Abstract
Purpose:
To identify potential predictors of response and resistance mechanisms in patients with hormone receptor–positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC) treated with the cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor abemaciclib ± endocrine therapy (ET), baseline and acquired genomic alterations in circulating tumor DNA (ctDNA) were analyzed and associated with clinical outcomes.
Experimental Design:
MONARCH 3: postmenopausal women with HR+, HER2− ABC and no prior systemic therapy in the advanced setting were randomly assigned to abemaciclib or placebo plus nonsteroidal aromatase inhibitor (NSAI). nextMONARCH: women with HR+, HER2− metastatic breast cancer that progressed on/after prior ET and chemotherapy were randomly assigned to abemaciclib alone (two doses) or plus tamoxifen. Baseline and end-of-treatment plasma samples from patients in MONARCH 3 and nextMONARCH (monotherapy arms) were analyzed to identify somatic genomic alterations. Association between genomic alterations and median progression-free survival (mPFS) was assessed.
Results:
Most patients had ≥1 genomic alteration detected in baseline ctDNA. In MONARCH 3, abemaciclib+NSAI was associated with improved mPFS versus placebo+NSAI, regardless of baseline alterations. ESR1 alterations were less frequently acquired in the abemaciclib+NSAI arm than placebo+NSAI. Acquired alterations potentially associated with resistance to abemaciclib ± NSAI included RB1 and MYC.
Conclusions:
In MONARCH 3, certain baseline ctDNA genomic alterations were prognostic for ET but not predictive of abemaciclib response. Further studies are warranted to assess whether ctDNA alterations acquired during abemaciclib treatment differ from other CDK4/6 inhibitors. Findings are hypothesis generating; further exploration is warranted into mechanisms of resistance to abemaciclib and ET.
Translational Relevance.
This study investigated genomic alterations in the circulating tumor DNA (ctDNA) of patients in the phase III MONARCH 3 and phase II nextMONARCH studies. This study is the first to explore genomic alterations in ctDNA samples from patients with hormone receptor–positive, HER2-negative advanced breast cancer treated with abemaciclib ± NSAI and the relationship between baseline or treatment-emergent genomic alterations and clinical outcomes. The most frequent baseline genomic alterations, similar in both studies, were previously associated with endocrine resistance and may additionally drive resistance to cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors plus endocrine therapy (ET). In MONARCH 3, abemaciclib plus NSAI was associated with improved median progression-free survival compared with placebo plus NSAI, regardless of baseline genomic alterations. Acquired alterations potentially associated with resistance to abemaciclib monotherapy or abemaciclib plus NSAI included RB1 and MYC. These findings are hypothesis generating, and further exploration is warranted into mechanisms of resistance to abemaciclib and ET. Understanding potential mechanisms of intrinsic and acquired resistance will help inform future drug development and clinical trials.
Introduction
Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) have changed the treatment landscape of hormone receptor–positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC; ref. 1). Three CDK4/6i, palbociclib, ribociclib and abemaciclib, have been approved for use with endocrine therapy (ET), including nonsteroidal aromatase inhibitors (NSAI) or fulvestrant, in the advanced setting (2–6). Phase III studies have demonstrated significant prolongation of progression-free survival (PFS) with abemaciclib when used as initial therapy for ABC in combination with NSAI (6), and PFS and overall survival (OS) in combination with fulvestrant following progression on ET (5, 7). In addition, abemaciclib is the only CDK4/6i FDA approved as monotherapy following disease progression after ET and chemotherapy in the metastatic setting, and for the adjuvant treatment of HR+, HER2−, node-positive, early breast cancer at high risk of recurrence and a Ki-67 score ≥20% (8).
Despite the efficacy of CDK4/6i, intrinsic resistance occurs in some patients, while others whose tumors initially respond to therapy may develop resistance during treatment, resulting in disease progression (9). While putative mechanisms of resistance have been evaluated, most current evidence comes from preclinical studies with limited clinical evidence of acquired genomic alterations associated with resistance (9–14). Resistance to CDK4/6i currently falls into two main categories: (i) cell-cycle alterations, for example, loss of the Rb tumor suppressor protein, or (ii) alterations in upstream oncogenic signal transduction (13). Greater understanding of the mechanisms of resistance to CDK4/6i will guide development of novel targeted therapeutic strategies aimed at overcoming or circumventing resistance and improving clinical outcomes.
Circulating tumor DNA (ctDNA) analysis is a noninvasive technique used to identify genomic alterations in cancer. This information may be useful for predicting treatment response, identifying mechanisms of resistance, or monitoring disease progression (15, 16). In this study, genomic alterations were analyzed in ctDNA from patients with HR+, HER2− ABC treated with abemaciclib in the MONARCH 3 and nextMONARCH studies.
MONARCH 3 (NCT02246621) was a phase III study of abemaciclib or placebo plus NSAI in postmenopausal women with HR+, HER2− ABC with no prior systemic therapy in the advanced setting. The primary endpoint of PFS was significantly prolonged in the abemaciclib group [median PFS (mPFS), 28.2 months] versus placebo arm (mPFS, 14.8 months; ref. 17). The phase II nextMONARCH trial (NCT02747004) evaluated the safety and efficacy of abemaciclib plus tamoxifen or two different doses of abemaciclib monotherapy (150 or 200 mg) in women with previously treated HR+, HER2− metastatic breast cancer (MBC) that progressed after prior chemotherapy and ET. In the abemaciclib monotherapy arms, mPFS was similar: 6.5 months in the abemaciclib 150 mg arm and 7.4 months in the abemaciclib 200 mg arm (18).
Here, we analyzed baseline and end-of-treatment (EOT) genomic alterations in ctDNA and association with clinical outcomes to identify potential predictors of response and mechanisms of resistance to abemaciclib among patients treated with abemaciclib plus NSAI (MONARCH 3) or abemaciclib monotherapy (nextMONARCH).
Materials and Methods
MONARCH 3 study design and patients
The MONARCH 3 study design was reported previously (6) and is summarized in Supplementary Fig. S1. MONARCH 3 was a phase III, randomized, double-blind trial of abemaciclib or placebo plus NSAI in women with HR+, HER2− ABC. The trial enrolled 493 postmenopausal women randomized 2:1 to receive oral abemaciclib (150 mg twice daily) or placebo, both in combination with NSAI (anastrozole or letrozole).
Eligible postmenopausal women had HR+, HER2– metastatic disease or locoregionally recurrent breast cancer not amenable to resection or radiotherapy with curative intent. Patients must have had either measurable or nonmeasurable bone-only disease as defined by RECIST Version 1.1 (RECIST V1.1), no prior systemic therapy for advanced disease, adequate organ function, and an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤1. Exclusion criteria included visceral crisis, lymphangitic spread or leptomeningeal carcinomatosis; inflammatory breast cancer; evidence or history of central nervous system (CNS) metastases; or prior treatment with everolimus or a CDK4/6i.
nextMONARCH study design and patients
The nextMONARCH study design was reported previously (19) and is summarized in Supplementary Fig. S1. nextMONARCH was a phase II, randomized, open-label study that evaluated efficacy and tolerability of abemaciclib ± tamoxifen in 234 women with previously treated HR+, HER2− MBC that progressed on or after prior ET.
Eligible women had prior treatment with ≥2 chemotherapy regimens (≥1 for MBC) and must have had measurable disease as defined by RECIST V1.1 and ECOG PS ≤1. Exclusion criteria included presence of visceral crisis; evidence or history of CNS metastases or thromboembolic disease; or prior treatment with a CDK4/6i.
Enrolled patients were randomized 1:1:1 to: (i) abemaciclib 150 mg every 12 hours plus tamoxifen (n = 78), (ii) abemaciclib 150 mg every 12 hours (n = 79), or (iii) abemaciclib 200 mg every 12 hours plus prophylactic loperamide (n = 77).
Both studies received ethical/Institutional Review Board approval, were conducted in accordance with the Declaration of Helsinki, and patients provided informed consent before enrollment.
Plasma sample collection and ctDNA analysis
As per study protocols and in accordance with country-specific guidelines, plasma samples were to be collected at baseline and EOT (follow-up) from patients enrolled in MONARCH 3 and nextMONARCH. This analysis focuses on the abemaciclib and placebo arms of MONARCH 3 and the abemaciclib monotherapy arms (B and C) of nextMONARCH.
ctDNA analyses were conducted on three populations: the translational research population (TR) - patients with a valid ctDNA sample at baseline; TR2 - patients with a valid ctDNA sample at both baseline and EOT; and TR3 - the subset of MONARCH 3 patients in TR2 with a valid EOT ctDNA sample and progressive disease (PD; Supplementary Fig. S1). For TR3, PD must have occurred while receiving abemaciclib/placebo and NSAI or within 60 days of discontinuation if one drug was stopped early.
Alterations at the gene level that were not present at baseline but acquired by EOT were identified in the TR2 population. Specific genes were also analyzed at the individual variant level, for example, ESR1 variants D538G, Y537S, etc. Synonymous mutations were excluded from analysis. Acquired gene alterations in MONARCH 3 patients who discontinued because of PD while on both study drugs, that is, abemaciclib or placebo plus NSAI, were identified in the TR3 population.
ctDNA was analyzed using the Guardant360 73-gene next-generation sequencing–based assay (Guardant Health; refs. 20–22), which has been validated with high rates of sensitivity and specificity (23). Potential tumor-related (somatic) genomic alterations were identified. Genomic alterations included point mutations [i.e., single-nucleotide variants (SNV)], insertions/deletions (INDEL), amplifications [i.e., copy-number alterations (CNA)], and fusions.
Statistical analyses
To assess baseline genomic alterations, data were dichotomized by presence/absence of a somatic alteration and treated as binary variables. To assess acquired genomic alterations, data were further subsetted into patients without a baseline somatic alteration on the gene of interest and then dichotomized by presence/absence of a somatic alteration on that same gene at EOT. Where applicable, rates of acquired genomic alterations by treatment arm were compared using a likelihood ratio χ2 test and P values were reported accordingly.
Clinical outcomes included PFS and objective response rate [ORR; percentage of patients with a best response of complete or partial response as per RECIST V1.1]. ORR was reported as the separate percentage of responders ± detectable genomic alterations. The Kaplan–Meier method was used to estimate mPFS and 95% confidence intervals (CI) in patients ± detectable genomic alterations and where appropriate, P values were reported using the log-rank test. HRs and 95% CIs were derived from a univariate Cox proportional hazards regression model. In MONARCH 3, this analysis modeled the effect of treatment within patients ± detectable genomic alterations separately. In nextMONARCH, this analysis modeled the effect of presence/absence of detectable genomic alterations.
Additionally for MONARCH 3, the predictive effect of each baseline genomic alteration on PFS was assessed by likelihood ratio test comparing a multivariate Cox proportional hazards model with the following factors: treatment arm, indicators for gene alteration(s) at baseline (yes/no) for each of EGFR, TP53, FGFR1, NF1, CCND1, MYC, PIK3CA, and ESR1; and treatment-by-biomarker interaction for the gene of interest to the model with the same factors excluding the treatment-by-biomarker interaction. The predictive effect of any genomic alteration at baseline, alterations in cell-cycle genes, and alterations in MAPK genes was assessed by likelihood ratio test comparing a multivariate Cox proportional hazards model with the following factors: treatment arm, presence of any alteration in group of genes, and biomarker-by-treatment interaction to the model with the same factors excluding the treatment-by-biomarker interaction.
Data cut-off dates were October 31, 2018 for MONARCH 3 and June 28, 2019 for nextMONARCH. These trials were not powered for retrospective biomarker analyses and no adjustments were made for multiplicity. Statistical analyses were conducted using SAS Version 9.3 or higher or R Version 3.4.4 or higher.
Data availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org/ourmember/lilly/.
Results
Patients
In MONARCH 3, 493 patients were randomized (2:1) to receive NSAI plus abemaciclib (n = 328) or placebo (n = 165) and comprise the intent-to-treat (ITT) population. An evaluable baseline ctDNA sample (TR population) was obtained from 295 patients (201 abemaciclib, 94 placebo) and 210 patients (131 abemaciclib, 79 placebo) had evaluable baseline and EOT ctDNA samples (TR2 population). In nextMONARCH, 156 patients received abemaciclib monotherapy (ITT population). An evaluable baseline ctDNA sample (TR population) was obtained from 139 patients and 79 patients had both evaluable baseline and EOT ctDNA samples (TR2 population; Supplementary Fig. S1). Baseline characteristics in both studies were similar among the respective ITT and TR populations (Supplementary Tables S1 and S2).
Genomic alterations in baseline ctDNA
A total of 81% of patients in MONARCH 3 and 90% of patients in nextMONARCH had at least one genomic alteration detected in baseline ctDNA.
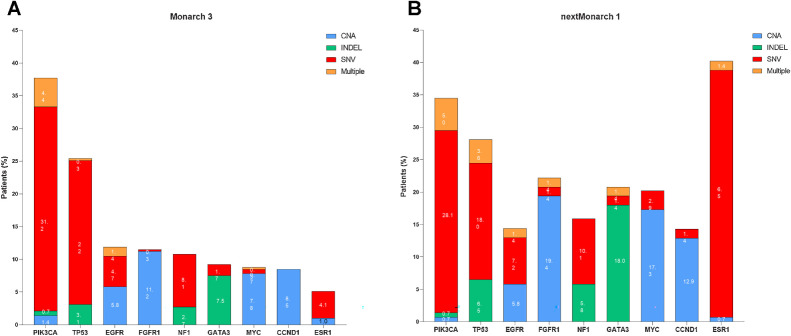
The most frequently altered genes at baseline were PIK3CA (37.6%), TP53 (25.4%), EGFR (11.9%), FGFR1 (11.5%), NF1 (10.8%), GATA3 (9.2%), MYC (8.8%), and CCND1 (8.5%) in MONARCH 3 (Fig. 1A) and ESR1 (40.3%), PIK3CA (34.5%), TP53 (28.1%), FGFR1 (22.3%), GATA3 (20.9%), and MYC (20.1%) in nextMONARCH (Fig. 1B).
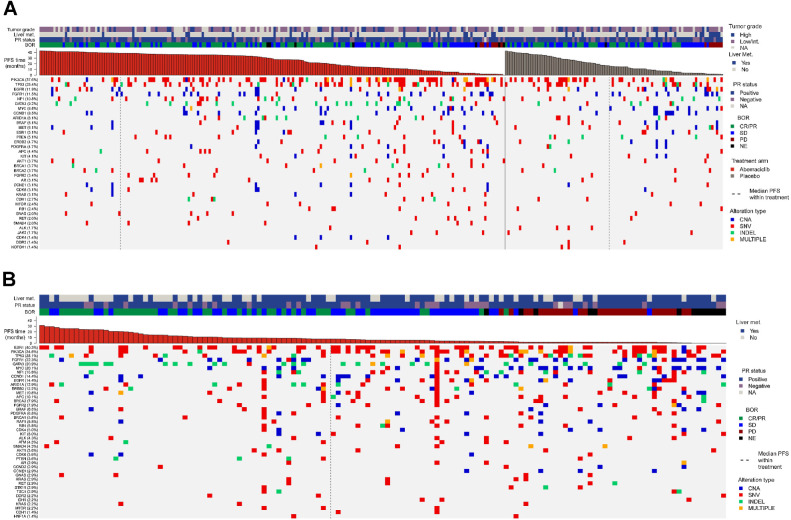
Figure 1.
Gene alterations at baseline. Heat maps of somatic alterations at baseline by gene (TR population) for MONARCH 3 (A) and nextMONARCH (B). CNA, copy-number alterations; INDEL, insertions/deletions; SNV, single-nucleotide variant; TR, translational research.
In both studies, the most common types of baseline alterations were SNV for patients with PIK3CA, TP53, NF1, and ESR1 alterations, CNA for patients with FGFR1, CCND1, and MYC alterations, and INDEL for patients with GATA3 alterations (Fig. 2A and B).
Figure 2.
Frequency of gene alterations at baseline. Bar graphs representing frequency of gene alterations at baseline by gene and type of alteration in MONARCH 3 (A; n = 295, TR population) and nextMONARCH (B; n = 139, TR population). CNA, copy-number alterations; INDEL, insertions/deletions; SNV, single-nucleotide variant; TR, translational research.
At baseline, 44 different PIK3CA variants were identified in MONARCH 3 and 69 variants in nextMONARCH. The most frequent baseline PIK3CA variants in both studies were common strong activating hotspot mutations, H1047R, E545K, E542K, and H1047L and weaker activating mutations including E726K (Supplementary Fig. S2; refs. 24, 25).
Association between baseline genomic alterations and clinical outcome
mPFS in the MONARCH 3 abemaciclib and placebo arms was 28.2 and 14.8 months (HR, 0.52; 95% CI, 0.42–0.66), respectively, in the ITT population, and 38.7 and 16.5 months (HR, 0.45; 95% CI, 0.33–0.61), respectively, in the TR population (Fig. 3). mPFS with abemaciclib monotherapy in nextMONARCH was 7.4 months in both the ITT and TR populations (Fig. 4).
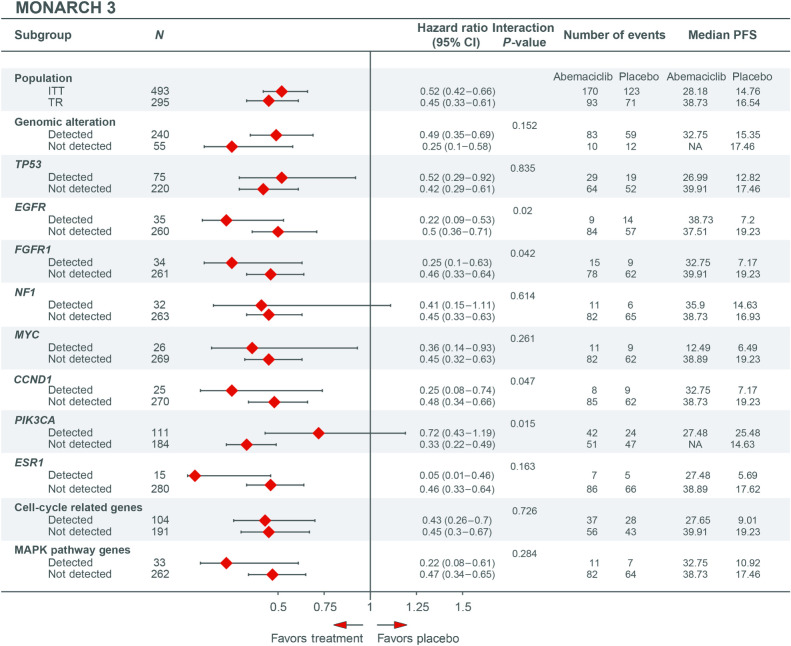
Figure 3.
Forest plots of PFS for patients with and without specific genomic alterations at baseline in MONARCH 3 (TR population). Cell cycle–related genes consist of CCND1, CCND2, CDK4, CDK5, CDKN2A, CCNE1, RB1, and TP53. MAPK genes consist of ARAF, BRAF, HRAS, KRAS, MAPK1, MAP2K1, MAP2K2, MAP3K1, NRAS, and RAF1 (CRAF). CI, confidence interval; ITT, intent-to-treat; NA, not achieved; PFS, progression-free survival; TR, translation research.
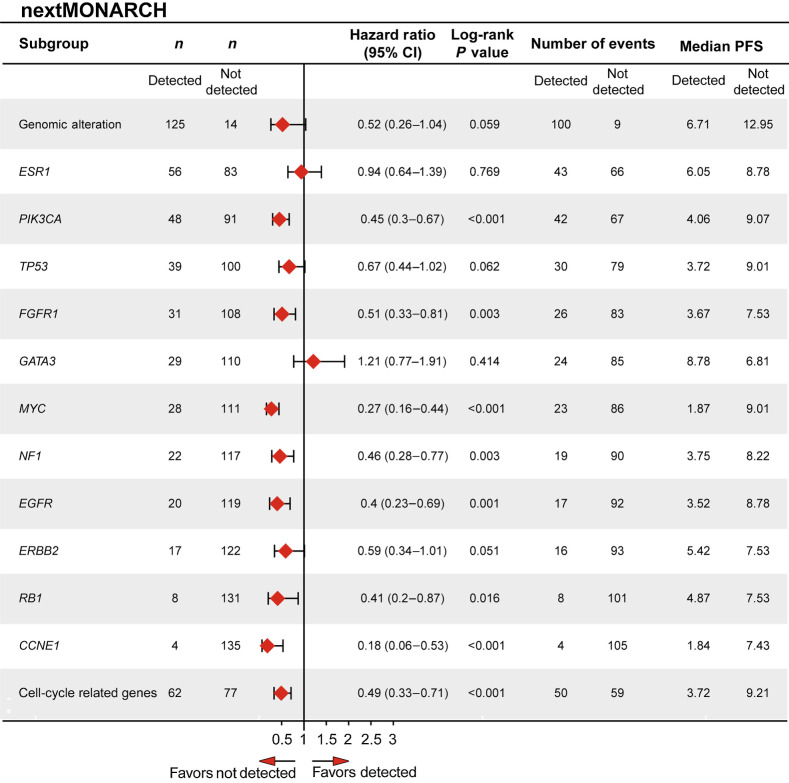
Figure 4.
Forest plot of PFS for patients with and without specific genomic alterations at baseline in nextMONARCH (TR population). Cell cycle–related genes consist of CCND1, CCND2, CDK4, CDK5, CDKN2A, CCNE1, RB1, and TP53. CI, confidence interval; ITT, intent-to-treat; NA, not achieved; PFS, progression-free survival; TR, translation research.
In MONARCH 3, patients treated with abemaciclib had a longer mPFS than those treated with placebo irrespective of whether a baseline alteration was detected (32.8 vs. 15.4 months; HR, 0.49; 95% CI, 0.35–0.69) or not detected (not reached vs. 17.5 months; HR, 0.25; 95% CI, 0.1–0.58). A nominally significant interaction effect between the presence/absence of an alteration and efficacy of abemaciclib plus NSAI versus placebo plus NSAI was observed for EGFR, FGFR1, CCND1, and PIK3CA (Fig. 3); however, these results should be interpreted with caution because of the exploratory nature of the analysis. In the placebo group, alterations in EGFR, FGFR1, MYC, CCND1, ESR1, cell cycle–related genes (CCRG), and MAPK pathway genes were associated with a mPFS less than 12 months (Fig. 3; Supplementary Fig. S3A). In nextMONARCH, mPFS was shorter in patients with a detectable baseline alteration than those with no baseline alteration detected (6.7 vs. 13.0 months; HR, 0.5; 95% CI, 0.26–1.04; Fig. 4). Baseline genomic alterations in PIK3CA, FGFR1, MYC, NF1, EGFR, RB1, CCNE1, or CCRGs were associated with a mPFS less than 5 months. Patients with detected alterations in TP53 or ERBB2 trended toward a shorter mPFS, while patients with a GATA3 alteration had numerically longer mPFS (Fig. 4; Supplementary Fig. S3B). Given that there is no control arm in nextMONARCH, these effects cannot be clearly attributed as prognostic or predictive. A similar trend was also evident for OS in nextMONARCH (Supplementary Fig. S4). Gene amplifications were the most frequent baseline EGFR alterations in MONARCH 3 and nextMONARCH (7.2% and 7.1%, respectively; Supplementary Fig. S2).
In MONARCH 3, ESR1 alterations were rare at baseline but were associated with numerically shorter mPFS in abemaciclib (27.5 months) and placebo (5.7 months) groups compared with those without such alterations (abemaciclib: 38.9 months; placebo: 17.6 months). In nextMONARCH, mPFS was similar with and without ESR1 alterations detected (6.1 vs. 8.8 months; HR, 0.94; 95% CI, 0.64–1.39). In nextMONARCH, there was an apparent association between having ESR1 mutation at baseline and having liver metastases (nominal P = 0.0075). ESR1 mutations, less common at baseline in MONARCH 3, were not associated with liver metastases (nominal P = 0.2478).
In MONARCH 3, ORR was numerically higher in patients treated with abemaciclib versus placebo, regardless of whether a baseline alteration was detected (54.3% vs. 47.4%) or not (64.9% vs. 16.7%; Supplementary Fig. S5A). In nextMONARCH, ORR was generally numerically higher in patients without detected alterations, with the exception of ESR1 (detected: 33.9% vs. not: 28.9%) and GATA3 alterations (detected: 44.8% vs. not: 27.3%; Supplementary Fig. S5B).
Regarding baseline mutant allele frequency (MAF), in MONARCH 3, treatment benefit was consistent regardless of highest baseline MAF (highest baseline MAF >median: HR, 0.49; ≤median: HR, 0.50), although having a highest baseline MAF >median did appear to be prognostic of shorter mPFS overall (Supplementary Fig. S6A). Similarly, in nextMONARCH, the subgroup with highest baseline MAF >median also had a somewhat shorter mPFS (5.2 vs. 9.2 months in the ≤median subgroup; Supplementary Fig. S6B).
Acquired genomic alterations
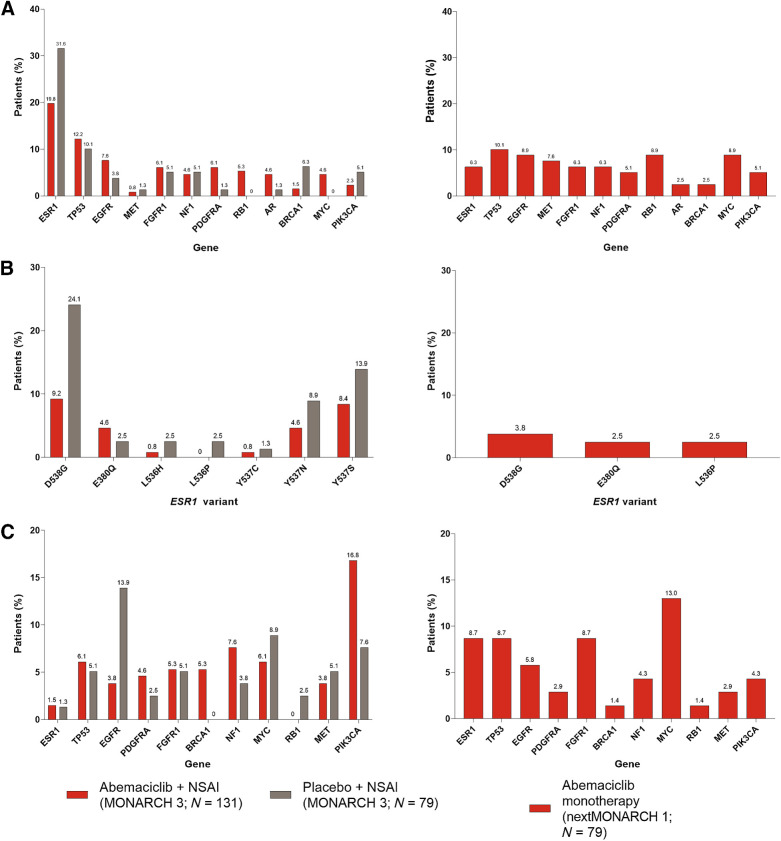
In MONARCH 3, the most commonly acquired alterations, were ESR1 (20%), TP53 (12%), and EGFR (8%) in the abemaciclib arm and ESR1 (32%), TP53 (10%), and BRCA1 (6%) in the placebo arm (Fig. 5A). Acquired alterations more frequent in the abemaciclib versus placebo arm included RB1 (5% vs. 0%, P = 0.009), MYC (5% vs. 0%, P = 0.016), APC (4% vs. 0%, P = 0.029), and BRCA2 (4% vs. 0%, P = 0.029). In nextMONARCH, alterations in TP53 (10%), EGFR (9%), RB1 (9%), and MYC (9%) were the most commonly acquired. Acquired alterations in ESR1 (6%) and AR (3%) were also found. In MONARCH 3, the most frequent ESR1 alterations were D538G (9.2% abemaciclib plus NSAI; 24.1% placebo plus NSAI) and Y537S (8.4% abemaciclib plus NSAI; 13.9% placebo plus NSAI). D538G (3.8%) was the most frequent ESR1 alteration in nextMONARCH (Fig. 5B). Acquired ESR1 mutations were not associated with liver metastases in either nextMONARCH (nominal P = 1.0) or MONARCH 3 (nominal P = 0.5278).
Figure 5.
Genomic alterations in the abemaciclib and placebo groups in MONARCH 3 and the abemaciclib monotherapy group in nextMONARCH (TR2 population). A, Acquired genomic alterations. *, P < 0.05 abemaciclib versus placebo in MONARCH 3. B, The frequency of individual ESR1 mutations (found in ≥2 patients) acquired during treatment. C, Genomic alterations detected at baseline but not detected at EOT. TR2 population consists of patients with a valid ctDNA sample at both baseline and EOT. NSAI, nonsteroidal aromatase inhibitor.
Certain baseline alterations were undetectable at EOT in a proportion of patients (Fig. 5C). For example, in MONARCH 3, PIK3CA alterations became undetectable in 16.8% of patients treated with abemaciclib compared with 7.6% in the placebo arm. In nextMONARCH this was observed in 4.3% of patients. This should be considered if ctDNA testing is done to identify PIK3CA mutations for use of alpelisib.
Acquired alterations in patients with progressive disease
Most patients in the TR2 population of both studies discontinued because of PD: 157 (74.8%) in MONARCH 3 [88 (67.2%) in the abemaciclib arm and 69 (87.3%) in the placebo arm] and 69 (87.3%) in nextMONARCH (Supplementary Table S3). The TR3 population consists of the subset of MONARCH 3 patients in TR2 with a valid EOT ctDNA sample and PD within 2 months of discontinuation of all study treatment (abemaciclib and NSAI; Supplementary Fig. S1). As in the TR2 population, ESR1 alterations were the most frequently acquired alterations in the TR3 population (abemaciclib: 19.2%; placebo: 30.4%). D538G and Y537S were the most frequently acquired individual ESR1 mutations in the TR3 population (Supplementary Fig. S7). Acquired genomic alterations in the TR3 population are displayed in Supplementary Fig. S8.
Association between acquired alterations and PFS
In the MONARCH 3 TR2 population, mPFS was 20.8 months in the abemaciclib and 14.6 months in the placebo group (HR, 0.61; 95% CI, 0.44–0.84). In the nextMONARCH TR2 population, mPFS was 7.4 months with abemaciclib monotherapy.
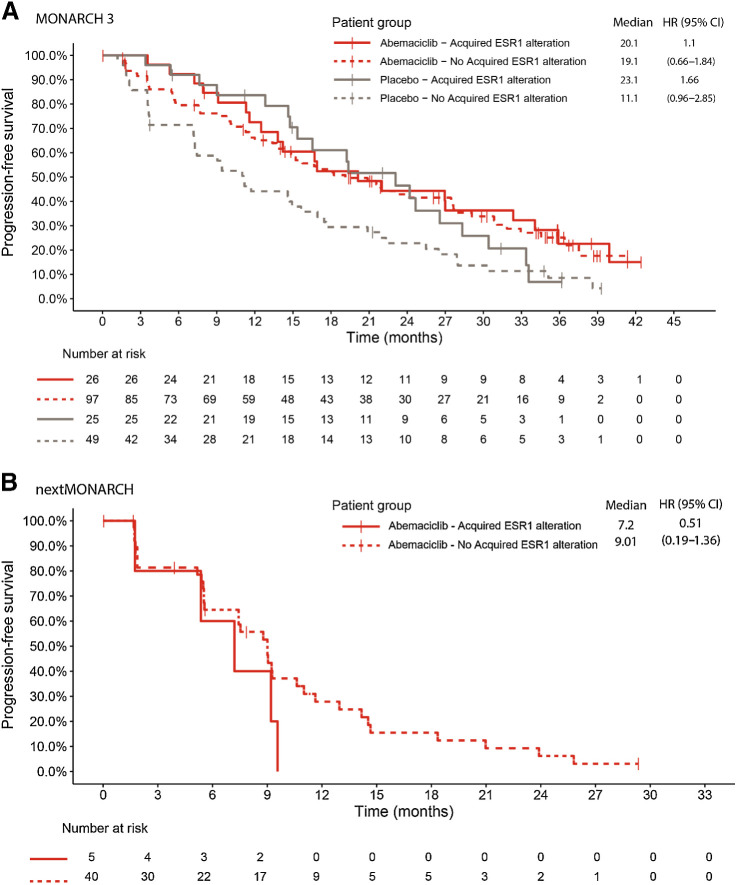
In MONARCH 3, mPFS was similar between patients with and without ESR1 alterations acquired during abemaciclib treatment (20.1 vs. 19.1 months; HR, 1.1; 95% CI, 0.66–1.84). In contrast, in the placebo arm, mPFS was longer in patients with ESR1 alterations acquired while on treatment compared with those without acquired alterations (23.1 vs. 11.1 months; HR, 1.66; 95% CI, 0.96–2.85; Fig. 6A). In nextMONARCH, mPFS was similar between patients with and without ESR1 alterations acquired during abemaciclib monotherapy (7.2 vs. 9.0 months; HR, 0.51; 95% CI, 0.19–1.36; Fig. 6B).
Figure 6.
PFS in patients with and without acquired ESR1 alterations in MONARCH 3 (A) and nextMONARCH (B). CI, confidence interval; HR, hazard ratio.
Examination of the association between the most commonly acquired gene alteration (ESR1) in MONARCH 3 and the time to second disease progression (PFS2) showed no significant difference between patients with versus without acquired ESR1 alterations (Supplementary Fig. S9).
In the abemaciclib arm of MONARCH 3, mPFS was shorter for patients with alterations in FGFR1 (HR, 0.33; 95% CI, 0.16–0.70), NF1 (HR, 0.23; 95% CI, 0.09–0.54), and PDGFRA (HR, 0.44; 95% CI, 0.21–0.92) acquired while on treatment compared with those without such acquired alterations (Supplementary Table S4).
Discussion
Abemaciclib has demonstrated efficacy in both the metastatic and adjuvant settings in HR+, HER2− breast cancer (5, 7, 17, 26–28). However, a small proportion of patients with MBC exhibit primary resistance to abemaciclib and other CDK4/6i, and most develop acquired resistance. Therefore, a greater understanding of the mechanisms of resistance is critically needed (11, 29, 30).
In vitro preclinical studies in breast cancer cell lines treated with CDK4/6i have identified genomic alterations potentially involved in resistance, including loss of RB1 and amplification of CCNE1, CCNE2, and CDK6 (9, 31, 32, 33). However, the clinical relevance of such findings in patients treated with abemaciclib is unclear. This study is the first to explore genomic alterations in ctDNA samples from patients with HR+, HER2− ABC treated with abemaciclib ± NSAI and the relationship between baseline or treatment-emergent genomic alterations and clinical outcomes. Though direct comparisons between the two studies cannot be made, given the differences in study populations, the analysis from MONARCH 3 provides data from a large, randomized, phase III study, while nextMONARCH allows for analysis in the context of monotherapy rather than combination with ET.
Most patients in MONARCH 3 and nextMONARCH had at least one baseline genomic alteration. While baseline gene alterations were prognostic in the abemaciclib arms of MONARCH 3 and nextMONARCH, in MONARCH 3, patients receiving abemaciclib plus NSAI consistently had improved mPFS compared with those receiving placebo plus NSAI, irrespective of baseline genomic alterations, consistent with results in the ITT population (6, 34).
Alterations in the estrogen receptor (ER) gene ESR1 were rarely present at baseline in the MONARCH 3 population (5%; initial therapy for advanced disease) but highly prevalent in the heavily pretreated nextMONARCH population (40%), reflecting the association of ESR1 mutations with exposure to ET (35). In previous studies, the detection of ESR1 mutations has been associated with inferior PFS in patients receiving aromatase inhibitor (AI)-containing therapies (36, 37). In MONARCH 3, though the frequency was low, patients in the placebo arm with baseline ESR1 alterations had a shorter mPFS than those without such alterations. Notably, patients with alterations derived substantial benefit from the addition of abemaciclib to NSAI. In nextMONARCH, mPFS was similar between patients with and without baseline ESR1 alterations receiving abemaciclib monotherapy suggesting benefit of abemaciclib despite ET resistance in this population (38). This is similar to MONARCH 2 data, where benefit from abemaciclib plus fulvestrant was observed regardless of ESR1 mutation status in an ET-resistant population (39).
The ESR1 alterations most frequently observed in this study occurred within the ligand-binding domain, at D538G and Y537S, consistent with other studies of patients on NSAI (40). While mPFS was similar between patients with and without ESR1 alterations acquired during abemaciclib treatment (both studies), mPFS was longer in patients with acquired ESR1 alterations on placebo plus NSAI (MONARCH 3), suggesting longer exposure to ET monotherapy is associated with the acquisition of ESR1 alterations. To determine whether the presence of ESR1 mutations conferred shorter PFS on the next line of therapy after initial disease progression, we evaluated PFS2 in MONARCH 3. No difference in PFS2 was observed between patients with and without acquired ESR1 alterations in MONARCH 3.
In the PALOMA-3 study of palbociclib or placebo plus fulvestrant, 12.8% of patients without a baseline ESR1 mutation had an acquired mutation at progression, with evidence of selection of ESR1 Y537S in both arms of the study (12). In contrast, fewer MONARCH 3 patients with PD acquired ESR1 alterations in the abemaciclib arm (19.2%) compared with placebo (30.4%; Supplementary Fig. S7), mainly driven by higher rates of acquisition of the ESR1 D538G alteration in patients with PD in the placebo arm (24.1%) compared with the abemaciclib arm (9.2%). Fulvestrant has demonstrated antitumor activity in ESR1-mutant disease preclinically (41, 42), in the metastatic setting (43), and in patients receiving therapy with AI plus palbociclib who experienced rising ESR1 ctDNA levels and were switched from AI to fulvestrant (while maintaining palbociclib; ref. 44). Given that abemaciclib may delay PD related to ESR1 mutation, further studies should evaluate the optimal CDK4/6i partner for selective estrogen receptor degraders and other ET.
Several baseline genomic alterations were associated with mPFS <12 months in the placebo arm of MONARCH 3, including ESR1, MYC, CCND1, EGFR, FGFR1, CCRGs, and MAPK pathway genes. In nextMONARCH, genomic alterations associated with a mPFS <5 months included CCNE1, MYC, EGFR, FGFR1, CCRGs, NF1, PIK3CA, and RB1.
Mutations in TP53, RB1, and NF1 have been previously associated with poor outcomes in patients with HR+, HER2− ABC, regardless of treatment (45). Our analyses are the first to suggest baseline EGFR alterations (Supplementary Fig. S2C and S2D) may also be associated with poor prognosis in patients with HR+, HER2− ABC, although maintain a benefit with abemaciclib plus NSAI. In the exploratory analyses from MONALEESA-2 and MONALEESA-7 trials, patients with altered receptor tyrosine kinase genes, including EGFR, derived a PFS benefit from ribociclib (46, 47).
In the MONALEESA-2 trial, PIK3CA (33%) and TP53 (12%) alterations were found in baseline ctDNA, with prolonged PFS with ribociclib plus letrozole regardless of PIK3CA and TP53 alteration status (46, 48). Similarly, in our analysis, TP53 and PIK3CA alterations were frequently observed at baseline, and patients with and without TP53 or PIK3CA alterations benefited from combined abemaciclib plus NSAI. In contrast, in nextMONARCH, patients without a detected TP53 or PIK3CA alteration had a longer mPFS than those with an alteration.
Mutations in FGFR1 and FGFR2 have been associated with resistance to ET and CDK4/6i (49–51). In MONALEESA-2, baseline FGFR1 alterations were associated with a poor prognosis. Patients with baseline FGFR1 amplification treated with ribociclib plus letrozole had a shorter mPFS (10.6 months) than patients with wild-type FGFR1 (24.8 months; ref. 50). While baseline FGFR1 alterations were associated with a shorter mPFS in both treatment arms of MONARCH 3 and with abemaciclib monotherapy in nextMONARCH, patients in MONARCH 3 benefited from the addition of abemaciclib to NSAI regardless of mutation status.
Limited clinical data on acquired resistance during CDK4/6i treatment have been reported (9, 12). Acquired genomic alterations potentially associated with emerging resistance to abemaciclib ± NSAI included alterations in RB1, MYC, or EGFR. However, these were seen in <10% of patients and could be impacted by small sample size, therefore further evaluation in a larger patient population is warranted. Acquired TP53 alterations were found in 10% of patients in both treatment arms of MONARCH 3 and the abemaciclib monotherapy arms of nextMONARCH.
Using whole-exome sequencing of metastatic tumor biopsies, Wander and colleagues (9) identified genomic alterations that could potentially drive resistance to CDK4/6i. These include loss of RB1, activating alterations in AKT1, RAS, aurora kinase A (AURKA), CCNE2, ERBB2 and FGFR2, and loss of ER expression. Loss of RB is a mechanism of both intrinsic and acquired resistance to CDK4/6i. However, this is uncommon and does not account for most of the acquired resistance observed in HR+, HER2− ABC. ctDNA analysis from the PALOMA-3 study revealed RB1 mutations in 5% of patients who acquired a mutation during palbociclib plus fulvestrant treatment, suggesting this is not the predominant mechanism of resistance to CDK4/6i (12). In this study, acquired RB1 alterations were detected in <10% of patients receiving abemaciclib ± NSAI.
In summary, we investigated genomic alterations potentially associated with resistance to abemaciclib ± NSAI in women with HR+, HER2− ABC using ctDNA analysis from MONARCH 3 and nextMONARCH. The most frequent baseline alterations in our study have been previously associated with endocrine resistance. Importantly, in MONARCH 3, abemaciclib plus NSAI was associated with improved mPFS compared with placebo plus NSAI, regardless of baseline genomic alterations. In addition, potential mechanisms of acquired resistance were explored. Finally, this is the first study to evaluate impact of genomic alterations on CDK4/6i monotherapy. Limitations of this study include that evaluable samples were not available for all patients and that interpretation of nextMONARCH data is limited by the lack of a control arm for comparison, and thus, confirmation if these findings reflect prognostic or predictive association of these alterations is not possible. These findings are hypothesis generating and need validation in suitably powered prospective studies. Understanding potential mechanisms of intrinsic and acquired resistance will help inform future drug development and clinical trials.
Supplementary Material
Supplementary Data
Acknowledgments
This work was supported by Eli Lilly and Company (no grant numbers apply).
The authors would like to acknowledge Deirdre Elmhirst, PhD, Rx Communications Group, for medical writing support funded by Eli Lilly and Company and Dwayne Byrne, PhD, Eli Lilly and Company for project management support and scientific communication expertise.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
M.P. Goetz reports other support from Lilly during the conduct of the study as well as other support from ARC Therapuetics, AstraZeneca, Biotheranostics, BlueprintMedicines, Novartis, RNA Diagnostics, Sanofi Genzyme, Seattle Genetics, Sermonix, Engage Health Media and grants from Pfizer, Sermonix, Loxo, Atossa Therapeutics, and AstraZeneca outside the submitted work. E.P. Hamilton reports grants from AbbVie, Acerta Pharma, ADC Therapeutics, AKESOBIO Australia, Amgen, Aravive, ArQule, Artios, Arvinas, AtlasMedx, BeiGene, Black Diamond, Bliss BioPharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Cascadian Therapeutics, Clovis, Compugen, Context Therapeutics, Cullinan, Curis, CytomX, Dana-Farber Cancer Institute, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Eisai, Elucida Oncology, EMD Serono, Fochon Pharmaceuticals, FujiFilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, Inspirna, InvestisBio, Jacobio, Karyopharm, K-Group Beta, Kind Pharmaceuticals, Leap Therapeutics, Lycera, Mabspace Biosciences, Macrogenics, MedImmune, Merus, Millennium, Molecular Templates, Myriad Genetic Laboratories, Nucana, OncoMed, Onconova Therapeutics, Oncothyreon, ORIC Pharmaceuticals, Orinove, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Prelude Therapeutics, Profound Bio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicine, Rgenix, Sermonix Pharmaceuticals, Shattuck Labs, Silverback Therapeutics, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Zenith Epigenetics, Zymeworks; grants and other support from Lilly, Accutar Biotechnology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Gilead Sciences, Loxo Oncology, Mersana, Novartis, Olema, Orum Therapeutics, Pfizer, Roche/Genentech, SeaGen, Stemline Therapeutics; and other support from Entos, Greenwich LifeSciences, Jazz Pharmaceuticals, Medical Pharma Services, Theratechnologies, Tubulis, Verascity Science, and Zentalis Pharmaceuticals outside the submitted work. M. Campone reports personal fees from Pierre Fabre Oncology, Sanofi, Novartis, Servier, Daiichi-Sankyo, Pet-Therapy, and Lilly and nonfinancial support from Pfizer, Roche, and AstraZeneca outside the submitted work. S.A. Hurvitz reports other support from Lilly during the conduct of the study as well as grants from Ambrx, Arvinas, AstraZeneca, Bayer, Celcuity, Cytomx, Daiichi-Sankyo, Dantari, Genentech/Roche, G1 Therapeutics, Gilead, Greenwich Life Sciences, GSK, Immunomedics, Eli Lilly, Loxo, Macrogenics, Novartis, Orinove, Orum, Pfizer, Phoenix Molecular Design, Pieris, PUMA, Radius, Sanofi, SeaGen, and Zymeworks outside the submitted work. J. Cortes reports personal fees from Lilly during the conduct of the study as well as personal fees from Roche outside the submitted work; in addition, J. Cortes has a patent for Pharmaceutical Combinations of a PI3K Inhibitor and a Microtubule Destabilizing Agent (Javier Cortes Castan, Alejandro Piris Gimenez, Violeta Serra Elizalde) WO 2014/199294 A issued and research funding to the institution from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/sol;Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Iqvia, and Queen Mary University of London. S. Johnston reports grants and personal fees from Eli Lilly, Pfizer, and Puma Biotechnology and personal fees from AstraZeneca, Novartis, Sanofi Genzyme, and Eisai outside the submitted work. A. Llombart-Cussac reports grants and personal fees from Eli Lilly, Pfizer, Menarini, and AstraZeneca; grants and nonfinancial support from Roche Genentech; grants from Novartis, MSD, and Daiichi Sankyo; and personal fees from MedSIR during the conduct of the study. P.A. Kaufman reports grants and personal fees from Lilly and Sermonix, grants from Pfizer Sanofi, and personal fees from AstraZeneca during the conduct of the study as well as grants and personal fees from Eisai, grants from Zymeworks, and personal fees from Daiichi Sankyo outside the submitted work. M. Toi reports grants, personal fees, and other support from Eli Lilly during the conduct of the study as well as grants and personal fees from Chugai, Takeda, Pfizer, Taiho, Eisai, AstraZeneca, Shimadzu, Yakult, and Nippon Kayaku; grants from JBCRG, KBCRN, Astellas, AFI Technology, Luxonus, Shionogi, GL Science, and Sanwa Shurui; grants, personal fees, and other support from Daiichi-Sankyo; personal fees from Kyowa-Kirin, MSD, Exact Science, Devicore Japan; and other support from Bertis, Terumo, and Knasai Medical Net outside the submitted work; in addition, M. Toi reports serving as chair of the board of directors of the Japanese Breast Cancer Society (no salary) and a member of the board of directors of JBCRG (no salary) and KBCRN (no salary). G. Jerusalem reports grants from Eli Lilly and other support from Eli Lilly during the conduct of the study as well as personal fees and other support from Novartis, Amgen, Roche, Pfizer, Bristol Myers Squibb, and Lilly, AstraZeneca and personal fees from Daiichi Sankyo, Abbvie, Seagen, and Diaccurate outside the submitted work. H. Graham reports personal fees from Eli Lilly during the conduct of the study as well as personal fees from Eli Lilly outside the submitted work. V.M. Jansen reports personal fees and other support from Eli Lilly and Company and personal fees from Mersana Therapeutics and Elevation Oncology, Inc outside the submitted work. L.M. Litchfield reports personal fees from Eli Lilly and Company during the conduct of the study as well as personal fees from Eli Lilly and Company outside the submitted work; in addition, L.M. Litchfield reports ownership of Eli Lilly and Company stock. M. Martin reports personal fees from Lilly, Pfizer, AstraZeneca, Merck, Amgen, and Menarini; personal fees and other support from Daiichi-Sankyo; grants from PUMA and Novartis; and grants and personal fees from Roche outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
M.P. Goetz: Conceptualization, supervision, investigation, writing–review and editing. E.P. Hamilton: Conceptualization, supervision, investigation, writing–review and editing. M. Campone: Investigation, writing–review and editing. S.A. Hurvitz: Investigation, writing–review and editing. J. Cortes: Investigation, writing–review and editing. S. Johnston: Investigation, writing–review and editing. A. Llombart-Cussac: Investigation, writing–review and editing. P.A. Kaufman: Investigation, writing–review and editing. M. Toi: Investigation, writing–review and editing. G. Jerusalem: Investigation, writing–review and editing. H. Graham: Formal analysis, writing–review and editing. H. Wang: Formal analysis, writing–review and editing. V.M. Jansen: Conceptualization, writing–review and editing. L.M. Litchfield: Conceptualization, supervision, investigation, writing–review and editing. M. Martín: Investigation, writing-review and editing.
References
- 1. Preusser M, De Mattos-Arruda L, Thill M, Criscitiello C, Bartsch R, Ruhstaller T, et al. CDK4/6 inhibitors in the treatment of patients with breast cancer: summary of a multidisciplinary round-table discussion. ESMO Open 2018;3:e000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eggersmann TK, Degenhardt T, Gluz O, Wuerstlein R, Harbeck N. CDK4/6 inhibitors expand the therapeutic options in breast cancer: palbociclib, ribociclib and abemaciclib. BioDrugs 2019;33:125–35. [DOI] [PubMed] [Google Scholar]
- 3. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im S-A, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. [DOI] [PubMed] [Google Scholar]
- 4. Slamon DJ, Neven P, Chia SKL, Im S-A, Fasching PA, DeLaurentiis M, et al. Ribociclib (RIB)+ fulvestrant (FUL) in postmenopausal women with hormone receptor-positive (HR+), HER2-negative (HER2–) advanced breast cancer (ABC): results from MONALEESA-3. J Clin Oncol 36:15s, 2018. (suppl; abstr 1000). [Google Scholar]
- 5. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–84. [DOI] [PubMed] [Google Scholar]
- 6. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–46. [DOI] [PubMed] [Google Scholar]
- 7. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 2020;6:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Royce M, Osgood C, Mulkey F, Bloomquist E, Pierce WF, Roy A, et al. FDA approval summary: abemaciclib with endocrine therapy for high-risk early breast cancer. J Clin Oncol 2022;40:1155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wander SA, Cohen O, Gong X, Johnson GN, Buendia-Buendia JE, Lloyd MR, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov 2020;10:1174–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Álvarez-Fernández M, Malumbres M. Mechanisms of sensitivity and resistance to CDK4/6 inhibition. Cancer Cell 2020;37:514–29. [DOI] [PubMed] [Google Scholar]
- 11. McCartney A, Migliaccio I, Bonechi M, Biagioni C, Romagnoli D, De Luca F, et al. Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front Oncol 2019;9:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 2018;8:1390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spring L, Griffin C, Isakoff SJ, Moy B, Wander SA, Shin J, et al. Phase II study of adjuvant endocrine therapy with CDK 4/6 inhibitor, ribociclib, for localized ER+/HER2-breast cancer (LEADER). J Clin Oncol 38:15s, 2020(suppl; abstr 531). [Google Scholar]
- 14. Wander SA, Juric D, Supko JG, Micalizzi DS, Spring L, Vidula N, et al. Phase Ib trial to evaluate safety and anti-tumor activity of the AKT inhibitor, ipatasertib, in combination with endocrine therapy and a CDK4/6 inhibitor for patients with hormone receptor positive (HR+)/HER2 negative metastatic breast cancer (MBC)(TAKTIC). J Clin Oncol 38:15s, 2020(suppl; abstr 1066). [Google Scholar]
- 15. Rohanizadegan M. Analysis of circulating tumor DNA in breast cancer as a diagnostic and prognostic biomarker. Cancer Genet 2018;228:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buono G, Gerratana L, Bulfoni M, Provinciali N, Basile D, Giuliano M, et al. Circulating tumor DNA analysis in breast cancer: is it ready for prime-time? Cancer Treat Rev 2019;73:73–83. [DOI] [PubMed] [Google Scholar]
- 17. Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamiton E, Cortes J, Dieras V, Ozyilkan O, Chen S, Petrakova K, et al. nextMONARCH 1: Phase 2 study of abemaciclib plus tamoxifen or abemaciclib alone in HR+, HER2-advanced breast cancer [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4–8; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2019;79(4 Suppl):Abstract nr PD1-11. [Google Scholar]
- 19. Hamilton E, Cortes J, Ozyilkan O, Chen SC, Petrakova K, Manikhas A, et al. nextMONARCH: abemaciclib monotherapy or combined with tamoxifen for metastatic breast cancer. Clin Breast Cancer 2021;21:181–90.33148479 [Google Scholar]
- 20. Kim ST, Banks KC, Lee S-H, Kim K, Park JO, Park SH, et al. Prospective feasibility study for using cell-free circulating tumor DNA–guided therapy in refractory metastatic solid cancers: an interim analysis. JCO Precis Oncol 2017;1:PO.16.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Odegaard JI, Vincent JJ, Mortimer S, Vowles JV, Ulrich BC, Banks KC, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue-and plasma-based methodologies. Clin Cancer Res 2018;24:3539–49. [DOI] [PubMed] [Google Scholar]
- 22. Zill OA, Banks KC, Fairclough SR, Mortimer SA, Vowles JV, Mokhtari R, et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin Cancer Res 2018;24:3528–38. [DOI] [PubMed] [Google Scholar]
- 23. Lanman RB, Mortimer SA, Zill OA, Sebisanovic D, Lopez R, Blau S, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 2015;10:e0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang M, Jang H, Nussinov R. PI3K driver mutations: a biophysical membrane-centric perspective. Cancer Res 2021;81:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filipenko ML, Os'kina NA, Oskorbin IA, Mishukova OV, Ovchinnikova LK, Gershtein ES, et al. Association between the prevalence of somatic mutations in PIK3CA gene in tumors and clinical and morphological characteristics of breast cancer patients. Bull Exp Biol Med 2017;163:250–4. [DOI] [PubMed] [Google Scholar]
- 26. Martin M, Hegg R, Kim SB, Schenker M, Grecea D, Garcia-Saenz JA, et al. Treatment with adjuvant abemaciclib plus endocrine therapy in patients with high-risk early breast cancer who received neoadjuvant chemotherapy: a prespecified analysis of the monarche randomized clinical trial. JAMA Oncol 2022;8:1190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnston S, O'Shaughnessy J, Martin M, Huober J, Toi M, Sohn J, et al. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. NPJ Breast Cancer 2021;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020;38:3987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberto M, Astone A, Botticelli A, Carbognin L, Cassano A, D'Auria G, et al. CDK4/6 inhibitor treatments in patients with hormone receptor positive, Her2 negative advanced breast cancer: potential molecular mechanisms, clinical implications and future perspectives. Cancers 2021;13:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sammons S, Shastry M, Dent S, Anders C, Hamilton E. Practical treatment strategies and future directions after progression while receiving CDK4/6 inhibition and endocrine therapy in advanced HR(+)/HER2(-) breast cancer. Clin Breast Cancer 2020;20:1–11. [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell 2018;34:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res 2016;76:2301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang C, Li Z, Bhatt T, Dickler M, Giri D, Scaltriti M, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 2017;36:2255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin M, Garcia-Saenz JA, Manso L, Llombart A, Cassinello A, Atienza M, et al. Abemaciclib, a CDK4 and CDK6 inhibitor for the treatment of metastatic breast cancer. Future Oncol 2020;16:2763–78. [DOI] [PubMed] [Google Scholar]
- 35. Lei JT, Gou X, Seker S, Ellis MJ. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat 2019;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol 2016;2:1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turner NC, Swift C, Kilburn L, Fribbens C, Beaney M, Garcia-Murillas I, et al. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res 2020;26:5172–7. [DOI] [PubMed] [Google Scholar]
- 38. Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015;12:573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tolaney SM, Toi M, Neven P, Sohn J, Grischke E-M, Llombart-Cussac A, et al. Clinical significance of PIK3CA and ESR1 mutations in circulating tumor DNA: analysis from the MONARCH 2 study of abemaciclib plus fulvestrant. Clin Cancer Res 2022;28:1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Najim O, Seghers S, Sergoynne L, Van Gaver H, Papadimitriou K, Wouters K, et al. The association between type of endocrine therapy and development of estrogen receptor-1 mutation in patients with hormone-sensitive advanced breast cancer: a systematic review and meta-analysis of randomized and non-randomized trials. Biochim Biophys Acta Rev Cancer 2019;1872:188315. [DOI] [PubMed] [Google Scholar]
- 41. Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 2013;45:1446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 2013;45:1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 2016;34:2961–8. [DOI] [PubMed] [Google Scholar]
- 44. Bidard FC, Hardy-Bessard A-C, Bachelot T, Pierga J-Y, Canon J-L, Clatot F, et al. Fulvestrant-palbociclib vs continuing AI-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- mBC patients: results of PADA-1, a UCBG-GINECO randomized phase 3 trial. [abstract]. In: Proceedings of the 2021 San Antonio Breast Cancer Symposium; 2021 Dec 7–10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2022;82(4 Suppl):Abstract nr GS3-05. [Google Scholar]
- 45. Bertucci F, Ng CK, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, et al. Genomic characterization of metastatic breast cancers. Nature 2019;569:560–4. [DOI] [PubMed] [Google Scholar]
- 46. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018;29:1541–7. [DOI] [PubMed] [Google Scholar]
- 47. Bardia A, Su F, Solovieff N, Im SA, Sohn J, Lee KS, et al. Genomic profiling of premenopausal HR+ and HER2- metastatic breast cancer by circulating tumor dna and association of genetic alterations with therapeutic response to endocrine therapy and ribociclib. JCO Precis Oncol 2021;5:PO.20.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738–48. [DOI] [PubMed] [Google Scholar]
- 49. Pandey K, An HJ, Kim SK, Lee SA, Kim S, Lim SM, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int J Cancer 2019;145:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Formisano L, Lu Y, Servetto A, Hanker AB, Jansen VM, Bauer JA, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun 2019;10:1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mao P, Cohen O, Kowalski KJ, Kusiel JG, Buendia-Buendia JE, Cuoco MS, et al. Acquired FGFR and FGF alterations confer resistance to estrogen receptor (ER) targeted therapy in ER+ metastatic breast cancer. Clin Cancer Res 2020;26:5974–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org/ourmember/lilly/.