Abstract
Pneumonia is a multifaceted illness with a wide range of clinical manifestations, degree of severity and multiple potential causing microorganisms. Despite the intensive research of recent decades, community-acquired pneumonia remains the third-highest cause of mortality in developed countries and the first due to infections; and hospital-acquired pneumonia is the main cause of death from nosocomial infection in critically ill patients. Guidelines for management of this disease are available worldwide, but there are questions which generate controversy, and the latest advances make it difficult to stay them up to date. A multidisciplinary approach can overcome these limitations and can also aid to improve clinical results. Spanish medical societies involved in diagnosis and treatment of pneumonia have made a collaborative effort to actualize and integrate last expertise about this infection. The aim of this paper is to reflect this knowledge, communicated in Fifth Pneumonia Day in Spain. It reviews the most important questions about this disorder, such as microbiological diagnosis, advances in antibiotic and sequential therapy, management of beta-lactam allergic patient, preventive measures, management of unusual or multi-resistant microorganisms and adjuvant or advanced therapies in Intensive Care Unit.
Keywords: Community-acquired pneumonia, aetiology, management, therapeutic failure, nosocomial pneumonia, healthcare-associated pneumonia, epidemiology, diagnosis stewardship, prevention
Abstract
La neumonía es una enfermedad polifacética con una amplia gama de manifestaciones clínicas, niveles de gravedad y microorganismos causantes potenciales. A pesar de la intensa investigación de las últimas décadas, la neumonía adquirida en la comunidad sigue siendo la tercera causa de mortalidad en los países desarrollados y la primera debida a infección; y la neumonía adquirida en el hospital es la principal causa de muerte por infección no torno, como el diagnóstico microbiológico, los avances en la terapia antibiótica y secuencial, el manejo del paciente alérgico a betalactámicos, las medidas preventivas, el manejo de microorganismos inusuales o multirresistentes y las terapias coadyuvantes o avanzadas en la Unidad de Cuidados Intensivos.
Palabras clave: Neumonía adquirida en la comunidad, etiología, manejo, fracaso terapéutico, neumonía nosocomial, neumonía asociada a la asistencia sanitaria, epidemiología, diagnóstico, administración, prevención
INTRODUCTION
Community-acquired pneumonia (CAP) is the infection with the higher mortality in industrialized countries. Not taking account COVID-19 (coronavirus disease 2019), it has an incidence of 1.2 cases per 1000 adults in Europe and 2.4 in USA. Higher rate of pneumococcal vaccination in Europe is believed to cause this difference. At extreme ages (under 5 and over 70-year-old) the incidence increases [1]. Hospital-acquired pneumonia (HAP) is also an important cause of morbidity, decreased quality of life, increased sanitary spending and mortality [2–6]. HAP is a pulmonary inflammatory process of infectious origin that develops after more than 48 hours from hospital admission time, was not previously incubating and was absent at time of admission. Ventilator-associated pneumonia (VAP) is a significant sub-set of HAP that appears in patients with an artificial airway more than 48-72 hours after tracheal intubation [7–9]. HAP is the main cause of death from nosocomial infection in critically ill patients (with an incidence of 5 to 10 cases per 1000 hospital admissions), while VAP affects 10-25% of all patients in intensive care units (ICU), with a higher mortality than HAP: 20-30% vs 20-50% respectively [10, 11].
Despite international guidelines implemented in all health systems, there is variability in the diagnostic and therapeutic management of these entities. Moreover, morbidity and mortality remain high and a multiprofessional approach is necessary to improve these rates [12, 13]. Finally, new information from clinical trials and epidemiological studies arises regularly so frequent actualization of knowledge is necessary.
Since 2019, an annual meeting of Pneumonia has been held by main Medical Societies involved in diagnosis and treatment of this disease in Spain. The fifth meeting happened on 14 November 2023 [14]. Experts of different medical specialities related to CAP, HAP and VAP presented the latest advances in their respective fields of action. The aim of the present paper is to synthesize the main ideas of each presentation showed in the meeting regarding the scientific program.
MATERIAL AND METHODS
Design. The Study Group of Infection in the Critically Ill Patient of the Spanish Society of Infectious Diseases and Clinical Microbiology (GEIPC-SEIMC) called experts of different Spanish Medical Societies involved in diagnosis and treatment of CAP, HAP, and VAP (listed in this document’s affiliation) to make a narrative review of their respective field of knowledge and to present their conclusions in different workshops in the Annual Meeting of Pneumonia.
Search strategy. Between July and November 2023, the experts performed a bibliographic search of their corresponding topics in PubMed ((http://www.ncbi.nlm.nih.gov/pubmed/, accessed on 1 November 2023), Embase (http://www.elsevier.com/online-tools/embase/, accessed on 1 November 2023) and Scopus (http://www.elsevier.com/onlinetools/scopus, ac- cessed on 1 November 2023). They chose the most relevant and current articles in their opinion for each issue, to prepare a presentation of 45 minutes for the meeting.
Drafting. On 14 November 2023, two medical writers (CMRL and CGC) attended and then, between November and December, they wrote a text with the main ideas exposed in the meeting.
Revision. Between January and February of 2024, all the experts had the opportunity to read the complete text and raise objections and changes.
RESULTS
Microbiological diagnosis. Targeted treatment has always been a great challenge in planning clinical work algorithms for infectious diseases, especially in sepsis with respiratory origin or HAP/VAP [8]. The time elapsed from the start of empirical antibiotics to the selection of targeted treatment in intensive care units (ICUs) is marked mainly by the microbiological results obtained. This time is conditioned by clinical identification of infection, obtention of specimens for microbiological testing and laboratory processing of samples. Clinicians initiate empirical treatment, which becomes targeted treatment when microbiological results are available. That empirical treatment can be appropriate or inappropriate, so microbiological results can be used to maintain the same therapy, deescalate therapy or to escalate spectrum based on identification and susceptibility testing [15].
Due to the potential appearance of resistant bacteria and possible therapeutic failures associated with an incorrect choice of antibiotic, the microbiological diagnostic techniques have evolved mainly in two aspects: response time and kind of information provided. Molecular biology has been decisive to improve them. It provides results on samples of the respiratory tract and blood in few hours and this information allows to transform an empirical treatment into a targeted one earlier. Polymerase Chain Reaction (PCR) is an example of these molecular techniques and has greatly developed in last years. Main advantages given by these procedures are [12]: i) High negative predictive value for studied microorganisms, ii) High positive predictive value for genotypic markers of resistance and iii) Increased detection yield compared to standard culture. However, they also have several limitations: i) Clinical significance of qualitative detection debatable (sample dependent), ii) Interpretation of (semi)quantitative result, iii) Interpretation of mixed detections and iv) Colonization microorganisms of doubtful clinical significance.
Despite these rapid techniques have dramatically improved time response of microbiological laboratory, it is early to make general recommendations about their use. In any case, progress in reducing time for identification of microorganism and resistance mechanisms is evident. Potential impact is especially relevant for sicker patients, such as ones with VAP or immunosuppressed. However, conventional bacteriological culture is still essential for the correct interpretation of results and decision making. Moreover, the development and improvement of conventional microbiology techniques continue to play an important role in obtaining better results [16].
Actualization in pneumonia. New documents driven by Spanish medical societies. Eleven medical societies collaborated in 2022 to prepare two documents to actualize knowledge about CAP, HAP, and VAP [17, 18]. These societies were GEIPC-SEIMC (critical patient infection study group, Spanish Society of Clinical Microbiology and Infectious Diseases), SEQ (Spanish Society of Chemotherapy), Infurgsemes-SEMES (Emergency Department Infection Study Group, Spanish Society of Emergency Medicine), GEVAC-SEIMC (Vaccines Study Group, Spanish Society of Clinical Microbiology and Infectious Diseases), GTEIS-SEMICYUC (Working Group on Infectious Diseases and Sepsis-Spanish Society of Intensive Care Medicine, Critical Care and Coronary Units), GEMARA-SEIMC (Task Force on Mechanisms of Action and Antimicrobial Resistance, Spanish Society of Clinical Microbiology and Infectious Diseases), GEIRAS-SEIMC (Healthcare-associated Infection Study Group, Spanish Society of Clinical Microbiology and Infectious Diseases), SEPAR (Spanish Society of Pneumology and Thoracic Surgery), SEGG (Spanish Society of Geriatrics and Gerontology), SEDAR-GTIPO (Perioperative Infections Task Force, Spanish Society of Anaesthesiology, Resuscitation and Pain Therapy), and SEHAD (Spanish Society of Hospital at Home). This collab-orative effort was preceded by a SWOG and CAME analysis to perform a good strategy, as can be seen in Table 1.
Table 1.
Strengths, Weakness, Opportunities and Treats (SWOT) and Correct, Adapt, Maintain y Explore (CAME) analysis.
| SWOT analysis | |
|---|---|
| Strengths | Weakness |
| Motivation, capacitation, values, and compromise (attitude and aptitude). Effort culture. |
Toxic competitivity between different medical specialities. Apathy. |
| Opportunities | Treats |
| Strategic alliances between study groups (SG). Strategic alliances between SG and Pharmaceutical Industry. Execution of inversions and grants. |
SG with same fields of interest and competition between them. |
| CAME analysis | |
| Correct the Weakness | Adapt to the Threats |
| Empowerment SG. Stimulation of members of SG. Renovation of board of directors. To improve attraction of new members. Generational replacement. Diffusion in social networks. |
Stimulation of own identity and mark. Create differential value. To search for alliances. |
| Maintain the Strengths | Explore the Opportunities |
| Awards for young investigation and publications. Awards for clinical cases. Relationship among Sepsis Code and with foundations. |
Consensus documents and recommendations. Own clinical trials and epidemiological studies. Derived publications. To improve web page. |
Experts wrote about ten important issues about community-acquired and nosocomial pneumonia.
Most relevant issues for CAP
Changing aetiology. Thanks to introduction of syndromic panels [19], proportion of pneumonia caused by identified bacteria grew from 15-30% to 62-71%, compared to classic methods, such as cultures [20, 21]. Also, the impact of COVID-19 made Haemophilus influenzae and Staphylococcus aureus were more frequent than Streptococcus pneumoniae [22].
Diagnostic procedures [23–25]. Primary care, outpatient clinic and long-term facilities: Only rapid antigen detection of SARS-CoV-2 in nasopharyngeal swab is recommended for vulnerable patients, such as aged ones. Emergency department. Recommended: rapid antigen detection of SARS-CoV-2 in nasopharyngeal swab. In severe cases: gram stain and culture of respiratory secretions, blood culture, urinary antigen test for S. pneumoniae and Legionella pneumophila and molecular tests for detection of bacterial and viral pathogens. In patients empirically treated for methicillin-resistant S. aureus (MRSA): nares screening for MRSA. Procalcitonin is not recommended to determine initiation of antibacterial therapy.
Use of corticosteroid therapy. Risks (corticosteroid induced hyperglycaemia) and potential benefits (to avoid ICU admission and reduction of treatment failure) must be balanced towards a personalized medicine [26, 27]. A deeper review will follow in next sections, but the most important remarks are: Influenza pneumonia. Clinicians must avoid corticosteroid [28], refractory septic shock (in context of respiratory focus origin). Corticosteroid therapy has demonstrated benefit [29]. Other situations such as COVID-19, autoimmune disease, or concurrent asthma of chronic obstructive pulmonary disease (COPD), employment of corticosteroid can be considered [30, 31].
Recommended initial treatment is shown in Table 2 [32]. The duration of treatment should be individualized according to clinical stability with a minimum of 5 days. Risk factors for multidrug resistant (MDR) bacteria include prior respiratory isolation of MRSA or Pseudomonas aeruginosa, severe COPD, bronchiectasis, or recent hospitalization and receipt of parenteral antibiotics (in the last 3 months).
Table 2.
Initial treatment strategies for patients with CAP. Adapted from Candel et al. [17].
| Primary care regimen | Hospital admission regimen | ICU admission regimen |
|---|---|---|
| Oral amoxicillin 1g/8h or oral amoxicillin-clavulanic 875/125 mg/8h (if asthma or COPD) or cefditoren 400mg/12h (alternative) | Ceftriaxone 2g/24h iv or cefotaxime 2g/8h iv or ceftaroline 600mg/12h iv (if post-influenza pneumonia or risk of S. aureus) | Ceftriaxone 2g/24h iv or cefotaxime 2g/8h iv or ceftaroline 600mg/12h iv |
| Plus | Plus | |
| Macrolide (oral azithromycin 500mg/24h/ 3 days or clarithromycin 500mg/12h) | Oral/iv macrolide (azithromycin 500mg/24h /3 days or clarithromycin 500mg/12h) | Macrolide (azithromycin 500mg/24h iv or clarithromycin 500mg/12h iv) or quinolone (levofloxacin 500mg/12h or moxifloxacin 400mg/24h) |
| or | or | |
| Levofloxacin500mg/12h (1-2 days) and then 500mg/24h or Moxifloxacin 400mg/24h | Levofloxacin 500mg/12h iv (1-2 days) and then 500mg/24h or moxifloxacin 400mg/24h iv | If risk factors for MDR bacteria: Meropenem 1g/8h iv + Levofloxacin 500 mg/12h iv + Ceftaroline 600mg/12h iv or Linezolid 600mg/12 h iv |
Main risk factors for readmission. CAP related [33–35]: worsening signs and symptoms of CAP, treatment failure, clinical instability at discharge, PSI (pneumonia severity index) ≥ 4, leucocytosis over 12000/mm3, and multidrug-resistant bacteria. Non-CAP related [36–38]: comorbidities, age over 65 years, Charlson comorbidity score over 2, coronary heart disease, COPD, non-metastatic cancer, complicated diabetes, chronic kidney disease, ≥3 previous admissions, chronic respiratory failure, heart failure, chronic liver disease, and discharge to hospital at home unit. Dementia was a protective factor for readmission, despite aspiration risk.
Other issues were the Spanish recommendations for pneumococcal vaccination (Table 3) and new advances given by artificial intelligence are available, such as machine learning for the prediction of sepsis [39] and interpretation of chest radiographs [40].
Table 3.
Pneumococcal vaccination guidelines in Spain. Adapted from Candel et al. [17]
| Population group | Recommended pattern | Modifications in autonomous regions |
|---|---|---|
| Over 65 years without risk factors | PPSV23v (1 dose) | PCV20v or PCV13v (1 dose) |
| Over 18 years with chronic pathology: chronic cardiovascular and respiratory disease, severe neurological and neuromuscular disease, chronic liver disease, diabetes mellitus, celiac disease, institutionalized persons. | PPSV23v 1 dose + Revaccination each 5 years |
PCV20v or PCV13v (1 dose) |
| Over 18 years high risk groups: immunodeficiencies and complement system deficiencies, immunosuppressive treatment, asplenia or severe splenic dysfunction, HIV infection, chronic renal failure and nephrotic syndrome, transplant, CSF fistula, cochlear implant, history of invasive pneumococcal disease, liver cirrhosis and chronic alcoholism, Down syndrome. | PCV13v (1 dose) + PPSV23v (1 dose) (at least 8 weeks) |
PCV20v (1 dose) + PPSV23v (1 dose) (at least 8 weeks) |
PPSV23v: 23-valent Pneumococcal polysaccharide vaccine. PCV13v: 13-valent pneumococcal conjugate vaccine, PCV20v: 20-valent pneumococcal conjugate vaccine. HIV: Human Immunodeficiency Virus. CSF fistula: cerebrospinal fluid fistula.
Most remarked issues in HAP and VAP (some of them are showed in other parts of the present document).
Molecular techniques. They are very useful for rapid diagnosis of HAP and VAP. They detect a wide range of micro-organisms, with a variety of commercial panels. The range of answer time goes from 20 to 120 minutes. They have demonstrated an improvement in health, mortality, and a good cost/ benefit profile. They are especially helpful for immunocom-promised patients and for detection of viruses such as SARSCoV-2 [41–45].
Nowadays, two main guidelines recommend different samples to reach microbiological diagnosis in VAP. The American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guideline [46] recommends tracheal aspiration, which is the easiest, safest, and cheapest way, although it is vulnerable to upper respiratory tract contamination, so sometimes it is difficult to differentiate colonization from real infection and a derived risk of overuse of antibiotics exists. On the other hand, the International ERS/ESICM/ESCMID/ALAT (European Respiratory Society, European Society of Intensive Care Medicine, European Society of Clinical Microbiology and Infectious Diseases, Latin American Thoracic Association) guideline [47] suggest the use of bronchoscopy with broncho-alveolar lavage (BAL), that requires trained staff, but it obtains a lower respiratory tract sample, has higher specificity, easily distinguishes infection from colonization and is safe. Finally, mini-BAL is a reasonable alternative when bronchoscopy is not available [48–51].
Immunosuppressed patients can benefit from a wide range of microbiological procedures [18]. Some of them are: i) Microbiological stains or respiratory secretions, with immediate results and low costs. Despite these advantages, there are derived risk of false negative results, and they are also observer dependent, ii) Traditional culture of respiratory specimens and blood, which are time dependent and have medium-low performance, iii) Detection of fungal antigens, such as galactomannan in respiratory samples or blood; and (1-3)-β-D-glucan or cryptococcal antigen in serum sample. More useful in neutropenic patients and some techniques are not completely validated, iv) PCR (polymerase chain reaction) in respiratory samples, nasopharyngeal swab, or blood. It is very sensitive but with risk of false positive (colonizing microorganisms) and false negative (inadequate sample, microorganisms not included in the panel), v) Direct fluorescent antibodies directed against certain microorganisms, vi) Detection of soluble antigens in urine.
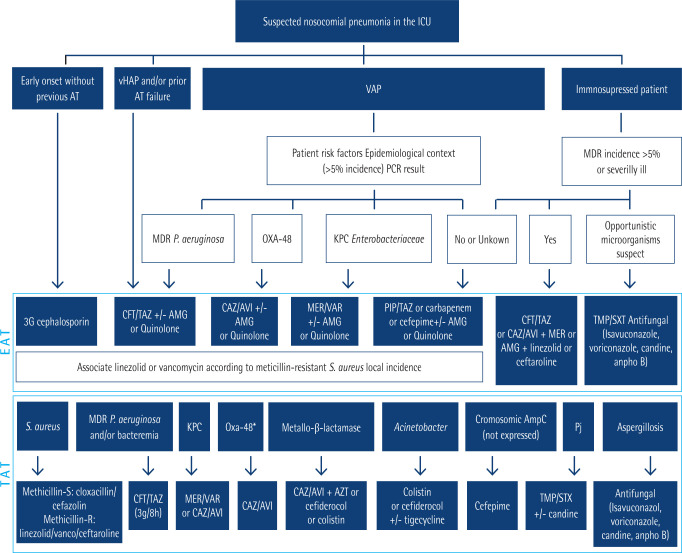
The prior PANNUCI algorithm for antibiotic treatment of pneumonia [52] has been updated [18]. It is shown in Figure 1. Use of antibiotics for hospital at home (HaH) is an useful tool to improve quality of life of some patients with HAP that requires intravenous treatment for a long time and reach clinical stability. New devices such as electronic and elastomeric pumps allow a safe administration at home [53–55]. Finally, a review of causes of therapeutic failure and recommendations to solve them can be seen in Table 4 [18].
Figure 1.
Modified PANNUCI algorithm from empirical to targeted treatment on nosocomial pneumonia in ICUs in European countries (both immunocompetent and immunosuppressed). Adapted from Candel et al. [18]
AT: antimicrobial therapy; vHAP: ventilated hospital-acquired pneumonia; VAP: ventilator-associated pneumonia; MDR: multidrug resistant; PCR: polymerase chain reaction; CFT/TAZ: ceftolozane/tazobactam; CAZ/AVI: ceftazidime/avibactam; PIP/TAZ: piperacillin/tazobactam; AMG: aminoglycoside; AZT: Aztreonam; EAT: empirical antimicrobial treatment; TAT: targeted treatment; OXA-48: OXA-48 Carbapenemase; KPC: Klebsiella pneumoniae Carbapenemase; MER-VAR: meropenem-vaborbactam; IMI-REL: imipenem-relebactam; ESBL-E: extended spectrum beta-lactamase-producing enterobacteria; PJ: Pneumocystis jirovecii. * If Oxa-48 susceptible to CAZ/AVI.
Table 4.
Causes of therapeutic failure in patients with HAP-VAP. Adapted from Candel et al. [18]
| Cause | Recommendation |
|---|---|
| Inadequate antibiotic treatment | Escalate based on microbiological results. |
| Sub-therapeutic antibiotic concentrations | Increase antimicrobial dosing. Use extended or continuous antibiotic infusions to optimize PK/PD parameters |
| New pathogens isolated | Antimicrobial treatment according to microbiological data |
| Undrained pyogenic focus (i.e., empyema) | Therapeutic drainage |
| Drug fever | Change antibiotic treatment |
| A non-infectious illness presenting as HAP | Management as appropriate |
PK/PD: pharmacokinetics/pharmacodynamics.
Allergy to beta-lactam antibiotics. Allergy to beta-lactam antibiotics is a frequent problem among patients and in Spain it is estimated that a 10-12% of the population has some type of hypersensitivity and women are more often affected. Overall, 17% of all these adverse drug reactions are severe and 0.6% are the cause of death [56, 57]. Clinical spectrum of allergic reactions is very wide. They include cutaneous reactions, anaphylaxis, blood dyscrasias and kidney diseases. Amoxicillin-clavulanic is the antibiotic more often involved, although more of the reactions are mild. Cases caused by piperacillin-tazobactam and meropenem are less frequent, but their occurrence has been increased in last years and their severity is higher [58]. Moreover, people who are allergic to beta-lactam antibiotics have worse outcomes: they are more days in hospital, have higher rates of hospital readmission, costs, ICU stay and mortality; have more postsurgical complications and higher risk of Clostridioides difficile diarrhoea and multidrug resistant bacterial infections [56, 59, 60]. In addition, alternative antibiotics such as quinolones have an unfavourable adverse reaction profile, with publication of safety notes by regulatory agencies [61].
Despite being a frequent problem among general population, different studies have demonstrated that only 18.3% to 28.6% of people who claim being allergic to beta-lactam are really allergic to them [62–64]. This false label limits possibilities of treatment and potentially causes worse outcomes as has been mentioned.
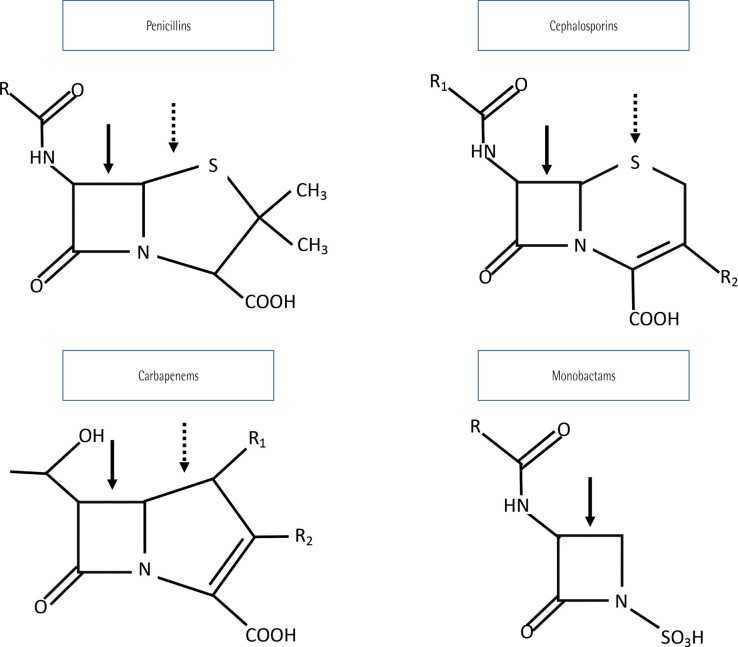
Chemical structure of beta-lactam antibiotics is important because it determines cross reactivity between different molecules. They have a shared beta-lactam ring, a specific ring for each group and one or more lateral chains, that can be similar between different beta-lactam antibiotics, even if they are of different families. Generic chemical structure is showed in Figure 2 [65–67].
Figure 2.
Beta-lactam structure
Solid arrow: beta-lactam ring. Dashed arrow: group ring. R, R1, R2: lateral chains.
Owing to their different chemical structure, hypersensitivity to penicillin, for example, does not mean hypersensitivity to other beta-lactam antibiotics. In fact, cross reactivity between first generation cephalosporins and penicillin is less than 10%. Lateral chain is a good predictor of cross reactivity, so that different beta-lactam antibiotics with a similar lateral chain have a relative risk of cross reactivity of 3 (confidence interval -CI- 1.6-5.5), whereas second and third generation cephalosporins, such as ceftriaxone, have a very low chance of cross reactivity with penicillin, due to their different lateral chains [65, 66, 68, 69]. In fact, 75 - 97% of penicillin allergic patients tolerate cephalosporins, and 99% tolerate aztreonam and carbapenems. Although aztreonam has been classically considered secure in these patients, it shares the same lateral chain than ceftazidime and cefiderocol, so cross reactions are expected between them and its use in patients allergic to these specific cephalosporines is not recommended. In the case of carbapenems, a history of penicillin allergy contraindicates their use, but in practice it is important to know result of historical cutaneal allergy test against penicillin, which has a high negative predictive value [64]: in case it is negative, use of carbapenems is considered secure; in case it is positive or unknown, carbapenem should be administrated in a gradual way, with increasing concentration and with narrow surveillance of allergic reactions [70–73] .
So, beta-lactam allergy is a heterogeneous problem because a patient can be allergic to a specific family (e.g. aminopenicillins) but can tolerate other families (e.g. carbapenems). Moreover, false label of beta-lactam allergy is a frequent issue that can be dangerous because it can lead to worse outcomes as has been mentioned. So, it is very important to take a detailed clinic history and consultation with allergology if available [74]. PEN-FAST is an instrument designed to stratify risk in these patients and it evaluates time since allergic reaction, clinic manifestations and severity of them, and treatment required for reaction [75]. So, after a careful evaluation, a patient can be classified as not allergic if he has an adverse reaction such as vomiting or diarrhoea; mild reaction probably not mediated by IgE, so cephalosporins of third generation and newer, carbapenems and aztreonam can be used; and severe reactions, that can be mediated by IgE, such as anaphylaxis, or not mediated by IgE, such as interstitial nephritis. In these last cases, only aztreonam can be employed. In all cases, a subsequent evaluation by allergy service is strongly recommended. Other instruments have been developed to evaluate the risk of cross reaction and they have demonstrated an increment in utilization of beta-lactam without a higher rate of adverse effects [76, 77].
Recommended empirical antibiotic treatment is shown in Table 5. Recommended duration of treatment is 3-5 days in CAP, 8 days in HAP-VAP, and individualized until drainage in the case of lung abscess. Clinicians also must consider antibiotic coverage of S. aureus and/or P. aeruginosa if needed. If a beta-lactam antibiotic is used, they may perform a previous controlled exposition trial [56]. As it has been stated, it is important to eliminate false allergy labels and to stratify risk. Allergy consultation, if available, is strongly recommended. Alternatives with non-beta-lactam antibiotics, such as moxifloxacin, omadacycline, eravacycline or plazomicin may be considered for some patients with multidrug resistant bacteria [78–80]. Currently, the optimized management of beta-lactam allergy is included in stewardship strategies or programs.
Table 5.
Empirical treatment in patients allergic to beta-lactam antibiotics. Adapted from Barberán et al. [79]
| Low risk of allergic reaction | High risk of allergic reaction | |
|---|---|---|
| CAP | Levofloxacin 750 mg/24 h vo If severe: Ceftriaxone 2 g/24 h iv plus Azithromycin 500 mg/24 h vo or Levofloxacin 750 mg/24 h iv |
Levofloxacin 750 mg/24 h iv |
| Early HAP-VAP (less than 5 days in hospital) | Ceftriaxone 2 g/24 h iv or Levofloxacin 750 mg/24 h iv |
|
| Late or severe HAP-VAP | Ceftazidime 2 g/8 h iv or Meropenem 1-2 g/8 h iv. Consider add: Linezolid 600 mg/12 h iv or Vancomycin 30 mg/kg/d in 2-3 doses iv |
Aztreonam 2 g/8 h plus Linezolid 600 mg/12 h iv or Vancomycin 30 mg/kg/d in 2-3 doses iv |
| Lung abscess or aspiration pneumonia | Ceftriaxone 2 g/24 h iv plus Clindamycin 600 mg/8 h iv |
Aztreonam 2 g/8 h plus Clindamycin 600 mg/8 h iv |
Zero Pneumonia. The Zero Pneumonia (ZN) project is a multifactorial intervention proposal based on the simultaneous application of a package of measures to prevent VAP. Its intention is to reduce the infectious complications in Spain. The project is sponsored by the Quality Agency of the Ministry of Health, Social Policy, and Equality (MSPSI) with the collaboration of the Spanish Society of Intensive Care Nursing and Coronary Units (SEEIUC) and the Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC). Zero Bacteriemia project was developed previously, and its structure was used to implement ZN project. It is an ambitious project that involves the MSPSI Quality Agency, the Health Departments of the Autonomous Communities (AACC) and the management team of several national hospitals. It also includes the collaboration of doctors and nurses from most ICUs in the country and different working groups of the SEMICYUC. The main objective of the ZN project is to reduce the national VAP rate to less than 9 episodes per 1,000 days of mechanical ventilation, which means a 40% reduction respect to previous rates (2000-2008) and a 25% reduction compared to 2009 and 2010 rates.
To achieve it, medical doctor and nurses, appointed by their respective societies, defined ten recommendations. Seven of them have the highest scientific evidence and are obligatory [81]. They are: Training of staff in airway manipulation, control of pneumotamponade, oral hygiene with chlorhexidine (0.12-0.2% every 6-8h), hand hygiene, avoid supine position whenever possible, promoting the process of respiratory mechanical weaning, avoid the scheduled change of humidifiers and tracheal tubes. Another three recommendations are highly recommended, but at the beginning they were not mandatory: Selective decontamination of the digestive tract, aspiration of subglottic secretions, use of systemic antibiotics during intubation in patients with a low level of consciousness.
The applications of these measures made incidence of VAP decrease until 5.41 episodes per 1,000 days of mechanical ventilation in 2019. However, COVID-19 pandemic made it rise to 19.99 in 2021. Therefore, the latest ZN guideline made all recommendations mandatory. This change, along with control of COVID-19 pandemic, made incidence of VAP decreases to 8.55 [81].
Finally, aetiology of VAP has maintained without great changes in the last 10 years, with P. aeruginosa and K. pneumoniae predominating among Gram-negative Bacilli (GNB), and methicillin-susceptible S. aureus among Gram Positive Cocci (GPC). As resistance mechanisms associated with GNB, extended-spectrum beta-lactamase (ESBL) producing Enterobacterales, multi-resistant Pseudomonas spp, and metal-lo-beta-lactamase producing GNB stand out. Meropenem and linezolid are the most used antibiotics, whereas utilization of piperacillin/tazobactam has decreased and the appearance of new molecules have allowed the development of new therapeutic regimens [14, 29, 81].
Therapeutic optimisation in community-acquired pneumonia
Community-acquired pneumonia in intensive care unit (ICU). Pneumonia is an important cause of death worldwide. It was the first cause of death at the end of twentieth century and nowadays is the second cause, only overcome by cardiovascular diseases [82]. Moreover, it frequently causes the death of aged people [83]. Among CAP that require ICU admission, mortality have maintained high (around 30%), despite efforts to reduce it [84–87]. Apart from distress syndrome, which severity is correlated with likelihood of death, other factors related with higher risk of decease are advance age, previous antibiotic therapy, comorbidities, multiorgan failure and an inadequate empiric antibiotic treatment [88]. Another factor related with higher mortality is delayed intubation in patients who needed it [89].
The main identified aetiology of CAP is S. pneumoniae, identified in more than 80% of isolates, but its prevalence has become slightly lower in last years. It is followed by other microorganism such as Streptococcus spp., Haemophilus spp., enterobacteria, Pseudomonas spp. and others [21,90,91]. However, in 50% of pneumonias, etiologic agent is not identified. This lack of identification is related with antibiotic treatment before obtaining sample and non-invasive tests [92]. Decreased susceptibility to penicillin of S. pneumoniae is observed in some pneumococcal pneumonia. An intermediate susceptibility can be observed in 5-10% of cases, while high resistance is confirmed in less than 4% of all cases [93]. S. pneumoniae has a decreased susceptibility to ceftriaxone in 5-10% of isolates, depending on studied region. In contrast, quinolones and ceftaroline maintains an excellent profile, with susceptibilities of almost all studied isolates [94].
Severe CAP pneumonia is defined as a CAP with a major criterion or at least 3 minor criteria established by the American Thoracic Society and Infectious Diseases Society of America -ATS/IDSA- [84,95]. These criteria are shown in table 6.
Table 6.
ATS/IDSA criteria to define severe CAP. Adapted from File et al. [84]
| Major criteria | Minor Criteria |
|---|---|
| Septic shock treated with vasopressors Respiratory failure necessitating mechanical ventilation |
Respiratory rate ≥ 30 bpm Confusion or disorientation Hypothermia (temperature < 36ºC) Hypotension necessitating aggressive fluid resuscitation Leukopenia (<4000 cells/mm3). Thrombocytopenia (<100000 platelets/mm3). Uremia: BUN ≥ 20 mg/dL. Ratio PaO2to FiO2 ≤ 250 Multilobar (≥2) infiltrates |
bpm: breath per minute, BUN: blood urea nitrogen level, PaO2: partial pressure of arterial oxygen, FiO2: fraction of inspired oxygen.
Recommended empiric antibiotic treatment can be read in table 2. Guidelines worldwide recommend a combination of a beta-lactam plus macrolide or quinolone [29,96–98]. Due to potential serious adverse effects of quinolones [61] and proven benefits of macrolide combinations of 3 days, this last is the preferred option. Election of beta-lactam is an important issue with new evidence emerging in last years. Traditionally, choice of election has been ceftriaxone or cefotaxime. An ideal antibiotic in severe CAP has these qualities: to reach an adequate serum concentration, to compensate distribution volume of critic patient, to create a high gradient to tissue, to reduce quickly bacterial charge and to achieve enough concentration to avoid mutant selection [99]. Two relatively news beta-lactam antibiotics have properties like the ones described. Ceftobiprole demonstrated a better rate of improvement than comparator [100]. Ceftaroline also proved better outcomes than ceftriaxone in different settings and in presence of bacteriemia [101–103]. Duration of treatment must be tailored to patient evolution, but in many cases five days is enough. Procalcitonin serum levels (PCT) can guide this, but there is not a specific threshold to take a decision. Dynamic changes, considering clinical state of patient, may be helpful [96,104]. If viral aetiology is demonstrated, in absence of signs of bacterial coinfection, antibiotic therapy can be safely stopped. Signs of bacterial coinfection are positive culture, radiographic findings suggestive of bacterial origin, high white-cell count (>15000/mm3), high c reactive protein (>150 mg/L) and/or PCT over 0.25 ng/mL.
So, to make mortality rates become lower in severe CAP, an energic approach is needed. Focus on vulnerable patients, early intubation in people who need it and wisely use of antibiotic therapy might aid to achieve this objective.
Sequential treatment in community-acquired pneu-monia. Antibiotic stewardship can be defined as a set of strategies to promote the responsible use of antimicrobials for the purpose of protecting public health [105]. A lot of interventions can contribute to this objective, but some of them are easier to implement than others and with stronger evidence of cost-saving results, such as formulary restrictions, batching of intravenous antimicrobials, therapeutic substitutions, and intravenous-to-oral conversions -sequential therapy- [106]. This last one intervention is also a basic element of stewardship programs in pneumonia treatment [107]. There are three kinds of intravenous-to-oral conversions [108]:
Sequential therapy. To replace the same antibiotic with an oral formulation. E. g. substitution of levofloxacin iv (intravenous) for levofloxacin, moxifloxacin (or perhaps or in the near future, delafloxacin po (per oral)).
Switch therapy. To substitute an antibiotic for an equivalent with an oral formulation. The best candidate for sequential therapy since ceftriaxone, by in vitro activity and with the best pharmacodynamic profile, is cefditoren at a dose of 400 mg every 12 hours. Its absorption improves if taken with food [109].
Step down therapy. To replace an antibiotic for another of other class with an oral formulation. E. g. substitution of ceftriaxone iv for levofloxacin po.
Sequential therapy is a universal recommendation in current guidelines for the treatment of pneumonia [95,96]. To do so, patient must have reached clinical stability, defined with the following criteria [29,110]: Resolution of vital sign abnormalities (heart rate less than 100 beats per minute, respiratory rate less than 24 breaths per minute, systolic blood pressure more than 90 mmHg, arterial oxygen saturation more or equal to 90% with usual oxygen flow for the patient, temperature less than 37.8ºC, normal mental status) and the ability to eat.
In a study conducted in Japan, sequential therapy was performed in 30.1% of patients, and was more frequent among mild patients and in people treated by pulmonologists [111]. Early intravenous-to-oral conversion of antibiotic therapy is safe, with the same rate of mortality, recurrent infections, and treatment success than exclusive intravenous therapy; and it is associated with a shorter length of hospital stay and lower costs [112,113].
Despite its advantages, there are resistances to implant sequential therapy. Some identified barriers are wrong concepts, practical issues, factors related to organization and insufficient medical education [114]. To overcome these barriers, it is necessary to establish a clear hospital program, with the identification of patients who can benefit from early switching from intravenous treatment to make recommendations to physicians and to maintain an open channel of two-way communication to create an appropriate culture. Finally, it is important a surveillance of these patients to implement improvement measures [108].
Characteristics of an ideal oral antibiotic to implement a sequential therapy are [115] similar antimicrobial spectrum, high bioavailability, favourable pharmacokinetic characteristics (oral route, administration every 12 to 24 hours), low resistance selection and, if possible, low cost. In table 7, available options to swich from an intravenous to an oral antibiotic treatment are shown. Due to high rate of pneumococcal resistance of cefixime, almost 70% of isolates, this antibiotic is considered inappropriate. Finally, cefuroxime in usual dosage (500 mg twice a day) does not reach enough serum concentration to be active against S. pneumoniae [61,116–119].
Table 7.
Available options for sequential treatment of pneumonia. Adapted from Barberán et al. [116]
| Advantages | Disadvantages | |
|---|---|---|
| Cefditoren | Almost no pneumococcal resistance Administration each 12 hours Dose of 400 mg each 12 hours has an optimal pharmacokinetic profile, even against S. pneumoniaewith decreased penicillin susceptibility Lowest risk of resistance selection |
Bioavailability of 20%. It can improve with food [109] |
| Amoxicillin-clavulanate | Low resistance rate Bioavailability of 60% |
Commercial formulation (875/125 mg) needs to be given each 8 ours High dose of amoxicillin (2000 mg twice a day) is needed for S. pneumoniae with decreased penicillin susceptibility and to avoid resistance selection Highest ecological impact |
| Levofloxacin | Administration each 12 -24 hours Highest bioavailability (>95%) |
Risk of severe side effects: QT syndrome, tendonitis, retinal detachment, aortic dissection, dysglicemia, psychiatric side effects High dose (500 mg twice a day) is needed to avoid resistance selection |
Therapeutic optimisation in healthcare-associated pneumonia
Antibiotic therapy in hospital-acquired pneumonia and ventilation associated pneumonia. Empiric antibiotic treatment for HAP and VAP in ICU is tailored to microbiologic results of samples, which usually last 24 to 48 hours to be known with traditional microbiological methods [120]. In Spain there is intensive surveillance of aetiology of infections in ICUs [121]. This surveillance shows that the most frequent microorganisms are P. aeruginosa (17.56%), S. aureus (10.43%) and K. pneumoniae (10.32%). Among the ten most common microorganisms isolated are also S. maltophilia (5.49%), A. fumigatus (2.63%) and cytomegalovirus -CMV- (2.09%), which are not routinely covered by empirical treatments. Moreover, around 20% of all empiric treatments for bloodstream infections do not treat the aetiology properly and the main predictor of empiric treatment failure is the isolation of a resistant microorganism [122]. Delayed appropriate treatment is associated with higher length of antibiotic treatment, hospital stay, disability and costs [123]. An early BAL is recommended for these infections to obtain a good sample for microbiological analysis and to adjust empiric treatment as soon as possible [120].
Current guidelines recommend empiric treatment with an antibiotic against methicillin-resistant S. aureus -MRSA- (linezolid or vancomycin) with a combination of two antibiotics with action against P. aeruginosa (beta-lactam plus fluoroquinolone or aminoglycoside or polymyxin) [6,7]. European guideline adapts empiric antibiotic coverage attending to severity and local epidemiology [124]. As antibiotic consumption in Spain shows, these recommendations are often followed [121]. A common suggested combination is meropenem plus linezolid plus amikacin, but due to predominant profiles of resistance of microorganisms, there is a high risk of a functional monotherapy of aminoglycoside, which is a not recommended option [7,121] . The most important risk factors for infection by a multidrug resistant microorganism are [125–134]: antibiotic pressure, immunosuppression, comorbidity, hospitalization-length of stay, severity of illness, local epidemiology, colonization of the patient by resistant microorganism and diagnostic and therapeutic invasive procedures performed.
There are some strategies that can help to design a more precise empiric treatment. These approaches are a potent tool to anticipate classical microbiological results, such as surveil-lance of colonization by bacteria of interest [135–138]. In table 8 the most used techniques are show, knowledge about local and regional epidemiology, and trends in resistance profile. Among enterobacteria in Spain, the most frequent kind of carbapenemase is OXA-48, but other types such as KPC are also rising [139]. In contrast, frequency of carbapenemase production by PAER is lower [140], previous antibiotic pressure increases risk of antibiotic resistance, especially in case of PAER [141], microscopic examination of samples and detection of resistance genes by molecular techniques such as PCR [120,142].
Table 8.
Surveillance of bacteria of interest. Adapted from Parente et al. [135]
| Bacteria | Sample | PPV | NPV |
|---|---|---|---|
| MRSA | Nasal exudate | Moderate | Very high |
| CPE | Perineal exudate | Moderate | Very high, combined with local epidemiology |
| PAER | Respiratory or perineal | High | Low |
CPE: carbapenemase producing enterobacteria, PAER: P. aeruginosa, PPV: positive predictive value, NVP: negative predictive value.
There are recently commercialized beta-lactam antibiotics that have a higher rate of susceptibility than older ones against PAER. Also, aminoglycosides and colistin maintain low rates of resistance [143], but new beta-lactams have demonstrated better results than combinations of older ones [144–150]. In table 9 there is a review of mechanisms of resistance and susceptibility of different recently developed beta-lactams [151,152].
Table 9.
Mechanisms of resistance and susceptibility of new beta-lactams. Adapted from Doi et al. [151]
| Carbapenemase | |||||||
|---|---|---|---|---|---|---|---|
| Class A | Class B | Class D | |||||
| KPC, GES | MBL: VIM, NDM, IMP | OXA-48 | PAER MR | Aanetobacter | S. maltophilia | ||
| Ceftazidime-avibactam | S | R | S | S | R | R | |
| Ceftolozane-tazobactam | R | R | R | S | R | R | |
| Imipenem-relebactam | S | R | R | S | R | R | |
| Meropenem-vaborbactam | S | R | R | R | R | R | |
| Cefiderocol | S | S | S | S | S | S | |
R: resistant, S: susceptible, PAER MR: P. aeruginosa multi-drug resistant.
Attending to all aspects commented in last paragraphs, a new approach to empiric treatment has been developed by Spanish Medical Societies, that is available in Figure 1 [18]. As it is shown, a suggested empirical treatment guided by clues given by patient profile, local epidemiology, gram staining and molecular techniques is strongly recommended. Empiric treatment against PAER must include a beta-lactam antibiotic, although double antibiotic empiric regimen is optional and it may be prescribed in case or risk of therapeutic failure, to achieve synergic action and to optimize pharmacokinetic and pharmacodynamic properties [153].
Although European Guidelines recommends a duration of treatment of less than 7 days [6], length of treatment is not well determined in case of multidrug resistant microorganisms [18]. Regarding the duration of antibiotic treatment for P. aeruginosa pneumonia, a recent clinical trial failed to demonstrate non-inferiority of 8 days versus 15 days. Moreover, the shorter length of treatment was associated with increased recurrencies [154].
Finally, benefit of nebulized antibiotics for VAP has been argued in last years. Pneumonia is an infection of high inoculum and due to bronchi obstruction and atelectasis, nebulized antibiotics fails to achieve enough concentration in target tissues of animal models [155–158]. In humans, different devices have been used to give these treatments, and membrane inhalers are preferred in ventilated patients [159]. Experts advise to avoid nebulized antibiotics for treatment of HAP and VAP, due to the lack of effectiveness in reducing mortality and length of ICU stay, and a high rate of respiratory adverse effects [160,161]. Whereas inhaled antibiotics for treatment are not recommended, amikacin may prevent VAP if given to patients recently intubated [162].
HAP-VAP by producing-carbapenemase enterobacteria non metallo-beta-lactamase (non-MBL CRE). Infectious Diseases Society of America (IDSA) in 2023 has proposed several recommendations for the treatment of infections caused by resistant bacteria [163]. Ceftazidime-avibactam and meropenem-vaborbactam are two current alternatives in the treatment of HAP-VAP caused by non-MBL CRE, especially carbapenemases OXA-48 like and KPC types.
Ceftazidime-avibactam has excellent activity against bacteria that produce β-lactamases of Ambler class A and C, as well as some of group D (OXA), including extended-spectrum β-lactamases (ESBL), AmpC, KPC-type carbapenemases and OXA-48 [164]. Data on the effectiveness of ceftazidime-avibactam in critically ill patients, such as mechanically ventilated patients, are limited. In 2020, a retrospective observational cohort study in central Greece compared critically ill and mechanically ventilated patients (41 subjects) suffering from CRE infections receiving ceftazidime-avibactam to 36 patients who received other appropriate available antibiotic therapy, such as polymyxin B, tigecycline and aminoglycosides. There was a statistically significant improvement in the Sequential Organ Failure Assessment (SOFA) score on days 4 and 10 in the ceftazi-dime-avibactam group compared to that in the control group. Ceftazidime-avibactam was better than other treatments in all evaluated outcomes: microbiological eradication, clinical cure, and mortality. Illness severity was also associated with mortality. In conclusion, a ceftazidime-avibactam-containing regime was more effective than other available antibiotic agents for the treatment of CRE infections in the high-risk, mechanically ventilated ICU population evaluated [165].
Despite these encouraging results, resistance to ceftazi-dime-avibactam has developed in recent years, such as KPC-2 and KPC-3 variants. Resistance caused by the plasmid with a mutation in the blaKPC-3 gene (D179Y variant, described in the ST258 clone) is a challenge for microbiology laboratory. It reduces the MIC to carbapenems and other beta-lactams, which can lead to false negative result in carbapenemase immuno-chromatography detection kits. This mutation produces changes in the KPC Ω-loop zone (165–179 positions), it increases the affinity for ceftazidime and meropenem and it restricts binding to avibactam [166].
Meropenem-vaborbactam is another novel antibiotic. Vaborbactam is a serineβ-Lactamase inhibitor, derived from boronic acid. It is defined as Ambler class A inhibitor (especially KPC) and C, but it does not inhibit B and D classes [167]. A Phase 3, multinational, open-label, randomized controlled trial (TANGO II) was conducted from 2014 to 2017 to evaluate the efficacy and safety of meropenem-vaborbactam monotherapy versus best available therapy (BAT) for CRE. Eligible patients were randomized 2:1 to meropenem-vaborbactam (2g/2g over 3 h-8h for 7-14 days) or BAT (mono or combination therapy with polymyxins, carbapenems, aminoglycosides, tigecycline; or ceftazidime-avibactam alone). Efficacy endpoints included clinical cure, day-28 all-cause mortality, microbiologic cure, and overall success (clinical cure + microbiologic eradication). Meropenem-vaborbactam was better than BAT for cure rates and test cure, but there was not a statistically significant difference for day-28-all-cause mortality [145]. As in the case of ceftazidime-avibactam, resistance to this new drug has been described, such as OmpK35 and 36 mutations [168].
Cefiderocol has potent in vitro and in vivo activity against multidrug-resistant (MDR) gram-negative bacilli, including carbapenem-resistant isolates (including A, B, C and D Ambler beta-lactamase classification). Exceptional reduced susceptibility during treatment to cefiderocol have al ready been reported [169].
Imipenem-relebactam is a new combination of a beta-lactam and a beta-lactamase inhibitor. Relebactam has the power to inhibit type A (KPC, GES, IMI) and C (AmpC, PCD) beta-lactamases, but it is useless against type B and D. It also inhibits ESBL. This combination has demonstrated non-inferiority compared to piperacillin-tazobactam in HAP-VAP, with or without bacteraemia [170]. Its safety profile is comparable to that of imipenem-cilastatin. It is a useful alternative in the treatment of HAP-VAP caused by non-MBL CRE type A, in a targeted treatment setting or in settings of high prevalence and clinical suspicion as empirical treatment [171].
Metallo-beta-lactamase-producing Pseudomonas aeruginosa. Infections caused by multidrug-resistant Gram-negative bacteria are becoming a worldwide problem due to their increasing incidence and associated high mortality. Carbapenem-resistant bacteria such as K. pneumoniae, P. aeruginosa and A. baumannii are the most important in clinical practice [172,173]. P. aeruginosa is presented as one of the main microorganisms causing HAP/VAP in the last few years [18]. Metallo-beta-lactamase (MBL) production has been the cause of therapeutic failures with the antibiotics available in the therapeutic arsenal. However, the appearance of new antimicrobials and the rescue of old known drugs have provided alternatives for this type of isolates [173]. Several teams from the CIBER for Infectious Diseases (CIBERINFEC) led by the Balearic Islands Health Research Institute (IdISBa)/Son Espases Hospital have analysed the evolution of antibiotic resistance in P. aeruginosa. The results have recently been published in The Lancet Regional Health-Europe [140]. The work reveals that, in 2022, bacteria showed lower resistance to all the antibiotics evaluated, both the oldest and the newest, which implies that the bacteria were more susceptible to these treatments. Additionally, a significant decrease in the prevalence of multidrug resistance (resistance to three or more families of antibiotics) and extensive resistance (resistance to all, except 1 or 2 families) bacterial profiles was found in 2022 compared to 2017. However, a significant increase in the proportion of strains with the most dangerous mechanism, the production of carbapenemases, has been described. Moreover, it is associated with the dissemination of the hypervirulent epidemic strain ST235. This strain, along with ST175, and others associated with high frequency to MBL production, are great challenges for antibiotic management [140]. Alternatives currently available in MBL-producing P. aeruginosa isolates are: cefiderocol, fosfomycin, high doses of amikacin and synergistic combinations [173]. The combination of ceftazidime/avibactam with aztreonam is an attractive alternative in MBL-producing enterobacterales. However, in the case of P. aeruginosa, due to the coexistence of collateral mechanisms, such as overexpression of efflux pumps or loss of porins, it is not the preferred alternative if other drugs are available [174].
The novel beta-lactam cefiderocol is stable against different serine- and metallo-beta-lactamases, and, due to its iron channel-dependent uptake mechanism, is not impacted by porin channel loss. Furthermore, the periplasmic level of cefiderocol is not affected by upregulated efflux pumps. The potential for on-treatment resistance development currently appears to be low, although more clinical data are required. Information from surveillance programs, real-world compassionate use, and clinical studies demonstrate that cefiderocol is an important treatment option for MBL-producing P. aeruginosa infections, including pneumonia [140,173–175].
Acinetobacter baumannii and Stenotrophomonas maltophilia. A. baumannii complex and S. maltophilia are two opportunistic bacterial species that cause nosocomial infection (mainly HAP-VAP and bacteraemia). A. baumannii is associated with resistance mechanisms that the World Health Organization (WHO) introduced in the “WHO priority list for research and development of new antibiotics for antibiotic-resistant bacteria” (Priority 1: critical) [176]. Especially in HAP-VAP infections, combinations of ampicillin-sulbactam together with cefiderocol, tigecycline or colistin have been proposed to increase the probability of therapeutic success [177]. IDSA and ESCMID recommendations in high-risk and severe ill patients suggest the combination of at least of two antibiotics with in vitro activity. Table 10 shows the differences between the recommendations for treatment of A. baumannii Carbapenems Resistant (CR) provided by IDSA and ESCMID [163,177,178]. Among the future options, it is worth highlighting the trials that are being carried out with sulbactam/durlobactam.
Table 10.
Differences in the IDSA and ESCMID A. baumannii CR infections treatment recommendations. Adapted from Tamma et al. [163] and Carrara et al. [178]
| IDSA | ESCMID |
|---|---|
| The use of high doses of ampicillin-sulbactam is recommended (6-9g/day) in combination with another antibiotic at least until clinical improvement is observed. Associate minocycline, tigecycline, polymyxin B or cefiderocol. do not associate fosfomycin, rifampicin or meropenem. It is recommended to use ampicillin-sulbactam, even if it is in-vitro resistant. |
For patients with A. baumannii CR pneumonia sensitive to sulbactam, suggests ampicillin-sulbactam (Low level of evidence) |
| Consider the use of polymyxin B in combination with another antibiotic, because of limitations of this antibiotic: narrow therapeutic range, suboptimal pulmonary penetration, potential clinical failure, and emergency of resistance during treatment. | For patients with A. baumannii CR resistant to sulbactam, polymyxin or high doses of tigecycline are recommended if they are active in vitro. There is not enough evidence and a preferred antibiotic could not be recommended. |
| High doses of minocycline or tigecycline can be used with at less another antibiotic. Tigecycline is associated with higher mortality rates and should not be used in presence of bacteriemia. |
We conditionally advise against the use of cefiderocol for treatment of infections caused by A. baumannii CR (low level of evidence). |
| Cefiderocol should be limited to the treatment of A. baumannii CR if other treatments fail, or it is resistant. It is recommended to prescribe it in combined treatment. | Neither combinations are recommended: polymyxin-meropenem (high level of evidence) nor polymyxin-rifampicin (moderate level of evidence). |
| The use of nebulized treatment is not recommended for respiratory infections. | In high risk and severe-ill patients, a combination of two antibiotics with in vitro activity among available therapies should be used: polymyxins, aminoglycosides, tigecycline, sulbactam. (very low level of evidence). If meropenem MIC is less than 8mg/L, combined therapy with meropenem extended infusion is suggested (good practice). |
Infections caused by the opportunistic pathogen S. maltophilia in immunocompromised patients are complicated to treat due to antibiotic resistance and the ability of the bacteria to produce biofilm [179]. These bacteria colonize the surface of medical devices such as urinary catheters, endoscopes, and ventilators; they can cause respiratory tract infections. Low outer membrane permeability due to multidrug-resistant efflux systems and the two chromosomally encoded β-lactamases present are a challenge for antimicrobial treatment. Moreover, there is a wide spread of antibiotic resistance genes among S. maltophilia that contribute to enhanced resistance to multiple antibiotics, such as penicillin, quinolones, and carbapenems.
Nevertheless, tetracycline derivatives, fluoroquinolones, trimethoprim-sulfamethoxazole (TMP-SMX) and cefiderocol are considered promising antibiotics. Due to the adaptive nature of the intrinsically resistant mechanism and its ability to acquire new resistance via mutation and horizontal gene transfer, it remains a challenge for clinicians [179,180]. The combination of ceftazidime-avibactam plus aztreonam could be a good option when there is resistance to TMP-SMX and fluoroquinolones [181]. Table 11 displays the differences between the recommendations for treatment of S. maltophilia treatment provided by IDSA and ESCMID [163,178,182].
Table 11.
Differences in the IDSA and ESCMID S. maltophilia infections treatment recommendations. Adapted from Tamma et al. [163] and Carrara et al. [178]
| IDSA | ESCMID |
|---|---|
| We recommend the use of 2 of the following antibiotics in combination: TMP-SMX, minocycline, tigecycline, cefiderocol or levofloxacin. | Consider combined therapy in severe infections, especially in immunocompromised patients. |
| We recommend the combination ceftazidime-avibactam plus aztreonam in clinical instability, intolerance, or resistance to other alternatives. | In patients with infections resistant to TMP-SMX or if it cannot be used, perform combined treatment based on in vitro activity. |
| Use TMP-SMX 8-12mg/kg (TMP) in combination therapy, at least until clinical improvement. | Use TMP-SMX at 15mg/Kg/day (TMP) in 3-4 doses adjusted to renal function. |
| High doses of minocycline (200mg/12h) in combination therapy is reasonable, until clinical improvement. Tigecycline is a sensible option. | Levofloxacin monotherapy is non-inferior to TMP-SMX monotherapy. If fluoroquinolones are used, emergence of resistance during treatment may appear. |
| We recommend cefiderocol in combined therapy until clinical improvement. | In patients with limited options consider second-line agents based on in vitro test. |
| Use levofloxacin as part of combination therapy. It is not advised leave it on monotherapy after clinical improvement. |
Current debates in respiratory sepsis
Steroids. Role of corticosteroids in severe pneumonia is controversial, as available evidence suggests [183–185]. In severe viral respiratory infections, causing pathogen is an important issue. As previously it has been mentioned, severe influenza pneumonia does not benefit from corticosteroid treatment [186], whereas dexamethasone is a corner stone for treatment of severe COVID-19 pneumonia [30]. However, some unanswered questions persist about the effectiveness of corticosteroids for this last entity. There is contradictory evidence about reduction of mortality in ventilated patients. A retrospective study of prospectively collected data conducted in 70 ICUs (mainly Spanish), included mechanically ventilated COVID-19-associated acute respiratory distress syndrome (ARDS) patients admitted in 2020. Patients exposed to corticosteroids at admission were matched with patients without, through propensity score matching. Primary outcome was all-cause ICU mortality. Secondary outcomes were to compare in-hospital mortality, ventilator-free days at 28 days, respiratory superinfection, and length of stay between them. ICU mortality did not differ between patients treated with and without corticosteroids and untreated patients. In survival analysis, corticosteroid treatment at ICU admission was associated with short-term survival benefit (HR 0.53; 95% CI 0.39–0.72), although beyond the 17th day of admission, this effect switched and there was an increased ICU mortality (long-term HR 1.68; 95% CI 1.16– 2.45). The sensitivity analysis reinforced the results. Subgroups of age less of 60 years, severe ARDS and corticosteroids plus tocilizumab could have greatest benefit from corticosteroids. Short-term courses of corticosteroids decreased ICU mortality without long-term negative effects. Longer length of stay was observed with corticosteroids among non-survivors both in the ICU and in hospital. There were no significant differences for the remaining secondary outcomes [187]. So, it seems that long term treatment of corticosteroid in ICU does not give any benefit.
CAP requiring intensive care unit admission, as it was previously mentioned, is associated with significant acute and long-term morbidity and mortality. Some papers support it use [27,184], whereas others show lack of benefit [31,185,188,189]. Recently, hydrocortisone has shown utility in a randomized clinical trial, but it only showed benefit in patients with spontaneous ventilation, unknown microorganism, younger than 65 years, women, milder pneumonia, and patients with strong inflammatory reaction -C reactive protein more than 15 mg/dL- [27]. So, patient’s phenotype plays an important role to reach benefit from corticosteroid therapy in severe CAP [31,190].
Some meta-analyses have been performed to try to solve this question, but they lack enough validity due to risk of bias or because they included too old studies [191–195]. It seems that corticosteroid can aid to avoid death in patients in ICU with septic shock [196].
The controversy over the impact of corticosteroids on CAP still persists. The limitations of the studies and meta-analyses do not allow us to give a definitive answer. New machine learning techniques might resolve this controversy, which may allow evaluating the impact of corticosteroids according to different clinical phenotypes based on large real-life databases [197–199].
Use of vasoactive amines. Sepsis is an organic dysfunction caused by a dysregulated patient’s answer to infection and it can cause death. Sepsis and septic shock are important and prevalent health issues worldwide and they kill between one and three of each six affected patients. Sepsis caused death of 11 million people in 2017, which is 20% of total worldwide mortality. Early identification and proper management in first hours are key to improve outcomes. The main priority is to correct hypoperfusion [200,201].
Current guidelines recommend offering 30 mL/kg of intravenous crystalloid within the first three hours, with a low quality of evidence. They also recommend considering additional fluids which must be guided by frequent reassessment of hemodynamic status [201]. However, there is a risk of under or over-resuscitation in some patients, so alternative approaches have been proposed. To offer 10 mL/kg of intravenous crystalloid within first hour seems a safer method. After this, a revaluation of the patient must be done to assess signs of hypovolemia or congestion and to tailor therapy to those signs [202].
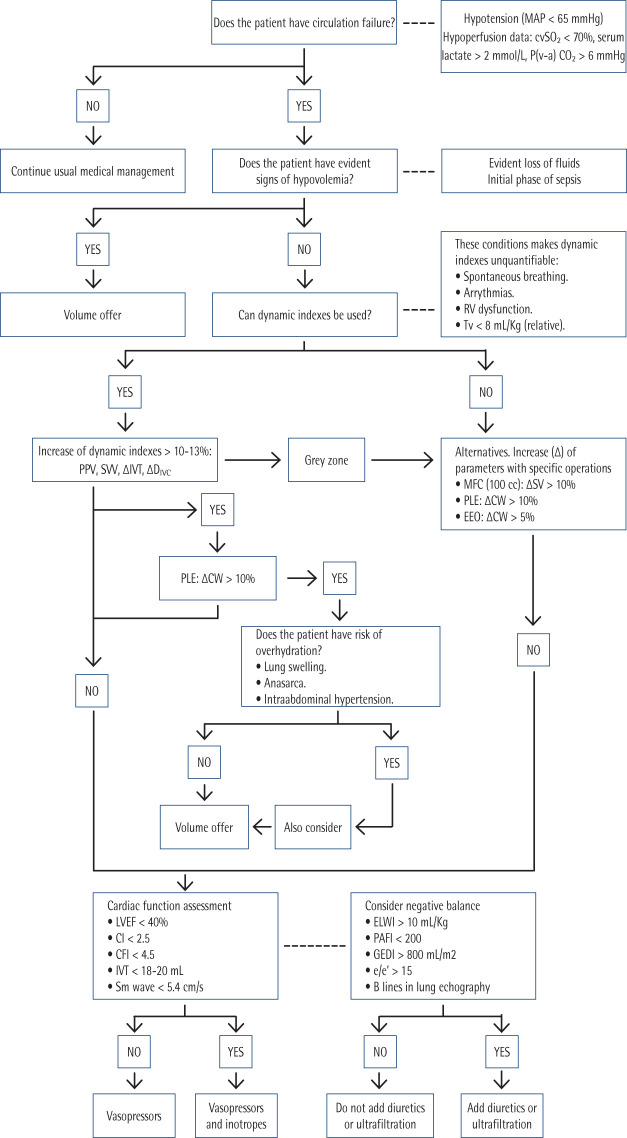
Objectives of hemodynamic reanimation are to assure an adequate perfusion pression and to correct hypoperfusion data [203], as can be seen in Figure 3. Mean arterial pressure (MAP) objective is 65 mmHg. Higher objectives, such as 85 mmHg, increase risk of atrial fibrillation, but in patients with previous chronic hypertension this objective can reduce rate of use of renal replacement therapies [204]. Early use of vasopressors is advised to achieve this personalized objective. Norepinephrine is the first choice because it potentiates the efficacy of volume expansion, and it is associated with lower mortality, shorter time to achieve target MAP and less volume of intravenous fluids [205,206]. Dose of norepinephrine must be tailored to patient’s response until doses between 0.25-0.5 µg/Kg/min. Norepinephrine perfusion can be delivered by a peripheric venous access, such as a vein in antecubital fossa with a wide catheter (number 18 or wider). This approach allows to begin with vasopressor administration earlier until a central venous access is secured [201,207]. Although norepinephrine is the preferred option to achieve MAP objective in septic shock, there are other drugs with different profiles of effects over adrenergic and other vascular wall and heart receptors [208]. There is strong evidence against use of dopamine in septic shock because it is associated with higher mortality and risk of arrhythmias [209].
Figure 3.
Algorithm of hemodynamic management.
MAP: mean arterial pressure, cvSO2: central venous oxygen saturation, P(v-a)CO2: partial pression veno-arterial of CO2, Tv: tidal volume, PPV: pulse pression variation, SVV: systolic volume variation, IVT: integral of velocity with respect to left ventricular outflow tract flow, DIVC: inferior vein cava diameter, MFC: mini-fluid challenge, PLE: passive leg elevation, EEO: end-expiratory occlusion, SV: systolic volume, CW: cardiac waste, LVEF: left ventricle ejection fraction, CI: cardiac index, CFI: cardiac function index, ELWI: extravascular lung water index, GEDI: global end-diastolic global volume index, PAFI: relationship between oxygen arterial partial pression and oxygen inspired fraction, e/e’: echocardiogram e/e’ index.
If objective MAP is not achieved despite an adequate fluid resuscitation and optimized norepinephrine perfusion, guidelines recommend adding vasopressin (0.01 – 0.03 U/min, fixed dose) instead of increasing norepinephrine doses. In case this combination is not enough, epinephrine must be considered. Also, corticosteroids can be employed if hypotension persists. Terlipressin must be avoided because it increases risk of peripheral and mesenteric ischemia. End of vasoactive drug perfusion may be considered if patient is stable and without hypoper-fusion signs for at least six hours, and catecholamines are the first drugs to be progressively withdrawn [201].
Patients with myocardial disfunction will also need inotropes. There are two choices: to use a combination of dobutamine and norepinephrine or epinephrine alone. Neither has demonstrate superiority over the other option, but the employment of dobutamine and norepinephrine allows to adjust each drug independently from the other and prevents potential lactic acidosis produced by epinephrine [201]. Levosimendan is associated with higher frequency of supraventricular tachyarrhythmias and lower rates of successful weaning from mechanical ventilation, so it must be avoided [210].
These facts are summarized in an algorithm (Figure 3). As can be seen, therapy must be tailored to patient’s characteristics and response to therapies. A personalized approach is key to get best results.
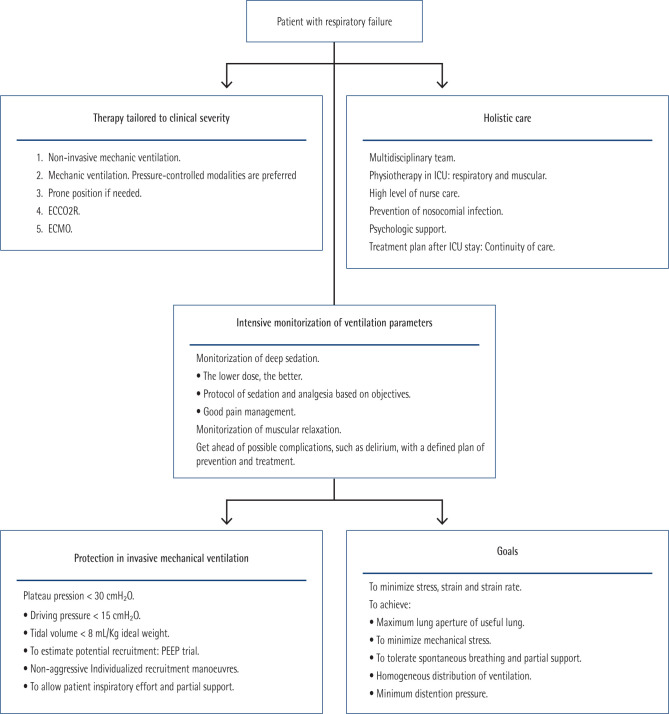
Ventilatory support. Severe pneumonia is a leading cause of acute respiratory distress syndrome -ARDS-. Classic definition of ARDS attends only to relationship between oxygen arterial partial pression and oxygen inspired fraction -PAFI- [211]. Emerging evidence in recent years, as well as the experience with the COVID-19 pandemic, have made evident the need for other parameters in addition to PAFI to adapt ventilatory support to patients with ARDS [212]. In fact, a great variability in mortality between different ICU services was observed during COVID-19 pandemic. These variations were attributed to heterogeneous organization and level of training, early use of respiratory support and prevention of secondary infections [213]. It also showed importance of longitudinal assessment of patients. Several phenotypes were recognized in patients with COVID-19, and an increase of dead space and mechanical power were associated with poorer prognosis [214]. Precision medicine, along with tailored therapies to single characteristics of each patient, has been recognized as an important requirement to improve outcomes in intensive care. To realise this, it is necessary an exhaustive monitoring and data-driven decision-making [215]. Current guidelines recognize importance of different phenotypes based on systemic inflammatory response, lung radiographic morphology, clinical features, and longitudinal changes in respiratory parameters; but they are not very flexible, and they lack enough detailed recommendations to tackle these longitudinal evolutionary changes [216]. The identification of these phenotypes may allow better outcomes. It is also important to recognize ARDS in illness that do not affect lungs primarily, because in these cases a delayed diagnosis is frequent [216,217]. Moreover, in non-invasive approaches, election of interface is very important. For example, in COVID-19 pandemic, continuous positive airway pressure -CPAP- was better than high flow nasal oxygen -HFNO- [218].
A protective strategy to perform invasive mechanical ventilation is key to prevent harm derived of medical procedures and to improve clinical results. Periodical evaluations are needed to tailor ventilatory parameters to patient’s evolution. Common mistakes to avoid are [219]: Breath-stacking or expiratory dysynchrony, excessive or insufficient ventilator assistance, excessive or insufficient sedation and excessive or insufficient PEEP (positive end-expiratory pressure).
Finally, considering these concepts, a unified approach is proposed to treat patients with ARDS (Figure 4).
Figure 4.
Unified approach to treat ARDS.
ECCO2R: extracorporeal carbon dioxide removal. ECMO: extracorporeal membrane oxygenation. PEEP: positive end-expiratory pressure.
Extracorporeal membrane oxygenation (ECMO). ECMO provides circulatory (venous and/or arterial) and/or respiratory support for a short period of time (days or weeks) in patients with cardiac or respiratory failure refractory to conventional treatments [220]. Clinicians can indicate ECMO in these settings when all other available treatments fail [220]: Pneumonia of any aetiology, aspiration syndromes, alveolar proteinosis, obstetric pathology, inhalation syndromes, airway obstruction, pulmonary contusion, bronchopleural fistula, bridge therapy, intraoperative respiratory support, asthmatic status, pulmonary haemorrhage or massive haemoptysis, hypercapnia (pH < 7.20) and/or PaCO2 > 80 mmHg, inability to maintain plateau pressure < 30 cmH2O, pulmonary vasculitis.
ECMO contraindications in ARDS are [220]: lung disease without predictable recovery of lung function if lung transplant is not indicated, contraindications for anticoagulation treatment, age > 65 years (more limited evidence). It is a relative contraindication, multiorgan failure with SOFA > 15 points, mechanical ventilation more than 7 days (special consideration with plateau pressure >30 cmH2O, impossibility of pressure>10 mmH2O, FiO2 > 0.9). It is a relative contraindication, severe pharmacological immunosuppression (neutrophils < 400/mm3), coma after cardiac arrest, comorbidities (active malignant disease, obesity, chronic heart disease, non-transplantable lung disease, cirrhosis with ascites, irreversible neurological disease), haemorrhagic or potentially haemorrhagic central nervous system lesions, impossible cannulation.
Patients with severe bacterial pneumonia can benefit from this technique [221,222] and it also has demonstrated utility in severe COVID-19 [223–225].
Although it is a live saving procedure for severe ill patients, it is associated with potential lethal complications such as catheter-related bacteraemia (14-44 ‰ catheter days), VAP (20-60‰ days of ventilation), catheter-related urinary tract infection (1-14‰ days of catheterization) and it also affects the pharmacokinetics and pharmacodynamics of some drugs (for example lipophilic drugs) [220,226–231]. In Spain, nosocomial infections were more frequent in patients with COVID-19 pneumonia [226]. Moreover, diagnosis of infectious complications is difficult due to frequent absence of fever, blood dyscrasias caused by technique and hypotension. Biomarkers, such as procalcitonin and lactate, are useful to recognize infectious complications [232].
Nowadays, ECMO could be consider as an essential technique that contributes to improving the patient’s condition with refractory SDRA for clinical recovery.
Approach to fungal pneumonia
Pneumonia by Pneumocystis jirovecii in patients without human immunodeficiency virus (HIV). P. jirovecii pneumonia (PJP) in patients without HIV infection is an important problem to clinicians nowadays. Its prevalence is rising because there is a growing number of vulnerable patients each year, diagnosis is usually delayed because of low grade of suspicion and, therefore, mortality is higher than in patients with HIV infection [233]. Risk factors for developing PJP are [119,234–237]:
Acute lymphoblastic leukaemia.
Allogenic stem cell transplant.
Solid organ transplant. In those patients, PJP usually develops in the first two months from transplant. Additional risk factors for those patients are age more than 65 years, CMV infection in the year preceding the transplant, immunosuppressive therapy containing tacrolimus and lymphopenia in the 50 days prior to transplant (<750 mm3).
Autologous stem cell transplant for underlying hemato-logic malignancy. • Chimeric antigen receptor-modified T-cell therapy.
Primary immunodeficiency: severe combined immunodeficiency, idiopathic CD4 T-lymphopenia, hyper IgM syndrome.
Patients receiving high-dose corticosteroid treatment (equivalent to 20 mg or more of prednisone daily for more than one month) who have additional cause of immuno-deficiency, such as malignancy or additional immunosuppressive medications.
Patients with COVID-19 and low count of lymphocytes, prior immunosuppression, more days of illness, high doses of corticosteroids or long courses of them.
Other immunosuppressive therapies, such as anti-CD20 monoclonal antibody, cytotoxic chemotherapy, mTOR inhibition, calcineurin inhibition, phosphatidylinositol 3-kinase inhibition, etc.
Previous episode of PJP.
Although there is not a complete consensus for all these groups of patients, they should take PJP chemoprophylaxis, usually with low dose of trimethoprim-sulfamethoxazole (e.g. 800/160 mg by mouth thrice a week) [119,234].
Patients with PJP usually suffer from sudden shortness of breath with respiratory failure, non-productive cough, and fever. They also have serum lactate-dehydrogenase (LDH) levels higher than patients with PCP and HIV. Chest X-ray can be initially normal or with interstitial infiltrates. Traditional microbiological methods for diagnosis are special staining of respiratory samples (toluidine blue, Giemsa, silver-methenamine) and immunofluorescence. This last one, made in BAL sample, is considered gold-standard test. Recently, molecular techniques such as PCR of respiratory specimens and detection of β-D-glucan in serum are available [233]. However, it is necessary to facilitate the diagnosis, so innovative approaches have been developed in last years:
Detection of P. jirovecii by molecular techniques (PCR) in oral wash samples in immunocompromised patients or nasopharyngeal swabs. Both methods have a high negative predictive value (NPV) near to 100%, but a lower positive predictive value -70-80%- [238,239].
PCR in respiratory samples is an extremely sensitive technique, so it is vulnerable to false positives. Cycle threshold (Ct) is useful to distinguish false from true positives. Lower Ct -less than 30- is associated with illness, whereas higher Ct -more than 35- is associated with colonization [240].
Detection of high serum concentration of β-D-glucan (>200 pg/mL with Fungitell®) is correlated with likelihood of illness in oncologic patients, and it has a high negative predictive value in patients with negative PCR [241].
There are several commercial kits to detect β-D-glucan in serum, with different thresholds. Serum levels associated with PCP are higher than the ones registered with candidemia [242].
This emerging knowledge can be summarized as [241,243–246]: Immunofluorescence performed in BAL remains gold-standard test. Immunofluorescence stains made in induced sputum is also accepted as diagnostic proof of PJP. In patients unable to endure a bronchoscopy, serum β-D-glucan has a high NPV that allows rule-out PJP in patients with low to moderate grade of suspicion. In fact, Bigot et al propose to do this test prior to bronchoscopy and to avoid it if test result is negative [245]. Patients with high grade of suspicion may need molecular techniques in respiratory samples. In patients with both negative tests, PCP is very unlikely. If both tests are positive, PCP is probable. Finally, in patients with serum positive β-D-glucan and negative PCR in respiratory sample, it must be reconsidered to perform a bronchoscopy and other fungal infections should be discarded.
These concepts are aligned with the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/ MSEGERC) consensus to define invasive fungal disease [247]. Host factors -immunosuppression-, clinical criteria -symptoms, signs, and radiological findings -, and microbiologic criteria must be fulfilled to achieve a diagnosis. According to the degree of certainty, two categories can be distinguished:
Proven PCP. Patient with clinical and radiological criteria, who also has a positive immunofluorescence staining in tissue or respiratory sample.
Probable PCP. Patient with predisposing host factors, clinical and radiological criteria who also has a positive molecular technique in respiratory specimens or positive detection of β-D-glucan. In this last scenario, clinicians should have a high grade of suspicion for another invasive fungal infection, because it could be a false positive.
As it has been shown, there is still some uncertainty to make a sure diagnosis of PCP. Novel approaches such as detecting circulating DNA of P. jirovecii in serum and machine learning tools have been demonstrated itself promising and their routine employment may be near [248,249].
Despite new diagnostic tools developed in last years, treatment of PCP has remained without changes and high dose of trimethoprim-sulfamethoxazole (15 mg/kg/d iv) continues being treatment of choice for most patients [119]. However, this high dose is often associated with negative adverse events which can be treatment limiting, so a clinical trial is nowadays active to compare standard dose with low dose (10 mg/kg/d) [250]. In patients not infected with HIV, the use of corticosteroids in moderate-severe pneumonia with hypoxemia remains controversial.
Invasive aspergillosis. The clinical presentation of aspergillosis lung disease is determined by the interaction between fungus (Aspergillus spp., with their virulence factors and/or resistance to antifungals) and their host (generally dependent on its immune status and previous structural bronchopulmonary local involvement). Invasive pulmonary aspergillosis (IPA) develops in severely immunocompromised patients, such as onco-haematology patients -especially those with neutropenia-, and its incidence is rising in the non-neutropenic, including lung transplant recipients, the critically ill patients and patients on corticosteroid treatment [251].
A high index of suspicion is required in patients without the typical risk factors (severe and prolonged neutropenia, HIV infection, high dose steroids, cirrhosis, alteration of T lymphocyte function, acute myeloid leukaemia, and allogeneic hematopoietic cell transplantation) as early presentation is usually silent and non-specific and pyrexia is uncommon. This high index of suspicion is very important because timely treatment is crucial for survival. Recently, acute viral infections have been associated with IPA leading to the concepts of influenza-associated IPA (IAPA) and COVID-19-associated IPA (CAPA) [251,252]. These viral infections may affect patients without underlying disease. Invasive aspergillosis has also been diagnosed in normal hosts after massive exposure to fungal spores (mainly A. fumigatus). Chronic pulmonary aspergillosis affects patients without obvious immune compromise, but with an underlying lung condition such as chronic obstructive pulmonary disease (COPD) or sarcoidosis, prior or concurrent tuberculous or non-tuberculous mycobacterial disease [252–254]. An invasive form of aspergillosis is seen in lung and heart transplant recipients that involves the trachea and bronchi, and in particular, the bronchial anasthosmosis.. Asymptomatic colonisation of the respiratory tract needs close monitoring as it can lead to clinical disease especially with future immunosuppression [253,254]. The halo sign is the radiological representation of lung infarction that follows angioinvasion by hyphae. The nodule represents the coagulation necrosis, and the halo is the oedema and haemorrhage that surround the zone of infarction. Although not specific, its presence in persistently febrile neutropenic patients must be interpreted as suggestive of an invasive disease. An important contribution to the management of IPA was made by studies showing the importance of the halo sign as the earliest detectable mark of disease [252].
Several tests are available for the diagnosis of IPA mainly in two types of clinical sample: BAL and serum. In both of them, specific PCR, lateral flow device, determination of β-1-3 D-glucan and galactomannan can be performed. In BAL samples the culture could be recommended to study susceptibility. [254].
Currently, there is not scientific evidence to support the superiority of one antifungal over another for the treatment of CAPA/IAPA. Therefore, it is recommended to follow the treatment indications in current national and international guidelines, considering the peculiarities of critical patients and, in particular, of patients with severe viral pneumonia due to influenza or COVID-19 [253,254]. It is recommended to include voriconazole, isavuconazole or liposomal amphotericin B as first-line drugs for the treatment of CAPA/IAPA patients. Surgery must be considered in case of great vessel affectation or massive haemoptysis. The antifungal treatment of CAPA/IAPA patients is recommended until diagnosis is confirmed [254]. New antifungal could be in the future therapeutic arsenal to treat Aspergillus spp. resistant isolates such as fosmanogepix, olorofim, opelconazol, ibrexafungerp and rezafungin [255,256].
CONCLUSIONS
Targeted treatment has always been a great challenge in planning clinical work algorithms, especially in sepsis with respiratory origin or HAP/VAP. Molecular biology techniques in microbiology could contribute to obtain quicker results to achieve therapeutic success. New documents that give answers to important questions about CAP, HAP, and VAP are available. Interdisciplinary teams generate knowledge that might improve clinical results. Beta-lactam allergy in patients with pneumonia is associated with worse outcomes due to therapeutical limitations. False label of beta-lactam allergy must be removed as soon as possible, and profile of hypersensitivity must be defined to allow use of beta-lactam antibiotics which lack of cross-reactions in a particular patient. Multidisciplinary projects such as Zero Pneumonia are useful to prevent VAP. Severe CAP is a main cause of mortality despite efforts done in last decades. A timely approach is essential to change this, and a tailored use of antibiotics may help to achieve better endings. Sequential therapy is strongly recommended to treat pneumonia, because it is a safe alternative and is associated with lower cost and length of stay than exclusive intravenous therapy. Several options are available. Alternatives currently available in MBL-producing P. aeruginosa isolates are: cefiderocol, aztreonam-avibactam, high doses of amikacin and synergistic combinations tailored to antibiotic in vitro test results, including fosfomycin, colistin or others, systemically and/or nebulized. New beta-lactam antibiotics are available to treat HAP and VAP, with better outcomes than older alternatives. A personalized approach must be employed to choose the best empiric treatment available, and clinicians must bear in mind specific patient profile, local epidemiology, and results of stains and molecular methods such as PCR. Nebulized antibiotics are not recommended for treatment, whereas they may be useful for prophylaxis of VAP. Ceftazidime-avibactam, meropenem-vaborbactam and cefiderocol are current alternatives in the treatment of HAP-VAP caused by non-MBL CRE, especially carbapenemases OXA-48 like and KPC types. A. baumannii complex and S. maltophilia are two opportunistic bacterial species that cause nosocomial infection (mainly HAP-VAP and bacteraemia). The IDSA and ESCMID recommendations could be of help to elaborate an individualized treatment in multi-resistant isolates. The controversy over the impact of corticosteroids on CAP still persists. It can be resolved by applying new machine learning techniques that could identify phenotypes that benefit from this treatment. Sepsis and septic shock are illness with a high associated prevalence and mortality. Early correction of hypoperfusion with intravenous fluids and promptly vasoactive therapy can enhance patient’s results. An adapted therapy to patients with severe pneumonia and respiratory failure might improve clinical results. A holistic approach and intensive monitorization allow get ahead of potential problems. ECMO provides circulatory and/or respiratory support for patients with refractory SDRA. It allows patients to heal when other methods are ineffective. In fungal world, IPA caused by Aspergillus spp remains a great challenge in the ICU for both its diagnosis and treatment, in part due to the difficulty of differentiating between colonization and infection. PCP in patients without HIV infection is an illness that affects immunosuppressed patients. Its diagnosis is difficult and requires a high grade of suspicion. Consequently, late diagnosis is often performed, and prognosis is poorer than in patients with HIV. New diagnostic approaches, such as PCR in respiratory samples and detection of β-D-glucan in serum, may allow an earlier diagnosis. High doses of trimethoprim -sulfamethoxazole remain best treatment available. Prevention is key, so selected patients with predisposing conditions, some belonging to new risk groups, should receive chemoprophylaxis.
ACKNOWLEDGEMENTS
Addenda. Collaborative authors and Medical societies in the 5th edition of Pneumonia Day: Angel Estella (Hospital U. de Jerez), GEIPC-SEIMC, GTEIS-SEMICYUC. Susana Sancho (Hospital U. i Politècnic La Fe. Valencia), GEIPC-SEIMC, GTEIS-SEMICYUC. Montserrat Rodríguez-Aguirregabiria (Hospital U. La Paz. Madrid), GEIPC-SEIMC, GTEIS-SEMICYUC. José Luis del Pozo (Clínica Universidad de Navarra. Pamplona), GEIPC-SEIMC. Almudena Burillo (Hospital General U. Gregorio Marañón. Madrid) GEIPC-SEIMC. Andrés Canut (Hospital U. de Araba. Vitoria), GEIPC-SEIMC. David Navarro (Hospital Clínico Universitario. Valencia), SEIMC. Mercedes Nieto (Hospital Clínico San Carlos. Madrid), GEIPC-SEIMC, GTEIS-SEMICYUC. Juan Manuel García-Lechuz (Hospital U. Miguel Servet. Zaragoza), GEIPC-SEIMC. Rafael Zaragoza (Hospital U. Doctor Peset. Valencia), GEIPC-SEIMC, GTEIS-SEMICYUC. Cruz Soriano (Hospital U. Ramón y Cajal. Madrid), GEIPC-SEIMC, GTEIS-SEMICYUC. Jesús Fortún (Hospital U. Ramón y Cajal. Madrid), SEIMC. José Barberán (Hospital U. HM Monteprincipe. Madrid). SEQ, SEIMC, SEMI. Pedro Rascado (Complejo Hospitalario Universitario de Santiago. A Coruña), GEIPC-SEIMC, GTEIS-SEMICYUC. David Andaluz (Complejo Asistencial Universitario de Palencia), GEIPC-SEIMC, GTEIS-SEMICYUC. Helena Barrasa (Hospital U. de Araba. Vitoria), GEIPC-SEIMC, GTEIS-SEMICYUC. Federico Gordo (Hospital Universitario del Henares. Madrid), GEIPC-SEIMC, GTEIS-SEMICYUC. Alejandro Rodríguez (Hospital U. Joan XXIII. Tarragona), GEIPC-SEIMC, GTEIS-SEMICYUC. José Ricardo Gimeno (Hospital U. i Politècnic La Fe. Valencia), GTEIS-SEMICYUC. Emilio Maseda (Hospital Quirón Sur. Madrid), GEIPC-SEIMC, SEDAR-GTIPO. Francisco López-Medrano (Hospital U. 12 de Octubre. Madrid), GESITRA-SEIMC. Ana Fernandez-Cruz (Hospital U. Puerta de Hierro. Majadahonda, Madrid), GEMICOMED-SEIMC.
FUNDING
None to declare
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Torres A, Cilloniz C, Niederman MS, Menéndez R, Chalmers JD, Wunderink RG, et al. Pneumonia. Nat Rev Dis Primers. 2021. Apr 8;7(1):25. [DOI] [PubMed] [Google Scholar]
- 2.Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Micro-biol Infect Dis [Internet]. 2017. Nov 10;36(11):1999–2006. Available from: http://link.springer.com/10.1007/s10096-016-2703-z [DOI] [PubMed] [Google Scholar]
- 3.Metersky ML, Wang Y, Klompas M, Eckenrode S, Bakullari A, Eldridge N. Trend in Ventilator-Associated Pneumonia Rates Between 2005 and 2013. JAMA. 2016. Dec 13;316(22):2427. [DOI] [PubMed] [Google Scholar]
- 4.Cillóniz C, Dominedò C, Torres A. An overview of guidelines for the management of hospital-acquired and ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria. Curr Opin Infect Dis. 2019. Dec;32(6):656–62. [DOI] [PubMed] [Google Scholar]
- 5.Dominedò C, Ceccato A, Niederman M, Cillóniz C, Gabarrús A, Martin-Loeches I, et al. Predictive Performance of Risk Factors for Multidrug-Resistant Pathogens in Nosocomial Pneumonia. Ann Am Thorac Soc. 2021. May;18(5):807–14. [DOI] [PubMed] [Google Scholar]
- 6.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017. Sep 10;50(3):1700582. [DOI] [PubMed] [Google Scholar]
- 7.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Executive Summary: Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016. Sep 1;63(5):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres A, Barberán J, Ceccato A, Martin-Loeches I, Ferrer M, Menéndez R, et al. Neumonía intrahospitalaria. Normativa de la Sociedad Española de Neumología y Cirugía Torácica (SEPAR). Actualización 2020. Arch Bronconeumol. 2020. Mar;56:11–9.34629620 [Google Scholar]
- 9.Erb CT, Patel B, Orr JE, Bice T, Richards JB, Metersky ML, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia. Ann Am Thorac Soc. 2016. Dec;13(12):2258–60. [DOI] [PubMed] [Google Scholar]
- 10.Bassetti M, Righi E, Vena A, Graziano E, Russo A, Peghin M. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug-resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr Opin Crit Care. 2018. Oct;24(5):385–93. [DOI] [PubMed] [Google Scholar]
- 11.Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia. Curr Opin Pulm Med. 2013. May;19(3):216–28. [DOI] [PubMed] [Google Scholar]
- 12.Vacas-Córdoba M, Cardozo-Espinola C, Puerta-Alcalde P, Cilloniz C, Torres A, García-Vidal C. Empirical treatment of adults with hospital-acquired pneumonia: lights and shadows of the 2016 Clinical Practice ATS/IDSA Guidelines. Rev Esp Quimioter. 2017. Sep;30 Suppl 1:30–3. [PubMed] [Google Scholar]
- 13.Vallés J, Martin-Loeches I, Torres A, Diaz E, Seijas I, López MJ, et al. Epidemiology, antibiotic therapy and clinical outcomes of health-care-associated pneumonia in critically ill patients: a Spanish cohort study. Intensive Care Med. 2014. Apr 18;40(4):572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GEIPC-SEIMC . V Jornada Anual de Neumonía [Internet]. 2023. [cited 2023 Dec 8]. Available from: https://jornada-anual-neumonia.com/
- 15.Kollef MH, Shorr AF, Bassetti M, Timsit JF, Micek ST, Michelson AP, et al. Timing of antibiotic therapy in the ICU. Crit Care. 2021;25(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey JD, Beskow LM, Brown J, Brown SM, Gayat É, Ng Gong M, et al. Use of pragmatic and explanatory trial designs in acute care research: lessons from COVID-19. Lancet Respir Med. 2022. Jul;10(7):700–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candel FJ, Salavert M, Basaras M, Borges M, Cantón R, Cercenado E, et al. Ten Issues for Updating in Community-Acquired Pneumonia: An Expert Review. J Clin Med. 2023. Oct 30;12(21):6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candel FJ, Salavert M, Estella A, Ferrer M, Ferrer R, Gamazo JJ, et al. Ten Issues to Update in Nosocomial or Hospital-Acquired Pneumonia: An Expert Review. J Clin Med. 2023. Oct 14;12(20):6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caméléna F, Péan de Ponfilly G, Pailhoriès H, Bonzon L, Alanio A, Poncin T, et al. Multicenter Evaluation of the FilmArray Blood Culture Identification 2 Panel for Pathogen Detection in Bloodstream Infections. Microbiol Spectr. 2023. Feb 14;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serigstad S, Markussen D, Grewal HMS, Ebbesen M, Kommedal Ø, Heggelund L, et al. Rapid syndromic PCR testing in patients with respiratory tract infections reduces time to results and improves microbial yield. Sci Rep. 2022. Jan 10;12(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadsby NJ, Musher DM. The Microbial Etiology of Community-Acquired Pneumonia in Adults: from Classical Bacteriology to Host Transcriptional Signatures. Clin Microbiol Rev. 2022. Dec 21;35(4):e0001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serigstad S, Markussen DL, Ritz C, Ebbesen MH, Knoop ST, Kom-medal Ø, et al. The changing spectrum of microbial aetiology of respiratory tract infections in hospitalized patients before and during the COVID-19 pandemic. BMC Infect Dis. 2022. Sep 30;22(1):763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentilotti E, De Nardo P, Cremonini E, Górska A, Mazzaferri F, Canziani LM, et al. Diagnostic accuracy of point-of-care tests in acute community-acquired lower respiratory tract infections. A systematic review and meta-analysis. Clin Microbiol Infect. 2022. Jan;28(1):13–22. [DOI] [PubMed] [Google Scholar]
- 24.Bellew S, Grijalva CG, Williams DJ, Anderson EJ, Wunderink RG, Zhu Y, et al. Pneumococcal and Legionella Urinary Antigen Tests in Community-acquired Pneumonia: Prospective Evaluation of Indications for Testing. Clin Infect Dis. 2019. May 30;68(12):2026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim P, Deshpande A, Rothberg MB. Urinary Antigen Testing for Respiratory Infections: Current Perspectives on Utility and Limitations. Infect Drug Resist. 2022. Apr;Volume 15:2219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wick KD, McAuley DF, Levitt JE, Beitler JR, Annane D, Riviello ED, et al. Promises and challenges of personalized medicine to guide ARDS therapy. Crit Care. 2021. Dec 23;25(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dequin PF, Meziani F, Quenot JP, Kamel T, Ricard JD, Badie J, et al. Hydrocortisone in Severe Community-Acquired Pneumonia. N Engl J Med. 2023. May 25;388(21):1931–41. [DOI] [PubMed] [Google Scholar]
- 28.Nedel WL. Corticosteroids for severe influenza pneumonia: A critical appraisal. World J Crit Care Med. 2016;5(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019. Oct 1;200(7):e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med . 2021. Feb 25;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno G, Ruiz-Botella M, Martín-Loeches I, Gómez Álvarez J, Jiménez Herrera M, Bodí M, et al. A differential therapeutic consideration for use of corticosteroids according to established COVID-19 clinical phenotypes in critically ill patients. Med Intensiva. 2023. Jan;47(1):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menéndez R, Cilloniz C, España PP, Almirall J, Uranga A, Méndez R, et al. Neumonía adquirida en la comunidad. Normativa de la Sociedad Española de Neumología y Cirugía Torácica (SEPAR). Actualización 2020. Arch Bronconeumol. 2020. Mar;56:1–10. [Google Scholar]
- 33.Capelastegui A, España Yandiola PP, Quintana JM, Bilbao A, Diez R, Pascual S, et al. Predictors of Short-term Rehospitalization Following Discharge of Patients Hospitalized With Community-Acquired Pneumonia. Chest. 2009. Oct;136(4):1079–85. [DOI] [PubMed] [Google Scholar]
- 34.Jang JG, Ahn JH. Reasons and Risk Factors for Readmission Following Hospitalization for Community-acquired Pneumonia in South Korea. Tuberc Respir Dis (Seoul). 2020;83(2):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mather JF, Fortunato GJ, Ash JL, Davis MJ, Kumar A. Prediction of Pneumonia 30-Day Readmissions: A Single-Center Attempt to Increase Model Performance. Respir Care. 2014. Feb;59(2):199–208. [DOI] [PubMed] [Google Scholar]
- 36.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and Timing of 30-Day Readmissions After Hospitalization for Heart Failure, Acute Myocardial Infarction, or Pneumonia. JAMA. 2013. Jan 23;309(4):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen DT, Huynh ST, Nguyen HN. Short-Term Readmission Following Community-Acquired Pneumonia: A Cross-Sectional Study. Hosp Pharm. 2022. Dec 25;57(6):712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halm EA, Fine MJ, Kapoor WN, Singer DE, Marrie TJ, Siu AL. Instability on Hospital Discharge and the Risk of Adverse Outcomes in Patients With Pneumonia. Arch Intern Med. 2002. Jun 10;162(11):1278. [DOI] [PubMed] [Google Scholar]
- 39.Fleuren LM, Klausch TLT, Zwager CL, Schoonmade LJ, Guo T, Roggeveen LF, et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020. Mar 21;46(3):383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quah J, Liew CJY, Zou L, Koh XH, Alsuwaigh R, Narayan V, et al. Chest radiograph-based artificial intelligence predictive model for mortality in community-acquired pneumonia. BMJ Open Respir Res. 2021. Aug 9;8(1):e001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akarapipad P, Bertelson E, Pessell A, Wang TH, Hsieh K. Emerging Multiplex Nucleic Acid Diagnostic Tests for Combating COVID-19. Biosensors (Basel). 2022. Nov 7;12(11):978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leber AL, Everhart K, Daly JA, Hopper A, Harrington A, Schreckenberger P, et al. Multicenter Evaluation of BioFire FilmArray Respiratory Panel 2 for Detection of Viruses and Bacteria in Nasopharyngeal Swab Samples. J Clin Microbiol. 2018. Jun;56(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SH, Ruan SY, Pan SC, Lee TF, Chien JY, Hsueh PR. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J Microbiol Immunol Infect. 2019. Dec;52(6):920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson J, Schechter-Perkins EM, Mitchell P, Mace S, Tian Y, Williams K, et al. Multi-center evaluation of the cobas ® Liat ® Influenza A/B & RSV assay for rapid point of care diagnosis. J Clin Virol. 2017. Oct;95:5–9. [DOI] [PubMed] [Google Scholar]
- 45.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019. Oct 1;200(7):e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Executive Summary: Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016. Sep 1;63(5):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017. Sep 10;50(3):1700582. [DOI] [PubMed] [Google Scholar]
- 48.Estella A. [Analysis of 208 flexible bronchoscopies performed in an intensive care unit]. Med Intensiva. 2012;36(6):396–401. [DOI] [PubMed] [Google Scholar]
- 49.Fujitani S, Yu VL. Diagnosis of Ventilator-Associated Pneumonia: Focus on Nonbronchoscopic Techniques (Nonbronchoscopic Bronchoalveolar Lavage, Including Mini-BAL, Blinded Protected Specimen Brush, and Blinded Bronchial Sampling) and Endotracheal Aspirates. J Intensive Care Med. 2006. Jan 30;21(1):17–21. [DOI] [PubMed] [Google Scholar]
- 50.Morris AC, Kefala K, Simpson AJ, Wilkinson TS, Everingham K, Kers-lake D, et al. Evaluation of the effect of diagnostic methodology on the reported incidence of ventilator-associated pneumonia. Thorax. 2009. Jun 1;64(6):516–22. [DOI] [PubMed] [Google Scholar]
- 51.Flanagan PG, Findlay GP, Magee JT, Ionescu A, Barnes RA, Smithies M. The diagnosis of ventilator-associated pneumonia using non-bronchoscopic, non-directed lung lavages. Intensive Care Med. 2000. Jan;26(1):20–30. [DOI] [PubMed] [Google Scholar]
- 52.Zaragoza R, Vidal-Cortés P, Aguilar G, Borges M, Diaz E, Ferrer R, et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit Care. 2020. Jun 29;24(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.González Ramallo VJ, Mirón Rubio M, Estrada Cuxart O, García Leoni ME. Usefulness of Hospital at Home in nosocomial infections: advantages and limitations. Rev Esp Quimioter. 2017. Sep;30 Suppl 1:61–5. [PubMed] [Google Scholar]
- 54.Mujal A, Sola J, Hernandez M, Villarino MA, Machado ML, Baylina M, et al. Safety and effectiveness of home intravenous antibiotic therapy for multidrug-resistant bacterial infections. Eur J Clin Microbiol Infect Dis. 2015. Jun 6;34(6):1125–33. [DOI] [PubMed] [Google Scholar]
- 55.Esteban-Cartelle B, Vicente-Oliveros N, Pérez Menéndez-Conde C, Serrano DR, Martín-Dávila P, Fortún-Abete J, et al. Antibiotic stability in portable elastomeric infusion devices: A systematic review. Am J Health Syst Pharm. 2022. Aug 5;79(16):1355–68. [DOI] [PubMed] [Google Scholar]
- 56.Paño-Pardo JR, Moreno Rodilla E, Cobo Sacristan S, Cubero Saldaña JL, Periañez Párraga L, del Pozo JL, et al. Management of patients with suspected or confirmed antibiotic allergy. Executive summary of guidance from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Society of Allergy and Clinical Immunology (SEAIC), the Spanish Society of Hospital Pharmacy (SEFH) and the Spanish Society of Intensive Medicine and Coronary Care Units (SEMICYUC). Enferm Infecc Microbiol Clin. 2023. Mar 41(3):181–6. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz Román C, Vilá Indurain B. Reacciones adversas a medicamentos: alergia a antibióticos, AINE, otros [Internet]. 2021. [cited 2023 Dec 9]. Available from: https://www.aeped.es/sites/default/files/documentos/21_ra_medicamentos_criterios-correg_21012020.pdf
- 58.Agencia Española del Medicamento y Productos Sanitarios . Informe sobre sospechas de reacciones adversas notificadas a medicamentos de uso humano o acontecimientos adversos ocurridos después de la vacunación [Internet]. 2023. [cited 2023 Dec 9]. Available from: https://www.aemps.gob.es/medicamentos-de-uso-humano/farmacovigilancia-de-medicamentos-de-uso-humano/informacion-de-sospechas-de-reacciones-adversas-a-medicamentos-de-uso-humano/informacion/
- 59.Strazzulla A, Postorino MC, Belfeki N, Iordache L, de Pontfarcy A, Pitsch A, et al. Risk of Multidrug Resistant Bacteria Acquisition in Patients with Declared β-Lactam Allergy during Hospitalization in Intensive Care Unit: A Retrospective Cohort Study (2007-2018). J Immunol Res. 2022;2022:8906316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saunders H, Shrestha R, Khadka S, Helgeson SA. Patient-reported penicillin allergy and intensive care unit outcomes in sepsis. J Allergy Clin Immunol Pract. 2023. Oct; [DOI] [PubMed] [Google Scholar]
- 61.Agencia Española del Medicamento y Productos Sanitarios . Quinolonas y fluoroquinolonas de administración sistémica: nuevas restricciones de uso [Internet]. 2018. [cited 2023 Dec 9]. Available from: https://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2018/docs/NI_MUH_FV-14-2018-quinolonas-fluoroquinolonas.pdf
- 62.Doña I, Blanca-López N, Torres MJ, García-Campos J, García-Núñez I, Gómez F, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22(5):363–71. [PubMed] [Google Scholar]
- 63.Moreno E, Laffond E, Muñoz-Bellido F, Gracia MT, Macías E, Moreno A, et al. Performance in real life of the European Network on Drug Allergy algorithm in immediate reactions to beta-lactam antibiotics. Allergy. 2016. Dec;71(12):1787–90. [DOI] [PubMed] [Google Scholar]
- 64.Iuliano S, Senn L, Moi L, Muller YD, Ribi C, Buss G, et al. Management of Beta-Lactam Antibiotics Allergy: A Real-Life Study. Front Allergy. 2022;3:853587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girard JP. Common antigenic determinants of penicillin G, ampicillin and the cephalosporins demonstrated in men. Int Arch Allergy Appl Immunol. 1968;33(5):428–38. [DOI] [PubMed] [Google Scholar]
- 66.Assem ES, Vickers MR. Tests for penicillin allergy in man. II. The immunological cross-reaction between penicillins and cephalospor-ins. Immunology. 1974. Aug 27(2):255–69. [PMC free article] [PubMed] [Google Scholar]
- 67.Suárez C, Gudiol F. Antibióticos betalactámicos. Enferm Infecc Microbiol Clin. 2009. Feb;27(2):116–29. [DOI] [PubMed] [Google Scholar]
- 68.Pichichero ME, Pichichero DM. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. J Pediatr. 1998. Jan;132(1):137–43. [DOI] [PubMed] [Google Scholar]
- 69.Novalbos A, Sastre J, Cuesta J, De Las Heras M, Lluch-Bernal M, Bombín C, et al. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001. Mar;31(3):438–43. [DOI] [PubMed] [Google Scholar]
- 70.Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010. Nov;126(5):994–9. [DOI] [PubMed] [Google Scholar]
- 71.Romano A, Guéant-Rodriguez RM, Viola M, Pettinato R, Guéant JL. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med. 2004. Jul 6;141(1):16–22. [DOI] [PubMed] [Google Scholar]
- 72.Frumin J, Gallagher JC. Allergic Cross-Sensitivity Between Penicillin, Carbapenem, and Monobactam Antibiotics: What are the Chances? Ann Pharmacother. 2009. Feb 3;43(2):304–15. [DOI] [PubMed] [Google Scholar]
- 73.Terico AT, Gallagher JC. Beta-Lactam Hypersensitivity and Cross-Re-activity. J Pharm Pract. 2014. Dec 14;27(6):530–44. [DOI] [PubMed] [Google Scholar]
- 74.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016. May 15;62(10):e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trubiano JA, Vogrin S, Chua KYL, Bourke J, Yun J, Douglas A, et al. Development and Validation of a Penicillin Allergy Clinical Decision Rule. JAMA Intern Med. 2020. May 1;180(5):745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Collins CD, Bookal RS, Malani AN, Leo HL, Shankar T, Scheidel C, et al. Antibiotic Use in Patients With β-Lactam Allergies and Pneumonia: Impact of an Antibiotic Side Chain–Based Cross-Reactivity Chart Combined With Enhanced Allergy Assessment. Open Forum Infect Dis. 2022. Jan 1;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brockow K, Wurpts G, Trautmann A. Patients with questionable penicillin (beta-lactam) allergy: Causes and solutions. Allergol Select. 2022. Jan 1;6(01):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKinnell JA, Dwyer JP, Talbot GH, Connolly LE, Friedland I, Smith A, et al. Plazomicin for Infections Caused by Carbapenem-Resistant Enterobacteriaceae. N Engl J Med. 2019. Feb 21;380(8):791–3. [DOI] [PubMed] [Google Scholar]
- 79.Barberán J, Mensa J, Fariñas C, Llinares P, Olaechea P, Palomar M, et al. [Recommendations of antimicrobial treatment in patients allergic to beta-lactam antibiotics]. Rev Esp Quimioter. 2008. Mar;21(1):60–82. [PubMed] [Google Scholar]
- 80.Lutgring JD, Balbuena R, Reese N, Gilbert SE, Ansari U, Bhatnagar A, et al. Antibiotic Susceptibility of NDM-Producing Enterobacterales Collected in the United States in 2017 and 2018. Antimicrob Agents Chemother. 2020. Aug 20;64(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.SEMICYUC . Proyecto Neumonia Zero [Internet]. 2011. [cited 2024 Jan 11]. Available from: https://semicyuc.org/proyecto-neumonia-zero/
- 82.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012. Dec 15;380(9859):2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rijkers GT, Pelton SI. The old man’s friend. Pneumonia. 2018. Dec 25;10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.File TM, Ramirez JA. Community-Acquired Pneumonia. N Engl J Med. 2023. Aug 17;389(7):632–41. [DOI] [PubMed] [Google Scholar]
- 85.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996. Jan 10;275(2):134–41. [PubMed] [Google Scholar]
- 86.Metersky ML, Waterer G, Nsa W, Bratzler DW. Predictors of In-Hospital vs Postdischarge Mortality in Pneumonia. Chest. 2012. Aug;142(2):476–81. [DOI] [PubMed] [Google Scholar]
- 87.Walden AP, Clarke GM, McKechnie S, Hutton P, Gordon AC, Rello J, et al. Patients with community acquired pneumonia admitted to European intensive care units: an epidemiological survey of the GenOSept cohort. Crit Care. 2014. Apr 1;18(2):R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cilloniz C, Ferrer M, Liapikou A, Garcia-Vidal C, Gabarrus A, Ceccato A, et al. Acute respiratory distress syndrome in mechanically ventilated patients with community-acquired pneumonia. Eur Resp J. 2018. Mar;51(3):1702215. [DOI] [PubMed] [Google Scholar]
- 89.Kolditz M, Ewig S, Klapdor B, Schütte H, Winning J, Rupp J, et al. Community-acquired pneumonia as medical emergency: predictors of early deterioration. Thorax. 2015. Jun;70(6):551–8. [DOI] [PubMed] [Google Scholar]
- 90.Kontou P, Kuti JL, Nicolau DP. Validation of the Infectious Diseases Society of America/American Thoracic Society criteria to predict severe community-acquired pneumonia caused by Streptococcus pneumoniae. Am J Emerg Med. 2009. Oct;27(8):968–74. [DOI] [PubMed] [Google Scholar]
- 91.Prina E, Ranzani OT, Polverino E, Cillóniz C, Ferrer M, Fernandez L, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015. Feb;12(2):153–60. [DOI] [PubMed] [Google Scholar]
- 92.Musher DM, Abers MS, Bartlett JG. Evolving Understanding of the Causes of Pneumonia in Adults, With Special Attention to the Role of Pneumococcus. Clin Infect Dis. 2017. Oct 30;65(10):1736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tleyjeh IM, Tlaygeh HM, Hejal R, Montori VM, Baddour LM. The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2006. Mar 15;42(6):788–97. [DOI] [PubMed] [Google Scholar]
- 94.Sader HS, Mendes RE, Le J, Denys G, Flamm RK, Jones RN. Antimicrobial Susceptibility of Streptococcus pneumoniae from North America, Europe, Latin America, and the Asia-Pacific Region: Results From 20 Years of the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis. 2019. Mar 15;6(Supple-ment_1):S14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007. Mar 1;44(Supplement_2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin-Loeches I, Torres A, Nagavci B, Aliberti S, Antonelli M, Bassetti M, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 2023. Jun 4;49(6):615–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eljaaly K, Alshehri S, Aljabri A, Abraham I, Al Mohajer M, Kalil AC, et al. Clinical failure with and without empiric atypical bacteria coverage in hospitalized adults with community-acquired pneumonia: a systematic review and meta-analysis. BMC Infect Dis. 2017. Dec 2;17(1):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu M, Yang X, Tian J, Fan H, Zhang Y. Antibiotic Treatment of Pulmonary Infections: An Umbrella Review and Evidence Map. Front Pharmacol. 2021. Oct 19;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006. Jun;34(6):1589–96. [DOI] [PubMed] [Google Scholar]
- 100.Scheeren TWL, Welte T, Saulay M, Engelhardt M, Santerre-Henriksen A, Hamed K. Early improvement in severely ill patients with pneumonia treated with ceftobiprole: a retrospective analysis of two major trials. BMC Infect Dis. 2019. Dec 26;19(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eckburg PB, Friedland HD, Llorens L, Smith A, Witherell GW, Laudano JB, et al. Day 4 Clinical Response of Ceftaroline Fosamil Versus Ceftriaxone for Community-Acquired Bacterial Pneumonia. Infect Dis Clin Pract (Baltim Md). 2012. Jul;20(4):254–60. [Google Scholar]
- 102.Taboada M, Melnick D, Iaconis JP, Sun F, Zhong NS, File TM, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016. Apr;71(4):862–70. [DOI] [PubMed] [Google Scholar]
- 103.Cilloniz C, Mendez R, Peroni H, Garcia-Vidal C, Rico V, Gabarrus A, et al. Impact on in-hospital mortality of ceftaroline versus standard of care in community-acquired pneumonia: a propensity-matched analysis. Eur J Clin Microbiol Infect Dis. 2022. Feb 12;41(2):271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodríguez AH, Avilés-Jurado FX, Díaz E, Schuetz P, Trefler SI, Solé-Violán J, et al. Procalcitonin (PCT) levels for ruling-out bacterial coinfection in ICU patients with influenza: A CHAID decision-tree analysis. J Infect. 2016. Feb;72(2):143–51. [DOI] [PubMed] [Google Scholar]
- 105.Dyar OJ, Huttner B, Schouten J, Pulcini C, ESGAP (ESCMID Study Group for Antimicrobial stewardshiP) . What is antimicrobial stew-ardship? Clin Microbiol Infect. 2017. Nov;23(11):793–8. [DOI] [PubMed] [Google Scholar]
- 106.Goff DA, Bauer KA, Reed EE, Stevenson KB, Taylor JJ, West JE. Is the ‘low-hanging fruit’ worth picking for antimicrobial stewardship programs? Clin Infect Dis. 2012. Aug;55(4):587–92. [DOI] [PubMed] [Google Scholar]
- 107.Viasus D, Vecino-Moreno M, De La Hoz JM, Carratalà J. Antibiotic stewardship in community-acquired pneumonia. Expert Rev Anti Infect Ther. 2017. Apr;15(4):351–9. [DOI] [PubMed] [Google Scholar]
- 108.Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014. Apr;5(2):83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.AEMPS . Ficha técnica de cefditoren [Internet]. 2020. [cited 2023 Dec 22]. Available from: https://cima.aemps.es/cima/dochtml/ft/65975/FT_65975.html#5.2
- 110.Aliberti S, Zanaboni AM, Wiemken T, Nahas A, Uppatla S, Morlacchi LC, et al. Criteria for clinical stability in hospitalised patients with community-acquired pneumonia. Eur Respir J. 2013. Sep;42(3):742–9. [DOI] [PubMed] [Google Scholar]
- 111.Kimura T, Ito M, Onozawa S. Switching from intravenous to oral antibiotics in hospitalized patients with community-acquired pneumonia: A real-world analysis 2010-2018. J Infect Chemother. 2020. Jul;26(7):706–14. [DOI] [PubMed] [Google Scholar]
- 112.Athanassa Z, Makris G, Dimopoulos G, Falagas ME. Early switch to oral treatment in patients with moderate to severe community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(17):2469–81. [DOI] [PubMed] [Google Scholar]
- 113.Deshpande A, Klompas M, Guo N, Imrey PB, Pallotta AM, Higgins T, et al. Intravenous to Oral Antibiotic Switch Therapy Among Patients Hospitalized With Community-Acquired Pneumonia. Clin Infect Dis. 2023. Jul 26;77(2):174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Engel MF, Postma DF, Hulscher MEJL, Teding van Berkhout F, Emmelot-Vonk MH, Sankatsing S, et al. Barriers to an early switch from intravenous to oral antibiotic therapy in hospitalised patients with CAP. Eur Respir J. 2013. Jan;41(1):123–30. [DOI] [PubMed] [Google Scholar]
- 115.Menendez R, Montull B, Mendez R. Antibiotic choice, route and duration: minimising the harm associated with antibiotics. Eur Respir J. 2014. Mar;155–67. [Google Scholar]
- 116.Barberan J, Pueyo J. Cefditoren en las infecciones de vías respiratorias bajas adquiridas en la comunidad. Rev Esp Quimioter. 2009. Jan 1;22(3):144–50. [PubMed] [Google Scholar]
- 117.Sempere J, González-Camacho F, Domenech M, Llamosí M, Del Río I, López-Ruiz B, et al. A national longitudinal study evaluating the activity of cefditoren and other antibiotics against non-susceptible Streptococcus pneumoniae strains during the period 2004–20 in Spain. J Antimicrob Chemother. 2022. Mar 31;77(4):1045–51. [DOI] [PubMed] [Google Scholar]
- 118.Cantón R, Barberán J, Linares M, Molero JM, Rodríguez-GonzálezMoro JM, Salavert M, et al. Decalogue for the selection of oral antibiotics for lower respiratory tract infections. Rev Esp Quimioter. 2022. Jan 20;35(1):16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soriano Viladomiu A, Mensa Pueyo J, López Suñé E, Zboromyrska Y, Llinares Mondejar P, Barberán López J. Guía de Terapéutica antimicrobiana: 2023. 33rd ed. Barcelona: Antares; 2023. [Google Scholar]
- 120.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020. May 10;46(5):888–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grupo de Trabajo de Enfermedades Infecciosas y Sepsis de la SEMICYUC (Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias) . Estudio Nacional de Vigilancia de Infección Nosocomial en Servicios de Medicina Intensiva. Informe 2022. [Internet]. 2022 [cited 2023 Dec 15]. Available from: https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202022.pdf
- 122.Kadri SS, Lai YL, Warner S, Strich JR, Babiker A, Ricotta EE, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021. Feb;21(2):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bonine NG, Berger A, Altincatal A, Wang R, Bhagnani T, Gillard P, et al. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status From Serious Gram-negative Bacterial Infections. Am J Med Sci. 2019. Feb;357(2):103–10. [DOI] [PubMed] [Google Scholar]
- 124.Martin-Loeches I, Rodriguez AH, Torres A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr Opin Crit Care. 2018. Oct 24(5):347–52. [DOI] [PubMed] [Google Scholar]
- 125.Mazzeffi M, Gammie J, Taylor B, Cardillo S, Haldane-Lutterodt N, Amoroso A, et al. Healthcare-Associated Infections in Cardiac Surgery Patients With Prolonged Intensive Care Unit Stay. Ann Thorac Surg. 2017. Apr;103(4):1165–70. [DOI] [PubMed] [Google Scholar]
- 126.van Vught LA, Klein Klouwenberg PMC, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA. 2016. Apr 12;315(14):1469–79. [DOI] [PubMed] [Google Scholar]
- 127.Timsit JF, Bassetti M, Cremer O, Daikos G, de Waele J, Kallil A, et al. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med. 2019. Feb;45(2):172–89. [DOI] [PubMed] [Google Scholar]
- 128.Detsis M, Karanika S, Mylonakis E. ICU Acquisition Rate, Risk Factors, and Clinical Significance of Digestive Tract Colonization With Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae: A Systematic Review and Meta-Analysis. Crit Care Med. 2017. Apr;45(4):705–14. [DOI] [PubMed] [Google Scholar]
- 129.Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012. Dec;38(12):1930–45. [DOI] [PubMed] [Google Scholar]
- 130.Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2018;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu WM, Yuan Z, Zhou HY. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020. Jan 31;9(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brink AJ. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019. Dec;32(6):609–16. [DOI] [PubMed] [Google Scholar]
- 133.Brink AJ, Richards GA. The role of multidrug and extensive-drug resistant Gam-negative bacteria in skin and soft tissue infections. Curr Opin Infect Dis. 2020. Apr;33(2):93–100. [DOI] [PubMed] [Google Scholar]
- 134.Averbuch D, Tridello G, Hoek J, Mikulska M, Akan H, Yanez San Segundo L, et al. Antimicrobial Resistance in Gram-Negative Rods Causing Bacteremia in Hematopoietic Stem Cell Transplant Recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin Infect Dis. 2017. Nov 13;65(11):1819–28. [DOI] [PubMed] [Google Scholar]
- 135.Parente DM, Cunha CB, Mylonakis E, Timbrook TT. The Clinical Utility of Methicillin-Resistant Staphylococcus aureus (MRSA) Nasal Screening to Rule Out MRSA Pneumonia: A Diagnostic Meta-analysis With Antimicrobial Stewardship Implications. Clin Infect Dis. 2018. Jun 18;67(1):1–7. [DOI] [PubMed] [Google Scholar]
- 136.Hoang S, Georget A, Asselineau J, Venier AG, Leroyer C, Rogues AM, et al. Risk factors for colonization and infection by Pseudomonas aeruginosa in patients hospitalized in intensive care units in France. PLoS One. 2018;13(3):e0193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Falcone M, Bassetti M, Tiseo G, Giordano C, Nencini E, Russo A, et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care. 2020. Jan 30;24(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garnacho-Montero J, Amaya-Villar R. The problem of multi-resistance in gram-negative bacilli in intensive care units: Treatment and prevention strategies. Med Intensiva. 2022. Jun;46(6):326–35. [DOI] [PubMed] [Google Scholar]
- 139.Gracia-Ahufinger I, López-González L, Vasallo FJ, Galar A, Siller M, Pitart C, et al. The CARBA-MAP study: national mapping of carbapenemases in Spain (2014–2018). Front Microbiol. 2023. Sep 8;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sastre-Femenia MÀ, Fernández-Muñoz A, Gomis-Font MA, Taltavull B, López-Causapé C, Arca-Suárez J, et al. Pseudomonas aeruginosa antibiotic susceptibility profiles, genomic epidemiology and resistance mechanisms: a nation-wide five-year time lapse analysis. Lancet Reg Health Eur. 2023. Nov;34:100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of Exposure to Antipseudomonal β-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Pharmacotherapy. 2019. Mar;39(3):261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Burillo A, Marín M, Cercenado E, Ruiz-Carrascoso G, Pérez-Granda MJ, Oteo J, et al. Evaluation of the Xpert Carba-R (Cepheid) Assay Using Contrived Bronchial Specimens from Patients with Suspicion of Ventilator-Associated Pneumonia for the Detection of Prevalent Carbapenemases. PLoS One. 2016. Dec 16;11(12):e0168473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wheatley RM, Botelho J. Chasing resistance: analyzing the fight against hospital infections. Lancet Reg Health Eur. 2023. Nov;34:100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pogue JM, Kaye KS, Veve MP, Patel TS, Gerlach AT, Davis SL, et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2020. Jul 11;71(2):304–10. [DOI] [PubMed] [Google Scholar]
- 145.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, et al. Effect and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect Dis Ther. 2018. Dec;7(4):439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bassetti M, Giacobbe DR, Patel N, Tillotson G, Massey J. Efficacy and Safety of Meropenem-Vaborbactam Versus Best Available Therapy for the Treatment of Carbapenem-Resistant Enterobacteriaceae Infections in Patients Without Prior Antimicrobial Failure: A Post Hoc Analysis. Adv Ther. 2019. Jul;36(7):1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin Infect Dis. 2018. Jan 6;66(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Castón JJ, Cano A, Pérez-Camacho I, Aguado JM, Carratalá J, Ramasco F, et al. Impact of ceftazidime/avibactam versus best available therapy on mortality from infections caused by carbapenemase-producing Enterobacterales (CAVICOR study). J Antimicrob Chemother. 2022. Apr 27;77(5):1452–60. [DOI] [PubMed] [Google Scholar]
- 149.Falcone M, Daikos GL, Tiseo G, Bassoulis D, Giordano C, Galfo V, et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients With Bloodstream Infections Caused by Metalloβ-lactamase-Producing Enterobacterales. Clin Infect Dis. 2021. Jun 1;72(11):1871–8. [DOI] [PubMed] [Google Scholar]
- 150.Kollef MH, Nová ček M, Kivistik Ü, Réa-Neto Á, Shime N, Martin-Loeches I, et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2019. Dec;19(12):1299–311. [DOI] [PubMed] [Google Scholar]
- 151.Doi Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin Infect Dis. 2019. Nov 13;69(Suppl 7):S565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs. 2019. Feb;79(3):271–89. [DOI] [PubMed] [Google Scholar]
- 153.Mensa J, Barberán J, Soriano A, Llinares P, Marco F, Cantón R, et al. Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev Esp Quimioter. 2018. Feb;31(1):78–100. [PMC free article] [PubMed] [Google Scholar]
- 154.Bouglé A, Tuffet S, Federici L, Leone M, Monsel A, Dessalle T, et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia in adults: a randomized, controlled, open-label trial. Intensive Care Med. 2022. Jul;48(7):841–9. [DOI] [PubMed] [Google Scholar]
- 155.Dhanani JA, Diab S, Chaudhary J, Cohen J, Parker SL, Wallis SC, et al. Lung Pharmacokinetics of Tobramycin by Intravenous and Nebulized Dosing in a Mechanically Ventilated Healthy Ovine Model. Anesthesiology. 2019. Aug;131(2):344–55. [DOI] [PubMed] [Google Scholar]
- 156.Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette CH, Rouby JJ. Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med. 2002. Nov 15;166(10):1375–81. [DOI] [PubMed] [Google Scholar]
- 157.Ferrari F, Lu Q, Girardi C, Petitjean O, Marquette CH, Wallet F, et al. Nebulized ceftazidime in experimental pneumonia caused by partially resistant Pseudomonas aeruginosa. Intensive Care Med. 2009. Oct;35(10):1792–800. [DOI] [PubMed] [Google Scholar]
- 158.Li Bassi G, Motos A, Fernandez-Barat L, Aguilera Xiol E, Chiurazzi C, Senussi T, et al. Nebulized Amikacin and Fosfomycin for Severe Pseudomonas aeruginosa Pneumonia: An Experimental Study. Crit Care Med. 2019. Jun;47(6):e470–7. [DOI] [PubMed] [Google Scholar]
- 159.Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010. Jul;55(7):845–51. [PubMed] [Google Scholar]
- 160.Rello J, Solé-Lleonart C, Rouby JJ, Chastre J, Blot S, Poulakou G, et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect. 2017. Sep;23(9):629–39. [DOI] [PubMed] [Google Scholar]
- 161.Niederman MS, Alder J, Bassetti M, Boateng F, Cao B, Corkery K, et al. Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with Gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial. Lancet Infect Dis. 2020. Mar;20(3):330–40. [DOI] [PubMed] [Google Scholar]
- 162.Ehrmann S, Barbier F, Demiselle J, Quenot JP, Herbrecht JE, Roux D, et al. Inhaled Amikacin to Prevent Ventilator-Associated Pneumonia. N Engl J Med. 2023. Nov 30;389(22):2052–62. [DOI] [PubMed] [Google Scholar]
- 163.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis. 2023; [DOI] [PubMed]
- 164.AEMPS . Ficha técnica de Ceftazidima-Avibactam [Internet]. 2021. [cited 2024 Jan 11]. Available from: https://cima.aemps.es/cima/dochtml/ft/1161109001/FT_1161109001.html
- 165.Tsolaki V, Mantzarlis K, Mpakalis A, Malli E, Tsimpoukas F, Tsirogianni A, et al. Ceftazidime-Avibactam To Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically Ill Mechanically Ventilated Patients. Antimicrob Agents Chemother. 2020;64(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. Emergence of Ceftazidime-Avibactam Resistance and Restoration of Carbapenem Susceptibility in Klebsiella pneumoniae Carbapenemase-Producing K pneumoniae: A Case Report and Review of Literature. Open Forum Infect Dis. 2017;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.AEMPS (Agencia Española del Medicamento y Productos Sanitarios) . Ficha tecnica Meropenem-Vaborbactam AEMPS-CIMA [Internet]. 2023. [cited 2024 Feb 16]. Available from: https://cima.aemps.es/cima/dochtml/ft/1181334001/FT_1181334001.html
- 168.Sader HS, Huband MD, Castanheira M, Flamm RK. Pseudomonas aeruginosa Antimicrobial Susceptibility Results from Four Years (2012 to 2015) of the International Network for Optimal Resistance Monitoring Program in the United States. Antimicrob Agents Chemother. 2017;61(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang C, Yang D, Wang Y, Ni W. Cefiderocol for the Treatment of Multidrug-Resistant Gram-Negative Bacteria: A Systematic Review of Currently Available Evidence. Front Pharmacol. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Titov I, Wunderink RG, Roquilly A, Rodríguez Gonzalez D, David-Wang A, Boucher HW, et al. A Randomized, Double-blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults With Hospital-acquired or Ventilator-associated Bacterial Pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis. 2021. Dec 6;73(11):e4539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.AEMPS (Agencia Española del Medicamento y Productos Sanitarios) . Informe de Posicionamiento Terapéutico de imipenem/ cilastatina/relebactam (Recarbrio®) en el tratamiento de la neumonía adquirida en el hospital/neumonía asociada a ventilación mecánica con o sin bacteriemia y en el tratamiento de infecciones debidas a organismos aerobios Gram-negativos en adultos con opciones de tratamiento limitadas [Internet]. 2022. [cited 2024 Feb 16]. Available from: https://www.aemps.gob.es/informa/informes-de-posicionamiento-terapeutico/informe-de-posicionamiento-terapeutico-de-imipenem-cilastatina-relebactam-recarbrio-en-el-tratamiento-de-la-neumonia-adquirida-en-el-hospital-neumonia-asociada-a-ventilacion-mecanica-con-o-sin/
- 172.Langendonk RF, Neill DR, Fothergill JL. The Building Blocks of Antimicrobial Resistance in Pseudomonas aeruginosa: Implications for Current Resistance-Breaking Therapies. Front Cell Infect Microbiol. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karakonstantis S, Kritsotakis EI, Gikas A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection. 2020;48(6):835–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morroni G, Bressan R, Fioriti S, D’Achille G, Mingoia M, Cirioni O, et al. Antimicrobial Activity of Aztreonam in Combination with Old and New β-Lactamase Inhibitors against MBL and ESBL Co-Producing Gram-Negative Clinical Isolates: Possible Options for the Treatment of Complicated Infections. Antibiotics (Basel). 2021. Nov 3;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Canton R, Doi Y, Simner PJ. Treatment of carbapenem-resistant Pseudomonas aeruginosa infections: a case for cefiderocol. Expert Rev Anti Infect Ther. 2022;20(8):1077–94. [DOI] [PubMed] [Google Scholar]
- 176.Savoldi A, Carrara E, Tacconelli E. Gross national income and antibiotic resistance in invasive isolates: analysis of the top-ranked antibiotic-resistant bacteria on the 2017 WHO priority list—authors’ response. J Antimicrob Chemother. 2020;75(7):2018. [DOI] [PubMed] [Google Scholar]
- 177.Shields RK, Paterson DL, Tamma PD. Navigating Available Treatment Options for Carbapenem-Resistant ‘Acinetobacter baumannii-calcoaceticus’ Complex Infections. Clin Infect Dis. 2023;76(Supplement_2):S179–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022. Apr;28(4):521–47. [DOI] [PubMed] [Google Scholar]
- 179.Flores-Treviño S, Bocanegra-Ibarias P, Camacho-Ortiz A, Morfín-Otero R, Salazar-Sesatty HA, Garza-González E. ‘Stenotrophomonas maltophilia’ biofilm: its role in infectious diseases. Expert Rev Anti Infect Ther. 2019;17(11):877–93. [DOI] [PubMed] [Google Scholar]
- 180.Majumdar R, Karthikeyan H, Senthilnathan V, Sugumar S. Review on ‘Stenotrophomonas maltophilia’: An Emerging Multidrug-resistant Opportunistic Pathogen. Recent Pat Biotechnol. 2022;16(4):329–54. [DOI] [PubMed] [Google Scholar]
- 181.Mojica MF, Ouellette CP, Leber A, Becknell MB, Ardura MI, Perez F, et al. Successful Treatment of Bloodstream Infection Due to Metallo-β-Lactamase-Producing Stenotrophomonas maltophilia in a Renal Transplant Patient. Antimicrob Agents Chemother. 2016. Sep;60(9):5130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pintado V, Ruiz-Garbajosa P, Aguilera-Alonso D, Baquero-Artigao F, Bou G, Cantón R, et al. Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) on the diagnosis and antimicrobial treatment of infections due to carbapenem-resistant Gram-negative bacteria. Enferm Infecc Microbiol Clin. 2023;41(6):360–70. [DOI] [PubMed] [Google Scholar]
- 183.Rodríguez A, Moreno G, Bodi M, Gomez J, Martín-Loeches I. Corticosteroids and RCTs against the supposed undervaluation of real data evidence. Crit Care. 2021. Aug 18;25(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005. Feb 1;171(3):242–8. [DOI] [PubMed] [Google Scholar]
- 185.Meduri GU, Shih MC, Bridges L, Martin TJ, El-Solh A, Seam N, et al. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022;48(8):1009–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moreno G, Rodríguez A, Reyes LF, Gomez J, Sole-Violan J, Díaz E, et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018. Sep;44(9):1470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moreno G, Carbonell R, Martin-Loeches I, Solé-Violán J, Correig i Fraga E, Gómez J, et al. Corticosteroid treatment and mortality in mechanically ventilated COVID-19-associated acute respiratory distress syndrome (ARDS) patients: a multicentre cohort study. Ann Intensive Care. 2021;11(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Snijders D, Daniels JMA, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010. May 1;181(9):975–82. [DOI] [PubMed] [Google Scholar]
- 189.Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. The Lancet. 2011. Jun;377(9782):2023–30. [DOI] [PubMed] [Google Scholar]
- 190.Wittermans E, van der Zee PA, Qi H, van de Garde EMW, Blum CA, Christ-Crain M, et al. Community-acquired pneumonia subgroups and differential response to corticosteroids: a secondary analysis of controlled studies. ERJ Open Res. 2022. Jan;8(1):489–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stern A, Skalsky K, Avni T, Carrara E, Leibovici L, Paul M. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017. Dec 13;12(12):CD007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Saleem N, Kulkarni A, Snow TAC, Ambler G, Singer M, Arulkumaran N. Effect of Corticosteroids on Mortality and Clinical Cure in Community-Acquired Pneumonia: A Systematic Review, Meta-analysis, and Meta-regression of Randomized Control Trials. Chest. 2023. Mar;163(3):484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu JY, Tsai YW, Hsu WH, Liu TH, Huang PY, Chuang MH, et al. Efficacy and safety of adjunctive corticosteroids in the treatment of severe community-acquired pneumonia: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2023. Jul 8;27(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen S, Hu C. Effect of corticosteroids on mortality in patients with community-acquired pneumonia. Crit Care. 2023. Sep 19;27(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiang CH, See XY, Wang TH, Chang YC, Lo JE, Liu WT, et al. Effects of corticosteroids on severe community-acquired pneumonia: a closer look at the evidence. Crit Care. 2023. Aug 29;27(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Martin-Loeches I, Nagavci B, Torres A. Final approval for corticosteroids in severe CAP? For sure, in septic shock. Crit Care. 2023. Sep 4;27(1):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luo M hao, Wan Z, Tu G wei, Luo Z. Comments on “Efficacy and safety of adjunctive corticosteroids in the treatment of severe community-acquired pneumonia: a systematic review and meta-analysis of randomized controlled trials”. Crit Care. 2023. Sep 6;27(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pitre T, Rochwerg B, Zeraatkar D. Corticosteroids in Community-Acquired Pneumonia. Chest. 2023. Jan;163(1):e47–8. [DOI] [PubMed] [Google Scholar]
- 199.Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016. Aug;21(4):125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.World Health Organization (WHO) . Sepsis [Internet]. 2023. [cited 2023 Dec 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/sepsis
- 201.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021. Nov;47(11):1181–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Karavana V, Smith I, Kanellis G, Sigala I, Kinsella T, Zakynthinos S, et al. 37th International Symposium on Intensive Care and Emergency Medicine (part 1 of 3). Crit Care. 2017. Mar 21;21(S1):57. [Google Scholar]
- 203.Mesquida J, Borrat X, Lorente JA, Masip J, Baigorri F. Objetivos de la reanimación hemodinámica. Med Intensiva. 2011. Nov;35(8):499–508. [DOI] [PubMed] [Google Scholar]
- 204.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus Low Blood-Pressure Target in Patients with Septic Shock. N Engl J Med. 2014. Apr 24;370(17):1583–93. [DOI] [PubMed] [Google Scholar]
- 205.Adda I, Lai C, Teboul JL, Guerin L, Gavelli F, Monnet X. Norepinephrine potentiates the efficacy of volume expansion on mean systemic pressure in septic shock. Crit Care. 2021. Dec 21;25(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li Y, Li H, Zhang D. Timing of norepinephrine initiation in patients with septic shock: a systematic review and meta-analysis. Crit Care. 2020. Aug 6;24(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Clark S, Barton G, Dean P, Mei Lau Y, Baldwin A. Guidance For: The use of Vasopressor Agents by Peripheral Intravenous Infusion in Adult Critical Care Patients [Internet]. 2023. [cited 2023 Dec 22]. Available from: https://ics.ac.uk/asset/3B607648%2D9292%-2D4EC7%2DB913D5FCCA6F7D55/
- 208.Andaluz-Ojeda D, Cantón-Bulnes ML, Pey Richter C, Garnacho-Montero J. Fármacos vasoactivos en el tratamiento del shock séptico. Med Intensiva. 2022. May;46:26–37. [DOI] [PubMed] [Google Scholar]
- 209.Avni T, Lador A, Lev S, Leibovici L, Paul M, Grossman A. Vasopressors for the Treatment of Septic Shock: Systematic Review and Meta-Analysis. PLoS One. 2015;10(8):e0129305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gordon AC, Perkins GD, Singer M, McAuley DF, Orme RML, Santhakumaran S, et al. Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N Engl J Med. 2016. Oct 27;375(17):1638–48. [DOI] [PubMed] [Google Scholar]
- 211.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012. Oct;38(10):1573–82. [DOI] [PubMed] [Google Scholar]
- 212.Gattinoni L, Marini JJ. Isn’t it time to abandon ARDS? The COVID-19 lesson. Crit Care. 2021. Dec 6;25(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Domecq JP, Lal A, Sheldrick CR, Kumar VK, Boman K, Bolesta S, et al. Outcomes of Patients With Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med. 2021. Mar 1;49(3):437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bos LDJ, Sjoding M, Sinha P, Bhavani S V, Lyons PG, Bewley AF, et al. Longitudinal respiratory subphenotypes in patients with COVID-19-related acute respiratory distress syndrome: results from three observational cohorts. Lancet Respir Med. 2021. Dec;9(12):1377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, et al. Redefining critical illness. Nat Med. 2022. Jun 17;28(6):1141–8. [DOI] [PubMed] [Google Scholar]
- 216.Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023. Jul;49(7):727–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ferguson ND, Frutos-Vivar F, Esteban A, Gordo F, Honrubia T, Peñuelas O, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11(5):R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Grieco DL, Maggiore SM, Roca O, Spinelli E, Patel BK, Thille AW, et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021. Aug;47(8):851–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, et al. Lung-and Diaphragm-Protective Ventilation. Am J Respir Crit Care Med. 2020. Oct 1;202(7):950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fernández-Mondéjar E, Fuset-Cabanes MP, Grau-Carmona T, López-Sánchez M, Peñuelas Ó, Pérez-Vela JL, et al. Empleo de ECMO en UCI. Recomendaciones de la Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias. Med Intensiva [Internet]. 2019. Mar [cited 2024 Jan 11];43(2):108–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0210569118302845 [DOI] [PubMed] [Google Scholar]
- 221.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009. Oct 17;374(9698):1351–63. [DOI] [PubMed] [Google Scholar]
- 222.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018. May 24;378(21):1965–75. [DOI] [PubMed] [Google Scholar]
- 223.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020. Oct 10;396(10257):1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Urner M, Barnett AG, Bassi GL, Brodie D, Dalton HJ, Ferguson ND, et al. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. BMJ. 2022. May 4;e068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Castaño M, Sbraga F, Pérez de la Sota E, Arribas JM, Cámara ML, Voces R, et al. Oxigenación con membrana extracorpórea en el paciente COVID-19: resultados del Registro Español ECMO-COVID de la Sociedad Española de Cirugía Cardiovascular y Endovascular. Cirugía Cardiovascular. 2022. Mar;29(2):89–102. [Google Scholar]
- 226.SEMICYUC . Informe ENVIN-UCI 2020-21 [Internet]. 2021. [cited 2024 Jan 11]. Available from: https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202021.pdf
- 227.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013. Sep;15(3):172–8. [PubMed] [Google Scholar]
- 228.Vaquer S, de Haro C, Peruga P, Oliva JC, Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care. 2017. Dec;7(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jamal JA, Economou CJP, Lipman J, Roberts JA. Improving antibiotic dosing in special situations in the ICU: burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr Opin Crit Care. 2012. Oct;18(5):460–71. [DOI] [PubMed] [Google Scholar]
- 230.Grasselli G, Scaravilli V, Di Bella S, Biffi S, Bombino M, Patroniti N, et al. Nosocomial Infections During Extracorporeal Membrane Oxygenation: Incidence, Etiology, and Impact on Patients’ Outcome. Crit Care Med. 2017. Oct;45(10):1726–33. [DOI] [PubMed] [Google Scholar]
- 231.Bouglé A, Bombled C, Margetis D, Lebreton G, Vidal C, Coroir M, et al. Ventilator-associated pneumonia in patients assisted by veno-arterial extracorporeal membrane oxygenation support: Epidemiology and risk factors of treatment failure. PLoS One. 2018. Apr 13;13(4):e0194976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Luna CM, Blanzaco D, Niederman MS, Matarucco W, Baredes NC, Desmery P, et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med. 2003. Mar;31(3):676–82. [DOI] [PubMed] [Google Scholar]
- 233.Hänsel L, Schumacher J, Denis B, Hamane S, Cornely OA, Koehler P. How to diagnose and treat a patient without human immunodeficiency virus infection having Pneumocystis jirovecii pneumonia? Clin Microbiol Infect. 2023. Aug 1;29(8):1015–23. [DOI] [PubMed] [Google Scholar]
- 234.Zhou S, Aitken SL. Prophylaxis Against Pneumocystis jirovecii Pneumonia in Adults. JAMA. 2023. Jul 11;330(2):182–3. [DOI] [PubMed] [Google Scholar]
- 235.Iriart X, Challan Belval T, Fillaux J, Esposito L, Lavergne RA, Cardeau-Desangles I, et al. Risk factors of Pneumocystis pneumonia in solid organ recipients in the era of the common use of posttrans-plantation prophylaxis. Am J Transplant. 2015. Jan;15(1):190–9. [DOI] [PubMed] [Google Scholar]
- 236.Viceconte G, Buonomo AR, D’Agostino A, Foggia M, Di Fusco A, Pinchera B, et al. Risk Factors for Pneumocystis jirovecii Pneumonia in Non-HIV Patients Hospitalized for COVID-19: A Case-Control Study. J Fungi (Basel). 2023. Aug 11;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Amstutz P, Bahr NC, Snyder K, Shoemaker DM. ‘Pneumocystis jirovecii’ Infections Among COVID-19 Patients: A Case Series and Literature Review. Open Forum Infect Dis. 2023. Feb 3;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Goterris L, Mancebo Fernández MA, Aguilar-Company J, Falcó V, Ruiz-Camps I, Martín-Gómez MT. Molecular Diagnosis of Pneumocystis jirovecii Pneumonia by Use of Oral Wash Samples in Immunocompromised Patients: Usefulness and Importance of the DNA Target. J Clin Microbiol. 2019. Dec;57(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lieu A, Lee TC, Lawandi A, Tellier R, Cheng MP, Dufresne PJ. Micro-biological characterization of ‘Pneumocystis jirovecii’ pneumonia using quantitative PCR from nasopharyngeal specimens: a retrospective study in a Canadian province from 2019 to 2023. J Clin Microbiol. 2023. Nov 21;61(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Veintimilla C, Álvarez-Uría A, Martín-Rabadán P, Valerio M, Machado M, Padilla B, et al. Pneumocystis jirovecii Pneumonia Diagnostic Approach: Real-Life Experience in a Tertiary Centre. J Fungi (Basel). 2023. Mar 28;9(4):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Morjaria S, Frame J, Franco-Garcia A, Geyer A, Kamboj M, Babady NE. Clinical Performance of (1,3) Beta-D Glucan for the Diagnosis of Pneumocystis Pneumonia (PCP) in Cancer Patients Tested With PCP Polymerase Chain Reaction. Clin Infect Dis. 2019. Sep 27;69(8):1303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Friedrich R, Rappold E, Bogdan C, Held J. Comparative Analysis of the Wako β-Glucan Test and the Fungitell Assay for Diagnosis of Candidemia and Pneumocystis jirovecii Pneumonia. J Clin Microbiol. 2018. Sep;56(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Apostolopoulou A, Fishman JA. The Pathogenesis and Diagnosis of Pneumocystis jiroveci Pneumonia. J Fungi (Basel). 2022. Nov 5;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Del Corpo O, Butler-Laporte G, Sheppard DC, Cheng MP, McDonald EG, Lee TC. Diagnostic accuracy of serum (1-3)-β-D-glucan for Pneumocystis jirovecii pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect. 2020. Sep;26(9):1137–43. [DOI] [PubMed] [Google Scholar]
- 245.Bigot J, Vellaissamy S, Senghor Y, Hennequin C, Guitard J. Usefulness of ß-d-Glucan Assay for the First-Line Diagnosis of Pneumocystis Pneumonia and for Discriminating between Pneumocystis Colonization and Pneumocystis Pneumonia. J Fungi (Basel). 2022. Jun 24;8(7):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Burzio C, Balzani E, Corcione S, Montrucchio G, Trompeo AC, Brazzi L. Pneumocystis jirovecii Pneumonia after Heart Transplantation: Two Case Reports and a Review of the Literature. Pathogens. 2023. Oct 21;12(10):1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lagrou K, Chen S, Masur H, Viscoli C, Decker CF, Pagano L, et al. Pneumocystis jirovecii Disease: Basis for the Revised EORTC/ MSGERC Invasive Fungal Disease Definitions in Individuals Without Human Immunodeficiency Virus. Clin Infect Dis. 2021. Mar 12;72(Suppl 2):S114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Moreno A, Epstein D, Budvytiene I, Banaei N. Accuracy of Pneumocystis jirovecii Plasma Cell-Free DNA PCR for Noninvasive Diagnosis of Pneumocystis Pneumonia. J Clin Microbiol. 2022. May 18;60(5):e0010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Li X, Xiong X, Liang Z, Tang Y. A machine learning diagnostic model for Pneumocystis jirovecii pneumonia in patients with severe pneumonia. Intern Emerg Med. 2023. Sep;18(6):1741–9. [DOI] [PubMed] [Google Scholar]
- 250.Sohani ZN, Butler-Laporte G, Aw A, Belga S, Benedetti A, Carignan A, et al. Low-dose trimethoprim-sulfamethoxazole for the treatment of Pneumocystis jirovecii pneumonia (LOW-TMP): protocol for a phase III randomised, placebo-controlled, dose-comparison trial. BMJ Open. 2022. Jul 21;12(7):e053039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270–7. [DOI] [PubMed] [Google Scholar]
- 252.Nucci M, Nouer SA, Cappone D, Anaissie E. Early diagnosis of invasive pulmonary aspergillosis in hematologic patients: an opportunity to improve the outcome. Haematologica. 2013;98(11):1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Koulenti D, Papathanakos G, Blot S. Invasive pulmonary aspergillosis in the ICU: tale of a broadening risk profile. Curr Opin Crit Care. 2023;29(5):463–9. [DOI] [PubMed] [Google Scholar]
- 254.Peral J, Estella Á, Nuvials X, Rodríguez A, Seijas I, Soriano C, et al. Managing the Next Wave of Influenza and/or SARS-CoV-2 in the ICU—Practical Recommendations from an Expert Group for CAPA/ IAPA Patients. J Fungi (Basel). 2023;9(3):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs. 2021. Oct;81(15):1703–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Douglas AP, Smibert OC, Bajel A, Halliday CL, Lavee O, McMullan B, et al. Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021. Intern Med J. 2021. Nov;51 Suppl 7:143–76. [DOI] [PubMed] [Google Scholar]