Abstract
Introduction
Trials evaluating the role of intravascular imaging in percutaneous coronary intervention (PCI) for complex coronary artery disease have yielded mixed results. This study aimed to compare the outcomes of intravascular imaging specifically intravascular ultrasound (IVUS) with those from conventional coronary angiography in complex PCI.
Methods
Comprehensive electronic search of MEDLINE, EMBASE, and Cochrane databases was performed until March 2023 for randomized clinical trials (RCTs) comparing intravascular imaging with coronary angiography in patients undergoing complex PCI. Complex PCI was defined per each study, and included PCI for American College of Cardiology/American Heart Association (ACC/AHA) type B2/C lesions, unprotected left main coronary artery disease, or multivessel stenting. The primary study outcome was major adverse clinical events (MACE).
Results
The meta-analysis included 10 RCTs with a total of 6615 patients (3576 in the intravascular imaging group and 3039 in the coronary angiography group). The weighted mean-follow up was 28.9 months. Compared with coronary angiography, intravascular imaging reduced MACE (8% vs. 13.3%; relative risk [RR] 0.63; 95% confidence interval [CI] 0.54–0.73), cardiac death (RR 0.47; 95% CI 0.31–0.73), definite/probable stent thrombosis (RR 0.48; 95% CI 0.24–0.97), target vessel revascularization (RR 0.62; 95% CI 0.46–0.83), and target lesion revascularization (RR 0.61; 95% CI 0.47–0.79). There was no difference between both groups in all-cause death (RR 0.79; 95% CI 0.53–1.18) and myocardial infarction (RR 0.80; 95% CI 0.61–1.04).
Conclusion
In patients undergoing complex PCI, intravascular imaging—specifically IVUS—reduced MACE by decreasing the incidence of cardiac death, stent thrombosis, and target vessel and target lesion revascularization.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40119-024-00364-7.
Keywords: Intravascular imaging, IVUS, Complex PCI, PCI, CA
Key Summary Points
| Why carry out this study? |
| The role of routine use of intravascular imaging in complex percutaneous coronary intervention (PCI) remains unclear. |
| Our study aimed to compare the outcomes of intravascular imaging (specifically intravascular ultrasound) with conventional coronary angiography in complex percutaneous coronary intervention (PCI). |
| What was learned from the study? |
| Complex PCI guided by intravascular imaging reduced the risk of major adverse cardiac events, cardiac death, definite/probable stent thrombosis, and target vessel and target lesion revascularization compared with coronary angiography. |
| Further efforts should be directed towards identifying the barriers behind the low use of intravascular imaging especially in complex coronary artery interventions. |
Introduction
Despite evolutions in the development of drug-eluting stents (DES) and technical advances in equipment, percutaneous coronary intervention (PCI) for complex coronary anatomy continues to pose a significant challenge. According to the American College of Cardiology/American Heart Association (ACC/AHA) lesion morphology classification, class B2 and C lesions are considered to represent complex anatomy, and include features such as ostial location, extensive calcification, chronic total occlusion (CTO), or long diffuse lesions. Complex PCI, including PCI for patients with complex coronary lesions, unprotected left main (LM) coronary artery disease, or multivessel disease, is associated with worse clinical outcomes due to the high risk of complications and higher rates of target lesion failure [1–7]. Intravascular imaging, using intravascular ultrasound (IVUS) or optical coherence tomography (OCT), was developed to overcome the limitations of conventional coronary angiography [8, 9]. Intravascular imaging enables meticulous assessment of coronary vessels and provides detailed information on the blood vessel wall, coronary plaque, and stent morphological characteristics; thus it enables a patient-tailored approach when managing patients with coronary artery disease (CAD) [10]. Yet, intravascular imaging is still not widely used in real-world clinical practice in part because of lack of experience in interpreting images, prolonged procedure times, and concerns about reimbursement [9, 11]. The use of intravascular imaging has been recommended by major scientific cardiology societies, to guide and optimize stent implantation in selected cases including complex PCI [12–14]. Several randomized clinical trials (RCTs) have evaluated the role of intravascular imaging compared with coronary angiography for guiding complex PCI [15–24]; however, many studies were underpowered. Therefore, we performed a systematic review and meta-analysis of RCTs comparing the outcomes of intravascular imaging-guided versus coronary angiography-guided complex PCI.
Methods
Data Sources and Search Strategy
A comprehensive electronic search of MEDLINE, EMBASE, and Cochrane databases was performed through March 2023 for RCTs that compared the safety and efficacy of intravascular imaging with either IVUS or OCT compared with coronary angiography in complex PCI. The following search terms were used: “intravascular imaging” OR “IVUS” OR “coronary angiography” OR “DES” AND “CAD” OR “coronary artery disease”. Additional screening of the bibliographies of the retrieved articles, ClinicalTrials.gov, and prior meta-analyses to identify other related studies that did not appear in the initial search. This study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25] (Supplemental Table 1) and the details of the systemic review were prospectively registered at PROSPERO (ID 411453).
Selection Criteria
This study included RCTs that evaluated the safety and efficacy of intravascular imaging versus coronary angiography in complex PCI. Complex PCI was defined as per each study (Supplemental Table 2). Only studies conducted in human subjects were included and there was no language restriction. Conference abstracts, review articles, case reports, and cohort and non-randomized trials were excluded.
Data Extraction
Data that met the inclusion criteria were extracted by two investigators independently (MH and SM) which included the study features, baseline characteristics, and clinical outcomes. Any discrepancy between investigators was resolved by consensus.
Outcomes
The study’s primary outcome was major adverse cardiac events (MACE) as defined by each individual study (Supplemental Table 3). The secondary outcomes included cardiac death, all-cause death, definite/probable stent thrombosis, target vessel revascularization (TVR), target lesion revascularization (TLR), myocardial infarction (MI), post-procedural minimal luminal diameter (MLD), procedural time, and fluoroscopy time. Definite/probable stent thrombosis was defined according to the Academic Research Consortium (ARC) [26, 27]. MI was defined per each study (Supplemental Table 4). Clinical outcomes were reported with the longest follow-up period and on an intention-to-treat basis.
Assessment of Quality of Included Studies
The Cochrane bias risk assessment tool was used to evaluate the quality of the included trials, which included various criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias [28]. Studies were then classified into low risk, high risk, or unclear risk of bias (Supplemental Table 5).
Statistical Analysis
Data were pooled by using random effects model utilizing the Mantel–Haenszel method. I2 statistic was used to assess the statistical heterogeneity among the included studies. I2 values of less than 25% were considered low degree of heterogeneity, 25–50% were considered moderate degree of heterogeneity, and greater than 50% were considered a high degree of heterogeneity [29]. Outcomes were reported as risk ratios (RR) for categorical variables and mean differences (MD) for continuous variables. The following sensitivity analyses were conducted: excluding studies with high risk of bias, including studies with consistent MACE definitions, including studies with consistent follow-up at 1- and 2-years outcome, and including studies exclusively using second-generation DES. Subgroup analyses including studies reporting LM coronary artery PCI and CTO PCI were also conducted. P values less than 0.05 were considered significant. Publication bias was assessed by using funnel plots. Statistical analyses were conducted using RevMan 5.4 software (Cochrane Collaboration, Oxford, UK).
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Included Studies
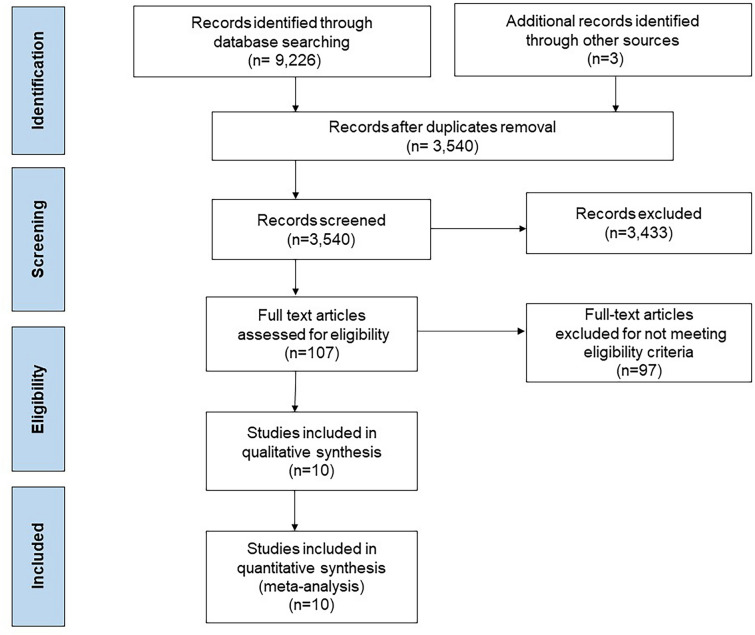
The detailed study selection process is shown in Fig. 1. The final analysis included 10 RCTs with a total of 6615 patients: 3576 in the intravascular imaging group and 3039 in the coronary angiography group [15–24]. The characteristics of the included studies are outlined in Tables 1 and 2. The baseline coronary angiographic data are shown in Table 3. The weighted mean follow-up was 28.9 months. The weighted mean age was 64.9 years, and 73.3% of the patients were men. Complex PCI was defined per each study (Supplemental Table 2), and included PCI for type B2/C lesions, unprotected LM coronary artery disease, or multivessel stenting. Most included studies included only patients undergoing complex PCI [15–19, 21–24], while ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions) included patients undergoing both complex and non-complex PCI [20]. HOME DES IVUS, Tan et al., and Liu et al. were single-center studies [15, 22, 24], while all other studies were multicenter [16–21, 23]. The quality of included studies appears in Supplemental Table 5. All of the included studies were open-label [15–24]. The HOME DES IVUS and Tan et al. studies had unclear risk of outcome assessment bias [15, 22]. In addition, Tan et al. had unclear risk of allocation bias [22]. The other studies were considered to be at low risk for bias. Inspection of the funnel plot suggested no evidence of publication bias (Supplemental Fig. 1).
Fig. 1.
Study flowsheet
Table 1.
Characteristics of the included studies
| Study | Year of publication | No. of centers | Country | Group 1 (intravascular imaging-guided) | Group 2 (coronary-guided angiography) | Longest follow-up duration | Inclusion criteria | Primary outcome | Stent type |
|---|---|---|---|---|---|---|---|---|---|
| HOME DES IVUS | 2010 | Single-center | Czech Republic | 105 | 105 | 18 months | Complex coronary lesions or complex patient characteristics such as type B2 and C according to the American Heart Association, proximal left anterior descending artery, left main disease, reference vessel diameter < 2.5 mm, lesion length > 20 mm, in-stent restenosis, insulin-dependent diabetes mellitus, and acute coronary syndrome were included in this study | To assess the role of IVUS guidance during implantation of DES on long-term outcome in patients with high clinical and angiographic | CYPHER (sirolimus-eluting stents) and TAXUS (paclitaxel-eluting stents) |
| Kim et al. [23] | 2013 | Multicenter | South Korea | 269 | 274 | 12 months | Age > 20 years and had a de novo lesion requiring a stent ≥ 28 mm in length in a vessel with a distal reference diameter ≥ 2.5 mm by visual angiographic estimation | MACE | Endeavor Sprint zotarolimus-eluting stents (E-ZES) or everolimus-eluting stent (EES) (Xience V, Abbott Vascular, Santa Clara, California) |
| AVIO | 2013 | Multicenter | Italy | 142 | 142 | 24 months | Complex lesions defined as one of the following: long length (> 28 mm); CTO, i.e., a total occlusion of duration more than 3 months; lesions involving a bifurcation; small vessels (≤ 2.5 mm) and patients requiring 4 or more stents | Post-procedure in lesion minimal lumen diameter | DES |
| AIR-CTO | 2015 | Multicenter | China | 115 | 115 | 2 years | Patients aged 18–80 years, who had at least one CTO lesion (defined as TIMI grade 0 and occlusion duration > 3 months) that had been successfully recanalized (defined as a wire-crossed CTO lesion and at the distal true lumen according to angiograms) | In-stent late lumen loss at 1-year follow-up | Either first- or second-generation DES |
| Tan et al. [22] | 2015 | Single-center | China | 61 | 62 | 2 years | Unprotected left main coronary artery, defined as at least 50% stenosis by visual assessment in the LM vessel without bypass grafts to the left anterior descending artery or left circumflex artery | MACE | Sirolimus-eluting stents (Firebird-2, Microport, Shanghai, China) or sirolimus-eluting stents (Excel, Jiwei, Shandong, China) |
| CTO-IVUS | 2015 | Multicenter | South Korea | 201 | 201 | 12 months | Patients with CTO who were aged 20–80 years and had typical symptomatic angina or positive test results for functional evaluation of ischemia | Cardiac death | Zotarolimus-eluting stents or Nobori biolimus-eluting stents |
| Liu et al. [24] | 2019 | Single-center | China | 167 | 169 | 12 months |
Adults, aged 18–75 years Unprotected left main coronary artery lesions and plan for DES implantation |
MACE, defined as cardiac death, MI, or TVR | DES |
| IVUS-XPL | 2020 | Multicenter | South Korea | 700 | 700 | 5 years | Patients with typical chest pain or evidence of myocardial ischemia were eligible for enrollment if implantation of an everolimus-eluting stent for a long coronary lesion (implanted stent ≥ 28 mm in length) was indicated on the basis of angiographic lesion length estimation | MACE, defined as cardiac death, target lesion-related MI, or ischemia-driven TLR at 5 years | Everolimus-eluting stent |
| ULTIMATE | 2021 | Multicenter | China | 724 | 724 | 3 years | Patients with silent ischemia, stable or unstable angina, or MI with more than 24 h between onset of chest pain and admission and de novo coronary lesions requiring DES implantation | TVF at 3 years after the index procedure, including cardiac death, target vessel MI, and clinically driven TVR | Second-generation DES |
| RENOVATE-COMPLEX-PCI | 2023 | Multicenter | South Korea | 1092 | 547 | 3 years | Patients 19 years of age or older who were undergoing PCI for complex coronary artery lesions, defined as true bifurcation lesions according to the Medina classification system with a side-branch diameter of at least 2.5 mm; a chronic total occlusion; unprotected left main coronary artery disease; long coronary artery lesions that would involve an expected stent length of at least 38 mm; multivessel PCI involving at least two major epicardial coronary arteries being treated at the same time; a lesion that would necessitate the use of multiple stents (at least three planned stents); a lesion involving in-stent restenosis; a severely calcified lesion; or ostial lesions of a major epicardial coronary artery | TVF, defined as the composite of death from cardiac causes, target vessel-related MI, or clinically driven TVR | Polymer-coated everolimus-eluting stents |
IVUS intravascular ultrasound, DES drug-eluting stent, CTO chronic total occlusion, MI myocardial infarction, MACE major adverse cardiac events, TLR target lesion revascularization, TVF target vessel failure, TVR target vessel revascularization, PCI percutaneous coronary intervention, TIMI thrombolysis in myocardial infarction, LM left main
Table 2.
Baseline characteristics of the studies population
| Studies | Groups | Age in years, mean (± SD) | Male % | Hypertension % | Diabetes mellitus % | Dyslipidemia % | Current smoking % | Previous PCI % | Previous MI % | Left ventricular ejection fraction % |
|---|---|---|---|---|---|---|---|---|---|---|
| HOME DES IVUS 2010 | Intravascular imaging-guided group | 59.4 ± 13 | 73 | 67 | 42 | 63 | 40 | 17 | 37 | – |
| Coronary angiography-guided group | 60.2 ± 11 | 71 | 71 | 45 | 66 | 35 | 14 | 32 | – | |
| Kim et al. [23] | Intravascular imaging-guided group | 62.8 ± 9.3 | 65.8 | 61.3 | 31.6 | 61.3 | 21.6 | – | 1.1 | 55.3 ± 23.9 |
| Coronary angiography-guided group | 64.3 ± 8.7 | 54.7 | 65.8 | 29.9 | 61.7 | 17.2 | – | 2.9 | 54 ± 25 | |
| AVIO 2013 | Intravascular imaging-guided group | 63.9 ± 10.1 | 82.4 | 70.4 | 23.9 | 70.4 | 34.5 | – | – | 55.3 ± 8.5 |
| Coronary angiography-guided group | 63.6 ± 11.0 | 76.8 | 66.9 | 26.8 | 76.8 | 31 | – | – | 55.9 ± 8.6 | |
| AIR-CTO 2015 | Intravascular imaging-guided group | 67 ± 10 | 88.7 | 74.8 | 29.6 | 21.9 | 39.1 | 20 | 20.9 | 55 ± 11 |
| Coronary angiography-guided group | 66 ± 11 | 80 | 70.4 | 27 | 27.8 | 39.1 | 20.9 | 30.4 | 56 ± 12 | |
| Tan et al. [22] | Intravascular imaging-guided group | 76.54 ± 4.95 | 62.3 | 41 | 34.4 | – | 44.3 | – | 16.4 | 55.32 ± 5.02 |
| Coronary angiography-guided group | 75.85 ± 3.49 | 69.3 | 46.8 | 29.5 | – | 46.8 | – | 21 | 53.33 ± 7.14 | |
| CTO-IVUS 2015 | Intravascular imaging-guided group | 61.0 ± 11.1 | 80.6 | 62.7 | 34.8 | – | 35.3 | 15.4 | 8 | 56.9 ± 13.1 |
| Coronary angiography-guided group | 61.4 ± 10.1 | 80.6 | 63.7 | 33.8 | – | 34.3 | 15.9 | 8 | 56.7 ± 11.4 | |
| Liu et al. [24] | Intravascular imaging-guided group | 65.3 ± 10.6 | 63.5 | 69.5 | 33.5 | 37.7 | 37.1 | 19.8 | 17.4 | 55.6 ± 11.7 |
| Coronary angiography-guided group | 64.9 ± 11.2 | 63.9 | 72.2 | 30.8 | 37.9 | 35.5 | 16.6 | 14.2 | 58.4 ± 10.5 | |
| IVUS-XPL 2020 | Intravascular imaging-guided group | 63.0 ± 9.0 | 69 | 65 | 32 | 68 | 22 | 11 | 5 | 63 ± 9.8 |
| Coronary angiography-guided group | 64.0 ± 9.0 | 69 | 64 | 36 | 66 | 26 | 10 | 4 | 62.3 ± 10.2 | |
| ULTIMATE 2021 | Intravascular imaging-guided group | 65.2 ± 10.9 | 73.9 | 70.7 | 30 | 53.7 | – | – | – | – |
| Coronary angiography-guided group | 65.9 ± 9.8 | 73.2 | 72 | 31.2 | 55.2 | – | – | – | – | |
| RENOVATE-COMPLEX-PCI 2023 | Intravascular imaging-guided group | 65.3 ± 10.3 | 79.6 | 62.5 | 36.1 | 51.3 | 19.4 | 24.5 | 6.9 | 58.4 ± 11.9 |
| Coronary angiography-guided group | 66.0 ± 10.0 | 78.8 | 59 | 40.8 | 51.2 | 17.4 | 23.2 | 7.7 | 59.3 ± 11.0 |
MI myocardial infarction, PCI percutaneous coronary intervention, SD standard deviation
Table 3.
Baseline quantitative coronary angiographic data of the studies population
| Studies | Groups | Coronary artery lesion | Reference vessel diameter (mm) [mean ± SD] | Min luminal diameter (mm) [mean ± SD] | Diameter stenosis % [mean ± SD] | Lesion length (mm) [mean ± SD] | |||
|---|---|---|---|---|---|---|---|---|---|
| LAD % | LCX % | RCA % | Left main % | ||||||
| HOME DES IVUS 2010 | Intravascular imaging-guided group | 56 | – | 29 | 3 | 3.17 ± 0.43 | 1.1 ± 0.40 | 82.3 ± 7.6 | 18.1 ± 7.3 |
| Coronary angiography-guided group | 54 | – | 24 | 4 | 2.95 ± 0.34 | 0.97 ± 0.37 | 79.2 ± 9.3 | 17.6 ± 6.7 | |
| Kim et al. [23] | Intravascular imaging-guided group | 62.1 | 15.2 | 22.7 | – | 2.82 (2.58–3.16)a | 0.95 (0.73–1.23)a | – | 29.6 (23.2–42.8)a |
| Coronary angiography-guided group | 67.5 | 12.8 | 19.7 | – | 2.80 (2.56–3.15)a | 0.93 (0.70–1.22)a | – | 30.6 (24.2–40.9)a | |
| AVIO 2013 | Intravascular imaging-guided group | 53.3 | – | – | – | 2.67 ± 0.46 | 0.76 ± 0.46 | 71.6 ± 15.8 | 27.4 ± 15.9 |
| Coronary angiography-guided group | 48.6 | – | – | – | 2.62 ± 0.41 | 0.65 ± 0.45 | 75.5 ± 16.1 | 25.5 ± 15.0 | |
| AIR-CTO 2015 | Intravascular imaging-guided group | 44.3 | 20.9 | 34.8 | 0 |
Proximal: 2.95 ± 0.37 Distal: 2.26 ± 0.41 |
– | – | 28.48 ± 17.76 |
| Coronary angiography-guided group | 36.5 | 14.8 | 46.1 | 2.6 |
Proximal: 2.89 ± 0.34 Distal: 2.25 ± 0.44 |
– | – | 29.21 ± 19.11 | |
| Tan et al. [22] | Intravascular imaging-guided group | – | – | – | 100 | – | – | – | – |
| Coronary angiography-guided group | – | – | – | 100 | – | – | – | – | |
| CTO-IVUS 2015 | Intravascular imaging-guided group | 41.8 | 14.4 | 43.8 | – | 2.69 ± 0.44 | – | – | 36.3 ± 17.1 |
| Coronary angiography-guided group | 46.8 | 15.9 | 37.3 | – | 2.64 ± 0.55 | – | – | 35.5 ± 17.0 | |
| Liu et al. [24] | Intravascular imaging-guided group | 55.7 | 44.3 | 62.3 | 100 | – | – | – | – |
| Coronary angiography-guided group | 52.7 | 49.7 | 58 | 100 | – | – | – | – | |
| IVUS-XPL 2020 | Intravascular imaging-guided group | 66 | 13 | 22 | – | 2.89 ± 0.46 | 0.83 ± 0.43 | 71.2 ± 14.4 | 34.9 ± 10.8 |
| Coronary angiography-guided group | 60 | 16 | 25 | – | 2.84 ± 0.45 | 0.82 ± 0.43 | 71.4 ± 14.4 | 35.2 ± 10.5 | |
| ULTIMATE 2021 | Intravascular imaging-guided group | – | – | – | – | – | – | – | – |
| Coronary angiography-guided group | – | – | – | – | – | – | – | – | |
| RENOVATE-COMPLEX-PCI 2023 | Intravascular imaging-guided group | 44.2 | 19.3 | 27.4 | 10.1 |
Proximal: 3.2 ± 0.5 Distal: 2.7 ± 0.5 |
0.44 ± 0.37 | 85.4 ± 11.5 | 28.4 ± 15.9 |
| Coronary angiography-guided group | 43.2 | 18.5 | 26.4 | 9 |
Proximal: 3.1 ± 0.5 Distal: 2.7 ± 0.4 |
0.44 ± 0.36 | 85.2 ± 11.7 | 26.8 ± 14.8 | |
LAD left anterior descending, LCX left circumflex, RCA right coronary artery, SD standard deviation
aMedian (interquartile range)
Primary Outcome
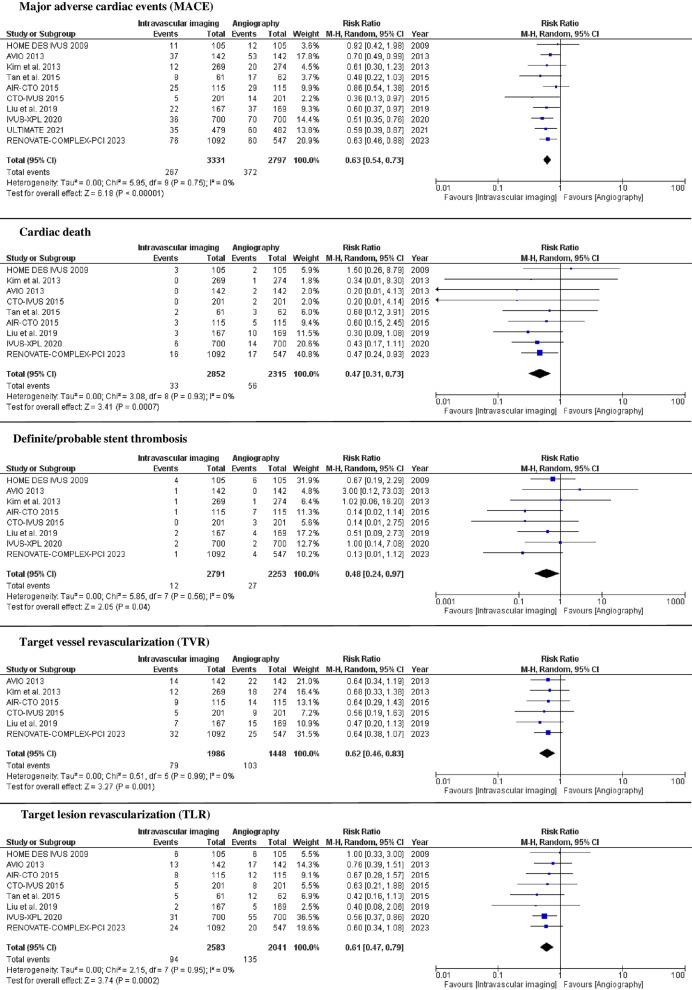
The primary outcome was reported in all included studies [15–24]. The definition of MACE was adopted per each study and was reported in Supplemental Table 3 [15–24]. Intravascular imaging reduced MACE compared with coronary angiography (8% vs. 13.3%; RR 0.63; 95% confidence interval [CI] 0.54 – 0.73), with low degree of heterogeneity (I2 = 0%) (Fig. 2). Sensitivity analyses excluding studies with high risk of bias (RR 0.63; 95% CI 0.54–0.73, I2 = 0%), excluding studies including OCT (RR 0.63; 95% CI 0.53–0.74, I2 = 0%), including studies with consistent MACE definition (i.e., composite of cardiac death, MI, or ischemia-driven repeat revascularization) (RR 0.64; 95% CI 0.54–0.74, I2 = 0%), including studies at 1-year follow-up (RR 0.64; 95% CI 0.47–0.86, I2 = 0%), including studies at 2-years follow-up (RR 0.71; 95% CI 0.55–0.93, I2 = 0%), and including studies exclusively using second-generation DES (RR 0.57; 95% CI 0.47–0.70, I2 = 0%) showed similar results (Supplemental Fig. 2). Subgroup analyses including studies reporting LM coronary artery PCI (RR 0.62; 95% CI 0.50–0.76, I2 = 0%) and CTO PCI (RR 0.66; 95% CI 0.55–0.79, I2 = 0%) showed similar results (Supplemental Fig. 4). Other subgroup analyses including patients undergoing IVUS (RR 0.64; 95% CI 0.55–0.74, I2 = 0%) and OCT (RR 0.49; 95% CI 0.28–0.85, I2 = 0%) showed similar results (Supplemental Fig. 4).
Fig. 2.
Forest plot for MACE, cardiac death, definite/probable stent thrombosis, target vessel revascularization, and target lesion revascularization among intravascular imaging versus coronary angiography groups. CI confidence interval, M–H Mantel–Haenszel
Secondary Outcomes
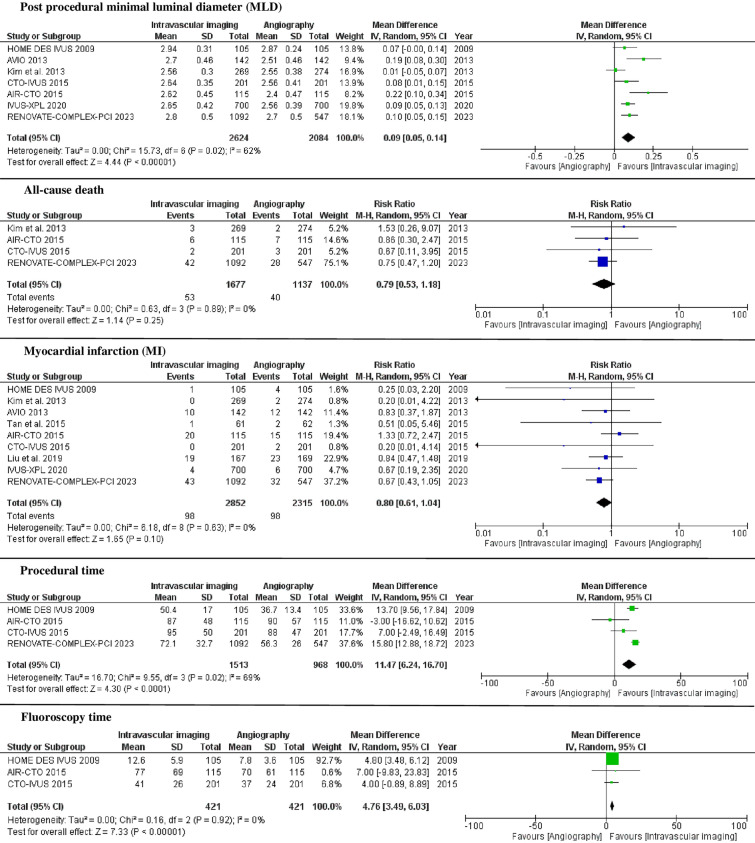
Compared with coronary angiography, intravascular imaging reduced the incidence of cardiac death (1.2% vs. 2.4%, RR 0.47; 95% CI 0.31–0.73; I2 = 0%), definite/probable stent thrombosis (0.4 vs. 1.2, RR 0.48; 95% CI 0.24–0.97; I2 = 0%), TVR (4% vs. 7.1%, RR 0.62; 95% CI 0.46–0.83; I2 = 0%), and TLR (3.6% vs. 6.6%, RR 0.61; 95% CI 0.47–0.79; I2 = 0%). Intravascular imaging also showed higher post-procedural MLD (MD 0.09; 95% CI 0.05–0.14; I2 = 62%) compared with angiography. There was no difference between intravascular imaging and coronary angiography groups in all-cause death (3.2% vs. 3.5%, RR 0.79; 95% CI 0.53–1.18; I2 = 0%) and MI (3.4% vs. 4.2%, RR 0.80; 95% CI 0.61–1.04; I2 = 0%). Intravascular imaging required longer procedural time (MD 11.47; 95% CI 6.24–16.70; I2 = 69%) and fluoroscopy time (MD 4.76; 95% CI 3.49–6.03; I2 = 0%) (Figs. 2, 3).
Fig. 3.
Forest plot for post-procedural minimal luminal diameter, all-cause death, MI, procedural time, and fluoroscopy time among intravascular imaging versus coronary angiography groups. CI confidence interval, IV inverse variance, M–H Mantel–Haenszel
Discussion
In this meta-analysis of 10 RCTs, including 6615 patients, we evaluated the role of intravascular imaging-guided versus angiography-guided complex PCI. The principal study findings are (1) compared with coronary angiography, complex PCI guided by intravascular imaging was associated with a lower risk of MACE; (2) this benefit was driven by a lower incidence of cardiac death, definite/probable stent thrombosis, and target vessel and target lesion revascularization; (3) there was no difference between angiography- or intravascular imaging-guided complex PCI in all-cause death or MI.
Intravascular imaging-guided PCI was compared with coronary angiography-guided PCI in prior meta-analyses [9, 30–34]. However, the present meta-analysis is the only one focusing on complex PCI. Prior individual RCTs have shown that the use of intravascular imaging was associated with a reduction of MACE in complex coronary artery lesions [15–19, 23]. Our analysis not only showed a decreased risk of MACE but also showed reduced risk of cardiac death, TVR, and TLR, and resulted in higher post-procedural MLD. Moreover, this current analysis suggested a numerical reduction in the incidence of MI that did not reach a statistically significant difference. In the current meta-analysis, we included the totality of available RCTs, including the recent RENOVATE-COMPLEX-PCI trial. RENOVATE-COMPLEX-PCI involved 1639 patients with a median follow-up of 2.1 years; it demonstrated that intravascular-guided imaging showed a lower risk of a composite of cardiac death, target vessel-related MI, or TVR/TLR that was consistent with prior study results. Moreover, RENOVATE-COMPLEX-PCI is the only study that included either IVUS or OCT for intravascular-guided imaging, while other studies used only IVUS [21].
Complex coronary artery lesions are challenging to manage and necessitate careful consideration of the best treatment strategy. Coronary angiography has some drawbacks as it provides only a 2-dimensional view of the complex 3-dimensional coronary artery lumen. It also lacks a detailed understanding of plaque morphology and vessel size [32]. There are different intravascular imaging modalities, including IVUS and OCT which are the most common and widely used intravascular imaging techniques. OCT can provide higher spatial resolution with better tissue characterization, while IVUS allows better tissue penetration that enables full-thickness visualization with lower resolution which helps the operator with decision-making in the PCI optimization [35, 36]. Both intravascular imaging techniques are complementary tools and the use of one of these tools depends on the individual’s expertise [37]. In addition, previous studies have shown that OCT was noninferior to IVUS [38]. The mechanism of intravascular imaging to improve outcomes is related to multiple factors. Intravascular imaging can provide a high-resolution cross-sectional image with detailed tomographic structural information of the anatomy of the coronary artery, such as plaque morphology and vessel size [9]. Furthermore, intravascular imaging encourages optimal coronary stent sizing while avoiding stent malposition and underexpansion [39, 40]. Moreover, it allows for the detection of complications such as edge dissections that may be missed with coronary angiography [41]. The use of intravascular imaging in calcific lesions is essential to assess the lesion morphology, as it can help quantify the calcium distribution and determine the need for atherectomy [42–44]. Intravascular imaging may also improve the safety and efficacy of atherectomy for calcific lesions [42–44]. The role of intravascular imaging use in LM coronary interventions has been robustly established, allowing assessment of disease distribution and plaque morphology that may help guide decisions around the need for an upfront two- versus one-stent approach [45, 46].
The inconsistent use of intravascular imaging amongst operators in routine clinical practice may be related to increased procedural time, operator experience, and concerns of higher costs related to intravascular imaging when compared with coronary angiography [47]. However, intravascular imaging has proven overall cost-effectiveness as it improves the overall burden on healthcare system by lowering costs for hospitalizations and urgent TVR [48, 49].
Our study had few limitations. First, studies included in the current analysis included various forms of complex coronary lesions and we could not ascertain outcome per types of complex lesions. Second, the use of OCT was evaluated only in one study, which might limit the generalizability of the study results to OCT. Third, the included studies used various types of DES which could alter the study outcomes, so we conducted a sensitivity analysis including studies exclusively using second-generation DES. Fourth, the mean follow-up time was 28.9 months; longer follow-up could alter the observed outcomes. Fifth, there was a lack of patient-level data that prohibited more granular analyses.
Conclusions
Among patients undergoing complex PCI, intracoronary imaging guidance reduced the risk of MACE compared with angiography guidance, an effect that was driven by reducing the incidence of cardiac death, definite/probable stent thrombosis, and target vessel and target lesion revascularization. Further efforts should be directed towards identifying the barriers behind the low use of intravascular imaging especially in complex coronary artery interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contribution
All authors including Mohamed Hamed, Sheref Mohamed, Mohamed Mahmoud, Jonathan Kahan, Amr Mohsen, Faisal Rahman, Waleed Kayani, Fernando Alfonso, Emmanuel S. Brilakis, Islam Y. Elgendy, Mamas A. Mamas, and Ayman Elbadawi contributed to the study conception and design, material preparation, data collection, statistical analysis, writing the article, critical revision of the article and final approval of the article.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Declarations
Conflict of Interest
Mohamed Hamed, Sheref Mohamed, Mohamed Mahmoud, Jonathan Kahan, Amr Mohsen, Faisal Rahman, Waleed Kayani, Fernando Alfonso, Emmanuel S. Brilakis, Islam Y. Elgendy, Mamas A. Mamas, and Ayman Elbadawi have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Suero JA, Marso SP, Jones PG, et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. 2001;38(2):409–414. doi: 10.1016/S0735-1097(01)01349-3. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Marso SP, Dai D, et al. Comparative effectiveness of drug-eluting versus bare-metal stents in elderly patients undergoing revascularization of chronic total coronary occlusions: results from the National Cardiovascular Data Registry, 2005–2008. JACC Cardiovasc Interv. 2012;5(10):1054–1061. doi: 10.1016/j.jcin.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Van den Branden BJ, Rahel BM, Laarman GJ, et al. Five-year clinical outcome after primary stenting of totally occluded native coronary arteries: a randomised comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (PRISON II study) EuroIntervention. 2012;7(10):1189–1196. doi: 10.4244/EIJV7I10A190. [DOI] [PubMed] [Google Scholar]
- 4.Rathore S, Katoh O, Matsuo H, et al. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries: procedural outcomes and predictors of success in contemporary practice. Circ Cardiovasc Interv. 2009;2(2):124–132. doi: 10.1161/CIRCINTERVENTIONS.108.838862. [DOI] [PubMed] [Google Scholar]
- 5.Ramadan R, Boden WE, Kinlay S. Management of left main coronary artery disease. J Am Heart Assoc. 2018;7(7):e008151. [DOI] [PMC free article] [PubMed]
- 6.Park DW, Park SJ. Intravascular ultrasound-guided percutaneous coronary intervention for left main disease: does procedural fine-tuning make a relevant clinical benefit? Circ Cardiovasc Interv. 2017;10(5):e005293. [DOI] [PubMed]
- 7.Yamamoto K, Shiomi H, Morimoto T, et al. Optimal intravascular ultrasound-guided percutaneous coronary intervention in patients with multivessel disease. JACC Asia. 2023;3(2):211–25. [DOI] [PMC free article] [PubMed]
- 8.Gaster AL, Slothuus Skjoldborg U, Larsen J, et al. Continued improvement of clinical outcome and cost effectiveness following intravascular ultrasound guided PCI: insights from a prospective, randomised study. Heart. 2003;89(9):1043–1049. doi: 10.1136/heart.89.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darmoch F, Alraies MC, Al-Khadra Y, et al. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(5):e013678. doi: 10.1161/JAHA.119.013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Franco AC, Nissen SE. Coronary intravascular ultrasound: implications for understanding the development and potential regression of atherosclerosis. Am J Cardiol. 2001;88(10A):7M–20M. doi: 10.1016/S0002-9149(01)02109-9. [DOI] [PubMed] [Google Scholar]
- 11.Elgendy IY, Ha LD, Elbadawi A, et al. Temporal trends in inpatient use of intravascular imaging among patients undergoing percutaneous coronary intervention in the United States. JACC Cardiovasc Interv. 2018;11(9):913–915. doi: 10.1016/j.jcin.2018.01.254. [DOI] [PubMed] [Google Scholar]
- 12.Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e4–e17. doi: 10.1161/CIR.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 13.Räber L, Mintz SG, Koskinas CK, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14(6):656–77. [DOI] [PubMed]
- 14.Johnson WT, Räber L, Di Mario C, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2019;15(5):434–451. doi: 10.4244/EIJY19M06_02. [DOI] [PubMed] [Google Scholar]
- 15.Jakabcin J, Spacek R, Bystron M, et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv. 2010;75(4):578–583. doi: 10.1002/ccd.22244. [DOI] [PubMed] [Google Scholar]
- 16.Chieffo A, Latib A, Caussin C, et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165(1):65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Kim BK, Shin DH, Hong MK, et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8(7):e002592. doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 18.Tian NL, Gami SK, Ye F, et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10(12):1409–1417. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- 19.Hong SJ, Mintz GS, Ahn CM, et al. Effect of Intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13(1):62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Gao XF, Ge Z, Kong XQ, et al. 3-year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14(3):247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Choi KH, Song YB, et al. Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med. 2023:388(18):1668–79. [DOI] [PubMed]
- 22.Tan Q, Wang Q, Liu D, et al. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36(5):549–553. doi: 10.15537/smj.2015.5.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JS, Kang TS, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6(4):369–376. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Liu XM, Yang ZM, Liu XK, et al. Intravascular ultrasound-guided drug-eluting stent implantation for patients with unprotected left main coronary artery lesions: a single-center randomized trial. Anatol J Cardiol. 2019;21(2):83–90. doi: 10.14744/AnatolJCardiol.2018.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Applegate RJ, Sacrinty MT, Little WC, et al. Incidence of coronary stent thrombosis based on academic research consortium definitions. Am J Cardiol. 2008;102(6):683–688. doi: 10.1016/j.amjcard.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 Consensus Document. Circulation. 2018;137(24):2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elgendy IY, Mahmoud AN, Elgendy AY, et al. Outcomes with intravascular ultrasound-guided stent implantation: a meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016;9(4):e003700. doi: 10.1161/CIRCINTERVENTIONS.116.003700. [DOI] [PubMed] [Google Scholar]
- 31.Buccheri S, Franchina G, Romano S, et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and Bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv. 2017;10(24):2488–2498. doi: 10.1016/j.jcin.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 32.Shin DH, Hong SJ, Mintz GS, et al. Effects of intravascular ultrasound-guided versus angiography-guided new-generation drug-eluting stent implantation: meta-analysis with individual patient-level data from 2,345 randomized patients. JACC Cardiovasc Interv. 2016;9(21):2232–2239. doi: 10.1016/j.jcin.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Elgendy IY, Gad M, Mintz GS. Meta-analysis of intravascular ultrasound-guided drug-eluting stent implantation for left main coronary disease. Am J Cardiol. 2020;1(128):92–93. doi: 10.1016/j.amjcard.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Elgendy IY, Mahmoud AN, Elgendy AY, et al. Intravascular ultrasound-guidance is associated with lower cardiovascular mortality and myocardial infarction for drug-eluting stent implantation—insights from an updated meta-analysis of randomized trials. Circ J. 2019;83(6):1410–1413. doi: 10.1253/circj.CJ-19-0209. [DOI] [PubMed] [Google Scholar]
- 35.Bhogal S, Hashim H, Merdler I, et al. Impact of IVUS and OCT on physician decision-making during post-PCI optimization. Cardiovasc Revasc Med. 2023;55:96–98. doi: 10.1016/j.carrev.2023.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Barus P, Piasecki A, Gumiezna K, et al. Multimodality OCT, IVUS and FFR evaluation of coronary intermediate grade lesions in women vs. men. Front Cardiovasc Med. 2023;10:1021023. doi: 10.3389/fcvm.2023.1021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamié D, Costa JR, Damiani LP, et al. Optical coherence tomography versus intravascular ultrasound and angiography to guide percutaneous coronary interventions. Circ Cardiovasc Interv. 2021;14(3):e009452. doi: 10.1161/CIRCINTERVENTIONS.120.009452. [DOI] [PubMed] [Google Scholar]
- 38.Kang D-Y, Ahn J-M, Yun S-C, et al. Optical coherence tomography-guided or intravascular ultrasound-guided percutaneous coronary intervention: the OCTIVUS randomized clinical trial. Circulation. 2023;148(16):1195–1206. doi: 10.1161/CIRCULATIONAHA.123.066429. [DOI] [PubMed] [Google Scholar]
- 39.Lee PH, Ahn JM, Chang M, et al. Left main coronary artery disease: secular trends in patient characteristics, treatments, and outcomes. J Am Coll Cardiol. 2016;68(11):1233–1246. doi: 10.1016/j.jacc.2016.05.089. [DOI] [PubMed] [Google Scholar]
- 40.Carrie D, Eltchaninoff H, Lefevre T, et al. Early and long-term results of unprotected left main coronary artery stenosis with paclitaxel-eluting stents: the FRIEND (French multicentre RegIstry for stenting of uNprotecteD LMCA stenosis) registry. EuroIntervention. 2011;7(6):680–688. doi: 10.4244/EIJV7I6A110. [DOI] [PubMed] [Google Scholar]
- 41.Alberti A, Giudice P, Gelera A, et al. Understanding the economic impact of intravascular ultrasound (IVUS) Eur J Health Econ. 2016;17(2):185–193. doi: 10.1007/s10198-015-0670-4. [DOI] [PubMed] [Google Scholar]
- 42.Ali ZA, Maehara A, Genereux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388(10060):2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 43.Allali A, Traboulsi H, Sulimov DS, et al. Feasibility and safety of minimal-contrast IVUS-guided rotational atherectomy for complex calcified coronary artery disease. Clin Res Cardiol. 2021;110(10):1668–1679. doi: 10.1007/s00392-021-01906-y. [DOI] [PubMed] [Google Scholar]
- 44.Sakakura K, Yamamoto K, Taniguchi Y, et al. Intravascular ultrasound enhances the safety of rotational atherectomy. Cardiovasc Revasc Med. 2018;19(3 Pt A):286–291. doi: 10.1016/j.carrev.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Ladwiniec A, Walsh SJ, Holm NR, et al. Intravascular ultrasound to guide left main stem intervention: a NOBLE trial substudy. EuroIntervention. 2020;16(3):201–209. doi: 10.4244/EIJ-D-19-01003. [DOI] [PubMed] [Google Scholar]
- 46.Cortese B, Piraino D, Gentile D, et al. Intravascular imaging for left main stem assessment: an update on the most recent clinical data. Catheter Cardiovasc Interv. 2022;100(7):1220–1228. doi: 10.1002/ccd.30440. [DOI] [PubMed] [Google Scholar]
- 47.Leesar MA, Hage FG. IVUS guidance on optimal stent deployment: new insights and perspectives. JACC Cardiovasc Interv. 2022;15(2):217–219. doi: 10.1016/j.jcin.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, Liew D, Duffy SJ, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a health economic analysis. Circ Cardiovasc Qual Outcomes. 2021;14(5):e006789. doi: 10.1161/CIRCOUTCOMES.120.006789. [DOI] [PubMed] [Google Scholar]
- 49.Mueller C, Hodgson JM, Schindler C, et al. Cost-effectiveness of intracoronary ultrasound for percutaneous coronary interventions. Am J Cardiol. 2003;91(2):143–147. doi: 10.1016/S0002-9149(02)03099-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.