Abstract
Background:
Antibody-mediated rejection has long been known to be one of the major organ failure mechanisms in xenotransplantation. In addition to the porcine α1,3-galactose (α1,3Gal) epitope, N-Glycolylneuraminic acid (Neu5Gc), a sialic acid, has been identified as an important porcine antigen against which most humans have pre-formed antibodies. Here we evaluate GalTKO.hCD46 lungs with an additional cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) gene knock-out (Neu5GcKO) in a xenogeneic ex vivo perfusion model
Methods:
Eleven GalTKO.hCD46.Neu5GcKO pig lungs were perfused for up to 6 h with fresh heparinized human blood. Six of them were treated with histamine (H) blocker famotidine and 1-thromboxane synthase inhibitor Benzylimidazole (BIA) and five were left untreated. GalTKO.hCD46 lungs without Neu5GcKO (n = 18: eight untreated and 10 BIA+H treated) served as a reference. Functional parameters, blood, and tissue samples were collected at pre-defined time points throughout the perfusion
Results:
All but one Neu5GcKO organs maintained adequate blood oxygenation and “survived” until elective termination at 6 h whereas two reference lungs failed before elective termination at 4 h. Human anti-Neu5Gc antibody serum levels decreased during the perfusion of GalTKO.hCD46 lungs by flow cytometry (∼40% IgM, 60% IgG), whereas antibody levels in Neu5GcKO lung perfusions did not fall (IgM p = .007; IgG p < .001). Thromboxane elaboration, thrombin generation, and histamine levels were significantly reduced with Neu5GcKO lungs compared to reference in the untreated groups (p = .007, .005, and .037, respectively); treatment with BIA+H masked these changes. Activation of platelets, measured as CD62P expression on circulating platelets, was lower in Neu5GcKO experiments compared to reference lungs (p = .023), whereas complement activation (as C3a rise in plasma) was not altered. MCP-1 and lactotransferin level elevations were blunted in Neu5GcKO lung perfusions (p = .007 and .032, respectively). Pulmonary vascular resistance (PVR) rise was significantly attenuated and delayed in untreated GalTKO.hCD46.Neu5GcKO lungs in comparison to the untreated GalTKO.hCD46 lungs (p = .003)
Conclusion:
Additional Neu5GcKO in GalTKO.hCD46 lungs significantly reduces parameters associated with antibody-mediated inflammation and activation of the coagulation cascade. Knock-out of the Neu5Gc sialic acid should be beneficial to reduce innate immune antigenicity of porcine lungs in future human recipients.
Keywords: lung transplantation, Neu5Gc, swine, xenotransplantation
1 |. INTRODUCTION
Xenotransplantation is a promising treatment option for end-stage organ failure patients, with the potential to solve the limited availability of human donor organs.1–5 Recent progress in genetic engineering, such as CRISPR/Cas9 technology, coupled with advances in understanding the rejection mechanisms leading to xenograft failure have contributed to increasing xenogeneic organ survival in various models.6–8
It is well understood that pre-formed human antibodies against porcine antigens lead to acute antibody-mediated rejection and remain a hurdle to successful xenotransplantation.9,10 The introduction of genetically modified pigs with the α1,3 galactosyltransferase gene “knocked out” (GalTKO) has contributed to overcoming hyperacute rejection (HAR) of porcine xenografts.11–13 Removal of the porcine αGal epitope eliminates one major trigger to initiate a response by the immune system towards wild-type pig tissues, and provides the opportunity to better-understand secondary xenoantigens. In that context, additional porcine carbohydrate epitopes such as Neu5Gc have been identified that are recognized by other “non-Gal” anti-pig antibodies, and are thought to promote GalTKO xenograft injury and failure.14,15
Unlike most mammals, humans cannot synthesize the glycocalyx sialic acid N-glycolylneuraminic acid (Neu5Gc) due to a loss-of-function mutation in the gene encoding the sialic acid-modifying enzyme cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH).16–20 Consequently, humans instead express Neu5Gc’s precursor, N-acetylneuraminic acid (Neu5Ac).18 Presumably provoked by diet-based intake of Neu5Gc in animal tissues or by its expression on intestinal microbes, most adult humans generate antibodies against Neu5Gc that react with Neu5Gc surface antigen in CMAH-expressing pig cells and organs,21–23 making Neu5Gc, like Gal, a canonical carbohydrate “xeno-antigen” for pig-to-human xenografts.18,24
Cytidine monophospho-N-acetylneuraminic acid hydroxylase has been successfully knocked-out in pigs, and the associated reduction in Neu5Gc expression has been demonstrated to lead to less human antibody binding in the in vitro setting.21 In this study, we evaluated GalTKO.hCD46 pig lungs with and without an additional Neu5GcKO gene modification, using an established ex vivo model of pig lung perfusion with fresh human blood to measure the impact of Neu5GcKO on pulmonary function, anti-Neu5Gc antibody levels, and biochemical markers of lung xenograft injury.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Twenty three genetically engineered pigs with α1,3-galactosyl transferase knockout and transgenic for human membrane cofactor (GalTKO.hCD46), including eight pigs with an additional CMAH knock out (GalTKO.hCD46.Neu5GcKO), were generated and provided by Revivicor, Inc (Blacksburg, VA, USA). All donor pigs had type O blood and were homozygous for expression of human CD46. All procedures were approved by the University of Maryland School of Medicine IACUC and were conducted in compliance with NIH guidelines for the care and use of laboratory animals. In most cases both lungs where perfused ex vivo in side-by-side circuits; in these paired experiments one lung was assigned for treatment and the other was not treated (see “Experimental Groups”, below). In three instances (one with a GalTKO.hCD46.Neu5GcKO lung and two with GTKO.hCD46 lungs) the left lung was used for an in vivo transplant procedure, and the right lung was perfused ex vivo, with treatment.
2.2 |. Lung harvest
Induction of anesthesia and surgical organ dissection were performed as previously described.25,26 Briefly, animals were anesthetized with ketamine (10 mg/kg) (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (1 mg/kg) (Rompun; Bayer Pharmaceuticals, Shawnee Mission, KS, USA), and kept under general anesthesia (isoflurane 0.5%–3%) throughout the surgical procedure. After intubation, median sternotomy, and heparinization (500 units/kg), 0.5 mg prostaglandin E1 (PGE1) (ProstinVR Pediatric; Pfizer; New York, NY, USA) was administrated directly into the cranial vena cava. The main pulmonary artery was cannulated, cardiac inflows diverted, and lungs were flushed with 50 ml/kg Perfadex at 4°C (Vitrolife, Goteburg, Sweden) containing 1 mg/L Treprostinil (Remodulin; United Therapeutics, Silver Spring, MD, USA) and 10 mg/L Nitroglycerine (Baxter; Deerfield, IL, USA). The heart-lung block was removed and placed on iced saline. Subsequently, the lungs were separated from the heart, the left and right lungs surgically separated, and their pulmonary arteries (PAs) and airways instrumented.
2.3 |. Lung perfusion
The lung xenografts were individually perfused via the PA using circuits fashioned from silicon tubing and polyurethane connectors as previously described.26,27 PA flow was measured and recorded with a flowmeter (Transonic Systems Inc., Model TTFM 73–0146). The Digimed System Integrator (Micro-Med) continuously recorded PA and airway pressures via transducers integrated into the perfusion (Ismatec MCP rollerpump, IDEX) and ventilator (Harvard apparatus respirator, model 613) circuits, respectively, using PowerLab 16/35 and LabChart 7 Pro (AD Instruments). Lungs were ventilated at 12 breaths/min (tidal volume approximately 7–10 ml/kg lung donor weight). GalTKO.hCD46 and GalTKO.hCD46.Neu5GcKO lungs were electively terminated at 4 and 6 h, respectively, if lung failure criteria were not reached. Pulmonary vascular resistance (PVR) was measured by dividing pulmonary arterial pressure (mm Hg) by pulmonary artery flow (L/min). Lung failure was defined as rise in PVR >600 mm Hg*min/L, development of gross tracheal edema (prohibiting lung ventilation or associated with lack of oxygenation), or loss of perfusate volume (>85% of starting reservoir volume) by massive intraparenchymal sequestration.
2.4 |. Perfusate preparation
Fresh whole blood was collected from two healthy human blood donors (∼450 ml each) into a blood collection bag containing 64 ml of CPDA-1. Two units (∼240 ml each) of type-compatible thawed plasma were added for each unit of blood to obtain an initial perfusate volume of ∼2 L with a pre-perfusion hematocrit of ∼16%. Heparin (3 IU/ml blood, Heparin Sodium Injection, Sagent), calcium chloride (1.3–1.6 mg/ml blood, American Regent, Inc.) to neutralize the CPDA chelating agent, and 8.4% sodium bicarbonate (up to 0.8 mg/ml blood, target pH of 7.36–7.44, Hospira, Inc.) were added to the blood pool. All blood components were thoroughly mixed before circuit priming (1 L per circuit).
2.5 |. Experimental groups
Both GalTKO.hCD46 “reference” lungs (n = 18) and GalTKO. hCD46.Neu5GcKO “experimental” lungs (n = 11) were perfused with heparinized human blood. Reference experiments were done prior to the perfusion of the CMAHKO lung perfusions. Due to the high rate of “survival” to elective termination in the reference group, perfusion time was consequently extended from 4 h in the reference group to 6 h for the experimental group (Table 1).
TABLE 1.
List of the experiments included in the study and their parameters
| Exp | Genotype | Protective treatment | Pig ID | Survival in min | Reason for end of study |
|---|---|---|---|---|---|
| Exp 01 | GalTKO.hCD46.Neu5GcKO | - | 720–01 | >360 | elective |
| Exp 02 | GalTKO.hCD46.Neu5GcKO | - | 720–02 | >360 | elective |
| Exp 03 | GalTKO.hCD46.Neu5GcKO | - | 724–02 | >360 | elective |
| Exp 04 | GalTKO.hCD46.Neu5GcKO | - | 724–01 | >360 | elective |
| Exp 05 | GalTKO.hCD46.Neu5GcKO | - | 724–04 | >360 | elective |
| Exp 06 | GalTKO.hCD46.Neu5GcKO | H Blocker + BIA | 720–01 | >360 | elective |
| Exp 07 | GalTKO.hCD46.Neu5GcKO | H Blocker + BIA | 720–02 | >360 | elective |
| Exp 08 | GalTKO.hCD46.Neu5GcKO | H Blocker + BIA | 724–02 | 330 | PVR elevation, TE |
| Exp 09 | GalTKO.hCD46.Neu5GcKO | H Blocker + BIA | 724–03 | >360 | elective |
| Exp 10 | GalTKO.hCD46.Neu5GcKO | H Blocker + BIA | 724–01 | >360 | elective |
| Exp 11 | GalTKO.hCD46.Neu5GcKO | H Blocker + BIA | 724–04 | >360 | elective |
| Exp 12 | GalTKO.hCD46 | - | 564–01 | >240 | elective |
| Exp 13 | GalTKO.hCD46 | - | 564–02 | >240 | elective |
| Exp 14 | GalTKO.hCD46 | - | 564–04 | >240 | elective |
| Exp 15 | GalTKO.hCD46 | - | 564–03 | >240 | elective |
| Exp 16 | GalTKO.hCD46 | - | 609–3 | >240 | elective |
| Exp 17 | GalTKO.hCD46 | - | 712–02 | >240 | elective |
| Exp 18 | GalTKO.hCD46 | - | XX | 200 | PVR elevation |
| Exp 19 | GalTKO.hCD46 | - | 556–01 | 45 | LOP |
| Exp 20 | GalTKO.hCD46 | H Blocker + BIA | 609–3 | >240 | elective |
| Exp 21 | GalTKO.hCD46 | H Blocker + BIA | 712–02 | >240 | elective |
| Exp 22 | GalTKO.hCD46 | H Blocker + BIA | XX | >240 | elective |
| Exp 23 | GalTKO.hCD46 | H Blocker + BIA | 478–06 | >240 | elective |
| Exp 24 | GalTKO.hCD46 | H Blocker + BIA | 664–03 | >240 | elective |
| Exp 25 | GalTKO.hCD46 | H Blocker + BIA | 664–07 | >240 | elective |
| Exp 26 | GalTKO.hCD46 | H Blocker + BIA | 664–08 | >240 | elective |
| Exp 27 | GalTKO.hCD46 | H Blocker + BIA | 664–02 | >240 | elective |
| Exp 28 | GalTKO.hCD46 | H Blocker + BIA | 664–09 | >240 | elective |
| Exp 29 | GalTKO.hCD46 | H Blocker + BIA | 664–04 | >240 | elective |
| Fig S2 | Supplemental Data | ||||
| Exp 30 | GalTKO.hCD46.Neu5GcKO | DDAVP | 727–01 | 225 | PVR elevation |
| Exp 31 | GalTKO.hCD46.Neu5GcKO | DDAVP | 726–04 | >360 | elective |
| Exp 32 | GalTKO.hCD46.Neu5GcKO | DDAVP + aGPIb + H Blocker + BIA | 727–01 | >360 | elective |
| Exp 33 | GalTKO.hCD46.Neu5GcKO | DDAVP + aGPIb + H Blocker + BIA | 726–04 | 316 | PVR elevation |
The last four experiments are excluded from the statistical analysis due to the small number, but selected assay results are reported in Supplemental Figure 2.
Abbreviations: GalTKO, α1,3 galactosyltransferase Knockout; Neu5GcKO, N-Glycolylneuraminic acid knockout; BIA, Benzylimidazole; PVR, peripheral vascular resistance; TE, tracheal edema; H blocker, Histamine receptor blocker; DDAVP, Desmopressin; GP1B, platelet glycoprotein 1B
10 of the 18 GalTKO.hCD46 and six of the 11 GalTKO.hCD46.Neu5GcKO lungs were treated with a thromboxane synthase inhibitor (1-Benzylimidazole; BIA) and histamine receptor blocker (famotidine) to inhibit elaboration of thromboxane and block histamine effects, respectively, to target two mediators of innate immune injury previously observed consistently in the lung model. The untreated and treated (BIA+H Rx) groups were then separately compared against each other.
Four experiments using GalTKO.hCD46.Neu5GcKO lungs (Table 1) were conducted after additionally treating the pig lung donor with Desmopressin (DDAVP®) given over 2 days prior to lung procurement as previously described, to stimulate vWF depletion in pulmonary vascular endothelium.28 DDAVP treatment alone (n = 2, one lung in each pair) was compared to additional treatment with 6B4, a monoclonal antibody Fab that blocks binding of human GP1B to porcine vWF (n = 2), added to the blood perfusate of the contralateral lung.28 These four experiments are excluded from the statistical analysis, but associated data are reported as supplemental information.
2.6 |. Sampling regimen
Baseline blood samples were taken after blood preparation (“pre” sample), and after drug addition (where indicated) and circulating the blood in the perfusion circuit for at least 5 min prior to initiation of lung perfusion (time 0 sample). Further samples were collected at 5, 15, 30, 60, 120, and 240 min after lung perfusion was initiated. All blood samples were stored at −70°C. Lung tissue samples were collected pre-perfusion and at 10, 30, 60, 120, and 240 min of perfusion. In the Neu5GcKO experiments, additional blood and tissue samples were collected at 360 min.
2.7 |. Hematologic analysis
Blood cell counts were enumerated by standard automated techniques (Antech Diagnostics and Hemavet 950FS Hematology Analyzer, Drew Scientific in duplicate) in blood samples collected in ethylenediaminetetraacetic acid (EDTA).
2.8 |. Enzyme-linked immunosorbent assays
Beta-thromboglobulin (βTG) and prothrombin fragments 1+2 (F1+2), were measured by commercial enzyme-linked immunosorbent assay (ELISA) using plasma samples collected in CTAD tubes (Becton Dickinson, Franklin Lakes, NK, USA). EDTA plasma was used to measure histamine (ELISA kit Starfish) and C3a levels (Microvue Complement C3a Plus, Quidel). Blood, collected in EDTA tubes containing 100 μL of meclofenamate (10 μg/ml, Sigma-Aldrich), was used for thromboxane (Thromboxane B2 EIA Kit).
Additionally, human blood serum samples were tested for anti-Neu5Gc IgG antibodies using Maxisorp 96-well flat bottom plates (Nunc, cat #44–2404-21) coated with 1 μg/ml of Neu5Gc-PAA (Glycotech, cat #08–051) or PAA (Glycotech, cat #08–000). Affinity purified horseradish peroxidase (HRP) - conjugated goat anti-human IgG (Jackson, cat #109–035-008) was used to detect the anti-Neu5Gc IgG antibodies. All ELISA plates were measured using Spectramax M3 (Molecular Devices).
2.9 |. Flow cytometry
Pig aortic endothelial cells (PAECs) and peripheral blood mononuclear cells (PBMCs, gating on lymphocytes) from GalTKO.hCD46 and GalTKO.hCD46.Neu5GcKO pigs were tested for Neu5Gc antigen expression using anti-Neu5Gc antibody (Biolegend, Cat# 146901). Our previously described protocol to quantify anti-non-Gal xenoreactive antibodies was modified to specifically test for anti-Neu5Gc antibodies in human plasma samples.29 Human plasma samples obtained at 0, 60, and 240 min after perfusion were incubated with human embryonic kidney (HEK) cells that were genetically modified to express porcine CMAH (HEK-pCMAH, provided by Curie Ahn30) or control HEK cells. Anti-Neu5Gc antibody deposition was detected using goat anti-human IgM-PE (Southern Biotech, Cat32020–09) and anti-human IgG (Invitrogen, cat# 62–8411). All samples were acquired on a FACSVerse (BD Biosciences).
2.10 |. Measurement of inflammatory mediators
Levels of MCP-1 and lactoferrin were quantified in plasma samples taken before the start of perfusion and at fixed time points during the perfusion using the commercial Search Light Chemiluminescent Protein Array service available through Endogen (Rockford, IL, USA). This evaluation was only done for untreated lungs. Results are expressed as fold increase relative to baseline levels before initiation of perfusion.
2.11 |. Cell culture
Human embryonic kidney cells and HEK-pCMAH cells were cultured using DMEM (Gibco, Cat#11965092) supplemented with 10% FBS (Gibco). HEK 293 and HEK 295 CMAH cells were harvested once they reached a confluency of 80%–90%.
2.12 |. Statistical analyses
Statistical analysis was conducted using GraphPad Prism version 9.0.0 for Windows, GraphPad Software, San Diego, California USA. Unless otherwise noted, all data are presented as mean and standard error of the mean (SEM) for all variables except for survival time, which is expressed as median. Continuous variables were checked for normality using Shapiro-Wilk test and by observing the histogram. Group comparison was conducted using the two-way ANOVA test to compensate for the multiple reading at different time points. Post hoc analysis was conducted using Fischer’s LSD test to compare the untreated GalTKO.hCD46 against the untreated GalTKO.hCD46.Neu5GcKO lungs. Similarly, the BIA+H-treated GalTKO.hCD46 group was compared to the treated GalTKO.hCD46.Neu5GcKO lungs. Two-tailed p-values <.05 were considered statistically significant. The DDAVP-treated lung groups were excluded from the statistical analysis due to n = 2.
3 |. RESULTS
3.1 |. Graft survival
All but two GalTKO.hCD46 control grafts and one GalTKO.hCD46.Neu5GcKO lung “survived” with function compatible with supporting life until elective termination at 4 h (GalTKO.hCD46) or 6 h (GalTKO.hCD46.Neu5GcKO). A rise in pulmonary vascular resistance let to the early termination of two GalTKO.hCD46 lungs at 45 and 200 min and of one GalTKO.hCD46.Neu5GcKO lung at 330 min of perfusion (Figure 1).
FIGURE 1.
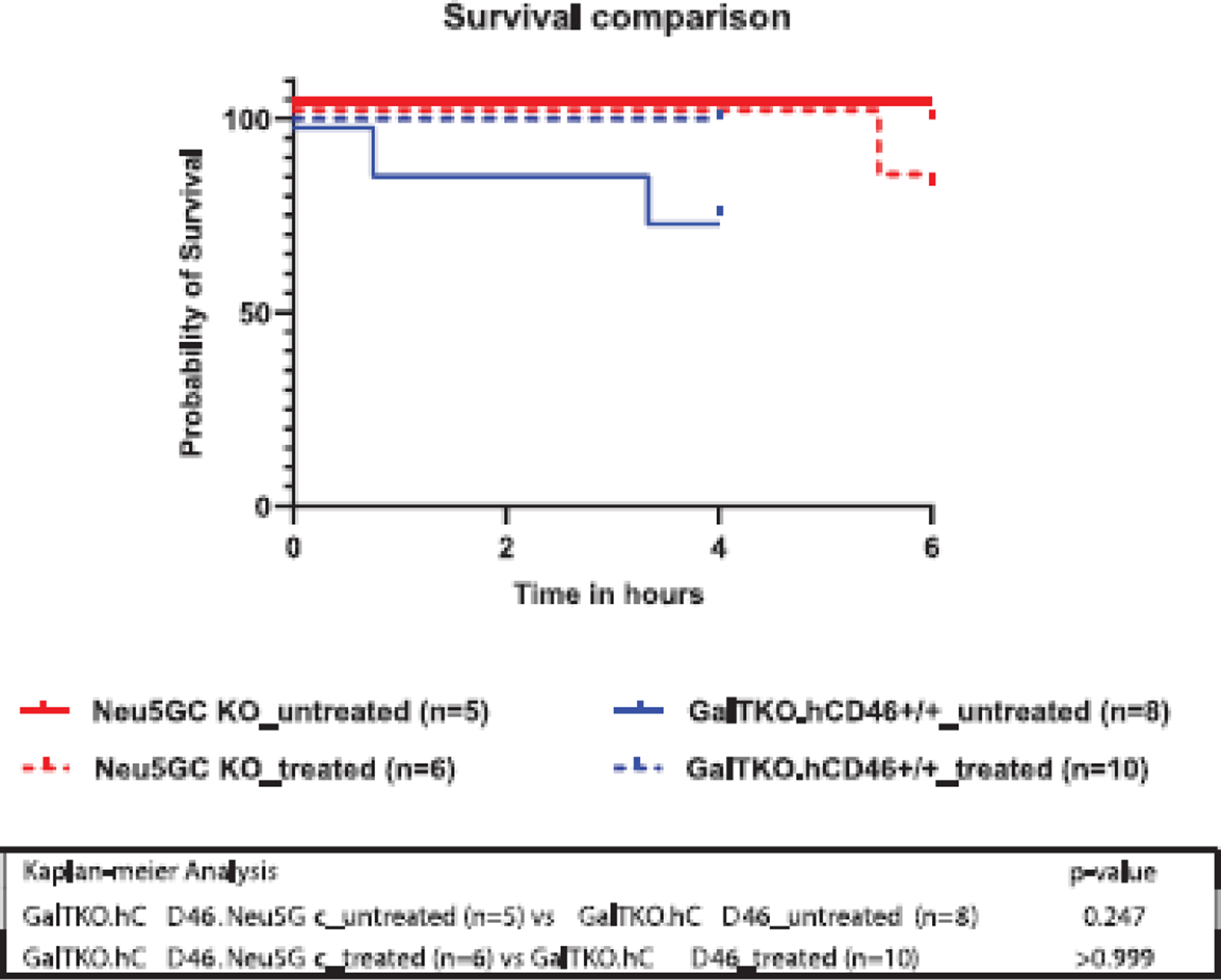
Cumulative lung survival. Survival of ex vivo perfused porcine lungs, based on pre-defined lung failure endpoint criteria,25 is illustrated for each experimental group. All but one GalTKO.hCD46.Neu5GcKO lungs “survived” to the elective termination at 6 h of perfusion where the experiment was electively terminated. Two GalTKO.hCD46 lungs failed prior to elective termination after 4 h due to a rise in PVR at 45 and 200 min of perfusion.
3.2 |. Porcine phenotype
Pig aortic endothelial cells (PAECs) isolated from GalTKO.hCD46 pigs demonstrated abundant expression of the Neu5Gc antigen compared to PBMCs isolated from GalTKO.hCD46.Neu5GcKO pigs or from humans (Figure 2A). Similarly, pig peripheral blood mononuclear cells (PBMCs) isolated from GalKO.hCD46 demonstrated strong expression of the Neu5Gc antigen compared to PBMCs isolated from GalTKO.hCD46.Neu5GcKO pigs or from humans (Figure 2B).
FIGURE 2.
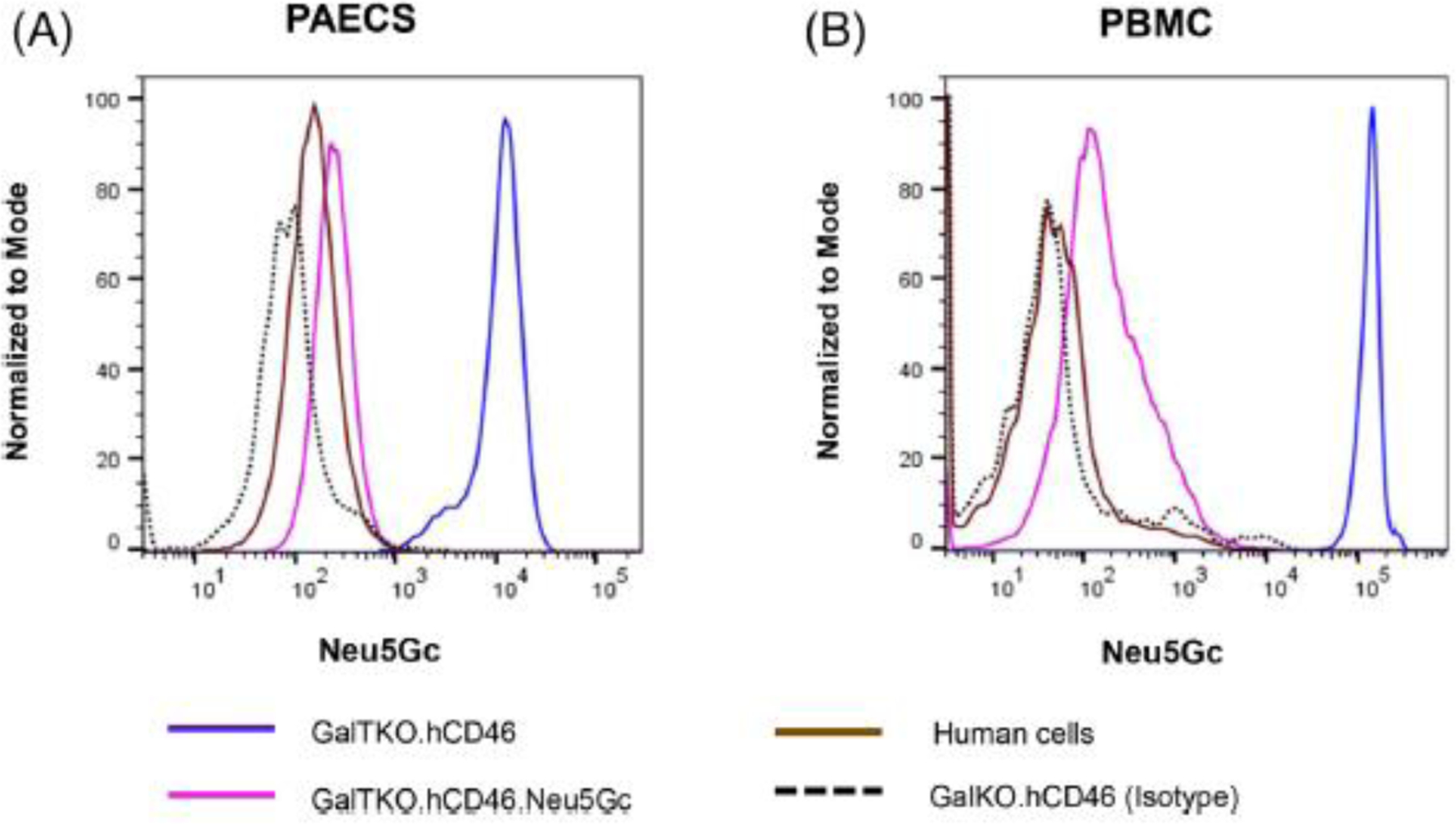
Pig phenotype verification. Neu5Gc expression of Pig aortic endothelial (PAECs) and peripheral blood lymphocytes (PBMC) were assessed by flow cytometry. (A) Pig aortic endothelial cells (PAECs) isolated from GalTKO.hCD46 pigs demonstrated abundant expression of the Neu5Gc antigen compared to the same cells isolated from GalTKO.hCD46.Neu5GcKO or from human (HAECs). Anti-Neu5Gc antibody isotype against GalTKO.hCD46 was used as an internal control. (B) Similarly, pig peripheral blood mononuclear cells (PBMCs) isolated from GalKO.hCD46 demonstrated strong expression of Neu5Gc antigen compared to PBMCs isolated from GalTKO.hCD46.Neu5GcKO pigs or from humans.
3.3 |. Pulmonary function parameters
Untreated GalTKO.hCD46 lungs exhibited a significant rise in pulmonary vascular resistance (PVR) during the whole time of perfusion, whereas untreated GalTKO.hCD46.Neu5GcKO lungs maintained very low and stable PVR (p = .003, Figure 3A). When the perfusate was treated with BIA+H, the trend toward lower PVR associated with GalTKO.hCD46.Neu5GcKO lungs was not significant (p = .097).
FIGURE 3.
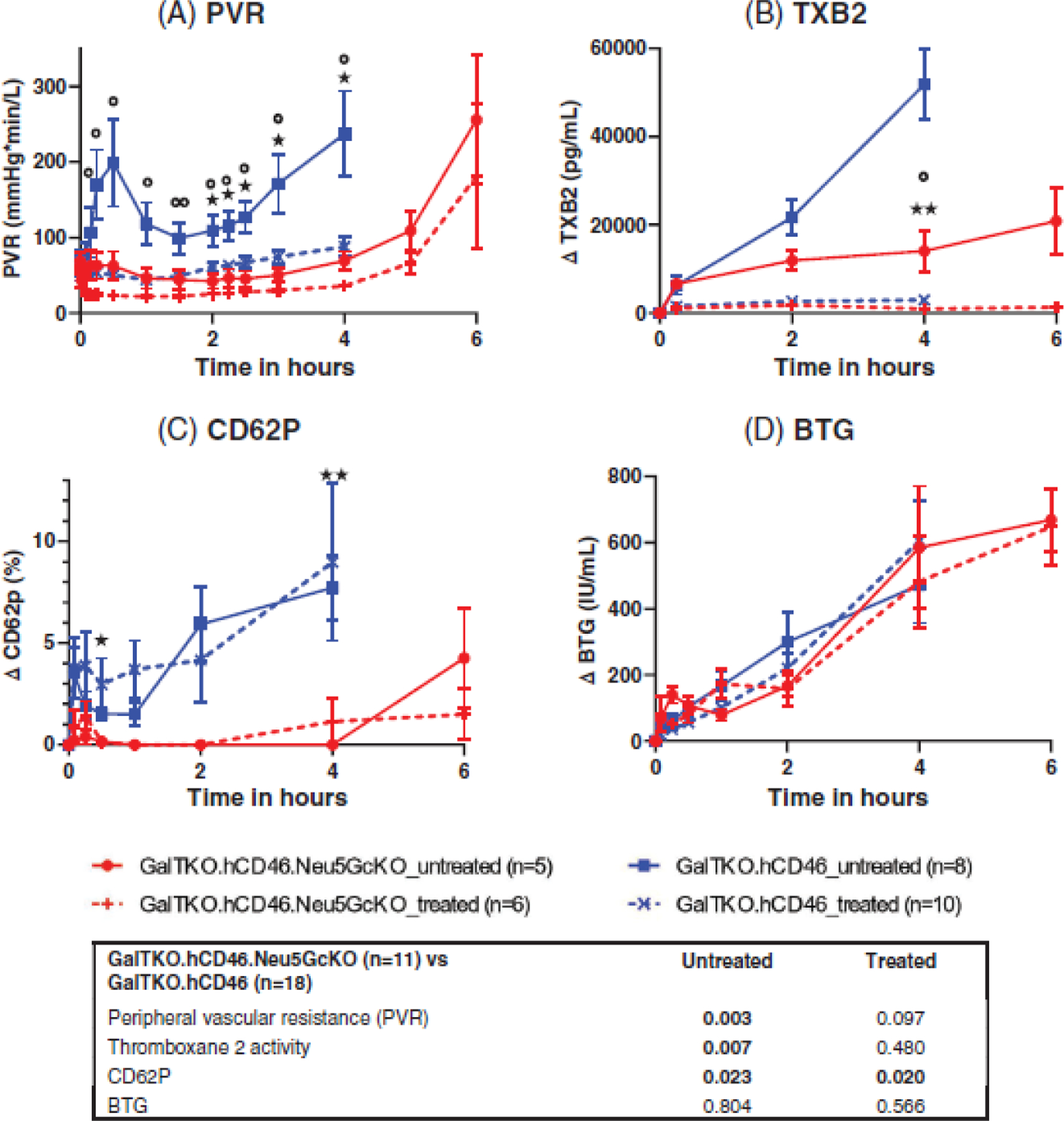
(A) Pulmonary vascular resistance (PVR) is expressed as a function of perfusion time. Time 0 represents measurements obtained during the first minute of lung perfusion. Lungs in the untreated GalTKO.hCD46.Neu5GcKO group maintained low and very stable PVR values during the first 4 h of perfusion, while the untreated GalTKO.hCD46 group displayed bi-phasic PVR elevation, with a significant PVR rises at 30 min, and again during the last 2 h of the 4-h perfusion interval, a physiologic profile typical of thromboxane elaboration in other models.27 Treatment with H blocker and BIA maintained lower PVR in both groups. (B) Plasma thromboxane B2 levels (TXB2): The elaboration in plasma thromboxane B2 levels observed in the GalTKO.hCD46 group throughout xeno lung perfusions was significantly mitigated in the GalTKO.hCD46.Neu5GcKO group. (C) CD62P and (D) β-Thromoboglobulin elaboration (BTG) as indicator of platelet activation: CD62P was significantly higher in both the untreated and treated GalTKO.hCD46 groups. * p < .05 and **p < .01 for GalTKO.hCD46.Neu5GcKO versus GalTKO.hCD46. *p < .05 and **p < .01 for GalTKO.hCD46.Neu5GcKO_treated versus GalTKO.hCD46_treated.
3.4 |. Thromboxane plasma levels
Elaboration of plasma thromboxane B2 levels observed in the untreated GalTKO.hCD46 group (∆TBX 51.9±8.1 at 4 h) was significantly mitigated in the untreated GalTKO.hCD46.Neu5GcKO group (14.1±4.7 ng/ml at 4 h; p = .007, Figure 3B). As expected, thromboxane elaboration was prevented by BIA administration in association with both lung phenotypes, resulting in no difference between the BIA+H-treated groups (p = .480).
3.5 |. Platelet sequestration and activation
In all groups, platelet sequestration of about 45%–70% was detected at 5 min with no statistically significant difference associated with the Neu5GcKO lung phenotype either with or without treatment (p = .819 and .116, respectively). Substantial intra-group variability in platelet sequestration was observed, and platelet levels appeared to rise over time beyond 30–60 min in all groups. However, we have recently shown that platelet counts measured by hemocytometry (as measured here) or even by manual counting are falsely elevated in ex vivo perfusion models due to detection of platelet-sized erythrocyte fragments that appear in increasing numbers during pig organ perfusion with human blood.31 In this context, relatively high apparent rebound in platelet levels in association with Neu5GcKO lungs without BIA and antihistamine is difficult to interpret; additional treatment with DDAVP and 6B4 demonstrated no consistent effect with high inter-experiment variability (Figure S1).
The GalTKO.hCD46 group demonstrated significantly higher levels of the platelet activation compared to GalTKO.hCD46.Neu5GcKO group throughout perfusion, when measured with CD62P (p = .023, Figure 3C) but not with βTG (p = .08, Figure 3D). Within the Neu5GcKO group, no consistent effect of BIA/antihistamine treatment on BTG or CD62P levels was evident.
3.6 |. Inflammation and coagulation cascade activation
Untreated GalTKO.hCD46 lungs were associated with higher thrombin formation at intervals beyond the first hour when compared to GalTKO.hCD46.Neu5GcKO (p = .005, Figure 4A). Within the Neu5GcKO group, no consistent effect of BIA+H on kinetics of F1+2 elaboration was evident. All experimental groups showed similar kinetics of complement activation, measured by plasma C3a (Figure 4B) and neutrophil sequestration (Figure 4C). Histamine elaboration was significantly delayed in association with the Neu5GcKO lung phenotype in both the untreated and treated groups (p = .037 and .035; Figure 4D); histamine levels were increased as expected in association with histamine receptor blockade.
FIGURE 4.
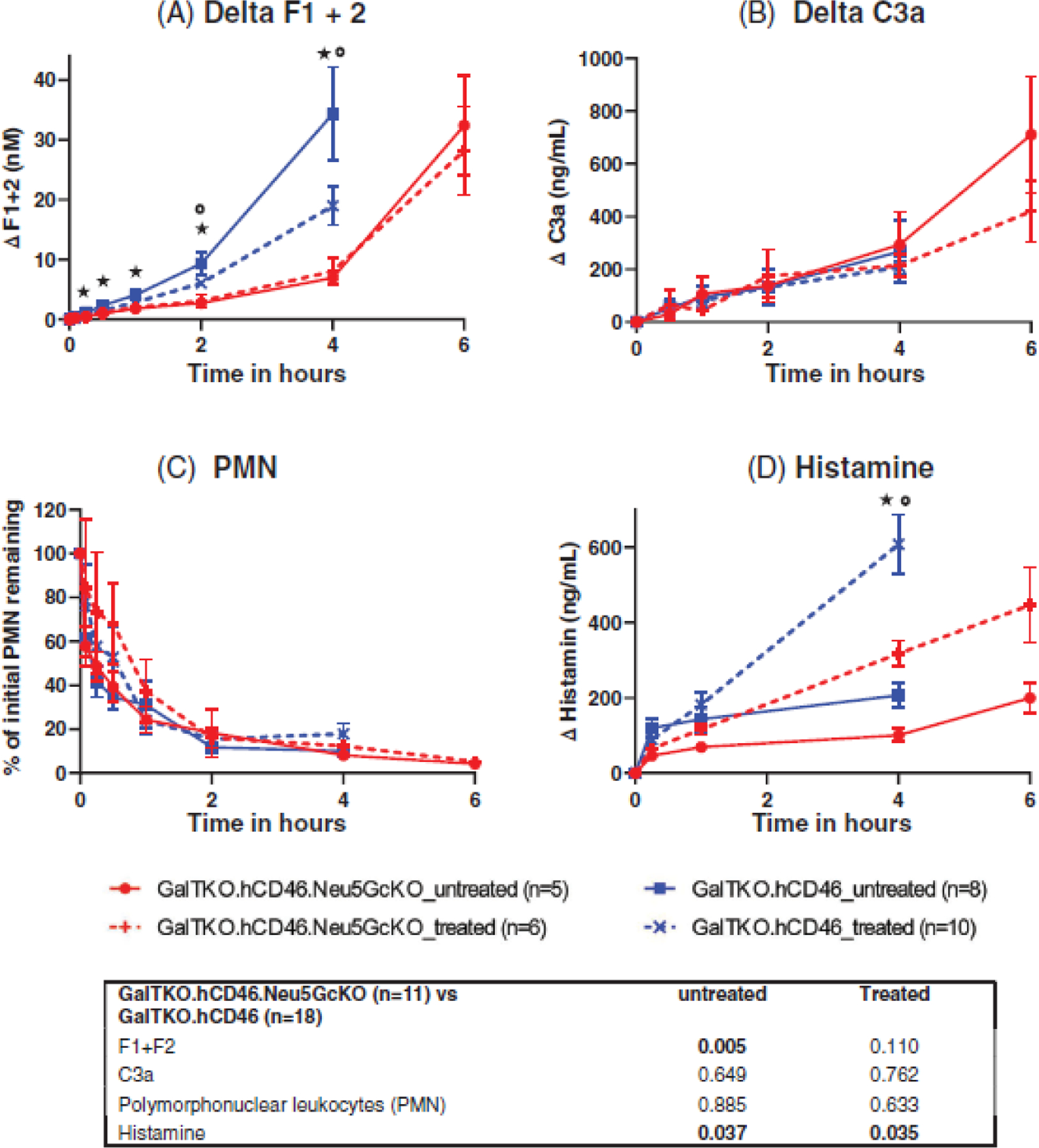
(A) Coagulation Cascade Activation (F1+F2): both GalTKO.hCD46 groups showed increased thrombin formation, though only the untreated showed a statistically significant rise. (B) complement activation (C3a) and (C) neutrophil sequestration were similar in all groups. (D) Histamine level increased significantly more in association with GalTKO.hCD46 lungs when compared to the GalTKO.hCD46.Neu5GcKO groups. Histamine receptor blockade exacerbate this difference in the treated groups for both lung phenotypes. * p < .05 and **p < .01 for GalTKO.hCD46.Neu5GcKO versus GalTKO.hCD46. *p < .05 and **p < .01 for GalTKO.hCD46.Neu5GcKO_treated versus GalTKO.hCD46_treated.
3.7 |. Plasma cytokines and inflammatory markers
Monocyte chemoattractant protein 1 (MCP1), a proinflammatory cytokine, declined in the untreated Neu5GcKO lungs and remained below pre-perfusion levels through the end of the experiment, but rose in association with untreated GalTKO.hCD46 lungs (p = .007, Figure 5A). Lactotransferin (LTF), as a marker for macrophage-associated inflammation, was elaborated in both untreated groups, but significantly higher levels were detected throughout the perfusion of GalTKO.hCD46 group when compared to GalTKO.hCD46.Neu5GcKO group (p = .032, Figure 5B).
FIGURE 5.
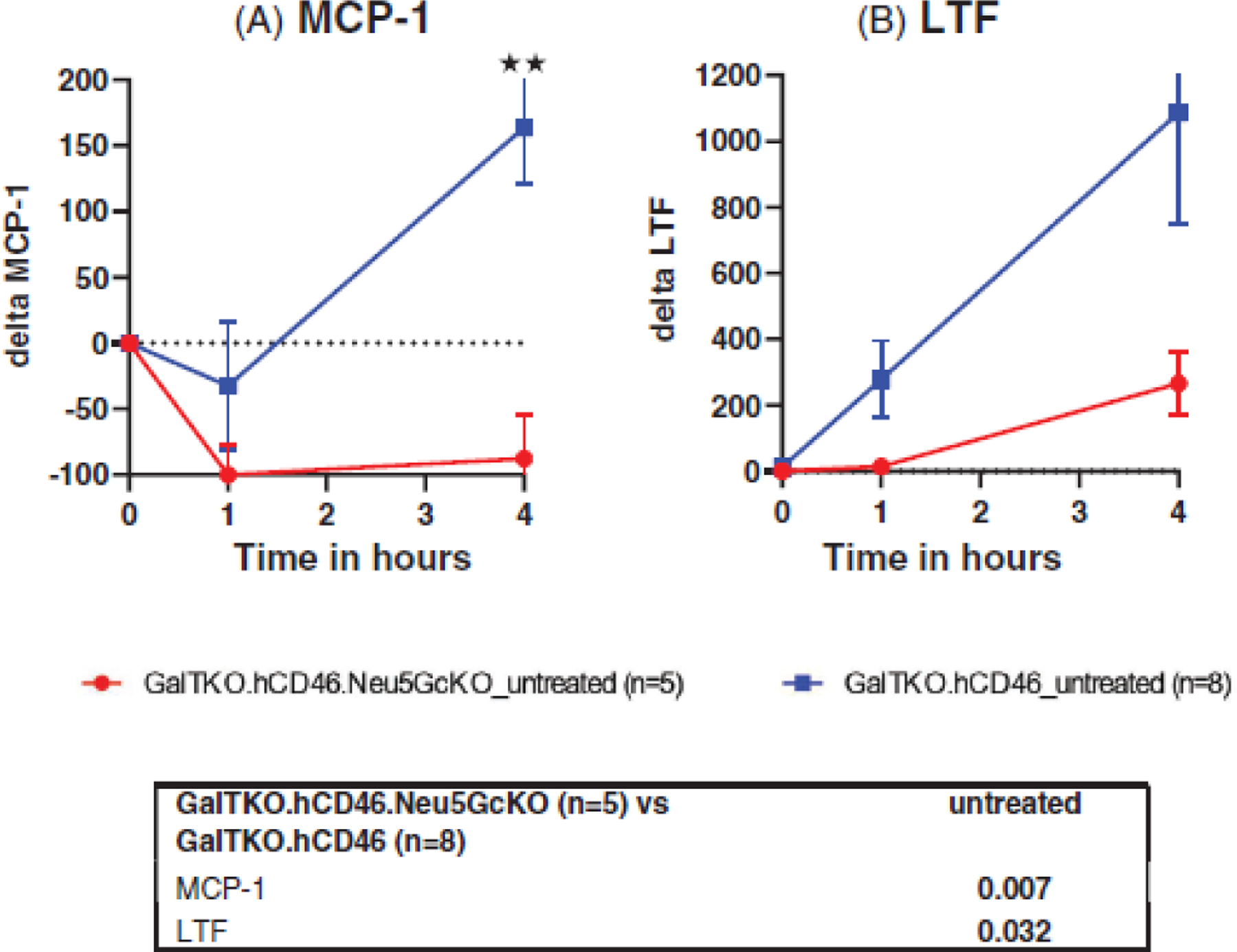
Plasma cytokines and inflammatory markers. Monocyte chemoattractant protein 1 (MCP1) and Lactotransferin (LTF) were measured in the untreated groups as prototypical markers of monocyte-associated and macrophage-associate inflammation, respectively. (A) MCP-1 levels declined below reference pre-perfusion levels in association with Neu5GcKO and remained low through elective study termination, but rose significantly in the GalTKO.hCD46 group. (B) LTF showed higher levels throughout the perfusion of GalTKO.hCD46 group when compared to GalTKO.hCD46.Neu5GcKO group. *p < .05 and **p < .01 for GalTKO.hCD46.Neu5GcKO versus GalTKO.hCD46.
3.8 |. Anti-Neu5Gc antibody levels
Levels of anti-Neu5Gc IgM in human blood were similar in both GalTKO.hCD46.Neu5GcKO and GalTKO.hCD46 before the perfusion (53±31 vs. 55±24, p = .921. Table 2). Similar levels of anti-Neu5Gc IgG were also noticed (73±17 vs. 66±23, p = .592). After starting perfusion of the lungs, anti-Neu5Gc IgM serum levels decreased by approximately 40% during the first hour of ex vivo perfusion of untreated GalTKO.hCD46 lungs, whereas levels did not fall during perfusion of untreated Neu5GcKO lungs (p = .0007) as demonstrated in Figure 6(A). Similarly, human anti-Neu5Gc IgG serum levels decreased by about 60% during the ex vivo perfusion of untreated GalTKO.hCD46 lungs, whereas levels did not fall during perfusion of untreated Neu5GcKO lungs (p = <.001, Figure 6B).
TABLE 2.
Absolute levels of IgM and IgG in perfusate plasma
| IgM level |
IgG level |
||||||
|---|---|---|---|---|---|---|---|
| Pre | 1 h | 4 h | Pre | 1 h | 4 h | ||
| GalTKO.hCD46.Neu5GcKO | Exp 01 | 96.0 | 87.8 | 76.1 | 55.4 | 50.4 | 12.4 |
| Exp 02 | 75.4 | 75.9 | 83.2 | 59.4 | 55.7 | 63.3 | |
| Exp 03 | 38.1 | 34.8 | 26.6 | 70.2 | 74.1 | 75.4 | |
| Exp 04 | 19.4 | 17.8 | 27.6 | 84.0 | 85.1 | 86.0 | |
| Exp 05 | 39.0 | 48.3 | 64.9 | 96.2 | 94.6 | 99.9 | |
| Avg ± SD | 54 ± 31 | 53 ± 29 | 56 ± 27 | 73 ± 17 | 72 ± 19 | 67 ± 34 | |
| GalTKO.hCD46 | Exp 12 | 47.9 | 33.0 | 43.8 | 91.4 | 34.5 | 37.5 |
| Exp 13 | 111.1 | 47.6 | 41.5 | 82.0 | 36.6 | 28.3 | |
| Exp 14 | 28.3 | 15.3 | 30.4 | 39.1 | 19.5 | 23.5 | |
| Exp 15 | 50.9 | 35.1 | 31.8 | 61.0 | 13.7 | 20.4 | |
| Exp 16 | 59.0 | 11.0 | 32.4 | 85.0 | 69.3 | 67.6 | |
| Exp 17 | 42.4 | 30.7 | 36.0 | 27.0 | 3.0 | 5.7 | |
| Exp 18 | 53.5 | 28.1 | 39.2 | 84.2 | 13.7 | 31.1 | |
| Exp 19 | 48.0 | 37.2 | 44.1 | 60.5 | 44.8 | 37.9 | |
| avg ± SD | 55 ± 24 | 30 ± 12 | 37 ± 06 | 66 ± 24 | 29 ± 21 | 31 ± 18 | |
GalTKO: α1,3 galactosyltransferase Knockout. Neu5GC: N-Glycolylneuraminic acid. IgM (p = .921) and IgG (p = .592) levels before perfusion did not differ significantly between groups.
FIGURE 6.
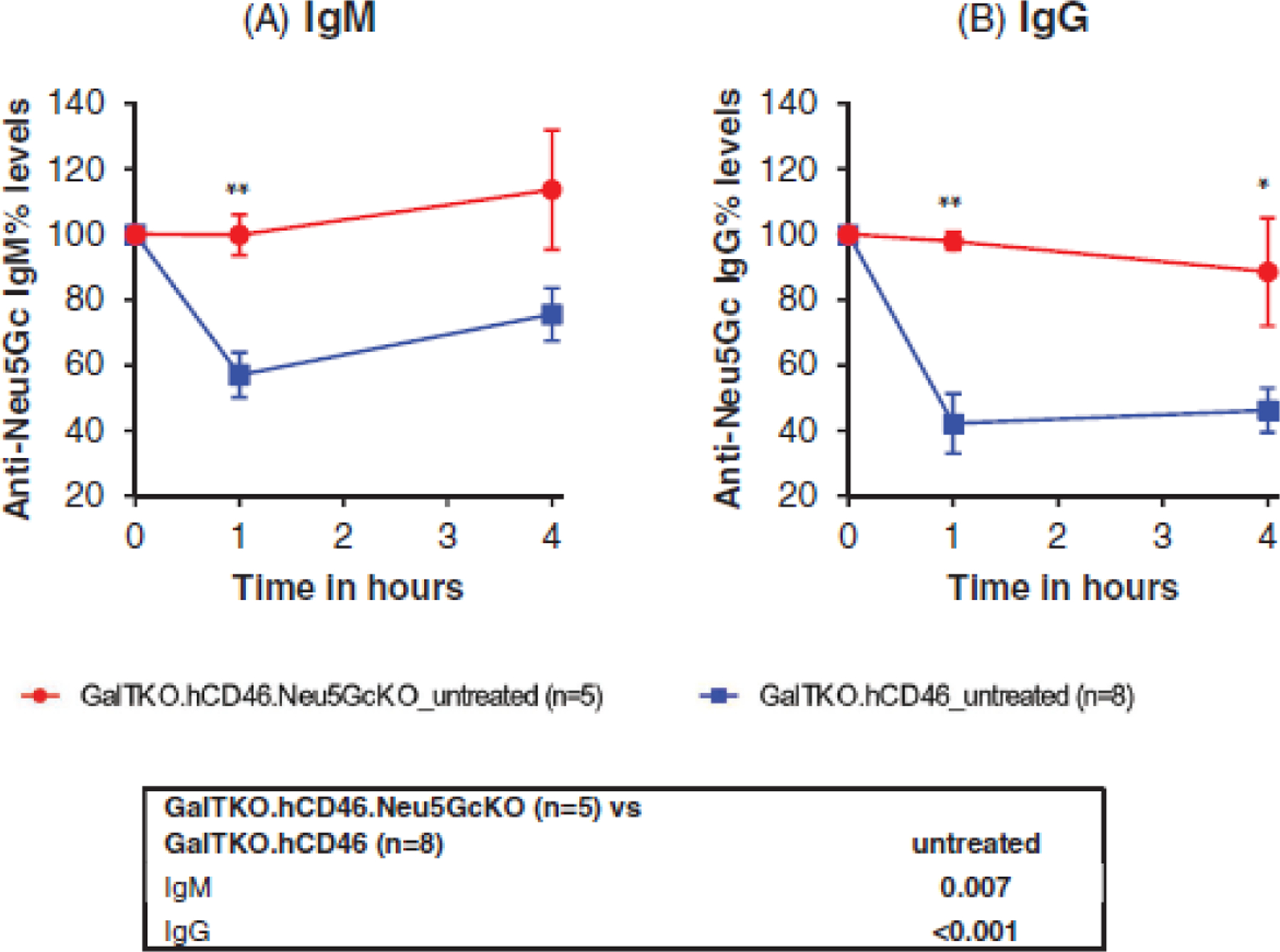
Anti-Neu5Gc antibodies level. In the GalTKO.hCD46 group, both (A) IgM and (B) IgG human anti-Neu5Gc antibodies titers dropped significantly during lung xenografts’ perfusion indicating the binding of those antibodies to Neu5Gc antigen expressed in such grafts. However, in the GalTKO.hCD46.Neu5GcKO group both IgG and IgM antibodies levels maintained their baseline levels throughout the perfusion indicating the lack of binding of those antibodies to their antigenic target. *p < .05 and **p < .01 for GalTKO.hCD46.Neu5GcKO versus GalTKO.hCD46.
4 |. DISCUSSION
It is well known that the majority of human anti-pig xeno-antibodies target the galactose-α(1,3)-galactose, or “Gal” epitope.1,32 The introduction of galactosyl transferase knockout (GalTKO) pigs has significantly reduced hyperacute rejection as a major obstacle in xenotransplantation33 and implicated “non-Gal” xenoreactive antibody directed at Neu5Gc as playing a major rule in GalTKO organ xenograft failure.12,34,35 Here we confirm that the CMAHKO was associated with efficient elimination of the Neu5Gc xenoantigen, since adsorption of anti-Neu5Gc antibody by GalTKO.hCD46 lungs was not observed in association with the Neu5GcKO lungs. We also demonstrate that Neu5Gc is a physiologically important “non-Gal” xenoantigen since lung inflammation (thromboxane and histamine release, platelet activation, fibrin formation) and injury (rise in pulmonary vascular resistance) were significantly attenuated or delayed in association with absence of the Neu5Gc antigen.
Perfusion with human blood is the best available model for predicting clinical behavior of CMAHKO organ xenografts. Like most mammals, human cells lack the Neu5Gc sialic acid moiety in their glycocalyx, the pericellular matrix of glycoproteins decorating the cell membrane. Humans are not the only species that lack Neu5Gc as many new world primates share this feature with humans.36 In contrast, macaques and baboons, the old-world primates species that are most commonly used for preclinical xenotransplant studies, are not useful to examining the role of Neu5Gc-antibodies in xenograft rejection since they express the Neu5Gc antigen on their cells and thus do not have preformed antibodies against the Neu5Gc on pig organs. Further, removal of the Neu5Gc antigen in pigs appears to unveil a “fourth antigen” on pig cells that is associated with a strongly positive CDC crossmatch when tested against baboon serum.37 These features significantly reduce the utility of the baboon model for studying the effects of the Neu5Gc antigen KO in the in vivo setting, as Neu5Gc-expressing pig organs would be predicted to perform better in human recipients than in baboons.37 Our results ex vivo human blood perfusion model predict that Neu5Gc-expressing organs are likely to suffer injury mediated by anti-Neu5Gc-antibodies and will exhibit earlier failure in human recipients than Neu5GcKO organs with otherwise identical genetics. The rapid elevation in pulmonary vascular resistance that we observed in the untreated GalTKO.hCD46 lungs, coupled with consumption of the Neu5Gc antibodies and increase in histamine and platelet activation strongly support this hypothesis.
Swine lung vasculature is particularly prone to release large amounts of von Willebrand Factor (vWF).38 Non-human primate and human platelets bind pig VWF through their GPIb receptors in the absence of shear stress, resulting in over-activation and adhesion to pig xenograft endothelium.39 To mitigate clotting cascade activation, various methods have been validated to block this “non-physiologic” interaction40: pre-depletion of pvWF from pig endothelium before organ procurement;41 blood treatment with a humanized aGPIb Fab fragment, 6B4, which selectively blocks the binding site of platelet GP1B by VWF; and genetically modifying pvWF to remove or “humanize” the epitopes that interact non-physiologically with human GP1b.28 Accordingly, in this study we treated four pairs of lungs with 1-deamino-8-d-arginine vasopressin (DDAVP), an analog of vasopressin, to deplete porcine endothelial VWF-stores according to a clinically well-established practice,42 and treated the human blood perfusate for one lung in each pair with GP1b.43 Although we failed to detect any statistically significant difference associated with these interventions in these two pairs of experiments, platelet activation and sequestration tended to be attenuated, as previously reported in association with different pig genetics. Further investigation appears to be warranted. Overall, we conclude that CMAHKO is associated with reduced platelet activation, likely by directly reducing FcR-mediated platelet binding triggered by anti-Neu5Gc antibody and indirectly by decreasing complement-mediated endothelial activation driven by complement-fixing anti-Neu5Gc antibody, even though no decrease in complement activation (as elaboration of C3a) was observed.
Of note, treatment with histamine (H) blocker famotidine and the thromboxane synthase inhibitor 1-benzylimidazole (BIA) resulted in substantial reduction in the pulmonary vascular resistance for GalTKO.hCD46.Neu5GcKO lungs (Figure 3A). This effect has been previously reported by our group in separate work studying different pig lung phenotypes.45 The current observation demonstrates that Gal- and Neu5Gc-independent elaboration of thromboxane occurs when GalTKO.hCD46. Neu5GcKO lungs are perfused with human blood. The upstream trigger for thromboxane release and the injury exhibited by GalTKO.hCD46.Neu5GcKO lungs within 8 h of ex vivo perfusion may be attributable to incomplete control of complement activation,46,47 vWF- 28 or selectin-mediated48 platelet adhesive interactions,49,50 innate immune cell activation via absence of HLA-E51 or human CD47,52 or incompatibility between human pro- and anti-coagulant molecules and pig thromboregulatory molecules.1,52,53 Evaluating each of these hypotheses separately may be limited by logistical constraints regarding production of informative pig phenotypes. Based on our prior reports showing that each of these pathways contributes to lung xenograft injury we presume each plays a significant role. In that context, here we show that Neu5Gc is a physiologically important target of human anti-GalTKO antibody, and conclude that CMAHKO will likely confer clinically valuable protection of pig lungs – and presumably other organs – expressing additional mechanism-directed transgenes.
Of note from a technical perspective, a detectable signal above background was observed during the Neu5Gc assay (Figure 2) when comparing the Neu5GcKO PAECs to the isotype control or to the HAECs. We suspect this weak “signal” is attributable to requirement for fetal bovine serum in order for the pCMAH-expressing HEK cell line to grow well in cell culture. FBS contains Neu5Gc antigen that is apparently transferred onto the cultured CMAHKO cells, and is detected by flow cytometry.44 We attribute the apparent rise in the anti-Neu5Gc levels seen in both lung phenotypes at 240 min, particularly for IgM, to hemoconcentration associated with gradual loss of vascular barrier function and accumulation of interstitial edema in lungs of both xenograft phenotypes due to injury mechanisms that were incompletely controlled by the interventions studied here.
Limitations for this study include the lack of some secondary data in the DDAVP/6B4-treated groups due to the relatively small number of CMAHKO lungs available; small numbers may also explain the inability to reach the statistical significance in some measurements for the study overall, such as βTG as a proxy for platelet activation. We chose not to perform immunohistochemical evaluation of complement deposition because we have previously observed that CD46 expression is associated with a very weak residual signal in tissue, and lung IHC is prone to significant artifacts (high background, low sensitivity and specificity). Because CD46 expression is likely to have modulated any effect of anti-Neu5Gc antibody binding on activation of the complement cascade in the reference group, we felt it futile to look for reduced complement activation in the CMAHKO group. We are confident that the platelet counting methods used in our lab when these experiments were performed overestimate residual platelets after the first 20–30 min of perfusion. This weakness is fully accounted in our discussion.31 Finally, changing the study protocol by increasing the length of experiments from 4 h in the reference lungs to 6 h for the GalTKO.hCD46.Neu5GcKO lungs may have masked differences in survival and physiologic and biochemical readouts between groups if some reference lungs had failed beyond 240 min, as expected, and biochemical parameters been measured in association with reference lungs after 4 h.
We conclude that Neu5Gc is an antigenic target in pig lungs expressing this carbohydrate, and presumably contributes to antibody-mediated rejection when GalTKO.hCD46 porcine lungs are perfused with human blood. These findings confirm our prior observations in a similar liver xeno ex vivo perfusion model.44 Together, these observations strongly support our working hypothesis that the Neu5GcKO genetic modification is very likely to be associated with improved protection from antibody mediated graft injury and organ dysfunction as xenotransplantation studies progress from nonhuman primate to human recipients. This conclusion supports incorporation of the CMAHKO into porcine xenograft donors intended for translation into the clinic.
4.1 |. Conclusion
Knock-out of the Neu5Gc sialic acid significantly reduces parameters associated with antibody-mediated inflammation and activation of the coagulation cascade. This should be beneficial to reduce innate immune antigenicity of porcine lungs in future human recipients.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the help of Jagdeece Ramsoondar from Revivicor’s in providing critical components for the study. The work described in this manuscript was directly supported by NIH U19AI090959 during performance of the experiments described, and indirectly by U01 AI153612 during manuscript preparation and completion of data collection and analysis. Additional support was received from United Therapeutics and Lung Biotechnology PBC through gifts and sponsored research agreements, and from Revivicor through provision of the genetically modified pigs. Ryan Chaban is supported by the Benjamin Research Fellowship from the German Research Foundation (DFG).
Funding information
NIH, Grant/Award Numbers: U19AI090959, U01 AI153612; German Research Foundation (DFG)
Abbreviations:
- aGal
α1,3 galactosyltransferase
- BIA
1-Benzylimidazole
- CMAH
cytidine monophospho-N-acetylneuraminic acid hydroxylase
- DDAVP
Desmopressin (1-desamino-8-D-arginine vasopressin)
- F1+2
Prothrombin fragments 1 + 2
- GalTKO
galactosyltransferase knockout
- GP1B
platelet glycoprotein 1B
- Neu5Gc
N-Glycolylneuraminic acid
- PVR
pulmonary vascular resistance
Footnotes
CONFLICT OF INTEREST
Richard N. Pierson has served without compensation on Revivicor’s Scientific Advisory Board. David L. Ayares is Executive VP and CSO and a full-time employee of Revivicor, Inc. Lars Burdorf and Kasinath Kuravi are employees of Revivicor, Inc. Revivicor, Inc. is a wholly owned subsidiary of United Therapeutics, Inc. and Lung Biotechnologies PBC.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Pierson RN 3rd, Fishman JA, Lewis GD, et al. Progress toward cardiac xenotransplantation. Circulation. 2020;142:1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DKC, Pierson RN 3rd. The future of cardiac xenotransplantation. Nat Rev Cardiol. 2022. doi: 10.1038/s41569-022-00684-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DKC. The 2021. IXA Keith Reemtsma Lecture: moving xenotransplantation to the clinic. Xenotransplantation. 2021: e12723. doi: 10.1111/xen.12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichart B, Längin M, Radan J, et al. Pig-to-non-human primate heart transplantation: the final step toward clinical xenotransplantation. J Heart Lung Transplant. 2020;39:751–757. [DOI] [PubMed] [Google Scholar]
- 5.Mohiuddin MM, DiChiacchio L, Singh AK, Griffith BP. Xenotransplantation: a step closer to clinical reality? Transplantation. 2019;103:453–454. [DOI] [PubMed] [Google Scholar]
- 6.Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–433. [DOI] [PubMed] [Google Scholar]
- 7.Mohiuddin MM, Goerlich CE, Singh AK, et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation. 2022; 29:2e12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DK, Ezzelarab MB, Hara H, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation. 2016;23:83–105. [DOI] [PubMed] [Google Scholar]
- 10.Rose AG, Cooper DK, Human PA, Reichenspurner H, Reichert B. Histopathology of hyperacute rejection of the heart: experimental and clinical observations in allografts and xenografts. J Heart Lung Transplant 1991;10: 223–234. [PubMed] [Google Scholar]
- 11.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;1:29–31. [DOI] [PubMed] [Google Scholar]
- 13.Galili U Discovery of the natural anti-Gal antibody and its past and future relevance to medicine. Xenotransplantation. 2013;20:138–47. [DOI] [PubMed] [Google Scholar]
- 14.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation. 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basnet NB, Ide K, Tahara H, Tanaka Y, Ohdan H. Deficiency of N-glycolylneuraminic acid and Galα1–3Galβ1–4GlcNAc epitopes in xenogeneic cells attenuates cytotoxicity of human natural antibodies. Xenotransplantation. 2010;17:440–8. [DOI] [PubMed] [Google Scholar]
- 16.Salama A, Evanno G, Harb J, Soulillou JP. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015;22:85–94. [DOI] [PubMed] [Google Scholar]
- 17.Chou HH, Takematsu H, Diaz S, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman MO, Gagneux P. Absence of Neu5Gc and presence of Anti-Neu5Gc antibodies in humans-an evolutionary perspective. Front Immunol. 2019;10:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou HH, Takematsu H, Diaz S, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95(20):11751–6. doi: 10.1073/pnas.95.20.11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou HH, Hayakawa T, Diaz S, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. 2002;99(18):11736–41. doi: 10.1073/pnas.182257399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R, Wang Y, Chen L, et al. Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically-deleting three major glycan antigens, GGTA1/β4GalNT2/CMAH. Acta Biomater. 2018;72:196–205. [DOI] [PubMed] [Google Scholar]
- 22.Tangvoranuntakul P, Gagneux P, Diaz S, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175:228–36. [DOI] [PubMed] [Google Scholar]
- 24.Perota A, Galli C. N-Glycolylneuraminic Acid (Neu5Gc) null large animals by targeting the CMP-Neu5Gc hydroxylase (CMAH). Front Immunol. 2019;10:2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen BN, Azimzadeh AM, Schroeder C, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011;18:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdorf L, Azimzadeh AM, Pierson RN 3rd. Xenogeneic lung transplantation models. Methods Mol Biol. 2020;2110:173–196. [DOI] [PubMed] [Google Scholar]
- 27.Brigham KL, Ogletree M, Snapper J, Hinson J, Parker R. Prostaglandins and lung injury. Chest. 1983;83:70S–72S. [DOI] [PubMed] [Google Scholar]
- 28.Connolly MR, Kuravi K, Burdorf L, et al. Humanized von Wille-brand factor reduces platelet sequestration in ex vivo and in vivo xenotransplant models. Xenotransplantation. 2021;28: e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azimzadeh AM, Byrne GW, Ezzelarab M, et al. Development of a consensus protocol to quantify primate anti-non-Gal xenoreactive antibodies using pig aortic endothelial cells. Xenotransplantation. 2014;21:555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurh S, Kang B, Choi I, et al. Human antibody reactivity against xenogeneic N-glycolylneuraminic acid and galactose-α−1,3-galactose antigen. Xenotransplantation. 2016;23:279–92. [DOI] [PubMed] [Google Scholar]
- 31.Habibabady ZA, Sendil S, Ellett F, et al. Human erythrocyte fragmentation during ex-vivo pig organ perfusion. Xenotransplantation. 2022;29:e12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper DK, Good AH, Koren E, Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. [DOI] [PubMed] [Google Scholar]
- 33.Tector AJ, Mosser M, Tector M, Bach JM. The possible role of Anti-Neu5Gc as an obstacle in xenotransplantation. Front Immunol. 2020;11:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao B, Long C, Lee W, et al. Anti-Neu5Gc and anti-non-Neu5Gc antibodies in healthy humans. PLoS ONE. 2017;12:e0180768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig non-Gal antigens in naïve and sensitized baboons. Xenotransplantation. 2006;13:400–7. [DOI] [PubMed] [Google Scholar]
- 36.Peri S, Kulkarni A, Feyertag F, Berninsone PM, Alvarez-Ponce D. Phylogenetic Distribution of CMP-Neu5Ac Hydroxylase (CMAH), the enzyme synthetizing the proinflammatory human xenoantigen Neu5Gc. Genome Biol Evol. 2018;10:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Hara H, Ayares D, Cooper DKC. The problem of the “4th xenoantigen” after pig organ transplantation in non-human primates may be overcome by expression of human “protective” proteins. Xenotransplantation. 2021;28:e12658. [DOI] [PubMed] [Google Scholar]
- 38.Holzknecht ZE, Coombes S, Blocher BA, et al. Immune complex formation after xenotransplantation: evidence of type III as well as type II immune reactions provide clues to pathophysiology. Am J Pathol. 2001;158:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaca JG, Lesher A, Aksoy O, Ruggeri ZM, Parker W, Davis RD. The role of the porcine von Willebrand factor: baboon platelet interactions in pulmonary xenotransplantation. Transplantation. 2002;74:1596–603. [DOI] [PubMed] [Google Scholar]
- 40.Burdorf L, Riner A, Rybak E, et al. Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor. Xenotransplantation. 2016;23:222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YT, Lee HJ, Lee SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15:27–35. [DOI] [PubMed] [Google Scholar]
- 42.Kang HJ, Lee G, Kim JY, et al. Pre-treatment of donor with 1-deamino-8-d-arginine vasopressin could alleviate early failure of porcine xenograft in a cobra venom factor treated canine recipient. Eur J Cardiothorac Surg. 2005;28:149–56. [DOI] [PubMed] [Google Scholar]
- 43.Fontayne A, Meiring M, Lamprecht S, et al. The humanized anti-glycoprotein Ib monoclonal antibody h6B4-Fab is a potent and safe antithrombotic in a high shear arterial thrombosis model in baboons. Thromb Haemost. 2008;100:670–7. [PubMed] [Google Scholar]
- 44.Cimeno A, Hassanein W, French BM, et al. N-glycolylneuraminic acid knockout reduces erythrocyte sequestration and thromboxane elaboration in an ex vivo pig-to-human xenoperfusion model. Xenotransplantation. 2017;24. doi: 10.1111/xen.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burdorf L, Harris D, Dahi S, et al. Thromboxane and histamine mediate PVR elevation during xenogeneic pig lung perfusion with human blood. Xenotransplantation. 2019;26:e12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeder C, Allan JS, Nguyen BN, et al. Hyperacute rejection is attenuated in GalT knockout swine lungs perfused ex vivo with human blood. Transplant Proc. 2005;37:512–3. [DOI] [PubMed] [Google Scholar]
- 47.Chan J, Chaban R, Pierson RN, et al. Clin Chest Med. 2022. [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laird CT, Hassanein W, O’Neill NA, et al. P- and E-selectin receptor antagonism prevents human leukocyte adhesion to activated porcine endothelial monolayers and attenuates porcine endothelial damage. Xenotransplantation. 2018;25:e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeiffer S, Zorn GL 3rd, Zhang JP, et al. Hyperacute lung rejection in the pig-to-human model. III. Platelet receptor inhibitors synergistically modulate complement activation and lung injury. Transplantation. 2003;75:953–9. [DOI] [PubMed] [Google Scholar]
- 50.Pfeiffer S, Zorn GL 3rd, Blair KS, et al. Hyperacute lung rejection in the pig-to-human model 4: evidence for complement and antibody independent mechanisms. Transplantation. 2005;79: 662–71. [DOI] [PubMed] [Google Scholar]
- 51.Laird CT, Burdorf L, French BM, et al. Transgenic expression of human leukocyte antigen-E attenuates GalKO.hCD46 porcine lung xenograft injury. Xenotransplantation. 2017;24(2). doi: 10.1111/xen.12294 [DOI] [PubMed] [Google Scholar]
- 52.Miura S, Habibabady ZA, Pollok F. et al. Effects of human TFPI and CD47 expression and selectin and integrin inhibition during GalTKO.hCD46 pig lung perfusion with human blood. Xenotransplantation. 2022;29(2):e12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robson SC, Young VK, Cook NS, et al. Thrombin inhibition in an ex vivo model of porcine heart xenograft hyperacute rejection. Transplantation. 1996;61:862–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


