Abstract
Introduction
Transcatheter aortic valve implantation (TAVI) plays a vital role in patients with symptomatic aortic stenosis. Despite the mortality benefit of TAVI, embolic stroke remains a feared complication. As a result, transcatheter cerebral embolic protection (TCEP) devices have been developed to reduce this risk. Given the ongoing debate of TCEP in TAVI, we performed a systematic review and meta-analysis of all randomized controlled trials to date to identify outcomes of periprocedural stroke using the Sentinel™ cerebral protection system (CPS).
Methods
MEDLINE, Cochrane, and Scopus databases were utilized from inception until 12/2023. PRISMA criteria was utilized. Keywords included “cerebral embolic protection”, “sentinel cerebral protection system”, “transcatheter aortic valve implantation”, and “transcatheter aortic valve replacement”. Primary outcome was periprocedural stroke. Secondary outcomes included periprocedural disabling and non-disabling stroke, all-cause mortality, transient ischemic attack, delirium, acute kidney injury, vascular complications, bleeding, and pacemaker implantation. Risk ratios (RR) were measured via Mantel–Haenszel method with fixed analysis. Heterogeneity was assessed via chi-squared and Higgin’s I2 test.
Results
Four trials with 3528 patients were assessed. SAPIEN 3 was the most common bioprosthetic valve used. The average age was 79.4 years with 41.9% of the sample size being females. The most prevalent comorbidities were hypertension, diabetes mellitus, and coronary artery disease. There was no difference in periprocedural stroke in patients who underwent TAVI with the Sentinel™ CPS compared to no TCEP (RR 0.75, P = 0.12). Periprocedural disabling strokes were less likely in those who underwent TAVI with the Sentinel™ CPS compared to no TCEP (RR 0.41, P = 0.02) with a number needed to treat (NNT) of 123. All other outcomes did not reach statistical significance.
Conclusions
In our analysis, there was no difference between TAVI with the Sentinel™ CPS compared to TAVI without TCEP in regard to risk of periprocedural stroke; however, it was associated with a decreased risk of periprocedural disabling stroke.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40119-024-00359-4.
Keywords: Cerebral embolic protection, Sentinel cerebral protection system, Transcatheter aortic valve implantation, Transcatheter aortic valve replacement, Periprocedural stroke
Key Summary Points
| The role of transcatheter cerebral embolic protection (TCEP) devices in decreasing risk of periprocedural stroke during transcatheter aortic valve implantation (TAVI) remains controversial. |
| The Sentinel™ cerebral protection system (CPS) is the only US Food and Drug Administration (FDA)-approved TCEP device. |
| We performed a systematic review and meta-analysis to identify the risk of periprocedural stroke in patients undergoing TAVI with the Sentinel™ CPS. |
| Our meta-analysis demonstrated that the Sentinel™ CPS was not associated with a lower risk of periprocedural stroke; however, it was associated with a lower risk of periprocedural disabling stroke when compared to no TCEP, and not associated with a difference in other outcomes. |
Introduction
Transcatheter aortic valve implantation (TAVI) has played an important role in the treatment of adult patients with symptomatic aortic stenosis with a largely expanding target population. Randomized controlled trials (RCTs) have evaluated patients with low, intermediate, and high surgical risk as determined by the Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score and found that TAVI has a favorable mortality benefit. It is also associated with shorter hospitalizations, quicker return to functional status, lower risk of atrial fibrillation, less bleeding, and less pain when compared to surgical aortic valve replacement (SAVR) [1]. Factors including patient age, comorbidities, life expectancy, vascular anatomy, and patient values are discussed when assessing route of valve replacement for patients being evaluated for TAVI [2].
Despite the mortality benefit of TAVI, embolic stroke remains a feared complication. The incidence of 30-day risk of stroke following TAVI is reported to be 1–11%, with a median of 4% [3]. As a result, transcatheter cerebral embolic protection (TCEP) devices have been developed and evaluated for their role in mitigating or reducing the risk of cerebral embolic strokes. These devices include Sentinel™ (Boston Scientific), TriGUARD 3™ (Venus Medtech), Embrella™ (Edwards LifeSciences), and others. RCTs have reported a decrease in new diffusion-weighted magnetic resonance imaging (DW-MRI) findings of ischemic lesions with these devices; however, these findings did not reach statistical significance [4, 5]. Specifically, the SENTINEL safety trial met non-inferiority criteria for 30-day major adverse cardiac and cerebrovascular events (MACCE) when comparing Sentinel™ use to control with no TCEP [6].
Given the lack of consensus in the use of TCEP in TAVI, we performed a systematic review and meta-analysis to identify outcomes of periprocedural stroke using the Sentinel™ cerebral protection system (CPS) (Boston Scientific, Marlborough, MA, USA).
Methods
A systematic search of MEDLINE, Cochrane, and Scopus databases was done utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [7] (Supplementary Materials). RCTs that evaluated stroke with utilization of the Sentinel™ CPS in patients undergoing TAVI were included from inception until December 2023. Keywords included “cerebral embolic protection”, “sentinel cerebral protection system”, “transcatheter aortic valve implantation”, and “transcatheter aortic valve replacement”. Inclusion criteria were all patients undergoing TAVI regardless of valve type, including self-expandable and balloon-expandable. Exclusion criteria were studies written in any language other than English, trials that assessed TCEP devices that were not the Sentinel™ CPS, and studies that were not RCTs. Studies were confirmed by multiple authors (Wissam Harmouch, Barbara Karnkowska, and Ravi Thakker). The primary outcome for the meta-analysis was periprocedural stroke. Secondary outcomes included periprocedural disabling and non-disabling stroke, all-cause mortality, transient ischemic attack (TIA), delirium, acute kidney injury, vascular complications, bleeding, and pacemaker implantation up to 30 days from discharge. The statistical method performed was the Mantel–Haenszel test with a fixed analysis model and effect measure of risk ratio (RR). Heterogeneity was assessed via chi-squared and Higgin’s I2 test. Publication bias was assessed via visual inspection of the funnel plot. RevMan 5.0 (Cochrane Collaboration, Oxford, UK) was utilized to perform statistical analysis. This article did not require ethics approval, as it is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Included Studies, Summary, and Baseline Characteristics
After screening 199 studies, four RCTs were included in our analysis as they met inclusion and exclusion criteria (Fig. 1). A total of 3528 patients were assessed and included patients from the United States, Europe, and Australia. Edwards SAPIEN 3 was the most common valve utilized. A summary of the four RCTs can be found in Table 1. Baseline characteristics of the cumulative patients assessed can be found in Table 2. In summary, the average age of patients was 79.4 years, female distribution of patients was 41.9%, mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score was 3.8%, mean EuroSCORE II was 4.6%, and mean Logistic EuroSCORE was 15.5%. There were 1208 patients with diabetes mellitus (34.2%), 2753 patients with hypertension (87%), 408 patients with peripheral vascular disease (11.6%), 1974 patients with coronary artery disease (57%), 1191 patients with previous coronary vascularization via percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery (34.4%), 458 patients with previous cerebral vascular events or transient ischemic attack (13%), and 1145 patients with atrial fibrillation or atrial flutter (32.5%). Hypertension was not reported in one trial, coronary artery disease was not reported in one trial, and previous coronary vascularization was not reported in one trial.
Fig. 1.

Study flowsheet
Table 1.
Summary of included studies
| PROTECTED TAVR [11] | SENTINEL [6] | MISTRAL-C [5] | CLEAN-TAVI [4] | |
|---|---|---|---|---|
| Sample size (N) | 3000 (1501 TCEP vs. 1499 control) | 363 (121 TCEP, 123 safety, 119 control) | 65 (32 TCEP vs. 33 control) | 100 (50 TCEP vs. 50 control) |
| Trial timeline | 02/2020–01/2022 | 03/2016–06/2016 | 11/2013–07/2015 | 04/2013–06/2014 |
| TAVR device utilized |
SAPIEN 3: 64% Evolut R/Evolut PRO: 24.1% ACURATE: 6.9% PORTICO: 2.7% Lotus Edge: 1% |
SAPIEN 3: 52.4% Evolut R: 25.9% SAPIEN XT: 17.8% CoreValve: 4% |
SAPIEN 3: 54% CoreValve: 25% SAPIEN XT: 15% Ballon Dilation: 5% PORTICO: 1% |
CoreValve 100% |
| Geographic region | United States, Europe, and Australia | United States and Germany | Netherlands | Germany |
| Inclusion criteria |
Patient with aortic valve stenosis and treated with an approved TAVR device via transfemoral access Subject has recommended artery diameter sizes for filter placement (9–15 mm for brachiocephalic artery and 6.5–10 mm in left common carotid artery as determined by CT) |
Severe symptomatic aortic stenosis with TAVR planned High-surgical-risk patients Vascular anatomy suitable for the Sentinel TCEP device |
Patients at high risk for SAVR Aortic arch anatomy size appropriate for Sentinel CPS Brachiocephalic trunk measured 9–15 mm and left common carotid measured 6.5–10 mm |
Symptomatic patients with severe aortic stenosis Increased risk for SAVR; determined by STS PROM and logistic EuroSCORE The aortic annulus was required to have a size between 20 and 29 mm via CT |
| Exclusion criteria |
Arterial stenosis > 70% in either left common carotid or brachiocephalic artery Brachiocephalic or left carotid artery with significant stenosis, ectasia, dissection, or aneurysm at the aortic ostium or within 3 cm of the ostium Compromised blood flow to right upper extremity Excessive tortuosity in access vessels Uncorrected bleeding disorder Contraindication to anticoagulation or antiplatelet therapy |
Contraindications for right radial or brachial artery access Inability to undergo MRI brain evaluation for any reason |
Excessive tortuosity Arterial stenosis > 70% of left common carotid or brachiocephalic artery Presence of permanent pacemaker or implantable cardiac defibrillator History of prior stroke |
Anatomy unsuitable for safe CoreValve implantation Preexisting permanent pacemaker Stroke within the last 12 months Carotid artery stenosis > 70% Significant stenosis of the right subclavian artery or the brachiocephalic trunk Expected non-compliance to follow-up visits Participation in another clinical study Severe renal failure (GFR < 30 ml/min/1.73 m2) Pregnancy |
| Primary endpoint | Stroke within 72 h after TAVR or before discharge |
Primary safety endpoint, including MACCE at 30 days Primary efficacy endpoint was reduction in new lesion volume in protected brain territories on MRI at 2–7 days post-procedure |
Incidence of new brain lesions found by DW-MRI, new lesion, and total lesion volumes 5–7 days after TAVR | Numerical difference in new positive post-procedure DW-MRI brain lesions 2 days after TAVR in TCEP protected territories |
| Primary outcomes |
A total of 34 strokes occurred in the TCEP group vs. 43 in the control Disabling strokes: 8 in TCEP group vs. 20 in control group Non-disabling strokes: 26 in TCEP group vs. 23 in control group |
MACCE at 30 days was lower in TCEP group compared to control, but not statistically significant New lesion volume was smaller in the TCEP group compared to the control, but not statistically significant |
TCEP group had numerically fewer new lesions and smaller total lesion volume | Number of new lesions was lower in the filter group compared to the control group. New lesion volume after TAVR was lower in the filter group compared to the control group |
| Stroke outcome | 2.3% TCEP group vs. 2.9% control, P = 0.30 | 5.6% TCEP vs. 9.1% control, P = 0.25 | 0% TCEP group vs. 6% control, P = NS | 10% TCEP group vs. 10% control, P = NS |
| Limitations | Small number of end points, short-term follow-up, non-ideal stroke reporting, TCEP group contained greater percent of female patients | Variability in MRI technique and acquisition, participant noncompliance with follow-up, small sample size, non-randomization of various valve devices | Small sample size, high MRI dropout rate, higher STS PROM score in control group | Single-center study, short-term follow-up, only one TAVI device utilized, interventional team could not be blinded |
TAVR transcatheter aortic valve replacement, CPS cerebral protection system, TCEP transcatheter cerebral embolic protection, CI confidence interval, MACCE major adverse cardiac and cerebrovascular events, SAVR surgical aortic valve replacement, DW-MRI diffusion-weighted magnetic resonance imaging, STS PROM Society of Thoracic Surgeons Predicted Risk of Mortality, CT computed tomography, NS not significant, MRI magnetic resonance imaging
Table 2.
Baseline characteristics of the studies' populations
| PROTECTED TAVR [11] | SENTINEL [6] | MISTRAL-C [5] | CLEAN-TAVI [4] | Cumulative | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Sample size, n | 3000 | 363 | 65 | 100 | 3528 |
| Age (years); mean | 78.9 | 83.4 | 82 | 79.7 | 79.4 |
| Female sex; (%) | 40% | 52.10% | 48% | 57% | 41.9% |
| STS PROM score; % mean | 3.4 | 6 | 4.8 | 5.4 | 3.8 |
| EuroSCORE II; % mean | 4.5 | – | – | – | 4.6 |
| Logistic EuroSCORE (mean); % mean | – | – | – | 15.5 | 15.5 |
| Clinical characteristics; no./total no. (%) | |||||
| Diabetes mellitus | 1023/3000 (34.1%) | 127/363 (35%) | 13/65 (20%) | 45/100 (45%) | 1208/3528 (34.2%) |
| Hypertension | 2618/3000 (87.3%) | – | 44/65 (68%) | 91/100 (91%) | 2753/3165 (87%) |
| Peripheral vascular disease | 327/3000 (10.9%) | 55/363 (15.2%) | 20/65 (31%) | 6/100 (6%) | 408/3528 (11.6%) |
| Coronary artery disease | 1730/3000 (57.7%) | 193/363 (53.2%) | – | 51/100 (51%) | 1974/3463 (57%) |
| Previous coronary revascularization (PCI or CABG) | 1043/3000 (34.8%) | 125/363 (34.4%) | – | 23/100 (23%) | 1191/3463 (34.4%) |
| History of cerebrovascular events or transient ischemic attacks | 394/3000 (13.1%) | 48/363 (13.2%) | 12/65 (19%) | 4/100 (4%) | 458/3528 (13%) |
| Atrial fibrillation or atrial flutter | 980/3000 (32.7%) | 115/363 (31.7%) | 16/65 (28%) | 34/100 (34%) | 1145/3528 (32.5%) |
| Congestive heart failure | – | – | – | 92/100 (92%) | 92/100 (92%) |
| NYHA class I | – | – | – | 10/100 (10%) | 10/100 (10%) |
| NYHA class II | – | – | 11/65 (17%) | 26/100 (26%) | 37/165 (22.4%) |
| NYHA class III and IV | – | 295/355 (83.1%) | 43/65 (66%) | 64/100 (64%) | 402/520 (77.3%) |
STS PROM Society of Thoracic Surgeons Predicted Risk of Mortality. PCI percutaneous coronary intervention, CABG coronary artery bypass graft, NYHA New York Heart Association
"." represents non-available information
Outcomes
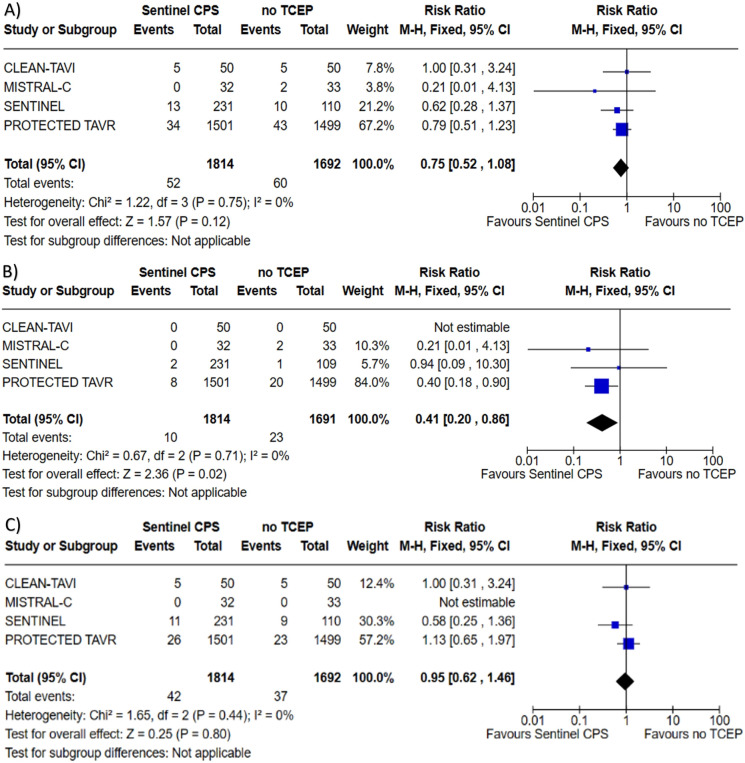
All four trials reported outcome of periprocedural stroke with an aggregate of 3506 patients assessed. Risk of publication bias for periprocedural stroke was low as assessed via visual inspection of the funnel plot (Fig. 2).There was no difference found with the use of Sentinel™ CPS in TAVI for stroke when compared to no TCEP; 2.87 vs. 3.55%, RR 0.75, (95% confidence interval (CI) 0.52–1.08, P = 0.12), heterogeneity I2 = 0% (P = 0.75). All four trials reported outcome of periprocedural disabling stroke with 3505 patients assessed. The use of Sentinel™ CPS in TAVI was associated with a lower risk of disabling stroke compared to no TCEP; 0.55 vs. 1.36%, RR 0.41 (95% CI 0.20–0.86, P = 0.02), number needed to treat (NNT) = 123, heterogeneity I2 = 0% (P = 0.71). All four trials reported outcome of periprocedural non-disabling stroke with 3506 patients assessed. There was no difference found with the use of Sentinel™ CPS in TAVI for non-disabling stroke when compared to no TCEP; 2.32 vs. 2.19%, RR 0.95 (95% CI 0.62–1.46, P = 0.80), heterogeneity I2 = 0% (P = 0.44). Forest plots depicting the results of periprocedural stroke, disabling stroke, and non-disabling stroke can be found in Fig. 3.
Fig. 2.
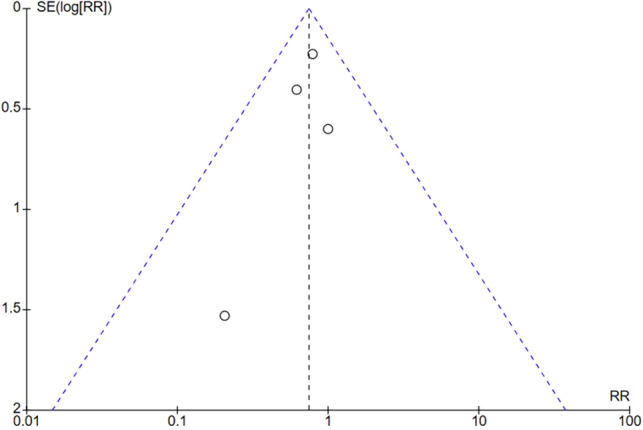
Funnel plot of included RCTs for outcome of periprocedural stroke. RR risk ratios, RCT randomized controlled trial, SE(log[RR]) standard error of the log of the risk ratio
Fig. 3.
Forrest plots for A Periprocedural stroke, B periprocedural disabling stroke, and C periprocedural non-disabling stroke among patients undergoing TAVI with the Sentinel™ CPS versus TAVI with no TCEP. TAVI transcatheter aortic valve implantation, CPS cerebral protection system, TCEP transcatheter cerebral embolic protection, CI confidence interval, M–H Mantel–Haenszel method
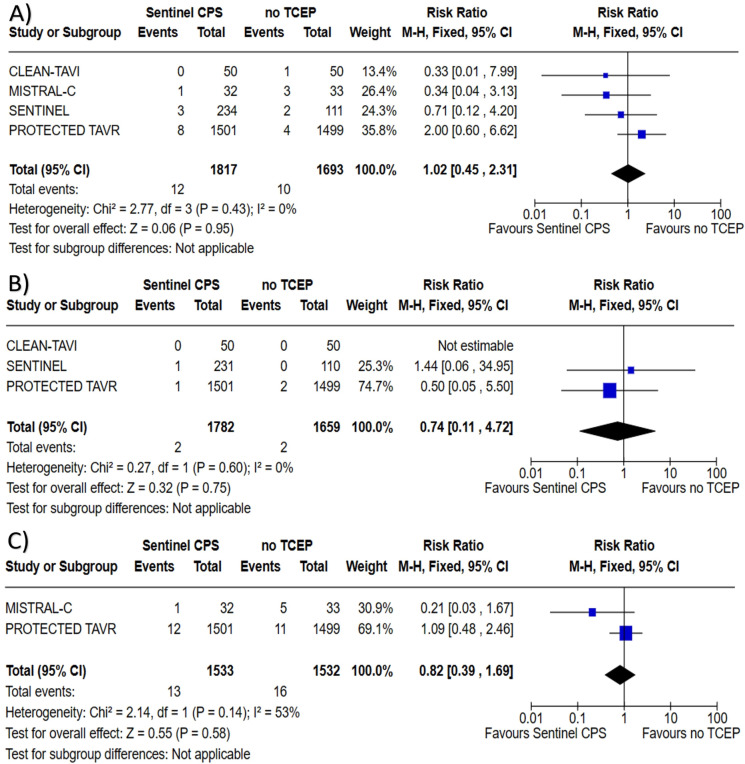
All four trials reported outcome of all-cause mortality with a total of 3510 patients assessed. There was no difference found with the use of Sentinel™ CPS in TAVI for all-cause mortality when compared to no TCEP; 0.66 vs. 0.59%, RR 1.02 (95% CI 0.45–2.31, P = 0.95), heterogeneity I2 = 0% (P = 0.43). Three trials reported outcome of TIA with a total of 3441 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for TIA when compared to no TCEP; 0.11 vs. 0.12%, RR 0.74 (95% CI 0.11–4.72, P = 0.75), heterogeneity I2 = 0% (P = 0.60). Two trials reported outcome of delirium with a total of 3065 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for delirium when compared to no TCEP; 0.85 vs. 1.04%, RR 0.82 (95% CI 0.39–1.69, P = 0.58), heterogeneity I2 = 53% (P = 0.14). Forest plots regarding all-cause mortality, TIA, and delirium can be found in Fig. 4.
Fig. 4.
Forrest plots for A all-cause mortality, B transient ischemic attack, and C delirium among patients undergoing TAVI with the Sentinel™ CPS versus TAVI with no TCEP. TAVI transcatheter aortic valve implantation, CPS cerebral protection system, TCEP transcatheter cerebral embolic protection, CI confidence interval, M-H Mantel–Haenszel method
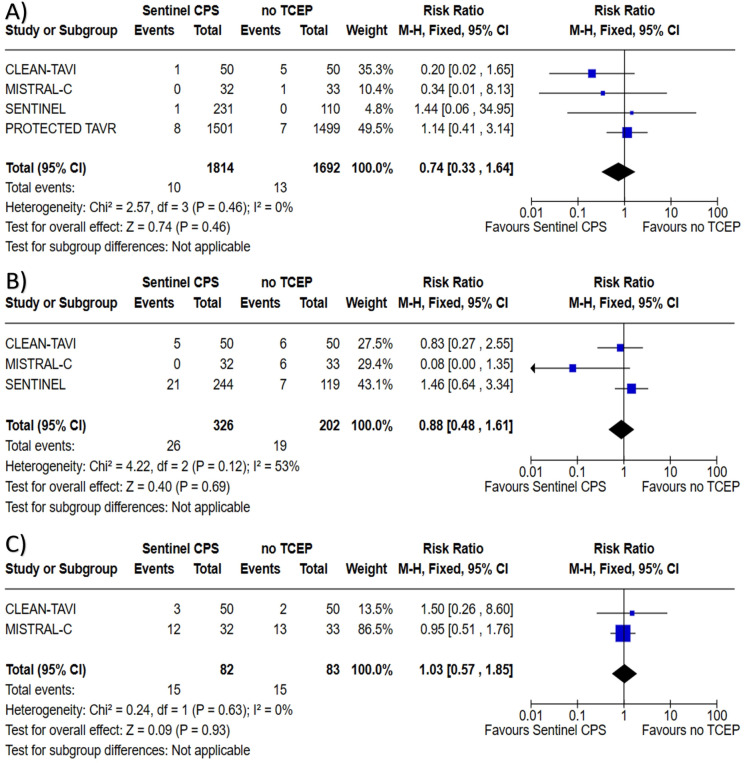
All four trials reported outcome of acute kidney injury with a total of 3506 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for acute kidney injury when compared to no TCEP; 0.55 vs. 0.77%, RR 0.74 (95% CI 0.33–1.64, P = 0.46), heterogeneity I2 = 0% (P = 0.46). Three trials reported outcome of major vascular complications with a total of 528 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for major vascular complications when compared to no TCEP; 7.98 vs. 9.41%, RR 0.88 (95% CI 0.48–1.61, P = 0.69), heterogeneity I2 = 53% (P = 0.12). Two trials reported outcome of minor vascular complications with a total of 165 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for minor vascular complications when compared to no TCEP; 18.29 vs. 18.07%, RR 1.03 (95% CI 0.57–1.85, P = 0.93), heterogeneity I2 = 0% (P = 0.63). Forest plots regarding outcomes of acute kidney injury, and major and minor vascular complications can be found in Fig. 5.
Fig. 5.
Forrest plots for A acute kidney injury, B major vascular complications, and C minor vascular complications among patients undergoing TAVI with the Sentinel™ CPS versus TAVI with no TCEP. TAVI transcatheter aortic valve implantation, CPS cerebral protection system, TCEP transcatheter cerebral embolic protection, CI confidence interval, M–H Mantel–Haenszel method
Two trials reported outcome of major or life-threatening bleeding with a total of 165 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for major or life-threatening bleeding when compared to no TCEP; 9.76 vs. 14.46%, RR 0.67 (95% CI 0.29–1.57, P = 0.36), heterogeneity I2 = 22% (P = 0.26). Two trials reported outcome of minor bleeding with a total of 165 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for minor bleeding when compared to no TCEP; 25.61 vs. 27.71%, RR 0.94 (95% CI 0.62–1.42, P = 0.76), heterogeneity I2 = 10% (P = 0.29). Two trials reported outcome of permanent pacemaker implantation with a total of 165 patients assessed. There was no difference found with the use of the Sentinel™ CPS in TAVI for permanent pacemaker implantation when compared to no TCEP; 23.17 vs. 15.66%, RR 1.48 (95% CI 0.78–2.79, P = 0.23), heterogeneity I2 = 0% (P = 0.95). Forest plots regarding outcomes of bleeding and pacemaker implantation can be found in Fig. 6.
Fig. 6.
Forrest plots for A major or life-threating bleeding, B minor bleeding, and C permanent pacemaker implantation among patients undergoing TAVI with the Sentinel™ CPS versus TAVI with no TCEP. TAVI transcatheter aortic valve implantation, CPS cerebral protection system, TCEP transcatheter cerebral embolic protection, CI confidence interval, M–H Mantel–Haenszel method
Discussion
In this meta-analysis of four RCTs including 3528 patients, we evaluated periprocedural stroke in patients undergoing TAVI using the Sentinel™ CPS. The main findings of the analysis are: (1) there was no difference in association of periprocedural stroke in TAVI with the Sentinel™ CPS compared to TAVI without TCEP; (2) TAVI with the Sentinel™ CPS compared to TAVI without TCEP was associated with a lower risk of periprocedural disabling stroke; (3) there was no difference in the other secondary outcomes, including non-disabling stroke, all-cause mortality, TIA, delirium, acute kidney injury, vascular complications, bleeding events, or need of pacemaker implantation in patients undergoing TAVI with the Sentinel™ CPS.
Debate on Stroke with the Sentinel™ CPS
Regarding stroke, we found that utilization of the Sentinel™ CPS was not associated with a difference in the outcome of periprocedural stroke, but it was associated with a statistically significant reduction in risk of disabling stroke. These are important findings as the risk of stroke remains a major concern in TAVI. In the PARTNER trial published in 2011, the rates of major stroke were 3.8% in the TAVI group at 30 days [8]. Most of these strokes occurred 1–3 days post-procedure [9].
The occurrence of periprocedural stroke has been further explored by assessment of new ischemic regions in the brain in the CLEAN-TAVI trial published by Haussig et al. where they found that the Sentinel™ CPS group had fewer new lesions via DW-MRI as well as smaller volumes of these lesions at 2 days post-TAVI when compared to the control group [4]. Van Mieghem et al. in MISTRAL-C aimed to add to these findings by evaluating new DW-MRI findings post-TAVI, as well as clinical manifestations of these lesions with a longer post-procedural follow-up of 5 and 7 days [5]. The group that underwent TAVI with the Sentinel™ CPS had 100% capture of embolic debris. Additionally, there was a reduced number of new lesions and lesion size in the Sentinel™ CPS group compared to the group without TCEP. They also found that there was diminished neurologic deterioration with the use of the Sentinel™ CPS. However, the study was limited due to poor follow-up with DW-MRI given that only 57% of randomized patients had an assessment at 5-days post-procedure. Additionally, both the CLEAN-TAVI [4] and MISTRAL-C [5] trials had a very small sample sizes; hence, the findings may not be applicable to all comers. Due to the clinical benefit of capturing periprocedural embolic debris in these two RCTs, they were included in our meta-analysis and further highlighted the lower risk of disabling stroke with use of the Sentinel™ CPS.
MACCE, including risk of stroke were studied as primary outcomes in a few trials, including SENTINEL [6], Seeger et al. [10], and PROTECTED-TAVR [11]. These studies were conflicting, as some favored the use of the Sentinel™ CPS due to decreased stroke rates and increased stroke-free survival, while others showed no benefit. Moreover, Kapadia et al. in 2017 found that the Sentinel™ CPS met non-inferiority criteria as MACCE was 7.3% in the Sentinel™ CPS arm compared to the historical performance of 18.3%, P < 0.001; however, it did not prove to be superior to the control group (MACCE = 9.9%, P = 0.405) [6]. This trial was largely underpowered to detect statistically significant endpoints, as the sample size was only 363 patients.
Given the low power to detect a statistically significant reduction in risk of periprocedural stroke in initial RCTs, Seeger et al. performed a prospective study with propensity score matched (PSM) groups and found that the odds of stroke occurred less with the Sentinel™ CPS than without, 2.1 vs. 6.8%, odds ratio (OR) 0.3, P = 0.01 [10]. This led to a pooled analysis with PSM patients from the CLEAN-TAVI [4] and SENTINEL [6] safety trials to assess risk of periprocedural stroke. These results were in favor of the Sentinel™ CPS with the OR for stroke being 0.35, P = 0.0028 and OR for all-cause mortality being 0.34, P = 0.0013 [12].
Regarding studies that showed no benefit of the Sentinel™ CPS, an observational study that used the Society for Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry assessed the risk of periprocedural stroke in 123,186 patients and found no difference for in-hospital stroke, RR 0.9 (95% CI 0.68–1.13) when compared to no TCEP [13]. Regarding RCTs, PROTECTED TAVR [11] was the largest RCT to evaluate the Sentinel™ CPS with 3000 patients enrolled. It demonstrated no difference in the incidence of periprocedural stroke with the use of the Sentinel™ CPS (2.3% TCEP vs. 2.9% control, P = 0.30) [11].
Overall, the conflicting results of individual trials and studies made it unclear whether this dual-filter device provided benefit in protection from embolic stroke. By pooling data from individual RCTs, our meta-analysis was able to elucidate significant findings in favor of the Sentinel™ CPS in decreasing the risk of periprocedural disabling stroke. The major limitation of most of these individual trials to detect a significant effect was their small sample size; therefore, it is possible that by aggregating the individual RCTs and increasing the power by means of a meta-analysis, the true significant effect of the Sentinel™ CPS was able to be detected. On the other hand, aggregating the data for secondary outcomes did not reveal any significant findings. This suggests that these outcomes did not have a significant difference with the Sentinel™ CPS or that further RCTs with larger sample sizes are needed to clarify findings of the Sentinel™ CPS on these secondary outcomes.
In comparing our findings with other meta-analysis, we found contrasting results. In 2020, Ndunda and group performed a meta-analysis with sensitivity analysis on three RCTs that were included in our study, but also a PSM cohort study that was not included in our study [14]. In their primary analysis, they found results in favor of the Sentinel™ CPS with reduced risk of stroke (RR 0.51, P = 0.002), overall mortality (RR 0.34, P = 0.03), and risk of bleeding (RR 0.50, P = 0.04) at 30 days [14]. Despite these promising results, skepticism stemmed from the results of the sensitivity analysis, which dissolved the significance that was initially seen for risk of stroke and 30-day mortality. The authors attributed the difference between the primary analysis and sensitivity analysis due to exclusion of the only non-RCT, Seeger et al. [10], and argued that the true difference was only seen with higher power to account for stroke as the rare event. The study was promising but left unanswered questions. We performed our meta-analysis with exclusion of Seeger et al. [10] to limit the potential for bias from non-RCTs. We also included results from PROTECCTED TAVR [11], an RCT by Kapadia and group that was published after the results of the meta-analysis by Ndunda and group [14] were published.
In June 2023, Wolfrum and group performed a meta-analysis [15] on four RCTs and one PSM study to investigate the efficacy of the Sentinel™ CPS. They included the same RCTs that Ndunda et al. [14] incorporated in their meta-analysis, but also data from PROTECTED TAVR [11] with an aggregate of 4066 patients included. Data from PROTECTED TAVR [11] carried the most weight, totaling 73.7% of the patient population, while the next largest study, Seeger et al. [10], represented 13.77%. Final analysis not only showed that the Sentinel™ CPS significantly reduced risk of stroke (RR 0.67, P = 0.02), but also reduced risk of disabling stroke (RR 0.33, P = 0.001) [15]. These findings were in correlation with previous meta-analysis; however, there was significant heterogeneity of the trials evaluated as most were RCTs; however, a non-randomized prospective study was also included. This could have led to the same limitation that Ndunda and group [14] encountered from their sensitivity analysis. Nonetheless, we found similar findings in our meta-analysis with regards to disabling stroke.
Regarding secondary outcomes, such as bleeding, vascular and procedural complications, we did not find a significant benefit with the Sentinel™ CPS regarding these measures. Interestingly, Wolfrum et al. [15] found a reduced risk of major or life-threatening risk of bleeding with the Sentinel™ CPS (RR 0.37, P = 0.02); however, there was no difference with regards to all-cause mortality, major vascular complications, and acute kidney injury. In our meta-analysis, we assessed outcomes of major or life-threatening bleeding and minor bleeding as independent outcomes and found no significant difference between the Sentinel™ CPS group and no TCEP group. When comparing our findings with Wolfrum et al. [15], we found that three studies were included in their analysis for this outcome while we had two studies that were among their three. The study that was not included in our meta-analysis was Seeger et al. [10]. This study accounted for the majority of their major bleeding population, accounting for 63.5% of the patients which likely contributed to the significant findings in the Sentinel™ CPS arm in terms of major or life-threating bleeding outcomes. We did not include Seeger et al. [10] in our analysis.
Subgroup Analysis and Future Direction
We found a NNT of 123 patients to prevent the event of a disabling stroke. This finding necessitates the use of subgroup analysis to determine which patients would derive the most benefit from the Sentinel™ CPS. Unfortunately, PROTECTED TAVR [11] was the only trial that performed subgroup analysis on the Sentinel™ CPS and found no difference in the risk of stroke when analyzing based on patient demographics, comorbidities, surgical risk, valvular anatomy, and procedural variables. Future studies are needed to assess high-risk variables, such as peripheral vascular disease, atrial fibrillation, previous stroke, valve calcification, and procedural variables, such as pre- and post-dilation among other variables to determine which patients would benefit the most from the Sentinel™ CPS to prevent periprocedural disabling stroke.
Limitations
One major limitation of the trials, except for PROTECTED TAVR [11], was the use of small sample sizes, thus limiting the power of the endpoints. Furthermore, a relatively short follow-up period was employed by all the trials included in this analysis. This could represent a pitfall for these trials as stroke symptoms may be subtle and only detected after extensive outpatient examination, or by the patient over a longer period of time. Additionally, because various TAVI valves were used in the trials, the results may be biased in that each TAVI valve may have a different inherent embolization risk. Furthermore, the Sentinel™ CPS is currently the only US Food and Drug Administration (FDA)-approved device for embolic protection during TAVI, therefore limiting the ability to compare outcomes to other TCEP devices. The Sentinel™ device does not provide protection for the left vertebral artery, which could be a potential avenue for embolization. Lastly, regarding secondary outcomes, not all trials reported these outcomes, which could have played a role in the discrepancy revealed in our discussion between previous works and ours. Specifically, major or life-threatening bleeding was only reported in CLEAN-TAVI [4] and MISTRAL-C [5], resulting in a low sample size to detect significant differences. Overall, further work is needed to close these gaps and include analysis on valvular and procedural variables to determine independent stroke events.
Conclusions
Overall, our analysis demonstrated that the use of the Sentinel™ CPS in TAVI had no difference in the risk of periprocedural stroke, but had a significant decreased risk of periprocedural disabling stroke when compared to TAVI without TCEP, helping add some clarity on the debate of TCEP in TAVI.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Wissam Harmouch: concept and design, drafting the manuscript, editing the manuscript, aggregating the edits and revisions. Barbara Karnkowska: literature review, writing portions of the manuscript. Ravi Thakker: concept and design, literature review, writing portions of the manuscript. Peter Rasmussen: writing portions of the manuscript. Mostafa Shalaby, Wissam Khalife, Haider Alwash, Afaq Motiwala, Paul Kumfa, Syed Gilani, Hani Jneid and Umamahesh Rangasetty: editing and revising the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Wissam Harmouch, Barbara Karnkowska, Ravi Thakker, Peter Rasmussen, Mostafa Shalaby, Wissam Khalife, Haider Alwash, Afaq Motiwala, Paul Kumfa, Syed Gilani, Hani Jneid, and Umamahesh Rangasetty have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Siontis GC, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, Søndergaard L, Jüni P, Windecker S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J. 2016;37(47):3503–3512. doi: 10.1093/eurheartj/ehw225. [DOI] [PubMed] [Google Scholar]
- 2.Burke CR, Kirkpatrick JN, Otto CM. Goals of care in patients with severe aortic stenosis. Eur Heart J. 2020;41(8):929–932. doi: 10.1093/eurheartj/ehz567. [DOI] [PubMed] [Google Scholar]
- 3.Auffret V, Regueiro A, Del Trigo M, Abdul-Jawad Altisent O, Campelo-Parada F, Chiche O, Puri R, Rodés-Cabau J. Predictors of early cerebrovascular events in patients with aortic stenosis undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68(7):673–684. doi: 10.1016/j.jacc.2016.05.065. [DOI] [PubMed] [Google Scholar]
- 4.Haussig S, Mangner N, Dwyer MG, Lehmkuhl L, Lücke C, Woitek F, Holzhey DM, Mohr FW, Gutberlet M, Zivadinov R, Schuler G, Linke A. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA. 2016;316(6):592–601. doi: 10.1001/jama.2016.10302. [DOI] [PubMed] [Google Scholar]
- 5.Van Mieghem NM, van Gils L, Ahmad H, van Kesteren F, van der Werf HW, Brueren G, Storm M, Lenzen M, Daemen J, van den Heuvel AF, Tonino P, Baan J, Koudstaal PJ, Schipper ME, van der Lugt A, de Jaegere PP. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention. 2016;12(4):499–507. doi: 10.4244/EIJV12I4A84. [DOI] [PubMed] [Google Scholar]
- 6.Kapadia SR, Kodali S, Makkar R, Mehran R, Lazar RM, Zivadinov R, Dwyer MG, Jilaihawi H, Virmani R, Anwaruddin S, Thourani VH, Nazif T, Mangner N, Woitek F, Krishnaswamy A, Mick S, Chakravarty T, Nakamura M, McCabe JM, Satler L, Zajarias A, Szeto WY, Svensson L, Alu MC, White RM, Kraemer C, Parhizgar A, Leon MB, Linke A, SENTINEL Trial Investigators Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;69(4):367–377. doi: 10.1016/j.jacc.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 9.Huded CP, Tuzcu EM, Krishnaswamy A, et al. Association between transcatheter aortic valve replacement and early postprocedural stroke. JAMA. 2019;321:2306–2315. doi: 10.1001/jama.2019.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seeger J, Gonska B, Otto M, Rottbauer W, Wöhrle J. Cerebral embolic protection during transcatheter aortic valve replacement significantly reduces death and stroke compared with unprotected procedures. JACC Cardiovasc Interv. 2017;10(22):2297–2303. doi: 10.1016/j.jcin.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Kapadia SR, Makkar R, Leon M, Abdel-Wahab M, Waggoner T, Massberg S, Rottbauer W, Horr S, Sondergaard L, Karha J, Gooley R, Satler L, Stoler RC, Messé SR, Baron SJ, Seeger J, Kodali S, Krishnaswamy A, Thourani VH, Harrington K, Pocock S, Modolo R, Allocco DJ, Meredith IT, Linke A, PROTECTED TAVR Investigators Cerebral embolic protection during transcatheter aortic-valve replacement. N Engl J Med. 2022;387(14):1253–1263. doi: 10.1056/NEJMoa2204961. [DOI] [PubMed] [Google Scholar]
- 12.Seeger J, Kapadia SR, Kodali S, Linke A, Wöhrle J, Haussig S, Makkar R, Mehran R, Rottbauer W, Leon M. Rate of peri-procedural stroke observed with cerebral embolic protection during transcatheter aortic valve replacement: a patient-level propensity-matched analysis. Eur Heart J. 2019;40(17):1334–1340. doi: 10.1093/eurheartj/ehy847. [DOI] [PubMed] [Google Scholar]
- 13.Butala NM, Makkar R, Secemsky EA, Gallup D, Marquis-Gravel G, Kosinski AS, Vemulapalli S, Valle JA, Bradley SM, Chakravarty T, Yeh RW, Cohen DJ. Cerebral embolic protection and outcomes of transcatheter aortic valve replacement: results from the transcatheter valve therapy registry. Circulation. 2021;143(23):2229–2240. doi: 10.1161/CIRCULATIONAHA.120.052874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndunda PM, Vindhyal MR, Muutu TM, Fanari Z. Clinical outcomes of sentinel cerebral protection system use during transcatheter aortic valve replacement: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2020;21(6):717–722. doi: 10.1016/j.carrev.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Wolfrum M, Handerer IJ, Moccetti F, et al. Cerebral embolic protection during transcatheter aortic valve replacement: a systematic review and meta-analysis of propensity score matched and randomized controlled trials using the Sentinel cerebral embolic protection device. BMC Cardiovasc Disord. 2023;23:306. doi: 10.1186/s12872-023-03338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.