Abstract
Very well-differentiated adenocarcinoma of intestinal type is a distinct subtype of gastric cancer characterized by anastomosing glands with a hand-in-hand pattern and low-grade cytologic atypia resembling intestinal metaplasia. This is a slow-growing neoplasm with an indolent clinical course; however, a subset demonstrates transformation into adenocarcinoma with higher-grade histology, typically diffuse-type carcinoma, and behaves aggressively. This study aimed to better characterize the genomic and pathologic features, with a focus on factors associated with diffuse-type transformation. A total of 58 cases with (n=31) and without (n=27) diffuse-type transformation were analyzed for molecular and pathologic features. First, comprehensive deep DNA sequencing was conducted in 18 cases (discovery cohort), followed by a digital droplet polymerase chain reaction of hot spot RHOA mutations in 40 cases (validation cohort). In total, RHOA mutations were the most common alteration (34%), followed by loss of ARID1A (12%), p53 alterations (10%), and CLDN18::ARHGAP26/6 fusions (3.4%). FGFR2 amplification was identified in an advanced case with a p53 alteration. Altered p53 expression was recognized only in higher-grade components and was significantly associated with advanced disease (P=0.0015) and diffuse-type transformation (P=0.026). A mixed mucin phenotype was also strongly correlated with advanced disease (P<0.001) and diffuse-type transformation (P<0.001). Decreased E-cadherin expression was frequently observed (74%) in poorly cohesive components. This study demonstrated that a subset of RHOA-mutant diffuse-type gastric cancers develops through the transformation of very well-differentiated adenocarcinoma of intestinal type. Our observations suggest a mixed mucin phenotype as a risk factor and alterations in p53 and E-cadherin as drivers of diffuse-type transformation.
Key Words: diffuse-type transformation, gastric cancer, mucin phenotype, p53, RHOA
Very well-differentiated gastric adenocarcinoma of intestinal type is a unique variant of gastric cancer and is characterized by fused or anastomosing neoplastic glands with low-grade cytologic atypia, mimicking intestinal metaplasia.1,2 Typically, this neoplasm grows slowly and remains an intramucosal lesion for a long follow-up period.1 However, in a subset of cases, a focus of poorly cohesive carcinoma develops within the lesion and undergoes diffuse-type transformation with aggressive behavior.1,2 Therefore, careful clinical management is necessary, and indicators of diffuse-type transformation must be determined for this neoplasm.
A recent study reported frequent RHOA mutations and occasional CLDN18::ARHGAP fusion in this neoplasm.3 This observation suggests that these genomic alterations underlie sequential progression from very well-differentiated gastric adenocarcinoma of intestinal type to diffuse-type adenocarcinoma, because both RHOA mutations and CLDN18::ARHGAP fusion are also characteristic of diffuse-type gastric cancer.4,5 To test this, RHOA mutations and CLDN18::ARHGAPs should be analyzed and demonstrated in advanced diffuse-type gastric cancer cases that contain an intramucosal, very well-differentiated component. Previous studies have focused on early lesions and lacked demonstration of such cases. In addition, molecular indicators of progression into advanced disease remain unclear.
Given the limited information regarding the molecular features and factors related to diffuse-type transformation of this neoplasm, this study aimed to perform pathologic and molecular evaluations for the largest cohort (n=58) of cases with diffuse-type transformation (n=31) and advanced disease (n=13). First, comprehensive deep DNA sequencing was performed to identify novel pathogenic mutations in the discovery cohort (n=18). Second, the profiles of RHOA mutations, CLDN18::ARHGAP fusion, as well as other clinicopathologic features in the entire cohort were determined (n=58). This study demonstrated that a subset of RHOA-mutant or CLDN18::ARHGAP fusion-positive very well-differentiated adenocarcinoma of intestinal type underwent a diffuse-type transformation and progressed to advanced cancer. Pathologic and molecular factors associated with diffuse-type transformation have also been identified.
MATERIALS AND METHODS
Case Selection and Clinicopathologic Data
The study was approved by the institutional review boards of each participating institute. Overall, 58 cases of very well-differentiated gastric adenocarcinomas of intestinal type resected between 2008 and 2020 were identified from the pathology archive of the University of Tokyo Hospital. The cohort included 33 endoscopically resected and 25 surgically resected cases. Demographic data, endoscopic findings, and clinical follow-up data were obtained by reviewing the medical records.
All hematoxylin and eosin (H&E)-stained sections were reviewed by experienced gastrointestinal pathologists (H.R. and U.T.). All cases were very well-differentiated adenocarcinoma of the intestinal type component that met the criteria described previously.1,2 Briefly, very well-differentiated gastric adenocarcinomas of intestinal type are composed of neoplastic epithelium with low-grade nuclear atypia, differentiating toward intestinal-type cells (such as absorptive cells, goblet cells, and Paneth cells), and simple tall mucin-producing cells akin to foveolar-type epithelia. Architecturally, glandular structures are commonly seen with tortuousness, branching, or anastomosing, recapitulating the shapes of W, H, Y, or X at a low-power view. Characteristically, the neoplastic glands are usually sparsely distributed and lack back-to-back crowding. Pathologic features, including tumor size, depth of invasion, lymphovascular invasion, lymph node metastasis, and the presence or absence of poorly cohesive carcinoma components (ie, diffuse-type transformation), were evaluated.
Immunohistochemical Staining
In this study, 3-μm-thick formalin-fixed, paraffin-embedded (FFPE) sections were subjected to immunohistochemistry using the Ventana BenchMark XT automated immunostainer (Roche Diagnostics, Basel, Switzerland). The antibodies used and staining conditions are summarized in Supplementary Table 1, http://links.lww.com/PAS/B800.
The mucin phenotype was determined based on the expression patterns of intestinal and gastric phenotypic markers.1 Tumors were categorized into intestinal (ie, positive for MUC2, CD10, or CDX-2 in ≥10% neoplastic cells) or gastric (ie, positive for MUC5AC or MUC6 in ≥10% neoplastic cells) mucin phenotypes. Tumors positive for both gastric and intestinal markers were classified as mixed-type.
Loss of ARID1A and MLH1 expression was defined as the complete absence of nuclear staining within the tumor cells. p53 staining was classified as a mutation pattern when diffuse and strong nuclear staining was observed in neoplastic cells or when it was completely negative.6,7 Otherwise, p53 staining status was judged as a wild-type pattern exhibiting scattered nuclear staining in varying intensities in <50% of neoplastic cells. FGFR2 immunostaining was defined as positive when strong membranous staining was observed in ≥10% of neoplastic cells. E-cadherin was evaluated for the presence of decreased membranous expression. CLDN18 immunohistochemistry was performed in advanced cases (n=13), and its membranous expression was evaluated based on the staining intensity (0, no staining; 1+, weak staining; 2+, moderate staining; and 3+, strong staining) and proportion. CLDN18 positivity was defined as moderate (2+) or strong (3+) staining using cutoff values at 75% (eligibility criteria in recent clinical trials8,9).
Targeted Deep DNA Sequencing
Targeted DNA sequencing was performed on genomic DNA extracted from FFPE tumor samples of the discovery cohort (n=18, samples resected between 2011 and 2019). Using H&E-stained slides as a guide, FFPE tumor regions were enriched and macrodissected for further analyses. A custom-designed panel (SureSelect; Agilent Technologies, Santa Clara, CA) composed of 67 gastric cancer-associated genes (Supplementary Table 2, http://links.lww.com/PAS/B801), including those significantly mutated in The Cancer Genome Atlas (TCGA) gastric cancer study.5 Library construction, sequencing, and mutation calling were performed per our published methods.10 Sequencing was conducted on a HiSeq 2500 (Illumina, San Diego, CA) at the National Cancer Center Research Institute (NCCRI), Japan. The sequencing depth of coverage of >500 and >200 were achieved for tumor and corresponding normal samples, respectively.
RNA-Sequencing
In the discovery cohort, RNA-sequencing was conducted in 7 of 18 tumors with adequate quality and amount of extracted RNA. FFPE tumor sections with 10 µm thickness were macrodissected, and the total RNA was isolated using a miRNeasy FFPE kit (Qiagen, Hilden, Germany). The quality and quantity of the RNA were determined using a Bioanalyzer 2100 (Agilent Technologies). A targeted RNA-sequencing library was prepared from 250 ng of total RNA using a TruSight RNA PanCancer Library Kit (Illumina), which covers 1385 cancer-related genes. The library was subjected to paired-end sequencing of 151-bp fragments on a MiSeq (Illumina) in NCCRI, Japan. At least 6 million reads per sample were obtained, and the paired-end reads were mapped and aligned to known RNA sequences in the RefSeq, Ensembl, and LincRNA databases with the BWA-MEM program. After selecting the best hits with the appropriate spacing and orientation, gene expression values were calculated as reads per kilobase of exon per million mapped reads.
Droplet Digital Polymerase Chain Reaction (PCR) for RHOA Mutation Analysis
To identify the tumor area, H&E-stained sections of FFPE tissue were reviewed from each sample, and macrodissection was performed on serial unstained sections. Genomic DNA was extracted from the FFPE sections using the QIAamp DNA Micro Kit (Qiagen). The QX200 Droplet Digital PCR System (ddPCR; Bio-Rad Laboratories, Hercules, CA) was used to detect hotspot RHOA mutations (p.G17E and p.Y42C). All samples were screened using the following components: ×2 ddPCR Supermix for Probes (No dUTP; Bio-Rad), ×20 ddPCR Mutation Detection Assays (RHOA p.G17E c.50G>A Assay ID: dHsaMDS435917233; or RHOA p.Y42C c.125A>G Assay ID: dHsaMDS460820307; Bio-Rad), HaeIII (4 U per reaction; Toyobo, Osaka, Japan), and 250 ng DNA. A nontemplate control containing water was added to the reaction mixture instead of DNA and was analyzed to assess potential carryover contamination. The final volume reactions of 20 μL were loaded into sample wells of a DG8 cartridge (Bio-Rad) with 70 μL of Droplet Generation Oil for Probes (Bio-Rad). Droplets were generated by the QX200 Droplet Generator (Bio-Rad), and 40 μL of the generated droplets were manually transferred with a multichannel pipette into a 96-well PCR plate and amplified in a C1000 Touch Thermal Cycler (Bio-Rad). The thermal cycling conditions were as follows: 95°C for 10 minutes, 40 cycles of 94°C for 30 seconds, 53°C for 1 minutes, followed by 98°C for 10 minutes, and cooling to 4°C. Droplets were analyzed using the QX200 Droplet Reader (Bio-Rad). Data analysis was performed with QuantaSoft version 1.7.4.0917 (Bio-Rad), which uses the number of positive and negative droplets to calculate the concentration of the target and reference DNA sequences and their Poisson-based 95% CIs. No false-positive droplets were detected in the negative controls.
Fluorescence in Situ Hybridization (FISH)
FFPE sections were subjected to FISH for CLDN18::ARHGAPs rearrangement, which was performed on a dual-color CLDN18 split assay using commercially available probes (GSP Lab Inc., Hyogo, Japan) as per the manufacturer’s instructions. This probe includes a Texas Red-labeled 430-kb DNA fragment of the proximal region of CLDN18 and a FITC-labeled DNA fragment corresponding to the 740-kb distal (3′) region of CLDN18. FISH for FGFR2 was also performed using the FGFR2/CEN10q Dual-Color FISH Probe (#GC018; GSP Lab Inc.). The FGFR2/CEP10 ratio of >2.0 was interpreted as positive for amplification.11,12
Reverse-Transcription (RT)-PCR Analysis of CLDN18::ARHGAP Fusion Transcripts
RT-PCR was conducted to detect 3 types of fusion transcripts, including CLDN18 exon 5-ARHGAP26 exon 12, CLDN18 exon 5-ARHGAP26 exon 10, and CLDN18 exon 5-ARHGAP6 exon 2. Briefly, RNA was extracted from FFPE sections, and total RNA was subjected to complementary DNA (cDNA) synthesis using Thermo SuperScript IV VILO Master Mix with ezDNase Enzyme (Thermo Fisher Scientific, Tokyo, Japan). The sequences of the PCR primers used are listed in Supplementary Table 3, http://links.lww.com/PAS/B802. In the PCR, AmpliTaq Gold (Thermo Fisher Scientific) was used for polymerase, and the annealing temperature was set to 58.5°C for CLDN18 exon 5-ARHGAP26 exon 12 and CLDN18 exon 5-ARHGAP6 exon 2. Amplified PCR products were bidirectionally sequenced by the Sanger method.
Statistical Analysis
The Fisher exact test or the χ2 test was used to test the significance of associations between categorical variables. For all tests, P<0.05 was considered significant.
RESULTS
Clinicopathologic Findings
The clinicopathologic characteristics of our cohort are presented in Table 1. Of the 58 patients, 43 were male (74%) and 15 were female (26%), with a mean age of 66.7 (range: 41 to 91) years. Approximately three-quarters (45/58; 78%) were early cancers (mean tumor size, 2.8 cm), and the rest (13/58) were advanced cancers (mean tumor size, 7.8 cm). Lymphovascular invasions were present in 4.4% (2/45) of early cases, which were all submucosal invasive tumors, and in 77% (10/13) of advanced cases. Lymph node metastasis was found in 14% (1/7) of submucosal invasive early cancers and 92% (12/13) of advanced cancers. Intestinal metaplasia was always present in the background non-neoplastic mucosa, and the majority was incomplete type (51/58; 88%).
TABLE 1.
Clinicopathologic Characteristics of 58 Tumors in This Study
| Entire cohort (n=58) | Discovery cohort (n=18) | Validation cohort (n=40) | |
|---|---|---|---|
| Sex | |||
| Female | 15 | 10 | 5 |
| Male | 43 | 8 | 35 |
| Age (y) | |||
| Mean | 66.7 | 67.4 | 66.4 |
| Range | 41–91 | 49–84 | 41–91 |
| Tumor size (cm) | |||
| Mean | 4.0 | 2.2 | 4.9 |
| Range | 0.4–16.0 | 0.6–5.0 | 0.4–16.0 |
| Tumor depth | |||
| Mucosa | 38 | 16 | 22 |
| Submucosa | 7 | 2 | 5 |
| Advanced | 13 | 0 | 13 |
| Lymphovascular invasion | |||
| Absent | 45 | 17 | 28 |
| Present | 13 | 1 | 12 |
| Lymph node metastasis | |||
| Absent | 45 | 18 | 27 |
| Present | 13 | 0 | 13 |
Representative images of tumor histology are shown in Figure 1. Very well-differentiated gastric adenocarcinoma of the intestinal type was always observed in the mucosal layer. High-grade (conventional) tubular adenocarcinoma was also noted in the mucosa in 27 of 58 cases: 24% (9/38) intramucosal, 71% (5/7) submucosal, and 100% (13/13) advanced cases, respectively. Poorly cohesive carcinoma component was noted in 31 of 58 cases: 34% (13/38) intramucosal, 71% (5/7) submucosal, and 100% (13/13) advanced cases, respectively. All 13 advanced cancers presented diffuse-type histology in the invasive area, which contained predominantly poorly cohesive carcinoma components (Fig. 1G). A minority of advanced cases (2/13; 15%) had a mixture of poorly cohesive carcinoma and high-grade tubular adenocarcinoma components in the submucosa or deeper area.
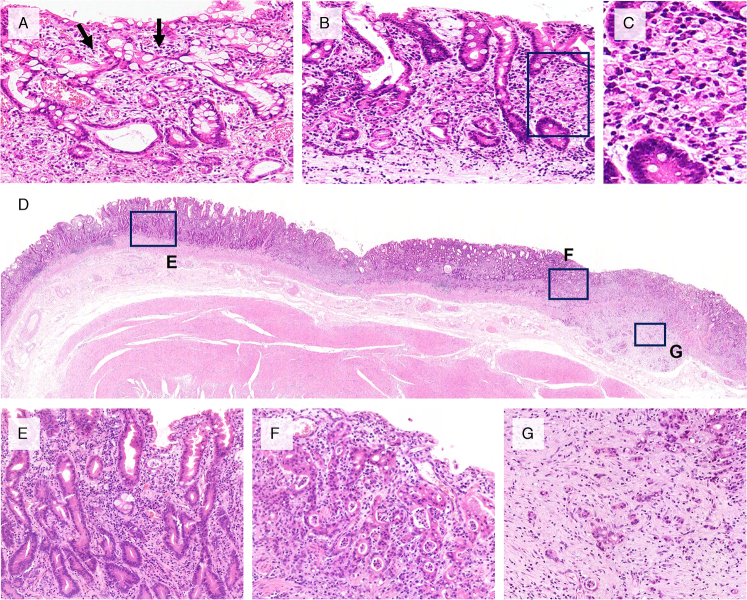
FIGURE 1.
Representative histology of very well-differentiated gastric adenocarcinoma of intestinal type and its transformation into diffuse-type advanced tumors. A, Hand-in-hand pattern (arrows) is seen in this typical example of very well-differentiated gastric adenocarcinoma. Tumor cells show low-grade cytologic atypia mimicking intestinal metaplasia. B, Another example of an intramucosal, very well-differentiated adenocarcinoma composed of tortuous neoplastic glands in the left, with signet-ring cell transformation in the right. This tumor had an RHOA p.G17E mutation. C, Frame in (B) a high-power view of a signet-ring cell component. D–G, An advanced case with the RHOA p.G17E mutation. D, A low-power view. E, Intramucosal component of very well-differentiated gastric adenocarcinoma. F, Tubule-forming component with high-grade cytologic atypia was also seen in the intramucosal layer. G, Poorly cohesive carcinoma component invading into the submucosal layer, exhibiting diffuse-type histology with stromal fibrosis.
Genetic Aberrations in the Discovery Cohort
First, mutation profiles were searched by deep DNA sequencing using a next-generation sequencer, covering exons of 67 genes, in the discovery cohort (n=18). The mutation distribution is displayed in Supplementary Figure 1, http://links.lww.com/PAS/B804. The most prevalent gene that mutated in this cohort was RHOA (6/18), followed by ARID1A (3/18), FAT4 (2/18), CYLC1 (2/18), SMARCA4 (2/18), and MBD6 (2/18). RHOA mutations consisted of RHOA p.Y42C (n=3), p. G17E (n=1), p.L57V (n=1), and p.L69V (n=1). In most RHOA-mutated tumors (4/6), RHOA had a higher variant allele frequency, obtained through deep RNA-bait sequencing (×500 and ×200 for tumor and normal samples, respectively), than any other mutated gene. This suggests that somatic RHOA mutations were acquired in the initial stage of tumorigenesis of very well-differentiated gastric adenocarcinoma of intestinal type. No somatic mutations in TP53 and CDH1 were detected (0/18) in the discovery cohort.
RHOA Hotspot Mutations in the Validation Cohort
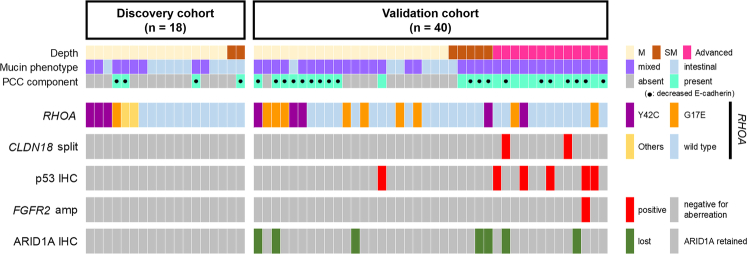
In our discovery cohort, recurrent RHOA mutations and the scarcity of other recurrently mutated genes were noted. Therefore, we then sought to determine the incidence of RHOA mutations in a larger cohort (n=40, validation cohort). In this analysis, 2 major hotspot RHOA mutations (p.G17E and p.Y42C) in our discovery cohort and an earlier publication3 were examined by the ddPCR method. Of the 40 cases in the validation cohort, 14 (35%) harbored RHOA mutations: p.G17E in 9 and p.Y42C in 5 cases. In the entire cohort (discovery cohort+validation cohort), RHOA mutations were detected in 34% (20/58) of the patients. An overview of the molecular aberrations, including key mutations in all 58 tumors, is presented in Figure 2.
FIGURE 2.
Genetic and pathologic landscape of 58 very well-differentiated gastric adenocarcinomas of intestinal type. Recurrent RHOA mutations were identified in both the discovery cohort and the validation cohort. Advanced tumors frequently harbor p53 aberrations. CLDN18::ARHGAP fusions were detected in RHOA-wild-type tumors. ARID1A loss and p53 aberration did not coexist. IHC indicates immunohistochemistry; M, intramucosal tumor; PCC, poorly cohesive carcinoma; SM, tumor with submucosal invasion.
CLDN18::ARHGAP Fusions
In the screening by CLDN18 split FISH, 2 of the 58 (3.4%) cases had split signals of CLDN18. The fusions were confirmed to be CLDN18::ARHGAP26 (n=1) and CLDN18::ARHGAP6 (n=1) by RT-PCR and Sanger sequencing (Supplementary Fig. 2, http://links.lww.com/PAS/B805). Cases positive for both fusions were advanced tumors with diffuse-type transformation, and both lacked RHOA mutations or abnormal p53 expression. CLDN18 split FISH signals were present not only in diffuse-type components but also in the intramucosal component of very well-differentiated histology.
Immunohistochemical Findings
In this study, 17 (29%) tumors showed an intestinal mucin phenotype, whereas the rest (71%; 41/58) had a mixed mucin phenotype that showed the expression of both intestinal and gastric markers (Supplementary Fig. 3, http://links.lww.com/PAS/B806).
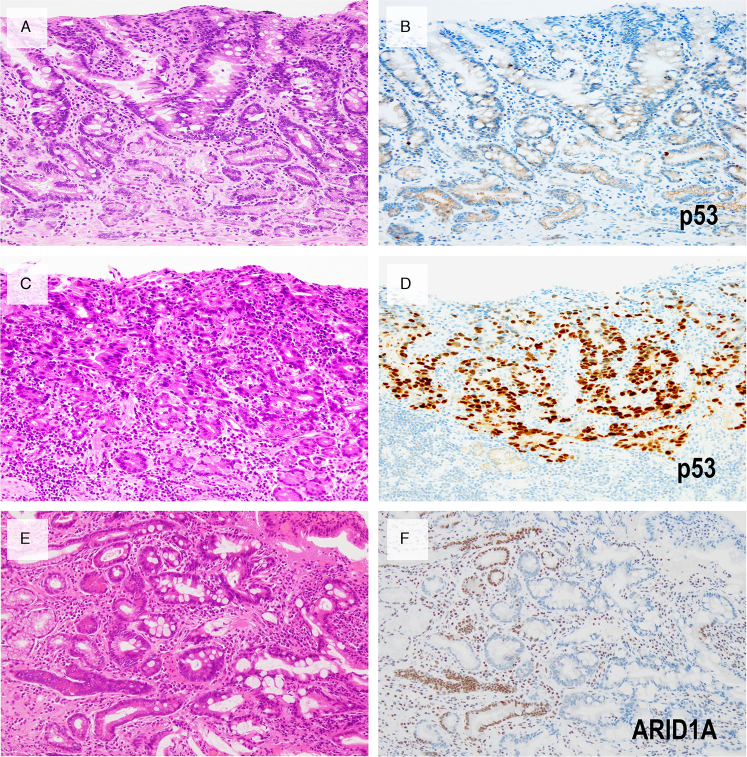
A mutant pattern of p53 staining was observed in 6 of 58 (10%) cases. Of these, 5 showed a diffuse overexpression pattern and 1 showed a completely absent (null) pattern. In all 6 cases, altered p53 staining was exclusively noted in components with high-grade cytologic atypia and diffuse-type histology, whereas very well-differentiated components always showed a wild-type p53 staining pattern (Fig. 3). Thus, a TP53 mutation is associated with a higher-grade or diffuse-type transformation. p53 immunohistochemical patterns were consistent with the TP53 mutation status in the discovery cohort; all tumors (18/18) showed a wild-type pattern of p53 immunohistochemistry, and all lacked somatic TP53 mutations. The incidence of p53 abnormal expression was comparable between RHOA-mutated (11.1%; 2/18) and RHOA-wild-type (11.8%; 4/34) tumors.
FIGURE 3.
A representative case with abnormal p53 expression and another case with ARID1A loss. Very well-differentiated component with typical low-grade histology (A) shows a wild-type pattern of p53 staining (B), whereas the accompanying high-grade tubular component (C) demonstrates diffuse and strong p53 staining (D). E and F, Biopsy pictures of another case with typical low-grade histology and ARID1A loss. Nuclear expression of ARID1A is lost in cells forming tortuous neoplastic glands. Note the retained expression in non-neoplastic glands and stromal cells.
Loss of ARID1A expression (Figs. 3E, F) was observed in 7 of 58 (12%) cases. When present, loss of expression was always noted in both the intramucosal very well-differentiated and higher-grade/diffuse-type components. Mutual exclusivity was observed between loss of ARID1A expression and p53 mutant-type staining in the entire cohort (n=58; Fig. 2). All tumors showed preserved MLH1 expression (58/58). Among 13 advanced tumors, 9 (69%) showed positivity for CLDN18 at the 75% cutoff.
Factors Related to Diffuse-type Transformation
The histologic and molecular features were compared between tumors with and without poorly cohesive carcinoma (Table 2). Advanced stage (P<0.001), p53 mutant pattern staining (P=0.026), and mixed mucin phenotype (P<0.001) were significantly associated with the presence of a poorly cohesive carcinoma component. Notably, all tumors with p53 abnormal expression had poorly cohesive components. In contrast, RHOA mutation status was not associated with the presence of a poorly cohesive component. ARID1A-lost tumors tended to have a poorly cohesive component; however, this did not reach statistical significance (P=0.11). Thus, the p53 alteration was suggested to be associated with diffuse-type transformation. A mixed mucin phenotype was an indicator of poorly cohesive carcinoma development but was not associated with RHOA mutation, p53, or ARID1A alterations (Table 3).
TABLE 2.
Associations Between Molecular Features, Poorly Cohesive Carcinoma Component, and Tumor Stage in Very Well-Differentiated Gastric Adenocarcinoma of Intestinal Type
| Tumor stage | PCC component | |||||
|---|---|---|---|---|---|---|
| Early | Advanced | Present | Absent | |||
| (n=45) | (n=13) | P | (n=31) | (n=27) | P | |
| PCC component | <0.001* | — | ||||
| Present | 18 | 13 | 31 | 0 | ||
| Absent | 27 | 0 | 0 | 27 | ||
| p53 immunohistochemistry | 0.0015 | 0.026 | ||||
| Mutation pattern | 1 | 5 | 6 | 0 | ||
| Wild-type pattern | 44 | 8 | 25 | 27 | ||
| RHOA | 0.88 | 0.92 | ||||
| G17E | 8 | 2 | 5 | 5 | ||
| Y42C | 7 | 1 | 5 | 3 | ||
| Other mutation | 2 | 0 | 1 | 1 | ||
| Wild type | 27 | 10 | 20 | 18 | ||
| ARID1A expression | 0.65 | 0.11 | ||||
| Retained | 40 | 11 | 25 | 26 | ||
| Loss | 5 | 2 | 6 | 1 | ||
| Mucin phenotype | <0.001 | <0.001 | ||||
| Mixed | 28 | 13 | 29 | 12 | ||
| Intestinal | 17 | 0 | 2 | 15 | ||
χ2 test (others: the Fisher exact test).
PCC indicate poorly cohesive carcinoma.
TABLE 3.
Association Between Molecular Genetic Features and Mucin Phenotype in Very Well-Differentiated Gastric Adenocarcinoma of Intestinal Type
| Mucin phenotype | |||
|---|---|---|---|
| Mixed (n=41) | Intestinal (n=17) | P | |
| p53 immunohistochemistry | 0.17 | ||
| Mutation pattern | 6 | 0 | |
| Wild-type pattern | 35 | 17 | |
| RHOA, n (%) | 0.77 | ||
| G17E | 8 (80) | 2 | |
| Y42C | 6 (75) | 2 | |
| Other mutation | 2 (100) | 0 | |
| Wild type | 25 (66) | 13 | |
| ARID1A | |||
| Retained | 34 | 10 | 0.32 |
| Loss | 7 | 0 | |
E-cadherin expression was invariably retained in very well-differentiated adenocarcinoma of intestinal type, whereas it was decreased in poorly cohesive components in 23 of 31 (74%) cases (Supplementary Table 4, http://links.lww.com/PAS/B803; Supplementary Fig. 4, http://links.lww.com/PAS/B807). Decreased E-cadherin expression in the poorly cohesive component was more frequently observed in p53-wild-type tumors than in p53-altered tumors (84% vs. 33%; P=0.026), suggesting that E-cadherin alteration, especially in p53-wild-type tumors, plays a role in diffuse-type transformation.
FGFR2 Amplification and Overexpression
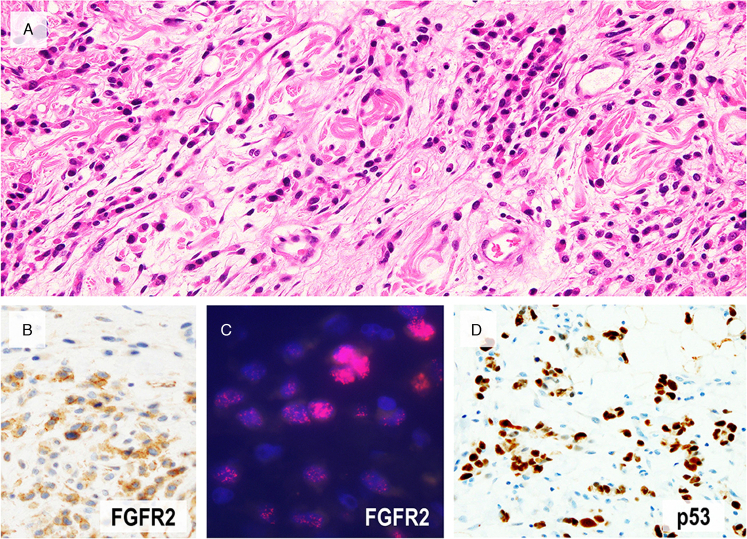
Of the 7 cases in which RNA-sequencing was performed, one showed FGFR2 amplification, which was confirmed by FISH, and was positive for FGFR2 immunohistochemistry (Fig. 4). In FISH and immunohistochemistry, FGFR2 amplification or overexpression was recognized in tumor cells invading the submucosa or a deeper area, but not in the intramucosal, very well-differentiated component. FGFR2 immunohistochemistry was subsequently performed on the representative slide of all 58 cases, and FGFR2 overexpression could not be detected in other cases.
FIGURE 4.
A case with FGFR2 amplification. (A) A representative image of invasive area showing diffuse-type histology. In this advanced tumor with FGFR2 amplification, overexpression by immunohistochemistry (B) and amplification by FISH (C, red signals) was confirmed in diffuse-type components but not in very well-differentiated component. This tumor showed diffuse positivity for p53 (D).
Relationship Between Very Well-Differentiated Gastric Adenocarcinoma of Intestinal Type and Diffuse-Type Gastric Cancer
Finally, we assessed the RHOA mutation patterns (“p.G17E/p.Y42C ratio,” Supplementary Fig. 5, http://links.lww.com/PAS/B808) and investigated the relationship between diffuse-type gastric cancers and very well-differentiated gastric adenocarcinomas of intestinal type. In our cohort (n=58), very well-differentiated gastric adenocarcinoma of intestinal type had a G17E/Y42C ratio of 1.25, and that in the cohort of Hashimoto et al3 (n=44) was 1.17. The values were remarkably comparable between the 2 independent cohorts of very well-differentiated gastric adenocarcinoma of intestinal type. By contrast, the G17E/Y42C ratios in 2 publicly available cohorts of diffuse-type gastric cancer were much lower (range: 0.20 to 0.50; Supplementary Fig. 5, http://links.lww.com/PAS/B808) than those of very well-differentiated gastric adenocarcinoma of intestinal type.
DISCUSSION
This study has expanded our understanding of the carcinogenic process and associated molecular alterations of very well-differentiated gastric adenocarcinoma of intestinal type by analyzing the largest case series, including both early and advanced tumors. First, recurrent RHOA mutations and CLDN18::ARHGAP fusion in this neoplasm were validated. Second, several cases harboring these alterations that underwent a diffuse-type transformation and developed advanced cancer were illustrated. In addition, factors related to the tumor progression of this neoplasm, including alterations of p53 and E-cadherin, FGFR2 amplification, and a mixed mucin phenotype, were identified.
Interestingly, recent studies have reported recurrent RHOA mutations in very well-differentiated gastric adenocarcinoma of intestinal type and diffuse-type gastric cancer in common. The former is usually an intramucosal carcinoma with indolent behavior, whereas the latter often appears as an advanced disease with aggressive behavior. Our observations demonstrated that a subset of RHOA-mutant diffuse-type cancer was derived from intramucosal, very well-differentiated adenocarcinoma of intestinal type. However, the extent of the role of a very well-differentiated subtype as a precursor lesion of diffuse-type gastric cancer remains unknown. On the basis of the results of the present study and earlier publications, the G17E/Y42C ratio was much higher in very well-differentiated adenocarcinoma of intestinal type than in diffuse-type carcinoma (Supplementary Fig. 5, http://links.lww.com/PAS/B808). The large difference in the G17E/Y42C ratios may suggest that very well-differentiated gastric adenocarcinoma of intestinal type is only a minor precursor lesion of diffuse-type gastric cancers. In fact, most RHOA-mutant diffuse-type gastric cancers originate from intramucosal precursors other than very well-differentiated gastric adenocarcinoma of intestinal type, such as conventional tubular and pure signet-ring cell carcinoma,13 which is probably rich in p.Y42C alteration. Alternatively, very well-differentiated gastric adenocarcinoma of intestinal type with the RHOA p.G17E mutation may have less potential for progression into diffuse-type cancer than those with the RHOA p.Y42C mutation. However, this is less likely because 2 of 3 RHOA-mutated advanced tumors in our cohort had p.G17E and because poorly differentiated components were frequently detected in RHOA p.G17E-positive early cases.
It is important to recognize this diagnostically challenging neoplasm because 53% (31/58) of the cases in this study had high-grade transformation. Similarly, Hashimoto et al3 described that discohesive cells were present in 22 of 44 cases, and Okamoto et al2 described that focal signet-ring cells were present in 16 of 25 lesions. As for factors related to tumor progression, p53 alteration was closely associated with the presence of poorly cohesive components and advanced disease. In our cohort, all tumors exhibiting p53 alterations harbored poorly differentiated components. In addition, p53 alteration was noted in 38% (5/13) of advanced cancers, whereas it was only 2.2% (1/45) in early cancers. Notably, altered p53 was recognized only in higher-grade components, whereas very well-differentiated components always showed a wild-type pattern of p53 immunostaining. This finding is in line with those of earlier publications analyzing early cancers that have reported p53 abnormalities in very well-differentiated gastric adenocarcinomas of intestinal type at low frequencies (0% in 2 studies and 12.4% in 1 study).1,3,6 Therefore, p53 alteration is considered a secondary event, involving the progression of very well-differentiated gastric adenocarcinoma of intestinal type into more aggressive cancer. In addition, decreased E-cadherin expression was also frequently observed in poorly cohesive components, and is considered to be a factor associated with diffuse-type transformation. This finding is in line with a previous study by Zheng et al,14 which reported that E-cadherin expression tends to be lower in the diffuse-type component than in the co-existing intestinal-type component. FGFR2 amplification was exclusively found within the invasive components of an advanced tumor and is likely a secondary event associated with tumor progression. Previous studies have reported enriched FGFR2 amplification in diffuse-type tumors.12,15 Our observation suggests that very well-differentiated gastric adenocarcinoma of intestinal type could be one of the precursors of FGFR2-amplified diffuse-type gastric cancer, although the data are limited.
By contrast, RHOA mutation and CLDN18::ARHGAP fusion are early events and are considered drivers of cancer initiation because these aberrations were observed in both very well-differentiated and higher-grade components. In addition, loss of ARID1A expression was noted in the entire lesion, including the intramucosal very well-differentiated component. Given the diagnostic difficulty of very well-differentiated gastric adenocarcinoma of intestinal type because of its subtle cytologic atypia and resemblance to intestinal metaplasia,1 ARID1A immunohistochemistry can be useful, especially for challenging cases in small biopsy specimens (Figs. 3E, F).
In our series, a mixed mucin phenotype was strongly correlated with poorly differentiated components and advanced disease. In addition, all advanced tumors, regardless of p53 status, showed a mixed mucin phenotype. Our results suggest the importance of a mixed mucin phenotype as a risk factor for progression into aggressive tumors, and this finding agrees with that of a previous study.1 We found that CLDN18 positivity was relatively high in cases with advanced cancer, suggesting the possible benefit of CLDN18-targeted therapy.8,9 This observation is likely associated with the presence of gastric-type mucin as well as intestinal-type mucin (mixed mucin phenotype) in these cases, since CLDN18 is a gastric phenotypic marker.16
In contrast, one-third (20/58) of the tumors have none of the alterations (RHOA mutation, CLDN18::ARHGAP fusion, p53 alteration, decreased E-cadherin expression, FGFR2 amplification/overexpression, or ARID1A loss). This is partly due to the limited number of genes analyzed in our discovery cohort, in which we focused on known gastric cancer-associated genes (n=67). In addition, only FFPE samples were available because of the rarity of very well-differentiated gastric adenocarcinoma of intestinal type; therefore, RNA-sequencing could be performed only in 7 cases of the discovery cohort. In the RHOA mutation analysis in the validation cohort, we examined hotspot mutations; thus, minor mutations could have been missed in this study. Further molecular studies are necessary to identify novel molecular abnormalities associated with cancer initiation and progression.
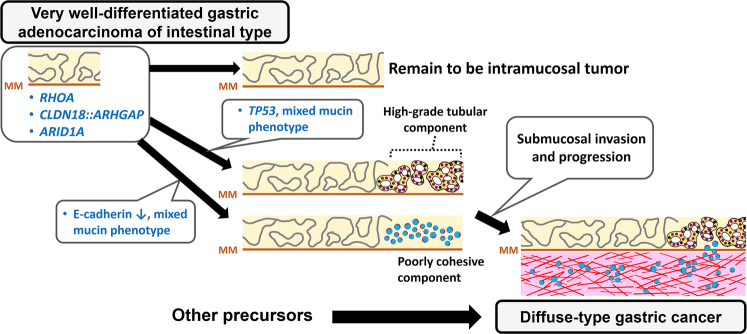
We summarized our proposed carcinogenic process and the associated molecular alterations of very well-differentiated gastric adenocarcinoma of intestinal type in Figure 5 based on our observations. We confirmed RHOA mutations (34.5%) and CLDN18::ARHGAP fusion (3.4%) in the largest case series of very well-differentiated adenocarcinoma of intestinal type. A subset of cases was demonstrated to undergo diffuse-type transformation and progression into advanced disease. The mixed mucin phenotype is considered a risk factor for diffuse-type transformation. Tumor progression is driven by subsequent p53 or E-cadherin alteration and, less frequently, by FGFR2 amplification. Although very well-differentiated gastric adenocarcinoma of intestinal type shares specific molecular abnormalities and sequentially progresses to diffuse-type cancer, given their significant differences in biological behavior and clinical management, further studies are necessary to identify novel molecular abnormalities and better stratify this unique neoplasm for the optimization of clinical management.
FIGURE 5.
Schematic illustration of the natural history of very well-differentiated gastric adenocarcinoma of intestinal type. RHOA mutation, CLDN18::ARHGAP fusion, and ARID1A loss are early events because they are present in the intramucosal low-grade components. TP53 mutation/p53 alteration is the major secondary event significantly associated with high-grade or diffuse-type transformation. Decreased E-cadherin expression is also associated with diffuse-type transformation. Tumor with mixed mucin phenotype is more likely to develop high-grade or diffuse-type transformation. Our analysis of the G17E/Y42C ratio suggested that diffuse-type gastric cancer more frequently develops from precursors other than very well-differentiated gastric adenocarcinoma. MM indicates muscularis mucosae.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Kei Sakuma, Minato Murata, Kimiko Takeshita, Tomoko Urushidate (The University of Tokyo), and Erika Arakawa (NCCRI) for their excellent technical assistance.
Footnotes
This work was supported by Japan Agency for Medical Research and Development (AMED) under Grant JP21cm0106502h0006 (TU).
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
This retrospective study was approved by the Institutional Review Board of Tokyo University Hospital (no. G3521 and G10106) and National Cancer Center (no. G2008-003), Japan, and the requirement to obtain written informed consent was waived.
H.R., T.S., and T.U. conceived and designed the study. H.R., Y.A., and A.K. performed sequencing experiments. H.R., Y.A., A.K., S.Y., H.N., N.H., A.N., F.H., Y.T., T.S., and T.U. analyzed and interpreted data. M.F. and Y.S. provided the clinical information. H.R. and T.U. wrote the manuscript, which was approved by all the authors.
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website www.ajsp.com.
Contributor Information
Hirofumi Rokutan, Email: rokutan.h@gmail.com;rokutan-tky@umin.ac.jp.
Yasuhito Arai, Email: yarai@ncc.go.jp.
Akiko Kunita, Email: kunita@m.u-tokyo.ac.jp.
Satoshi Yamasaki, Email: s_ymsk@saitama-med.ac.jp.
Hiromi Nakamura, Email: hiromwat@ncc.go.jp.
Natsuko Hama, Email: nhama@ncc.go.jp.
Atsuhito Nakayama, Email: nakayama-atsuhito870@g.ecc.u-tokyo.ac.jp.
Fumie Hosoda, Email: fhosoda1020@gmail.com.
Yasushi Totoki, Email: totoki@cgi.med.osaka-u.ac.jp.
Mitsuhiro Fujishiro, Email: mtfujish@gmail.com.
Yasuyuki Seto, Email: seto-tky@umin.ac.jp.
Tatsuhiro Shibata, Email: tshibata@ims.u-tokyo.ac.jp.
Tetsuo Ushiku, Email: usikut@g.ecc.u-tokyo.ac.jp.
REFERENCES
- 1.Ushiku T, Arnason T, Ban S, et al. Very well-differentiated gastric carcinoma of intestinal type: analysis of diagnostic criteria. Mod Pathol. 2013;26:1620–1631. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto N, Kawachi H, Yoshida T, et al. “Crawling-type” adenocarcinoma of the stomach: a distinct entity preceding poorly differentiated adenocarcinoma. Gastric Cancer. 2013;16:220–232. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto T, Ogawa R, Tang TY, et al. RHOA mutations and CLDN18-ARHGAP fusions in intestinal-type adenocarcinoma with anastomosing glands of the stomach. Mod Pathol. 2019;32:568–575. [DOI] [PubMed] [Google Scholar]
- 4.Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–587. [DOI] [PubMed] [Google Scholar]
- 5.The Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo HY, Bae YS, Kim JH, et al. Distinct expression profile of key molecules in crawling-type early gastric carcinoma. Gastric Cancer. 2017;20:612–619. [DOI] [PubMed] [Google Scholar]
- 7.Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–1253. [DOI] [PubMed] [Google Scholar]
- 8.Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401:1655–1668. [DOI] [PubMed] [Google Scholar]
- 9.Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rokutan H, Hosoda F, Hama N, et al. Comprehensive mutation profiling of mucinous gastric carcinoma. J Pathol. 2016;240:137–148. [DOI] [PubMed] [Google Scholar]
- 11.Su X, Zhan P, Gavine PR, et al. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer. 2014;110:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn S, Lee J, Hong M, et al. FGFR2 in gastric cancer: protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod Pathol. 2016;29:1095–1103. [DOI] [PubMed] [Google Scholar]
- 13.Ushiku T, Ishikawa S, Kakiuchi M, et al. RHOA mutation in diffuse-type gastric cancer: a comparative clinicopathology analysis of 87 cases. Gastric Cancer. 2016;19:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng HC, Li XH, Hara T, et al. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori Y, Itoh H, Uchino S, et al. Immunohistochemical detection of K-sam protein in stomach cancer. Clin Cancer Res. 1996;2:1373–1381. [PubMed] [Google Scholar]
- 16.Shinozaki A, Ushiku T, Morikawa T, et al. Epstein-Barr virus-associated gastric carcinoma: a distinct carcinoma of gastric phenotype by claudin expression profiling. J Histochem Cytochem. 2009;57:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]