Abstract
The best-characterized receptors for adenoviruses (Ads) are the coxsackievirus-Ad receptor (CAR) and integrins αvβ5 and αvβ3, which facilitate entry. The αv integrins recognize an Arg-Gly-Asp (RGD) motif found in some extracellular matrix proteins and in the penton base in most human Ads. Using a canine adenovirus type 2 (CAV-2) vector, we found that CHO cells that express CAR but not wild-type CHO cells are susceptible to CAV-2 transduction. Cells expressing αMβ2 integrins or major histocompatibility complex class I (MHC-I) molecules but which do not express CAR were not transduced. Binding assays showed that CAV-2 attaches to a recombinant soluble form of CAR and that Ad type 5 (Ad5) fiber, penton base, and an anti-CAR antibody partially blocked attachment. Using fluorescently labeled CAV-2 particles, we found that in some cells nonpermissive for transduction, inhibition was at the point of internalization and not attachment. The transduction efficiency of CAV-2, which lacks an RGD motif, surprisingly mimicked that of Ad5 when tested in cells selectively expressing αvβ5 and αvβ3 integrins. Our results demonstrate that CAV-2 transduction is augmented by CAR and possibly by αvβ5, though transduction can be CAR and αvβ3/5 independent but is αMβ2, MHC-I, and RGD independent, demonstrating a transduction mechanism which is distinct from that of Ad2/5.
At least 100 different adenoviruses (Ads) have been isolated, approximately half of which are different human serotypes (21). The presumed tropism of an Ad is often based on the clinical symptoms that are caused by the infection. However, the tropisms of many serotypes are poorly understood. For example, Ad serotypes 2 and 5 (Ad2/5) cause mild upper respiratory tract infections but seem to poorly infect epithelial cells lining the respiratory tract (60). Ads that give rise to symptoms similar to those caused by Ad2/5 may have different tropisms and modes of entry. Ad2/5, which are the best characterized, have icosahedral capsids with the external surface composed mainly of hexon, penton base, and fiber (21, 44). The fiber is an elongated thread-like molecule that projects from the penton base and initiates binding to the cellular surface.
Ad entry into the cytoplasm can be functionally divided into attachment, internalization, and permeabilization of the membrane. The C-terminal knob domain of many Ads attaches to the coxsackievirus-Ad receptor (CAR) (5, 42, 53), followed by internalization and permeabilization in clathrin-coated pits implicating dynamin (55) and αvβ5 and αvβ3 integrins (58), though Ad2 preferentially used the former for permeabilization (57). Recently, the crystal structure of the Ad12 fiber knob in complex with CAR and Ad5 surface plasmon resonance analysis defined a surface exposed knob loop in contact with one face of CAR (7, 26). Simultaneously, a mutational analysis identified several amino acids in the knob AB loop from Ad5, Ad9, and Ad41 that were critical for CAR binding (43). The cellular function of CAR has not been identified. The cytoplasmic and transmembrane domains of CAR are not essential for coxsackievirus and Ad2 infection (56). Ad3, Ad7, and Ad35 from subgroup B do not use CAR to attach to and enter cells, and the receptor for these viruses has not yet been described. In addition, in Ad5, the trimeric C-terminal spherical fiber knob domain appears to interact with the α2 domain of major histocompatibility complex class I (MHC-I) (20). Arnberg et al. have identified α(2-3)-linked sialic acid saccharides on glycoproteins as the receptor for Ad37 (1).
The αv integrins recognize a conserved Arg-Gly-Asp (RGD) motif (32) found in some extracellular matrix proteins and the Ad2/5 penton base. The three-dimensional structure of a recombinant soluble αvβ5 integrin bound to the penton base of Ad2 and Ad12 has been described, and a 20-Å RGD-binding cleft was found in the globular domain (9). On some cells that lack CAR, integrins may be involved in Ad2 attachment. Huang et al. have shown that Ad2 attaches to hematopoietic cells via αMβ2 integrins and enters via αvβ5 and that CHO cells expressing αMβ2 are more susceptible to Ad transduction (24).
We have generated replication-defective vectors from canine adenovirus type 2 (CAV-2) (28), which normally causes mild upper respiratory tract infections in dogs. In vitro and in vivo results using CAV-2 vectors demonstrated that CAV-2 did not mimic Ad5 tropism or transduction efficiency. For example, CAV-2 vectors transduce the airway epithelium of C57BL/6 mice poorly when compared to BALB/c mice. Ad5 vectors, on the other hand, transduce the airway epithelium of C57BL/6 mice more readily than in BALB/c mice. The goal of the present study was to identify the cell surface molecules used by CAV-2 to attach to and transduce cells. We assayed CAV-2 attachment and transduction using cell lines that express surface molecules that were involved in human Ad attachment and entry. Based on our results, we conclude that CAV-2 attaches to and enters cells using a mechanism that is distinct from that of the well-characterized Ad2/5 pathway. CAV-2 bound to and uses CAR to enter cells, though in some cells CAV-2 transduction could be CAR and αv integrin independent. Unlike Ad2/5, CAV-2 did not use the α2 domain of the MHC-I molecule or αMβ2 integrins to enter cells. Though the CAV-2 virion lacks an RGD motif, the CAV-2–αv integrin interaction appeared to play a role during attachment and transduction.
MATERIALS AND METHODS
Cell lines.
Chinese hamster ovary (CHO)-derived cells were grown in α minimal essential medium (α-MEM; Gibco) supplemented with 10% fetal bovine serum (FBS; BioWhittaker). CHO-pcDNA3.1 cells (referred to as CHOpc) and CHOK1 cells (ECACC 85051005) were used as a control for Ad5 and CAV-2 transduction and binding. CHO cells were transfected with pcDNA3.1 (Clontech), and a subclone, CHOpc cells, was selected for neomycin resistance. CHO-hCAR cells (5) constitutively express the human homologue of CAR, while CHO-mCAR cells (6) express the murine homologue. Daudi cells are a human B-lymphoblastoid line, while E8.1 cells are Daudi cells constitutively expressing the MHC-I molecules (37). Daudi cells lack the β2 microglobulin gene, but the MHC-I α chain is synthesized but not expressed on the cell surface. E8.1 cells have been transfected with the β2 microglobulin gene and permanently express the β2 microglobulin protein: as a result, the heterodimer αvβ2 microglobulin is expressed at the cell surface. The α2 domain is one of the three structural immunoglobulin-like domains of the α chain. The CS-1 hamster melanoma cell line (13), a gift from D. Cheresh (The Scripps Research Institute, La Jolla, Calif.) does not express β3 and β5 integrins and was grown in RPMI medium (RPMI; Gibco) supplemented with 10% FBS. CS-1/β3 and CS-1/β5 cells (57), which are derived from CS-1 cells and constitutively express β3 and β5 integrins, were grown in RPMI–10% FBS and 400 μg of Geneticin per ml (Sigma). M21-L4 (αv expressing) and M21-L12 (αv deficient) cells (58), also a gift from D. Cheresh, are human melanoma cells and were grown in Dulbecco modified Eagle medium (DMEM)–10% FBS–20 mM HEPES. DK28Cre cells (28) were used to propagate CAV-2 vectors and contain the CAV-2 E1 region stably integrated in the genome with the E1A region under the control of the cytomegalovirus (CMV) promoter and the E1B region under the control of its own promoter. DK28Cre cells express the canine homolog of CAR (15), as assayed by immunocytochemistry (see below). HeLa and MRC-5 cells (human lung fibroblast) were grown in DMEM supplemented with 10% FBS. THP-1 cells (human monocytic leukemia line), Jurkat (human T-lymphocyte), and Ramos (human B-lymphocyte) cells were purchased from the American Type Culture Collection and were grown in RPMI supplemented with 10% FBS. GMO2894 cells, a gift from V. Kalatzis (Hôpital Necker-Enfants Malades, Paris, France) are transformed human skin fibroblasts and were grown in Ham's F-12 medium (Gibco) supplemented with 10% FBS. Primary peripheral blood macrophage-derived human dendritic cells were sorted using an anti-CD14 monoclonal antibody (MAb) and cultured in RPMI supplemented with 10% FBS (HyClone) 1,000 U of interleukin-4 per ml, 50 ng of granulocyte-macrophage colony-stimulating factor, Glutamax (Gibco), and nonessential amino acids (Gibco). All cells were grown at 37°C with 5% CO2. See Table 1 for a summary of cell lines.
TABLE 1.
Characteristics of cell lines used in this study
| Cell type | CAR, integrin, and MHC-I contenta
|
Transducibilityb
|
|||||
|---|---|---|---|---|---|---|---|
| CAR | αv | β3 | β5 | MHC-I | CAVGFP | AdGFP | |
| HeLa | P | P | P | P | P | +++ | +++ |
| DK28Cre | P | P | +++ | +++ | |||
| CHOpc | L/N | P | P | P | P | − | − |
| CHO-hCAR | P | P | P | P | P | +++ | +++ |
| CHO-mCAR | P | P | P | P | P | +++ | +++ |
| Daudi | P | L/N | L/N | N | − | − | |
| E8.1 | P | L/N | L/N | P | − | ++ | |
| Ramos | P | L/N | L/N | P | − | − | |
| Jurkat | P | L/N | L/N | P | ++ | ++ | |
| THP-1 | L/N | L/N | L/N | P | + | − | |
| Dendritic cellsc | L/N | P | L/N | P | P | − | +++ |
| MRC-5 | P | P | P | P | ++ | +++ | |
| GM02894 | P | P | P | P | ++ | +++ | |
| M21-L12 | L/N | L/N | L/N | L/N | P | ++ | +++ |
| M21-L4 | P | P | P | P | P | +++ | +++ |
| CS-1 | P | P | L/N | L/N | P | +++ | +++ |
| CS-1/β3 | P | P | P | L/N | P | ++ | + |
| CS-1/β5 | P | P | L/N | P | P | +++ | +++ |
P, present; L/N, little or none.
+++, >40% of the cells were GFP positive, using a multiplicity of infection of 103 particles/cell; ++, 10 to 40% of the cells were GFP positive; +, 1 to 10% of the cells were GFP positive; −, ≤1% of the cells were GFP positive.
Dendritic cells express αMβ2 integrins.
Antibodies and blocking reagents.
Recombinant fibers from Ad3, Ad5, and Ad37 (fib3, fib5, and fib37) and Ad2 penton base (pb2) were expressed in Sf9 cells using baculovirus intermediate transfer vectors as previously described (20). The soluble CAR (sCAR) protein consists of the CAR extracellular domain fused to the Fc region of rabbit immunoglobulin, made in mammalian cells, purified on protein A-Sepharose and dialyzed against phosphate-buffered saline (PBS). P1F6 (Chemicon; MAb 1961) is a functional-blocking MAb against αvβ5 integrins. To test for CAR expression, we used RmcB (22), which is a mouse MAb that recognizes CAR; it poorly inhibits Ad2 attachment but can inhibit group B coxsackievirus attachment or rabbit anti-CAR serum. 69-6-5 (a gift from José Luis, CNRS 6032, Marseille, France) is a rat MAb (29) directed against αv integrins, and it recognizes αvβ3, αvβ5, αvβ6, and probably αvβ1 and αvβ8 integrins.
Vectors.
CAVGFP and AdGFP have been described previously (28). Briefly, CAVGFP is an E1-deleted, replication-defective vector derived from CAV-2 (Toronto strain A26/61) harboring a green fluorescent protein (GFP) expression cassette. The expression cassette contains the CMV early promoter, a synthetic intron, the green fluorescent protein (EGFP) cDNA, and a simian virus 40 poly(A) signal. AdGFP is an E1, E3-deleted, Ad5-derived vector that contains the same GFP expression cassette as CAVGFP. Vectors were purified, the titers were determined, and the vectors were stored as previously described (28). CAVGFP stocks contained 2.7 × 1012 to 5.6 × 1012 particles per ml, with a particle/infectious unit ratio of approximately 3 to 1. The AdGFP stock contained 5.6 × 1012 particles per ml, with a particle/infectious unit ratio of approximately 7 to 1. Vector concentration as determined by the optical density at 260 nm (OD260) was done using two dilutions of two aliquots of each virus-vector stock as described previously (35). Briefly, 5 to 10 μl of vector was mixed with 90 μl of 0.1% (wt/vol) sodium dodecyl sulfate, 10 mM Tris-Cl (pH 7.2), and 1 mM EDTA and heated for 10 min at 56°C. The OD260 was measured and multiplied by the extinction coefficient 9.09 × 10−13 OD · ml · cm−1 · virion−1 in order to determine the number of vector particles/ml.
CAV-Cy3, fluorescently labeled CAVGFP, was covalently labeled with the fluorescent marker Cy3 (Molecular Probes) as described for human Ad vectors (30). Briefly, 1012 particles of CAVGFP were incubated for 30 to 60 min with Cy3, dialyzed against PBS at 4°C, and stored in PBS–10% glycerol at −80°C. GFP expression from CAV-Cy3 was similar to that of the mock-treated vector, demonstrating that Cy3 labeling did not significantly damage the capsid.
35S-labeled CAV (35S-CAV) was generated using 10 15-cm-diameter plates containing a confluent monolayer of DK28Cre cells infected with 100 particles of CAVGFP per cell. The supernatant was replaced 6 h later with 10 ml of l-methionine-free α-MEM (Sigma M-3911) and 2 mCi of [35S]methionine (NEN-709A). Ten hours later, 10 ml of complete medium (DMEM and 10% FBS) was added. The vector was recovered 40 h postinfection and purified as described above. The specific activity of 35S-CAV was 7.5 × 103 particles/cpm.
Transduction assays.
Approximately 105 cells were plated in 12-well (for adherent cells) or 24-well (for suspension cells) plates and incubated with twofold dilutions of vector beginning with 103 particles per cell. To maximize transduction efficiency the plates were gently agitated at 37°C and in 5% CO2 overnight. A minimum of 104 cells was assayed for GFP expression by flow cytometry, i.e., fluorescence-activated cell sorting (FACS; FACSCalibur; Becton Dickinson), approximately 40 h posttransduction. All assays were done at least in duplicate. Images were taken using a CoolSnap camera and the associated software. The exposure time was 1 s for all images.
Blocking assays.
All assays, unless otherwise noted, were performed using 105 cells and medium at 0 to 4°C. Adherent cells (in 250 μl of DMEM) were blocked with 1010 particles of an Ad5 or CAV-2 vector (minimum of 105 particles/cell). Up to 2 μg of fib3, fib5, fib37, and pb2 was used in blocking assays. Blocking agents were incubated with cells in 24-well plates at 4°C with gentle agitation for 90 min. The blocking agent(s) was removed by two PBS rinses, followed by the addition of approximately 5 × 109 particles of 35S-CAV. Suspension cells (in 100 μl of medium) were processed in Eppendorf tubes and centrifuged between rinses. Controls were treated the same way as test samples, but blocking agents were not included. 35S-CAV binding was determined by dissolving the cells in 0.4 ml of Optiphase scintillation fluid (Wallace) and counting for 60 s in a Wallace 1450 Microbeta Plus liquid scintillation counter. All experiments were done in triplicate.
Increasing amounts (0.6, 1.5, and 4.2 μg) of sCAR were incubated with 109 particles of 35S-CAV in 100 μl of DMEM for 90 min on ice prior to incubation with 105 CHO-hCAR or Ramos cells prechilled on ice. The cells were agitated gently for 90 min and rinsed twice with DMEM, and the bound 35S-CAV particles were counted.
35S-CAV attachment to Daudi, E8.1, CHO-derivative, and CS-1 cells and their derivatives was assayed using 5 × 105 cells because the 35S-CAV stock had gone through two half-lives. In order to determine background levels, cells were blocked with 5 × 105 particles of CAV-2 per cell. Since cells were assayed at different times, the relative levels of binding to each cell type are comparable (i.e., CHO-derived cells were done simultaneously) but not versus one another (i.e., CAV-2 binding to Daudi cells should not be compared with M21-L cells).
CAV attachment and entry assays using CAV-Cy3.
Approximately 2 × 105 cells were incubated with 109 particles of CAV-Cy3 in 100 μl of medium for suspension cells and 250 μl of medium for adherent cells for 90 min at 4°C with gentle agitation and transferred at t = 0 to 37°C for 1 h. Aliquots were removed at 0, 15, 30, and 60 min; rinsed in PBS; and fixed with 3% formaldehyde in PBS. Suspension cells were fixed to slides by cytospinning. Images were taken using a CoolSnap camera and the accompanying software.
RESULTS
We selected a series of cell lines (see Table 1) based on the presence or absence of cell surface proteins that have been reported to facilitate Ad2, Ad3, and Ad5 attachment and transduction. CAV-2 transduction was monitored using GFP expression from CAVGFP, and as a control, we used AdGFP, an Ad5 vector containing the same GFP expression cassette. CAV-2 attachment was assayed using 35S-CAV, and internalization was monitored using CAV-Cy3.
CAV-2 transduction: CAR. (i) CHO cells expressing CAR.
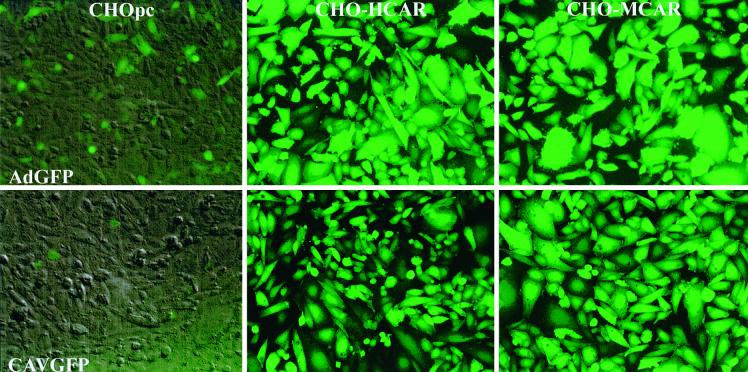
CHO cells are transduced poorly by Ad2/5 vectors, and the blockage is at the cell surface due to the lack of an appropriate receptor. Following the cloning of HCAR and MCAR cDNAs, plasmids coding for these cell surface molecules were transfected into NIH 3T3 (53) and CHO cells (5, 6). CHO-hCAR and CHO-mCAR cells, which constitutively express HCAR and MCAR, respectively, were shown to be susceptible to transduction by Ad5 vectors (5, 6). In order to determine if CAV-2 vectors can also use CAR, we incubated these cells with CAVGFP (Fig. 1). AdGFP transduced CHOpc cells poorly, while there was no transduction by CAVGFP. Ad5 and CAV-2 vectors readily transduced CHO-hCAR and CHO-mCAR cells (Fig. 1). These results demonstrated that the limiting factor for CAV-2 transduction of CHOpc cells is CAR and that CAV-2 vectors can use the human or murine homologue to transduce these cells. CHO cells express αvβ5 as their major integrins, some αvβ3 integrins, and no β2 or β6 integrins (24), suggesting that these latter integrins were not essential for CAV-2 attachment or entry.
FIG. 1.
CHO cells and derivatives expressing HCAR and MCAR: AdGFP versus CAVGFP. Confluent monolayers were incubated with 103 particles of each vector per cell. At 40 h posttransduction the cells were photographed using an inverted fluorescence microscope and a GFP filter with a bandwidth of 485 to 507 nm. In CHOpc cells the GFP expression is overlaid on a phase-contrast image in order to show the cell density. CAVGFP and AdGFP transduced >85% of CHO-hCAR and CHO-mCAR cells.
(ii) CAV-2 attachment to sCAR.
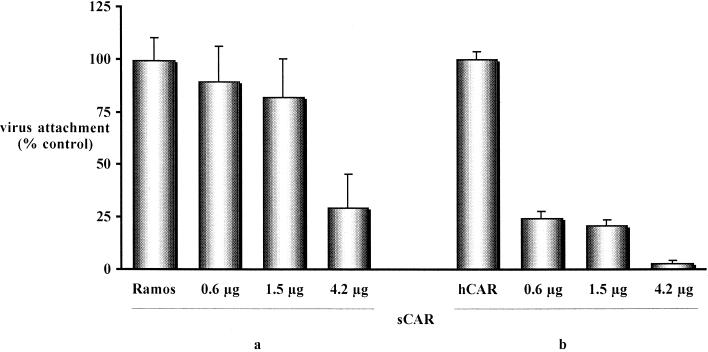
In order to determine if CAV-2 was capable of binding directly to CAR, we used a soluble form of CAR (sCAR) in which the extracellular domain of CAR was fused to the Fc region of rabbit immunoglobulin. Previously, we determined that 5 μg of sCAR was able to completely inhibit AdGFP transduction (5 × 109 particles; not shown). 35S-CAV was incubated for 60 min with 0.6 to 4.2 μg of sCAR prior to incubation of the vector with CHO-hCAR and Ramos cells. sCAR inhibited 35S-CAV binding in a dose-dependent manner in Ramos and CHO-hCAR cells (Fig. 2). The blocking effect was greater using CHO-hCAR cells, possibly due to a higher number of CARs on Ramos cells or because CAV-2 was binding to other cell surface molecules on Ramos cells. These data demonstrate that CAV-2 can bind directly to CAR, probably via the fiber knob. Importantly, by this assay we cannot exclude the possibility that CAV-2 binds to other cell surface molecules. Steric hindrance due to the attached sCAR may have inhibited CAV-2 attachment.
FIG. 2.
Inhibition of 35S-CAV attachment using sCAR. Increasing concentrations of sCAR were incubated with 35S-CAV (109 particles) prior to attachment to Ramos (a) and CHO-hCAR (b) cells. The data are the means of three points ± the standard deviation and are expressed as the percentage of virus bound to control cells.
CAV-2 transduction: MHC-I molecule and αMβ2 integrins. (i) Daudi cells expressing MHC-I.
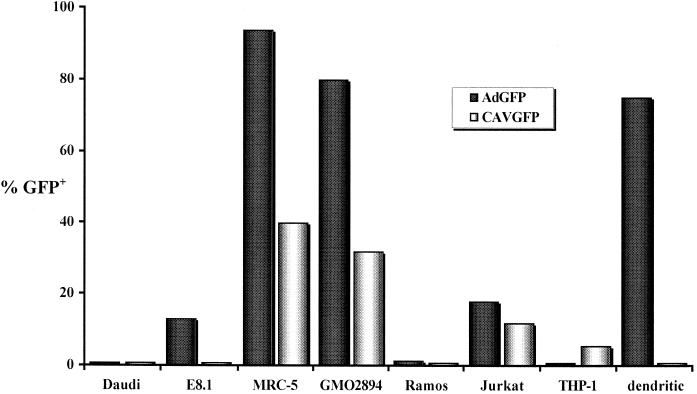
Daudi cells, which are resistant to Ad2/5 transduction, were transfected with the cDNA coding for the β2 microglobulin chain to generate E8.1 cells, which express MHC-I molecules on their surface. Hong et al. (20) used E8.1 cells to demonstrate that Ad2/5 use the MHC-I molecule to transduce human cells. Daudi and E8.1 cells express low levels of CAR and little or no αvβ5 integrins (Table 1 and data not shown). Daudi and E8.1 cells were incubated with 103 particles of AdGFP or CAVGFP per cell overnight with gentle agitation and then analyzed by FACS at 24 h postinfection. CAVGFP was not able to transduce Daudi or E8.1 cells, while AdGFP transduced E8.1 but not Daudi cells (Fig. 3). These results suggest that CAV-2 does not use the MHC-I α2 domain, unlike Ad2/5. These data do not exclude the possibility that this cell surface protein is a site of attachment for CAV-2 (see below) since transduction may be blocked postattachment.
FIG. 3.
Transduction efficiency of CAVGFP (open bars) versus AdGFP (shaded bars) using cells that express molecules shown to facilitate Ad2/5 transduction. A total of 104 cells were assayed at ca. 40 h posttransduction. The data are presented as the averages of two tests and give the percentage of GFP-positive cells. All cells showed a linear decrease in the percentage of GFP-positive cells when twofold serial dilutions of AdGFP and CAVGFP were used.
(ii) Dendritic cell αMβ2 integrins.
Rea et al. demonstrated that dendritic cells do not express HCAR or αvβ3 but do express integrins αvβ5 and αMβ2 and the MHC-I molecule (39). Moreover, as Nemerow and coworkers have shown, αMβ2 can be used by Ad2 to transduce cells (24). We tested primary human dendritic cells for the ability to be transduced by CAVGFP (Fig. 3). At 5 days postisolation, undifferentiated dendritic cells were incubated with AdGFP or CAVGFP. These cells showed the most dramatic difference in transduction efficiency with CAVGFP compared to AdGFP. As previously described (12), Ad5 vectors efficiently transduced human dendritic cells. A CAV-2 vector containing the same expression cassette was not able to transduce dendritic cells, even at an input of 6 × 103 particles/cell. If αMβ2 integrin is responsible for Ad5 transduction of dendritic cells, its expression is not sufficient for CAV-2 transduction. Furthermore, dendritic cells were one of the few cell types in which we were unable to detect CAV-2 binding (data not shown).
CAV-2 transduction: αv, β3, and β5 integrins. (i) CS-1 cells and derivatives: expression of β3 and β5 integrins.
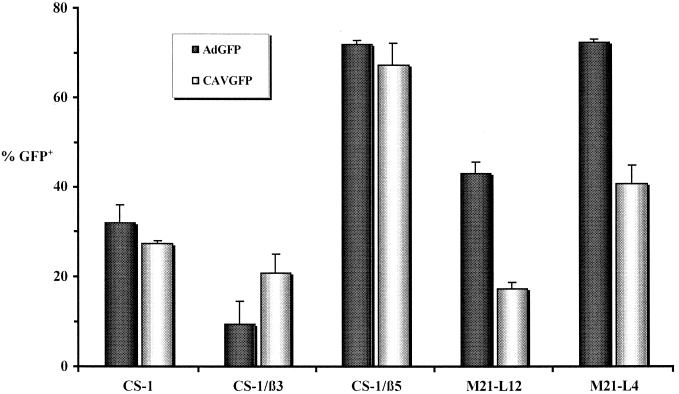
CS-1 cells do not express functional β3 or β5 integrins but do express the hamster homologue of CAR (Table 1) and contain an intracellular pool of αv integrin (51). In order to characterize the attachment and/or entry pathway of human Ads, Cheresh and coworkers generated stable polyclonal CS-1 lines expressing either αvβ3 or αvβ5 integrin dimer (57). CS-1/β3 and CS-1/β5 cells were selected by a functional assay based on the ability of the cells to adhere to immobilized vitronectin or Ad2 penton base. These researchers demonstrated that CS-1/β5 cells were more susceptible to Ad5 transduction than were CS-1/β3 and CS-1 cells and that Ad2 poorly permeabilized the latter.
We tested CS-1, CS-1/β3, and CS-1/β5 cells for the ability to be transduced by the CAVGFP. Figure 4 shows that CAVGFP and AdGFP transduce CS-1/β5 cells more readily than they transduce CS-1/β3 cells. These results are similar to those described for Ad2 by Wickham et al. (57). Surprisingly, AdGFP transduced CS-1 cells three- to fourfold more efficiently than CS-1/β3 cells. CAVGFP transduction was also greater in CS-1 cells than in CS-1/β3 cells.
FIG. 4.
Roles of β3, β5, and αv integrins. Transduction of CS-1 and M21-L and their derivatives by CAVGFP (open bars) and AdGFP (shaded bars) is shown. A minimum of 104 cells were assayed for GFP expression by FACS at ca. 40 h posttransduction. The data are presented as the percentage of GFP-positive cells per well and are the averages of four to six tests ± the standard deviation.
(ii) M21-L derivatives: expression of αv integrins.
M21 human melanoma cells express αv and β3/5 integrin chains. To establish the structural and functional properties of αvβ3/5 integrins on M21 cells, stable variant cell lines which express altered αv integrin levels were selected (8). M21-L cells fails to synthesize αv or its mRNA yet produce normal levels of the β3/5 integrins. In these cells, the β chain does not reach the cell surface but accumulates inside the cell. M21-L cells lacking αv are incapable of attaching to vitronectin, fibrinogen, or an RGD-containing heptapeptide, yet they attach normally to fibronectin, whereas the parental M21 cells attach to all of these adhesive proteins. M21-L cells were also used to demonstrate that Ad2 uses αvβ3 and αvβ5 integrins to be internalized into cells (58). We tested M21-L12 cells (αv deficient) and M21-L4 cells (αv expressing) for the ability to be transduced by CAVGFP. M21-L4 cells express 20-fold more αvβ3 than αvβ5 (57). Figure 4 shows that CAVGFP and AdGFP transduce M21-L4 cells approximately twofold more readily than they transduce M21-L12 cells.
CAV-2, which does not contain an RGD motif in its penton base, hexon, or fiber, unexpectedly mimicked the transduction efficiency of Ad5 when using CS-1 and M21-L cells selectively expressing αvβ3 or αvβ5 dimers. The transduction of CS-1 and M21-L cells suggests that, as is true for Ad2/5, CAV-2 does not require αvβ3 or αvβ5 expression for internalization. The enhanced sensitivity of CS-1/β5 and M21-L4 cells to CAV-2 transduction suggests that αvβ5 may play a facilitating role in transduction. However, in contrast to results reported for Ad5 (57), virus-binding studies suggest that the sensitivity of these cells to transduction by CAV-2 may be in part determined by virus attachment, as opposed to internalization (see Fig. 5 below). In a previous study with CS-1 cells to demonstrate Ad2 interaction with αvβ5/3, β-glucuronidase activity was assayed 5 h postincubation and not the percentage of transduced cells 40 h postincubation. In our assay, β3 expression had little to no enhancing effect on CAV-2 and Ad5 transduction in CS-1 cells. We have also found that the rate of internalization of CAV-2 is similar to that of Ad5 in CS-1 and M21-L cells (not shown), suggesting that the effect was not due to the difference in time of incubation of the cells and vectors.
FIG. 5.
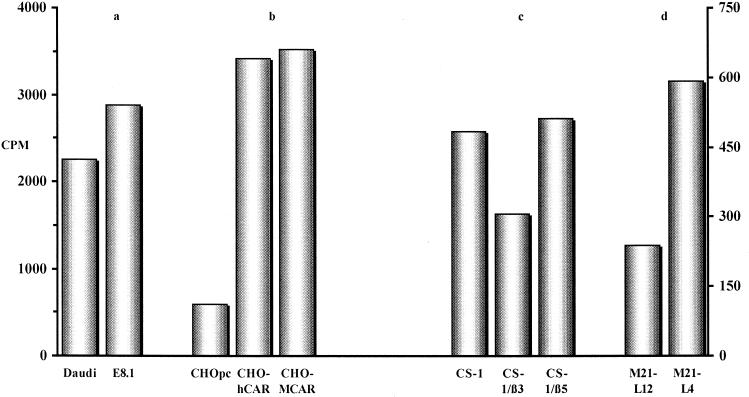
CAV-2 attachment to CAR, MHC-1 α2 domain, and αvβ5 integrins. Daudi (a), CHO (b), CS-1 (c), and M21-L (d) cells and their derivatives were incubated with 35S-CAV in order to determine the binding capacities relative to the parental cell line. The data are presented as the total counts per minute per cell type. The background counts (cells blocked with cold CAV-2 prior to incubation with 35S-CAV) were ca. 100 cpm for CHO- and CS-1-derived cells and 250 and 25 cpm for Daudi and E8.1 cells, respectively. For Daudi, E8.1, and the CHO derivatives, the y axis is on the left (0 to 4,000 cpm). For CS-1 and M21-L cells, the y axis is on the right (0 to 600 cpm). The data are the means of two samples, with an error of <10% for each sample.
CAV-2 transduction: fibroblasts and other hematopoietic cells.
Ad5 vectors are reported to transduce MRC-5 and THP-1 cells poorly, while a chimeric Ad5/3 vector containing the fiber knob of Ad3 showed a >10- and a 50-fold increase, respectively, in transduction efficiency (48). Huang et al. (24) reported that THP-1 cells do not bind radioactive recombinant Ad5 fiber, a finding consistent with our observation that these cells do not express CAR (Table 1). Using MAb 69-6-5, we found that THP-1 cells expressed little or no αv integrin (not shown). We demonstrate that AdGFP transduced THP-1 cells poorly (1.0%). However, THP-1 cells were fivefold more readily transduced by CAVGFP under the same conditions (Fig. 3). These results demonstrate that CAV-2 transduction can be CAR and αv integrin independent at a multiplicity of infection of 103 particles/cell in THP-1 cells.
In our study, AdGFP was efficient (85% at 102 particles/cell) in the transduction of MRC-5 cells, while CAVGFP generated 25% GFP-positive cells. MRC-5 cells express αvβ5 and low levels of CAR (not shown). In order to determine if other human fibroblast lines behave similarly, we tested GMO2894 cells, which were derived from skin fibroblasts. At 103 AdGFP particles/cell, 80% of the cells were GFP positive. Using CAVGFP, 32% of the cells were GFP positive at this multiplicity of infection. When more than approximately 50% of the cells in a plate are transduced, the likelihood of two particles transducing the same cell increases. In these conditions, the percentage of cells transduced does not increase by a factor of 2. At a lower vector input, our results with MRC-5 and GMO2894 correspond to six- to eightfold-lower transduction efficiencies for CAVGFP. The lower transduction efficiency of human fibroblasts by CAV-2 may be due to the lack of an RGD motif in the CAV-2 virion, and therefore reduced RGD integrin-dependent internalization, or to the presence of a second cell surface molecule that Ad5 can use but not CAV-2. Further experiments are needed to test this possibility.
Ramos and Jurkat cells express CAR and little or no αv integrins (Table 1 and reference 41) but nonetheless showed different sensitivities to transduction (Fig. 3). Ramos cells were resistant to Ad5 (as previously described) and CAV-2 transduction (<1.5%). However, AdGFP and CAVGFP were capable of transducing Jurkat cells (18 and 12%, respectively) at 103 particles/cell. The differences in transducibility may reflect the expression levels of CAR, other integrin dimers, or other receptors. Notably, Ramos cells are derived from B lymphocytes and Jurkat cells are from T lymphocytes.
Primary human hematopoietic cells such as monocytes, CD34+ cells, and resting or activated (with phytohemagglutinin and phorbol myristate acetate and maintained with interleukin-2) T cells were also resistant to CAV-2 transduction even at 104 particles/cell (not shown). This is noteworthy because during T-cell activation αv integrin expression is induced (23).
CAV-2 binding. (i) CAR, MHC-I α2 domain, and αv integrins.
In order to determine if CAR, the αv domain of MHC-I, or αv integrins were acting as sites of attachment for CAV-2, we incubated CHO-, Daudi-, CS-1-, and M21-L-derived cells with 35S-CAV and determined the total binding. Background levels were determined by blocking with 105 CAVGFP particles per cell prior to incubation with 35S-CAV.
In CHOpc cells, HCAR and MCAR expression increased CAV-2 binding more than fivefold (Fig. 5). These results confirm that CAV-2 used CAR as a site of attachment when expressed on the surface of CHO cells.
Expression of the MHC-I molecules on E8.1 cells modestly increased CAV-2 binding versus Daudi cells (approximately 1.3-fold). In an earlier report, a 10-fold difference in Ad5 binding was detected between Daudi and E8.1 cells (20). If the MHC-I molecule was acting as a CAV-2 attachment site, the interaction was small in magnitude. Furthermore, the small difference in CAV-2 binding may be due to a slightly higher level of CAR expression on E8.1 cells (not shown).
Due to our results concerning CAV-2 transduction of CS-1- and M21-L-derived cells, we also tested these cells for CAV-2 binding. CAV-2 binding was greater in CS-1/β5 cells than in CS-1/β3 cells. Roelvink et al. reported a twofold increase in Ad9 (a short fiber serotype) binding to CS-1/β5 versus CS-1 cells (41) and suggested that increased binding was due to the Ad9 penton base-integrin interaction. Whether increased transduction is due to the expression of αvβ5 or to other attachment molecules in CS-1 cells is uncertain at this time. If CAV-2 is interacting with α integrins, it must be in an RGD-independent fashion. CAV-2 binding to M21-L cells mimicked CAV-2 transduction (Fig. 4). However, we found that M21-L12 cells express little or no CAR, while M21-L4 cells express a significant amount (data not shown and Table 1), suggesting that increased transduction may have been due to attachment to this receptor.
Inhibition of CAV-2 binding.
Ad2/5 virion and recombinant fibers bind specifically to CAR on certain cell types. In order to identify the site of attachment of CAV-2, we tested a selection of cells in combination with blocking reagents for the ability to inhibit CAV-2 binding. We tested the ability of CAV-2 to bind to HeLa, CHO-hCAR, Ramos, Daudi, and E8.1 cells using 35S-CAV. Cells were incubated at 4°C with blocking agents that consisted of recombinant Ad fibers, Ad5 and CAV-2 capsids, MAbs, and recombinant Ad2 penton base. At 4°C, Ad2, Ad5, and recombinant fibers can effectively bind CAR but are not internalized (11, 36, 50).
(i) Ad5 and CAV-2 capsid cross-competition.
We detected 35S-CAV binding to each of the above cell types. In order to determine nonspecific CAV-2 attachment and the blocking efficiency of Ad5, we preincubated the cells with 5 × 105 particles/cell of CAVGFP or AdGFP (Fig. 6). The highest level of inhibition was detected when cells were preincubated with CAV-2. Preincubation with CAV-2 decreased 35S-CAV attachment to approximately 22% of the control in the three suspension cell lines. In the two adherent lines a more modest reduction in binding was found. Preincubation of Daudi, E8.1, and Ramos cells with Ad5 also produced a decrease in CAV-2 attachment but less than that found with CAV-2 preincubation in the respective cell lines.
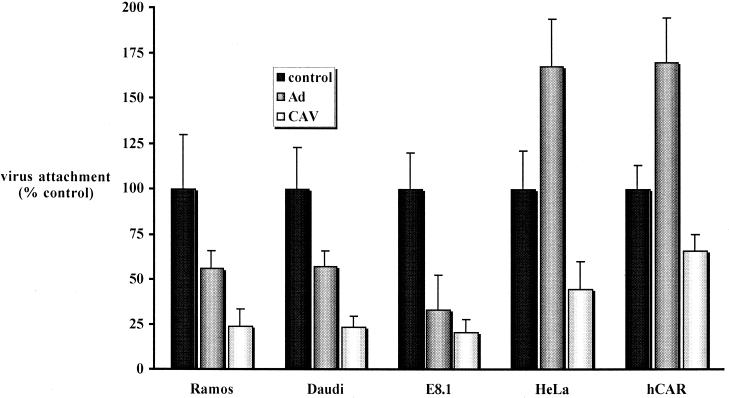
FIG. 6.
CAV-2 attachment in cells preincubated with Ad5 or CAV-2 virion. HeLa cells express approximately 5 × 103 CAR molecules per cell (18), and therefore a ratio of 5 × 105 viral particles/cell was used to block CAR attachment. The data are the means of three points ± the standard deviation and are expressed as the percentages of virus attachment to control cells.
Unexpectedly, a variable but reproducible increase of CAV-2 attachment was detected when HeLa and CHO-hCAR cells were preincubated with Ad5. It is unlikely that CAV-2 particles attached directly to the Ad5 capsid, and the results from the preincubation of Ramos, Daudi, and E8.1 cells with Ad5 argued against a direct CAV-2–Ad5 interaction. We suspected that this enhanced binding might have resulted from an Ad5-induced rearrangement or conformational change among surface molecules that exposed CAV-2 attachment sites. CHO-hCAR cells were fixed prior to blocking with Ad5 and incubation with 35S-CAV. Consistent with these possibilities, CAV-2 could still bind formalin-treated CHO-hCAR cells, but the paradoxical enhancement due to Ad5 was not observed (not shown). The nature of the formalin-sensitive molecules, whether CAR, integrins, or other unidentified receptors, remains to be identified. Although membrane fluidity is reduced at 4°C, Ad-induced integrin clustering (55) may have aided 35S-CAV binding. In addition, if preincubation with Ad5 specifically blocked CAV-2–CAR interaction, then CAV-2 binding was mediated by cell surface molecules other than CAR.
(ii) Inhibition of CAV-2–CAR interaction: fiber and RmcB cross-competition.
Ad2/5 binding can be blocked by competition with purified fibers, and fib2 and fib5 are interchangeable in CAR blocking assays (41, 47, 48). We tested fib5 on CHO-hCAR cells for the ability to block GFP expression from AdGFP. CHO-hCAR cells were chosen because CAV-2 attachment appeared to be CAR dependent (Fig. 5b). We found that a concentration of 0.5 μg/ml effectively blocked AdGFP transduction (not shown), while 2 μg of fib3 or fib37 per ml had no effect, as previously described (41, 47). We then tested whether fib5, fib3, or fib37 inhibited CAV-2 attachment to Ramos and CHO-hCAR cells.
Similar to Ad2/5 binding studies, fib3 and fib37 had no inhibitory effect on CAV-2 binding to Ramos cells (Fig. 7). The anti-CAR MAb RmcB also had no inhibitory effect; we have observed that RmcB inhibits Ad2/5 attachment poorly (unpublished data), probably because it attached to a distant epitope. Unlike the case with Ad2/5, fib5 had little if any effect on CAV-2 binding to Ramos cells. We did not detect an additive effect when RmcB was used in combination with fib5 (not shown). These data are consistent with the observation that sCAR did not fully inhibit CAV-2 attachment of these cells (Fig. 2) and suggested that CAV-2 bound to sites other than the fib5 site on CAR.
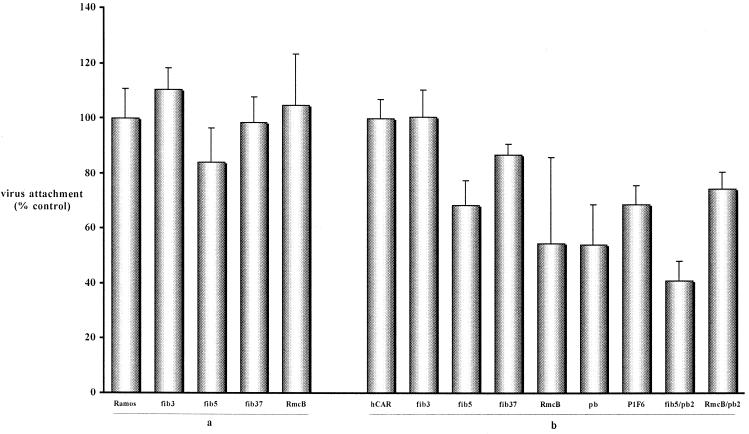
FIG. 7.
Ramos cells (a) and CHO-hCAR cells (b) blocked with selected reagents prior to incubation with 35S-CAV. The data are the means of three points ± the standard deviation and are expressed as the percentages of virus attachment to control cells.
On CHO-hCAR cells, RmcB and fib5 had a small inhibitory effect on CAV-2 binding. The blocking effect induced by RmcB and fib5 suggests that the CAV-2–CAR interaction site is close but not identical to the fib5 attachment site. Following the identification of sialic acid as an Ad37 receptor (1), our data obtained using fib37 suggest that this surface moiety is not a receptor for CAV-2. CAV-2 attachment was not inhibited by fib3. If Ramos and CHO cells were eventually found to express the Ad3 fiber receptor, we would predict that CAV-2 does not use the same cell surface moiety.
(iii) CAV-2 and αv integrins: penton base and P1F6 cross-competition.
In order to understand the interaction of CAV-2 with the αv integrins, we used a functional blocking MAb (P1F6) against αvβ5 and a recombinant penton base from Ad2 (pb2) to assay CAV-2 binding. pb2 and P1F6 were ineffective at blocking AdGFP transduction of HeLa cells at the highest concentration tested (2 μg/ml for pb2) (not shown). Ramos cells do not express αv integrins, and we did not detect inhibition of CAV-2 binding when pb2 or P1F6 was used alone or in combination with fib5 or RmcB (not shown).
In spite of the fact that CAV-2 does not have an RGD motif, we detected the inhibition of CAV-2 binding to CHO-hCAR cells using pb2 and P1F6 (Fig. 7). Preincubation of the cells with pb2 reduced CAV-2 binding to 54% of that of the control. P1F6 also had a small effect on CAV-2 binding. An additive effect was seen when fib5 and pb2 were combined, but not with pb2-RmcB. The inhibition of CAV-2 binding by pb2 and P1F6 suggested that αv integrins might play a role in CAV-2 attachment in some cells.
CAV-2 internalization.
Our initial question was which factors are necessary for CAV-2 attachment and entry in transducible cells. We have shown that CAV-2 can bind (Fig. 6 and 7) to several cell types without being able to direct transgene expression (Fig. 3). In order to address the question of whether transduction was blocked at the membrane or at some postattachment point in the trafficking of the virus, we used CAV-Cy3, a fluorescently labeled CAV-2 virion, to monitor virus entry into cells.
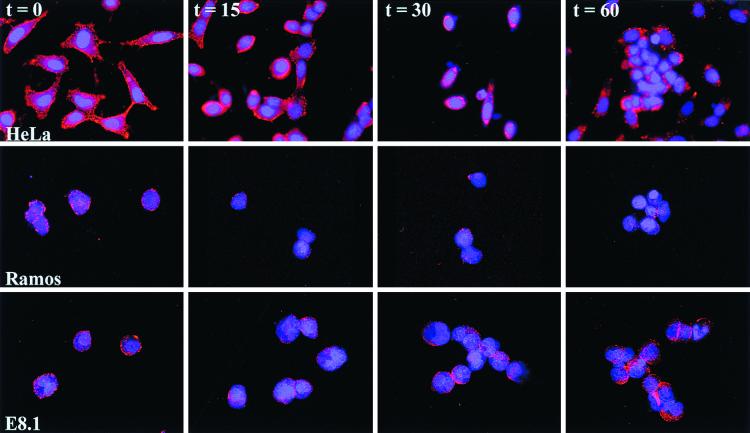
CAV-Cy3 was incubated with cells on ice in order to promote vector attachment but not internalization and was rinsed with cold medium to remove unbound vectors. The cell-vector suspension was warmed to 37°C, and an aliquot was removed and fixed at 15-min intervals. Attachment and entry were analyzed by fluorescence microscopy. Three cell types were tested: HeLa cells, which are permissive for CAV-2 transduction, and Ramos and E8.1 cells, which are not (Fig. 3). Like Ad2/5 (17, 41), CAV-2 was internalized within the first 15 min and then migrated and attached to the nuclear membrane within approximately 30 min (Fig. 8). At t = 0 min, we found that CAV-Cy3 attached to the external membrane in all the cells tested. In HeLa cells, at t = 15 min, CAV-Cy3 was found throughout the cytoplasm as well as near the nucleus. At t = 30 min, the majority of the fluorescent signal was found attached to the nuclear membrane. At t = 60 min, the CAV-2 capsid can again be found throughout the cytoplasm.
FIG. 8.
CAV-2 attachment and entry. CAV-Cy3 was incubated with Ramos, E8.1, and HeLa cells in order to identify the point of inhibition for CAV-2 transduction. Cells were fixed at the indicated times. CAV-Cy3 appears as red points, and the cell nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole).
Using Ramos and E8.1 cells, we were unable to detect a pattern different from that found at t = 0. The nucleus occupies a significant portion of the cytoplasm in Ramos and E8.1 cells, but cytospinning the cells to slides allows the membrane to be slightly displaced. This gave an unnatural look to the cells, but it demonstrated that CAV-Cy3 attached to the external cell membrane. These results demonstrate that CAV-2 vectors bind (as previously shown with 35S-CAV) but cannot be internalized by Ramos or E8.1 cells (or CHOpc cells; not shown) after attachment and an incubation of more than 30 min at 37°C. These data demonstrate that CAV-2 transduction, in these cell types, is inhibited at the point of internalization and not of postinternalization trafficking.
DISCUSSION
To our knowledge, this is the first study identifying cell surface molecules used by a nonhuman Ad to transduce cells. A phylogenetic comparison of several Ads found that CAV-2 is more closely related to murine adenovirus type 1 (MAV-1) and bovine adenovirus type 3 (PAV-3) than to the human serotypes included in the analysis (at least one from each subgenus) (3). Here we demonstrate that CAV-2 binds directly to HCAR and can use it to transduce cells. Considering our data, one might predict that the attachment site on CAR is conserved in mammals if human and nonhuman Ads are able to use it as a receptor.
Our data also suggest that CAV-2 attaches to other cell surface molecules. We detected a low and significant level of CAV-2 transduction in two cell lines (THP-1 and M21-L12 cells) that were CAR negative, and expression of this cell surface protein was not sufficient for CAV-2 transduction (e.g., Ramos cells). The relatively low binding and the efficient transduction of M21-L12 cells by CAV-2 suggest that there is at least a second receptor on these cells, which mediates CAV-2 (and Ad5) transduction. CAV-2 transduction is notably different from that of Ad2/5 because it cannot transduce E8.1 cells (MHC-I α2) or primary human dendritic cells (αMβ2). In addition, we did not detect CAV-2 binding to dendritic cells. Other primary human hematopoietic cells were also resistant to CAVGFP transduction. These data may have an important bearing on the potential of CAV-2 vectors for gene transfer. If an induced immune response, directed against viral capsid proteins or the transgene, is augmented by the transduction of professional antigen-presenting cells (dendritic cells), then CAV-2 vectors may have an innate advantage (versus Ad2/5 vectors) in the clinical setting.
Additional distinctions in the transduction efficiency of CAV-2 versus Ad5 were found in the CHO, THP-1, and fibroblast cell lines. As mentioned previously, however, THP-1 cells, a human monocytic line, were transducible, although poorly. Further experiments are needed to identify the differences between primary monocytes and THP-1 cells.
CAV-2, like Ad40 and Ad41 (52), does not have an RGD in the penton base. If the CAV-2 penton base (477 amino acids) is aligned with that of Ad5 (571 amino acids), a striking 77-amino-acid deletion (from amino acids 302 to 379 in Ad5) is found, with the Ad5 RGD motif (amino acids 340 to 342) located in the center of this deletion (not shown). Furthermore, the CAV-2 penton base does not contain the RGD-like RGE, an LDV (27, 31), or other integrin-interacting motifs (46). Therefore, one would expect the interaction of the CAV-2 capsid, particularly the penton base, with αv integrins, to be less important. There are 17 different known α integrins and 8 β integrins (25), forming at least 23 heterodimers. αvβ3/5 integrins contain an NPXY motif in the β subunit that may play a role in Ad entry via clathrin-coated pits (55). Mathias et al. demonstrated that soluble αvβ5 integrin dimers show more binding to Ad2, Ad5, Ad19, and Ad37 than to Ad12. These authors suggested that the relatively short RGD loop in the Ad12 penton base might bind less efficiently to integrins than do the longer RGD loops of the other Ad serotypes tested. Ad41 was not included in this study. Our results suggest a role for αv integrins but do not exclude a role for other capsid proteins in CAV-2 attachment. The data presented here support the suggestion by Nemerow and coworkers that the Ad2/5 penton base binding to αvβ5 integrins could be RGD independent (57). It was also noted in that study that αvβ5 could not be eluted off a penton base affinity column with an RGD-containing peptide. Our study is the first using an Ad that is naturally deficient in an RGD motif. Bai et al. mutated the RGD motif in the Ad2 penton base and found that the kinetics of virus propagation was delayed in adherent cells (2). Ad40 and Ad41 also have unusual propagation kinetics (52); whether this is due to the lack of an RGD motif is uncertain. This is not the case with CAV-2, however, where internalization, permeabilization, endosome escape, nuclear entry, and release of functional virus are essentially identical to that of Ad5 (M. Chillon et al., manuscript in preparation).
CAV-2, like Ad9, hemagglutinates mouse, rat, and human red blood cells (data not shown). Moreover, an anti-CAR MAb can inhibit Ad9-induced hemagglutination (42). Roelvink et al. demonstrated that Ad9 binding strategy is dependent upon the interaction of the penton base and integrins (41) and proposed that fiber length is the prime determinant of Ad attachment. The Ad2 fiber shaft is a left-handed triple-helical structure composed of β-strands interspersed with extended loops which contain 22 repeats, with 15 amino acids per repeat (Ad9 has 8 repeats). The CAV-2 fiber shaft contains 18 repeat motifs with 18 amino acids per repeat and six potential glycosylation sites (38). Preliminary electron microscopy data (not shown) suggest that the length of the CAV-2 fiber is close to that of the Ad2/5 fiber (37 nm) and probably 2.5 to 3 times the length of the Ad9 fiber (11 nm). Therefore, the CAV-2 fiber falls into the group of “long-shafted, CAR-recognizing fibers,” being above the minimum number of repeats, estimated to be 12 (41). Based on this model, one would expect CAV-2 to bind initially to CAR and to be internalized in a mechanism different from that of Ad2/5 due to the lack of an RGD motif. The lack of an RGD motif in the CAV-2 virion suggests that this motif may have a rather limited role in internalization and that other motifs in the CAV-2 capsid probably aid attachment and internalization.
Cotten and coworkers have estimated the net surface charges of Ad3, Ad5, Ad40, and CELO virus to be dominated by the exposed loops extending from the 720 hexons (4). The relative net charge was based on the antigenic sites (10, 54, 59), crystal structure of the hexon (40), and electron microscopy studies of the Ad virion (49). Based on this model, the CAV-2 capsid is approximately 10-fold-less negatively charged than Ad2/5. Supporting this calculation of the net surface charge of CAV-2 is the fact that we have been unable to increase CAV-2 transduction via Ad-CaPi coprecipitates (14) or incubation with other cationic DNA transfection reagents that increase Ad5 transduction (not shown). It is possible that the more neutral net charge of the CAV-2 capsid permits attachment to most of the cell types tested (unpublished data). Using CAV-Cy3, we were able to demonstrate that the inhibition of gene transfer was at the point of internalization and not due to permeabilization, cytoplasmic transport, or transgene expression (Fig. 8). The net charge may also be the reason that CAV-2 attachment and entry do not fit neatly into the model proposed by Roelvink et al. We suggest a mechanism in which the CAV-2 fiber binds CAR and, due to the lower level of repulsion between the capsid and the cell surface glycoproteins, is internalized via an RGD-independent integrin (possibly αv) pathway. This makes the model based on the length of the fiber as the primary determinant important only when the capsid is sufficiently negatively charged and the penton base contains an RGD motif.
In order to understand fully virus-disease etiology, it is necessary to determine which cell types and/or tissues are and (just as importantly) are not targeted by the virus. One of the possible uses of a CAV-2 vector is as a gene transfer tool in the clinic. We believe that this nonhuman Ad vector has some advantages over human Ad vectors. We have shown previously that sera from 98% of a random healthy cohort did not contain anti-CAV neutralizing antibodies and that CAV-2 vectors are able to direct efficient transgene expression in the lungs of BALB/c mice (28). Nonhuman Ad vectors derived from ovine (19), bovine (34), CELO (33), and avian (45) sources may also offer similar advantages. For example, there is also little or no preexisting neutralizing humoral immunity directed against the ovine capsid (19).
Significant work is being done to modify the tropism of Ad vectors for the clinic by targeting specific cell surface molecules. Nonhuman Ad vectors will have tropisms different from human Ad serotypes, as we found in the nasal cavities of rats and in human brain biopsies (C. Soudais, unpublished data). The tropism of CAV-2 makes these vectors less promiscuous and, in turn, one may be able to target specific cell types in a clinical setting. It is likely that modifying and shuffling Ad fiber will produce some interesting vectors, and it is now conceivable to exchange 1 of the 50 different human Ad fiber knobs with a CAV-like capsid or vice versa. This is assuming that the anti-Ad neutralizing antibodies and the long-lived CD4+ T-cell-based immunity found in the majority of potential patients (16) will not be directed against the human Ad fiber knob on a CAV-2 capsid. Finally, from the data presented here it is clear that the fiber knob is only one of the several players that determine Ad transduction and tropism.
ACKNOWLEDGMENTS
We thank José Luis for MAb 69-6-5; D. Cheresh for the CS-1, CS-1/β3, and CS-1/β5 cells; A. Abina for transduction of the PBMCs; K. Jooss for the human dendritic cells; and C. Laplace for the confocal microscopy.
Financial support was provided by the Association Française contre les Myopathies, Inserm (E.J.K.), EMBO (M.C.), the Association Française Lutte contre la Mucoviscidose (S.S.H.), the Fondation pour la Recherche Médicale (P.B. and S.S.H.), the Programme de Recherche Foundamentale en Microbiologie, Maladies Infectieuses et Parasitaires (P.B. and S.S.H.), and the National Institutes of Health and The American Heart Association (J.M.B.).
REFERENCES
- 1.Arnberg N, Edlund K, Kidd A H, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- 2.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey A, Mautner V. Phylogenetic relationships among adenovirus serotypes. Virology. 1994;205:438–452. doi: 10.1006/viro.1994.1664. [DOI] [PubMed] [Google Scholar]
- 4.Baker A, Saltik M, Lehrmann H, Killisch I, Mautner V, Lamm G, Christofori G, Cotten M. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997;4:773–782. doi: 10.1038/sj.gt.3300471. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson J M, Cunningham J, Droguett G, Kurt E, Krithivas A, Hong J, Horwitz M, Crowell R, Finberg R. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bewley M C, Springer K, Zhang Y B, Freimuth P, Flanagan J M. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 8.Cheresh D A, Spiro R C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 9.Chiu C Y, Mathias P, Nemerow G R, Stewart P L. Structure of adenovirus complexed with its internalization receptor, αvβ5 integrin. J Virol. 1999;73:6759–6768. doi: 10.1128/jvi.73.8.6759-6768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crompton J, Toogood C I, Wallis N, Hay R T. Expression of a foreign epitope on the surface of the adenovirus hexon. J Gen Virol. 1994;75:133–139. doi: 10.1099/0022-1317-75-1-133. [DOI] [PubMed] [Google Scholar]
- 11.Defer C, Belin M T, Caillet-Boudin M L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietz A B, Vuk-Pavlovic S. High-efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood. 1998;91:392–398. [PubMed] [Google Scholar]
- 13.Farishian R A, Whittaker J R. Tyrosine utilization by cultured melanoma cells: analysis of melanin biosynthesis using [14C]tyrosine and [14C]thiouracil. Arch Biochem Biophys. 1979;198:449–461. doi: 10.1016/0003-9861(79)90519-8. [DOI] [PubMed] [Google Scholar]
- 14.Fasbender A, Lee J, Walters R, Moninger T, Zabner J, Welsh M J. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J Clin Investig. 1998;102:184–193. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss H P, Lamers J, Poller W. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 16.Flomenberg P, Piaskowski V, Truitt R L, Casper J T. Characterization of human proliferative T cell response to adenovirus. J Infect Dis. 1995;171:1090–1096. doi: 10.1093/infdis/171.5.1090. [DOI] [PubMed] [Google Scholar]
- 17.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 18.Henry L J, Xia D, Wilke M E, Deisenhofer J, Gerard R D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann C, Loser P, Cichon G, Arnold W, Both G W, Strauss M. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J Virol. 1999;73:6930–6936. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwitz M S. Adenoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Raven Press; 1996. pp. 2149–2171. [Google Scholar]
- 22.Hsu K-H, Lonberg-Holm K, Alstein B, Crowell R L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Endo R I, Nemerow G R. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries M J. Integrin cell adhesion receptors and the concept of agonism. Trends Pharmacol Sci. 2000;21:29–32. doi: 10.1016/s0165-6147(99)01410-8. [DOI] [PubMed] [Google Scholar]
- 26.Kirby I, Davison E, Beavil A J, Soh C P, Wickham T J, Roelvink P W, Kovesdi I, Sutton B J, Santis G. Identification of contact residues and definition of the CAR-binding site of adenovirus type 5 fiber protein. J Virol. 2000;74:2804–2813. doi: 10.1128/jvi.74.6.2804-2813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komoriya A, Green L J, Mervic M, Yamada S S, Yamada K M, Humphries M J. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991;266:15075–15079. [PubMed] [Google Scholar]
- 28.Kremer E J, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol. 2000;74:505–512. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann M, Rabenandrasana C, Tamura R, Lissitzky J C, Quaranta V, Pichon J, Marvaldi J. A monoclonal antibody inhibits adhesion to fibronectin and vitronectin of a colon carcinoma cell line and recognizes the integrins alphavbeta3, alphavbeta5, and alphavbeta6. Cancer Res. 1994;54:2102–2107. [PubMed] [Google Scholar]
- 30.Leopold P L, Ferris B, Grinberg I, Worgall S, Hackett N R, Crystal R G. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 31.Makarem R, Humphries M J. LDV: a novel cell adhesion motif recognized by the integrin alpha4beta1. Biochem Soc Trans. 1991;19:380S. doi: 10.1042/bst019380s. [DOI] [PubMed] [Google Scholar]
- 32.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michou A I, Lehrmann H, Saltik M, Cotten M. Mutational analysis of the avian adenovirus CELO, which provides a basis for gene delivery vectors. J Virol. 1999;73:1399–1410. doi: 10.1128/jvi.73.2.1399-1410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittal S K, Prevec L, Graham F, Babiuk L. Development of a bovine adenovirus type 3-based expression vector. J Gen Virol. 1995;76:93–102. doi: 10.1099/0022-1317-76-1-93. [DOI] [PubMed] [Google Scholar]
- 35.Mittereder N, March K, Trapnell B C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quillet A, Presse F, Marchiol-Fournigault C, Harel-Bellan A, Benbunan M, Ploegh H, Fradelizi D. Increased resistance to non-MHC-restricted cytotoxicity related to HLA A, B expression. Direct demonstration using beta2-microglobulin-transfected Daudi cells. J Immunol. 1988;141:17–20. [PubMed] [Google Scholar]
- 38.Rasmussen U, Schlesinger Y, Pavirani A, Mehtali M. Sequence analysis of the canine adenovirus 2 fiber-encoding gene. Gene. 1995;159:279–280. doi: 10.1016/0378-1119(95)00110-r. [DOI] [PubMed] [Google Scholar]
- 39.Rea D, Schagen F H, Hoeben R C, Mehtali M, Havenga M J, Toes R E, Melief C J, Offringa R. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J Virol. 1999;73:10245–10253. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts M M, White J L, Grutter M G, Burnett R M. Three-dimensional structure of the adenovirus major coast protein hexon. Science. 1986;232:1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- 41.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roelvink P W, Mi Lee G, Einfeld D A, Kovesdi I, Wickham T J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 44.Shenk T. Adenoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Raven Publishers; 1996. pp. 2111–2148. [Google Scholar]
- 45.Sheppard M, Werner W, Tsatas E, McCoy R, Prowse S, Johnson M. Fowl adenovirus recombinant expressing VP2 of infectious bursal disease virus induces protective immunity against bursal disease. Arch Virol. 1998;143:915–930. doi: 10.1007/s007050050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonnenberg A. Integrins and their ligands. Curr Top Microbiol Immunol. 1993;184:7–35. doi: 10.1007/978-3-642-78253-4_2. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson S, Rollence M, White B, Weaver L, McClelland A. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J Virol. 1995;69:2850–2857. doi: 10.1128/jvi.69.5.2850-2857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevenson S C, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart P L, Burnett R M, Cyrklaff M, Fuller S D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- 50.Svensson U, Persson R. Entry of adenovirus 2 into HeLa cells. J Virol. 1984;51:687–694. doi: 10.1128/jvi.51.3.687-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas L, Chan P W, Chang S, Damsky C. 5-Bromo-2-deoxyuridine regulates invasiveness and expression of integrins and matrix-degrading proteinases in a differentiated hamster melanoma cell. J Cell Sci. 1993;105:191–201. doi: 10.1242/jcs.105.1.191. [DOI] [PubMed] [Google Scholar]
- 52.Tiemessen C T, Kidd A H. The subgroup F adenoviruses. J Gen Virol. 1995;76:481–497. doi: 10.1099/0022-1317-76-3-481. . (Erratum, 76:2413.) [DOI] [PubMed] [Google Scholar]
- 53.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toogood C I, Crompton J, Hay R T. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol. 1992;73:1429–1435. doi: 10.1099/0022-1317-73-6-1429. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Bergelson J M. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wickham T J, Filardo E J, Cheresh D A, Nemerow G. Integrin alphavbeta5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wickham T J, Mathais P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 59.Willcox N, Mautner V. Antigenic determinants of adenovirus capsids. II. Homogeneity of hexons, and accessibility of their determinants, in the virion. J Immunol. 1976;116:25–29. [PubMed] [Google Scholar]
- 60.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high-affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]