Abstract
We report the case of a 14‐year‐old patient with a known history of Crohn's disease who was incidentally diagnosed with an asymptomatic cecal lipoma. A routine surveillance colonoscopy as part of the management of the patient's Crohn's Disease revealed a well‐defined, submucosal, yellowish mass in the patient's cecum. Histopathological examination of a biopsy specimen revealed submucosal adipose tissue, consistent with the endoscopic images showing the characteristic appearance of the lipoma. A computed tomography examination further confirmed the diagnosis. While colonic lipomas are infrequent and typically manifest later in life, few cases report the coexistence of a cecal lipoma with Crohn's disease, particularly in the pediatric population. In this case, managing this dual condition posed a notable challenge. Here, we present the conservative approach to managing a pediatric patient with cecal lipoma and Crohn's disease. The decision to leave the lipoma in situ was based on the absence of symptoms and potential risks associated with surgical removal.
Keywords: cecum, Crohn's Disease, Gastrointestinal, Lipoma, Pediatric
1. INTRODUCTION
Colonic lipomas are rare, benign mesenchymal tumors of the gastrointestinal system that typically present later in life, predominantly in women, and are often incidentally found during colonoscopy. 1 The pathogenesis of lipomas remains unknown, with possible links to previous trauma and genetic predisposition. Colonic lipomas are seen in just 0.2%–4.4% of autopsies, with cecal manifestations occurring in <20% of cases. 2 Although typically asymptomatic when smaller than 2 cm in diameter, larger cecal lipomas can cause rectal bleeding, abdominal pain, intussusception, and bowel obstruction. 3 The coexistence of cecal lipomas and Crohn's disease is a rare occurrence. In this case report, we present a teenager with Crohn's disease who was incidentally diagnosed with an asymptomatic cecal lipoma, highlighting the challenges in its management.
2. CASE REPORT
A 14‐year‐old female patient with a known history of Crohn's disease predominantly in the esophagus, ascending colon, ileocecal valve, and terminal ileum presented for a surveillance colonoscopy following her diagnosis of Inflammatory Bowel Disease (IBD) 1 year prior. The patient was currently in remission with azathioprine and adalimumab maintenance therapy.
During the colonoscopy, an incidental finding of a well‐defined, submucosal, yellowish mass was observed in the cecum (Figure 1). The mass appeared asymptomatic, had no ulcerations, and exhibited no signs of luminal obstruction or intussusception. Given the large mass size, the patient's young age, and the absence of symptoms, a decision was made to perform biopsies rather than proceed with immediate endoscopic resection.
Figure 1.
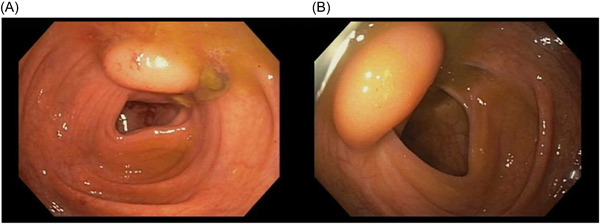
A wide‐based, smooth, dome‐shaped lesion with normal overlying mucosa (A) and minimal lumen distortion located just proximal to the ileocecal valve (B). This lesion exhibited the “pillow sign” in which the tip of the colonoscope compressed the lipoma, causing it to flatten and promptly regain its original shape upon pressure release.
Histopathological examination of the biopsy specimen suggested the diagnosis of a cecal lipoma. The examination revealed a submucosal proliferation of mature adipocytes coursed by paucicellular fibrous septa, consistent with the endoscopic images showing the characteristic appearance of the lipoma (Figure 2). A computed tomography (CT) scan of the abdomen and pelvis confirmed the finding, revealing a small, encapsulated lesion of fat attenuation (1.6 cm) associated with the ileocecal valve (Figure 3). Given the lipoma's benign nature and the absence of obstructive symptoms, the decision was made to treat conservatively and closely monitor the patient's condition.
Figure 2.
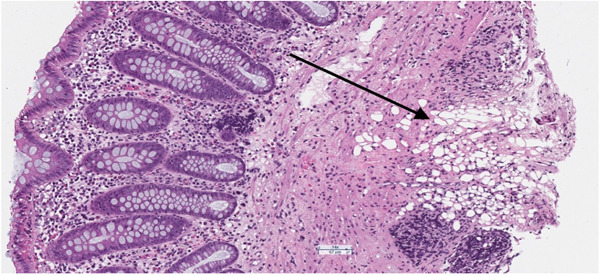
Hematoxylin and Eosin Stain ×10. Submucosal adipocytes extending into the muscularis mucosa consistent with a colonic lipoma.
Figure 3.
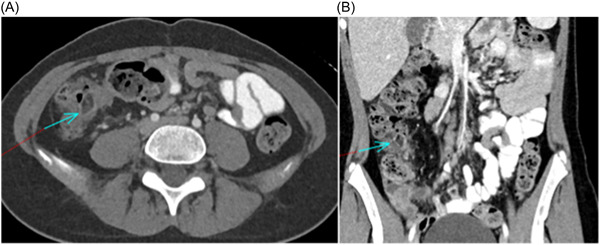
Axial (A) and coronal (B) CT images through the abdomen with ellipsoid low attenuation area in the cecum surrounded by inflammatory bowel wall changes and internal luminal stool.
3. DISCUSSION
Gastrointestinal lipomas are most likely found within the stomach, small bowel, and esophagus. 1 Whereas lipomas of the gastrointestinal tract are uncommon, colonic manifestations of such lipomas are even less common, with a reported incidence between 0.035% and 0.400%. 4 Colonic lipomas typically occur in the sixth and seventh decades of life, but rarely in children. 5 The coexistence of cecal lipomas and Crohn's disease is thus highly uncommon, especially in pediatric patients, and the management approach can be challenging.
The precise mechanisms behind the development of subcutaneous lipomas remain unclear. Nonetheless, previous theories have proposed a connection between soft tissue injury and the formation of lipomas. These theories suggest that either pseudolipomas may form due to the herniation of adipose tissue or preadipocytes may proliferate in response to cytokine release. 1 Increased inflammation and cytokine release in the setting of IBD may thus play a role in development of lipomata; however, this relationship has yet to be developed in literature. More likely, cecal lipomas and IBD may coexist in a patient without one directly causing or influencing the other. Thus, both conditions could be incidental findings. Of note, Siegal and Witz report two cases of large subserosal lipoma associated with Crohn's Disease in elderly women. 6 Thompson reports a case of a 50‐year‐old man with diffuse small‐intestinal lipomatosis and underlying Crohn's Disease. 7 In both cases, patients underwent surgical resection. Additionally, Lapsia et al. report a case of rectal lipoma in a 16‐year‐old female with Crohn's disease measuring 1.5 cm in diameter and treated with excision. 8 The decision to excise was based upon the concern for premalignant or malignant changes.
Differential diagnosis of cecal lipoma include pseudopolyps and sarcolipoma. In the setting of Crohn's disease, polypoid masses in the colon are most often identified as psudopolyps. 8 Psuedopolyps exhibit a firm, coarse texture, setting them apart from lipomas, which are commonly characterized by well‐defined, spherical shapes and a soft consistency. It is also important to distinguish lipoma from sarcolipoma as the latter poses a significant threat to life. Unlike sarcolipoma, lipoma typically exhibits a “cushion sign,” when pressing into the mass results in pillowing. 1 The “tenting sign” manifests when pulling a section of the tumor results in a tent‐like shape. However, the gold standard of differentiation continues to be biopsy with the “naked fat sign” being identified by the presence of mature fat cells in the biopsy specimen of the lipomatous tumor. 1 , 9 , 10 Radiographic evidence suggesting sarcolipoma as opposed to lipoma includes considerable lesion size, the existence of thick septa, the presence of nodular and/or globular areas resembling nonadipose masses, and a reduced proportion of fat content. 11
Colonic lipomas have been reported to become symptomatic after reaching a diameter greater than 2 cm with lipomas greater than 4 cm always producing associated symptoms. 9 Common symptoms include pain and diarrhea with possibility for subacute obstruction and intussusception. 12 Excision is typically warranted with lipoma greater than 2 cm. 10 Whereas sarcomatous change in cecal lipoma has never been reported, 3 growth of such lipomas over time have been reported to cause symptoms including intussusception. 13
In the present case, the patient with Crohn's disease was incidentally diagnosed with an asymptomatic cecal lipoma during colonoscopy. Considering the patient's young age, the absence of symptoms, size of lipoma <2 cm, and the desire to preserve bowel integrity in a patient with IBD, a decision was made to perform a biopsy and leave the lipoma in place. While surgical resection is considered the definitive treatment for symptomatic or complicated colonic lipomas, managing asymptomatic lipomas less than 2 cm remains controversial due to risk of bleeding, infection, and bowel perforation associated with intervention. 14 A systematic review by Crocetti et al. found an 8% complication rate associated with endoscopic excision of lipoma. 9 Additionally, the presence of IBD increases the risks associated with surgical intervention due to the inflamed and weakened state of the intestinal tissues in patients with Crohn's disease experiencing higher rates of postoperative complications. 15 However, surgical resection may be indicated when the possibility of malignancy cannot be excluded. Katsinelos et al. report a case of a 63‐year‐old woman who underwent hemicolectomy following an incidental finding of cecal lipoma with pseudomalignant features. 4
In the presented case, a biopsy of the lipoma allowed confirmation of the diagnosis and ruled out any malignancy or inflammatory changes associated with Crohn's disease. The decision to leave the lipoma in situ was based upon the absence of symptoms, small size <2 cm, and the potential risk associated with endoscopic removal. As of this report, no standard of care guidelines exists regarding long‐term monitoring of colonic lipoma. Regular follow‐up and imaging studies were planned to evaluate the lipoma for any changes or development of symptoms in concordance with the patient's Crohn's disease treatment plan. Removal should be considered if the lipoma is found to grow larger than 2 cm, become symptomatic, or begin to cause concern for obstruction or intussusception. 9
4. CONCLUSION
The coexistence of cecal lipomas and Crohn's disease is a rare occurrence, particularly in pediatric patients. In this case, the incidental finding of an asymptomatic cecal lipoma in a teenager with Crohn's disease presented a management challenge. The decision to perform a biopsy and leave the lipoma in place was based on the absence of symptoms, the patient's young age, and the desire to minimize the potential risks associated with surgical intervention in the setting of Crohn's disease.
Long‐term monitoring and regular follow‐up are essential to evaluate lipoma for any changes or the development of new symptoms. Further research and larger case studies are warranted to better understand the management and outcomes of cecal lipomas in patients with concurrent Crohn's disease.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Informed consent was obtained from the parents to publish this work.
ACKNOWLEDGMENTS
The authors have no funding to report.
Shargo RA, Ekblad M, Baran JV, et al. Concurrent cecal lipoma and Crohn's disease in a pediatric patient: a conservative approach. JPGN Rep. 2024;5:158‐161. 10.1002/jpr3.12061
REFERENCES
- 1. Erginoz E, Uludag SS, Cavus GH, Zengin K, Ozcelik MF. Clinicopathological features and management of colonic lipomas: case reports. Medicine (Baltimore). 2022;101(10):e29004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasso E, Guastella T. Giant submucosal lipoma cause colo‐colonic intussusception. Ann Ital Chir. 2012;83(6):559‐562. [PubMed] [Google Scholar]
- 3. Sekhar S, Venishetty H, Saritha C, Ali M. Large intestinal lipoma: a case report and review of literature. Int Surg J. 2016;3:985‐987. [Google Scholar]
- 4. Katsinelos P, Chatzimavroudis G, Zavos C, et al. Cecal lipoma with pseudomalignant features: a case report and review of the literature. World J Gastroenterol. 2007;13(17):2510‐2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nallamothu G, Adler DG. Large colonic lipomas. Gastroenterol Hepatol (NY). 2011;7(7):490‐492. [PMC free article] [PubMed] [Google Scholar]
- 6. Siegal A, Witz M. Gastrointestinal lipoma and malignancies. J Surg Oncol. 1991;47(3):170‐174. [DOI] [PubMed] [Google Scholar]
- 7. Thompson WM. Imaging and findings of lipomas of the gastrointestinal tract. Am J Roentgenol. 2005;184(4):1163‐1171. [DOI] [PubMed] [Google Scholar]
- 8. Lapsia S, Khlevner J, Morganstern J, Chawla A. Rectal lipoma in a pediatric patient with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56(5):e36‐7. [DOI] [PubMed] [Google Scholar]
- 9. Crocetti D, Sapienza P, Sterpetti AV, et al. Surgery for symptomatic colon lipoma: a systematic review of the literature. Anticancer Res. 2014;34(11):6271‐6276. [PubMed] [Google Scholar]
- 10. Jiang L, Jiang LS, Li FY, et al. Giant submucosal lipoma located in the descending colon: a case report and review of the literature. World J Gastroenterol. 2007;13(42):5664‐5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well‐differentiated liposarcoma. Radiology. 2002;224(1):99‐104. [DOI] [PubMed] [Google Scholar]
- 12. Geraci G, Pisello F, Arnone E, Sciuto A, Modica G, Sciumè C. Endoscopic resection of a large colonic lipoma: case report and review of literature. Case Rep Gastroenterol. 2010;4(1):6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siu S, Oliphant R, Benstock S, Keshava A, Rickard MJFX. Colonic lipoma causing intussusception: a case for colonoscopic surveillance? ANZ J Surg. 2019;89(4):428‐430. [DOI] [PubMed] [Google Scholar]
- 14. Ivekovic H, Rustemovic N, Brkic T, Ostojic R, Monkemuller K. Endoscopic ligation (“Loop‐And‐Let‐Go”) is effective treatment for large colonic lipomas: a prospective validation study. BMC Gastroenterol. 2014;14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakurai Kimura CM, Scanavini Neto A, Queiroz NSF, et al. Abdominal surgery in Crohn's disease: risk factors for complications. Inflamm Intest Dis. 2021;6(1):18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]


