Abstract
Background
Tularemia is an important reemerging disease with a multimodal transmission pattern. Treatment outcomes of current recommended antibiotic regimens (including ciprofloxacin and doxycycline) remain unclear. In this retrospective cohort study, we report clinical, laboratory, geographical, and treatment outcomes of laboratory-confirmed tularemia cases over an 11-year period in Northern Sweden.
Methods
Data from reported tularemia cases (aged >10 years at time of study) in Norrbotten county between 2011 and 2021 were collected through review of electronic medical records and participant questionnaires; 415 of 784 accepted participation (52.9%). Of these, 327 were laboratory-confirmed cases (serology and/or polymerase chain reaction). A multivariable logistic regression model was used to investigate variables associated with retreatment.
Results
Median age of participants was 54 years (interquartile range [IQR], 41.5–65) and 49.2% were female. Although ulceroglandular tularemia was the predominant form (n = 215, 65.7%), there were several cases of pulmonary tularemia (n = 40; 12.2%). Inflammatory markers were largely nonspecific, with monocytosis frequently observed (n = 36/75; 48%). Tularemia was often misdiagnosed on presentation (n = 158, 48.3%), with 65 (19.9%) receiving initial inappropriate antibiotics and 102 (31.2%) retreated. Persistent lymphadenopathy was infrequent (n = 22, 6.7%), with 10 undergoing surgical interventions. In multivariable analysis of variables associated with retreatment, we highlight differences in time until receiving appropriate antibiotics (8 [IQR, 3.25–20.75] vs 7 [IQR, 4–11.25] days; adjusted P = .076), and doxycycline-based treatment regimen (vs ciprofloxacin; adjusted P = .084), although this was not significant after correction for multiple comparisons.
Conclusions
We comprehensively summarize clinical, laboratory, and treatment outcomes of type B tularemia. Targeting tularemia requires clinical awareness, early diagnosis, and timely commencement of treatment for an appropriate duration.
Keywords: Francisella tularensis, doxycycline, ciprofloxacin, treatment, outcome
Treating tularemia is challenging because of a multitude of clinical presentations, and optimal treatment regimens remain unknown. We analyzed the outcomes of 327 laboratory-confirmed tularemia cases in Sweden and highlight the need for early diagnosis and initiating appropriate antibiotic treatment.
Tularemia is a reemerging zoonotic disease present in most countries in the Northern Hemisphere [1, 2]. Outbreaks of tularemia caused by the bacterium Francisella tularensis subsp. holarctica (“type B”) have occurred at irregular intervals in the northern parts of Sweden since first described in the country in 1931 [3, 4]. Although there is notable annual fluctuation, there has been an increasing trend in the number of cases seen across Sweden and the entire European Union over the latest 5-year period (2017–2021) [5, 6]. Increasing frequency of outbreaks in middle and southern parts of Sweden suggests tularemia is a reemerging infectious disease complexly linked with climate change [6–14].
Zoonotic, waterborne, airborne, and vector (primarily mosquito)-borne outbreaks of the disease have important health-related and socioeconomic burden on individuals and society through prolonged illness, absence from work, and healthcare costs [6].
Manifestations of the disease vary depending on inoculation dose, type of exposure (oral, ocular, pulmonary, cutaneous), and geographical location (type A in North America vs type B globally), with the latter causing debilitating symptoms, but rarely resulting in lethalities [4, 15, 16].
Recommended treatment regimens for tularemia include aminoglycosides (streptomycin and gentamicin), quinolones (ciprofloxacin), and tetracyclines (doxycycline), but knowledge of their in vivo effectiveness remains scarce [6, 17–20]. Based on limited available evidence, the World Health Organization recommends treatment with ciprofloxacin 500 mg twice daily for 10 days, or doxycycline 100 mg twice daily for 14 days for milder disease in adults, but comparisons of treatment outcomes are limited to small case series [18, 21, 22].
In this study, we comprehensively investigated clinical, microbiological, laboratory, and treatment outcomes in a cohort of tularemia cases in northern Sweden, which includes the second (2019) and third (2015) largest outbreaks recorded in Sweden.
METHODS
Study Setting
This study was performed in Norrbotten County, the largest (98 245 km2) and northernmost county in Sweden, with a sparse population of approximately 250 000 [23].
Study Design and Procedure
This is a retrospective cohort study involving electronic medical record (EMR) review of tularemia cases in Norrbotten in combination with a targeted questionnaire to further explore clinical details. Cases were identified via the National Infection Control database (SmiNet). Inclusion criteria were: (1) diagnosis 2011–2021; (2) alive at time of study; (3) available contact details; and (4) age ≥10 years at time of study consent. Exclusion criteria were: (1) nonresponse/declined study participation; (2) unavailable EMR documentation; (3) improbable clinical diagnosis; or (4) not meeting World Health Organization presumptive or confirmed tularemia case definition [18].
Eligible participants were sent a letter containing a questionnaire, consent form, study information sheet, and web link to a secure survey platform (EvaSysV8.1, Region Norrbotten). Nonrespondents were sent notifying mobile text messages and a second paper questionnaire.
Serology and Microbiology
Serological analysis was performed at the regional clinical microbiology laboratory at Sunderby hospital, Luleå, Sweden, using an immunochromatographic rapid test (VIRapid, Vircell, Granada, Spain) [24]; and the national reference laboratory for tularemia at Umeå University hospital, Umeå, Sweden, using a validated in-house enzyme-linked immunosorbent assay, together with polymerase chain reaction (PCR) and cultures [25].
Statistical Analysis
Data were collected from the regional EMR (VAS 49.0, Region Norrbotten), including documentation from general practitioners (GPs), hospitals, and specialist clinics. Integrated electronic prescriptions and microbiological data were reviewed. Geographical information of reported site of infection was mapped using Epi Info 7.2.5 (Centre for Disease Control). Statistical analysis was performed in SPSS 28.0 (IBM). Statistical methods included logistic regression (categorical variables) and Mann-Whitney U test (continuous and ordinal variables) for univariate analysis; a multivariable logistic regression model using a stepwise backwards elimination protocol (Supplementary Table 1) [26]. Holm-Šídák correction was applied, with a corrected P value <.05 considered significant.
Ethics
Ethics committee approval was obtained from The Swedish Ethical Review Authority (Dnr 2020-04411, 2021-02953) and Norrbotten County Research Council (Dnr 01690-2020). All study participants received written age-appropriate information about the study and provided written informed consent. Additionally, parental consent was obtained for individuals aged <15 years.
RESULTS
Study Participants
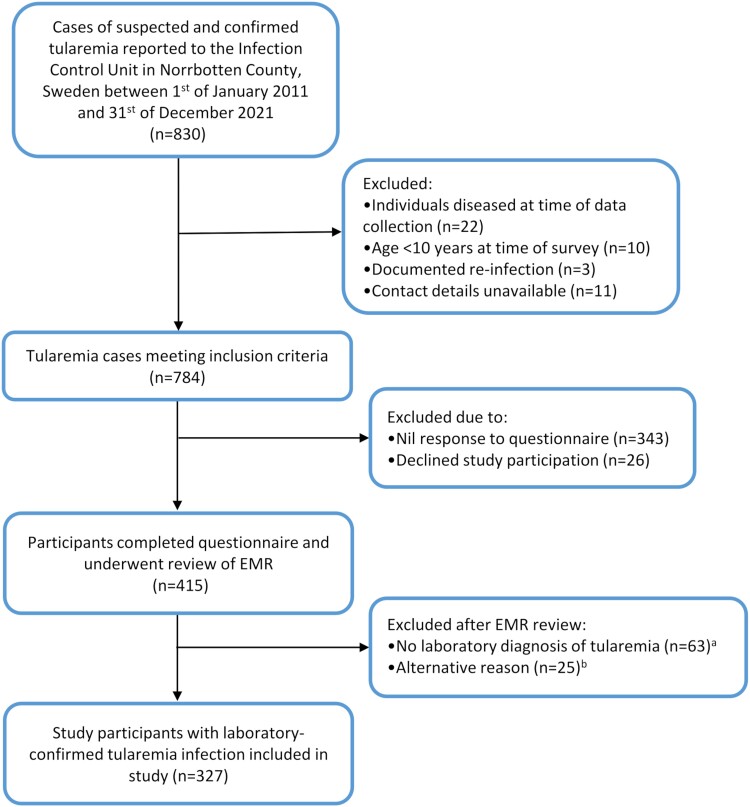
In total, 830 cases of suspected or confirmed tularemia were reported to the Department of Communicable Disease Control, Norrbotten County, Sweden, between 1 January 2011 and 31 December 2021 (incidence, 30.1 per 100 000 per year). Three cases of tularemia reinfection were reported, all without laboratory confirmation. Overall, there were 441 questionnaire respondents between 30 December 2020 and 16 October 2022 (56.3% response rate), with 26 declining study participation. A further 88 of 415 were excluded after EMR review (Figure 1).
Figure 1.
Study method and inclusion of participants. EMR, electronical medical record. aLack of electronical medical record documentation (n = 15); confirmed false-positive tularemia tests (n = 2; ×1 false-positive rapid test, ×1 false-positive PCR); misdiagnosis (later confirmed as mycoplasma infection via PCR; n = 1); incidental positive serology without evident clinical symptoms of tularemia infection (n = 5); age <10 y at time of survey (n = 2). bClinical diagnosis of tularemia without a positive tularemia serology, culture, and/or PCR and were therefore excluded from further analysis (of these, 15 had a single negative serology result and 1 had a negative PCR result). PCR, polymerase chain reaction.
Study participants had a median age of 54 years at time of diagnosis (interquartile range [IQR], 46–65) and equal gender distribution (Table 1). Compared with all reported adult cases (aged ≥18 years), adult study participants (respondents) were older (median, 55 [IQR, 45–65] vs 53 [IQR, 40–65] years; P = .034) and less likely to be male (50.7% vs 57.8%; P = .049), but did not vary with regard to time since diagnosis (median, 6 [IQR, 6–9] vs 6 [IQR, 6–9] years; P = .088).
Table 1.
Demographic, Microbiological, Clinical, and Laboratory Manifestations of Laboratory-Diagnosed Tularemia Cases and Associated Abnormal Values (n = 327)
| Laboratory-Diagnosed Tularemia Cases (n = 327) | Values Outside Laboratory Reference Range (%) | |
|---|---|---|
| Age (y) | 54 (IQR, 41.5–65) | |
| 10–19 | 24 (7.3%) | |
| 20–39 | 50 (15.3%) | |
| 40–59 | 128 (39.1%) | |
| 60–79 | 120 (36.7%) | |
| ≥80 | 5 (1.5%) | |
| Gender | ||
| Male | 166 (50.7%) | |
| Female | 161 (49.2%) | |
| Immunosuppression | ||
| Mildly immunocompromiseda | 3 (0.9%) | |
| Severely immunocompromisedb | 6 (1.8%) | |
| Diabetes mellitus | 1 (type 1, 0.3%); 17 (type 2, 5.2%) | |
| Pregnant at time of diagnosis | 0 (0%) | |
| Clinical manifestations | ||
| Ulceroglandular | 215 (65.7%) | |
| Glandular | 34 (10.4%) | |
| Pulmonary | 40 (12.2%) | |
| Typhoidal | 24 (7.3%) | |
| Oculoglandular | 1 (0.3%) | |
| Oropharyngeal | 0 (0%) | |
| Undifferentiatedc | 23 (7.0%) | |
| Microbiological diagnosis | ||
| Positive F. tularensis serology titer (n = 311) | 298 (95.8%) | |
| Seroconversion or significant rise in titer | 114 (36.7%) | |
| PCR positive (n = 46) | 44 (95.6%) | |
| Culture grown F. tularensis | 18 (39.1%) | |
| Laboratory findings | ||
| CRP (mg/L; n = 288) | 60 (30–108) | |
| Leukocytes (×106 cells/mL; n = 198)d | 7.6 (6.2–9.3) | >8.8: 58 (29.3%) |
| Neutrophils (×106 cells/mL; n = 71) | 5.1 (3.6–6.2) | >6.1: 21 (29.6%) |
| Lymphocytes c (×106 cells/mL; n = 76) | 1.6 (1.1–2.3) | <1.1: 16 (21%) > 3.5: 3 (3.9%) |
| Monocytes c (×106 cells/mL; n = 75) | 0.7 (0.5–1.0) | >0.8: 36 (48.0%) |
| Eosinophils (×106 cells/mL; n = 74) | 0.09 (0.080–0.090) | >0.5: 0 (0%) |
| Basophils (×106 cells/mL; n = 73) | 0.03 (0.010–0.050) | >0.1: 2 (2.7%) |
| Hemoglobin (g/dL; n = 212) | 137 (128–146) | <120 (female) or <130 (male): 40 (18.9%) |
| Platelets (×106/mL; n = 204) | 216 (168–300) | <150: 28 (13.7%) > 300: 50 (24.5%) |
| Procalcitonin (ng/mL; n = 17) | 0.21 (0.13–0.34) | >0.1: 15 (88.2%) > 0.25: 6 (35.3%) |
| Urine analysis (n = 33) | ||
| Hematuria | 18 (54.5%) | Trace: 4 (12.1%) 25: 4 (12.1%) 80: 8 (24.2%) 200: 2 (6%) |
| Proteinuria | 17 (51.5%) | Trace: 1 (3%) 0.3 (+): 10 (30.3%) 1.0 (++) 6 (18.2%) |
| Pyuria | 7 (21.2%) | 15: 5 (15.2%) 70: 1 500: 1 |
Abbreviations: CRP, C-reactive peptide; F. tularensis, Francisella tularensis; PCR, polymerase chain reaction.
aLow-dose corticosteroids (equivalent to <20 mg prednisolone; n = 2); methotrexate (≤0.4 mg/kg/week; n = 1).
bInfliximab and methotrexate (n = 2); Etanercept 50 mg and methotrexate 15 mg weekly (n = 1); golimumab monthly and methotrexate 15 mg weekly (n = 1); cyclosporine (n = 1); etanercept and sulfasalazine 500 mg 4 times per day (n = 1).
cTwelve participants (3.7%) had ulcers without documented lymphadenopathy and a further 11 (3.4%) had nonspecific mild symptoms.
dParticipants aged <18 years were excluded because of alternative cutoff values.
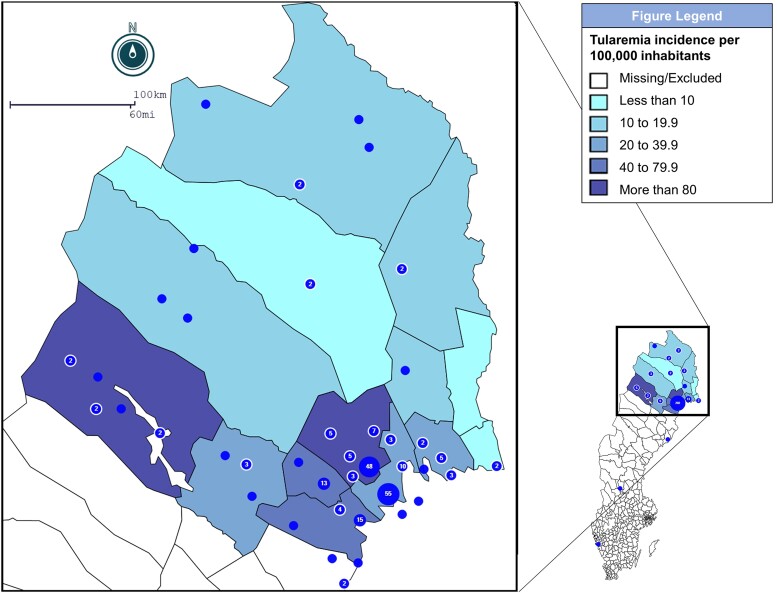
Geographical distribution (Figure 2) showed predominance of tularemia cases in coastal municipalities, as well as high incidence (>80 per 100 000 inhabitants) in certain highland areas popular among tourists, with a high density of lakes and waterways.
Figure 2.
Geographical distribution and epidemiological incidence of tularemia cases at municipality level in Norrbotten County over 11 y between 2011 and 2021 (n = 243). Incidence calculated using total number of reported cases (N = 830). Blue dots indicate self-reported location of exposure/illness by individual cases. Number in blue dots indicate cumulative number of cases in area.
Microbiological Analyses
Of 327 laboratory-confirmed cases, 298/311 (95.8%) had at least 1 positive serology, whereas 13/311 had 1 negative serology taken early on, combined with positive PCR and/or culture (Table 1). Overall, 114 (36.7%) had a documented seroconversion or significant rise in antibody titer [25]. F. tularensis PCR was positive in 43/43 (100%) samples from peripheral sites, 1/1 (100%) transbronchial needle aspirates, and 0/2 (0%) cerebrospinal fluid samples. F. tularensis (n = 18) was cultured from peripheral swabs (n = 13), blood (n = 3), pleural fluid (n = 1), and lymph node biopsy (n = 1).
Clinical Manifestations
Visible skin ulceration and regional lymphadenopathy were seen among 215 (65.7%) participants (ulceroglandular tularemia), whereas 34 (10.4%) had isolated tender and enlarged lymph nodes (glandular tularemia; Table 1). Lymphadenopathy was located in the lower extremities in 156 (62.7%), the upper extremities (axilla or chest) in 39 (15.7%), the head/neck region in 41 (16.5%), and in an unspecified location in 13 (5.2%) cases. A single case of oculoglandular tularemia was reported. Eight participants reported a transient pharyngitis, but symptoms were otherwise not consistent with oropharyngeal tularemia. Twenty-four (7.3%) participants had persistent fever without localized symptoms, suggestive of typhoidal tularemia. Other symptoms included diarrhea and/or vomiting (n = 17; 5.2%) and headache (n = 84; 25.7%). Onset of primary lymphadenopathy and/or skin lesion and fever generally occurred within the same time frame (median, 0 days; IQR, −2 to +1 days).
Respiratory symptoms (cough, shortness of breath, pleuritic chest pain) were described among 59 (18.0%) participants. Among these who had chest radiograph imaging, 25/40 (62.5%) had confirmed infiltrates and 5/40 (12.5%) cases had atypical imaging findings. Fifteen cases had confirmed infiltrates on computed tomography (CT) scans, with 14 scans reporting nodular infiltrates (5 involving multiple lobes), 4 suspected pulmonary abscesses, 8 enlarged thoracic lymph nodes >10 mm, and 4 necrotic lymph nodes. Six CT reports were highly suspicious for malignancy, with 3 undergoing positron emission tomography/CT scanning, and 1 CT-guided lung biopsy. Fourteen (93.3%) cases underwent repeat CT of the chest with partial or complete resolution of infiltrates/lymph nodes. No malignancy was identified during follow-up.
Secondary skin manifestations separate from the primary ulcer site and lymph node were self-reported among 44 (13.5%) participants. Rashes were documented in 34 (10.4%) cases, with characteristics reported in 16 cases. Of these, 2 were erythema nodosum, 6 vesicular, 2 pustular, 4 macular, and 2 papular. Sites of secondary rashes were lower limbs (n = 16; 61.9%), upper extremities (n = 12; 57.1%), trunk and/or back (n = 7; 33.3%), and/or head and/or neck (n = 6; 28.6%). Palmar involvement was described in a single case, whereas no mucosal involvement was reported. Median time until onset of rash was 10 days from symptom onset (n = 11; range, 4–20 days). In 19 cases, rashes occurred after initiation of antibiotic treatment (median, 3; range, 0–5 days), whereas 4 developed a rash before starting treatment.
Participants waited a median of 4 days (IQR, 3–7 days) from symptom onset until seeking healthcare. First point of contact were GPs (n = 229; 70.0%), after-hours GPs (n = 48; 14.7%), emergency departments (n = 40; 12.2%), or specialist clinics (n = 9; 2.75%); 227 (69.4%) had subsequent follow-up. Fifty-two (15.9%) participants required hospital admission, with indications including suspected severe illness (n = 16; 30.8%) and/or investigation of unknown fever (n = 43; 82.7%). Admitting teams included internal/respiratory medicine (n = 26; 50%), infectious diseases (n = 19; 36.5%), pediatrics (n = 2; 3.8%), and otorhinology (n = 3; 5.8%).
An initial alternative presumptive diagnosis was frequent at presentation (n = 158; 48.3%), including unspecified viral infection (n = 51; 13.1%), bacterial skin/soft-tissue infection (n = 31; 9.5%), bacterial respiratory infection (n = 16; 4.9%), Puumala virus infection (n = 11; 3.4%), undifferentiated fever/sepsis (n = 13; 3.4%), urinary tract infection (n = 9; 2.8%), other specified infections (8; 1.8%), meningitis (n = 5; 1.5%), eczema/urticaria (n = 3; 0.9%), thromboembolic events (n = 3; 0.9%), or acute surgical pathology (n = 2; 0.6%).
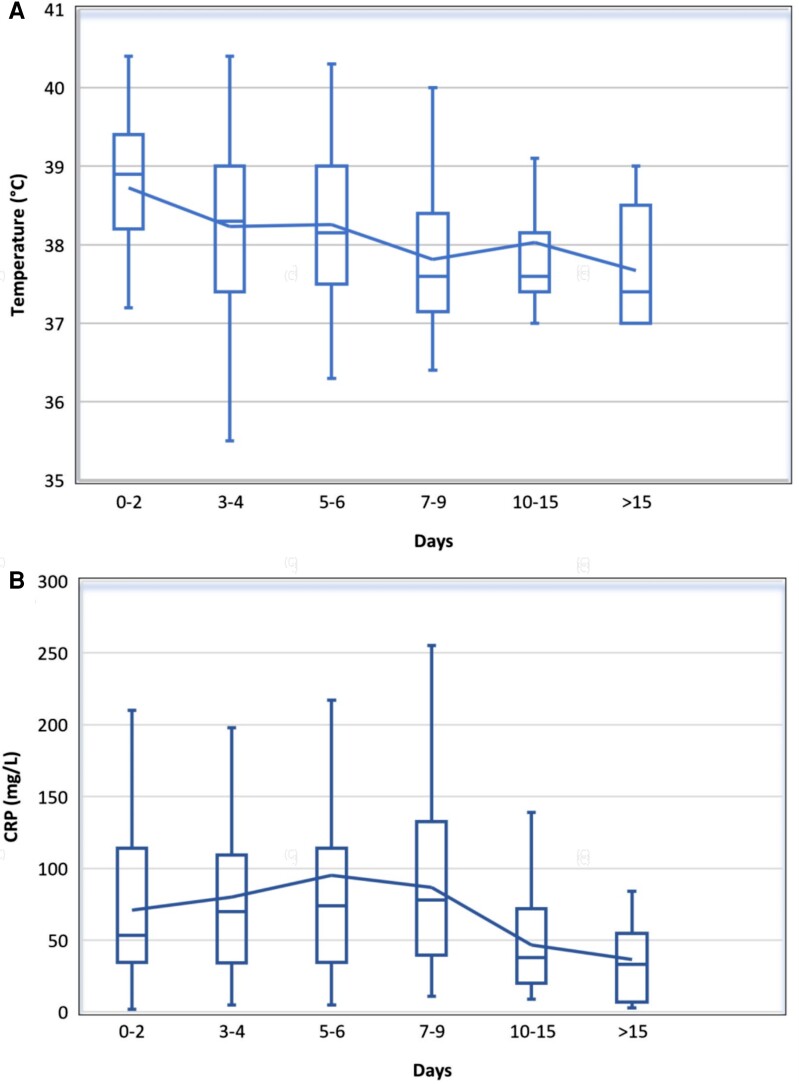
Of 230 participants with temperature recorded at time of the first healthcare visit, 137 (59.6%) had a temperature ≥38.0°C. Median temperature was 38.3 °C (IQR, 37.5–39.0). Participants presenting later had a lower median initial temperature (Figure 3A). Median self-reported duration of fever was 5 days (IQR, 4–10).
Figure 3.
Initial recorded (A) temperature (°C) and (B) CRP and at time from onset of tularemia symptoms (n = 289). Box diagram denotes median, interquartile range, range (extreme values not included). Line denotes mean values. CRP, C-reactive protein.
Laboratory Manifestations
Laboratory data (Table 1) were available for most patients because of capillary point-of-care testing in all primary care facilities. Among 289 (88.4%) participants who had an initial C-reactive protein (CRP) recorded, median CRP was highest on days 7 through 9 after symptom onset (78 mg/L; IQR, 40–133), and lower among participants who presented later, suggesting a spontaneous reduction over time and/or milder illness (Figure 3B).
Leukocyte counts remained within normal limits for most participants (≤8.8 × 106 cells/mL; n = 140; 70.7%), whereas monocytosis (>0.8 × 106 cells/mL) was present among 36 cases (48.0%). Procalcitonin was mildly elevated (>0.1 ng/mL) among most cases (n = 15; 88.2%), whereas few had levels above 0.25 ng/mL (n = 6; 35.3%).
Treatment Outcomes
A total of 321 (98.2%) participants received antibiotic treatment, which was started a median of 5 days after onset of symptoms (IQR, 3–9 days). Median time until appropriate antibiotic regimen (defined as a quinolone, tetracycline, or aminoglycoside) was 7 days (IQR, 4–14 days).
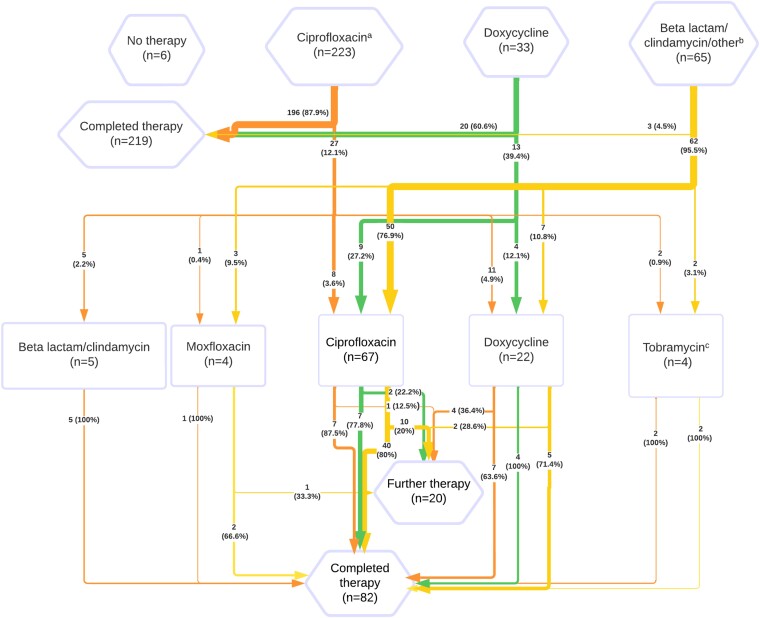
Initial antibiotic treatment (Figure 4) was most often ciprofloxacin (n = 223; 68.2%). Twenty-five participants (7.6%) had a previous documented allergy to antibiotics, predominantly beta-lactams (n = 20). Overall, 71 (21.7%) participants did not receive an appropriate first-line antibiotic, of which 6 (1.8%) received no documented antibiotic treatment.
Figure 4.
Antibiotic treatment (and retreatment) pathways for laboratory-confirmed tularemia cases (n = 327). Rectangles represents retreatment. aIncluding 2 cases combined with 5 d of tobramycin (not requiring further retreatment). bOther treatments included: rifampicin/isoniazid/pyrazinamide/ethambutol for tuberculosis regimen (n = 1), and erythromycin (n = 1). cIn combination with vancomycin (n = 1), ciprofloxacin (n = 2).
Retreatment
A second antibiotic agent was given to 102 (31.2%) of participants. Of these, 62 (60.7%) had received an inappropriate first-line antibiotic (Figure 4).
Among participants initially treated with ciprofloxacin, 27 (12.1%) received retreatment. Of these, 7 (25.9%) had <10 days of initial treatment. Reported causes for retreatment were suspected bacterial superinfection (n = 2), adverse drug reaction (n = 8), or suspected treatment failure (n = 17; nonmutually exclusive: persistent lymphadenitis [n = 9], persistent/recurrent fevers [n = 3], persistent arthralgia/suspected reactive arthritis [n = 2], persistent cough [n = 2], peri-/postinfectious lethargy [n = 2], relapsing myopericarditis [n = 1]).
Among participants receiving initial treatment with doxycycline, 13 (39.4%) were retreated. Four (30.8%) had received subtherapeutic doses, and 10 (76.9%) were medicated for <14 days. Among those successfully treated with doxycycline, only 1 (5%) had subtherapeutic dosing, whereas 5 (25%) had treatment <14 days. Reported causes for retreatment were optimization of antibiotics after tularemia diagnosis (n = 2), adverse drug reaction (n = 2), and suspected treatment failure (n = 9; peri-/postinfectious lethargy [n = 4], persistent lymphadenitis [n = 4]; persistent/recurrent fevers [n = 3], persistent cough [n = 3]).
Persistent lymphadenopathy (after 1 course of appropriate antibiotics) was present among 22 cases (6.7%); with 6 (27.3%) having spontaneously draining suppurative lymph nodes, 6 (27.3%) receiving fine-needle aspiration, and 4 (18.2%) incision and drainage. Three aspirates were F. tularensis PCR positive and 1 was culture positive; however, not all aspirates were sent for analysis.
Multivariable Analysis of Treatment Outcomes
Participants who experienced treatment failure after receiving an appropriate antibiotic regimen were compared with those who had successful treatment (Table 2). Variables were analyzed using multivariable analysis after correction for multiple comparisons and included time until receiving appropriate antibiotics (8 [IQR, 3.25–20.75] vs 7 [IQR, 4–11.25] days; adjusted P = .076), doxycycline-based treatment regimen (vs ciprofloxacin; adjusted P = .084), and participant age (years; adjusted P = .270). Thus, no significant correlations were identified.
Table 2.
Comparison of Characteristics of Participants With Completed Treatment After Appropriate Antibiotic Regimens vs Those Requiring Further Treatment Courses
| Completed Treatment After Appropriate Antibiotic Regimen (n = 267)a | Retreatment After Appropriate Antibiotic Regimen (n = 45)b | Missing Values | Univariate P Value |
Multivariable P Value (adjusted) |
|
|---|---|---|---|---|---|
| Gender | .506 | ||||
| Male | 133 (49.8%) | 25 (56.5%) | |||
| Female | 134 (50.2%) | 20 (43.5%) | |||
| Age (y) | 54 (IQR, 40–65) | 57 (IQR, 47–66) | .156 | .270 (.270) | |
| CRP (mg/L) | 55 (IQR, 30–102) | 67 (29.5–120.5) | 37 (11.9%) | .650 | |
| Leukocytes (×106 cells/mL) | 7.50 (IQR, 6.20–9.05) | 7.60 (IQR, 6.25–9.38) | 104 (33.3%) | .833 | |
| Days until seeking healthcare | 4 (IQR, 3–7) | 3 (IQR, 2–5.75) | 13 (4.2%) | .045 | |
| Days until appropriate antibiotic | 7 (IQR, 4–11.25) | 8 (IQR, 3.25–20.75) | 13 (4.2%) | .210 | .026 (0.076) |
| Respiratory symptoms | 47 (17.5%) | 8 (17.8%) | .928 | ||
| Year of treatment | .518 | ||||
| Nonepidemic year | 43 (16.1%) | 9 (20.0%) | |||
| Epidemic year (2012, 2015, 2019) | 224 (83.9%) | 36 (80.0%) | |||
| Initial appropriate regimen | .024 | .043 (.084) | |||
| Ciprofloxacin | 240 (89.9%) | 35 (77.8%) | |||
| Doxycycline | 27 (10.1%) | 10 (22.2%) | |||
| Antibiotic regimen with appropriate dose and duration | 18 (5.8%) | .500 | [.524]c | ||
| Ciprofloxacin ≥500 mg twice daily ≥10 d | 239 (89.5%) | 27 (60.0%) | |||
| Doxycycline ≥100 mg twice daily ≥14 d | 24 (9.0%) | 4 (8.8%) | |||
| Immunosuppression | 7 (2.6%) | 1 (2.2%) | .876 |
Logistic regression for categorical variables. Mann-Whitney U test for ordinal and continuous variables. Data reported in absolute numbers and percentages (in brackets), or median and interquartile range, as specified. Significance in multivariable analysis after Holm-Šídák correction applied for adjusted P values is displayed in brackets.
Abbreviations: CRP, C-reactive protein; IQR, interquartile range.
aDefined as regimen containing ciprofloxacin, aminoglycoside, or doxycycline, as per the World Health Organization.
bRetreatment despite a course of ciprofloxacin or doxycycline, individuals with “optimization” of antibiotics after diagnosis (n = 2) or adverse drug reactions requiring treatment with alternative regimen (n = 11) were excluded.
cValue included as alternative to “initial appropriate regimen” in multivariable analysis and not part of formal multivariable model.
DISCUSSION
In this retrospective cohort study of 327 tularemia cases in northern Sweden, we demonstrate that tularemia is a multifaceted disease with a multitude of differential diagnoses and wide range of clinical manifestations, including prolonged suppurative lymphadenitis and atypical respiratory infection. We further describe successful treatment outcomes among individuals receiving early treatment with ciprofloxacin and doxycycline and highlight time until appropriate treatment as a potential cause for requiring retreatment, as previously noted [27].
Our cohort had similar demographics to previous studies performed in northern Europe [3, 9, 11, 28–31] and identified men and women aged 40 to 60 years as having the highest burden of tularemia, although there was high prevalence of infection throughout all age groups. Our data support ulceroglandular tularemia transmitted by mosquitos as the predominant clinical form in this geographical region [6, 31], pulmonary tularemia as an important cause of pneumonia, and atypical disease often misdiagnosed as malignancy and/or tuberculosis [9, 32]. Immunosuppression was infrequent in our cohort (n = 8), with 4 cases admitted to the hospital, including 1 developing pulmonary tularemia and 2 cases with F. tularensis septicemia.
Our findings show secondary skin manifestations in approximately 15% of cases, consistent with previous studies [9, 33–35]. Children aged 10 to 15 years appeared to have frequent skin involvement (54.5%), as previously described [36]. Interestingly, secondary skin manifestations appeared in close relation to commencing appropriate antibiotics, and one could speculate about a correlation between the 2. We highlight that limited knowledge about these reactions can lead to potentially inappropriate allergy labeling, as evidenced by our cohort.
Reported laboratory values in our cohort identified CRP (50–150 mg/L in 45.3%), procalcitonin (>0.1 ng/mL in 88.2%; <0.5 ng/mL in 94.1%) and monocytes (>0.8 × 106 cells/mL in 48.0%) as nonspecific markers for tularemia infection in the context of high clinical suspicion [16]. Previous studies have predominantly reported normal differential counts in type B tularemia, with monocytosis >0.95 × 106 cells/mL present in 4% of cases compared with 30.6% in our cohort [9, 37]. Leukocyte differential count is not routinely performed in primary care settings in Sweden. Perhaps wider implementation could help guide diagnosis of atypical manifestations of tularemia.
Chronic suppurative lymphadenitis has previously been described in up to 30% of patients with ulceroglandular and glandular tularemia [1, 9]. We describe chronic suppuration in 8.8% of cases, often leading to surgical intervention and/or retreatment with multiple courses of antibiotics. Interestingly, among PCR-positive cases, culture negativity was associated with longer time since symptom onset (median, 10.5 days; IQR, 6.5–23.0 vs 6 days; IQR, 4.75–8.5; P = .029). It remains unclear whether replicating bacteria are present in the ulcer after completed treatment or whether persistent lymphadenitis is primarily driven by an inflammatory response, as highlighted by few PCR-positive cases after aspiration and only a single culture positive [38]. Rates of incision and drainage appear to be lower in our cohort than in studies from France (27%) and the United States (19%) [13, 39], perhaps potentially related to shorter time until institution of appropriate antibiotic treatment. Even higher rates (60%) have been reported in pediatric populations [40]. The clinical value of prolonged antibiotic treatment in drained suppurative lymphadenitis remains unclear and will require further studies, potentially stratified by PCR status and/or culture.
Our cohort appears to have lower retreatment rates after appropriate antibiotic regimens than previous studies (16.8% vs 38.6%) [13]. This could be explained by the short duration to receiving appropriate therapy, as observed in another Swedish study with 95.3% successful treatment rate after initiating therapy early (median, 3 days from symptom onset) [19]. We further show successful treatment in 3 of 4 (75%) of cases treated with moxifloxacin as a first- or second-line antitularemia agent, as supported by previous in vitro studies [17]. As previously described [6, 17], treatment with beta-lactams or clindamycin appears ineffective.
Comparisons between ciprofloxacin and doxycycline for treatment of tularemia have previously been limited by sample size [13, 18, 20, 22, 39]. In our cohort, we show a multivariable trend toward requiring retreatment in those receiving doxycycline compared with ciprofloxacin (adjusted P = .076); however, when accounting for appropriate dosing and duration of antibiotic therapy, there was no significant difference between groups (14.2% vs 10.2%; P = .524). Although this is consistent with previous studies showing that shorter treatment periods are associated with relapse [22], our study is likely underpowered for assessment of this outcome.
Interestingly, we observed a broad range of differential diagnoses among clinicians, leading to 19.9% of participants receiving empiric antibiotics ineffective against tularemia. Higher rates were observed during nonepidemic years, suggesting that improved awareness and high clinical suspicion is required among clinicians to promote overall outcomes given that initial serological results can be negative [9].
No deaths were recorded in our cohort, and only 3 deaths were reported to the Swedish Cause of Death Register between 1997 and 2022, suggesting that, although type B tularemia can cause serious morbidity, it is rarely fatal.
Our study was limited by its retrospective design. Despite being 1 of the largest descriptive studies of clinical outcomes in tularemia, the sample size limited statistical analysis. Antibiotic treatment outcomes are difficult to interpret in those receiving retreatment because cases often received overlapping antibiotic regimens. The addition of a questionnaire to study participants improved the quality of clinical data by assessing long-term outcomes, but because of the long time between infection and time of survey in some cases, recall bias and limited memory could have influenced these results. Respondents were slightly older and more likely to be female compared with overall reported cases, introducing a selection bias that could have skewed the results. While our results should be generally applicable to type B tularemia in northern Europe, the level of relevance remains unclear in settings where alternative clinical and microbiological forms dominate.
CONCLUSION
In conclusion, we comprehensively summarize clinical, laboratory, and treatment outcomes of type B tularemia. Targeting tularemia remains challenging because of its multifaceted presentation with a wide range of differential diagnosis and unclear optimal treatment regimens. We believe the present study can provide guidance regarding targeted treatment and highlight areas for future research. Ideally, a randomized controlled trial to evaluate effectiveness of antibiotic therapies, as well as the possible need for prolonged antibiotic treatment in suppurative lymphadenitis, should be conducted to improve clinical outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Martin Plymoth, Department of Clinical Microbiology, Sunderby Research Unit, Umeå University, Umeå, Sweden; Department of Infectious Diseases, Westmead Hospital, Sydney, New South Wales, Australia.
Robert Lundqvist, Department of Public Health and Clinical Medicine, Sunderby Research Unit, Umeå University, Umeå, Sweden.
Anders Nystedt, Department of Communicable Disease Control, County Council of Norrbotten, Luleå, Sweden.
Anders Sjöstedt, Department of Clinical Microbiology, Umeå University, Umeå, Sweden.
Tomas N Gustafsson, Department of Clinical Microbiology, Sunderby Research Unit, Umeå University, Umeå, Sweden.
Notes
Author contributions . Conceptualization: T. N. G., M. P., A. S., R. L., and A. N. Investigation: M. P., T. N. G., and R. L. Formal analysis: M. P., T. N. G., and R. L. Writing – Original Draft: M. P. and T. N. G. Writing – Review & Editing: T. N. G., A. S., M. P., A. N., and R. L. Supervision: T. N. G.
Acknowledgments . The authors thank the study participants for their valuable contributions.
Data Availability . Data not publicly available.
Financial support . This work was supported by generous funding from the County Council of Norrbotten (NLL-933177 to T. N. G.); Umeå University through a regional agreement with the County Council of Norrbotten (ALF Universitets-ST to T. N. G.); and from County Council of Västerbotten (RV-966950 and RV-939171 to A. S.).
References
- 1. Sjöstedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci 2007; 1105:1–29. [DOI] [PubMed] [Google Scholar]
- 2. Yeni DK, Büyük F, Ashraf A, Shah M. Tularemia: a re-emerging tick-borne infectious disease. Folia Microbiol 2021; 66:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tärnvik A, Sandström G, Sjöstedt A. Epidemiological analysis of tularemia in Sweden 1931–1993. FEMS Immunol Med Microbiol 1996; 13:201–4. [DOI] [PubMed] [Google Scholar]
- 4. Johansson A, Farlow J, Larsson P, et al. Worldwide genetic relationships among Francisella tularensis isolates determined by multiple-locus variable-number tandem repeat analysis. J Bacteriol 2004; 186:5808–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Centre for Disease Prevention and Control . The European Union One Health 2021 zoonoses report. EFSA J 2022; 20:e07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis 2016; 16:113–24. [DOI] [PubMed] [Google Scholar]
- 7. Dryselius R, Hjertqvist M, Mäkitalo S, et al. Large outbreak of tularaemia, central Sweden, July to September 2019. Euro Surveillance 2019; 24:1900603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Furberg M. Towards the limits—climate change aspects of life and health in Northern Sweden: studies of tularemia and regional experiences of changes in the environment. Umeå, Sweden: Umeå University Medical Disserations, 2016. Available at: https://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-126949. [Google Scholar]
- 9. Eliasson H, Bäck E. Tularaemia in an emergent area in Sweden: an analysis of 234 cases in five years. Scand J Infect Dis 2007; 39:880–9. [DOI] [PubMed] [Google Scholar]
- 10. Ma Y, Vigouroux G, Kalantari Z, Goldenberg R, Destouni G. Implications of projected hydroclimatic change for tularemia outbreaks in high-risk areas across Sweden. Int J Environ Res Public Health 2020; 17:6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desvars A, Furberg M, Hjertqvist M, et al. Epidemiology and ecology of tularemia in Sweden, 1984–2012. Emerging Infect Dis 2015; 21:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seiwald S, Simeon A, Hofer E, Weiss G, Bellmann-Weiler R. Tularemia goes west: epidemiology of an emerging infection in Austria. Microorganisms 2020; 8:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maurin M, Pelloux I, Brion JP, Del Bano JN, Picard A. Human tularemia in France, 2006–2010. Clin Infect Dis 2011; 53:e133–41. [DOI] [PubMed] [Google Scholar]
- 14. Faber M, Heuner K, Jacob D, Grunow R. Tularemia in Germany—a re-emerging zoonosis. Front Cell Infect Microbiol 2018; 8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones RM, Nicas M, Hubbard A, Sylvester MD, Reingold A. The infectious dose of Francisella tularensis (tularemia). Applied Biosafety 2005; 10:227–39. [Google Scholar]
- 16. Tärnvik A, Berglund L. Tularaemia. Eur Respir J 2003; 21:361–73. [DOI] [PubMed] [Google Scholar]
- 17. Caspar Y, Maurin M. Francisella tularensis susceptibility to antibiotics: a comprehensive review of the data obtained in vitro and in animal models. Front Cell Infect Microbiol 2017; 7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . WHO Guidelines on tularaemia. Geneva, Switzerland: WHO Press, World Health Organization, 2007. [Google Scholar]
- 19. Johansson A, Berglund L, Sjöstedt A, Tärnvik A. Ciprofloxacin for treatment of tularemia. Clin Infect Dis 2001; 33:267–8. [DOI] [PubMed] [Google Scholar]
- 20. Williams MS, Baker MR, Guina T, et al. Retrospective analysis of pneumonic tularemia in Operation Whitecoat human subjects: disease progression and tetracycline efficacy. Front Med 2019; 6:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hepburn MJ, Simpson AJ. Tularemia: current diagnosis and treatment options. Expert Rev Anti Infective Ther 2008; 6:231–40. [DOI] [PubMed] [Google Scholar]
- 22. Tärnvik A, Chu MC. New approaches to diagnosis and therapy of tularemia. Ann N Y Acad Sci 2007; 1105:378–404. [DOI] [PubMed] [Google Scholar]
- 23. Statistics Sweden . Population statistics—Quarter 2, 2023. Available at: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population/population-composition/population-statistics/pong/tables-and-graphs/population-statistics---month-quarter-half-year/population-statistics---quarter-2-2023/. Accessed 8 October 2023.
- 24. Kiliç S, Çelebi B, Yeşilyurt M. Evaluation of a commercial immunochromatographic assay for the serologic diagnosis of tularemia. Diagn Microbiol Infect Dis 2012; 74:1–5. [DOI] [PubMed] [Google Scholar]
- 25. Lindgren H, Eklund J, Eneslätt K, Sjöstedt A. Kinetics of the serological response up to one year after tularemia. Front Cell Infect Microbiol 2022; 12:1072703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Penn RL, Kinasewitz GT. Factors associated with a poor outcome in tularemia. Arch Intern Med 1987; 147:265–8. [PubMed] [Google Scholar]
- 28. Dahlstrand S, Ringertz O, Zetterberg B. Airborne tularemia in Sweden. Scand J Infect Dis 1971; 3:7–16. [DOI] [PubMed] [Google Scholar]
- 29. Eliasson H, Lindbäck J, Nuorti JP, Arneborn M, Giesecke J, Tegnell A. The 2000 tularemia outbreak: a case-control study of risk factors in disease-endemic and emergent areas, Sweden. Emerging Infect Dis 2002; 8:956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Svensson K, Bäck E, Eliasson H, et al. Landscape epidemiology of tularemia outbreaks in Sweden. Emerging Infect Dis 2009; 15:1937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christenson B. An outbreak of tularemia in the northern part of central Sweden. Scand J Infect Dis 1984; 16:285–90. [DOI] [PubMed] [Google Scholar]
- 32. Kravdal A, Stubhaug ØO, Wågø AG, et al. Pulmonary tularaemia: a differential diagnosis to lung cancer. ERJ Open Res 2020; 6:00093-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Şenel E, Satılmış Ö, Acar B. Dermatologic manifestations of tularemia: a study of 151 cases in the mid-Anatolian region of Turkey. Int J Dermatol 2015; 54:e33–7. [DOI] [PubMed] [Google Scholar]
- 34. Polat M, Karapınar T, Sırmatel F. Dermatological aspects of tularaemia: a study of 168 cases. Clin Exp Dermatol 2018; 43:770–4. [DOI] [PubMed] [Google Scholar]
- 35. Syrjälä H, Karvonen J, Salminen A. Skin manifestations of tularemia: a study of 88 cases in northern Finland during 16 years (1967–1983). Acta Dermato Venereologica 1984; 64:513–6. [PubMed] [Google Scholar]
- 36. Jounio U, Renko M, Uhari M. An outbreak of holarctica-type tularemia in pediatric patients. Pediatr Infect Dis J 2010; 29:160–2. [DOI] [PubMed] [Google Scholar]
- 37. Syrjälä H. Peripheral blood leukocyte counts, erythrocyte sedimentation rate and C-reactive protein in tularemia caused by the type B strain of Francisella tularensis. Infection 1986; 14:51–4. [DOI] [PubMed] [Google Scholar]
- 38. Wetzstein N, Kärcher I, Küpper-Tetzel CP, et al. Clinical characteristics in a sentinel case as well as in a cluster of tularemia patients associated with grape harvest. Int J Infect Dis 2019; 84:116–20. [DOI] [PubMed] [Google Scholar]
- 39. Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis 2012; 55:1283–90. [DOI] [PubMed] [Google Scholar]
- 40. Schöbi N, Agyeman PKA, Duppenthaler A,et al. Pediatric tularemia—a case series from a single center in Switzerland. Open Forum Infect Dis 2022; 9:ofac292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.