Abstract
Background
A substantial percentage of the US population is not up to date on guideline-recommended cancer screenings. Identifying interventions that effectively improve screening rates would enhance the delivery of such screening. Interventions involving health IT (HIT) show promise, but much remains unknown about how HIT is optimized to support cancer screening in primary care.
Objective
This scoping review aims to identify (1) HIT-based interventions that effectively support guideline concordance in breast, cervical, and colorectal cancer screening provision and follow-up in the primary care setting and (2) barriers or facilitators to the implementation of effective HIT in this setting.
Methods
Following scoping review guidelines, we searched MEDLINE, CINAHL Plus, Web of Science, and IEEE Xplore databases for US-based studies from 2015 to 2021 that featured HIT targeting breast, colorectal, and cervical cancer screening in primary care. Studies were dual screened using a review criteria checklist. Data extraction was guided by the following implementation science frameworks: the Reach, Effectiveness, Adoption, Implementation, and Maintenance framework; the Expert Recommendations for Implementing Change taxonomy; and implementation strategy reporting domains. It was also guided by the Integrated Technology Implementation Model that incorporates theories of both implementation science and technology adoption. Reporting was guided by PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews).
Results
A total of 101 studies met the inclusion criteria. Most studies (85/101, 84.2%) involved electronic health record–based HIT interventions. The most common HIT function was clinical decision support, primarily used for panel management or at the point of care. Most studies related to HIT targeting colorectal cancer screening (83/101, 82.2%), followed by studies related to breast cancer screening (28/101, 27.7%), and cervical cancer screening (19/101, 18.8%). Improvements in cancer screening were associated with HIT-based interventions in most studies (36/54, 67% of colorectal cancer–relevant studies; 9/14, 64% of breast cancer–relevant studies; and 7/10, 70% of cervical cancer–relevant studies). Most studies (79/101, 78.2%) reported on the reach of certain interventions, while 17.8% (18/101) of the included studies reported on the adoption or maintenance. Reported barriers and facilitators to HIT adoption primarily related to inner context factors of primary care settings (eg, staffing and organizational policies that support or hinder HIT adoption). Implementation strategies for HIT adoption were reported in 23.8% (24/101) of the included studies.
Conclusions
There are substantial evidence gaps regarding the effectiveness of HIT-based interventions, especially those targeting guideline-concordant breast and colorectal cancer screening in primary care. Even less is known about how to enhance the adoption of technologies that have been proven effective in supporting breast, colorectal, or cervical cancer screening. Research is needed to ensure that the potential benefits of effective HIT-based interventions equitably reach diverse primary care populations.
Keywords: cancer prevention, health information technology, implementation, implementation strategies, scoping review
Introduction
Background
For common cancer types such as cervical, colorectal, and breast cancer, routine screening provided in primary care settings can save lives [1]. Although evidence-based national guidelines exist for the provision of such screenings [1-4], patient receipt of guideline-concordant cancer screening is suboptimal nationally and varies substantially across clinical settings [5,6]. This is driven by multiple factors, including provider-level barriers such as the challenge of staying current on changing cancer screening guidelines [6] and the cognitive overload that providers can face when managing the needs of patients with complex conditions [7-11]. Patient-level barriers include lack of knowledge of screening recommendations [6], loss to follow-up [12], fear about screening procedures or outcomes, and financial and logistical challenges [13].
Understanding which interventions effectively address these challenges—and the barriers and facilitators to implementing such interventions—is needed to enhance the delivery of guideline-concordant cancer screening in primary care. The Community Preventive Services Task Force summary of evidence-based interventions for addressing barriers to guideline-concordant cancer screening [14] identifies health IT (HIT)–based interventions as showing particular promise [15-17]. Prior systematic reviews found that HIT-based interventions such as patient reminders and provider feedback tools can be effective in supporting cancer prevention care [15,17,18]. Such interventions can enhance provider-patient communication about cancer screening [19-22]. These interventions can also help care teams identify patients due for screening with automated reminders embedded in the electronic health record (EHR) that can appear either at the point of care [23] and during panel or population management [24].
Yet HIT-based interventions targeting numerous health outcomes are underused in primary care settings [23,25]. One recent systematic review involving 55 studies showed that clinical decision support tools were adopted in <35% of eligible encounters [26]. The adoption of such interventions is impeded by multilevel barriers, such as the challenges inherent to integrating new tools into clinical workflows [27], and lack of training in how to use such tools [28,29]. There is a need to understand best practices for enhancing the adoption of effective HIT-based interventions targeting cancer prevention, including how barriers to the adoption of such interventions can best be addressed in primary care [17,18,30,31].
Objectives
In 2020, the National Cancer Institute’s Consortium for Cancer Implementation Science (CCIS) “Technology in Implementation Science Action Group” identified a need for the scoping review presented here. This review aims to describe the specific knowledge gaps in this evidence base, that is, what is known and unknown about the implementation of effective HIT for cancer screening in primary care. Specifically, it aims to identify (1) HIT-based interventions that effectively support guideline concordance in breast, cervical, and colorectal cancer screening provision and follow-up in the primary care setting and (2) barriers or facilitators to the implementation of effective HIT in this setting. To refine the scope of this review, we focused on common cancer screenings that are in the purview of primary care: breast, colorectal, and cervical cancer screening. We note that earlier systematic reviews [15,17,18] assessed the effectiveness of HIT-based interventions at improving cancer screening rates in primary care, but the most recent included data up to June 2014 [15]. This review first summarizes related evidence accrued since 2014 and then assesses current knowledge on the adoption of such interventions. To our knowledge, this is the first scoping review to assess the implementation of HIT in cancer screening.
Methods
Overview
This scoping review was conducted by a multidisciplinary team of researchers from the CCIS with expertise in implementation science, health informatics, health services research, and cancer control. We followed the 6-stage scoping review methodology described by Arksey and O’Malley [32], with consideration of later modifications to this approach made by Levac et al [33]. This review was reported in accordance with the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) [34].
Ethical Considerations
Ethics approval from the George Mason University Institutional Review Board was not required for this review.
Research Questions
This scoping review was designed to answer two overarching questions: (1) What is known about how HIT-based interventions are used to enhance guideline concordance of cancer screening in primary care settings? (2) What is known about the barriers or facilitators to the implementation and dissemination of these interventions?
Identifying Relevant Studies
With assistance from a health sciences librarian, the first author (COJ) conducted a 3-step search process to identify relevant US-based peer-reviewed and gray literature studies. First, the following bibliographic databases were systematically searched: MEDLINE, CINAHL Plus, Web of Science, and IEEE Xplore. These databases were searched using a combination of search strings that included relevant controlled vocabulary (eg, Medical Subject Heading) and keywords with Boolean operators. The search terms were selected based on a review of the existing literature and refined based on the input of the coauthors. To ensure that the search yielded relevant studies, variations of the search strategy were pilot-tested by 3 authors (COJ, RG, and RX) and refined before the final search was conducted. Our final search strategy for bibliographic databases is provided in Multimedia Appendix 1.
Second, this search was supplemented by a review of gray literature (eg, study protocols, unpublished empirical trials, dissertations, reports, and government publications) to consider studies that might not be indexed in bibliographic databases. This search primarily consisted of targeted website searching of cancer, HIT, public health organizations, and funding agencies recommended by the authors (COJ, RG, KHC). Our final gray literature search strategy is provided in Multimedia Appendix 2. Additional gray literature databases (CQ Press Library, Policy File Index, Find Policy, and Harvard Kennedy School Think Tank Search), recommended by the health sciences librarian, were explored but did not yield useful results. Finally, we identified relevant studies with a snowball search technique, whereby the reference lists of sources selected for full-text review were also examined for additional studies to include in the final review sample.
Study Selection
Eligibility Criteria
Studies on HIT and cancer screening before January 2015 are covered in prior publications [15,17,18]. Our search was designed to build on that work, so it was limited to studies published from 2015 to 2021 (the time at which we started the review process). Studies were considered eligible for inclusion if they (1) were US-based, reported in the English language, and published between January 2015 and June 2021; (2) reported on activities conducted in the primary care setting; (3) focused on evidence-based cancer screening; (4) involved the use of HIT to support this screening; (5) were related to specific workflow steps involved in conducting cancer screening in primary care (identifying patients due for screening at the point of care or in panel management, obtaining results of past screenings through data exchange, or providing appropriate follow-up care); and (6) targeted screening for breast, colorectal, or cervical cancer. A checklist of these criteria was created to guide the selection of relevant studies and then pilot-tested in a subsample of articles (n=60) and refined (COJ, RG, and RX) to ensure that its criteria could be applied consistently. The checklist was supported by a glossary of key terms to ensure shared understanding across reviewers of potential studies. The final checklist and glossary are provided in Multimedia Appendices 3 and 4, respectively. All study designs were eligible for inclusion as long as the study included some description of how HIT was used to support breast, colorectal, and cervical cancer screening in primary care settings. If a study was an evidence review (eg, systematic review or narrative review), only studies included in the final sample of the review and published between January 2015 and June 2021 were assessed for potential inclusion. If multiple publications described a single intervention but described different approaches for using HIT, all applicable studies were assessed for inclusion.
Dual Screening Review
Results of the search strategies described above were imported and managed in Zotero [35]. The first author (COJ) removed duplicate studies. Then, reviewers in eight 2-person teams were assigned studies to dual screen [36] (team 1: COJ and RG; team 2: AH and HA; team 3: LD and RX; team 4: KR and JMF; team 5: KHC and EB; team 6: KAR and JC; team 7: MMK and ATR; and team 8: MIF and DJA). Dual screening was performed in 2 steps. First, study titles and abstracts were dual screened by each review team using the inclusion and exclusion checklist to assess eligibility. Second, studies included for full-text dual screening were assessed by the same review teams for final inclusion in the scoping review. Any discrepancies that emerged within a review team were reconciled by consensus. The first and senior authors (COJ and RG) provided final decisions for any studies that could not be reconciled by a review team.
Data Charting
A data charting form was developed using Qualtrics, a web-based survey software, to systematically extract information from studies selected for inclusion in the review analyses. The form was initially pilot-tested on 2 articles and refined (COJ, RG, and HA). Next, the review teams extracted information from their assigned studies. Extracted data elements included study citation, publication year, publication type, study design, study setting, sample composition by race or ethnicity, and cancer screening focus (breast, colorectal, and cervical cancer). Extracted characteristics of the relevant HIT tools involved in a given study included type, users, functions, purpose (intervention or implementation strategy supporting an intervention), and supported cancer screening activities. Data elements were extracted in multiple choice or free-text form, depending on the type of data. Multiple implementation frameworks [37-40] were used to guide data extraction. A check of at least 50% (49/101 studies) of extracted studies suggested that data charting quality was high and the agreement rate between the initial reviewers and the reviewers that conducted the quality check was >90%.
Multiple implementation frameworks [37-40] were used to guide data extraction. Specifically, the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework [37] guided the extraction of dissemination and implementation outcomes: target end users (clinical staff and patients) of HIT (Reach), HIT impact on cancer screening in primary care (Effectiveness), the rate of HIT adoption (Adoption), the extent to which a given HIT-based intervention was implemented (Implementation), and the extent to which sustainability of HIT adoption was measured (Maintenance). Assessment of the evidence on barriers and facilitators of HIT adoption was guided by the Integrated Technology Implementation Model (ITIM), which includes 12 inner and outer context concepts known to be central to the implementation and adoption of technology in health care settings, and is based on the Consolidated Framework for Implementation Research, adapted to HIT-based interventions [38]. Although technology frameworks have been used to investigate the usability and acceptance of HIT-based interventions [41-43], to our knowledge, the ITIM is the only model that incorporates theories of both implementation science and technology adoption. The Expert Recommendations for Implementing Change (ERIC) compilation [39] guided the categorization of discrete implementation strategies identified in the studies. The implementation strategies reporting the framework by Proctor et al [40] guided the extraction and analysis of implementation strategies used to support the HIT adoption.
Collating, Summarizing, and Reporting Results
Descriptive data were compiled and interpreted using Stata/MP (version 15.1; StataCorp LLC) to quantify the frequencies of extracted data in discrete fields. Free text data charted in Qualtrics were exported to Excel (Microsoft Corp) for qualitative content analysis [44,45]. Authors (COJ, JC, RX, and RG) reviewed and categorized free text for HIT characteristics, RE-AIM domains, implementation barriers, facilitators, and core elements of implementation strategies (eg, actor and target of action). Most analyses used an iterative process, which involved initial coding and identification of themes (ie, categories) by 2 reviewers, resolving discrepancies and refining categories through team discussion, and recoding the text using finalized categories. Multimedia Appendix 5 provides details about these procedures.
Consultation
Authors (RG, JC, RX, HA, and AH) were consulted at each stage of the scoping review to provide input on the search, data abstraction, and interpretation of the results. We also consulted with implementation science experts about the conceptual frameworks selected for this study.
Results
Literature Search
The search yielded an initial total of 618 studies (Figure 1). After removing duplicates, 485 titles and abstracts were assessed for inclusion. Among these, 350 studies were excluded as not meeting the inclusion criteria. Full-text review was conducted on 135 records that met the inclusion criteria. A snowball search yielded an additional 115 studies that were assessed for eligibility. A final total of 101 studies met the inclusion criteria. Multimedia Appendix 6 provides a complete list of these studies.
Figure 1.
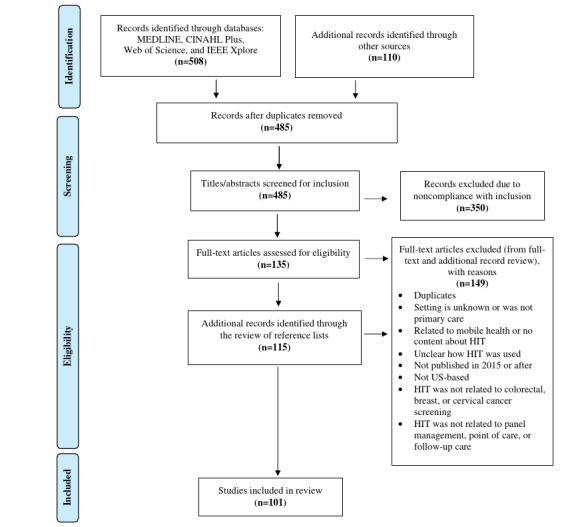
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. HIT: health IT.
Characteristics of the Included Studies
Included studies were published between January 2015 and June 2021 (Table 1). Most studies were peer-reviewed (92/101, 91.1%). Study design was mostly nonexperimental (descriptive: 18/101, 17.8% or observational: 15/101, 14.9%) in comparison to experimental (randomized controlled trials: 29/101, 28.7%), quasi-experimental (pre-post design: 21/101, 20.8%; nonrandomized controlled trials: 5/101, 5%; or other quasi-experimental studies: 3/101, 3%), and other studies (10/101, 9.9%). Most studies covered HIT targeting colorectal cancer screening (83/101, 82.2%), followed by breast cancer screening (28/101, 27.7%) and cervical cancer screening (19/101, 18.8%); these sum up >101 as some addressed more >1 type of cancer screening.
Table 1.
Characteristics of the included studies (N=101).
| Characteristics | Colorectal cancer (n=83), n (%)a | Breast cancer (n=28), n (%) | Cervical cancer (n=19), n (%) | Total, (N=101), n (%) | ||
| Publication yearb | ||||||
|
|
2015 | 13 (15.7) | 4 (14.3) | 1 (5.3) | 15 (14.9) | |
|
|
2016 | 15 (18.1) | 6 (21.4) | 3 (15.8) | 18 (17.8) | |
|
|
2017 | 15 (18.1) | 5 (17.9) | 4 (21.1) | 21 (20.8) | |
|
|
2018 | 16 (19.3) | 2 (7.1) | 3 (15.8) | 18 (17.8) | |
|
|
2019 | 10 (12) | 5 (17.9) | 4 (21.1) | 13 (12.9) | |
|
|
2020 | 9 (10.8) | 5 (17.9) | 3 (15.8) | 11 (10.9) | |
|
|
2021 | 5 (6) | 1 (3.6) | 1 (5.3) | 5 (5) | |
| Publication type | ||||||
|
|
Peer-reviewed article | 78 (94) | 26 (92.9) | 16 (84.2) | 92 (91.1) | |
|
|
Report | 1 (1.2) | 2 (7.1) | 2 (10.5) | 4 (4) | |
|
|
Study protocol | 3 (3.6) | —c | — | 3 (3) | |
|
|
Other | 1 (1.2) | — | 1 (5.3) | 2 (2) | |
| Study design | ||||||
|
|
Nonexperimental | |||||
|
|
|
Descriptive | 15 (18.1) | 5 (17.9) | 4 (21.1) | 18 (17.8) |
|
|
|
Observational | 11 (13.3) | 9 (32.1) | 4 (21.1) | 15 (14.9) |
|
|
Experimental | |||||
|
|
|
RCTd | 24 (28.9) | 7 (25) | 5 (26.3) | 29 (28.7) |
|
|
Quasi-experimental | |||||
|
|
|
Pre-post study design | 17 (20.5) | 5 (17.9) | 2 (10.5) | 21 (20.8) |
|
|
|
Non-RCT | 3 (3.6) | 1 (3.6) | 3 (15.8) | 5 (5) |
|
|
|
Other quasi-experimental | 3 (3.6) | — | — | 3 (3.0) |
|
|
Other study designs | 10 (12) | 1 (3.6) | 1 (5.3) | 10 (9.9) | |
aPercentages were calculated based on column totals.
bPublication year represents studies published from January 2015 to June 2021.
cNot available.
dRCT: randomized controlled trial.
Characteristics of the primary care settings where the research in the included studies was conducted are shown in Table 2. Approximately half of the included studies (52/101, 51.5%) reported on practice location. Most studies involving colorectal (22/83, 27%) or breast (6/28, 21%) cancer screening were conducted in urban areas, and most studies on cervical cancer screening (5/19, 26%) were conducted in rural areas. Studies on colorectal cancer screening were primarily conducted in federally qualified health centers (20/83, 24%); most of those on breast and cervical cancer screening were conducted in academic-based clinics (9/28, 32% and 5/19, 26%, respectively). More than half (59/101, 58.4%) of the included studies (colorectal: 47/83, 57%; breast cancer: 17/28, 61%; and cervical: 8/19, 42%) reported information on racial or ethnic minoritized participants (patients from racial or ethnic minority groups). Of these 59 studies, 34 (58%) reported that ≤50% of study participants were members of racial or ethnic minority populations.
Table 2.
Primary care practice characteristics of the included studies (N=101).
| Characteristics | Colorectal cancer (n=83), n (%)a | Breast cancer (n=28), n (%) | Cervical cancer (n=19), n (%) | Total, (N=101), n (%) | |
| Practice location | |||||
|
|
Urban | 22 (26.5) | 6 (21.4) | 2 (10.5) | 26 (25.7) |
|
|
Rural | 11 (13.3) | 5 (17.9) | 5 (26.3) | 15 (14.9) |
|
|
Combination of urban and rural | 11 (13.3) | 2 (7.1) | 3 (15.8) | 11 (10.9) |
|
|
Not reported | 39 (47) | 15 (53.6) | 9 (47.4) | 49 (48.5) |
| Practice type | |||||
|
|
Academic-based clinic | 17 (20.5) | 9 (32.1) | 5 (26.3) | 22 (21.8) |
|
|
Federally Qualified Health Centers | 20 (24.1) | 1 (3.6) | 3 (15.8) | 21 (20.8) |
|
|
Freestanding or other | 18 (21.7) | 4 (14.3) | 3 (15.8) | 21 (20.8) |
|
|
Hospital-based clinic | 10 (12) | 2 (7.1) | 1 (5.3) | 12 (11.9) |
|
|
Not reported | 18 (21.7) | 12 (42.9) | 7 (36.8) | 25 (24.8) |
| Sample percentage of racial or ethnic minority groups | |||||
|
|
≤50% | 25 (30.1) | 13 (46.4) | 6 (31.6) | 34 (33.7) |
|
|
>50% | 22 (26.5) | 4 (14.3) | 2 (10.5) | 25 (24.8) |
|
|
Not reported | 36 (43.4) | 11 (39.3) | 11 (57.9) | 42 (41.6) |
aPercentages were calculated based on column totals.
Characteristics of the HIT Interventions
Our definitions of HIT tool types and functions and the types of cancer screening activities they supported are provided in Multimedia Appendix 4. Of the 101 included studies, 66 (65.3%) reported on interventions involving 1 HIT tool and 35 (34.7%) reported on interventions involving >1 HIT tool (Table 3). In these studies, the HIT tool was either the intervention of focus, one component of a multicomponent intervention that also included non-HIT elements, or was used as an implementation strategy to support the intervention of focus.
Table 3.
Characteristics of the health IT (HIT) sources and functions used to promote cancer screening in primary care, as represented in the included studies (N=101).
| Characteristics | Colorectal cancer (n=83), n (%)a | Breast cancer (n=28), n (%) | Cervical cancer (n=19), n (%) | Total (N=101), n (%) | |||||
| Using single or multiple HIT tools | |||||||||
|
|
Single HIT tools | 53 (63.9) | 22 (78.6) | 14 (73.7) | 66 (65.3) | ||||
|
|
Multiple HIT tools | 30 (36.1) | 6 (21.4) | 5 (26.3) | 35 (34.7) | ||||
| HIT sources | |||||||||
|
|
EHRb based | 74 (89.2) | 20 (71.4) | 18 (94.7) | 85 (84.2) | ||||
|
|
Web based | 11 (13.3) | 9 (32.1) | 3 (15.8) | 18 (17.8) | ||||
|
|
Other or unclear | 15 (18.1) | 3 (10.7) | 3 (15.8) | 19 (18.8) | ||||
| HIT functions | |||||||||
|
|
CDSc panel management or outreach | 50 (60.2) | 7 (25) | 9 (47.4) | 57 (56.4) | ||||
|
|
CDS point of care | 41 (49.4) | 16 (57.1) | 12 (63.2) | 48 (47.5) | ||||
|
|
Risk identification | 13 (15.7) | 5 (17.9) | 6 (31.6) | 18 (17.8) | ||||
|
|
Patient decision aid | 13 (15.7) | 9 (32.1) | 2 (10.5) | 18 (17.8) | ||||
|
|
Provider assessment and feedback | 11 (13.3) | 1 (3.6) | 1 (5.3) | 12 (11.9) | ||||
|
|
Tracking patient adherence | 27 (32.5) | 4 (14.3) | 6 (31.6) | 30 (29.7) | ||||
|
|
Other | 3 (3.6) | —d | — | 3 (3.0) | ||||
| Cancer screening activities supported by HIT | |||||||||
|
|
Panel management | 50 (60.2) | 8 (28.9) | 7 (36.8) | 56 (55.4) | ||||
|
|
Point of care | 39 (47) | 15 (53.6) | 12 (63.2) | 45 (44.6) | ||||
|
|
Follow-up (referral) | 36 (43.4) | 7 (25.0) | 7 (36.8) | 41 (40.6) | ||||
|
|
Follow-up (abnormal or positive result) | 12 (14.5) | 2 (7.1) | 5 (26.3) | 17 (16.8) | ||||
|
|
Acquire previous results | 7 (8.4) | 2 (7.1) | 4 (21.1) | 10 (9.9) | ||||
|
|
Other | 21 (25.3) | 11 (39.3) | 5 (26.3) | 24 (23.8) | ||||
aPercentages were calculated based on column totals. Some studies featured >1 HIT source, function, and cancer screening activity. As a result, these categories are not mutually exclusive and will not necessarily sum to 100%. Refer to Multimedia Appendix 4 for definitions of the terms used in this table.
bEHR: electronic health record.
cCDS: clinical decision support.
dNot available.
Most of the included studies (85/101, 84.2%) involved EHR-based HIT tools (Table 3). Web-based (18/101, 17.8%) and other types of HIT tools (19/101, 18.8%) were less common. The HIT function most commonly involved in included studies was clinical decision support (CDS) across all cancer screening types (Table 3). CDS tools for panel management were most common in studies involving colorectal cancer screening (50/83, 60%). CDS at the point of care was commonly used in studies on breast (16/28, 57%) and cervical cancer screening (12/19, 63%). Other commonly studied HIT functions included risk identification (colorectal: 13/83, 16% and cervical: 6/19, 32%), patient decision aids (colorectal: 13/83, 16% and breast: 9/28, 32%), and tools for tracking patient adherence to recommended care (colorectal: 27/83, 33% and cervical: 6/19, 32%).
The cancer screening activities were primarily related to identifying patients for screening in panel management (colorectal: 50/83, 60%; breast: 8/28, 29%; and cervical: 7/19, 37%) and at the point of care (colorectal: 39/83, 47%; breast: 15/28, 54%; and cervical: 12/19, 63%). Other commonly supported cancer screening activities included follow-up care for referral (colorectal: 36/83, 43%; breast: 7/28, 25%; and cervical: 7/19, 37%) and for positive or abnormal screening results (colorectal: 12/83, 15% and cervical: 5/19, 26%; Table 3).
Reporting on RE-AIM Outcomes
Overview
A summary of reporting on RE-AIM outcomes is provided in Table 4.
Table 4.
Reporting on Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) outcomes for health IT (HIT) targeting cancer screening in primary care.
| RE-AIM domains | Data charted | Cancer screening type | ||
|
|
|
Colorectal cancer | Breast cancer | Cervical cancer |
| Reach | Was the number of targeted staff or patients for HIT-based intervention reported | Higha | High | High |
| Effectiveness | Did the HIT tools show positive results in the cancer screening intervention | High | Moderate | High |
| Adoption | Rate of HIT adoption | Low | Moderate | Low |
| Implementation | Barriers, facilitators, and implementation strategies used related to HIT | Moderate | Low | Low |
| Maintenance | Was the sustainment of HIT adoption measured | Low | Low | Low |
aLow: <25% of the included studies for each cancer screening type category, moderate: 25% to 50% of the included studies for each cancer screening type category, and high: >50% of the included studies for each cancer screening type category. Percentages were calculated with respect to the included studies for each cancer screening type category.
Effectiveness
Of the 101 included studies, 24 (23.8%) reported on the effectiveness of HIT targeting breast (14/28, 50% of breast cancer–relevant studies) and cervical cancer screening (10/19, 53% of cervical cancer–relevant studies; Multimedia Appendix 7 includes a table with these results). Of the 101 included studies, 54 (53.5%) reported the effectiveness of HIT targeting colorectal cancer screening (54/83, 65% of colorectal cancer–relevant studies). Among studies reporting on effectiveness, most-reported positive outcomes (improved screening rate) associated with the use of HIT (36/54, 67% of colorectal cancer–relevant studies; 9/14, 64% of breast cancer–relevant studies; and 7/10, 70% of cervical cancer–relevant studies). This evidence mostly represented CDS used during panel management (22/83, 27% of colorectal cancer–relevant studies) or at the point of care (5/28, 18% of breast cancer–relevant studies and 5/19, 26% of cervical cancer–relevant studies; Multimedia Appendix 7).
Reach, Adoption, and Maintenance
Among the 101 included studies, 79 (78.2%) reported on the reach of HIT-based interventions. Most of the studies focused on reach involved HIT for colorectal cancer screening (63/83, 76% of colorectal cancer–relevant studies studies). The reach of HIT-based interventions targeting breast cancer screening was reported in 82% (23/28) of the breast cancer–relevant studies and in 74% (14/19) of the studies targeting cervical cancer screening. Overall, 15.8% (16/101) of the studies reported on HIT adoption (colorectal: 10/83, 12%; breast: 9/28, 32%; and cervical: 1/19, 5%), and 2% (2/101) of the studies reported on maintenance of HIT-based interventions. Of those that reported on adoption, there was mostly a low rate of adoption (≤50%) across all cancer screening types (Multimedia Appendix 8 includes a table with these results).
Implementation
The proportion of studies reporting on the implementation of the HIT ranged from 25% to 50% for those related to colorectal cancer screening (Table 4). It was reported in <25% of the studies related to HIT targeting breast and cervical cancer screening. Implementation barriers, facilitators, and strategies related to HIT adoption across all cancer screening types are described further in the next 2 sections.
Implementation Barriers and Facilitators of HIT Adoption
A total of 34 studies reported on barriers and 37 studies reported on facilitators to implementing the HIT-based interventions of focus in primary care (Table 5). The most-reported barriers and facilitators were related to the ITIM constructs inner context (barriers: 17/34, 50% and facilitators: 14/37, 38%), nature of the innovation (barriers: 15/34, 44% and facilitators: 17/37, 46%), and outer context (barriers: 11/34, 32% and facilitators: 9/37, 24%). Inner context barriers included limited staff time to use the HIT and adoption competing with other clinic priorities. Inner context facilitators included having dedicated staff assigned to operate and manage a given HIT tool, and organizational policies supporting HIT adoption. Barriers related to the nature of the innovation included inaccurate cancer screening data reported by the HIT intervention and the burden of HIT development and maintenance. Facilitators related to the nature of the innovation included that HIT automation and customization features reduced staff resources and time needed in providing care. Outer context barriers included challenges involved with working with an EHR vendor to activate and update the tool and challenges with accessing screening results conducted outside the clinics. Outer context facilitators included Medicaid expansion including cancer screening as an incentivized metric and the clinic being a Federally Qualified Health Center, which necessitated responsiveness to such metrics. A table with more examples of barriers and facilitators is provided in Multimedia Appendix 9.
Table 5.
Reporting on the barriers and facilitators of health IT adoption aligned with the Integrated Technology Implementation Model (ITIM).
| ITIM constructs | Barriers (n=34), n (%)a | Facilitators (n=37), n (%) |
| Adoption or adopters | 2 (6) | 1 (3) |
| Communication | 6 (18) | 5 (14) |
| Economic environment | 5 (15) | 6 (16) |
| Facilitators (boundary spanner) | —b | 4 (11) |
| Implementation | 3 (9) | 9 (24) |
| Inner context | 17 (50) | 14 (38) |
| Interfacing systems | 5 (15) | 2 (5) |
| Leadership | 2 (6) | 2 (5) |
| Nature of the innovation | 15 (44) | 17 (46) |
| Outer context | 11 (32) | 9 (24) |
| Users (adopters) | 9 (26) | 4 (11) |
| Workflow | 9 (26) | 11 (30) |
aPercentages were calculated with respect to the total studies that reported barriers or facilitators. Some studies featured both barriers and facilitators to health IT adoption for cancer screening in primary care. As a result, these categories are not mutually exclusive and will not necessarily sum to 100%.
bNot available.
Implementation Strategies to Support HIT Adoption
Implementation strategies targeting HIT adoption were reported in 24% (24/101) of the included studies. Those reported were mapped to 22 implementation strategies from the ERIC compilation [39] (Multimedia Appendix 10). Of the studies reporting implementation strategies, >50% used ≥2 strategies and >50% reported strategies promoting HIT use for colorectal cancer screening. Common strategies to promote HIT use among all cancer screening types included central technical assistance, conducting small tests of change, and educational meetings. A table with more examples is available in Multimedia Appendix 10. Reported evidence mapped to the domains formulated by Proctor et al [40] (Table 6) and were mostly focused on describing implementation strategies to support HIT adoption for colorectal cancer screening (22/83, 27% of colorectal cancer–relevant studies) in comparison to breast (6/28, 21% of breast cancer–relevant studies) and cervical cancer screening (4/19, 21% of cervical cancer–relevant studies). Overall, less than half of the included studies, for each cancer screening type, reported evidence in accordance with each implementation strategy domain.
Table 6.
Reporting on the implementation strategies used to support health IT adoption.
| Implementation strategy domains by Proctor et al [40] | Data charted | Cancer screening type | ||
|
|
|
Colorectal cancer | Breast cancer | Cervical cancer |
| Actor | Who delivers the strategy | Moderatea | Low | Low |
| Action | Procedures to conduct the strategy | Moderate | Low | Low |
| Target of action | Intent of action | Low | Low | Low |
| Temporality | When does the strategy happen | Low | Low | Low |
| Dose | Frequency or intensity | Low | Low | Low |
| Implementation outcomes affected | What will the strategy change | Low | Low | Low |
| Justification | Purpose of the strategy | Moderate | Low | Low |
aLow: <25% of the included studies for each cancer screening type category, moderate: 25% to 50% of the included studies for each cancer screening type category, and high: >50% of the included studies for each cancer screening type category.
Discussion
Principal Findings
This scoping review summarizes the state of the science about the implementation of HIT-based interventions targeting breast, cervical, or colorectal cancer screening in primary care. Previous reviews identified the positive impact of HIT-based interventions throughout the cancer care continuum, including cancer screening [15,17,18]. This review adds to prior evidence by bringing an implementation science perspective; this is needed because the impact of HIT-based interventions is limited by the extent to which such interventions are effectively integrated into practice. This scoping review provides updated evidence up to 2021. This is not a systematic review; our goal was to identify knowledge gaps. Results indicate that key knowledge gaps related to the implementation of HIT in cancer screening in primary care include (1) the effectiveness of HIT targeting breast and cervical cancer screening, (2) HIT adoption in diverse primary care settings, (3) the implementation strategies that support the adoption of HIT, and (4) equitable reach or adoption of HIT. Addressing these evidence gaps may be critical to supporting the implementation of high-quality primary care [46].
Knowledge Gap 1: Limited Evidence on the Effectiveness of HIT Targeting Breast and Cervical Cancer Screening
This review emphasizes the need to improve the evidence on HIT effectiveness, especially HIT targeting breast and cervical cancer screening uptake, in diverse primary care settings. Effectiveness outcomes included, but were not limited to, improvements in cancer screening initiation by the patient or provider and patient completion of cancer screening. Although the use of HIT-based interventions was associated with improved screening outcomes for all 3 cancer types, there were far fewer studies of HIT effectiveness for breast and cervical cancer screening (a combined total of 24 studies) in comparison to the 54 studies involving colorectal cancer screening. Furthermore, most studies related to HIT targeting breast or cervical cancer prevention were conducted in academic medical centers and were not readily generalizable to other primary care settings. This limited evidence is concerning as both are common cancers, and evidence-based guidelines for such screenings are not met in many patient populations.
In addition, the lack of reporting on HIT effectiveness was especially common in studies in which HIT was part of a multicomponent intervention [14]; thus, even if the effectiveness of the overall intervention was reported, the impact of the HIT element of the intervention was not clear. More research is needed to establish the effectiveness of HIT targeting cancer screening in diverse primary care settings, including trials of the individual and combined effect of HIT within multicomponent interventions. The need for an improved understanding of the effectiveness of HIT is especially salient given that national programs (eg, Promoting Interoperability Program, formerly Meaningful Use) promote the use of HIT in health care settings [47] as a means to improve health outcomes.
Knowledge Gap 2: Limited Evidence of the Reach, Adoption, and Maintenance of Effective HIT Targeting Cancer Screening
The limited reporting on the reach, adoption, and maintenance of such interventions aligns with the known lack of reporting on these implementation outcomes in analyses of other interventions [48]; the need to improve such reporting is well known in implementation science. When HIT adoption is not reported, it is difficult to assess an intervention’s population-level impact. In particular, if a limited number of potential users adopt an intervention, even when there is good reach and it is highly effective, population-level impacts may be low. Where adoption was reported, its rates were generally low (≤50%), underscoring the need for further research on improving the uptake of effective HIT [49]. When implementation barriers and facilitators to HIT adoption were reported, most related to inner context, outer context, and the nature of the innovation (including a given HIT tool’s function). Future research should assess which combination of these contextual factors is associated with the adoption of HIT with varied functions when used in different workflow steps (ie, panel management, point of care, and follow-up care). To further understand how contextual factors impact care teams’ adoption of HIT for cancer screening, there is also a need for more widespread reporting on practice type, which was rarely noted in the studies included here. Similarly, few studies reported on the sustainment of tool adoption. This evidence gap is seen throughout the implementation science literature [50]; improved knowledge of how to sustain the use of effective interventions is critical to maximizing their impact. Knowledge gap 3 describes the need for evidence on how to improve the adoption and maintenance of HIT-based interventions targeting cancer screening in primary care. We also posit that the lack of evidence on such interventions’ reach is relevant to how such interventions support equity in cancer screening, as discussed in knowledge gap 4.
Knowledge Gap 3: Limited Evidence on Implementation Strategies That Support the Adoption of HIT Targeting Cancer Screening in Primary Care
A total of 24 studies (<25% of the included studies) reported on strategies used to support the adoption of HIT-based interventions, and few of these assessed the effectiveness of the strategies. This is complicated by the fact that in some cases a given HIT tool was considered the intervention or an intervention component, and in others, it was considered an implementation strategy for supporting the adoption of a clinical intervention. In the implementation science literature, the boundaries between clinical intervention and implementation intervention and between implementation intervention and implementation strategies are not always clear, adding complexity to this reporting.
Research is needed on how to support the adoption of HIT-based interventions targeting cancer screening using implementation strategies, how to use HIT as an implementation strategy, and what types of support strategies are used even in reports on HIT-based interventions’ impact. Reporting must strive to clearly differentiate between these approaches; the need for better reporting on implementation strategies is well known [51-53]. Although such reporting can be resource intensive, methods are emerging to facilitate it [40,54].
Research is also needed to specify how effectively different implementation strategies support the adoption of different HIT-based interventions in different care settings. Known barriers and facilitators to HIT adoption, in general, may also be impactful for HIT targeting cancer screening. For example, evidence indicates that barriers to HIT use include inadequate training for care teams on using EHR functions to their full potential [55-58]. Thus, effective implementation strategies for HIT targeting cancer prevention may involve training.
Knowledge Gap 4: Limited Evidence on the Reach and Equitable Implementation of HIT for Cancer Screening in Primary Care
The equitable reach of HIT tools for cancer screening is poorly described. A few studies specifically focused on racial or ethnic minority groups; many were conducted in federally qualified health centers, which often serve racial or ethnic minority groups. Relevant data were reported in just 58.4% (59/101) of the studies included here. However, where such data were reported, eligible patients reached by the HIT interventions had a lower percentage of non-White patients than would be expected for the populations served, suggesting inequities in reach or underreporting. This is concerning, as racial disparities in cancer screening persist [59-61], and previous research found that interventions targeting breast or cervical cancer screening are less likely to target patients considered most at risk, for example, those in socioeconomically and racial or ethnic minoritized groups [5]. Findings from this scoping review underscore the need to understand potential drivers of these inequities (eg, design flaws in algorithms used to identify eligible patients and clinician bias in applying the HIT tool) and solutions to mitigate these inequities. One step toward addressing this inequity must involve improved reporting on how HIT is used in diverse patient populations. The well-documented need to improve reporting of race or ethnicity in health care [62] likely exacerbates the lack of reporting on the comparative reach of the tools included in this review among different groups. Another step toward equitable reach of HIT is understanding and addressing barriers to the inclusion of racial or ethnic minoritized patients in research on HIT adoption and impact. Future research on HIT adoption for cancer screening should explore strategies that support documentation, recruitment, and retention of racial or ethnic minoritized patients [63].
Limitations
HIT-based interventions might be used to improve outcomes at each step of the cancer control continuum, such as risk assessment, prevention, detection, diagnosis, treatment, survivorship, and end-of-life care [15]. This review was limited to cancer screening. Furthermore, although breast, colorectal, and cervical cancer are highly prevalent cancers whose detection is in the purview of primary care, no other cancers recommended for screening in primary care (eg, lung cancer) were included; future research could assess whether the gaps identified in this study are seen for a broader set of cancers. This review was limited to US studies; therefore, the relevance of the findings is limited to the context of HIT policies and infrastructure as applicable to US primary care settings. Another potential limitation is that urban or rural status was defined based on what each study reported, and they may have used different methods for making this characterization.
In addition, the overlapping quality of some HIT characteristic categories (tool types and functions) made it difficult to execute related data charting. Similarly, content analysis of HIT functions was complicated when implementation strategies overlapped or when studies did not specify which cancers were targeted by the strategies. Our definition of effectiveness did not capture screening outcomes related to each clinical workflow (eg, an intervention using CDS for panel management showed improvements in colorectal cancer screening but did not clarify how improvements impacted screening initiation, completion, or follow-up care). Finally, we followed the PRISMA-ScR guidelines [34] to examine a broad array of literature to include studies that are heterogeneous in design and quality [64]. Although our search strategy followed an iterative process, it is possible that some relevant existing articles were not captured; we sought to mitigate this using a snowball search.
Conclusions
In what is, to our knowledge, the first scoping review of the implementation of HIT-based interventions for cancer screening in primary care settings, we identified critical knowledge gaps. Little is known about the effectiveness of HIT-based interventions specifically targeting guideline-concordant breast and cervical cancer screening. Clarity is needed on the individual and combined effectiveness of HIT when integrated into a multicomponent intervention targeting cancer screening. Even less is known about how to enhance the adoption of cancer-targeted HIT in primary care. The potential for inequities in the reach of HIT for cancer screening remains underexplored. Research is necessary on implementation strategies to promote equitable access, ensuring that the potential benefits of HIT for population health are realized across diverse patient populations.
Acknowledgments
The authors would like to extend their gratitude to the Consortium for Cancer Implementation Science (CCIS). This research was conducted as part of the National Cancer Institute’s CCIS Technology in Implementation Science Action Group. This research was supported by the National Cancer Institute of the National Institutes of Health (P50CA244289, P50CA244693, P50CA244688, P50CA244690, P50CA244431), through funding provided as part of the Cancer Moonshot. This research was also supported by a doctoral scholarship award from the Provost Office of George Mason University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CCIS
Consortium for Cancer Implementation Science
- CDS
clinical decision support
- EHR
electronic health record
- ERIC
Expert Recommendations for Implementing Change
- HIT
health IT
- ITIM
Integrated Technology Implementation Model
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews
- RE-AIM
Reach, Effectiveness, Adoption, Implementation, and Maintenance
Database search strategy.
Gray literature search strategy.
Inclusion and exclusion criteria checklist.
Glossary of key terms.
Stage 5 procedures for qualitative content analysis.
References to the included studies (N=101).
Reporting status of health IT (HIT) effectiveness by cancer screening activities and HIT functions as represented in the included studies.
Reporting of health IT adoption as represented in the included studies.
Barriers and facilitators (with examples) of health IT adoption aligned with the Integrated Technology Implementation Model.
Expert Recommendations for Implementing Change implementation strategies used to support health IT adoption (n=24 studies).
PRISMA-ScR checklist.
Footnotes
Authors' Contributions: COJ and RG conceptualized the research questions. COJ conceptualized and led the data collection methods and analysis. COJ conducted a 3-step search process to identify relevant US-based peer-reviewed and gray literature studies. To ensure that the search yielded relevant studies, variations of the search strategy were pilot-tested by 3 authors (COJ, RG, and RX) and refined before the final search was conducted. COJ and RG conceptualized the eligibility criteria, and COJ, RG, and RX participated in the pilot-testing of the eligibility criteria before study selection. All authors conducted dual screening and applied the eligibility criteria for study selection. COJ and RG developed the data charting form, and COJ, RG, and HA participated in pilot-testing the data charting form before data charting. All authors conducted data charting of the final sample of studies selected for inclusion. COJ, JC, RX, and RG synthesized the data and performed quality checks on the reported results. COJ led the manuscript development. All coauthors had the opportunity to read, edit, and approve the final manuscript.
Conflicts of Interest: None declared.
References
- 1.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Brawley OW, Wender RC. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2017 Mar 07;67(2):100–21. doi: 10.3322/caac.21392. [DOI] [PubMed] [Google Scholar]
- 2.Siu AL, U.S. Preventive Services Task Force Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016 Feb 16;164(4):279–96. doi: 10.7326/M15-2886. https://www.acpjournals.org/doi/abs/10.7326/M15-2886?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .2480757 [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling Jr JW, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. 2018 Aug 21;320(7):674–86. doi: 10.1001/jama.2018.10897.2697704 [DOI] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force. Davidson K, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021 May 18;325(19):1965–77. doi: 10.1001/jama.2021.6238.2779985 [DOI] [PubMed] [Google Scholar]
- 5.Martires KJ, Kurlander DE, Minwell GJ, Dahms EB, Bordeaux JS. Patterns of cancer screening in primary care from 2005 to 2010. Cancer. 2014 Jan 15;120(2):253–61. doi: 10.1002/cncr.28403. https://onlinelibrary.wiley.com/doi/10.1002/cncr.28403 . [DOI] [PubMed] [Google Scholar]
- 6.Suk R, Hong YR, Rajan SS, Xie Z, Zhu Y, Spencer JC. Assessment of US preventive services task force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022 Jan 04;5(1):e2143582. doi: 10.1001/jamanetworkopen.2021.43582. https://europepmc.org/abstract/MED/35040970 .2788175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007 May 01;5(3):196–201. doi: 10.1370/afm.679. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=17548846 .5/3/196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yawn B, Goodwin MA, Zyzanski SJ, Stange KC. Time use during acute and chronic illness visits to a family physician. Fam Pract. 2003 Aug;20(4):474–7. doi: 10.1093/fampra/cmg425. [DOI] [PubMed] [Google Scholar]
- 9.Beasley JW, Wetterneck TB, Temte J, Lapin JA, Smith P, Rivera-Rodriguez AJ, Karsh B. Information chaos in primary care: implications for physician performance and patient safety. J Am Board Fam Med. 2011;24(6):745–51. doi: 10.3122/jabfm.2011.06.100255. http://www.jabfm.org/cgi/pmidlookup?view=long&pmid=22086819 .24/6/745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickens CD. Multiple resources and mental workload. Hum Factors. 2008 Jun 01;50(3):449–55. doi: 10.1518/001872008x288394. [DOI] [PubMed] [Google Scholar]
- 11.Altmann EM, Gray WD. Forgetting to remember: the functional relationship of decay and interference. Psychol Sci. 2002 Jan 06;13(1):27–33. doi: 10.1111/1467-9280.00405. [DOI] [PubMed] [Google Scholar]
- 12.Jones RM, Woolf SH, Cunningham TD, Johnson RE, Krist AH, Rothemich SF, Vernon SW. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010 May;38(5):499–507. doi: 10.1016/j.amepre.2010.01.020. https://europepmc.org/abstract/MED/20347555 .S0749-3797(10)00097-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthukrishnan M, Arnold LD, James AS. Patients' self-reported barriers to colon cancer screening in federally qualified health center settings. Prev Med Rep. 2019 Sep;15:100896. doi: 10.1016/j.pmedr.2019.100896. https://linkinghub.elsevier.com/retrieve/pii/S2211-3355(19)30075-0 .S2211-3355(19)30075-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CPSTF findings for cancer prevention and control. The Guide to Community Preventive Services. [2022-06-09]. https://www.thecommunityguide.org/content/task-force-findings-cancer-prevention-and-control .
- 15.Tarver WL, Menachemi N. The impact of health information technology on cancer care across the continuum: a systematic review and meta-analysis. J Am Med Inform Assoc. 2016 Mar;23(2):420–7. doi: 10.1093/jamia/ocv064. https://europepmc.org/abstract/MED/26177658 .ocv064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesse BW, Hanna C, Massett HA, Hesse NK. Outside the box: will information technology be a viable intervention to improve the quality of cancer care? J Natl Cancer Inst Monogr. 2010 Apr 12;2010(40):81–9. doi: 10.1093/jncimonographs/lgq004. https://europepmc.org/abstract/MED/20386056 .lgq004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimbo M, Nease DE, Ruffin MT, Rana GK. Information technology and cancer prevention. CA Cancer J Clin. 2006 Jan 01;56(1):26–49. doi: 10.3322/canjclin.56.1.26. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0007-9235&date=2006&volume=56&issue=1&spage=26 .56/1/26 [DOI] [PubMed] [Google Scholar]
- 18.Brouwers MC, De Vito C, Bahirathan L, Carol A, Carroll JC, Cotterchio M, Dobbins M, Lent B, Levitt C, Lewis N, McGregor SE, Paszat L, Rand C, Wathen N. What implementation interventions increase cancer screening rates? A systematic review. Implement Sci. 2011 Sep 29;6:111. doi: 10.1186/1748-5908-6-111. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-6-111 .1748-5908-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreps GL. Strategic use of communication to market cancer prevention and control to vulnerable populations. Health Mark Q. 2008 Jul 02;25(1-2):204–16. doi: 10.1080/07359680802126327. [DOI] [PubMed] [Google Scholar]
- 20.Rupert DJ, Squiers LB, Renaud JM, Whitehead NS, Osborn RJ, Furberg RD, Squire CM, Tzeng JP. Communicating risk of hereditary breast and ovarian cancer with an interactive decision support tool. Patient Educ Couns. 2013 Aug;92(2):188–96. doi: 10.1016/j.pec.2013.04.008.S0738-3991(13)00166-3 [DOI] [PubMed] [Google Scholar]
- 21.Kinney AY, Boonyasiriwat W, Walters ST, Pappas LM, Stroup AM, Schwartz MD, Edwards SL, Rogers A, Kohlmann WK, Boucher KM, Vernon SW, Simmons RG, Lowery JT, Flores K, Wiggins CL, Hill DA, Burt RW, Williams MS, Higginbotham JC. Telehealth personalized cancer risk communication to motivate colonoscopy in relatives of patients with colorectal cancer: the family CARE randomized controlled trial. J Clin Oncol. 2014 Mar 01;32(7):654–62. doi: 10.1200/JCO.2013.51.6765. https://europepmc.org/abstract/MED/24449229 .JCO.2013.51.6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ElKefi S, Asan O. How technology impacts communication between cancer patients and their health care providers: a systematic literature review. Int J Med Inform. 2021 May;149:104430. doi: 10.1016/j.ijmedinf.2021.104430. https://europepmc.org/abstract/MED/33684711 .S1386-5056(21)00056-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yabroff KR, Zapka J, Klabunde CN, Yuan G, Buckman DW, Haggstrom D, Clauser SB, Miller J, Taplin SH. Systems strategies to support cancer screening in U.S. primary care practice. Cancer Epidemiol Biomarkers Prev. 2011 Dec;20(12):2471–9. doi: 10.1158/1055-9965.EPI-11-0783. https://europepmc.org/abstract/MED/21976292 .1055-9965.EPI-11-0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klabunde CN, Lanier D, Breslau ES, Zapka JG, Fletcher RH, Ransohoff DF, Winawer SJ. Improving colorectal cancer screening in primary care practice: innovative strategies and future directions. J Gen Intern Med. 2007 Aug 30;22(8):1195–205. doi: 10.1007/s11606-007-0231-3. https://europepmc.org/abstract/MED/17534688 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schapira MM, Sprague BL, Klabunde CN, Tosteson AN, Bitton A, Chen JS, Beaber EF, Onega T, MacLean CD, Harris K, Howe K, Pearson L, Feldman S, Brawarsky P, Haas JS, PROSPR consortium Inadequate systems to support breast and cervical cancer screening in primary care practice. J Gen Intern Med. 2016 Oct 23;31(10):1148–55. doi: 10.1007/s11606-016-3726-y. https://europepmc.org/abstract/MED/27251058 .10.1007/s11606-016-3726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kouri A, Yamada J, Lam Shin Cheung J, Van de Velde S, Gupta S. Do providers use computerized clinical decision support systems? A systematic review and meta-regression of clinical decision support uptake. Implement Sci. 2022 Mar 10;17(1):21. doi: 10.1186/s13012-022-01199-3. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-022-01199-3 .10.1186/s13012-022-01199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimbo M, Shultz CG, Nease DE, Fetters MD, Power D, Ruffin MT. Perceived barriers and facilitators of using a web-based interactive decision aid for colorectal cancer screening in community practice settings: findings from focus groups with primary care clinicians and medical office staff. J Med Internet Res. 2013 Dec 18;15(12):e286. doi: 10.2196/jmir.2914. https://www.jmir.org/2013/12/e286/ v15i12e286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Police R, Foster T, Wong K. Adoption and use of health information technology in physician practice organisations: systematic review. Inform Prim Care. 2010 Dec 01;18(4):245–58. doi: 10.14236/jhi.v18i4.780. https://access.portico.org/Portico/auView?auId=ark:%2F27927%2Fphw1p04t7w8 .780 [DOI] [PubMed] [Google Scholar]
- 29.Gold R, Bunce A, Davis JV, Nelson JC, Cowburn S, Oakley J, Carney S, Horberg MA, Dearing JW, Melgar G, Bulkley JE, Seabrook J, Cloutier H. "I didn't know you could do that": a pilot assessment of EHR optimization training. ACI open. 2021 Jun 27;5(1):e27–35. doi: 10.1055/s-0041-1731005. https://europepmc.org/abstract/MED/34938954 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cialdella-Kam L, Sabado P, Bispeck MK, Silverman S, Bernstein L, Krawiec V, Hawk E, O'Donnell JF. Implementing cancer prevention into clinical practice. J Cancer Educ. 2012 May 25;27(2 Suppl):S136–43. doi: 10.1007/s13187-012-0331-6. https://europepmc.org/abstract/MED/22367592 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesse BW, Kwasnicka D, Ahern DK. Emerging digital technologies in cancer treatment, prevention, and control. Transl Behav Med. 2021 Nov 30;11(11):2009–17. doi: 10.1093/tbm/ibab033. https://europepmc.org/abstract/MED/34850933 .6446311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005 Feb;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 33.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010 Sep 20;5:69. doi: 10.1186/1748-5908-5-69. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-5-69 .1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MD, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018 Oct 02;169(7):467–73. doi: 10.7326/M18-0850. https://www.acpjournals.org/doi/abs/10.7326/M18-0850?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .2700389 [DOI] [PubMed] [Google Scholar]
- 35.Your personal research assistant. Zotero. [2022-06-09]. https://www.zotero.org/
- 36.Waffenschmidt S, Knelangen M, Sieben W, Bühn S, Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol. 2019 Jun 28;19(1):132. doi: 10.1186/s12874-019-0782-0. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-019-0782-0 .10.1186/s12874-019-0782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999 Sep;89(9):1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoville RR, Titler MG. Guiding healthcare technology implementation: a new integrated technology implementation model. Comput Inform Nurs. 2015 Mar;33(3):99–E1. doi: 10.1097/CIN.0000000000000130.00024665-201503000-00004 [DOI] [PubMed] [Google Scholar]
- 39.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015 Feb 12;10:21. doi: 10.1186/s13012-015-0209-1. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-015-0209-1 .10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013 Dec 01;8:139. doi: 10.1186/1748-5908-8-139. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-8-139 .1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu PJ, Chau PY, Sheng OR, Tam KY. Examining the technology acceptance model using physician acceptance of telemedicine technology. J Manag Inf Syst. 2015 Dec 02;16(2):91–112. doi: 10.1080/07421222.1999.11518247. [DOI] [Google Scholar]
- 42.Venkatesh V, Morris MG, Davis GB, Davis FD. User acceptance of information technology: toward a unified view. MIS Q. 2003;27(3):425–78. doi: 10.2307/30036540. [DOI] [Google Scholar]
- 43.Holden RJ, Asan O, Wozniak EM, Flynn KE, Scanlon MC. Nurses' perceptions, acceptance, and use of a novel in-room pediatric ICU technology: testing an expanded technology acceptance model. BMC Med Inform Decis Mak. 2016 Nov 15;16(1):145. doi: 10.1186/s12911-016-0388-y. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-016-0388-y .10.1186/s12911-016-0388-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013 Sep 11;15(3):398–405. doi: 10.1111/nhs.12048. [DOI] [PubMed] [Google Scholar]
- 45.Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008 Apr;62(1):107–15. doi: 10.1111/j.1365-2648.2007.04569.x.JAN4569 [DOI] [PubMed] [Google Scholar]
- 46.National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division. Board on Health Care Services; Committee on Implementing High-Quality Primary Care. Implementing HPCRTFOHC. Robinson SK, Meisnere M, Phillips Jr RL, McCauley L. Implementing High-Quality Primary Care: Rebuilding the Foundation of Health Care. Washington, DC: National Academies Press; 2021. [PubMed] [Google Scholar]
- 47.Promoting interoperability. The Office of the National Coordinator for Health Information Technology. [2022-11-17]. https://www.healthit.gov/topic/meaningful-use-and-macra/promoting-interoperability .
- 48.Glasgow RE, Huebschmann AG, Brownson RC. Expanding the CONSORT figure: increasing transparency in reporting on external validity. Am J Prev Med. 2018 Sep;55(3):422–30. doi: 10.1016/j.amepre.2018.04.044.S0749-3797(18)31869-5 [DOI] [PubMed] [Google Scholar]
- 49.Yen PY, McAlearney AS, Sieck CJ, Hefner JL, Huerta TR. Health information technology (HIT) adaptation: refocusing on the journey to successful HIT implementation. JMIR Med Inform. 2017 Sep 07;5(3):e28. doi: 10.2196/medinform.7476. https://medinform.jmir.org/2017/3/e28/ v5i3e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hailemariam M, Bustos T, Montgomery B, Barajas R, Evans LB, Drahota A. Evidence-based intervention sustainability strategies: a systematic review. Implement Sci. 2019 Jun 06;14(1):57. doi: 10.1186/s13012-019-0910-6. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-019-0910-6 .10.1186/s13012-019-0910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson PM, Sales A, Wensing M, Aarons GA, Flottorp S, Glidewell L, Hutchinson A, Presseau J, Rogers A, Sevdalis N, Squires J, Straus S. Enhancing the reporting of implementation research. Implement Sci. 2017 Feb 08;12(1):13. doi: 10.1186/s13012-017-0546-3. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-017-0546-3 .10.1186/s13012-017-0546-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis CC, Boyd MR, Walsh-Bailey C, Lyon AR, Beidas R, Mittman B, Aarons GA, Weiner BJ, Chambers DA. A systematic review of empirical studies examining mechanisms of implementation in health. Implement Sci. 2020 Apr 16;15(1):21. doi: 10.1186/s13012-020-00983-3. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-020-00983-3 .10.1186/s13012-020-00983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis CC, Klasnja P, Powell BJ, Lyon AR, Tuzzio L, Jones S, Walsh-Bailey C, Weiner B. From classification to causality: advancing understanding of mechanisms of change in implementation science. Front Public Health. 2018;6:136. doi: 10.3389/fpubh.2018.00136. https://europepmc.org/abstract/MED/29868544 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haley AD, Powell BJ, Walsh-Bailey C, Krancari M, Gruß I, Shea CM, Bunce A, Marino M, Frerichs L, Lich KH, Gold R. Strengthening methods for tracking adaptations and modifications to implementation strategies. BMC Med Res Methodol. 2021 Jun 26;21(1):133. doi: 10.1186/s12874-021-01326-6. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-021-01326-6 .10.1186/s12874-021-01326-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JG, Rodriguez HP, Estlin KA, Morris CG. Impact of longitudinal electronic health record training for residents preparing for practice in patient-centered medical homes. Perm J. 2017;21:16–122. doi: 10.7812/TPP/16-122. https://www.thepermanentejournal.org/doi/10.7812/TPP/16-122?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .16-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Longhurst CA, Davis T, Maneker A, Eschenroeder Jr HC, Dunscombe R, Reynolds G, Clay B, Moran T, Graham DB, Dean SM, Adler-Milstein J, Arch Collaborative Local investment in training drives electronic health record user satisfaction. Appl Clin Inform. 2019 Mar 15;10(2):331–5. doi: 10.1055/s-0039-1688753. http://www.thieme-connect.com/DOI/DOI?10.1055/s-0039-1688753 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantaleoni JL, Stevens LA, Mailes ES, Goad BA, Longhurst CA. Successful physician training program for large scale EMR implementation. Appl Clin Inform. 2015 Dec 19;6(1):80–95. doi: 10.4338/ACI-2014-09-CR-0076. http://www.thieme-connect.com/DOI/DOI?10.4338/ACI-2014-09-CR-0076 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson KE, Kersey JA. Novel electronic health record (EHR) education intervention in large healthcare organization improves quality, efficiency, time, and impact on burnout. Medicine (Baltimore) 2018 Sep;97(38):e12319. doi: 10.1097/MD.0000000000012319. https://europepmc.org/abstract/MED/30235684 .00005792-201809210-00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong M, Hill L, Artiga S. Racial disparities in cancer outcomes, screening, and treatment. KFF. [2022-06-21]. https://www.kff.org/racial-equity-and-health-policy/issue-brief/racial-disparities-in-cancer-outcomes-screening-and-treatment/
- 60.Zavala VA, Bracci PM, Carethers JM, Carvajal-Carmona L, Coggins NB, Cruz-Correa MR, Davis M, de Smith AJ, Dutil J, Figueiredo JC, Fox R, Graves KD, Gomez SL, Llera A, Neuhausen SL, Newman L, Nguyen T, Palmer JR, Palmer NR, Pérez-Stable EJ, Piawah S, Rodriquez EJ, Sanabria-Salas MC, Schmit SL, Serrano-Gomez SJ, Stern MC, Weitzel J, Yang JJ, Zabaleta J, Ziv E, Fejerman L. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021 Jan 09;124(2):315–32. doi: 10.1038/s41416-020-01038-6. https://europepmc.org/abstract/MED/32901135 .10.1038/s41416-020-01038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell EP. Declines in cancer screening during COVID-19 pandemic. J Natl Med Assoc. 2020 Dec;112(6):563–4. doi: 10.1016/j.jnma.2020.12.004. https://europepmc.org/abstract/MED/33339569 .S0027-9684(20)30440-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Institute of Medicine (US) Subcommittee on Standardized Collection of Race/Ethnicity Data for Healthcare Quality Improvement. Ulmer C, McFadden B, Nerenz DR. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 63.Chinman M, Woodward EN, Curran GM, Hausmann LR. Harnessing implementation science to increase the impact of health equity research. Med Care. 2017 Sep;55 Suppl 9 Suppl 2(Suppl 9 2):S16–23. doi: 10.1097/MLR.0000000000000769. https://europepmc.org/abstract/MED/28806362 .00005650-201709001-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg AX, Hackam D, Tonelli M. Systematic review and meta-analysis: when one study is just not enough. Clin J Am Soc Nephrol. 2008 Jan 04;3(1):253–60. doi: 10.2215/CJN.01430307.3/1/253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Database search strategy.
Gray literature search strategy.
Inclusion and exclusion criteria checklist.
Glossary of key terms.
Stage 5 procedures for qualitative content analysis.
References to the included studies (N=101).
Reporting status of health IT (HIT) effectiveness by cancer screening activities and HIT functions as represented in the included studies.
Reporting of health IT adoption as represented in the included studies.
Barriers and facilitators (with examples) of health IT adoption aligned with the Integrated Technology Implementation Model.
Expert Recommendations for Implementing Change implementation strategies used to support health IT adoption (n=24 studies).
PRISMA-ScR checklist.


