Abstract
Background:
Implant-based breast reconstruction after nipple-sparing mastectomy (NSM) presents unique benefits and challenges. The literature has compared outcomes among total submuscular (TSM), dual-plane (DP), and prepectoral (PP) planes; however, a dedicated meta-analysis relevant to NSM is lacking.
Methods:
We conducted a systematic review of studies on immediate breast reconstruction after NSM using TSM, DP, or PP prosthesis placement in PubMed, Embase, and Cochrane databases. In total, 1317 unique articles were identified, of which 49 were included in the systematic review and six met inclusion criteria for meta-analysis. Pooled descriptive outcomes were analyzed for each cohort for all 49 studies. Fixed-effects meta-analytic methods were used to compare PP with subpectoral (TSM and DP) reconstructions.
Results:
A total of 1432 TSM, 1546 DP, and 1668 PP reconstructions were identified for descriptive analysis. Demographics were similar between cohorts. Pooled descriptive outcomes demonstrated overall similar rates of reconstructive failure (3.3%–5.1%) as well as capsular contracture (0%–3.9%) among cohorts. Fixed-effects meta-analysis of six comparative studies demonstrated a significantly lower rate of mastectomy flap necrosis in the PP cohort compared with the subpectoral cohort (relative risk 0.24, 95% confidence interval [0.08–0.74]). All other consistently reported outcomes, including, hematoma, seroma, infection, mastectomy flap necrosis, nipple -areola complex necrosis, and explantation were comparable.
Conclusions:
A systematic review of the literature and meta-analysis demonstrated the safety of immediate prepectoral breast reconstruction after NSM, compared with submuscular techniques. Submuscular reconstruction had a higher risk of mastectomy flap necrosis, though potentially influenced by selection bias.
Takeaways
Question: Which plane of reconstruction is best after nipple-sparing mastectomy?
Findings: A systematic review of six comparative studies identified a significantly lower rate of mastectomy flap necrosis after prepectoral reconstruction compared with the subpectoral cohort (RR 0.24). However, prepectoral reconstructions had fewer risk factors for complications. All other outcomes were equivocal.
Meaning: This novel meta-analysis shows similar complication rate profiles between different planes of reconstruction after nipple-sparing mastectomy.
INTRODUCTION
Nipple-sparing mastectomy (NSM) has become a readily described procedure due to establishment of its oncologic safety in certain patient populations and improved psychosocial outcomes from nipple preservation.1–5 However, NSM is a technically more challenging procedure and has additional considerations, particularly with regard to mastectomy flap and nipple–areola complex (NAC) necrosis due to preservation of the entire skin envelope.
Multiple planes for prosthesis placement in immediate breast reconstruction have been described, including submuscular techniques [dual-plane (DP) or total submuscular (TSM) planes] and, more recently, prepectoral (PP) reconstruction.5–10 Each of these different procedures has different benefits as well as risks that must be considered in light of case-specific concerns and the patient’s desired reconstructive goals.9,11 Subpectoral (SP) reconstruction provides an additional layer of soft tissue coverage, but can be associated with animation deformity as well as increased pain and upper extremity disability.6,12 On the other hand, PP reconstruction avoids the morbidity of subpectoral techniques, but is highly reliant on both mastectomy flap quality and thickness for successful reconstructive and aesthetic outcomes.13,14
Nipple-sparing mastectomy critically exaggerates the importance of these different considerations between implant planes. Preservation of the entirety of the skin envelope in NSM increases the importance of mastectomy flap quality and the concern for NAC or skin ischemia. Contributing factors include a larger surface area and longer skin flaps for perforating vessels to traverse, more difficult access that may result in retraction injury, challenges secondary to macromastia and ptosis, and decreased ability to excise larger areas concerning for hypoperfusion. In prepectoral techniques, which lack interpositional vascularized soft tissue, mastectomy flap and NAC necrosis can be particularly devastating.13
Although recent meta-analyses have compared different implant planes, a large-scale review specifically isolating these outcomes in NSM and investigating the optimal plane for breast reconstruction after NSM is lacking in the literature.6 The purpose of this study was to perform a systematic review and meta-analysis comparing outcomes between prepectoral and subpectoral implant-based breast reconstruction after NSM.
METHODS
Literature Review
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Review and Meta-analyses guidelines. A reproducible search strategy was developed and conducted through March 2022 to query the PubMed/MEDLINE, Embase, and Cochrane Database of Controlled Trials for studies on implant-based reconstruction after NSM. Both controlled vocabularies (eg, MeSH terms) and keywords in the title or abstract fields were searched. (See appendix, Supplemental Digital Content 1, Search strategy as tailored for search in PubMed is reported. Similar search strategies were used in other databases after modification for each engine’s specific language. http://links.lww.com/PRSGO/D203.)
Articles were independently screened by two reviewers at each stage. Inclusion criteria included original retrospective or prospective case series, cohort studies or controlled trials. Exclusion criteria included nonprimary literature or literature without outcomes (ie, abstracts, review articles, letters to the editor) and non-English articles. Only studies that reported separate outcomes for each plane of reconstruction were included; studies that only reported pooled outcomes for mixed cohorts (eg, a combined TSM and DP cohort) were excluded. Level of evidence was defined by ASPS guidelines.15 Study quality was assessed by two blinded independent reviewers using the methodological index for nonrandomized studies criteria. References from full-text articles were additionally searched for relevant articles.
Data Analysis
Relevant data were extracted from the included articles with data coding to accommodate differences in reporting of complications. Per-breast and per-patient outcomes were considered with separate denominators. If medians and interquartile range or ranges were reported, then a conversion to mean and SD was performed using established methods.16 Specifically, for infection, both major and minor were included. Necrosis and ischemia to the mastectomy flap were both included as “flap ischemia.” For capsular contracture, only Baker grades 3 or 4 were included, as these are generally indications for revision. NSM was analyzed in an intention-to-treat fashion; ie, if the NAC was lost or later removed due to positive margin, the procedure was still considered an NSM. Significant deviations from standard NSM technique such as robotic mastectomy were excluded.
Studies with overlapping patients were considered carefully. If multiple studies reported outcomes of overlapping patient populations, then the most complete data possible was included. When possible, complications were classified as major (if explicitly designated as such by papers or correlating to a Clavein–Dindo scale grade 3 or 4) or minor (if designated by papers or correlating to a Clavein–Dindo scale grade 1 or 2).
Data analysis was performed using R statistical software [R Core Team (2021) R Foundation for Statistical Computing, Vienna, Austria].17 Means were compared for variables when possible. Descriptive analysis was performed using pooled means and percentages between all planes. Meta-analytic comparison of outcomes between subpectoral (SP) (a composite cohort including both TSM and DP) and PP cohorts was performed. Due to low I2 values, fixed-effects analysis was performed. As only one study compared outcomes of TSM versus DP reconstructions, no meta-analysis was performed between these two planes.
RESULTS
Data Collection and Analysis
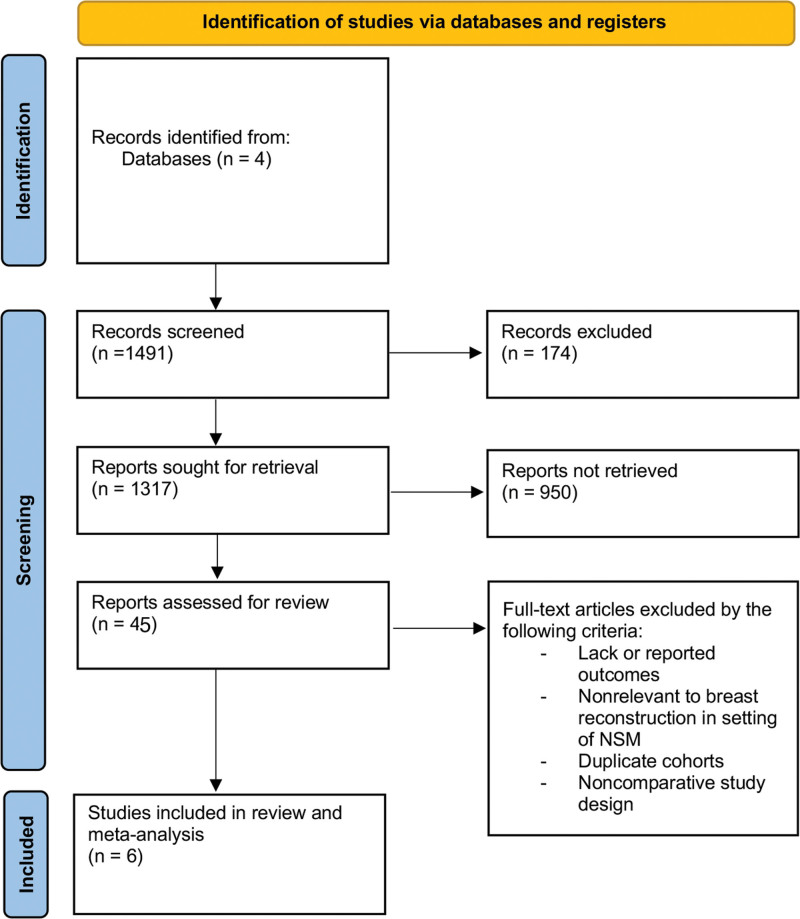
In total, 1317 unique articles were identified, of which 49 studies met inclusion criteria for noncomparative (descriptive) analysis and six studies met inclusion criteria for comparative meta-analysis (Fig. 1). Tables 1 and 2 contain summary information of included studies for the descriptive and comparative analyses, respectively. All comparative studies were level of evidence III retrospective cohort studies. Studies’ quality as per methodological index for nonrandomized studies criteria was on average 21 (range 19–23).
Fig. 1.
The Preferred Reporting Items for Systematic Review and Meta-analyses diagram outlining search strategy and results for systematic review at each stage.
Table 1.
Characteristics of Included Studies
| Study | LOE | Plane of Reconstruction | Patients (N) | Breasts (N) |
|---|---|---|---|---|
| Chen et al18 | III | TSM | 32 | 32 |
| Dayicioglu et al19 | III | TSM | 35 | 63 |
| Djohan et al20 | II | TSM | 8 | 15 |
| Li et al21 | III | TSM | 21 | 42 |
| Mori et al22 | III | TSM | 42 | 42 |
| Ozgur et al23 | III | TSM | 107 | 117 |
| Pallara et al24 | III | TSM | 56 | 56 |
| Radovanovic et al25 | III | TSM | 205 | 214 |
| Rancati et al26 | II | TSM | 22 | 30 |
| Ravazi et al27 | III | TSM | 70 | 102 |
| Sahin et al28 | III | TSM | 21 | 42 |
| Sgarzani et al29 | III | TSM | 26 | 26 |
| Shi et al30 | III | TSM | 35 | 37 |
| Verheyden et al31 | III | TSM | 20 | 30 |
| Yazar et al32 | III | TSM | 100 | 144 |
| Beier et al33 | III | DP | 53 | 73 |
| Dorfman et al34 | II | DP | 59 | 102 |
| Frey et al35 | III | DP | NR | 765 |
| Rodriguez-Feliz et al36 | III | DP | 14 | 27 |
| Ashikari et al37 | III | DP | 65 | 130 |
| Chen et al18 | III | DP | 27 | 27 |
| El Hage Chehade et al38 | III | DP | 63 | 92 |
| Folli et al39 | II | DP | 46 | 54 |
| Oven et al40 | III | DP | 15 | 29 |
| Ozgur et al23 | III | DP | 83 | 91 |
| Patzelt et al41 | III | DP | 64 | 128 |
| Sgarzani et al29 | III | DP | 28 | 28 |
| Tasoulis et al42 | III | DP | 18 | 36 |
| Imahiyerobo et al43 | III | TSM and DP, combined | 76 | 128 |
| Pallara et al24 | III | TSM and DP, combined | 106 | 106 |
| Peled et al44 | III | TSM and DP, combined | 91 | NR |
| Casella et al45 | II | PP | 46 | 92 |
| Cuomo et al46 | II | PP | 14 | 14 |
| de Vita et al47 | III | PP | 21 | 34 |
| Downs et al48 | III | PP | 45 | 79 |
| Fin et al49 | III | PP | 32 | 33 |
| Khalil et al50 | III | PP | 8 | 16 |
| Manrique et al51 | III | PP | 9 | 17 |
| Manrique et al52 | III | PP | 40 | 75 |
| Nahabedian et al53 | III | PP | 6 | 8 |
| Onesti et al54 | III | PP | 10 | 13 |
| Parus and Venturi55 | III | PP | 6 | 12 |
| Reitsamer et al56 | II | PP | 134 | 200 |
| Salibian et al3 | III | PP | 155 | 250 |
| Woo et al57 | III | PP | 21 | 23 |
LOE, level of evidence; NR, not reported.
Table 2.
Characteristics of Comparative Studies
| Study Name | LOE | Prepectoral | Subpectoral | Subpectoral Plane | Follow-up Period, mo | ||
|---|---|---|---|---|---|---|---|
| Patients, n | Breasts, n | Patients, n | Breasts, n | ||||
| Avila et al7 | III | 116 | 203 | 114 | 202 | DP | NR |
| ElSherif et al8 | III | NR | 205 | NR | 366 | TSM | 20 |
| Franceschini et al5 | III | 82 | 109 | 95 | 146 | TSM | 20 (PP), 16 (TSM) |
| Ng et al9 | III | 40 | 50 | 40 | 59 | DP | 21 (PP), 26.5 (DP) |
| Sbitany et al58 | III | 51 | 84 | 115 | 186 | DP | 11.1 (PP), 12.5 (DP) |
| Braun et al10 | III | 116 | 209 | 44 | 79 | DP | 16 (PP), 24 (DP) |
LOE, level of evidence, NR, not reported.
Patient and Surgical Factors of All Included Studies
A total of 4646 breasts in 2597 patients were represented in the included studies (Table 3). Prosthesis plane included TSM (1432 breasts, 845 patients), DP (1546 breasts, 833 patients), or PP (1668 breasts, 919 patients) reconstructions. Because not all studies reported both the total number of patients and the total number of breasts, overall conclusions about number of unilateral or bilateral nature of reconstruction could not always be designated.
Table 3.
Patient Demographics and Surgical Factors of All Included Studies
| Patient and Surgical Factors | TSM | DP | PP | |||
|---|---|---|---|---|---|---|
| Sample Size | ||||||
| Patients, n* | 845+ | 833+ | 919+ | |||
| Breasts, n | 1432 | 1546 | 1668 | |||
| Factor | Value | N Reporting | Value | N Reporting | Value | N Reporting |
| Age, mean (y) | 44.3 | 686 | 45.2 | 723 | 46.3 | 764 |
| BMI, mean | 23.5 | 251 | 23.7 | 630 | 25.0 | 610 |
| Diabetes, % (n) | 2.8 (7) | 248 | 3.7 (15) | 405 | 4.6 (18) | 391 |
| Tobacco use, % (n) | 23.9 (39) | 163 | 7.2 (46) | 635 | 5 (26) | 521 |
| XRT, preoperative, % (n) | 2.3 (11) | 475 | 2.6 (25) | 973 | 6.6 (72) | 1098 |
| XRT, adjuvant, % (n) | 27.2 (126) | 463 | 11.6 (89) | 766 | 11.3 (126) | 1116 |
| Mastectomy weight, mean (g) | 431.1 | 212 | 375.3 | 313 | 373.7 | 459 |
| Implant size, mean (mL) | 367.0 | 562 | 396.5 | 565 | 400.3 | 771 |
| ADM use, % (n) | 0 (0) | 609 | 96.9 (1310) | 1352 | 66.6 (97) | 1455 |
| Diagnosis | 757 | 499 | 799 | |||
| Prophylactic, % (n) | 20.1 (152) | 58.9 (294) | 40.8 (326) | |||
| Cancer, % (n) | 64.7 (453) | 26.5 (132) | 46.8 (374) | |||
| Staged | 1330 | 1324 | 1660 | |||
| TEE, % (n) | 35.1 (467) | 37.1 (491) | 41.3 (686) | |||
| DTI, % (n) | 64.1 (853) | 62.6 (829) | 58.4 (969) | |||
| Implant Type | 432 | 394 | 819 | |||
| Smooth, % (n) | 2.5 (11) | 35.3 (139) | 45.1 (369) | |||
| Textured, % (n) | 97.5 (421) | 57.4 (226) | 48.7 (399) | |||
| Mastectomy Incision | 700 | 953 | 952 | |||
| Radial, % (n) | 64.0 (448) | 15.6 (149) | 13.1 (125) | |||
| IMF, % (n) | 29.3 (205) | 57.3 (546) | 80.8 (769) | |||
| Wise pattern, % (n) | 3.7 (26) | 14.5 (138) | 2 (19) | |||
| Periareolar, % (n) | 2.6 (18) | 10.2 (97) | 2.9 (28) | |||
Sample size (patients) was not reported by all studies.
All three cohorts had a mean age under 50 years and a mean body mass index (BMI) at or below 25 kg per m2. Comorbidities such as diabetes and prior radiotherapy were relatively uncommon in all groups. Tobacco use was relatively high in the TSM cohort (23.9%) versus the DP and PP cohorts (7.2% and 5.0%, respectively), although tobacco use was also not reported consistently in the TSM cohort. The TSM cohort also had a high rate of adjuvant radiotherapy (27.2%).
Surgical characteristics included similar mean mastectomy weights between all cohorts (373.7–431.1 g). Mastectomy incision choice was most commonly inframammary fold (IMF) in the DP (57.3%) and PP (80.8%) cohorts, and a radial incision (64.0%) in the TSM cohort. Mean implant size was similar between all cohorts, between 367.0 and 400.3 mL. Acellular dermal matrix (ADM) use was included in almost all (96.9%) DP reconstructions reporting this outcome and in 66.6% of PP reconstructions. Single-stage DTI reconstructions were more common than staged reconstructions in all cohorts (64.1% of TSM, 62.6% of DP, and 58.4% of PP). Most reconstructions used textured implants (97.5% of TSM, 57.4% of DP, and 48.7% of PP), although implant type was also not universally reported.
Pooled rates of complications and outcomes are provided in Table 4 for reference, with statistical comparisons performed only in meta-analysis of comparative studies. Rates of notable complications included reconstructive failure (3.8% in TSM, 3.6% in DP, and 5.1% in PP), mastectomy flap necrosis (6.5% in TSM, 5.3% in DP, and 4.0% in PP cohorts), NAC necrosis (4.7% in TSM, 5.5% in DP, and 4.2% in PP), and seroma (2.6% in TSM, 3.6% in DP, and 6.5% in PP). Capsular contracture rates were 4.8% in TSM, 0.3% in DP, and 3.1% in PP. Rippling was notably high in the prepectoral cohort (10.6%) and lower in submuscular planes (0.9% in TSM, 4.8% in DP). Animation deformity was reported in 10.3% of TSM and 5.1% of DP reconstructions. BREAST-Q satisfaction scores were between 60 and 93 across all domains in all cohorts.
Table 4.
Descriptive Outcomes and Complications of All Included Studies
| Outcomes and Complications | TSM | DP | PP | |||
|---|---|---|---|---|---|---|
| Complications | % (n) | N Reporting | % (n) | N Reporting | % (n) | N Reporting |
| Mastectomy flap necrosis | 6.5 (54) | 837 | 5.3 (72) | 1366 | 4.0 (37) | 923 |
| NAC necrosis, any | 4.7 (27) | 577 | 5.5 (60) | 1095 | 4.2 (57) | 1362 |
| NAC necrosis, total | 0.9 (5) | 577 | 1.7 (15) | 893 | 1.2 (11) | 909 |
| Infection | 5.5 (64) | 1169 | 3.8 (54) | 1426 | 5.7 (83) | 1460 |
| Seroma | 2.6 (13) | 493 | 3.6 (34) | 940 | 6.5 (58) | 893 |
| Hematoma | 1.1 (5) | 453 | 1.4 (11) | 801 | 1.9 (20) | 1027 |
| Dehiscence | 2.3 (6.9) | 302 | 2.7 (19) | 705 | 0.6 (2) | 349 |
| Capsular contracture | 4.8 (18) | 376 | 0.3 (1) | 323 | 3.1 (21) | 684 |
| Rippling | 0.9 (1.1) | 117 | 4.8 (20) | 421 | 10.6 (68) | 641 |
| Animation deformity | 10.3 (12.1) | 117 | 5.1 (6) | 118 | 0 (0) | 34 |
| Reconstructive failure | 3.8 (23.2) | 611 | 3.6 (49) | 1352 | 5.1 (64) | 1263 |
| BREAST-Q * | Score | N Reporting | Score | N Reporting | Score | N Reporting |
| Satisfaction with breasts | 70.7 | 58 | 70.2 | 159 | 71.5 | 86 |
| Psychosocial wellbeing | 74.4 | 58 | 76.4 | 159 | 77.4 | 86 |
| Sexual wellbeing | 63.2 | 58 | 62.4 | 159 | 60.9 | 86 |
| Physical wellbeing | 66.8 | 58 | 69.1 | 159 | 78.3 | 86 |
| Overall satisfaction | 93 | 26 | 82.9 | 92 | 83.6 | 67 |
Scores represent weighted means.
Meta-analysis
Six studies compared subpectoral with prepectoral reconstructions and were included for meta-analysis. The SP cohort comprised a pooled cohort of TSM and DP patients (Table 2). Because only a single study compared TSM with DP cohorts, no meta-analysis could be performed between these planes.23 Table 5 details patient and surgical factors for the six comparative studies. Most demographics and risk factors were similar between the PP and SP cohorts, including age (46.4 versus 47.4 years), BMI (24.4 versus 24.3), diabetes (5.3% versus 3.5%) and preoperative (2.3% versus 1.6%) and adjuvant (11.3% versus 10.9%) radiation. Subpectoral reconstructions had a higher rate of tobacco use compared with prepectoral cases (6.7% versus 2.7%) as well as two-stage tissue expander reconstruction (58.0% versus 41.9%) as opposed to direct-to-implant.
Table 5.
Patient Demographics and Surgical Factors in Comparative Studies
| PP | SP | |||||||
|---|---|---|---|---|---|---|---|---|
| All SP | TSM | DP | ||||||
| Sample Size | ||||||||
| Patients, n* | 405+ | 408+ | 95+ | 313 | ||||
| Breasts, n | 860+ | 1038 | 512 | 526 | ||||
| Factor | Value | N Reporting | Value | N Reporting | Value | N Reporting | Value | N Reporting |
| Age, mean (y) | 46.4 | 405 | 47.4 | 408 | 44 | 95 | 47.1 | 313 |
| BMI, mean | 24.4 | 405 | 24.3 | 408 | 24.8 | 95 | 24.2 | 313 |
| Diabetes, % (n) | 5.3 (11) | 207 | 3.5 (7) | 199 | NR | 0 | 3.5 (7) | 199 |
| Tobacco use, % (n) | 2.2 (7) | 323 | 6.7 (21) | 313 | NR | 0 | 6.7 (21) | 313 |
| XRT, preoperative, % (n) | 2.3 (13) | 571 | 1.6 (8) | 486 | 0 (0) | 146 | 2.4 (8) | 340 |
| XRT, adjuvant, % (n) | 11.3 (51) | 452 | 10.9 (51) | 470 | 15.1 (22) | 146 | 9.0 (29) | 324 |
| Mastectomy weight, mean (g) | 372.4 | 272 | 356.3 | 198 | NR | 0 | 356.3 | 198 |
| Implant size, mean (mL) | 442.4 | 323 | 434.5 | 313 | NR | 0 | 434.5 | 313 |
| ADM use, % (n) | 75.1 (492) | 655 | 48.5 (1038) | 672 | 0 | 512† | 96.0 (503) | 526 |
| Diagnosis | 209 | 79 | 0 | 79 | ||||
| Prophylactic, % (n) | 62.2 (130) | 59.5 (47) | NR | 59.5 (47) | ||||
| Cancer, % (n) | 37.8 (79) | 40.5 (32) | NR | 40.5 (32) | ||||
| Staged | 860 | 1038 | 512 | 526 | ||||
| TE, % (n) | 41.9 (360) | 58.0 (602) | 41.6 (213) | 74.0 (389) | ||||
| DTI, % (n) | 57.4 (494) | 41.6 (432) | 58.4 (299) | 25.3 (133) | ||||
| Implant Type | 312 | 348 | 146 | 202 | ||||
| Smooth, % (n) | 52.6 (164) | 22.4 (78) | 0 (0) | 38.6 (78) | ||||
| Textured, % (n) | 46.2 (144) | 69.3 (241) | 100 (146) | 47.1 (95) | ||||
| Mastectomy Incision | 412 | 281 | 0 | 281 | ||||
| Radial, % (n) | 17.2 (71) | 3.9 (11) | NR | 8.9 (25) | ||||
| IMF, % (n) | 79.1 (326) | 85.4 (240) | NR | 85.4 (240) | ||||
| Wise pattern, % (n) | 1.0 (4) | 0 | NR | 0 | ||||
| Periareolar, % (n) | 0 | 0 | NR | 0 | ||||
Sample size (patients) was not reported by all studies. NR, not reported.
All TSM reconstructions were assumed to be performed without ADM if not specified.
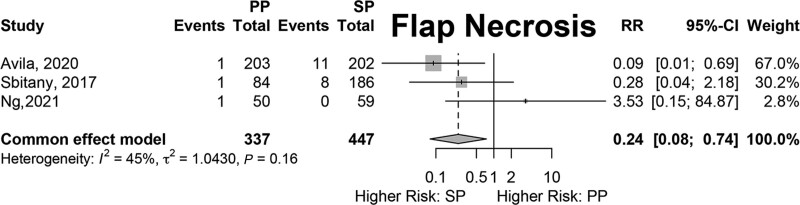
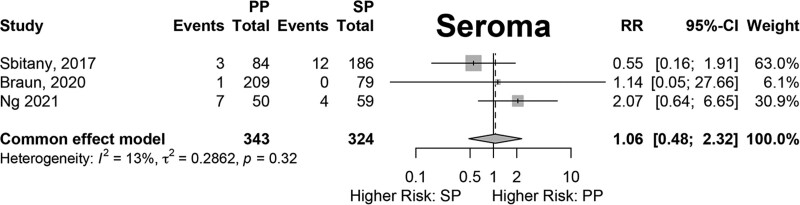
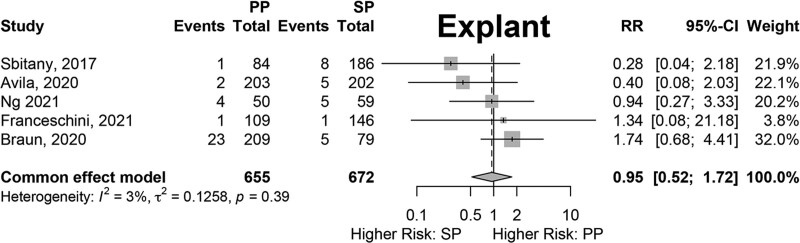
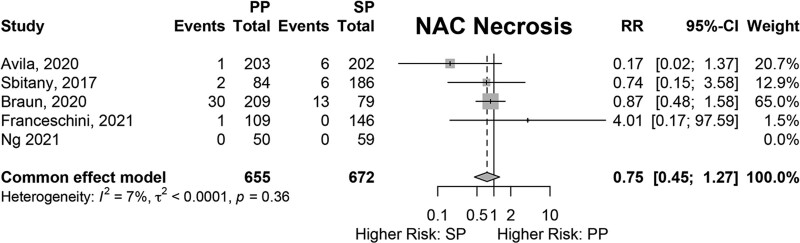
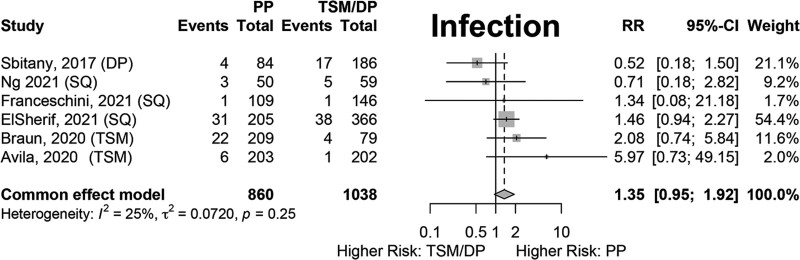
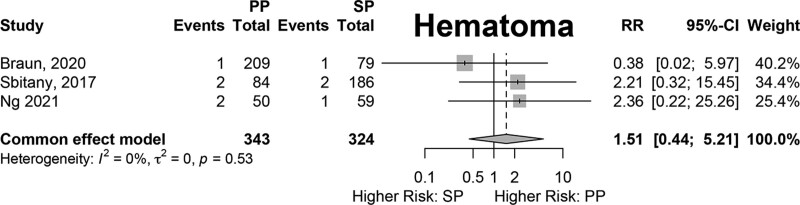
Results of fixed-effects meta-analysis of six comparative studies performed (Figs. 2–7) demonstrated a significantly decreased risk of mastectomy flap necrosis in prepectoral reconstruction compared with subpectoral [relative risk (RR) 0.24, 95% confidence interval (CI) 0.08–0.74] (Fig. 2). Rates of other complications were not different between cohorts (Figs. 3–7), including reconstructive failure (RR 0.95, 95% CI 0.52–1.72), NAC necrosis (RR 0.75, 95% CI 0.45–1.27), infection (RR 1.35, 95% CI 0.95–1.92), hematoma (RR 1.51, 95% CI 0.44–5.21), and seroma (RR 1.06, 95% CI 0.48–2.32). Publication bias for each outcome was assessed visually using funnel plots, which were felt to be symmetric for each outcome, indicating relative lack of significant publication bias, although the small number of included studies limits interpretation. [See figure, Supplemental Digital Content 2, funnel plots for publication bias. Funnel plots representing risk of bias from meta-analysis are shown. Outcomes include (a) mastectomy necrosis, (b) explant, (c) nipple-areolar complex necrosis, (d) infection, (e) hematoma, and (f) seroma. http://links.lww.com/PRSGO/D204.]
Fig. 2.
Fixed-effects meta-analysis results for mastectomy flap necrosis. A significantly lower rate of mastectomy flap necrosis was noted in the prepectoral group (RR 0.24).
Fig. 7.
Fixed-effects meta-analysis results for seroma. No significant difference was found between the prepectoral and subpectoral groups.
Fig. 3.
Fixed-effects meta-analysis results for explant. No significant difference was found between the prepectoral and subpectoral groups.
Fig. 4.
Fixed-effects meta-analysis results for nipple-areolar complex necrosis. No significant difference was found between the prepectoral and subpectoral groups.
Fig. 5.
Fixed-effects meta-analysis results for infection. No significant difference was found between the prepectoral and subpectoral groups.
Fig. 6.
Fixed-effects meta-analysis results for hematoma. No significant difference was found between the prepectoral and subpectoral groups.
DISCUSSION
This study provides the first systematic review and meta-analysis of implant-based breast reconstruction after NSM with comparison of outcomes between PP and SP reconstruction. A recent meta-analysis of breast reconstruction by Saldanha, et al compared outcomes between various planes of breast reconstruction.6 Their findings suggest lower rates of pain and upper extremity disability in PP reconstructions, with comparable rates of most measured complications across all implant planes. Specifically, rates of necrosis and infection were found to be similar between planes by two individual included studies, but with wide odds ratios and inability to perform meta-analysis.59,60 Although Saldanha’s review provided insight into many aspects of breast reconstruction, it included all types of mastectomy (NSM, skin-sparing, and simple mastectomy) without controlling for nipple preservation. At least four other recent reviews have used similar methodology.61–64 Murphy et al also recently reviewed ADM versus no-ADM reconstructions, but did not specifically stratify by plane of reconstruction or nipple preservation.65 Given the critical implications of preservation of the entirety of the skin envelope on ischemic complications of the mastectomy flap and NAC, which also influence infection and reconstructive failure, this study sought to specifically compare outcomes among different planes of reconstruction only in immediate reconstruction after NSM.
The total pool of patients for descriptive analysis was robust, incorporating 4646 reconstructed breasts, though studies ranged over a longer period (1998–2022). The overall sample of patients in the comparative meta-analysis was also large, incorporating up to 1898 reconstructions with more recent studies (range 2017–2021). Results of the meta-analysis demonstrate comparable rates of most complications between PP and SP reconstructions, apart from mastectomy flap necrosis being more common in the SP cohort.
Demographics and patient factors were overall similar between the cohorts in the meta-analysis. However, certain features of the SP cohort may potentially explain the choice to use this plane, such as the higher incidence of tobacco use (6.7%), which could be considered as a relative contraindication to PP reconstruction. Importantly, with the smaller sample in the comparative studies, many factors are not reported consistently enough in the included study to draw definitive conclusions.
The meta-analysis demonstrated significantly lower rates of mastectomy flap necrosis in PP compared with SP reconstructions. One potential explanation is preoperative and intraoperative selection bias given the retrospective, nonrandomized nature of the studies included in the meta-analysis. Preoperative decision-making may funnel poor candidates for PP reconstruction or those with a higher risk of potential mastectomy flap necrosis (smoking, ptosis, macromastia) into the SP cohorts. Additionally, intraoperative decision-making based on mastectomy flap quality is likely the most critical factor and would select for patients with potential mastectomy flap ischemia based on clinical or imaging evaluation to receive subpectoral reconstructions due to concerns about potential flap ischemia. More recently, indocyanine green (ICG) angiography has been used to assist intraoperative assessment of mastectomy flap perfusion, but this was only mentioned by two of the comparative studies (Avila et al, and Franceschini et al). Sbitany et al report eight cases of mastectomy flap necrosis in their DP cohort and only one in their PP cohort.58 However, the authors discuss that threatened flaps were treated with either delayed reconstruction or with submuscular reconstruction, potentially explaining their higher rate of flap necrosis in the DP cohort. The utilization of TE reconstruction (rather than DTI) may in some cases reflect a high-risk reconstruction. We identified a higher rate of TE reconstruction in the TSM cohort (58.0%) versus the pooled SP cohort (41.9%) in the comparative analysis (Table 5).
Learning curve within each study may also contribute to these findings given the later adoption of PP reconstruction compared with SP techniques. Avila et al report one incidence of mastectomy flap necrosis in their PP cohort and 11 in their SP cohort.7 However, closer examination of their data demonstrate that nine of the SP flap necroses occurred within a single year (2015). The occurrence of the majority of mastectomy flap necrosis early on in their study period may suggest contribution of a learning curve to the observed outcomes.
It is inherently logical that optimal candidates are chosen for PP reconstruction after NSM based on both preoperative and intraoperative factors. Although this bias is important to consider during interpretation of the data, it does not undermine the findings of this study, which demonstrate that with the appropriate indications, immediate PP reconstruction after NSM is safe compared with SP techniques. Such conclusions from larger samples as afforded by meta-analysis are useful to broaden the applicability of PP reconstruction to the unique challenges of NSM.
It remains important to keep in consideration that meta-analysis does not attempt to control for all possible patient factors and clinical scenarios. Treatment choices, as always, should be individualized. Careful preoperative patient selection plays a critical role in determining who is a candidate for PP reconstruction after NSM. Equally if not arguably more important is clinical and imaging assessment of the mastectomy flap quality, particularly with regards to NSM, in determination of the optimal reconstructive techniques.5,52,66
Long-term outcomes are also critical to consider in implant-based reconstruction. Capsular contracture rates across all three techniques were low, ranging from 0.3% to 4.8%. As would be expected, animation deformity was noted in 10.3% of TSM and 5.1% of DP reconstructions and remains a principle factor in advocating for PP reconstruction. On the other hand, rates of rippling were higher in PP reconstructions (10.6%) compared with TSM and DP planes (0.9% and 4.8%) in the descriptive analysis. Although this outcome could not be comparatively analyzed, the observation is similarly intuitive, given the decreased soft tissue coverage in the PP plane. However, rippling is influenced by many important variables that were not analyzed, including absolute subcutaneous tissue thickness of the mastectomy flaps, ADM use, patient BMI, implant cohesivity, and utilization of fat grafting. More importantly, this demonstrates that while PP reconstruction avoids animation deformity and has comparable short-term outcomes to SP reconstruction, additional aesthetic considerations such as a potential increased risk of rippling must be discussed with patients preoperatively. There is no “perfect” technique.
One potential confounding variable not assessed in this analysis is the use of ADM. ADM serves different roles in different implant planes. For example, PP reconstructions often rely on ADM for definition of the implant pocket and prosthesis support. Prepectoral reconstruction without ADM, however, has also been readily described.67 A recent systematic review identified similar complication rates between ADM- and no-ADM PP reconstructions, although these were not limited to NSM.67 The role of ADM in this technique continues to require further elucidation. Additionally, a wide variety of ADMs are available, with no consensus regarding the optimal material to be used.65 Furthermore, given the paucity of studies comparing TSM and DP reconstructions, this differentiation was not able to be analyzed in the meta-analysis. However, these comparative studies of these techniques have been well described in the literature, with a recent meta-analysis demonstrating lower overall rates of complications in TSM compared with DP reconstructions with mesh.65
Our study has several limitations that are important to consider when deriving conclusions and interpreting the results. Only six comparative studies were included in the meta-analysis; however, overall sample size was large. Most of the data from the literature are subject to selection bias with respect to which patients were appropriate to undergo PP reconstruction. Although several risk factors were comparable between PP and SP cohorts, certain factors such as smoking were higher in the TSM cohort, suggesting selection of certain higher-risk patients in TSM reconstruction. Intraoperative surgeon decision-making was also not controlled for, particularly with regard to the critical variable of mastectomy flap quality. Several studies included in the meta-analysis noted that the common practice of their surgeons changed over time, suggesting that time spent in practice may not have been equitable between cohorts.7,68 Additionally, the low incidence of smoking and diabetes and low BMI potentially limit the external applicability of the findings. Finally, long-term outcomes, including capsular contracture and patient-reported outcomes, were unable to be included in the meta-analysis due to low rates of reporting in comparative studies and remain critical endpoints in evaluation of implant-based techniques.
CONCLUSIONS
This systemic review and meta-analysis of patients undergoing breast reconstruction in the PP, DP, or TSM planes after NSM demonstrates comparable rates of complications between PP and SP cohorts in properly selected patients. A higher rate of mastectomy flap necrosis in the SP cohort was observed and may reflect selection bias of threatened flaps to receive SP reconstructions and the learning curve in performing NSM. Importantly, there was no difference in reconstructive failure and NAC necrosis between reconstructive planes. These findings suggest that in the appropriate patient, immediate PP breast reconstruction is a safe method of reconstruction in patients undergoing NSM that carries unique benefits and challenges compared with traditional mastectomy techniques.
DISCLOSURES
Dr. Salibian is a research consultant for Abbvie, Inc. All the other authors have no financial interest to declare in relation to the content of this article. Abbvie, Inc. did not have any involvement in the conception, design, or execution of this study.
Supplementary Material
Footnotes
Published online 14 May 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Agarwal S, Agarwal S, Neumayer L, et al. Therapeutic nipple-sparing mastectomy: trends based on a national cancer database. Am J Surg. 2014;208:93–98. [DOI] [PubMed] [Google Scholar]
- 2.Headon HL, Kasem A, Mokbel K. The oncological safety of nipple-sparing mastectomy: a systematic review of the literature with a pooled analysis of 12,358 procedures. Arch Plast Surg. 2016;43:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salibian AH, Harness JK, Mowlds DS. Staged suprapectoral expander/implant reconstruction without acellular dermal matrix following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:30–39. [DOI] [PubMed] [Google Scholar]
- 4.Palmieri B, Baitchev G, Grappolini S, et al. Delayed nipple-sparing modified subcutaneous mastectomy: rationale and technique. Breast J. 2005;11:173–178. [DOI] [PubMed] [Google Scholar]
- 5.Franceschini G, Scardina L, Di Leone A, et al. Immediate prosthetic breast reconstruction after nipple-sparing mastectomy: traditional subpectoral technique versus direct-to-implant prepectoral reconstruction without acellular dermal matrix. J Pers Med. 2021;11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saldanha IJ, Cao W, Broyles JM, et al. AHRQ Comparative Effectiveness Reviews. In: Breast Reconstruction After Mastectomy: A Systematic Review and Meta-Analysis. Rockville (MD): Agency for Healthcare Research and Quality; 2021. [PubMed] [Google Scholar]
- 7.Avila A, Bartholomew AJ, Sosin M, et al. Acute postoperative complications in prepectoral versus subpectoral reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2020;146:715e–720e. [DOI] [PubMed] [Google Scholar]
- 8.ElSherif A, Cocco D, Armanyous S, et al. Nipple-sparing mastectomy: are we providing proper prophylactic antibiotic coverage? Ann Surg Oncol. 2021;28:5486–5494. [DOI] [PubMed] [Google Scholar]
- 9.Ng EI, Quah GS, Graham S, et al. Immediate prepectoral implant reconstruction using TiLOOP Bra Pocket results in improved patient satisfaction over dual plane reconstruction. ANZ J Surg. 2021;91:701–707. [DOI] [PubMed] [Google Scholar]
- 10.Braun SE, Dreicer M, Butterworth JA, et al. Do nipple necrosis rates differ in prepectoral versus submuscular implant-based reconstruction after nipple-sparing mastectomy? Ann Surg Oncol. 2020;27:4760–4766. [DOI] [PubMed] [Google Scholar]
- 11.Young WA, Degnim AC, Hoskin TL, et al. Outcomes of >1300 nipple-sparing mastectomies with immediate reconstruction: the impact of expanding indications on complications. Ann Surg Oncol. 2019;26:3115–3123. [DOI] [PubMed] [Google Scholar]
- 12.Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg. 2011;128:1162–1169. [DOI] [PubMed] [Google Scholar]
- 13.Salibian AA, Frey JD, Choi M, et al. Optimizing the mastectomy flap to improve aesthetic outcomes. Aesthet Surg J. 2020;40:S1–S12. [DOI] [PubMed] [Google Scholar]
- 14.Frey JD, Salibian AA, Choi M, et al. The importance of tissue perfusion in reconstructive breast surgery. Plast Reconstr Surg. 2019;144(1S):21S–29S. [DOI] [PubMed] [Google Scholar]
- 15.ASPS. Evidence rating scales. Available at https://www.plasticsurgery.org/documents/medical-professionals/health-policy/evidence-practice/ASPS-Rating-Scale-March-2011.pdf. Published March 2011. Accessed April 18, 2024. [Google Scholar]
- 16.McGrath S, Zhao X, Steele R, et al. ; DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Team RC. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 18.Chen G, Zhang Y, Xue J, et al. Surgical outcomes of implant-based breast reconstruction using TiLoop bra mesh combined with pectoralis major disconnection. Ann Plast Surg. 2019;83:396–400. [DOI] [PubMed] [Google Scholar]
- 19.Dayicioglu D, Trotta R, Agoris C, et al. Duoderm-bra for nipple-sparing mastectomy. Ann Plast Surg. 2016;76:S280–S285. [DOI] [PubMed] [Google Scholar]
- 20.Djohan R, Scomacao I, Knackstedt R, et al. Neurotization of the nipple-areola complex during implant-based reconstruction: evaluation of early sensation recovery. Plast Reconstr Surg. 2020;146:250–254. [DOI] [PubMed] [Google Scholar]
- 21.Li XR, Zhang YJ, Wang JD, et al. Application of immediate breast reconstruction with silicon prosthetic implantation following bilateral mammary gland excision in treatment of young patients with early breast cancer. J Thorac Dis. 2013;5:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori H, Uemura N, Okazaki M, et al. Nipple malposition after nipple-sparing mastectomy and expander-implant reconstruction. Breast Cancer. 2016;23:740–744. [DOI] [PubMed] [Google Scholar]
- 23.Ozgur I, Kurul S, Bademler S, et al. Comparison of subpectoral versus dual-plane implant based immediate breast reconstruction after nipple-areola sparing mastectomy. Ann Chir Plast Esthet. 2021;66:447–458. [DOI] [PubMed] [Google Scholar]
- 24.Pallara T, Cagli B, Fortunato L, et al. Direct-to-implant and 2-stage breast reconstruction after nipple sparing mastectomy: results of a retrospective comparison. Ann Plast Surg. 2019;83:392–395. [DOI] [PubMed] [Google Scholar]
- 25.Radovanovic Z, Radovanovic D, Golubovic A, et al. Early complications after nipple-sparing mastectomy and immediate breast reconstruction with silicone prosthesis: results of 214 procedures. Scand J Surg. 2010;99:115–118. [DOI] [PubMed] [Google Scholar]
- 26.Rancati AO, Angrigiani CH, Hammond DC, et al. Direct to implant reconstruction in nipple sparing mastectomy: patient selection by preoperative digital mammogram. Plast Reconstr Surg Glob Open. 2017;5:e1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razavi SA, Hart AL, Carlson GW. Ischemic complications after bilateral nipple-sparing mastectomy and implant-based reconstruction: a critical analysis. Ann Plast Surg. 2021;86(6S Suppl 5):S526–S531. [DOI] [PubMed] [Google Scholar]
- 28.Sahin I, Isik S, Alhan D, et al. One-staged silicone implant breast reconstruction following bilateral nipple-sparing prophylactic mastectomy in patients at high-risk for breast cancer. Aesthetic Plast Surg. 2013;37:303–311. [DOI] [PubMed] [Google Scholar]
- 29.Sgarzani R, Pasquali S, Buggi F, et al. Sub-muscular reconstruction after NAC sparing mastectomy: direct to implant breast reconstruction with human ADM versus tissue expander. Aesthetic Plast Surg. 2021;45:413–420. [DOI] [PubMed] [Google Scholar]
- 30.Shi A, Wu D, Li X, et al. Subcutaneous nipple-sparing mastectomy and immediate breast reconstruction. Breast Care (Basel). 2012;7:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verheyden CN. Nipple-sparing total mastectomy of large breasts: the role of tissue expansion. Plast Reconstr Surg. 1998;101:1494–500; discussion 1501–1492. [DOI] [PubMed] [Google Scholar]
- 32.Yazar S, Bengur FB, Altinkaya A, et al. Nipple-sparing mastectomy and immediate implant-based reconstruction with or without skin reduction in patients with large ptotic breasts: a case-matched analysis. Aesthetic Plast Surg. 2021;45:956–967. [DOI] [PubMed] [Google Scholar]
- 33.Beier L, Faridi A, Neumann C, et al. Human acellular dermal matrix (Epiflex) in immediate implant-based breast reconstruction after skin- and nipple-sparing mastectomy and treatment of capsular fibrosis: results of a multicenter, prospective, observational NOGGO-AWOGyn study. Breast Care (Basel). 2021;16:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorfman RG, Mioton L, Stone E, et al. The effect of implant type on nipple position geometry and aesthetics following tissue expander reconstruction after nipple sparing mastectomy. Aesthet Surg J. 2018;38:605–613. [DOI] [PubMed] [Google Scholar]
- 35.Frey JD, Choi M, Salibian AA, et al. Comparison of outcomes with tissue expander, immediate implant, and autologous breast reconstruction in greater than 1000 nipple-sparing mastectomies. Plast Reconstr Surg. 2017;139:1300–1310. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Feliz J, Codner MA. Embrace the change: incorporating single-stage implant breast reconstruction into your practice. Plast Reconstr Surg. 2015;136:221–231. [DOI] [PubMed] [Google Scholar]
- 37.Ashikari RH, Ashikari AY, Kelemen PR, et al. Subcutaneous mastectomy and immediate reconstruction for prevention of breast cancer for high-risk patients. Breast Cancer. 2008;15:185–191. [DOI] [PubMed] [Google Scholar]
- 38.El Hage Chehade H, Headon H, Wazir U, et al. Nipple-sparing mastectomy using a hemi-periareolar incision with or without minimal medial-lateral extensions; clinical outcome and patient satisfaction: a single centre prospective observational study. Am J Surg. 2017;213:1116–1124. [DOI] [PubMed] [Google Scholar]
- 39.Folli S, Curcio A, Melandri D, et al. A New human-derived acellular dermal matrix for breast reconstruction available for the European market: preliminary results. Aesthetic Plast Surg. 2018;42:434–441. [DOI] [PubMed] [Google Scholar]
- 40.Oven SD, Scarlett WL. Reconstruction of large ptotic breasts after nipple-sparing mastectomy: a modified buttonhole technique. Ann Plast Surg. 2020;85:233–236. [DOI] [PubMed] [Google Scholar]
- 41.Patzelt M, Zarubova L, Vecerova M, et al. Risk comparison using autologous dermal flap and absorbable breast mesh on patient undergoing subcutaneous mastectomy with immediate breast reconstruction. Aesthetic Plast Surg. 2022;46:1145–1152. [DOI] [PubMed] [Google Scholar]
- 42.Tasoulis MK, Agusti A, Karakatsanis A, et al. The use of hydrodissection in nipple- and skin-sparing mastectomy: a retrospective cohort study. Plast Reconstr Surg Glob Open. 2019;7:e2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imahiyerobo TA, Jr, Small KH, Sackeyfio R, et al. transition from round to shaped implants in immediate breast reconstruction: our preferred approach and clinical outcomes. Aesthetic Plast Surg. 2017;41:284–292. [DOI] [PubMed] [Google Scholar]
- 44.Peled AW, Foster RD, Ligh C, et al. Impact of total skin-sparing mastectomy incision type on reconstructive complications following radiation therapy. Plast Reconstr Surg. 2014;134:169–175. [DOI] [PubMed] [Google Scholar]
- 45.Casella D, Di Taranto G, Marcasciano M, et al. Nipple-sparing bilateral prophylactic mastectomy and immediate reconstruction with TiLoop Bra mesh in BRCA1/2 mutation carriers: a prospective study of long-term and patient reported outcomes using the BREAST-Q. Breast. 2018;39:8–13. [DOI] [PubMed] [Google Scholar]
- 46.Cuomo R, Giardino FR, Neri A, et al. Optimization of prepectoral breast reconstruction. Breast Care (Basel). 2021;16:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Vita R, Buccheri EM, Villanucci A, et al. Breast reconstruction actualized in nipple-sparing mastectomy and direct-to-implant, prepectoral polyurethane positioning: early experience and preliminary results. Clin Breast Cancer. 2019;19:e358–e363. [DOI] [PubMed] [Google Scholar]
- 48.Downs RK, Hedges K. An alternative technique for immediate direct-to-implant breast reconstruction-a case series. Plast Reconstr Surg Glob Open. 2016;4:e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fin A, De Biasio F, Mura S, et al. Prepectoral implant-based breast reconstruction using meshed ADM. Plast Surg (Oakv). 2021;29:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalil HH, Malahias MN, Youssif S, et al. Nipple-sparing mastectomy and prepectoral implant/acellular dermal matrix wrap reconstruction in large ptotic breasts. Plast Reconstr Surg Glob Open. 2019;7:e2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manrique OJ, Arif C, Banuelos J, et al. Prepectoral breast reconstruction in nipple-sparing mastectomy with immediate mastopexy. Ann Plast Surg. 2020;85:18–23. [DOI] [PubMed] [Google Scholar]
- 52.Manrique OJ, Huang TC, Martinez-Jorge J, et al. Prepectoral two-stage implant-based breast reconstruction with and without acellular dermal matrix: do we see a difference? Plast Reconstr Surg. 2020;145:263e–272e. [DOI] [PubMed] [Google Scholar]
- 53.Nahabedian MY, Tsangaris TN. Breast reconstruction following subcutaneous mastectomy for cancer: a critical appraisal of the nipple–areola complex. Plast Reconstr Surg. 2006;117:1083–1090. [DOI] [PubMed] [Google Scholar]
- 54.Onesti MG, Di Taranto G, Ribuffo D, et al. ADM-assisted prepectoral breast reconstruction and skin reduction mastectomy: expanding the indications for subcutaneous reconstruction. J Plast Reconstr Aesthet Surg. 2020;73:673–680. [DOI] [PubMed] [Google Scholar]
- 55.Parus A, Venturi ML. A strategic approach to nipple-sparing mastectomy reconstruction with a wide-based inframammary fold flap. Plast Reconstr Surg Glob Open. 2020;8:e3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reitsamer R, Peintinger F, Klaassen-Federspiel F, et al. Prepectoral direct-to-implant breast reconstruction with complete ADM or synthetic mesh coverage—36-Months follow-up in 200 reconstructed breasts. Breast. 2019;48:32–37. [DOI] [PubMed] [Google Scholar]
- 57.Woo J, Seung IH, Hong SE. Funnel usefulness in direct-to-implant breast reconstruction using periareolar incision with prepectoral implant placement and complete coverage with acellular dermal matrix. J Plast Reconstr Aesthet Surg. 2020;73:2016–2024. [DOI] [PubMed] [Google Scholar]
- 58.Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 59.Nealon KP, Weitzman RE, Sobti N, et al. Prepectoral direct-to-implant breast reconstruction: safety outcome endpoints and delineation of risk factors. Plast Reconstr Surg. 2020;145:898e–908e. [DOI] [PubMed] [Google Scholar]
- 60.Kraenzlin F, Darrach H, Khavanin N, et al. Tissue expander-based breast reconstruction in the prepectoral versus subpectoral plane: an analysis of short-term outcomes. Ann Plast Surg. 2021;86:19–23. [DOI] [PubMed] [Google Scholar]
- 61.Xie J, Wang M, Cao Y, et al. ADM-assisted prepectoral breast reconstruction is not associated with high complication rate as before: a meta-analysis. J Plast Surg Hand Surg. 2023;57:7–15. [DOI] [PubMed] [Google Scholar]
- 62.Abbate O, Rosado N, Sobti N, et al. Meta-analysis of prepectoral implant-based breast reconstruction: guide to patient selection and current outcomes. Breast Cancer Res Treat. 2020;182:543–554. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Su Y, Xiu B, et al. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: a systematic review and meta analysis. Eur J Surg Oncol. 2019;45:1542–1550. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Xu G, Yu N, et al. prepectoral versus subpectoral implant-based breast reconstruction: a meta-analysis. Ann Plast Surg. 2020;85:437–447. [DOI] [PubMed] [Google Scholar]
- 65.Murphy D, O’Donnell JP, Ryan É J, et al. Immediate breast cancer reconstruction with or without dermal matrix or synthetic mesh support: a review and network meta-analysis. Plast Reconstr Surg. 2023;151:563e–574e. [DOI] [PubMed] [Google Scholar]
- 66.Salibian AA, Frey JD, Bekisz JM, et al. ischemic complications after nipple-sparing mastectomy: predictors of reconstructive failure in implant-based reconstruction and implications for decision-making. Plast Reconstr Surg Glob Open. 2019;7:e2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nolan IT, Farajzadeh MM, Boyd CJ, et al. Do we need acellular dermal matrix in prepectoral breast reconstruction? A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2023;86:251–260. [DOI] [PubMed] [Google Scholar]
- 68.King CA, Bartholomew AJ, Sosin M, et al. A Critical appraisal of late complications of prepectoral versus subpectoral breast reconstruction following nipple-sparing mastectomy. Ann Surg Oncol. 2021;28:9150–9158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.