Abstract
The Nociceptin/Orphanin FQ (N/OFQ) peptide and its receptor NOP are highly expressed within several regions of the mesolimbic system, including the ventral tegmental area (VTA). Evidence indicates that the N/OFQ-NOP receptor system is involved in reward processing and historically it has been proposed that activation of NOP receptors attenuates the motivation for substances of abuse. However, recent findings demonstrated that drug self-administration and relapse to drug-seeking are also attenuated after administration of NOP receptor antagonists. Here, to shed light on the mechanisms through which NOP receptor blockers modulate these processes, we utilized ex vivo patch-clamp recordings to investigate the effect of the selective NOP receptor antagonist LY2817412 on VTA dopaminergic (DA) function in male rats. Results showed that, similar to the endogenous NOP receptor agonist N/OFQ, LY2817412 reduced the spontaneous basal firing discharge of VTA DA neurons. Consistently, we found that NOP receptors are expressed both in VTA DA and GABA cells and that LY2817412 slice perfusion increased GABA release onto VTA DA cells. Finally, in the attempt to dissect the role of postsynaptic and presynaptic NOP receptors, we tested the effect of N/OFQ and LY2817412 in the presence of GABA receptors blockers. Results showed that the effect of LY2817412 was abolished following pretreatment with GABABR, but not GABAAR, blockers. Conversely, inhibition of DA neuronal activity by N/OFQ was unaffected by blockade of GABA receptors. Altogether, these results suggest that both NOP receptor agonists and antagonists can decrease VTA DA neuronal activity, but through distinct mechanisms of action. The effect of NOP receptor antagonists occurs through a GABABR-mediated mechanism while NOP receptor agonists seem to act via a direct effect on VTA DA neurons.
1. Introduction
The Nociceptin/Orphanin FQ (N/OFQ) peptide and its cognate receptor NOP are considered the fourth member of the opioid superfamily given their structural homologies with classical opioid peptides and receptors [1–3]. Accordingly, activation of the NOP receptor triggers the same intracellular signaling cascade of classical MOP, DOP and KOP opioid receptors leading to a generalized inhibition of neuronal activity and neurotransmitter release (see [4] for an extensive review). However, compared to other opioid receptors, NOP is expressed within different brain regions or neuronal populations, and therefore NOP receptor modulators possess distinguishable properties in regulating neural functions and behavior [5]. Given the elevated expression of N/OFQ and NOP receptors within the brain mesolimbic system [6, 7], its role in the regulation of reward processing and motivation has been widely scrutinized [8–10].
Earlier studies demonstrated that treatment with N/OFQ produces neither conditioned place preference (CPP) nor aversion (CPA), indicating that this peptide is devoid of intrinsic motivational properties [11–13]. On the other hand, in drug of abuse-induced CPP experiments it was shown that administration of N/OFQ counteracted the rewarding effects of cocaine, morphine, alcohol, and methamphetamine [11, 14–22]. Furthermore, it has been demonstrated that treatment with NOP receptor agonists reduced alcohol consumption as well as stress- and cue-induced reinstatement of alcohol-seeking in the rat [14, 23–30]. Similarly, NOP receptor activation decreased cocaine self-administration and cue-induced reinstatement of cocaine-seeking [31, 32]. Finally, it has recently been reported that treatment with NOP receptor agonists also diminished saccharin self-administration in rats and sucrose self-administration in mice [10, 31]. Altogether, these data suggest that the anti-rewarding properties of NOP receptor agonists are not specifically confined to drugs of abuse, but rather that NOP receptor activation might play a more general role in constraining the motivation for salient stimuli [10].
In the last few years, a number of selective NOP receptor antagonists have been tested to evaluate their ability to contrast addiction-like behaviors in laboratory animals. Interestingly, data showed that treatment with LY2940094 and LY2817412, two potent and selective NOP blockers, reduced alcohol intake and, stress and cue-induced reinstatement of alcohol-seeking in rodents [33–37]. Similar findings have been obtained with SB612111, another NOP receptor antagonist, that diminished alcohol and nicotine (but not cocaine) self-administration in rats [31, 38]. Finally, experiments in rats with a genetic deletion of the NOP receptor showed a reduced propensity to self-administer various substances of abuse, including alcohol, cocaine, heroin, and nicotine [34, 39]. Collectively, these findings demonstrate that the reduction of NOP receptor-mediated transmission confers resilience to addiction-like behavior in animal models.
Activation of dopaminergic (DA) neurons originating from the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc) is considered a key mechanism mediating drug reward and reinforcement [40, 41], while activation of the N/OFQ system has been shown to negatively modulate VTA DA transmission. For instance, intracerebroventricular (icv) or intra-VTA administration of N/OFQ significantly reduced the extracellular levels of DA in the NAc [42–46] and opposed the effects of cocaine and morphine on DA release [47–49]. Consistent with this finding earlier in situ hybridization studies showed that about half of VTA DA neurons co-express the NOP receptor, and the majority (∼75%) of the VTA NOP receptor-expressing neurons are DA [50, 51]. These findings were confirmed by ex vivo electrophysiological and in vivo fiber photometry recordings as they showed that application of N/OFQ directly hyperpolarized putative VTA DA neurons and reduced their activity [10, 52–54]. However, the effects of N/OFQ were not confined to DA cells, and peptide administration resulted also in a hyperpolarization of VTA GABA neurons, producing a decreased GABA inhibitory tone towards DA neurons [52, 53].
Recently it was shown that the VTA is a neurobiological site of action of NOP receptor antagonists as well. Indeed, intra-VTA administration of LY2817412 reduced both voluntary alcohol drinking [36] and stress- and cue-induced reinstatement of alcohol-seeking [37]. Moreover, administration of the NOP receptor antagonist LY2940094 abolished the increase in extracellular DA levels in the NAc of rats receiving alcohol [33]. Additionally, we recently showed that the reduction of nicotine self-administration produced by treatment with NOP antagonists is mediated by the VTA circuit, since LY2817412 microinjection into the VTA, but not into the NAc and the CeA, produced an effect resembling that of systemic injection [34, 39]. Collectively, these results support the idea of a complex modulation of mesolimbic DA transmission by NOP receptor signaling/transmission, possibly resulting by a combination of pre- and postsynaptic effects mediated by this receptor.
Although a number of possible hypotheses have been recently proposed to explain the mechanisms through which NOP receptor agonists and antagonists both attenuate addiction-like behaviors (e.g. see [33, 38]), the debate is still open. Here, in the attempt to disentangle the effects of NOP receptor agonists and antagonists and to gain insights into the mechanism through which NOP receptor blockade may regulate the reward circuitry we conducted an electrophysiological and molecular experiments to study the actions of LY2817412 on VTA DA activity.
2. Materials and Methods
2.1. Animals
Experiments were performed in 6–12 weeks old male Wistar Han rats (Charles River, Calco, Italy). Animals were housed in groups of four in a room with reversed artificial 12h/12h light/dark cycle (lights off at 8:00 a.m.), constant temperature (20–22°C) and humidity (45–55%). Food (4RF18, Mucedola, Settimo Milanese, Italy) and water were provided ad libitum. All the procedures were conducted in adherence to the guidelines of the European Community Council Directive for Care and Use of Laboratory Animals (2010/63/EU). Formal approval to conduct the experiments described was obtained from the Organism Responsible for Animal Welfare of the University of Camerino and the Italian Ministry of Health (Protocol number: 1D580.21)
Slice preparation and ex vivo electrophysiology
Rats were deeply anesthetized with isoflurane and decapitated during the early dark phase of their light/dark cycle. Brains were quickly removed and placed into an ice-cold N-methyl-D-glucamine (NMDG)-based cutting solution [55] containing (in mM): 92 NMDG, 20 HEPES, 25 glucose, 30 NaHCO3, 1.2 NaH2PO4, 2.5 KCl, 5 sodium ascorbate, 3 sodium pyruvate, 2 thiourea, 10 MgSO4, and 0.5 CaCl2, pH 7.4. Acute horizontal brain slices (200 μm thick) containing the VTA were obtained using a vibratome (Leica VT1000 S, Leica Biosystems Inc., IL, USA). After cutting, slices were incubated for additional 5 minutes in the same solution at ∼34° C and then transferred to a holding chamber filled with a solution (at room temperature) containing (in mM): 92 NaCl, 20 HEPES, 25 glucose, 30 NaHCO3, 1.2 NaH2PO4, 2.5 KCl, 5 sodium ascorbate, 3 sodium pyruvate, 2 thiourea, 1 MgSO4, and 2 CaCl2, pH 7.4. After >1 hour of recovery, a single slice was transferred to the recording chamber and continuously perfused at a flow rate of ∼2.0 ml/min with artificial cerebrospinal fluid (aCSF, in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 11 glucose, 26 NaHCO3, 2.4 CaCl2, pH 7.4. All solutions were saturated with 95% O2 and 5% CO2. The VTA was identified as being medial to the medial terminal nucleus of the accessory optic tract (MT). Neurons were visualized with infrared differential interference contrast (IR-DIC) optics on an Olympus BX51WI microscope (Olympus Corporation, MA, USA) and recordings were confined to neurons located within the lateral portion of the VTA. Putative VTA DA neurons were identified by conventional anatomical and electrophysiological parameters, such as a regular tonic spontaneous firing rate characterized by a frequency lower than 4 Hz [56, 57]. At the end of most recordings, the whole-cell configuration was obtained and neurons were further confirmed as putative VTA DA cells by the presence of a prominent Ih current evoked in response to a single hyperpolarizing voltage step from −60 to −120 mV [58]. The use of these conventional parameters to identify VTA DA neurons has been questioned [59, 60]; however, within the lateral portion of the VTA, DA neurons exhibit a prominent Ih and firing activity reminiscent of conventional DA neurons [61, 62]. Cell attached-electrophysiological recordings were carried out using borosilicate glass patch pipettes (2.5–3.0 MΩ; Harvard Apparatus, MA, USA) containing (in mM): 120 K-methanesulfonate, 15 KCl, 10 HEPES, 0.1 EGTA, 2 MgCl2, 5 Na2-phosphocreatine, 2 Mg-ATP, 0.3 Na-GTP (pH adjusted to 7.3 using KOH). Neuronal firing rate was assessed in tight-seal (GΩ seal resistance) cell-attached configuration. Recordings were performed at room temperature in voltage-clamp mode and the command voltage was set at that value in which no holding current is injected [63]. Whole cell IPSCs recordings were carried out using an internal solution contained (in mM): 125 Cs-methanesulfonate, 10 CsCl, 5 NaCl, 10 HEPES, 1 EGTA, 2 MgCl2, 2 Mg-ATP, 0.3 Na-GTP (pH adjusted to 7.3 using CsOH). Recordings were performed in voltage clamp mode holding the potential at +20 mV. At the end of each recording picrotoxin (100 μM) was perfused to verify that the recorded currents were of GABAergic nature. Recordings were conducted in gap-free mode using a Multiclamp 700B amplifier (2 kHz low-pass Bessel filter and 10 kHz digitization using a Digidata 1440A, Molecular Devices, CA, USA) with pClamp 10.7 software (Molecular Devices, CA, USA). In experiments involving pretreatment with LY2817412, GABA receptors antagonists or naloxone, brain slices were preincubated with the antagonists for at least 15 minutes prior the start of the baseline recording to ensure stable conditions during recordings.
2.2. RNAscope: Fluorescent in situ hybridization
Rats were deeply anesthetized with isoflurane and decapitated. Brains were quickly removed, and flash-frozen in isopentane on dry ice. Brains were sliced with a cryostat (Leica Biosystems GmbH, Heidelberg, Germany) and 20 μm coronal sections were collected at the level of the VTA [approximately at −5.6 mm to bregma; [64]]. Brain slices were mounted directly onto Superfrost Plus microscope slides (Fisher Scientific, Göteborg, Sweden) and kept at −80°C until use. In situ hybridization was performed according to the RNAscope Fluorescent Multiplex Kit User Manual (Advanced Cell Diagnostics, CA, USA). Briefly, brain sections were fixed by immersion in 10% neutral buffered formalin for 15 min at 4°C and then dehydrated in a series of 50%, 70%, and 100% ethanol at room temperature. A barrier around each brain section was made using a hydrophobic pen. Sections were incubated for 30 minutes at room temperature with the protease pretreatment IV solution from the RNAscope Fluorescent Multiplex Kit (Advanced Cell Diagnostics, CA, USA) and then washed in PBS and incubated with the target probes for 2 hours at 40°C using the HybEZ Hybridization System. Oprl1 (accession number NM_031569.4), Th (accession number NM_012740.3), and Gad1 (accession number NM_017007.1). probes were purchased from Advanced Cell Diagnostics. Sections were then incubated with a series of four amplifier probes at 40°C. During the last step, sections were incubated with fluorescently labeled probes Alexa 488 (green), Atto 550 (red), and Atto 647 (far red). Finally, sections were briefly incubated with DAPI to visualize nuclei in blue. Slides were cover-slipped, air dried, and stored at 4° C. Brains sections were examined with a confocal microscope (Zeiss LSM 800, Oberkochen, Germany) at 20x magnification to determine marker colocalization. Quantification of NOP colocalization with Th and Gad1 positive cells (N = 4 rats) was performed from two hemispheres of 3 sections. Positive cells expressing TH and GABA were detected using cell detection classifier with Qupath [65]. QuPath’s cell and subcellular detection functions were utilized to identify DAPI-labeled cells and color-coded probes within user-defined regions of interest. All detected cells underwent object classification based on their distinct staining and intensity thresholds. The quantification of Oprl1-positive cells in the Gad1 and Th channels involved considering the positive subcellular detections within each defined annotation. The measurement summary provided the counts of Oprl1 positive cells in Gad1 and Th counts, and the percentage of the colocalized cells were average across the entire set of sections of each rat.
2.3. Drugs
The NOP receptor antagonist LY2817412 was synthesized and kindly provided by Eli Lilly (IN, USA; [66]). LY2817412, picrotoxin, and CGP55845 were dissolved in 100% dimethyl sulfoxide (DMSO) and stocked at −20° C in 103x or 104x aliquots. The final concentration of DMSO in recording solutions was always lower than 0.21%. DMSO concentrations were maintained constant during the total duration of each recording (before, during, and after drug perfusion) and between groups. N/OFQ was a generous gift of Professor Remo Guerrini (University of Ferrara, Italy), and, similarly to tetrodotoxin (TTX), was dissolved in distilled water and stocked at −20° C in 103x stock solutions. All other compounds and reagents were purchased from Sigma-Aldrich (Milan, Italy) or Tocris (Bristol, UK).
2.4. Data analysis
Neuronal action potential firing frequency (1 minute time bins) was analyzed using a template detection protocol included in the pClamp 10.7 software. Data were normalized to the baseline condition and represented as a percentage (%) of baseline. Averaged data were expressed as mean ± standard error (SEM). Data were first examined for homogeneity of variances using Levene’s test, and for normality using Shapiro-Wilk test. When deviation from homogeneity of variances was detected, nonparametric analysis was used. In detail, concentration-dependence of the effects of LY2817412 was analyzed by using a one-way ANOVA, with “concentrations” as a between-subject factor (only neurons showing a stable response diverging more than 5% from baseline recordings were considered as “responsive cells”). For all the other electrophysiological experiments all recorded neurons were included in the analysis unless outliers were detected using ROUT method. Specifically, one recording was excluded from the experiment evaluating the effect of LY2817412 on VTA DA firing activity in presence of picrotoxin + CGP55845 pretreatment (Fig. 5B) because it was identified as an outlier. For the effects of N/OFQ or LY2817412 on firing activity, a two-way ANOVA was performed using “treatment” (N/OFQ or LY2817412) as within-subject factor and “pretreatment” with Vehicle/GABARs antagonists/Naloxone as between-subject factor. Newman-Keuls post hoc test was used where appropriate. To calculate effect sizes, Cohen’s d and partial eta squared (ηp2) were used. Significant difference was set at p < 0.05. Statistical analyses were performed using Prism 7 software (GraphPad Prism, CA, USA) and Statistica 7.0 software (Statsoft, OK, USA). n indicates the number of neurons recorded while N represents the number of animals used for any experimental group.
Figure 5. LY2817412 decreases VTA DA firing activity in a GABAB receptors-mediated manner.
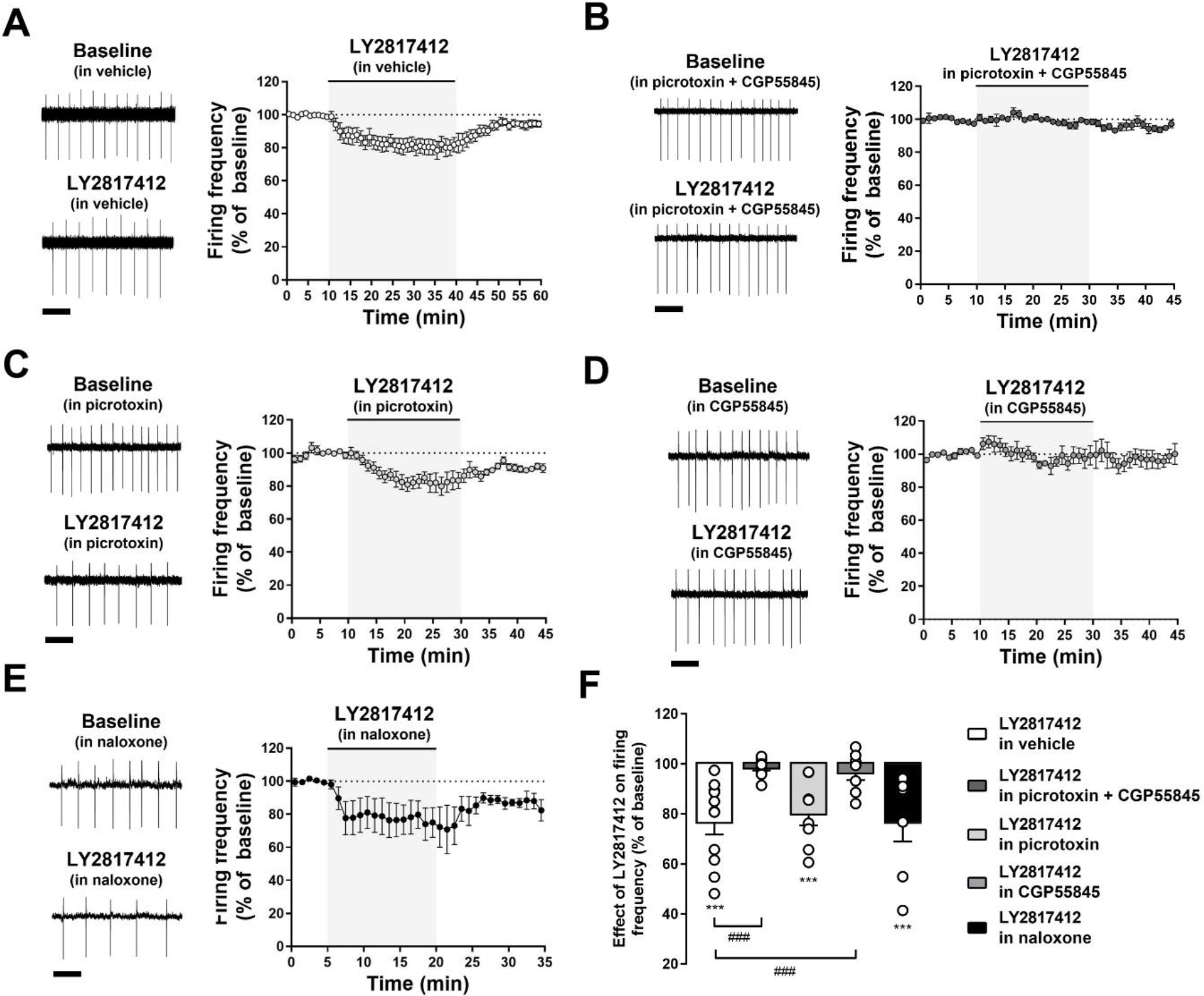
(A-E) The graphs show representative and averaged traces of the spontaneous firing from putative VTA DA neuron before, during, and after LY2817412 (1 μM) slice treatment in (A) absence, and in presence of (B) picrotoxin (100 μM) + CGP55845 (1 μM), (C) picrotoxin (100 μM), (D) CGP55845 (1 μM), or (E) naloxone (10 μM). Scale bar: 1 s. (F) Bar graphs represent the averaged maximal effects of LY2817412 on the firing rate of VTA DA neurons in the same experimental conditions. *** p < 0.001, ### p < 0.001.
3. Results
3.1. LY2817412 reduces the firing activity of putative VTA DA neurons
To establish whether NOP receptor blockade might affect VTA DA neuronal activity, we employed ex vivo cell-attached patch-clamp electrophysiological recordings monitoring the effect of the highly potent and selective NOP antagonist LY2817412 [see 36, 66 for complete pharmacological properties] on the spontaneous firing discharge of putative lateral VTA DA neurons. Contrary to expectations, LY2817412 bath perfusion induced a concentration-dependent and reversible decrease of the spontaneous firing discharge in most (∼72%) of putative VTA DA neurons recorded [(F(5, 44) = 2.914, p < 0.05 (n = 50/69, N = 37, in a range of concentrations between 0.1 nM and 10 μM)] (Fig. 1C).
Figure 1. LY2817412 decreases the spontaneous VTA DA firing activity.
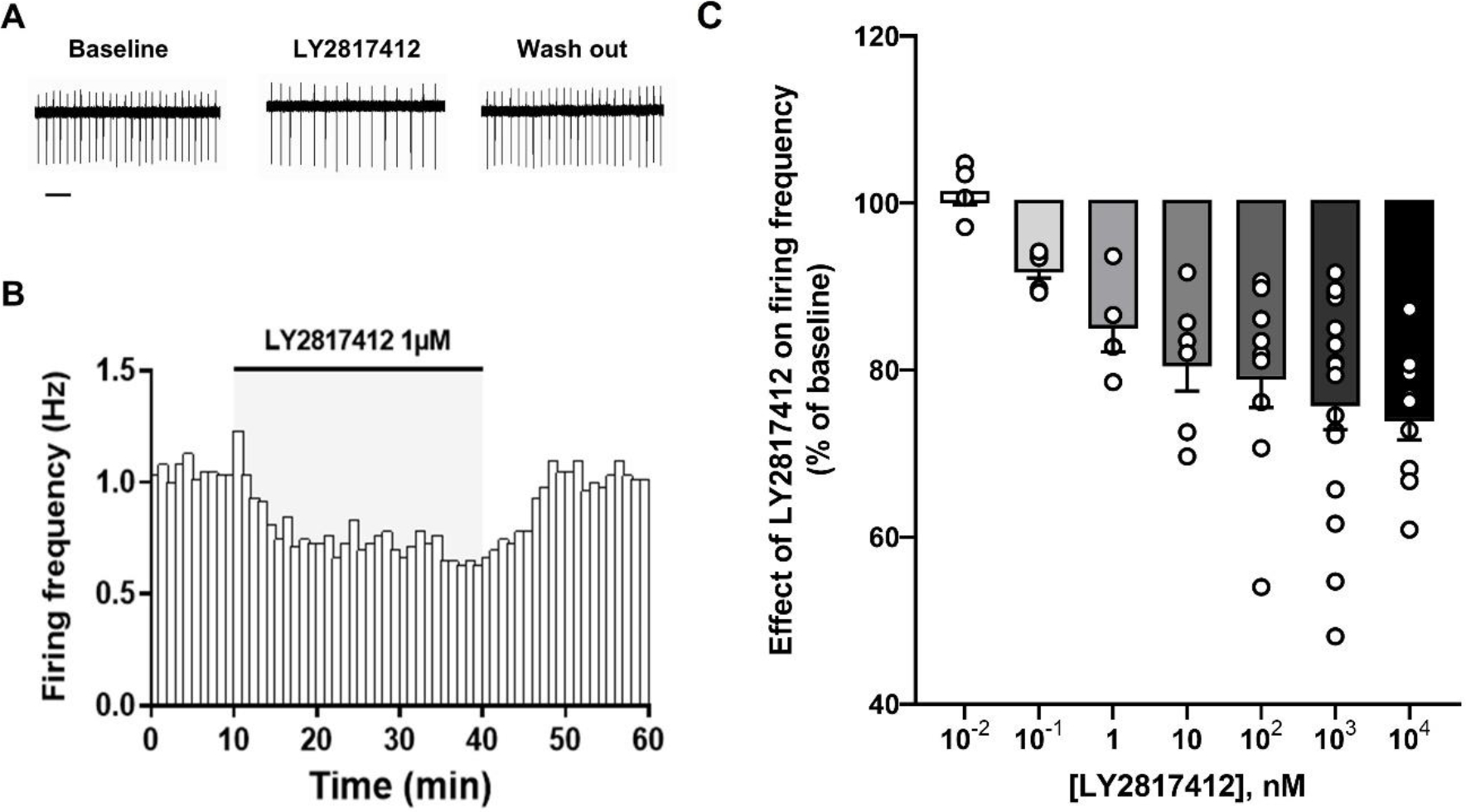
(A) Representative traces of spontaneous firing activity recorded in cell-attached configuration from a putative VTA DA neuron before (Baseline), during (LY2817412 1 μM), and after (Wash out) LY2817412 slice perfusion. Scale bar: 2 s. (B) The bar graph shows the temporal changes of firing rate (Hz) of the same representative putative VTA DA cell before, during and after LY2817412 slice perfusion. (C) The graph reports the concentration-dependent effect of LY2817412 on putative VTA DA neuronal firing rate. Note that, except for the 10−2 nM concentration, only data from LY2817412-responding cells are reported in this graph.
3.2. NOP receptors are expressed in both VTA DA and GABA neurons
To obtain anatomical information on NOP receptor localization in the VTA, we evaluated the expression of NOP receptors on Th- and Gad1-positive neurons, respectively. Results showed that the majority of Th-expressing (92.73 ± 1.76 %, N = 4; Fig. 2F) and Gad1-expressing (83.78 ± 6.25 %; N = 4; Fig. 2G) neurons were positive for NOP receptor mRNA signal, confirming that the receptor is strongly expressed in both DA and GABA neuronal populations (Fig. 2B–E). To functionally confirm this data, we analyzed the effects of LY2817412 on the properties of spontaneous inhibitory synaptic currents (sIPSCs) recorded from putative VTA DA neurons. LY2817412 exposure induced a significant increase in the frequency of sIPSCs (Baseline: 0.358 ± 0.057 Hz; LY2817412: 0.486 ± 0.099 Hz; t(10) = 2.373, p < 0.05, paired t-test; Coheńs d: 0.72; n = 11, N = 5; Fig. 3B) without affecting their amplitude (Baseline: 22.54 ± 1.75 pA; LY2817412: 22.08 ± 1.58 pA; t(10) = 0.3945, p > 0.05, paired t-test; Coheńs d: 0.12; n = 11, N = 5; Fig. 3C). Additionally, we evaluated the effect of LY2817412 on miniature IPSCs (mIPSCs) by bath-applying tetrodotoxin (TTX, 1 μM). Intriguingly, LY2817412 failed to modulate either the frequency (Baseline: 0.587 ± 0.123 Hz; LY2817412: 0.639 ± 0.167 Hz; p > 0.05, Wilcoxon matched-pairs signed rank test; Coheńs d: 0.29; n = 9, N = 4; Fig. 3E) or amplitude (Baseline: 14.45 ± 1.38 pA; LY2817412: 14.43 ± 1.96 pA; p > 0.05, Wilcoxon matched-pairs signed rank test; Coheńs d: 0.01; n = 9, N = 4; Fig. 3F) of mIPSCs. This suggests that NOP receptor blockade increases GABA release onto VTA DA neurons in an action potential-dependent manner and that a functional slice network is necessary to mediate such effect. Of note, recorded sIPSCs and mIPSCs were completely abolished by subsequent exposure to picrotoxin (100 μM), indicating they are mediated by GABA receptors (data not shown). Altogether, these results suggest that the effect of NOP modulators on VTA DA activity might not be limited to direct postsynaptic effects.
Figure 2. NOP receptors are expressed in VTA DA and GABA neurons.
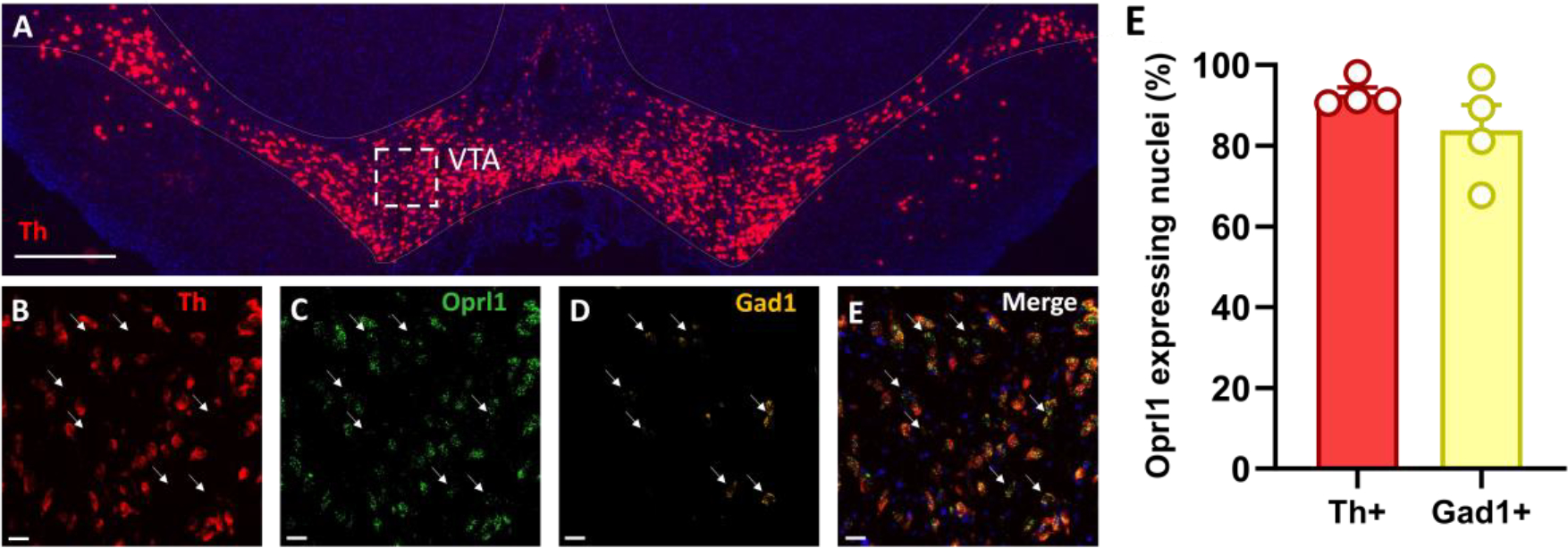
(A) Representative large-scale 2x magnification image showing the expression of Th (red) and DAPI (blue) mRNA through fluorescent in situ hybridization in VTA coronal slices. Scale bar: 500 μm. (B-E) Representative 20x magnification images from the white dashed-line box from panel A showing expression of Th (red), Oprl1 (green), Gad1 (yellow), and DAPI (blue) mRNA through fluorescent in situ hybridization in VTA coronal slices. White arrows point to the localization of Gad1-positive neurons. Scale bar: 25 μm. (F) Bar graphs represent the average percentage of Th- and Gad1-positive nuclei co-expressing Oprl1.
Figure 3. LY2817412 modulates GABA release onto VTA DA neurons.
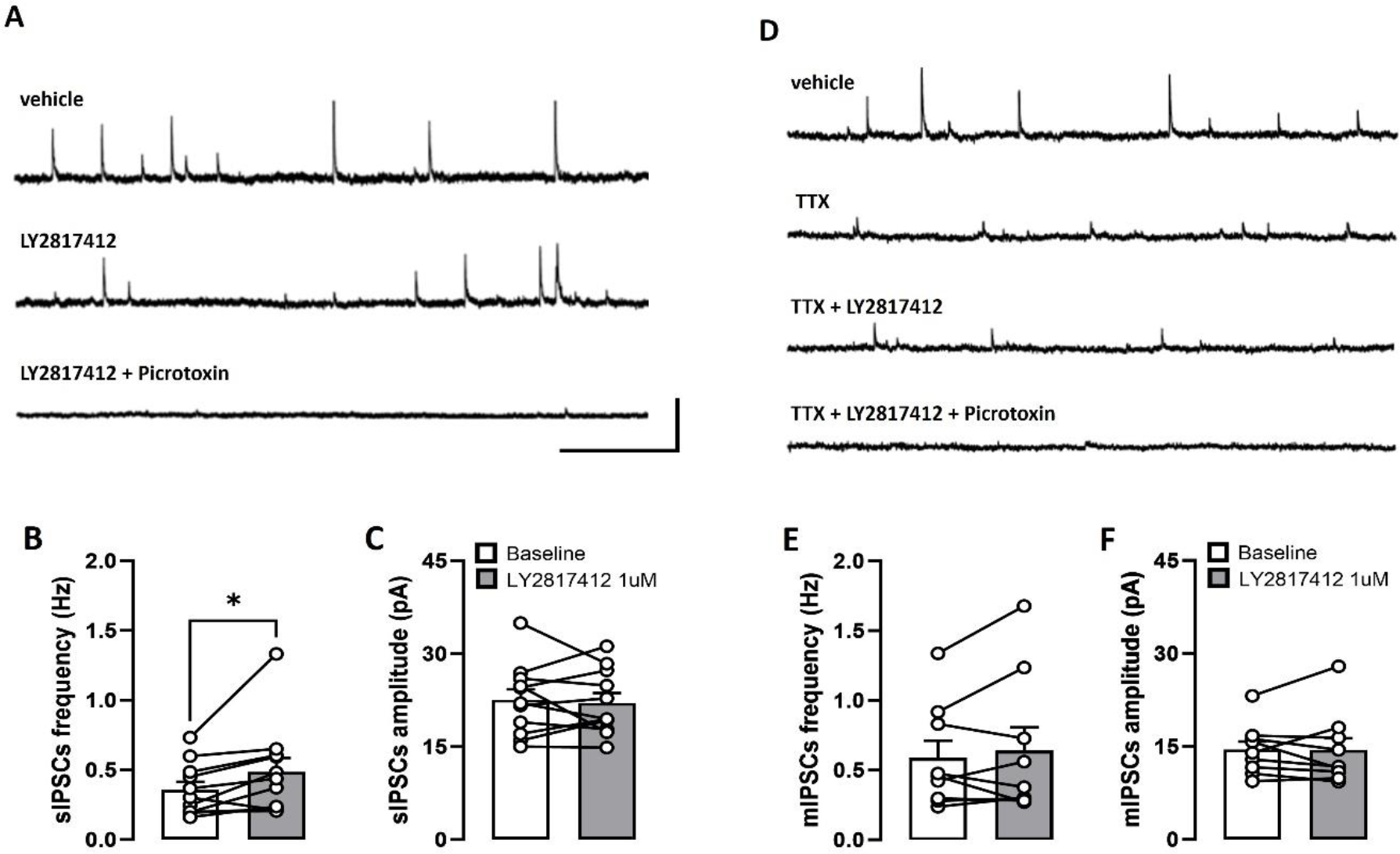
(A) Representative sIPSCs traces recorded from putative VTA DA neurons before (Baseline), during LY2817412 (1 μM), and during picrotoxin (100 μM) slice perfusion. Scale bars: 50 pA × 2 s. (B-C) Bar graphs showing the effects of LY2817412 (1 μM) on frequency (D) and amplitude (E) of sIPSCs. (D). Representative mIPSCs traces recorded from putative VTA DA neurons before (vehicle), during TTX (1 μM), TTX + LY2817412 (1 μM), and during TTX + LY2817412 + picrotoxin (100 μM) slice perfusion. Scale bars: 50 pA × 2 s. (E-F) Bar graphs showing the effects of LY2817412 (1 μM) on frequency (D) and amplitude (E) of mIPSCs.
3.3. N/OFQ and LY2817412 reduce VTA DA neuronal activity through different mechanisms of action.
Building on the evidence of the dual localization of NOP receptors, we explored to which extent the effects of NOP receptor agonists and antagonists on VTA DA activity were (or were not) mediated by GABA transmission.
First, we studied the effects of bath perfusion with N/OFQ (0.3 μM), on VTA DA neuronal firing activity in the absence (vehicle) or in presence of GABAA and GABAB receptor antagonists, picrotoxin (100 μM) and CGP55845 (1 μM; Fig. 4). Results showed that N/OFQ significantly reduced the firing discharge of putative VTA DA neurons in both experimental groups [treatment: (F(1,12) = 53.70, p < 0.001, ηp2 = 0.79; N/OFQ: 49.3 ± 11.7% of baseline (n = 7, N = 4, p < 0.001), from 1.05 ± 0.06 Hz to 0.5 ± 0.09 Hz; N/OFQ in picrotoxin and CGP55845: 48.0 ± 7.5% of baseline (n = 7, N = 6, p < 0.001), from 1.04 ± 0.22 Hz to 0.62 ± 0.21 Hz] with no significant differences found in the peak amplitude of N/OFQ response between groups [pretreatment: (F(1,12) = 0.01, p > 0.05, ηp2 = 0.02); treatment x pretreatment: (F(1,12) = 0.01, p > 0.05, ηp2 = 0.01)] (Fig.4B). This finding demonstrates that the reduction of the spontaneous firing discharge of VTA DA cells elicited by activation of NOP receptor is independent of GABA transmission.
Figure 4. Effects of N/OFQ on VTA DA neuronal firing discharge is not altered by blockade of GABARs.
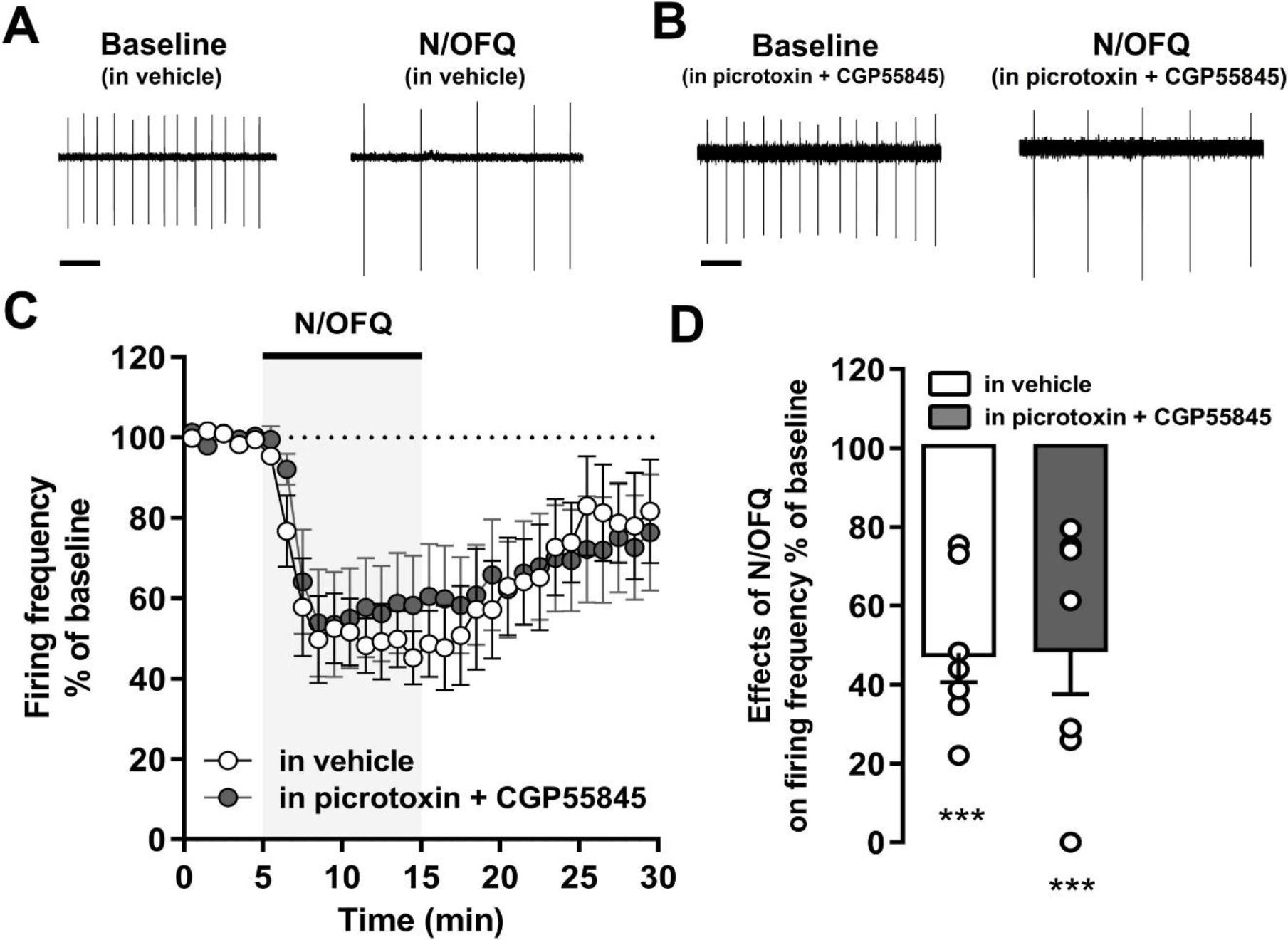
(A) Representative and averaged temporal changes of firing rate of putative VTA DA recorded neurons before, during, and after N/OFQ (0.3 μM) slice perfusion in absence, or in presence of picrotoxin (100 μM) and CGP55845 (1 μM). Scale bar: 1 s. (B) Bar graphs represent the averaged peak effects of N/OFQ in the firing rate of VTA DA neurons in absence, and in presence of picrotoxin and CGP55845 pretreatment. ** p < 0.01.
Next, using the same experimental settings, we evaluated the effects of LY2817412 (1 μM). Results showed that the inhibitory effect of the NOP antagonist on spontaneous VTA DA firing activity was significantly [treatment: (F(2, 74) = 27.69; p < 0.001; ηp2 = 0.42)] reduced by GABARs antagonism [pretreatment: (F(4, 37) = 4.91; p < 0.01; ηp2 = 0.26); treatment x pretreatment: (F(8, 74) = 4.09; p < 0.001; ηp2 = 0.24)] (Fig. 5). Specifically, pretreatment with the combination of picrotoxin and CGP55845 completely prevented the reduction of VTA DA neuronal firing produced by LY2817412 [LY2817412 in vehicle: 76.7 ± 5.0% of baseline (n = 11, N = 10, p < 0.01), from 1.68 ± 0.28 Hz to 1.34 ± 0.26 Hz; LY2817412 in picrotoxin and CGP55845: 94.2 ± 4.5% of baseline (n = 9, N = 6, p > 0.05), from 1.32 ± 0.21 Hz to 1.30 ± 0.20 Hz; LY2817412 in vehicle vs LY2817412 in picrotoxin and CGP55845: p < 0.001)] (Fig. 5F). This effect was replicated following selective inhibition of GABABRs with CGP55845 [LY2817412 in CGP55845: 96.6 ± 3.1% of baseline (n = 8, N = 4, p > 0.05), from 1.12 ± 0.19 Hz to 1.08 ± 0.18 Hz; LY2817412 in vehicle vs LY2817412 in CGP55845: p < 0.001)]. In contrast, the effect of LY2817412 was not prevented by selective blockade of GABAARs [(LY2817412 in picrotoxin: 80.2 ± 4.7% of baseline (n = 8, N = 5, p < 0.001), from 1.45 ± 0.48 Hz to 1.24 ± 0.41 Hz; LY2817412 in vehicle vs LY2817412 in picrotoxin: p > 0.05] (Fig. 5F). Of note, the effect of LY2817412 (1 μM) on basal VTA DA firing activity was not altered by pretreatment with the pan opioid antagonist naloxone [LY2817412 in naloxone 10 μM: 76.8 ± 7.8% of baseline (n = 7, N = 4, p < 0.001), from 1.12 ± 0.16 Hz to 0.88 ± 0.19 Hz; LY2817412 in vehicle vs LY2817412 in naloxone: p > 0.05] (Fig. 5F), confirming that it is selectively mediated by NOP receptors and not influenced by non-specific effects on other opioid receptors. Altogether these data indicate that NOP receptor antagonism decreases the spontaneous activity of VTA DA neurons through facilitation of GABABRs-mediated transmission.
4. Discussion
Results demonstrated that both activation and inhibition of NOP receptors reduced VTA DA neuronal activity ex vivo. To address the mechanisms underlying this phenomenon, we explored the contribution of GABA signaling to the effects of NOP agonist and antagonists on VTA DA firing activity. We found that NOP receptors are expressed both on VTA DA and GABA neurons, and that slice treatment with the NOP receptor antagonist LY2817412 increased the frequency of sIPSCs, and did not affect the properties of mIPSCs conveying onto VTA DA neurons. This set of data suggests that NOP antagonists might modulate VTA DA neuronal activity through a presynaptic, action potential-dependent, enhancement of GABA tone toward these cells. Furthermore, we found that the inhibitory effect of N/OFQ on VTA DA firing activity was not prevented by simultaneous blockade of GABAA and GABAB receptors. This is consistent with previously published results showing that the reduction of DA neuronal activity by NOP receptor agonism is mainly mediated by a direct hyperpolarization of DA neurons [52]. Conversely, pretreatment with a combination of GABAAR and GABABR blockers abolished the inhibitory effect of the NOP receptor antagonist LY2817412 on VTA DA activity. This result was replicated by preincubation with a GABABR blocker alone, whereas pretreatment with a GABAAR antagonist did not exert any significant effect. Altogether these data demonstrate that NOP receptor antagonism inhibits VTA DA activity through an enhancement of GABA transmission mediated by GABABRs.
Based on the current literature and on the present results we believe that the net effect of NOP receptor agonists on the firing rate of VTA DA neurons may result from at least two simultaneous, but opposed, effects: (1) direct postsynaptic hyperpolarization of VTA DA neurons; (2) presynaptic inhibition of GABA transmission and subsequent attenuation of the inhibitory inputs on downstream VTA DA cells. Accordingly, previous work has demonstrated that NOP receptor agonists do not solely directly decrease VTA DA firing, but also inhibit the activity of putative VTA GABA neurons and presynaptically reduce GABA release toward VTA DA cells [52, 53]. We therefore hypothesize that in the case of exogenous administration of N/OFQ or synthetic agonists the activation of NOP receptors located on DA neurons strongly prevails on NOP-mediated reduction of GABA transmission, resulting in direct inhibition of DA activity. This is also consistent with pharmacological data showing that the ability of the NOP receptor agonist SCH221510 to attenuate the motivation for sucrose self-administration is absent in NOP receptor knock-out mice, but it is completely restored following re-expression of NOP receptors selectively on VTA DA neurons [10].
Interestingly, here we demonstrated that occlusion of endogenous N/OFQ transmission following NOP receptor blockade leads to inhibition of VTA DA activity through GABABRs mediated mechanisms. Although additional experiments are needed to fully support this hypothesis, it is tempting to speculate that LY2817412-induced inhibition of DA transmission might depend on the following series of events: (1) removal of N/OFQ mediated endogenous inhibition of GABA transmission by NOP receptor blockade; (2) increased activity and neurotransmitter release from presynaptic GABA neurons; (3) enhanced activation of postsynaptic GABABRs in VTA DA neurons; (4) reduced firing activity of these neurons.
Notably, the effect of LY2817412 on DA neuronal activity, even at the highest concentration tested, appears less pronounced than those reported here and in other reports [52] of NOP agonists. If on one hand this can be seen as a limitation of the therapeutic potential of NOP antagonists, on the other hand there are some potential advantages associated with that. These advantages might include a decreased propensity for the emergence of side effects (e.g generalized decrease in motivation due to an excessive blunting of VTA DA activity [67, 68]) and reduction of tolerance promoted by desensitization/downregulation of NOP receptors that can arise in response to chronic exposure to agonists [69].
At present, we are unable to predict whether this mechanism involves actions on local GABA interneurons or involves the participation of NOP receptors localized on GABA terminals originating from extra-VTA brain regions, such as the NAc, the rostromedial tegmental nucleus (RMTg), or the lateral hypothalamus (LH) [70–72]. However, the lack of an effect of LY2817412 on mIPSCs properties indirectly supports the functional relevance the local network preserved in the brain slices on mediating such effect. It is also important to emphasize that our data do not rule out a potential contribution of VTA glutamatergic neurons and terminals. In fact, it has been recently reported that morphine increases VTA DA firing activity through removal of GABABR-mediated inhibition of glutamate release in the VTA [73]. Hence, it is theoretically possible that NOP receptor antagonism may attenuate VTA DA activity via GABABRs-mediated inhibition of the basal glutamatergic tone conveyed to VTA DA neurons. Further studies will aim to verify the contribution of these different components to the observed phenomenon.
A limitation to the present study is represented by the fact that our experiments were all conducted in drug naïve male rats. Considering that the N/OFQ-NOP receptor system is known to plastically adapt in response to chronic drug use [9], it is possible that the balance between the presynaptic and the postsynaptic effect of NOP modulators might be modified under these conditions. Furthermore, VTA NOP receptors might be differentially expressed and could differentially adapt in female rats, compared to males. Further experiments are needed to better elucidate these points. Nevertheless, we previously demonstrated that systemic or intra-VTA LY2817412 administration of LY2817412 reduces voluntary alcohol intake and cue- and stress-induced reinstatement of alcohol seeking in male and female rats, suggesting a similar modulatory effect of NOP antagonism on both sexes [36, 37].
Present data are in contrast with the results of previous studies showing that administration of the NOP receptor antagonist J-113397 (previously known as Compound B) increased VTA DA neuronal activity, enhanced DA release in the NAc, and promoted CPP [10, 42]. However, these effects of J-113397 were mediated by mechanisms unrelated to its NOP receptor activity, and possibly linked to the lower selectivity of this molecule compared to LY2817412 [36, 42, 66].
Previous work in laboratory animals has demonstrated that the motivation for substances of abuse can be attenuated by the administration of both NOP receptor agonists and antagonists. To resolve this conundrum, in earlier studies we hypothesized that NOP receptor agonists may act as a functional antagonist by facilitating the rapid desensitization of the NOP receptor system [9, 33, 38, 74]. This hypothesis stemmed from two observations: (1) exogenous administration of N/OFQ or synthetic NOP receptor ligands produce rapid receptor desensitization [see [69] for an extensive review]; (2) the effect of NOP receptor agonists on drugs of abuse is more pronounced after chronic administration and is maintained for several days after treatment discontinuation, while NOP receptor antagonists act after acute injection [27, 33, 36]. However, in the light of the present data we need to reconsider this hypothesis because, as shown here, NOP antagonists can attenuate mesolimbic DA activity also in a manner similar to NOP agonists, although they do so through a different mechanism. We speculate that this could represent a common factor explaining why both NOP receptor agonists and antagonists can attenuate the motivation for drugs of abuse [75].
In conclusion, in this study we demonstrate that NOP receptor antagonism decreases VTA DA activity through a GABABR-mediated mechanism of action. This finding provides important insights into the mechanisms subserving the effects of NOP receptor agonists and antagonists on drugs of abuse and provides new information on the role of the endogenous N/OFQ-NOP receptor system in regulating the function of the mesolimbic DA system.
Figure 6. Hypothetical mechanism of action of NOP agonist and antagonist on regulating the spontaneous VTA DA firing discharge.
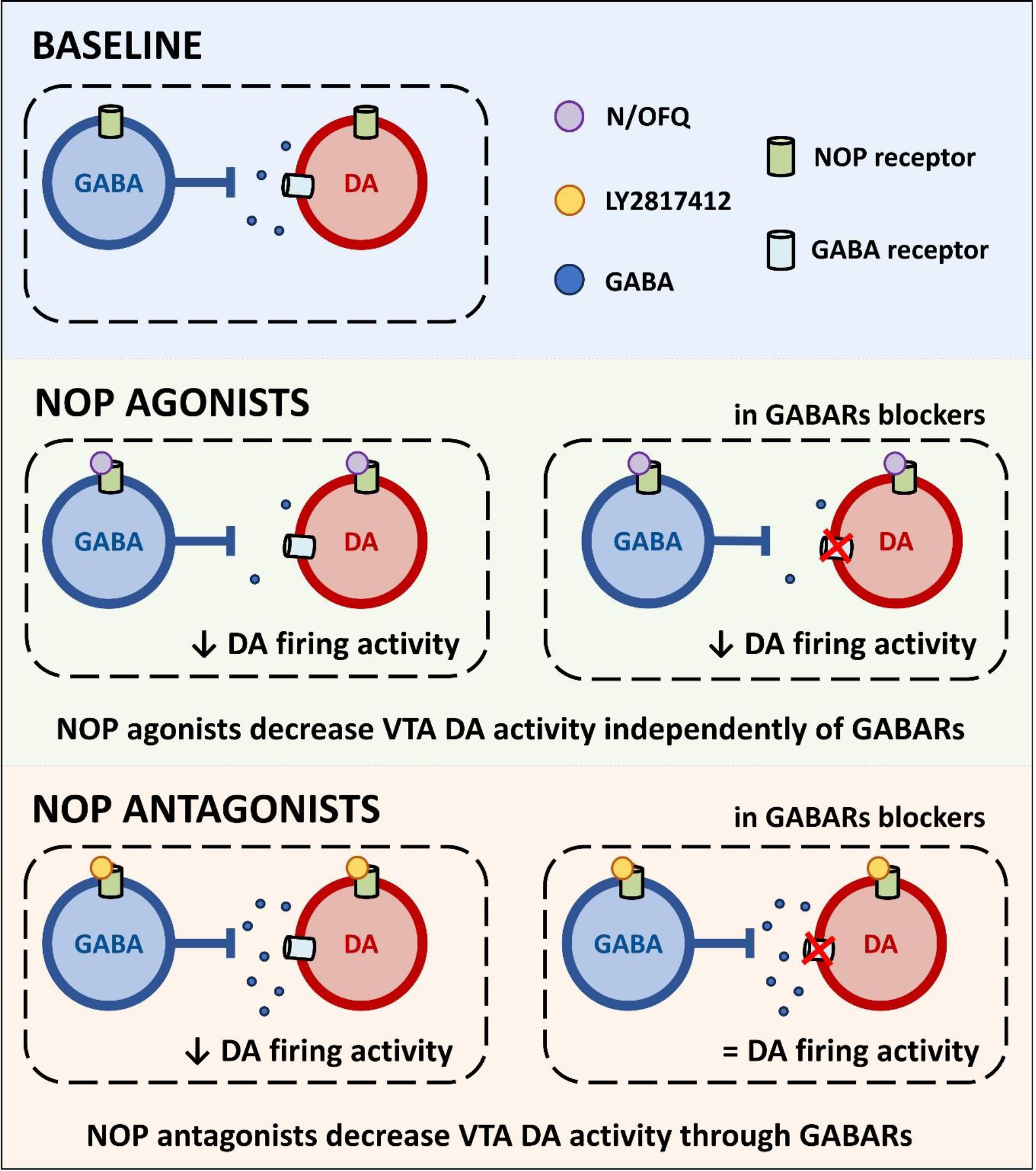
NOP receptor agonists produce a direct postsynaptic hyperpolarization of VTA DA neurons and a presynaptic inhibition of GABA transmission. The activation of NOP receptors located on DA neurons strongly prevails on NOP-mediated reduction of GABA transmission, resulting in direct inhibition of DA activity. NOP receptor antagonists leads to inhibition of VTA DA activity through a GABARs mediated mechanisms; in presence of GABARs antagonists the effect is abolished. Potential mechanism of NOP antagonists: (1) removal of N/OFQ-mediated endogenous inhibition of GABA transmission; (2) increased activity and neurotransmitter release from presynaptic GABA neurons; (3) enhanced activation of postsynaptic GABARs in VTA DA neurons resulting in a decreased firing activity of these neurons.
Acknowledgments
We wish to thank Linda M. Rorick-Kehn for the scientific inputs and thoughtful comments on the work. We also wish to thank Rina Righi, Agostino Marchi and Mariangela Fiorelli for animal care as well as Alfredo Fiorelli for his excellent technical support. Authors gratefully thank Marina Antonini for her experimental support.
Funding and Disclosure
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grants AA014351 (to FW and RC) and AA017447 (to MR and RC). The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Mollereau C, et al. , ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett, 1994. 341(1): p. 33–8. [DOI] [PubMed] [Google Scholar]
- 2.Meunier JC, et al. , Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature, 1995. 377(6549): p. 532–5. [DOI] [PubMed] [Google Scholar]
- 3.Reinscheid RK, et al. , Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science, 1995. 270(5237): p. 792–4. [DOI] [PubMed] [Google Scholar]
- 4.Winters BL, Christie MJ, and Vaughan CW, Electrophysiological Actions of N/OFQ. Handb Exp Pharmacol, 2019. 254: p. 91–130. [DOI] [PubMed] [Google Scholar]
- 5.Toll L, et al. , Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev, 2016. 68(2): p. 419–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal CR Jr., et al. , Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol, 1999. 412(4): p. 563–605. [PubMed] [Google Scholar]
- 7.Neal CR Jr., et al. , Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol, 1999. 406(4): p. 503–47. [PubMed] [Google Scholar]
- 8.Ciccocioppo R, et al. , NOP-Related Mechanisms in Substance Use Disorders. Handb Exp Pharmacol, 2019. 254: p. 187–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkin JM, et al. , The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther, 2014. 141(3): p. 283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker KE, et al. , A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward. Cell, 2019. 178(3): p. 653–671 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciccocioppo R, et al. , Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol, 2000. 404(1–2): p. 153–9. [DOI] [PubMed] [Google Scholar]
- 12.Devine DP, et al. , The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res, 1996. 727(1–2): p. 225–9. [DOI] [PubMed] [Google Scholar]
- 13.Le Pen G, et al. , The orphanin receptor agonist RO 64–6198 does not induce place conditioning in rats. Neuroreport, 2002. 13(4): p. 451–4. [DOI] [PubMed] [Google Scholar]
- 14.Economidou D, et al. , Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides, 2006. 27(12): p. 3299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotlinska J, et al. , Orphanin FQ/nociceptin but not Ro 65–6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol, 2002. 13(3): p. 229–35. [DOI] [PubMed] [Google Scholar]
- 16.Kuzmin A, et al. , Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther, 2003. 304(1): p. 310–8. [DOI] [PubMed] [Google Scholar]
- 17.Murphy NP, Lee Y, and Maidment NT, Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res, 1999. 832(1–2): p. 168–70. [DOI] [PubMed] [Google Scholar]
- 18.Rutten K, et al. , Effects of the NOP receptor agonist Ro65–6570 on the acquisition of opiate- and psychostimulant-induced conditioned place preference in rats. Eur J Pharmacol, 2010. 645(1–3): p. 119–26. [DOI] [PubMed] [Google Scholar]
- 19.Sakoori K and Murphy NP, Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl), 2004. 172(2): p. 129–36. [DOI] [PubMed] [Google Scholar]
- 20.Sakoori K and Murphy NP, Expression of morphine-conditioned place preference is more vulnerable than naloxone-conditioned place aversion to disruption by nociceptin in mice. Neurosci Lett, 2008. 443(2): p. 108–12. [DOI] [PubMed] [Google Scholar]
- 21.Zaveri NT, et al. , The Nociceptin Receptor (NOP) Agonist AT-312 Blocks Acquisition of Morphine- and Cocaine-Induced Conditioned Place Preference in Mice. Front Psychiatry, 2018. 9: p. 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaveri NT, et al. , A Novel and Selective Nociceptin Receptor (NOP) Agonist (1-(1-((cis)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indol-2-yl)methanol (AT-312) Decreases Acquisition of Ethanol-Induced Conditioned Place Preference in Mice. Alcohol Clin Exp Res, 2018. 42(2): p. 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Fardon R, et al. , Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport, 2000. 11(9): p. 1939–43. [DOI] [PubMed] [Google Scholar]
- 24.Kuzmin A, et al. , The nociceptin/orphanin FQ receptor agonist Ro 64–6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology, 2007. 32(4): p. 902–10. [DOI] [PubMed] [Google Scholar]
- 25.Economidou D, et al. , Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry, 2008. 64(3): p. 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Guglielmo G, et al. , MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol, 2015. 20(4): p. 643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccocioppo R, et al. , Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology, 2014. 39(11): p. 2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciccocioppo R, et al. , Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl), 1999. 141(2): p. 220–4. [DOI] [PubMed] [Google Scholar]
- 29.Ciccocioppo R, et al. , Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl), 2004. 172(2): p. 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aziz AM, et al. , The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacology (Berl), 2016. 233(19–20): p. 3553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cippitelli A, et al. , Potent and selective NOP receptor activation reduces cocaine self-administration in rats by lowering hedonic set point. Addict Biol, 2019: p. e12844. [DOI] [PubMed] [Google Scholar]
- 32.Li H, et al. , NOP Receptor Agonist Ro 64–6198 Decreases Escalation of Cocaine Self-Administration in Rats Genetically Selected for Alcohol Preference. Front Psychiatry, 2019. 10: p. 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rorick-Kehn LM, et al. , A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin Exp Res, 2016. 40(5): p. 945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallupi M, et al. , Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology, 2017. 42(3): p. 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunori G, et al. , NOP Receptor Antagonists Decrease Alcohol Drinking in the Dark in C57BL/6J Mice. Alcohol Clin Exp Res, 2019. 43(10): p. 2167–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borruto AM, et al. , NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br J Pharmacol, 2020. 177(7): p. 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borruto AM, et al. , NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats. Neuropsychopharmacology, 2021. 46(12): p. 2121–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cippitelli A, et al. , A key role for the N/OFQ-NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co-administration. Sci Rep, 2016. 6: p. 26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domi A, et al. , Genetic Deletion or Pharmacological Blockade of Nociceptin/Orphanin FQ Receptors in the Ventral Tegmental Area Attenuates Nicotine-Motivated Behaviour. Br J Pharmacol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Chiara G and Imperato A, Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther, 1988. 244(3): p. 1067–80. [PubMed] [Google Scholar]
- 41.Wise RA, Drug-activation of brain reward pathways. Drug Alcohol Depend, 1998. 51(1–2): p. 13–22. [DOI] [PubMed] [Google Scholar]
- 42.Koizumi M, et al. , Exogenous, but not endogenous nociceptin modulates mesolimbic dopamine release in mice. J Neurochem, 2004. 89(1): p. 257–63. [DOI] [PubMed] [Google Scholar]
- 43.Koizumi M, et al. , The NOP (ORL1) receptor antagonist Compound B stimulates mesolimbic dopamine release and is rewarding in mice by a non-NOP-receptor-mediated mechanism. Br J Pharmacol, 2004. 143(1): p. 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy NP, Ly HT, and Maidment NT, Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience, 1996. 75(1): p. 1–4. [DOI] [PubMed] [Google Scholar]
- 45.Murphy NP and Maidment NT, Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem, 1999. 73(1): p. 179–86. [DOI] [PubMed] [Google Scholar]
- 46.Shieh K and Pan J, Effects of orphanin FQ on central dopaminergic neuronal activities and prolactin secretion. Am J Physiol Regul Integr Comp Physiol, 2001. 280(3): p. R705–12. [DOI] [PubMed] [Google Scholar]
- 47.Di Giannuario A and Pieretti S, Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides, 2000. 21(7): p. 1125–30. [DOI] [PubMed] [Google Scholar]
- 48.Lutfy K, Do T, and Maidment NT, Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl), 2001. 154(1): p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez-DeRose J, et al. , Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur J Pharmacol, 2013. 699(1–3): p. 200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maidment NT, et al. , Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport, 2002. 13(9): p. 1137–40. [DOI] [PubMed] [Google Scholar]
- 51.Norton CS, et al. , Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol, 2002. 444(4): p. 358–68. [DOI] [PubMed] [Google Scholar]
- 52.Zheng F, Grandy DK, and Johnson SW, Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol, 2002. 136(7): p. 1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Driscoll JR, et al. , Differential Modulation of Ventral Tegmental Area Circuits by the Nociceptin/Orphanin FQ System. eNeuro, 2020. 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez J, et al. , Nociceptin/orphanin FQ neurons in the Arcuate Nucleus and Ventral Tegmental Area Act via Nociceptin Opioid Peptide Receptor Signaling to Inhibit Proopiomelanocortin and A10 Dopamine Neurons and Thereby Modulate Ingestion of Palatable Food. Physiol Behav, 2021. 228: p. 113183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ting JT, et al. , Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol, 2014. 1183: p. 221–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson SW and North RA, Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol, 1992. 450: p. 455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theile JW, et al. , GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience, 2011. 172: p. 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wanat MJ and Bonci A, Dose-dependent changes in the synaptic strength on dopamine neurons and locomotor activity after cocaine exposure. Synapse, 2008. 62(10): p. 790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margolis EB, et al. , The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol, 2006. 577(Pt 3): p. 907–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang TA, Placzek AN, and Dani JA, In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology, 2010. 59(6): p. 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lammel S, Lim BK, and Malenka RC, Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology, 2014. 76 Pt B(0 0): p. 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pignatelli M and Bonci A, Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron, 2015. 86(5): p. 1145–57. [DOI] [PubMed] [Google Scholar]
- 63.Perkins KL, Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods, 2006. 154(1–2): p. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paxinos G, and Watson C, The Rat Brain in Stereotaxic Coordinates, 4th Edn. San Diego, Academic Press., 1998. [Google Scholar]
- 65.Bankhead P, et al. , QuPath: Open source software for digital pathology image analysis. Sci Rep, 2017. 7(1): p. 16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toledo MA, et al. , Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7’-thieno[2,3-c]pyran) scaffold. J Med Chem, 2014. 57(8): p. 3418–29. [DOI] [PubMed] [Google Scholar]
- 67.Berridge KC, Venier IL, and Robinson TE, Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci, 1989. 103(1): p. 36–45. [DOI] [PubMed] [Google Scholar]
- 68.Robinson TE and Berridge KC, The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev, 1993. 18(3): p. 247–91. [DOI] [PubMed] [Google Scholar]
- 69.Donica CL, et al. , Cellular mechanisms of nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor regulation and heterologous regulation by N/OFQ. Mol Pharmacol, 2013. 83(5): p. 907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards NJ, et al. , Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat Neurosci, 2017. 20(3): p. 438–448. [DOI] [PubMed] [Google Scholar]
- 71.Jhou TC, et al. , The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron, 2009. 61(5): p. 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nieh EH, et al. , Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron, 2016. 90(6): p. 1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen M, et al. , Morphine disinhibits glutamatergic input to VTA dopamine neurons and promotes dopamine neuron excitation. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ubaldi M, Cannella N, and Ciccocioppo R, Emerging targets for addiction neuropharmacology: From mechanisms to therapeutics. Prog Brain Res, 2016. 224: p. 251–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volkow ND, Wise RA, and Baler R, The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci, 2017. 18(12): p. 741–752. [DOI] [PubMed] [Google Scholar]


