Abstract
Objective Glucose self-monitoring is critical for the management of diabetes in pregnancy, and increased adherence to testing is associated with improved obstetrical outcomes. Incentives have been shown to improve adherence to diabetes self-management. We hypothesized that use of financial incentives in pregnancies complicated by diabetes would improve adherence to glucose self-monitoring.
Study Design We conducted a single center, randomized clinical trial from May 2016 to July 2019. In total, 130 pregnant patients, <29 weeks with insulin requiring diabetes, were recruited. Participants were randomized in a 1:1:1 ratio to one of three payment groups: control, positive incentive, and loss aversion. The control group received $25 upon enrollment. The positive incentive group received 10 cents/test, and the loss aversion group received $100 for >95% adherence and “lost” payment for decreasing adherence. The primary outcome was percent adherence to recommended glucose self-monitoring where adherence was reliably quantified using a cellular-enabled glucometer. Adherence, calculated as the number of tests per day divided by the number of recommended tests per day×100%, was averaged from time of enrollment until admission for delivery.
Results We enrolled 130 participants and the 117 participants included in the final analysis had similar baseline characteristics across the three groups. Average adherence rates in the loss aversion, control and positive incentive groups were 69% (SE=5.12), 57% (SE = 4.60), and 58% (SE=3.75), respectively ( p =0.099). The loss aversion group received an average of $50 compared with $38 (positive incentive) and $25 (control).
Conclusion In this randomized clinical trial, loss aversion incentives tended toward higher adherence to glucose self-monitoring among patients whose pregnancies were complicated by diabetes, though did not reach statistical significance. Further studies are needed to determine whether use of incentives improve maternal and neonatal outcomes.
Key Points
Self-glucose monitoring is a critical part of diabetes management in pregnancy.
Loss aversion financial incentives may increase adherence to glucose self-monitoring in pregnancy.
The impact of testing incentives on maternal and neonatal outcomes requires further investigation.
Keywords: diabetes, pregnancy, financial incentives, loss aversion incentive, gestational diabetes, type 2 diabetes, glucose monitoring, adherence, cellular-enabled glucometer
Diabetes increases pregnancy complications, and glucose self-monitoring is critical for management due to increasing insulin demands throughout pregnancy. 1 2 3 Adherence to recommended glucose self-monitoring varies by type of diabetes, and increased adherence is associated with improved obstetrical outcomes. 4 5 6 Patients identify several limiters to diabetes self-management in pregnancy including time, physical and social constraints associated with recommended glucose monitoring. 7 8
Attempts have been made to use incentives to improve patient adherence to medication administration, weight loss, glucose monitoring and smoking cessation with mixed, and typically short lived, effects in non-pregnant patients (Reviewed by Mantzari et al and Vlaev et al 9 10 ). Incentives can be directed toward rewarding actions needed to improve an outcome (e.g., checking glucose or exercise) or toward rewarding an outcome (e.g., improved glycosylated hemoglobin A1c [HbA1C] or weight loss). While incentives have been shown to improve some behaviors during the course of management, these impacts are largely not sustained following the removal of incentive (Reviewed by Thirumurthy et al 11 ). However, due to its limited time course, pregnancy may provide a unique window where relatively short-term use of incentives improves both maternal and neonatal health. Indeed, financial incentives have been shown to improve rates of smoking cessation in pregnancy 12 13 and attendance at prenatal care visits. 14 15 Use of incentives in pregnancies complicated by diabetes has not been reported.
We sought to test the impact of two incentive strategies, positive incentive and loss aversion, on adherence to glucose monitoring in pregnancy. In the positive incentive scheme, a small incentive is provided on per action basis: this strategy has been used to improve adherence to medication 16 and glucose monitoring 17 outside of pregnancy. Since people tend to weigh the value of a loss greater than a similar value of gain, a loss aversion strategy may influence patient behavior. In this model, patients deposit money in an account and lose fractions of that money in response to decreases in adherence. This model has been associated with increased rates of smoking cessation 18 and increased rates of glucose testing among adolescents with diabetes. 19
We designed a randomized controlled trial to determine if adherence to recommend glucose self-monitoring could be improved through the use of patient level financial incentives. In this study, patients were randomized to control, positive incentive and loss aversion groups and overall adherence to recommend glucose testing over the entire period of enrollment was determined using a cellular-enabled glucometer. We hypothesized that both positive and loss aversion models would improve overall adherence to glucose monitoring in pregnancies complicated by diabetes.
Materials and Methods
From May 2016 to July 2019, we recruited pregnant patients with type 1, type 2, or gestational diabetes requiring insulin who were at less than 29 weeks gestation and referred to an academic perinatal diabetes clinic. As part of a broader quality improvement initiative, 20 our program offered remote glucometer monitoring to patients less than 30 weeks gestation. As part of this program, all participants received compatible test strips free of charge. Written informed consent was obtained, and participants were allocated in 1:1:1 ratio to one of three arms. The control group received $25 with enrollment. The positive incentive group received $0.10 for each test completed which was paid out at 2 to 4 week intervals (this was increased to 4 week intervals 6 months into the study due to administrative burdens of sending checks every 2 weeks). The loss aversion group had $100 placed into a department-specific account with enrollment with losses to their postdelivery payout based on overall adherence to testing. At the time this study was conceived, adherence was reported at >95% in at least one RCT of glucose management in pregnancy, 21 which we used as an ideal target. Since we desired to incentivize adherence greater than the 70% our preliminary data suggested, we defined ranges between 70 and 95%. Those completing more than 95% of recommended tests lost no funds and received the full $100 following delivery. Those completing 85 to 94% of recommended tests lost $25 and received $75 after delivery. Those completing 70 to 84% of recommended tests lost $50 and received $50 after delivery. Those completing fewer than 69% of tests lost $75 and received $25 after delivery. If participants in the positive incentive and loss aversion groups completed 70% of recommended tests over 25 weeks, they would both receive $50 in compensation. This study was reviewed and approved by the University of Iowa Institutional Review Board (no.: 201603724) and registered at clinicaltrials.gov (no.: NCT03338829 https://clinicaltrials.gov/ct2/show/NCT03338829 ). The study protocol ( Supplementary Material S1 , available in the online version) as submitted to our IRB and clinicaltrials.gov is included as a supplement. This randomized study follows CONSORT reporting guidelines for trial studies. 22
The primary outcome of the study was overall adherence to recommended glucose self-monitoring. At the time this study started, patients with type 1 diabetes did not regularly use continuous glucometers, and we recommended seven daily glucose checks (fasting, 1hour post breakfast, pre-lunch, 1hour post lunch, pre-dinner and 1hour post dinner and nightly). Patients with type 2 and gestational diabetes were advised to have four glucose tests daily (fasting and 1hour post breakfast, lunch and dinner). To determine adherence to testing, all participants were provided with a cellular-enabled glucometer as part of our broader care program (Telcare, now BioTel). This glucometer uploads all glucose values to a HIPAA compliant, web-based portal irrespective of patient access to cellular data or wireless internet. 23 All participants could track their testing adherence throughout pregnancy using this portal. Time stamps on glucose values were manually reviewed to ensure that tests were done throughout the course of day. Overall adherence from the date of enrollment to admission for delivery was determined by the number of tests daily divided by the # of recommended tests daily and averaged over the course of the study as previously described. 6 If participants were admitted to the hospital, hospital days were excluded in our adherence calculations. Secondary outcomes were HbA1c at birth, rates of preeclampsia, cesarean delivery, neonatal hypoglycemia, and large for gestational age neonates.
Based on our preliminary analysis of adherence to recommended glucose monitoring prior to the start of the study, we found a 70% adherence to glucose self-monitoring without incentives. We estimated that a 10% difference in adherence would be clinically meaningful. With a type I error rate of 5%, power of 80%, and an anticipated drop-out rate of 30%, we recruited 130 participants.
Participants were allocated 1:1:1 to control, positive incentive, and loss aversion group in blocks of 10 and stratified by diabetes type (type 1 vs. type 2 or GDM and A2) using a computer random number generator (by B.D.). Based on historic assessment that approximately 25% of our patient population had type 1 diabetes, our stratification strategy randomized 30 patients with type 1 diabetes to ensure equal representation among groups. Allocation was concealed with assignments in sequentially numbered, opaque sealed envelopes of equal weight and size that were numbered in advanced by assessor (S.A.W.) and opened sequentially by our study coordinator (D..F) at the time of participant enrollment. Prenatal care providers were blinded to group allocation. There was no record of incentive group in the medical record. By necessity, patients, study coordinator, and assessor were not blinded to treatment group.
Welch test was conducted to assess the impact of intervention group on levels of adherence at delivery. Chi-square tests, one-way analysis of variance, Kruskal–Wallis, and Welch tests were used to compare baseline characteristics and secondary outcomes between groups. All analyses were performed using SPSS version 26.
Results
Characteristics of Participants
From May 2016 to July 2019, 802 patients with diabetes were cared for by our perinatal diabetes clinic and assessed for eligibility ( Fig. 1 ). In total, 429 did not require insulin and 132 were not considered eligible for the remote glucometer, largely due to gestational age >32 weeks at first contact with our program. Eleven declined remote monitoring. Overall, 230 used remote monitoring as part of our clinic and of these, 63 were not eligible for our study due to language, early pregnancy loss prior to recruitment, and first contact after 29 weeks gestation. An additional 30 patients with a diagnosis of type 1 diabetes were not approached by our study group as we had reached our cap of 30 participants with this diagnosis prior to their encounter in clinic. In total, 137 patients were eligible for this study and seven declined. In total, 130 participants were ultimately recruited in the study ( Fig. 1 ). We excluded from the analysis participants who withdrew from the study due to relocation or pregnancy loss after recruitment. One participant was recruited while on glyburide and not included in the analysis.
Fig. 1.
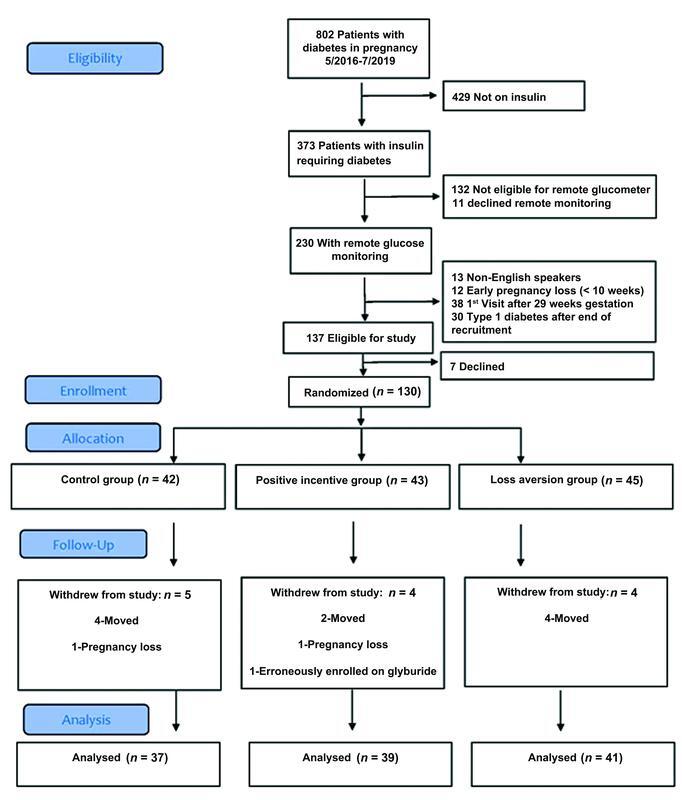
Flow diagram showing patient eligibility, recruitment, and exclusions.
Overall, 117 participants were included in the final analysis and the baseline characteristics of participants enrolled in the study were similar among the groups ( Table 1 ). While all enrolled participants used insulin, two patients in both the positive incentive and loss aversion group patients used metformin in addition to insulin. Additionally, nine patients used a concentrated form of insulin (500 U/mL) due to high insulin resistance. Approximately half of participants in each group were enrolled in public insurance. There were similarities in body mass index and chronic hypertension. On average, diabetes was similarly controlled at the time of enrollment based on HbA1c of 7.6, 7.8, and 7.2% for the control, positive and loss aversion groups.
Table 1. Baseline characteristics.
| Control | Positive incentive | Loss aversion | |
|---|---|---|---|
| N | 37 | 39 | 41 |
| Maternal age, years | 31 (0.92) | 30 (0.79) | 32 (0.93) |
| Self-reported Race/Ethnicity | |||
| White | 28 (76%) | 26 (67%) | 30 (73%) |
| Black | 4 (11%) | 7 (18%) | 7 (17%) |
| Hispanic/Latina | 4 (11%) | 3 (8%) | 3 (7%) |
| Asian | 0 (0%) | 1 (3%) | 1 (2%) |
| Multiracial | 1 (3%) | 2 (5%) | 0 (0%) |
| Medicaid | 21 (57%) | 22 (56%) | 22 (54%) |
| Nulliparous | 11 (30%) | 12 (31%) | 15 (37%) |
| Diabetes type: | |||
| Type 1 diabetes | 10 (27%) | 9 (23%) | 10 (24%) |
| Type 2 diabetes | 21 (57%) | 25 (64%) | 24 (59%) |
| Gestational diabetes, A2 | 6 (16%) | 5 (13%) | 7 (17%) |
| Gestational age at enrollment (weeks) | 15 (1.08) | 18 (1.16) | 16 (1.36) |
| Medication: | |||
| Insulin | 35 (95%) | 35 (90%) | 34 (83%) |
| Insulin and metformin | 0 (0%) | 2 (5%) | 2 (5%) |
| Insulin (U500) | 2 (5%) | 2 (5%) | 5 (12%) |
| BMI kg/m 2 | 36 (1.37) | 39 (1.36) | 37 (1.25) |
| Chronic hypertension | 17 (46%) | 16 (41%) | 17 (42%) |
| Depression | 11 (30%) | 15 (39%) | 17 (43%) a |
| HbA1c at first prenatal visit | 7.6 (0.33) a | 7.8 (0.32) a | 7.2 (0.27) |
Note: Data are expressed as mean (SE) or n (%).
Missing data for one participant.
Follow-up and Outcomes
We found that participants in the loss aversion group had increased rates of overall adherence to glucose self-monitoring ( Table 2 ), though this did not reach statistical significance. Average adherence in the loss aversion group was 69% (SE=3.75%) compared with 57% (SE=±5.12%) in the control group and 58% (SE4.6%) in the positive incentive group ( p =0.099). We noted a wide range of adherence in all three groups, though the only participants who did not obtain any glucose values in pregnancy were in the control group ( Fig. 2 ). Participants in the loss aversion group received $50 on average compared with $38 in the positive incentive group and the predetermined $25 in the control group.
Table 2. Comparison of adherence.
| Control | Positive incentive | Loss aversion | Overall p | Positive incentive vs. control p | Loss aversion vs. control p | |
|---|---|---|---|---|---|---|
| Primary outcome: overall % adherence | 57.31 (5.12) | 58.48 (4.60) | 69.08 (3.75) | 0.099 a | N/A | N/A |
| Average $ paid |
25.00 (0.00)
b, c
Median (IQR): 25.00 (25–25) |
38.13 (5.22)
d
Median (IQR): 30.80 (15.6–50.6) |
50.00 (4.54)
d
Median (IQR): 50.00 (25–75) |
<0.001 e | 0.022 | <0.001 |
Abbreviations: IQR, interquartile range; N/A, not available.
Note: Data are expressed as mean (standard error) or median (IQR).
p -Value for Welch's test as variable violated assumption of homogeneity of variance.
Differs from positive incentive.
Differs from loss aversion.
Differs from control.
p -Value is for Kruskal–Wallis test.
Fig. 2.
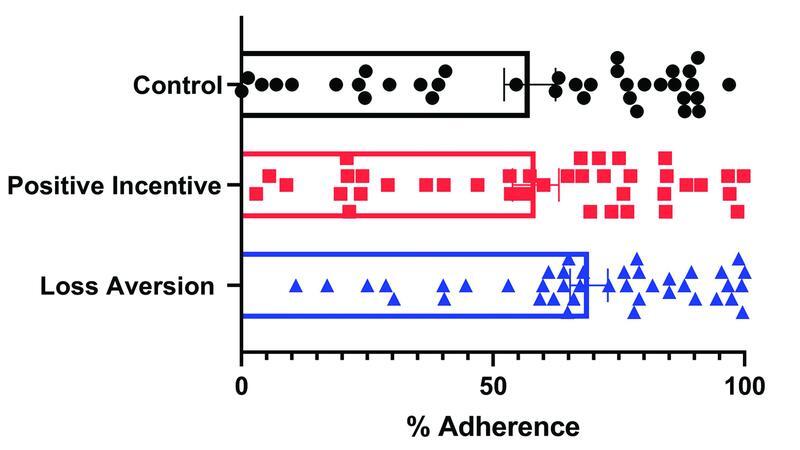
Graph demonstrating variation in adherence to self-glucose monitoring between participants in control, positive, and loss aversion groups.
On average, all groups showed improved HbA1c at the time of delivery compared with enrollment. There were no statistically significant differences in preeclampsia, gestational hypertension, gestational age at delivery, cesarean delivery, birthweight, neonatal hypoglycemia, and large for gestational age neonates ( Table 3 ).
Table 3. Delivery outcomes.
| Control | Positive incentive | Loss aversion | Overall p | |
|---|---|---|---|---|
| HbA1c at delivery | 5.8 (0.17) a | 6.0 (0.18) b | 5.8 (0.15) a | 0.587 |
| Average change in HbA1c from first obstetrician visit | −1.5 (0.29) c | −1.7 (0.39) d | −1.1 (0.23) e | 0.270 f |
| Gestational age at delivery (wk) | 37 (0.36) | 37 (0.37) | 37 (0.65) g | 0.559 |
| Preeclampsia | 11 (30%) | 10 (26%) | 8 (20%) | 0.573 |
| Gestational hypertension | 4 (11%) | 6 (15%) | 4 (10%) | – |
| Cesarean delivery | 25 (68%) | 22 (56%) | 22 (55%) h | 0.475 |
| Neonatal hypoglycemia | 19 (54%) g | 24 (63%) h | 30 (75%) h | 0.169 |
| Birth weight (g) | 3,349 (145) h | 3,301 (130) | 3,249 (168) h | 0.895 |
| Large for gestational age neonate | 10 (28%) h | 8 (21%) | 10 (25%) h | 0.759 |
Note: Data are expressed as mean (standard error) or n (%).
p -Values for analysis of variance for continuous outcomes and for Chi-square for categorical outcomes.
Missing data for nine participants.
Missing data for four participants.
Missing data for ten participants.
Missing data for five participants.
Missing data for 11 participants.
p -Value for Welch's test as variable violated assumption of homogeneity of variance.
Missing data for two participants.
Missing data for one participant.
A post hoc analysis was done to explore if there were differences in outcomes based on insurance status. Baseline demographics of these groups are provided in Supplementary Table S1 (available in the online version). No statistically significant differences in adherence to self-glucose monitoring were detected between the three groups for either publicly or privately insured participants ( Supplementary Tables S2 and S3 [available in the online version]).
Discussion
This work shows that the use of a loss aversion incentive model may improve adherence to glucose testing in pregnant patients with insulin-requiring diabetes. This study is the first we are aware of that tests the impact of loss aversion incentives on diabetes management in pregnancy. Prior work shows that the loss aversion model of incentives can improve adherence to glucose testing 19 ; our findings show a similar trend toward improved adherence with the loss aversion model in pregnancy though do not reach statistical significance. While the use of loss aversion models in diabetes is not associated with long-term behavior changes, the inherently limited time frame of pregnancy may make it a unique opportunity to impact important clinical outcomes.
In contrast to the trend to improved adherence with loss aversion incentives, we see no impact of positive incentives on adherence to glucose testing. Since the potential payout for someone enrolled for 25 weeks with four recommended daily glucose tests was approximately $70 and approximately $123 for seven recommended tests, we do not think the payment magnitude explains this difference in adherence. Rather, the impact of positive incentive may have been diminished by the interval of payment. The most effective payment intervals have been found to be less than 1 week. 9 We initially planned to pay our positive incentive group at 2 week intervals. However, due to several logistical difficulties with check production, payment intervals were increased to 4 weeks shortly after commencing enrollment. This payment timeframe may have been deferred enough to limit positive reinforcement.
We opted to target our financial incentives to reinforce process as opposed to outcome measures (such as HbA1c or neonatal outcome). Frequent insulin adjustments in response to glucose monitoring are necessary during pregnancy due to the known effects of increased insulin resistance. Use of incentives may reinforce constructive habits to improve clinical outcomes; however, this study is limited in ability to detect that change. There is a concern that rewarding recommended behavior may lead patients to be less willing to engage in positive behavior without rewards in the future (reviewed by Vlaev et al 10 ). We cannot address that concern with this work, though it should be noted that glucose monitoring requirements in pregnancy are different than in non-pregnant populations; thus, the impact of this concern may be less in pregnancy.
Over the course of this study, there were several changes that occurred in clinical management of diabetes in pregnancy. We recruited 30 participants with type 1 diabetes early in the study, at a time where continuous glucometers were not a routine part of our clinical practice. Thus, continuous glucometers did not likely affect self-glucose monitoring in this population. Additionally, until 2018, participants with any initial diagnosis of glucose intolerance in pregnancy, were classified as having gestational diabetes per ACOG guidelines 24 and oral medication agents were typically first line treatment choices in pregnant patients with type 2 and gestational diabetes. By recruiting participants requiring insulin therapy at less than 29 weeks gestation, most participants likely had type 2 diabetes or severe gestational diabetes in our population.
Strengths
A strength of this study is its randomized design. The incorporation of a cellular-enabled glucometer allowed accurate assessment of patient adherence to testing independent of patient wireless or cellular service access or data plan. The use of this meter allowed assessments and payments to be done without direct communication between patients, providers, and study assessors limiting potential bias. Additionally, all participants received care in the same integrated, multidisciplinary perinatal diabetes clinic. Clinical providers were not aware of study assignment of participants, since no record of group allocation was maintained in the clinical record. Overall, this work contributes to an emerging body of evidence suggesting that incentives may improve adherence to prenatal care recommendations. 14 25
Limitations
A limitation of this study is that it was powered to detect differences in adherence to glucose self-monitoring; thus its limited sample size prevents detection of differences in subsequent clinical outcomes. Unfortunately, our participants' actual adherence was lower and standard deviation greater than those utilized in our sample size calculation, thus limiting our power to detect 10% differences in our primary outcome. Given the small sample size, it is difficult to assess if there are any meaningful trends in the clinical outcomes observed. Additionally, we cannot specifically address how factors such as depression impact adherence to testing recommendations.
There is some historical controversy about whether glucose self-monitoring improves perinatal outcomes in patients with gestational diabetes (reviewed by Homko and Reece 26 ). However, glucose self-monitoring is considered a key component of diabetes management in pregnancy. 1 3 Our group has shown that a 10% increase in glucose testing is associated with decreased rates of cesarean delivery, neonatal hypoglycemia, and large for gestational age neonates 6 and others have shown decreased rates of preeclampsia with increased testing frequency. 4 Additionally, our study was completed within an integrated perinatal diabetes care clinic so results may not be generalizable to other settings.
Conclusion
Adverse maternal and neonatal outcomes are associated with inadequately controlled diabetes in pregnancy and adherence to testing is one component of diabetes care in pregnancy. 1 2 3 Our current work suggests that the use of cellular-enabled glucometers and loss aversion-based incentives may increase adherence to testing recommendations and improve perinatal diabetes care. Looking ahead, we would recommend further two arm studies, comparing control and loss aversion models, to determine if the use of incentives can improve maternal and neonatal outcomes in pregnancies complicated by diabetes.
Acknowledgments
The authors wish to acknowledge Ms. Dixie Bixler, Department of Obstetrics and Gynecology at the University of Iowa, who assisted with accounting and mailing of checks to participants. She was not directly compensated for her work.
Funding Statement
Funding This work was funded internally through the perinatal diabetes program at the University of Iowa Hospitals and Clinics.
Conflict of Interest None declared.
Note
A preliminary analysis of work was previously presented in poster format at the 2021 Society for Maternal Fetal Medicine Annual Meeting. This study is registered with Clinical Trial Registry at: https://clinicaltrials.gov/ct2/show/NCT03338829 . The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
References
- 1.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics . ACOG Practice Bulletin No. 201: pregestational diabetes mellitus. Obstet Gynecol. 2018;132(06):e228–e248. doi: 10.1097/AOG.0000000000002960. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(02):e49–e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association . 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43 01:S183–S192. doi: 10.2337/dc20-S014. [DOI] [PubMed] [Google Scholar]
- 4.Cosson E, Baz B, Gary F et al. Poor reliability and poor adherence to self-monitoring of blood glucose are common in women with gestational diabetes mellitus and may be associated with poor pregnancy outcomes. Diabetes Care. 2017;40(09):1181–1186. doi: 10.2337/dc17-0369. [DOI] [PubMed] [Google Scholar]
- 5.Sperling J D, Maggio L, Has P, Daley J, Khander A, Coustan D R. Prenatal care adherence and neonatal intensive care unit admission or stillbirth among women with gestational and preexisting diabetes mellitus. Am J Perinatol. 2018;35(02):103–109. doi: 10.1055/s-0037-1603343. [DOI] [PubMed] [Google Scholar]
- 6.Wernimont S A, Sheng J S, Tymkowicz A et al. Adherence to self-glucose monitoring recommendations and perinatal outcomes in pregnancies complicated by diabetes mellitus. Am J Obstet Gynecol MFM. 2019;1(03):100031. doi: 10.1016/j.ajogmf.2019.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carolan M, Gill G K, Steele C. Women's experiences of factors that facilitate or inhibit gestational diabetes self-management. BMC Pregnancy Childbirth. 2012;12:99. doi: 10.1186/1471-2393-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yee L M, McGuire J M, Taylor S M, Niznik C M, Simon M A. “I Was Tired of All the Sticking and Poking”: identifying barriers to diabetes self-care among low-income pregnant women. J Health Care Poor Underserved. 2015;26(03):926–940. doi: 10.1353/hpu.2015.0073. [DOI] [PubMed] [Google Scholar]
- 9.Mantzari E, Vogt F, Shemilt I, Wei Y, Higgins J P, Marteau T M. Personal financial incentives for changing habitual health-related behaviors: a systematic review and meta-analysis. Prev Med. 2015;75:75–85. doi: 10.1016/j.ypmed.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlaev I, King D, Darzi A, Dolan P. Changing health behaviors using financial incentives: a review from behavioral economics. BMC Public Health. 2019;19(01):1059. doi: 10.1186/s12889-019-7407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thirumurthy H, Asch D A, Volpp K G. The uncertain effect of financial incentives to improve health behaviors. JAMA. 2019;321(15):1451–1452. doi: 10.1001/jama.2019.2560. [DOI] [PubMed] [Google Scholar]
- 12.Higgins S T, Washio Y, Heil S Het al. Financial incentives for smoking cessation among pregnant and newly postpartum women Prev Med 201255(suppl):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cessation in Pregnancy Incentives Trial Team . Tappin D, Bauld L, Purves D et al. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ. 2015;350:h134. doi: 10.1136/bmj.h134. [DOI] [PubMed] [Google Scholar]
- 14.Adams J, van der Waal Z, Rushton S, Rankin J. Associations between introduction and withdrawal of a financial incentive and timing of attendance for antenatal care and incidence of small for gestational age: natural experimental evaluation using interrupted time series methods. BMJ Open. 2018;8(01):e017697. doi: 10.1136/bmjopen-2017-017697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal M B, Li Z, Robertson A D, Milstein A.Impact of financial incentives for prenatal care on birth outcomes and spending Health Serv Res 200944(5 Pt 1):1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raiff B R, Jarvis B P, Dallery J. Text-message reminders plus incentives increase adherence to antidiabetic medication in adults with type 2 diabetes. J Appl Behav Anal. 2016;49(04):947–953. doi: 10.1002/jaba.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen A P, Sewell T B, Riley E B et al. Financial incentives for home-based health monitoring: a randomized controlled trial. J Gen Intern Med. 2014;29(05):770–777. doi: 10.1007/s11606-014-2778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpern S D, French B, Small D S et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372(22):2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong C A, Miller V A, Murphy K et al. Effect of financial incentives on glucose monitoring adherence and glycemic control among adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA Pediatr. 2017;171(12):1176–1183. doi: 10.1001/jamapediatrics.2017.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syrop C H, Wernimont S A, Fleener D K, Kardos J M, Rubenstein L M, Andrews J I. Redesigned care delivery for insulin-requiring diabetes in pregnancy improves perinatal glycemic control while reducing neonatal intensive care admissions, length of stay, and costs. J Womens Health (Larchmt) 2021;30(04):557–568. doi: 10.1089/jwh.2020.8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Veciana M, Major C A, Morgan M A et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333(19):1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 22.Schulz K F, Altman D G, Moher D. CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wernimont S A, Sheng J S, Fleener D, Summers K M, Syrop C, Andrews J I. Cellular-enabled glucometers and maternal glucose control: a quality improvement initiative. J Diabetes Sci Technol. 2020;14(01):77–82. doi: 10.1177/1932296819856360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ACOG Committee on Practice Bulletins . ACOG practice bulletin. clinical management guidelines for obstetrician-gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105(03):675–685. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- 25.Till S R, Everetts D, Haas D M. Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes. Cochrane Database Syst Rev. 2015;(12):CD009916. doi: 10.1002/14651858.CD009916.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homko C J, Reece E A. Self-monitoring of blood glucose in gestational diabetes. J Matern Fetal Neonatal Med. 2002;12(06):389–395. doi: 10.1080/jmf.12.6.389.395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


