Abstract
Background
There is evidence of an association of severe coroanavirus disease (COVID-19) outcomes with increased body mass index (BMI) and male sex. However, few studies have examined the interaction between sex and BMI on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral dynamics.
Methods
Participants conducted RT-PCR testing every 24–48 hours over a 15-day period. Sex and BMI were self-reported, and Ct values from E-gene were used to quantify viral load. Three distinct outcomes were examined using mixed-effects generalized linear models, linear models, and logistic models, respectively: all Ct values (model 1), nadir Ct value (model 2), and strongly detectable infection (at least 1 Ct value ≤28 during their infection) (model 3). An interaction term between BMI and sex was included, and inverse logit transformations were applied to quantify the differences by BMI and sex using marginal predictions.
Results
In total, 7988 participants enrolled in this study and 439 participants (model 1) and 309 (models 2 and 3) were eligible for these analyses. Among males, increasing BMI was associated with lower Ct values in a dose-response fashion. For participants with BMIs greater than 29 kg/m2, males had significantly lower Ct values and nadir Ct values than females. In total, 67.8% of males and 55.3% of females recorded a strongly detectable infection; increasing proportions of men had Ct values <28 with BMIs of 35 and 40 kg/m2.
Conclusions
We observed sex-based dimorphism in relation to BMI and COVID-19 viral load. Further investigation is needed to determine the cause, clinical impact, and transmission implications of this sex-differential effect of BMI on viral load.
Keywords: SARS-CoV-2, Ct value, viral load, biological sex, body mass index
We observed an interaction between sex and BMI on viral load, and the highest viral loads were among males with high BMIs. For participants with BMIs over 29, males had significantly lower Ct values and nadir Ct values than females.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus that causes coronavirus disease 2019 (COVID-19), has resulted in significant morbidity and mortality globally since its emergence in 2019. While many SARS-CoV-2 infections are mild and self-resolving, severe COVID-19 has been associated with risk factors including male sex and increased body mass index (BMI) [1–3]. Globally, COVID-19 has resulted in more hospitalizations, severe disease, and mortality among males than females, despite similar rates of SARS-CoV-2 infection between these groups [4]. Furthermore, increased BMI has been associated with hospitalization, ventilation, and death in a dose-response manner, with the highest BMI categories having the highest risk of severe outcomes [2, 3, 5]. Despite these 2 characteristics being known risk factors for severe disease, few studies have examined the interaction between sex and BMI on SARS-CoV-2 infection course and COVID-19 outcomes.
The increased severity of COVID-19 among males has been hypothesized to be multifactorial, due to a combination of immune factors, hormonal factors, and ACE-2 expression, which differ between males and females [6–8]. Many of the same mechanisms have been proposed to account for the increased severity of COVID-19 associated with increased BMI [9]. Therefore, we hypothesized that there could be sex differences in the impact of BMI on SARS-CoV-2 infection.
Understanding the longitudinal viral dynamics of SARS-CoV-2 infection has important implications to characterize infection course and identify detection windows for diagnostics, as well as a potential role in infectivity and transmission. Using data from 2 large longitudinal cohort studies, we aimed to determine whether longitudinal viral dynamics and higher viral burdens in SARS-CoV-2–infected community-dwelling adults across the United States differed by sex and BMI.
METHODS
Study Population
We used data from 2 longitudinal cohort studies, Test Us At Home and Test Us At Home-Daily, funded by the National Institutes of Health Rapid Acceleration of Diagnostics (RADx) program. Both studies were approved by the Western-Copernicus Group (WCG) Institutional Review Board (20214875), and all participants provided written informed consent. Participants from across the continental United States were enrolled between 18 October 2021 and 3 February 2022, and these studies used a digital site-less approach where tests were shipped to participants' residences. Participants over 18 years old who tested positive for SARS-CoV-2 at least once by reverse transcription–polymerase chain reaction (RT-PCR) during the study period were included in this analysis.
All participants were asked to complete baseline and symptom surveys using a study app (MyDataHelps, CareEvolution LLC) and conduct self-sampling for RT-PCR testing every 48 hours over a 15-day period (Test Us At Home) or every 24 hours over a 10-day period (Test Us At Home-Daily). Participants were required to be asymptomatic on consenting to participate in the study; however, there was a 24–48-hour lag between consenting and starting the testing period due to a delay in the shipment of testing materials. During each testing session, a bilateral anterior nasal swab was collected by the participant and sent to a central laboratory, Quest Diagnostics, for Roche Cobas RT-PCR testing. Details on the study design, protocol, and participants were described elsewhere [10].
Variables
Exposures
Participants self-reported their biological sex (male, female, intersex, other), height, and weight on enrollment in the study. Participants who reported their sex as “intersex” or “other” were excluded from analyses due to the small sample size. Height and weight were used to calculate participant BMI.
Outcomes
Cycle threshold (Ct) values for the E-gene from Roche Cobas 6800 SARS-CoV-2 RT-PCR were used in analyses to quantify viral load. If a cycle threshold was only reported for the Open Reading Frame 1a/b (ORF1a/b) gene, a Ct value of 40 was used. Three distinct outcomes were examined: (1) Ct value at the test level; (2) nadir Ct value, defined at the participant level as the lowest Ct value over the infection course; and (3) strongly detectable infection, defined at the participant level as at least 1 Ct value less than or equal to 28.
Confounders
Vaccination status (< or >2 doses), history of previous SARS-CoV-2 infection, age, and comorbidities were self-reported on enrollment. Participants self-reported symptoms (fever, body aches, fatigue, rash, nausea, abdominal pain, diarrhea, loss of smell, runny nose, cough, headache, or other) immediately prior to each swab collection using the study app. Cases that tested positive on or after 1 January 2022 were assigned to the Omicron variant group, while samples collected before 20 December 2021 were assigned to the Delta variant group. Samples that were positive between 19 December and 31 December 2021 were sequenced and assigned to their respective variant accordingly. Those without sequencing results were excluded in adjusted analyses (n = 20).
Data Analysis
Descriptive statistics were tabulated at the participant level by sex. We analyzed 3 dependent variables: Ct value (model 1), nadir Ct value (model 2), and presence of strongly detectable infection, defined as at least 1 Ct value of 28 or less over the course of the infection (model 3). Models 2 and 3 excluded participants who had their lowest Ct value on the first day of the study or tested positive for the first time on the last study day to ensure we captured the peak viral load. For all 3 dependent variables, we analyzed the outcomes of 3 different model specifications: unadjusted bivariate model with sex or BMI (continuous) as the independent variable, an adjusted model including additional covariates, and an adjusted model with an interaction term between BMI and sex. Adjusted models included the following additional covariates: vaccination doses (0, 1, 2, or 3+ doses), previous infections (none or ≥1), number of symptoms on the day of sample collection, number of comorbidities, age (continuous), variant (Delta or Omicron), and day from nadir Ct-value squared (only included in model 1, which uses repeated measures) (Supplementary Figure 2) [11, 12]. Day from nadir Ct value was squared to account for the underlying distribution of Ct values across the duration of an infection, which is not a linear relationship. A generalized linear mixed-effects model with a random intercept at the study participant level was used to assess the relationship between sex and BMI on all Ct values (model 1). Nadir Ct value was evaluated at the participant level using an adjusted generalized linear model (model 2). A logistic model was used to estimate the predicted probability of a strongly detectable infection by sex and BMI (model 3). A Ct value of 28 was used as the cutoff point due to that being the approximate limit of detection for lower-sensitivity diagnostics (model 3) [13, 14]. Nonlinear combinations of predictions were used to quantify the differences by BMI and sex and calculate the accompanying 95% confidence intervals (CIs) using the Delta method, which uses Taylor series linearization. The analysis was rerun analyzing Ct values from the ORF1a/b gene as a sensitivity analysis. Additionally, a sensitivity analysis was run including participants with a BMI greater than 50 kg/m2. Statistical analyses were conducted using Stata version 17.0 (StataCorp LLC, College Station, Texas).
RESULTS
Study Population
In total, 439 eligible participants, including 121 males and 318 females, tested positive for SARS-CoV-2 during the study period and collected 1532 positive RT-PCR results (Figure 1). Among these participants, 309 participants (90 males and 219 females) did not have their lowest Ct values on the first day of the study, meaning that we were able to capture their nadir Ct value (peak viral load). Rates of asymptomatic infections were similar between males (27.3%) and females (24.2%), and more females than males reported having more than 3 symptoms (42.1% vs 34.7%) (Table 1). Approximately 22.0% of females and 25.6% of males were unvaccinated for SARS-CoV-2. Males in the study were slightly older than females, with 10.7% of males and 4.4% of females being over 65 years old. Additionally, 38.4% of females and 28.1% of males had a BMI greater than 30 kg/m2 (Supplementary Figure 1).
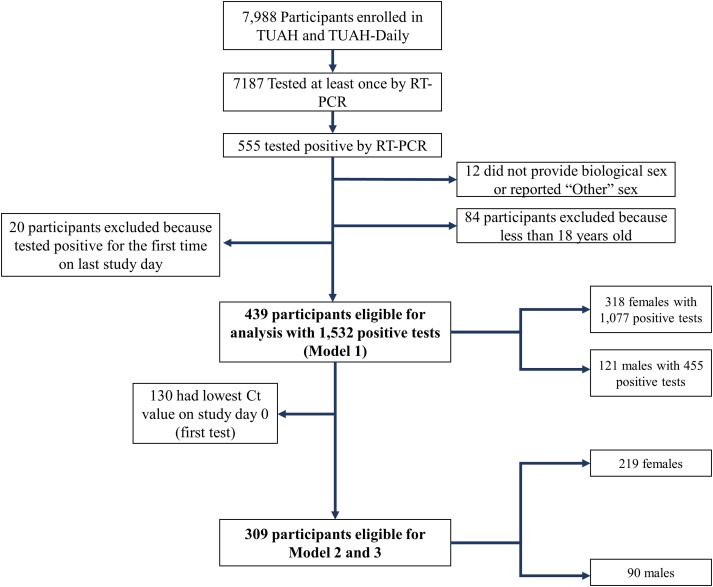
Figure 1.
CONSORT diagram of Test Us At Home participants. In total, 439 participants were eligible for model 1, which included 121 males and 318 females. Among these participants, 130 were excluded from models 2 and 3 because they had their lowest Ct value on the first study day. Therefore, 309 participants were eligible for model 2 and model 3. Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; Ct, cycle threshold; RT-PCR, reverse transcription–polymerase chain reaction; TUAH, Test Us At Home; TUAH-Daily, Test Us At Home-Daily.
Table 1.
Characteristics of Participants by Sex
| Female (n = 318) | Male (n = 121) | |
|---|---|---|
| Number of positive PCR tests per participant, mean (range) | 3.27 (2.18) | 3.69 (2.07) |
| Symptoms, n (%) | ||
| Asymptomatic | 77 (24.2%) | 33 (27.3%) |
| 1–2 symptoms | 107 (33.5%) | 46 (38.0%) |
| 3+ symptoms | 134 (42.1%) | 42 (34.7%) |
| Vaccination status, n (%) | ||
| Unvaccinated | 70 (22.0%) | 31 (25.6%) |
| 1 dose | 8 (2.5%) | 4 (3.3%) |
| 2 doses | 133 (41.8%) | 45 (37.2%) |
| 3 doses | 105 (33.0%) | 40 (33.1%) |
| Missing | 2 (0.6%) | 1 (0.8%) |
| BMI category (kg/m2), n (%) | ||
| <18.5 | 4 (1.3%) | 3 (2.5%) |
| 18.5–25 | 122 (38.4%) | 38 (31.4%) |
| 25–30 | 70 (22.0%) | 46 (38.0%) |
| >30 | 122 (38.4%) | 34 (28.1%) |
| Prior COVID-19 infections, n (%) | ||
| No previous infection | 281 (88.4%) | 109 (90.1%) |
| 1 previous infection | 32 (10.1%) | 9 (7.4%) |
| 2+ previous infections | 5 (1.6%) | 3 (2.5%) |
| Age category, n (%) | ||
| 18–44 y | 223 (70.1%) | 72 (59.5%) |
| 45–64 y | 71 (22.3%) | 32 (26.4%) |
| 65+ y | 14 (4.4%) | 13 (10.7%) |
| Missing | 10 (3.1%) | 4 (3.3%) |
| Comorbidities, n (%) | ||
| No comorbid conditions | 190 (59.7%) | 78 (64.5%) |
| 1 comorbidity | 80 (25.2%) | 26 (21.5%) |
| 2+ comorbidities | 48 (15.1%) | 17 (14.0%) |
| Variant, n (%) | ||
| Omicron | 245 (77.0%) | 99 (81.8%) |
| Delta | 52 (16.4%) | 20 (16.5%) |
| Missing | 21 (6.6%) | 2 (1.6%) |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
Cycle Threshold Values by Sex and BMI
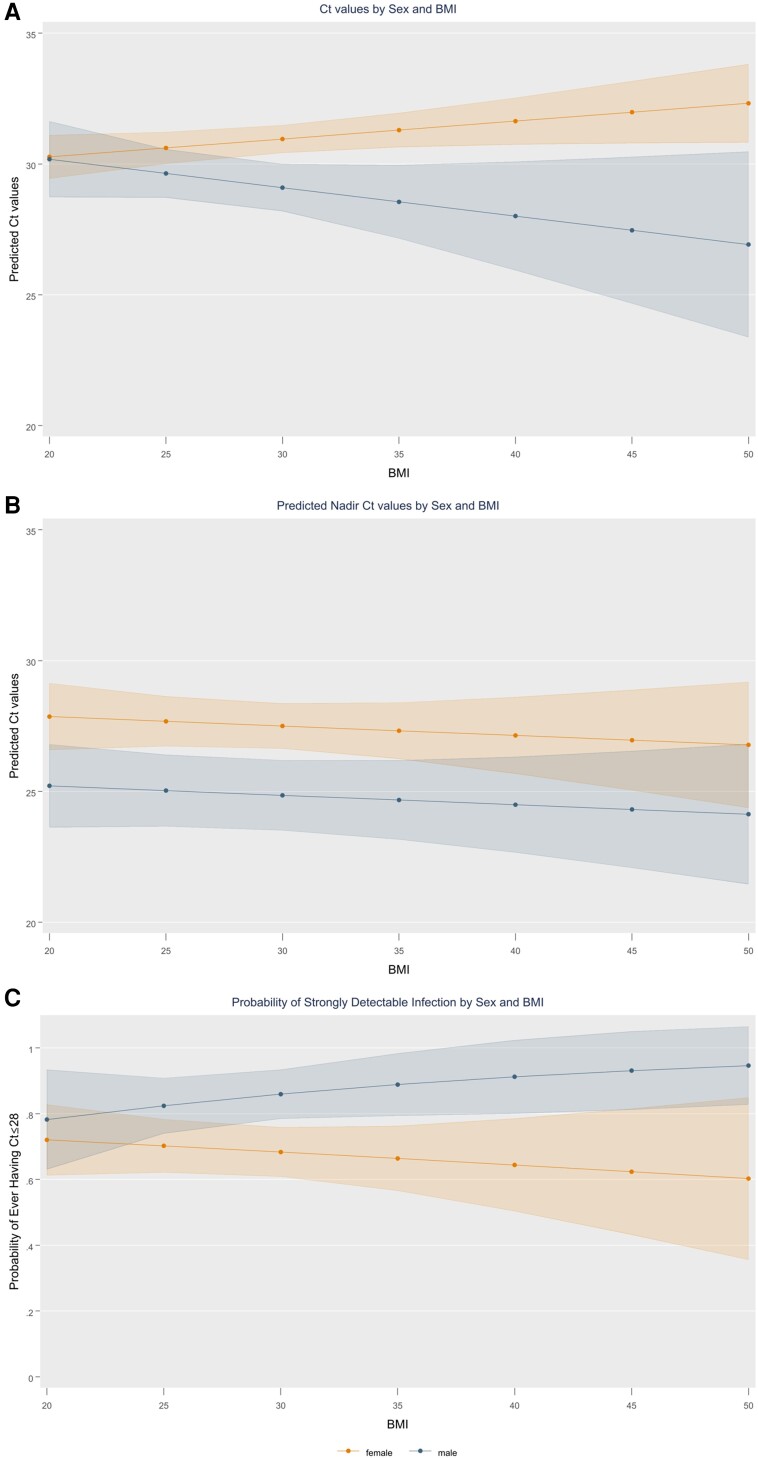
In unadjusted bivariate models, males had lower SARS-CoV-2 Ct values than females (−1.15; 95% CI, −2.15 to −.16) (Supplementary Table 1). After adjusting for covariates, males had Ct values that were 1.49 (95% CI, .49–2.50) points lower than females (Supplementary Table 2). Ct values did not significantly differ by BMI in unadjusted and adjusted models; however, it was observed that there was a significant interaction between sex and BMI, and the impact of BMI on Ct values differed significantly by sex (P = .039) (Figure 2A; Supplementary Figure 5). Among males, increasing BMI resulted in lower Ct values among males in a dose-response fashion. Among females, conversely, increasing BMI was not associated with lower Ct values. Males had significantly lower Ct values than females for BMIs greater than 28 kg/m2. For every 1-unit increase in BMI, Ct values increased by 0.07 (95% CI, .01–.14) for females and decreased by 0.11 (β = −0.11; 95% CI, −.26 to .05) for males (Supplementary Table 3). Ct values from females were higher than males by 1.85 points (95% CI, .81–2.91), 2.74 points (95% CI, 1.19–4.30), and 3.63 points (95% CI, 1.36–5.89) at BMIs of 30, 35, and 40 kg/m2, respectively (Table 2).
Figure 2.
Predicted Ct values, nadir Ct values, and strongly detectable infections by sex and BMI. The shaded error bar represents the 95% confidence interval. Panel A shows predicted Ct values among males and females by BMI. Panel B shows predicted nadir Ct values among males and females by BMI. Panel C shows the probability of a strongly detected infection among males and females by BMI. Strongly detected infection is defined as at least 1 Ct value below or equal to 28. Abbreviations: BMI, body mass index; Ct, cycle threshold.
Table 2.
Predicted Ct Values, Nadir Ct Values, and Probability of Strongly Detectable Infection by Sex and BMI
| BMI (kg/m2) | Model 1: Predicted Ct Values | Model 2: Predicted Nadir Ct Values | Model 3: Predicted Probability for Strongly Detectable Infection | |||||
|---|---|---|---|---|---|---|---|---|
| Male (95% CI) | Female (95% CI) | Δ (95% CI) | Male (95% CI) | Female (95% CI) | Male (95% CI) | Female (95% CI) | Δ (95% CI) | |
| 20 | 30.2 (28.7 to 31.6) | 30.3 (29.4 to 31.1) | −0.09 (−1.75 to 1.57) | 25.21 (23.62 to 26.80) | 27.86 (26.58 to 29.14) | 0.78 (.63 to .93) | 0.72 (.61 to .83) | 0.05 (−.11 to .21) |
| 25 | 29.6 (28.7 to 30.6) | 30.6 (30.0 to 31.2) | −0.97 (−2.08 to 0.13) | 25.03 (23.66 to 26.40) | 27.68 (26.73 to 28.63) | 0.82 (.74 to .91) | 0.70 (.62 to .78) | 0.11 (.01 to .21) |
| 30 | 29.1 (28.2 to 30.0) | 31.0 (30.4 to 31.5) | −1.85 (−2.91 to −.81) | 24.85 (23.51 to 26.19) | 27.50 (26.63 to 28.36) | 0.86 (.78 to .93) | 0.68 (.61 to .76) | 0.16 (.06 to .25) |
| 35 | 28.6 (27.2 to 30.0) | 31.3 (30.6 to 31.9) | −2.74 (−4.30 to −1.19) | 24.67 (23.15 to 26.18) | 27.32 (26.24 to 28.39) | 0.89 (.79 to .98) | 0.66 (.57 to .76) | 0.21 (.07 to .34) |
| 40 | 28.0 (25.9 to 30.1) | 31.6 (30.7 to 32.5) | −3.63 (−5.89 to −1.36) | 24.49 (22.66 to 26.32) | 27.14 (25.67 to 28.60) | 0.91 (.80 to 1.02) | 0.64 (.50 to .79) | 0.25 (.07 to .44) |
| 45 | 27.5 (24.7 to 30.3) | 32.0 (30.8 to 33.2) | −4.51 (−7.56 to −1.47) | 24.31 (22.07 to 26.54) | 26.96 (25.03 to 28.88) | 0.93 (.81 to 1.05) | 0.62 (.43 to .82) | 0.30 (.07 to .52) |
Abbreviations: BMI, body mass index; CI, confidence interval; Ct, cycle threshold; Δ, difference in Ct values between males and females.
Nadir Cycle Threshold Values by Sex and BMI
Increasing BMI and male sex were associated with 0.12 (95% CI, −.24 to −.01) and 1.86 (95% CI, −3.65 to −.06) point decreases in nadir Ct values in unadjusted bivariate models. In adjusted models, nadir Ct values were 2.65 points (95% CI, 1.05–4.24) lower among males compared with females (Table 2). We did not observe an interaction between sex and BMI for nadir Ct values, and every 1-point increase in BMI was associated with a decrease in Ct values of 0.04 (95% CI, −.14 to .07) among both females and males (Figure 2B). Nadir Ct values among males were 2.65 points (95% CI, 1.05–4.24) lower than among females for all BMIs.
Occurrence of Strongly Detectable Infections by Sex and BMI
Of the 439 participants, 67.8% of males and 52.2% of females recorded at least 1 Ct value of 28 or less during their infection—that is, a strongly detectable infection (P < .003). These findings were consistent when restricting to the 309 participants who had their nadir Ct value documented during the study, where 67.8% of males and 55.3% of females had at least 1 Ct value less than 28 during their infection. In bivariate and adjusted models, respectively, males had 1.72 (95% CI, 1.05–2.81) and 2.39 (95% CI, 1.30–4.36) times higher odds of having a strongly detectable infection compared with females (Table 2). BMI was not associated with having a strongly detectable infection in the unadjusted and adjusted model excluding the interaction term. On inclusion of the interaction term between BMI and sex, the probability of having a strongly detectable infection increased as BMI increased among males, while there was no significant change by BMI among females (Figure 2C). For participants with a BMI of 30 kg/m2, the probability of having a strongly detectable infection was 85.9% (95% CI, 78.5–93.4%) for males and 68.3% (95% CI, 60.9–75.9%) for females, for a difference of 15.9 (95% CI, 6.4–25.5) percentage points. As BMI increased, the probability of a strongly detectable infection continued to increase among males while staying approximately stable at around 65% for females, and the probability differed between females and males by 20.8 (95% CI, 7.2–34.5) and 25.4 (95% CI, 7.0–43.8) percentage points for BMIs of 35 and 40 kg/m2, respectively. The findings were consistent when Ct values from the ORF1a/b gene were used; sex and BMI maintained a significant interaction on ORF1a/b Ct values (Supplementary Figure 4).
DISCUSSION
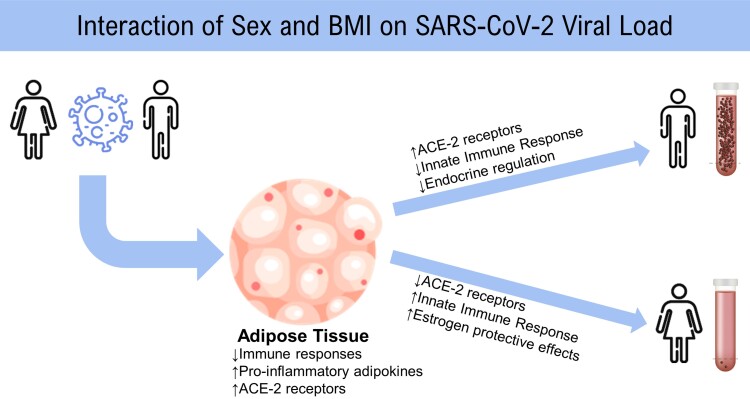
In this study evaluating longitudinal RT-PCR results from participants across the United States, we present some of the first evidence demonstrating a significant interaction between sex and BMI on Ct value. Viral load increased with increasing BMI among males; however, viral load remained stable across BMI among females. These results suggest that there is a considerable sex-based dimorphism in the effect of BMI on SARS-CoV-2 viral kinetics, with the highest viral loads seen among males with a high BMI (Figure 3). This finding was consistent when evaluating all Ct values as well as nadir Ct values, even after adjusting for day of positivity and other confounding variables. These findings bring insight to the following areas: (1) COVID-19 diagnostics, (2) sex-based differences in viral immunologic responses, and (3) sexual dimorphism in obesity.
Figure 3.
Hypothesized mechanisms explaining the roles of adipose tissue and sex on SARS-CoV-2 viral load. Abbreviations: ACE-2, angiotensin converting enzyme 2; BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Implications for COVID-19 Diagnostics and Public Health
We observed that approximately 65% of females did not have a viral load that was detectable at or under 28 cycles of RT-PCR, and, for all BMIs over 25 kg/m2, males were more likely to have strongly detectable infections than females. This finding is noteworthy, especially as 73% of US adults have BMIs greater than 25 kg/m2, and overweight (defined as a BMI ranging from 25 to 30 kg/m2) and obesity (defined as a BMI ≥30 kg/m2) disproportionally impact those who are non-Hispanic Black and Hispanic [15–17]. This has a potential impact on the detection and diagnostics for COVID-19, as these results may indicate that the window of detection with lower sensitivity diagnostics for females is shorter than that of males. Singanayagam et al [18] found that approximately 22% of samples with Ct values greater than 30 were culture positive, indicating that low-viral-load infections may still be infectious, which would mean that these undetected, low-viral-load infections carry clinical significance. However, more research is necessary to understand transmission risk and clinical manifestations of low-viral-load infections. Furthermore, COVID-19 viral load has been correlated with disease severity and mortality; however, previous studies have been conducted among hospitalized patients and often used Ct values at admission, rather than nadir Ct value or peak viral load, due to the delayed presentation of the patient [19–22]. Therefore, understanding the natural course of infection and implications for clinical severity is warranted.
Implications for Understanding Immunologic Response to SARS-CoV-2 Infection
Our findings of differential viral dynamics may be a result of sex-based immunologic differences. A strong immune response is necessary to control and eliminate a replicating virus, and lower Ct values (ie, higher viral load) have been correlated with higher inflammatory biomarkers and lower lymphocyte and T-cell counts [19]. Prior evidence points to differential immune responses to SARS-CoV-2 by sex, with females mounting a higher initial innate immune response than males. Obesity has also shown immunomodulatory effects in response to respiratory viruses [23]. In response to SARS-CoV-2, males have been found to have a lower increase in monocytes and dendritic cells compared with females, as well as a lower initial interferon (IFN)-α (IFN-α) response, which supports our findings indicating a higher viral load among males [1, 8, 24, 25]. Likewise, obesity can result in deficient IFN-α and IFN-γ responses to respiratory viral infections [23]. These similar mechanisms of immunomodulation may result in an additive suppressive effect on the immune response within males. Takahashi et al [6] also found differences in the immune response to SARS-CoV-2 infection between males and females; however, they found that viral load did not differ by sex among 48 SARS-CoV-2–positive patients after adjusting for BMI. They were not able to adjust for day of positivity or capture nadir Ct values due to the convenience-sampling study design, which may account for the discordant findings [6, 26]. Further, Yamamoto et al [27] found that SARS-CoV-2 antibody titers postvaccination decreased with increasing BMI among males, while there was no change in antibody titers by BMI among females, indicating that there may also be differences in adaptive immunity to SARS-CoV-2 by sex and BMI. This is important also in the context of long COVID, also known as post-acute sequelae of SARS-CoV-2 infection (PASC), which occurs in over 10% of SARS-CoV-2 infections. PASC is multifactorial and diverse in symptomology, and women represent approximately 80% of all patients with long COVID [28, 29]. While numerous mechanisms are under investigation, immune dysregulation is thought to play a role in the etiology of this condition. The significant interaction we observed between sex and BMI on SARS-CoV-2 viral dynamics may prompt additional inquiries into the interaction of sex and BMI on the risk of PASC.
Sexual Dimorphism in Obesity
Obesity is a disorder characterized by sexual dimorphism, with sex hormones serving as a key factor in body adiposity and metabolic syndrome [30]. The role and distribution of adipose tissue among males differ from that of females, especially in the premenopausal age range when estrogens result in more subcutaneous fat accumulation among females rather than visceral fat commonly found in males. Obesity results in a chronic inflammatory state that can dampen the innate immune response, and it has been proposed that the impact of obesity on the immune system also displays a sexual dimorphic pattern, as exhibited in our own findings [30, 31].
Strengths and Limitations
This study is the first study to report the interaction of BMI and sex on Ct values for SARS-CoV-2 and adds valuable knowledge to the field of clinical virology. The longitudinal study design adds rigor and enhances our ability to examine nadir Ct values, which would not otherwise be possible and is understudied in the literature. Further, our study sample includes participants from across the United States and includes Omicron and Delta SARS-CoV-2 specimens.
There are several limitations to our study. First, BMI in this study was self-reported; however, self-reported BMI has been found to be a valid measure in men and women of all sociodemographic groups [32]. Additionally, this analysis only included those who were over 18 years old; therefore, these results cannot be generalized to children. In the study cohort, the mean age was 39.1 (range, 18–77) years and the majority of participants had no comorbid conditions; therefore, these findings may not be applicable to older adults with more complex comorbidities or severe COVID-19 disease. Furthermore, history of previous infection was self-reported, so there may have been misclassification among individuals who were unknowingly previously infected. Due to sample-size limitations, we were also limited in our ability to perform subgroup analyses, nor were we able to explore the intersectionality of BMI, sex, and race using 3-way interaction terms [33]. Last, BMI is an imperfect measure and does not account for muscle mass or other nuances in body habitus [34, 35]. However, it is the most widely accepted measure of body habitus in the scientific literature and medical community. Furthermore, it is important to acknowledge that there may be additional gender-based differences in the experiences of obesity, including differences in discrimination, eating habits, anxiety, and stress, which have not been explored in this study and may play a role in chronic inflammatory states and immune suppression.
CONCLUSIONS
In conclusion, we observed an interaction between sex and BMI on viral load, and the highest viral loads were observed among males with high BMIs. Further investigation into the impact of BMI on sex-based immune responses to COVID-19 is needed to determine the cause of this differential effect among males and to understand the clinical, immunologic, and public health implications.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Carly Herbert, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; UMass Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Yukari C Manabe, Division of Infectious Disease, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Andreas Filippaios, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Honghuang Lin, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Biqi Wang, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Chad Achenbach, Division of Infectious Disease, Department of Medicine, Havey Institute for Global Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Vik Kheterpal, CareEvolution LLC, Ann Arbor, Michigan, USA.
Paul Hartin, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Thejas Suvarna, CareEvolution LLC, Ann Arbor, Michigan, USA.
Emma Harman, CareEvolution LLC, Ann Arbor, Michigan, USA.
Pamela Stamegna, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Lokinendi V Rao, Quest Diagnostics, Marlborough, Massachusetts, USA.
Nathaniel Hafer, UMass Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Program in Molecular Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
John Broach, Department of Emergency Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Katherine Luzuriaga, UMass Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Program in Molecular Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Katherine A Fitzgerald, Division of Infectious Diseases and Immunology, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
David D McManus, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Division of Cardiology, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Apurv Soni, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; UMass Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Division of Health System Science, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Notes
Author Contributions. C. H. and A. S. conceptualized the research question. C. H., A. S., H. L., B. W., Y. C. M., L. V. R., V. K., and C. A. contributed to the methodology design. A. F., T. S., V. K., E. H., and P. H. contributed to data collection and curation. C. H. and A. S. conducted formal analysis and wrote the original draft. D. D. M., K. A. F., K. L., J. B., and N. H. provided supervision. P. S. and P. H. provided administrative support. D. D. M., J. B., A. S., N. H., and K. L. contributed to funding acquisition. All authors contributed to reviewing and editing the manuscript.
Acknowledgments. The authors thank their study participants; their collaborators from the National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering and National Heart, Lung, and Blood Institute), who provided scientific input into the design of this study and interpretation of the results but could not formally join as coauthors due to institutional policies; and the Food and Drug Administration (Office of In Vitro Diagnostics and Center for Devices and Radiological Health) for their involvement in the primary Test Us At Home study.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Institute of Biomedical Imaging and Bioengineering; the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Data availability. Data are available at COVID Rapid Acceleration of Diagnostics (RADx) Data Hub: https://radx-hub.nih.gov/.
Financial support. The Test Us At Home study was funded by the National Institutes of Health (NIH) RADx Tech program as a Clinical Studies Core supplement (grant number 3U54HL143541-02S2 to D. D. M., J. B., and A. S.) and the National Institutes of Health Clinical and Translational Science Award (grant numbers UL1TR001453 to N. H. and K. L., TR001453 to C. H.).
References
- 1. Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open 2020; 10:e040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao M, Piernas C, Astbury NM, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol 2021; 9:350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID-19–related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March–December 2020. MMWR Morb Mortal Wkly Rep 2021; 70:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Health 50/50. The Sex, Gender and COVID-19 Health Policy Portal. Available at: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/policy-portal/. Accessed 15 June 2023.
- 5. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev 2020; 21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; 588:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viveiros A, Rasmuson J, Vu J, et al. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am J Physiol Heart Circ Physiol 2021; 320:H296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhindsa S, Zhang N, McPhaul MJ, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open 2021; 4:e2111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aghili SMM, Ebrahimpur M, Arjmand B, et al. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta-analysis. Int J Obes 2021; 45:998–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soni A, Herbert C, Pretz C, et al. Design and implementation of a digital site-less clinical study of serial rapid antigen testing to identify asymptomatic SARS-CoV-2 infection. J Clin Transl Sci 2023; 7:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kissler SM, Fauver JR, Mack C, et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N Engl J Med 2021; 385:2489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hay JA, Kissler SM, Fauver JR, et al. Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: a retrospective cohort study. eLife 2022; 11:e81849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith RL, Gibson LL, Martinez PP, et al. Longitudinal assessment of diagnostic test performance over the course of acute SARS-CoV-2 infection. J Infect Dis 2021; 224:976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soni A, Herbert C, Lin H, et al. Performance of rapid antigen tests to detect symptomatic and asymptomatic SARS-CoV-2 infection. Ann Intern Med 2023; 176:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Obesity is a common, serious, and costly disease. Published 20 July 2022. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed 19 June 2023.
- 16. Bryan S, Afful J, Carroll M, et al. NHSR 158. National Health and Nutrition Examination Survey 2017–March 2020 pre-pandemic data files. Atlanta, GA: Centers for Disease Control and Prevention, 2021. [Google Scholar]
- 17. Ogden CL, Fryar CD, Martin CB, et al. Trends in obesity prevalence by race and Hispanic origin—1999–2000 to 2017–2018. JAMA 2020; 324:1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance 2020; 25:2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther 2020; 9:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagman K, Hedenstierna M, Widaeus J, et al. Correlation of SARS-CoV-2 nasopharyngeal CT values with viremia and mortality in adults hospitalized with COVID-19. Open Forum Infect Dis 2022; 9:ofac463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelleni MT. SARS CoV-2 viral load might not be the right predictor of COVID-19 mortality. J Infect 2021; 82:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med 2020; 8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pérez-Galarza J, Prócel C, Cañadas C, et al. Immune response to SARS-CoV-2 infection in obesity and T2D: literature review. Vaccines 2021; 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agrawal S, Salazar J, Tran TM, Agrawal A. Sex-related differences in innate and adaptive immune responses to SARS-CoV-2. Front Immunol 2021; 12:739757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spiering AE, de Vries TJ. Why females do better: the X chromosomal TLR7 gene-dose effect in COVID-19. Front Immunol 2021; 12:756262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shattuck-Heidorn H, Danielsen AC, Gompers A, et al. A finding of sex similarities rather than differences in COVID-19 outcomes. Nature 2021; 597:E7–9. [DOI] [PubMed] [Google Scholar]
- 27. Yamamoto S, Mizoue T, Tanaka A, et al. Sex-associated differences between BMI and SARS-CoV-2 antibody titers following the BNT162b2 vaccine. Obesity (Silver Spring) 2022; 30:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertson MM, Qasmieh SA, Kulkarni SG, et al. The epidemiology of long coronavirus disease in US adults. Clin Infect Dis 2023; 76:1636–45. [DOI] [PubMed] [Google Scholar]
- 29. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM. Lifelines corona research initiative. Persistence of somatic symptoms after COVID-19 in The Netherlands: an observational cohort study. Lancet 2022; 400:452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 2015; 402:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bechmann N, Barthel A, Schedl A, et al. Sexual dimorphism in COVID-19: potential clinical and public health implications. Lancet Diabetes Endocrinol 2022; 10:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hodge JM, Shah R, McCullough ML, Gapstur SM, Patel AV. Validation of self-reported height and weight in a large, nationwide cohort of U.S. adults. PLoS One 2020; 15:e0231229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soni A, Fahey N, Bhutta ZA, et al. Early childhood undernutrition, preadolescent physical growth, and cognitive achievement in India: a population-based cohort study. PLoS Med 2021; 18:e1003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gutin I. In BMI we trust: reframing the body mass index as a measure of health. Soc Theory Health 2018; 16:256–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monaghan LF, Colls R, Evans B. Obesity discourse and fat politics: research, critique and interventions. Crit Public Health 2013; 23:249–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.