Scholar]
- 25.Blet A, Deniau B, Santos K, et al. ; AdrenOSS-1 Study Investigators: Monitoring circulating dipeptidyl peptidase 3 (DPP3) predicts improvement of organ failure and survival in sepsis: A prospective observational multinational study. Crit Care. 2021; 25:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deniau B, Picod A, Van Lier D, et al. : High plasma dipeptidyl peptidase 3 levels are associated with mortality and organ failure in shock: Results from the international, prospective and observational FROG-ICU cohort. Br J Anaesth. 2022; 128:e54–e57 [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 28.Cohen J: Statistical Power Analysis for the Behavioural Sciences. Second Edition. New York, NY, Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 29.Tibshirani R: Regression shrinkage and selection via the LASSO. J R Stat Soc. 1996; 58:267–288 [Google Scholar]
- 30.Liu S, He C, He W, et al. : Lactate-enhanced-qSOFA (LqSOFA) score is superior to the other four rapid scoring tools in predicting in-hospital mortality rate of the sepsis patients. Ann Transl Med. 2020; 8:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao YN, Kitamura K, Ito K, et al. : Glycine-extended adrenomedullin exerts vasodilator effect through amidation in the rat aorta. Regul Pept. 2003; 113:109–114 [DOI] [PubMed] [Google Scholar]
- 32.De la Torre-Prados MV, Garcia-de la Torre A, Enguix A, et al. : Mid-regional pro-adrenomedullin as prognostic biomarker in septic shock. Minerva Anestesiol. 2016; 82:760–766 [PubMed] [Google Scholar]
- 33.Önal U, Valenzuela-Sánchez F, Vandana KE, et al. : Mid-regional pro-adrenomedullin (MR-proADM) as a biomarker for sepsis and septic shock: Narrative review. Healthcare (Basel). 2018; 6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeed K, Wilson DC, Bloos F, et al. : The early identification of disease progression in patients with suspected infection presenting to the emergency department: A multi-centre derivation and validation study. Crit Care. 2019; 23:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elke G, Bloos F, Wilson DC, et al. ; SepNet Critical Care Trials Group: The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis—a secondary analysis of a large randomised controlled trial. Crit Care. 2018; 22:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oppert M, Engel C, Brunkhorst FM, et al. ; German Competence Network Sepsis (Sepnet): Acute renal failure in patients with severe sepsis and septic shock—a significant independent risk factor for mortality: Results from the German Prevalence Study. Nephrol Dial Transplant. 2008; 23:904–909 [DOI] [PubMed] [Google Scholar]
- 37.Deniau B, Rehfeld L, Santos K, et al. : Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: Dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur J Heart Fail. 2020; 22:290–299 [DOI] [PubMed] [Google Scholar]
- 38.Charles PE, Gibot S: Predicting outcome in patients with sepsis: New biomarkers for old expectations. Crit Care. 2014; 18:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierrakos C, Vincent JL: Sepsis biomarkers: A review. Crit Care. 2010; 14:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright SW, Hantrakun V, Rudd KE, et al. : Enhanced bedside mortality prediction combining point-of-care lactate and the quick Sequential Organ Failure Assessment (qSOFA) score in patients hospitalised with suspected infection in southeast Asia: A cohort study. Lancet Glob Health. 2022; 10:e1281–e1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wacker C, Prkno A, Brunkhorst FM, et al. : Procalcitonin as a diagnostic marker for sepsis: A systematic review and metaanalysis. Lancet Infect Dis. 2013; 13:426–435 [DOI] [PubMed] [Google Scholar]
- 42.Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. : Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet. 2004; 363:600–607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.