Abstract
In established T-cell lines, the membrane-fusing capacity of the human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins mediates cytopathic effects, both syncytium formation and single-cell lysis. Furthermore, changes in the HIV-1 envelope glycoproteins are responsible for the increased CD4+ T-cell-depleting ability observed in infected monkeys upon in vivo passage of simian-human immunodeficiency virus (SHIV) chimeras. In this study, a panel of SHIV envelope glycoproteins and their mutant counterparts defective in membrane-fusing capacity were expressed in primary human CD4+ T cells. Compared with controls, all of the functional HIV-1 envelope glycoproteins induced cell death in primary CD4+ T-cell cultures, whereas the membrane fusion-defective mutants did not. Death occurred almost exclusively in envelope glycoprotein-expressing cells and not in bystander cells. Under standard culture conditions, most dying cells underwent lysis as single cells. When the cells were cultured at high density to promote syncytium formation, the envelope glycoproteins of the passaged, pathogenic SHIVs induced more syncytia than those of the respective parental SHIV. These results demonstrate that the HIV-1 envelope glycoproteins induce the death of primary CD4+ T lymphocytes by membrane fusion-dependent processes.
Human immunodeficiency virus type 1 (HIV-1) infection of humans is characterized by progressive loss of CD4+ T lymphocytes, leading to the development of AIDS (2, 10, 21, 23). The cause of CD4+ T-cell depletion is unknown, although measurements of viral dynamics in HIV-1-infected humans suggest the possible contribution of viral cytopathic effects and immunological clearance to the death of infected cells (24, 51). Because most immunopathogenic mechanisms are dependent on CD4+ T-cell function, T-cell loss in vivo must be driven by nonimmunologic processes, most likely virus determined. Consistent with this model, the degree of CD4+ T-cell decline is directly related to the virus load and inversely related to the level of antiviral immune responses (5, 12, 25–27, 32, 46).
Several HIV-1 proteins have been reported to exert cytotoxic or cytostatic effects in tissue culture. The Tat regulatory protein has been reported to be toxic when added exogenously to cells (3, 36, 40) but has also been shown to be protective against apoptosis when expressed endogenously (40). Vpr arrests the cell cycle of infected cells at the G2/M phase, which can lead to caspase activation and apoptosis (49). The regulatory protein Nef has been suggested to induce apoptosis through a serine/threonine kinase-dependent signaling pathway (42). Studies of HIV-1 deletion mutants, however, have demonstrated that the expression of these regulatory proteins is either not necessary or insufficient for the major cytopathic effects of virus infection (14, 45, 47). Rather, the HIV-1 envelope glycoproteins apparently mediate most of the acute cytotoxic consequences accompanying virus production in the infected cell. The envelope glycoproteins support virus entry into host cells by binding the receptors, CD4 and chemokine receptors, and promoting the fusion of the viral and target cell membranes (1, 9, 13, 16–19, 22, 31, 39). Analogous membrane fusion events have been shown to contribute to HIV-1 cytopathic effects in immortalized cell lines (6, 35, 38). HIV-1 envelope glycoproteins expressed on the surfaces of infected cells can bind receptors on adjacent, uninfected cells, resulting in the fusion of cells and the formation of multinucleated syncytia (37, 48). Syncytia exhibit limited longevity and undergo apoptosis (34, 35, 50). In the context of a single infected cell, intracellular HIV-1 envelope glycoprotein-receptor interactions can trigger membrane fusion events that result in cell lysis (6). Modulation of the membrane-fusing capacity of the HIV-1 envelope glycoproteins has been shown to alter the cytopathic properties of the virus in tissue-cultured cells (33).
In vivo studies of the HIV-1 envelope glycoproteins have been conducted using simian-human immunodeficiency virus (SHIV)-infected rhesus macaques. SHIVs contain the HIV-1 tat, rev, vpu, and env genes cloned into the simian immunodeficiency virus provirus and thus express HIV-1 envelope glycoproteins. Two HIV-1 envelope glycoproteins that have been studied in this context are derived from the HXBc2 virus, a T-cell-tropic strain that uses the CXCR4 coreceptor (22, 41), and the 89.6 virus, a dual-tropic strain that can use either CCR5 or CXCR4 as a coreceptor (11, 18). SHIV chimeras containing these HIV-1 envelope glycoproteins do not cause severe CD4+ T-cell depletion or other known pathological consequences in infected macaques. However, after serial in vivo passage, pathogenic variants of both SHIV-HXBc2 and SHIV-89.6 emerged (28, 44). The passaged viruses, SHIV-HXBc2P and SHIV-89.6P, respectively, cause profound and rapid CD4+ T-cell depletion and, eventually, an AIDS-like disease in rhesus monkeys. The SHIV-89.6P determinants of CD4+ T-cell-depleting ability have been mapped to the exterior domains of the gp120 and gp41 envelope glycoproteins (29). Changes in these regions specified three properties: increased membrane fusogenicity, enhanced affinity of chemokine receptor binding, and increased resistance to neutralizing antibodies (20, 29). Envelope glycoprotein changes were also shown to be sufficient for the rapid CD4+ T-cell depletion induced by SHIV-HXBc2P (8). In this case, unlike that of SHIV-89.6P, large increases in virus replicative ability in vivo resulted from animal passage and likely contribute to increased pathogenicity.
To date, the cytopathic properties of the HIV-1 envelope glycoproteins have been studied almost exclusively in established cell lines. Here we study the consequences of HIV-1 envelope glycoprotein expression in primary human CD4+ T lymphocytes. The envelope glycoproteins from the parental and pathogenic SHIV variants were studied, as well as mutant derivatives of these envelope glycoproteins that are defective in membrane-fusing capacity.
MATERIALS AND METHODS
Cell lines and primary cell cultures.
293T cells were grown in Dulbecco modified Eagle medium–10% fetal calf serum (FCS) with antibiotics. Primary human CD4+ T cells were isolated from fresh blood using the RosetteSep negative selection system (StemCell Technologies, Vancouver, British Columbia, Canada). Whole human peripheral blood mononuclear cells (PBMC) were isolated from fresh blood by Ficoll-Paque (Amersham Pharmacia, Uppsala, Sweden) density centrifugation. All isolated primary cells were cultured (106 cells/ml) in RPMI–10% FCS with antibiotics. Activation of primary cells was achieved through an initial 3-day stimulation with 1 μg of purified phytohemagglutinin (Murex Biotech, Dartford, England)/ml and subsequent culturing with 10 U of recombinant human interleukin-2 (Collaborative Biomedical Products, Bedford, Mass.)/ml.
Viral vectors.
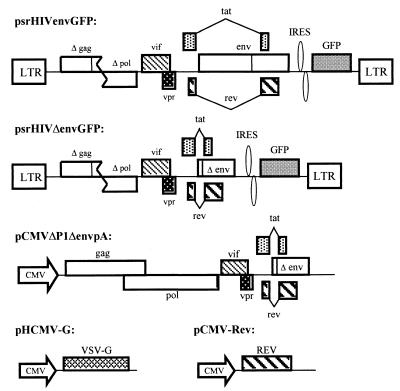
Replication-defective HIV-1 vectors that were capable of expressing HIV-1 envelope glycoproteins and green fluorescent protein (GFP) were constructed. The psrHIVenvGFP vector was modified from previously described vector v653rtatpC (43) through insertion of an internal ribosome entry sequence (NcoI-NcoI) and the enhanced GFP gene (Clontech) (NcoI-XhoI) in place of nef. The psrHIVenvGFP vector, in addition to the envelope glycoproteins and GFP, expresses the HIV-1 Tat, Rev, and Vif proteins and a C-terminally truncated, functionally defective Vpr protein derived from the HXBc2 strain. The KpnI-BamHI fragments of the coding sequences for the HXBc2, HXBc2-P, 89.6, and 89.6P envelope glycoproteins were cloned into the corresponding sites of this vector. Phenylalanine 522 in each of these envelope glycoproteins was changed to tyrosine using PCR site-directed mutagenesis to create the corresponding F/Y mutants. The psrHIVΔenvGFP construct was made by deletion of the env sequences while leaving the Rev-responsive element (HXBc2 nucleotide positions 6094 to 7655) intact. Recombinant viruses were produced by cotransfection of 293T cells with psrHIVenvGFP, pCMVΔP1ΔenvpA (43), pHCMV-G (52), and a Rev-expressing plasmid in a 10:10:2:1 ratio. At 12 h following transfection, the cells were washed and cultured in RPMI–10% FCS with antibiotics. Conditioned medium containing recombinant viruses was harvested and filtered (0.45-μm pore size) 24 h later.
Titration of recombinant virus.
Recombinant virus was titered on either Jurkat cells or activated primary human CD4+ T cells, depending on which target cells were to be used in the experiment. Cells were cultured at a density of 5 × 105 cells/ml in 24-well plates. Recombinant virus was added in serial dilutions while maintaining a constant volume of culture medium in each well. Forty-eight hours after transduction, the cells were collected, fixed in 3.7% formaldehyde, and analyzed by fluorescence-activated cell sorting (Becton Dickinson FACScan).
Immunofluorescence.
293T cells were cultured on chamber slides (Lab-Tek, Naperville, Ill.) with either psrHIVΔenvGFP recombinant virus or recombinant virus expressing the HXBc2P envelope glycoproteins. The slides were washed in phosphate-buffered saline (PBS) and fixed in 3.7% formaldehyde for 20 min at room temperature. The cells were then permeabilized in PBS–0.1% Triton X-100 for 15 min. Slides were then placed in blocking solution (5% bovine serum albumin, 0.2% Tween 20, and 0.2% Triton X-100 in PBS) for 1 h. Cells were stained with a 1:100 dilution of F105 anti-gp120 antibody in 1:5 blocking solution for 1 h at room temperature and then washed in buffer (0.2% Triton X-100 and 0.2% Tween 20 in PBS) three times for 10 min each. Rhodamine-conjugated goat anti-human antibody was added to the slides in a 1:200 dilution in 1:5 blocking solution for 1 h at room temperature. Cells were washed again, and slides were visualized on a Nikon TE300 inverted microscope.
Immunoprecipitation.
293T cells were transduced with equivalent amounts of titered virus, washed at 5 h after transduction, and incubated in [35S]methionine and [35S]cysteine in labeling media depleted of cysteine and methionine. At 24 h after transduction, the cells were pelleted and the supernatant was discarded. Cell pellets were resuspended in NP-40 lysis buffer (0.5% NP-40, 0.5 M NaCl, 10 mM Tris, pH 7.5) and were centrifuged to pellet cellular debris. Cellular lysates were precipitated in the presence of unlabeled cell lysate with anti-gp120 antibody F105 or anti-HIV patient sera and protein A-Sepharose beads at 4°C overnight. Immunoprecipitation was followed by three washes with wash buffer (0.5% NP-40, 5 M NaCl in PBS), one wash with PBS, and resuspension in Tris-HCl loading buffer containing 5% β-mercaptoethanol. Samples were then boiled for 5 min, analyzed on a sodium dodecyl sulfate–7.5% polyacrylamide gel, and exposed to film.
Transduction of cells and viability assay.
Either Jurkat cells or activated primary human CD4+ T cells were cultured in duplicate in 24-well plates at a density of 2.5 × 105 cells in 500 μl of medium. A sufficient titer of recombinant viruses was added to each well to achieve infection of 5% of the cells. The medium was changed at 5 h to remove excess input virus. At 5 h after transduction and at 24-h intervals thereafter, the cells in duplicate wells were centrifuged for 2 min at 1,500 rpm, washed, and stained in 100 μl of PBS with 250 ng of propidium iodide (Molecular Probes, Eugene, Oreg.) for 15 min at room temperature. Cells were then washed in PBS and fixed in 4% formaldehyde. Fluorescence-activated cell sorter analysis was performed immediately to quantify GFP expression and propidium iodide positivity.
Transduction of cells and syncytium assay.
Activated primary human CD4+ T cells were cultured in 96-well plates at a density of 4 × 105 cells in 200 μl of RPMI–10% FCS with interleukin-2 and antibiotics. All transductions were done in separate, duplicate wells with sufficient amounts of titered virus to achieve a 10% frequency of infection. Syncytia were counted by visual inspection using a Nikon TE300 inverted microscope at 42, 56, 66, and 78 h after transduction of the cultures.
RESULTS
Construction of an HIV-1 vector expressing envelope glycoproteins and GFP.
Although previous studies have examined the consequences of HIV-1 envelope glycoprotein expression in immortalized cell lines, similar studies of primary human T lymphocytes have been hampered by the relative resistance of these cells to transfection. We utilized a defective HIV-1 vector to express the HIV-1 envelope glycoproteins from nonpathogenic and pathogenic SHIV variants in primary CD4+ T cells enriched from human PBMC (Fig. 1). To examine the role of membrane fusion in any observed phenotypes, fusion-defective envelope glycoproteins (designated F/Y) containing an alteration of phenylalanine 522 were also expressed in these vectors (4). Phenylalanine 522 is located in the “fusion peptide” at the N terminus of gp41, and changes in this residue attenuate membrane-fusing ability without affecting receptor binding (4). Expression of the envelope glycoproteins in the HIV-1 vectors is under the control of the viral long terminal repeats and Tat and Rev proteins and therefore should achieve levels comparable to those in HIV-1-infected cells. The potential contribution of HIV-1 regulatory proteins expressed by the vectors (Tat, Rev, Vif, and a truncated, defective Vpr protein) to the observed phenotypes was controlled for by including a vector with env deleted in the study. As an aid in titering the recombinant viruses and in identifying transduced cells, a gene for GFP, which was expressed from an internal ribosome entry sequence, was included in the vectors. All recombinant viruses were pseudotyped with the vesicular stomatitis virus (VSV) G glycoprotein to equalize entry into target cells despite differences in the efficiency with which the HIV-1 envelope glycoproteins encoded by the vectors might mediate virus entry. Because the sequences encoding the VSV G glycoprotein are not packaged into the recombinant virions, the VSV G glycoprotein is not expressed in the target cells.
FIG. 1.
Defective HIV-1 vectors expressing HIV-1 envelope glycoproteins. The psrHIVenvGFP plasmid expressing the HIV-1 envelope glycoproteins is shown. The HXBc2, HXBc2P, 89.6, and 89.6P envelope glycoproteins and the corresponding 522 F/Y mutant envelope glycoproteins were individually encoded in this construct. The control vector psrHIVΔenvGFP, which contains a large env deletion, is also shown. Both psrHIVenvGFP and psrHIVΔenvGFP plasmids contain intact HIV-1 packaging signals. LTR, long terminal repeat; IRES, internal ribosome entry sequence. To generate recombinant HIV-1 vectors, one of the two plasmids described above was cotransfected into 293T cells along with the pCMVΔP1ΔenvpA, pHCMV-G, and pCMV-Rev plasmids. These three plasmids supply the HIV-1 gag and pol gene products, the VSV G glycoprotein, and the HIV-1 Rev protein, respectively, in trans. All three plasmids lack HIV-1 packaging signals and express gene products under the control of the cytomegalovirus immediate-early promoter (CMV).
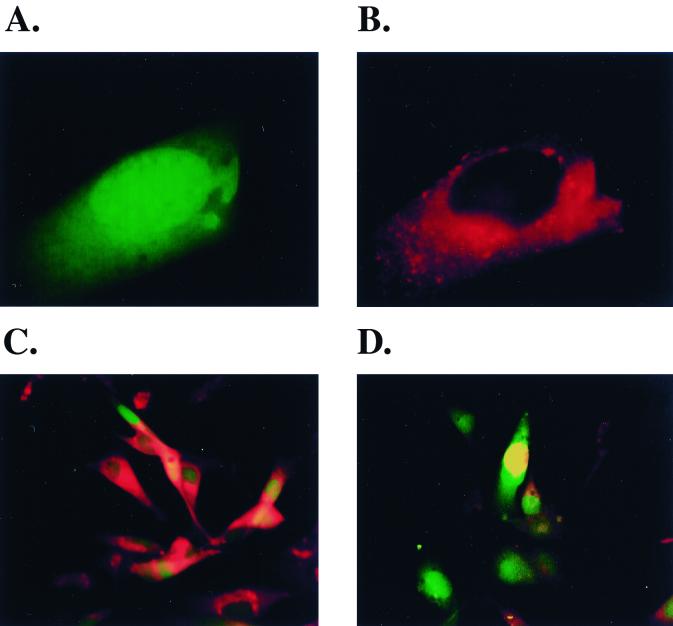
To verify the utility of GFP expression as a marker for envelope glycoprotein expression by the vectors, adherent 293T cells grown in slide chambers were infected with either the psrHIVHXBc2PGFP or the psrHIVΔenvGFP vector preparation. Because the intracellular expression of the HIV-1 envelope glycoproteins is typically more abundant than that on the cell surface, the transduced cells were permeabilized and stained with the F105 antibody, which efficiently recognizes all the variants of HIV-1 envelope glycoproteins used in this study. In cultures transduced with the psrHIVHXBc2PGFP vector, every GFP-positive cell exhibited significant staining with the F105 antibody (Fig. 2A to C). The pattern of F105 staining was consistent with the known abundance of the HIV-1 envelope glycoproteins in the endoplasmic reticulum of an expressing cell. In cultures transduced with the control psrHIVΔenvGFP vector, although many GFP-positive cells were evident, these did not stain with the F105 antibody (Fig. 2D). Thus, GFP expression allows a simple and nondisruptive determination of which cells in the culture are expressing HIV-1 envelope glycoproteins.
FIG. 2.
Utility of GFP fluorescence as a marker for cells expressing HIV-1 envelope glycoproteins. 293T cells were transduced with either the psrHIVHXBc2PGFP (panels A to C) or the psrHIVΔenvGFP (D) vector and then fixed and permeabilized 48 h later. (A) A single transduced cell (×85 magnification) demonstrating cytoplasmic GFP fluorescence. (B) The cell shown in A was labeled with the F105 anti-gp120 antibody and rhodamine-conjugated goat anti-human antibody. (C) The 293T cells transduced with the psrHIVHXBc2PGFP vector are shown (×50 magnification), demonstrating that every GFP-expressing cell also stains with the F105 antibody and rhodamine-conjugated secondary antibody. (D) The 293T cells transduced with the psrHIVΔenvGFP vector are shown at ×50 magnification. Note that the GFP-positive cells do not stain with a combination of the F105 antibody and the rhodamine-conjugated secondary antibody.
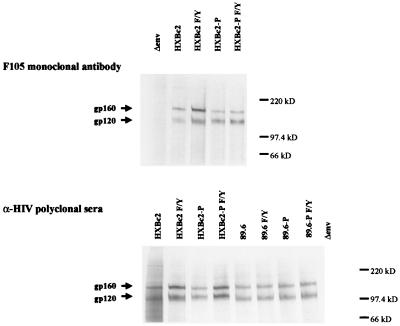
To determine if the different HIV-1 envelope glycoproteins were expressed at similar levels by the vectors, equivalent amounts of titered virus were used to transduce 293T cells. At 24 h after infection, the cells were radiolabeled and the cell lysates were precipitated by either the F105 anti-gp120 antibody or a mixture of sera from HIV-1-infected individuals. The expression levels of all of the HXBc2 envelope glycoprotein derivatives were similar, as were those of the 89.6 envelope glycoprotein variants (Fig. 3).
FIG. 3.
Levels of HIV-1 envelope glycoprotein expression in transduced cells. 293T cells were transduced with titered recombinant HIV-1 vectors expressing the HIV-1 envelope glycoproteins, labeled, and lysed. The cell lysates were precipitated with either the F105 anti-gp120 monoclonal antibody or with a mixture of sera from HIV-1-infected individuals. The bands corresponding to the gp160 envelope glycoprotein precursor and mature gp120 envelope glycoprotein are indicated.
Effects of HIV-1 envelope glycoprotein expression in Jurkat T lymphocytes.
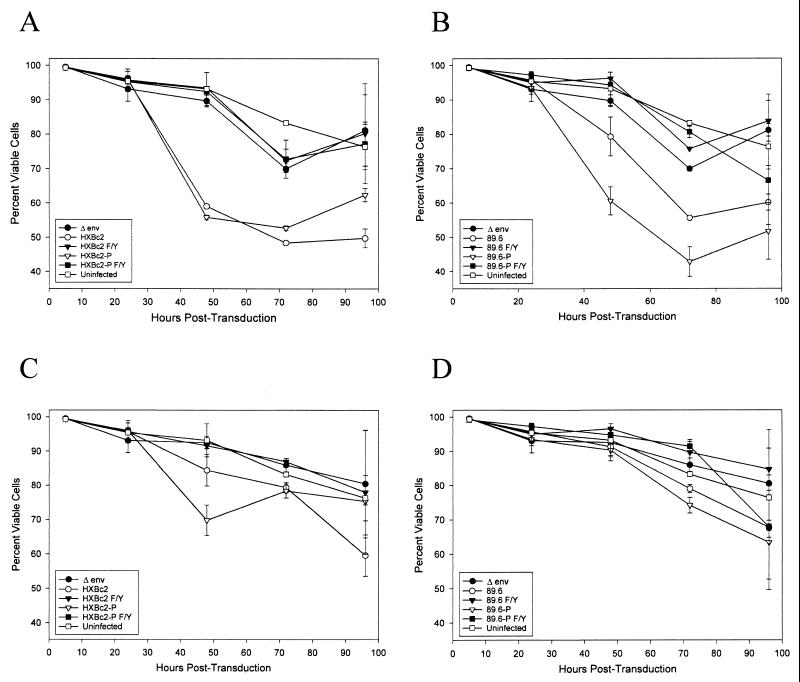
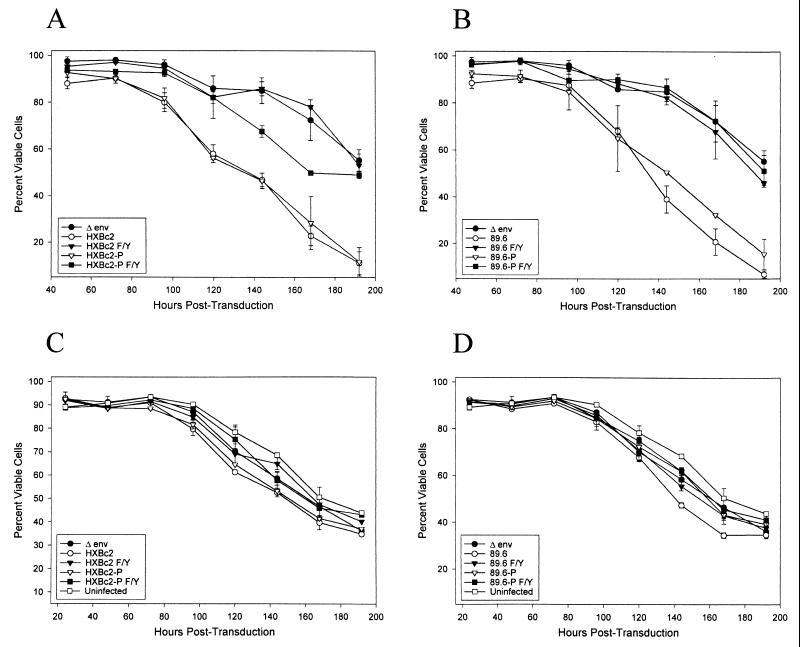
To examine the consequences of HIV-1 envelope glycoprotein expression in an established human CD4+ T-lymphocyte line, Jurkat cells were incubated with the HIV-1 vectors, washed after 5 h, and stained at successive time points with propidium iodide to determine cell viability. GFP+ cells, which express the HIV-1 envelope glycoproteins, were examined separately from GFP− cells. Cells expressing the HXBc2 and HXBc2P envelope glycoproteins exhibited significant decreases in viability compared with the psrHIVΔenvGFP- transduced control cells (Fig. 4A). Cells expressing the membrane fusion-defective envelope glycoproteins (HXBc2 F/Y and HXBc2P F/Y) did not differ in viability from the psrHIVΔenvGFP-expressing control cells or from uninfected cells. Expression of the 89.6 and 89.6P envelope glycoproteins resulted in significant losses of viability in the Jurkat cultures, with the 89.6P envelope glycoproteins exhibiting more toxicity than the 89.6 envelope glycoproteins (Fig. 4B). The viability of the cultures expressing the 89.6 F/Y and 89.6P F/Y envelope glycoproteins did not differ from that of the control cultures. Flow cytometric analysis of the Jurkat cells expressing the HXBc2, HXBc2P, 89.6, and 89.6P envelope glycoproteins indicated that the vast majority of the propidium iodide-positive cells were single cells and not syncytia (data not shown).
FIG. 4.
Cell viability in Jurkat lymphocytes expressing HIV-1 envelope glycoproteins. Jurkat lymphocytes were incubated with a sufficient amount of recombinant viruses expressing the indicated envelope glycoproteins to result in a 5% transduction efficiency. Control Jurkat lymphocytes (uninfected) that were mock infected were included. At the indicated times, the independent duplicate cultures were stained with propidium iodide. Flow cytometry was used to quantify cell viability in both the GFP-positive (A and B) and GFP-negative (C and D) populations.
The viability of the GFP− Jurkat cells was generally greater than that of the GFP+ cells (Fig. 4C and D). In the cultures expressing the HIV-1 envelope glycoproteins competent for membrane fusion, the viability of the GFP− cells was slightly decreased compared with that in cultures expressing the membrane fusion-defective envelope glycoproteins or control cultures. These results suggest that the death of bystander cells in the HIV-1 envelope glycoprotein-expressing Jurkat cultures is infrequent compared with the death of envelope glycoprotein-expressing cells and that such death is dependent on the membrane-fusing activity of the envelope glycoproteins. Thus, consistent with our previous study, almost all of the cytopathic effects associated with the expression of the HIV-1 envelope glycoproteins in Jurkat cells depend on the ability of the viral envelope glycoproteins to mediate the fusion of membranes.
Effects of HIV-1 envelope glycoprotein expression in primary human CD4+ T cells.
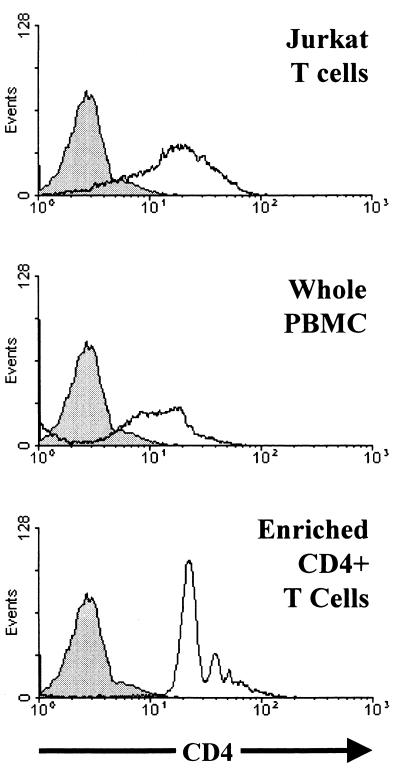
To examine the consequences of HIV-1 envelope glycoprotein expression in primary human T lymphocytes, CD4+ T cells were purified from human peripheral blood. A negative selection was used to avoid any artifactual effects of CD4-directed antibodies and achieved greater than 95% pure populations of CD4+ T lymphocytes (Fig. 5). The CD4+ T cells were activated, infected by the recombinant viruses expressing the various envelope glycoproteins, washed, and monitored for viability by propidium iodide staining at successive time points. Both GFP+ and GFP− subsets of the culture were studied.
FIG. 5.
CD4 expression in cells used in the study. The OKT4 anti-CD4 monoclonal antibody was used to stain the surface of Jurkat T lymphocytes, whole human PBMC isolated by standard Ficoll-Paque density centrifugation, and a PBMC preparation enriched for CD4+ T cells. The staining of 293T cells, which were used as a negative control, is shown in gray.
The expression of membrane fusion-competent HIV-1 envelope glycoproteins resulted in significant loss of viability in the GFP+ primary human CD4+ T lymphocytes (Fig. 6A and B and 7). As was observed for the envelope glycoprotein-expressing Jurkat cultures, most of the propidium iodide-positive cells were single cells and not syncytia (data not shown). Primary CD4+ T cells expressing the membrane fusion-defective F/Y envelope glycoproteins did not reproducibly differ in viability from control cells transduced with the psrHIVΔenvGFP vector.
FIG. 6.
Viability of primary CD4+ T cells expressing HIV-1 envelope glycoproteins. Primary human CD4+ T lymphocytes were isolated from the peripheral blood and activated. These cells were transduced with titered recombinant viruses, washed, and assayed at successive time points for cell viability using propidium iodide. Flow cytometry was used to quantify viability in GFP-positive (A and B) and GFP-negative (C and D) populations.
None of the HIV-1 envelope glycoproteins affected the viability of the GFP− primary CD4+ T cells (Fig. 6C and D). Thus, expression of HIV-1 envelope glycoproteins in primary T-cell cultures did not result in the death of bystander cells.
Syncytium formation in primary human CD4+ T cells.
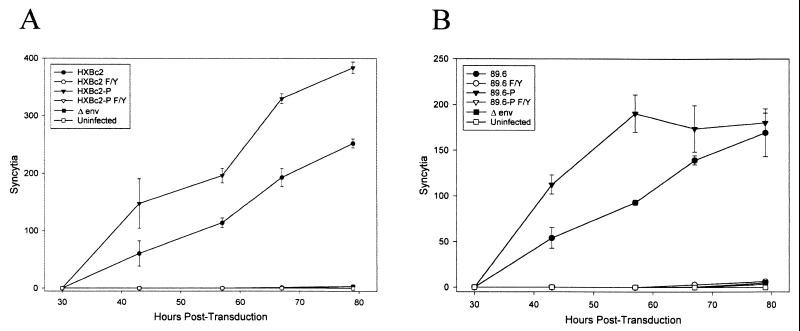
The envelope glycoproteins of some pathogenic SHIV variants have been reported to exhibit enhanced syncytium-forming ability, compared with the glycoproteins from the nonpathogenic parental SHIVs (29). To examine the syncytium-forming ability of the HIV-1 envelope glycoproteins used in this study, primary human CD4+ T cells were transduced with the panel of recombinant HIV-1 vectors and then cultured at high density to favor cell-cell interactions. The HXBc2P and 89.6P envelope glycoproteins derived from pathogenic SHIV induced more syncytia than the HXBc2 and 89.6 envelope glycoproteins, respectively (Fig. 8). No difference in the sizes of the syncytia induced by any of these membrane fusion-competent envelope glycoproteins was seen (data not shown). No syncytia were observed in the primary CD4+ T-cell cultures transduced with the psrHIVΔenvGFP vector or with vectors expressing the membrane fusion-defective envelope glycoproteins.
FIG. 8.
Syncytium formation in primary human CD4+ T cells. At various times after the transduction of primary CD4+ T cells with recombinant viruses, syncytia were visually scored. Syncytium formation was monitored in cultures expressing the HXBc2 envelope glycoproteins and derivatives (A) or the 89.6 envelope glycoproteins and derivatives (B).
DISCUSSION
Understanding the role of the HIV-1 envelope glycoproteins in causing the death of primary human CD4+ T lymphocytes has been hampered by the difficulty of transfecting these cells. In this study, we utilized defective HIV-1 vectors to express HIV-1 envelope glycoproteins in primary human CD4+ T cells. Because the envelope glycoproteins are expressed by the vectors in a context similar to that in the authentic provirus, the levels of glycoprotein expression achieved are expected to resemble those in HIV-1-infected cells. The use of GFP as a marker for the vector-transduced cells allowed an estimation of cell death in cells expressing the HIV-1 envelope glycoproteins and in bystander cells.
Primary human CD4+ T lymphocytes expressing functional HIV-1 envelope glycoproteins underwent single-cell lysis when cultured under standard conditions and became incorporated into multinucleated syncytia when cultured at high density. The ability of HIV-1 envelope glycoproteins to mediate membrane fusion was essential for the induction of both cytopathic processes in primary human CD4+ T lymphocytes. Thus, at least in this context, binding of the gp120 envelope glycoprotein to CD4 and/or the chemokine receptors is insufficient to lyse T cells. The importance of membrane fusion to the destruction of single primary CD4+ T cells implies that membrane damage contributes to the process of cell killing. Membrane fusion events mediated by the HIV-1 envelope glycoproteins have been suggested to be important for the induction of cytopathic effects in immortalized cell lines (6, 33, 37, 48). Our results demonstrate that important similarities between envelope glycoprotein-host cell interactions in immortalized lines and primary CD4+ T lymphocytes exist.
Under standard culture conditions, death occurred almost exclusively in the primary T cells expressing the HIV-1 envelope glycoproteins and not in bystander cells. Apparently, expression of the viral envelope glycoproteins at levels comparable to those expected for HIV-1-infected cells did not induce the death of surrounding cells, apart from those incorporated into syncytia. It is possible that bystander cell lysis can be mediated by the HIV-1 envelope glycoproteins in contexts (e.g., within virions or in the presence of cross-linking antibodies) different than those studied in this work. The contribution of bystander cell death to CD4+ T-cell depletion in vivo is uncertain. In HIV-1-infected individuals, the half-life of infected T cells that are not actively producing HIV-1 is as long as that expected of normal T cells (51), suggesting that most bystander cells are not specifically targeted for destruction. However, because of the relatively large size of the uninfected T-cell pool, loss of a small fraction of these cells, which could significantly contribute to cumulative CD4+ T-cell depletion, is not ruled out by the available data. Indeed, it has been suggested that loss of some uninfected CD4+ T lymphocytes may occur in monkeys that exhibit rapid and profound depletion of T cells during acute SHIV infection (29). However, a full understanding of the contribution of bystander cell loss in this model system awaits further studies.
Changes within the HIV-1 envelope glycoproteins have been shown to be important for the increased pathogenicity of SHIVs that have been passaged in monkeys (8, 29). Even when the levels of virus replication are taken into account, the envelope glycoprotein ectodomains of one of these passaged viruses, SHIV-89.6P, specify an increased ability to cause CD4+ T-cell depletion in vivo (29). Relative to the envelope glycoproteins of the nonpathogenic parental SHIVs, the SHIV-89.6P and SHIV-HXBc2P envelope glycoproteins more efficiently induced the formation of syncytia in primary CD4+ T lymphocytes. This increase in membrane fusogenicity was less manifest in the induction of cell death in suspensions of primary CD4+ T cells or Jurkat lymphocytes cultured at low density. In this setting, single-cell lysis rather than cell-cell fusion predominates as a form of cytopathic effect. Previous studies have suggested that more successful envelope glycoprotein-receptor interactions are required for the formation of a syncytium than for the lysis of a single cell (6, 7, 15, 33). Under the more-stringent conditions required for syncytium formation, differences in the fusogenic capacities of the nonpathogenic and pathogenic SHIV envelope glycoproteins may be more apparent. The relative contribution of syncytium formation and single-cell lysis to in vivo CD4+ T-lymphocyte destruction is unknown. As is the case for HIV-1-infected humans, syncytia have not been commonly observed in the lymph nodes of SHIV-infected monkeys (29). Syncytia formed in vivo may consist of few cells and therefore may be difficult to identify or may be too short-lived to accumulate. Alternatively, most CD4+ T-lymphocyte death in vivo may involve the lysis of single cells. Further work will be required to determine the precise roles that membrane fusion events mediated by the HIV-1 envelope glycoproteins play in the loss of CD4+ T lymphocytes in the infected host.
FIG. 7.
Propidium iodide (PI) staining of primary CD4+ T cells expressing HIV-1 envelope glycoproteins. Primary CD4+ T cells were transduced with recombinant viruses as described in the legend to Fig. 6. At 168 h after transduction, the cultures were stained with propidium iodide. Flow cytometry was used to quantify propidium iodide staining of the GFP-positive cells. The gates for propidium iodide and GFP were chosen based on the staining of untransduced cells.
ACKNOWLEDGMENTS
We acknowledge Marshall Posner for supplying the F105 monoclonal antibody. We thank Maris Handley at the Dana-Farber Cancer Institute flow cytometry core facility for excellent technical support and Yvette McLaughlin and Sheri Farnum for manuscript preparation. Thanks to Michelle LaBonte for helpful discussions and assistance.
This work was supported by grants from the National Institutes of Health (AI24755 and AI33832) and by Center for AIDS Research grant AI28691. Additional support was provided by the G. Harold and Leila Y. Mathers Foundation, the late William F. McCarty-Cooper, the Friends 10, and Douglas and Judith Krupp.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1991;28:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamert S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 3.Bartz S R, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergeron L, Sullivan N, Sodroski J. Target cell-specific determinants of membrane fusion within the human immunodeficiency virus type 1 gp120 third variable region and gp41 amino terminus. J Virol. 1992;66:2389–2397. doi: 10.1128/jvi.66.4.2389-2397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao J, Park I, Cooper A, Sodroski J. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J Virol. 1996;70:1340–1354. doi: 10.1128/jvi.70.3.1340-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J, Vasir B, Sodroski J. Changes in the cytopathic effects of human immunodeficiency virus type 1 associated with a single amino acid alteration in the ectodomain of the gp41 transmembrane glycoprotein. J Virol. 1994;68:4662–4668. doi: 10.1128/jvi.68.7.4662-4668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayabyab M, Karlsson G B, Etemad-Moghadam B A, Hofmann W, Steenbeke T, Halloran M, Fanton J W, Axthelm M K, Letvin N L, Sodroski J. Changes in the human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2) J Virol. 1999;73:976–984. doi: 10.1128/jvi.73.2.976-984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 10.Clavel F, Guetard D, Brun-Vezinet F, Chamaret S, Rey M A, Santos-Ferreira M O, Laurent A F, Dauguet C, Katlama C, Rouzioux C, et al. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 11.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conner R I, Mohri H, Cao Y, Ho D D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalgleish A G, Beverly P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4(T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 14.Dayton A I, Sodroski J G, Rosen C A, Goh W C, Haseltine W A. The transactivator gene of the human T-cell lymphotropic virus type III is required for replication. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 15.Dedera D, Ratner L. Demonstration of two distinct cytopathic effects with syncytium formation-defective human immunodeficiency virus type 1 mutants. J Virol. 1991;65:6129–6139. doi: 10.1128/jvi.65.11.6129-6136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 17.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 18.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Madon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Etemad-Moghadam B, Sun Y, Nicholson E K, Fernandes M, Liou K, Lee J, Sodroski J. Envelope glycoprotein determinants of increased fusogenicity in pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J Virol. 2000;74:4433–4440. doi: 10.1128/jvi.74.9.4433-4440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauci A S, Macher A M, Longo D L, Lane H C, Rook A H, Masur H, Gelman E P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 23.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B G, Palker T J, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 24.Ho D D, Nekumann A, Perelson A, Chen W, Leonard J, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–129. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 25.Ho D D, Moudgil T, Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989;321:1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- 26.Holodniy M, Katzenstein D A, Sengupta S. Detection and quantification of human immunodeficiency virus RNA in patient serum by use of the polymerase chain reaction. J Infect Dis. 1991;164:862–866. doi: 10.1093/infdis/163.4.862. [DOI] [PubMed] [Google Scholar]
- 27.Hsia K, Spector J A. Human immunodeficiency virus DNA is present in a high percentage of CD4+ lymphocytes of seropositive individuals. J Infect Dis. 1991;164:470–475. doi: 10.1093/infdis/164.3.470. [DOI] [PubMed] [Google Scholar]
- 28.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson G B, Halloran M, Li J, Park I, Gomila R, Reimann K A, Axthelm M K, Iliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 32.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular and immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowalski M, Bergeron L, Dorfman T, Haseltine W, Sodroski J. Attenuation of human immunodeficiency virus type 1 cytopathicity by a mutation affecting the transmembrane envelope glycoprotein. J Virol. 1991;65:281–291. doi: 10.1128/jvi.65.1.281-291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent-Crawford A G, Krust B, Muller S, Riviere Y, Rey Cuille M-A, Bechet J-M, Montagnier L, Hovanessian A G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 35.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Paule Kieny M, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retrovir. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 36.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 37.Lifson J D, Feinberg M B, Reyes G R, Rabin L, Banapour B, Chakrabar B, Wong-Staal F, Steimer K S, Engleman E G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV glycoprotein. Nature. 1986;323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Koga Y, Tanaka K, Sasaki M, Kimura G, Nomoto K. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J Virol. 1994;68:390–399. doi: 10.1128/jvi.68.1.390-399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 40.McCloskey T W, Ott M, Tribble E, Khan S A, Teichberg S, Paul M O, Pahwa S, Verdin E, Chirmule N. Dual role of HIV Tat in regulation of apoptosis in T cells. J Immunol. 1997;158:1014–1019. [PubMed] [Google Scholar]
- 41.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 42.Okada H, Takei R, Tashiro M. Nef protein of HIV-1 induces apoptotic cytolysis of murine lymphoid cells independently of CD95 (Fas) and its suppression serine/threonine protein kinase inhibitors. FEBS Lett. 1997;417:61–64. doi: 10.1016/s0014-5793(97)01255-6. [DOI] [PubMed] [Google Scholar]
- 43.Parolin C, Taddeo B, Palu B, Sodroski J. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology. 1996;222:415–422. doi: 10.1006/viro.1996.0438. [DOI] [PubMed] [Google Scholar]
- 44.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogel M, Wu L, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saag M S, Crain M J, Decker M D, Campbell-Hill S, Robinson S, Brown W E, Leuther M, Whitley R J, Hahn B H, Shaw G M. High level viremia in adults and children infected with human immunodeficiency virus: relation to the disease stage and CD4+ lymphocyte levels. J Infect Dis. 1991;164:72–80. doi: 10.1093/infdis/164.1.72. [DOI] [PubMed] [Google Scholar]
- 47.Sodroski J, Goh W C, Rosen C A, Tartar A, Portelle D, Burny A, Haseltine W A. Replicative and cytopathic potential of human T-lymphotropic virus type III (HTLV-III/LAV) with sor gene deletions. Science. 1986;240:1549–1553. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- 48.Sodroski J, Goh W C, Rosen C A, Campbell K, Haseltine W A. Role of the HTLV/LAV envelope in syncytia formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 49.Stewart S A, Poon B, Song J Y, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–3111. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terai C, Kornbluth R S, Pauza C D, Richman D D, Carson D A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Investig. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei X, Ghosh S, Taylor M, Johnson V, Emini E, Deutsch P, Lifson J, Bonhoeffer S, Nowak M, Hahn B, Saag M, Shaw G. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 52.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Griedmann T. A general method for the generation of high-titer, pan-tropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]