Key Points
Question
Among people with obesity trying to lose weight, is a wireless system that provides daily feedback on progress in lifestyle change and weight loss noninferior to the same system combined with human coaching?
Findings
In an adaptive, noninferiority randomized clinical trial of 400 adults with overweight or obesity, mean 6-month weight change was −2.8 (95% CI, −3.5 to −0.08) kg with the wireless system alone and −4.8 (95% CI, −5.5 to −4.1) kg with the wireless system with coaching (difference in weight change, −2.0 kg [90% CI, −2.9 to –1.1]). The CI around the difference in weight change estimate included the prespecified noninferiority threshold margin of −2.5 kg.
Meaning
A wireless system that provided daily feedback on progress in lifestyle change and weight loss was not noninferior to the same system with human coaching.
Abstract
Importance
Lifestyle interventions for weight loss are difficult to implement in clinical practice. Self-managed mobile health implementations without or with added support after unsuccessful weight loss attempts could offer effective population-level obesity management.
Objective
To test whether a wireless feedback system (WFS) yields noninferior weight loss vs WFS plus telephone coaching and whether participants who do not respond to initial treatment achieve greater weight loss with more vs less vigorous step-up interventions.
Design, Setting, and Participants
In this noninferiority randomized trial, 400 adults aged 18 to 60 years with a body mass index of 27 to 45 were randomized in a 1:1 ratio to undergo 3 months of treatment initially with WFS or WFS plus coaching at a US academic medical center between June 2017 and March 2021. Participants attaining suboptimal weight loss were rerandomized to undergo modest or vigorous step-up intervention.
Interventions
The WFS included a Wi-Fi activity tracker and scale transmitting data to a smartphone app to provide daily feedback on progress in lifestyle change and weight loss, and WFS plus coaching added 12 weekly 10- to 15-minute supportive coaching calls delivered by bachelor’s degree–level health promotionists viewing participants’ self-monitoring data on a dashboard; step-up interventions included supportive messaging via mobile device screen notifications (app-based screen alerts) without or with coaching or powdered meal replacement. Participants and staff were unblinded and outcome assessors were blinded to treatment randomization.
Main Outcomes and Measures
The primary outcome was the between-group difference in 6-month weight change, with the noninferiority margin defined as a difference in weight change of −2.5 kg; secondary outcomes included between-group differences for all participants in weight change at 3 and 12 months and between-group 6-month weight change difference among nonresponders exposed to modest vs vigorous step-up interventions.
Results
Among 400 participants (mean [SD] age, 40.5 [11.2] years; 305 [76.3%] women; 81 participants were Black and 266 were White; mean [SD] body mass index, 34.4 [4.3]) randomized to undergo WFS (n = 199) vs WFS plus coaching (n = 201), outcome data were available for 342 participants (85.5%) at 6 months. Six-month weight loss was −2.8 kg (95% CI, −3.5 to −2.0) for the WFS group and −4.8 kg (95% CI, −5.5 to −4.1) for participants in the WFS plus coaching group (difference in weight change, −2.0 kg [90% CI, −2.9 to –1.1]; P < .001); the 90% CI included the noninferiority margin of −2.5 kg. Weight change differences were comparable at 3 and 12 months and, among nonresponders, at 6 months, with no difference by step-up therapy.
Conclusions and Relevance
A wireless feedback system (Wi-Fi activity tracker and scale with smartphone app to provide daily feedback) was not noninferior to the same system with added coaching. Continued efforts are needed to identify strategies for weight loss management and to accurately select interventions for different individuals to achieve weight loss goals.
Trial Registration
ClinicalTrials.gov Identifier: NCT02997943
This noninferiority randomized trial compares the effects of a wireless system designed to provide daily feedback on progress in lifestyle change and weight loss vs the same system with additional human coaching on weight change among people with overweight and obesity.
Introduction
Obesity is a worsening public health problem. In the US, 42% of adults currently have obesity, which has increased from 31% in 2000 and is expected to reach 49% by 2030.1,2 Excess weight is associated with increased incidence of and mortality from prevalent conditions including cardiovascular disease, diabetes, and some cancers that are among the leading causes of preventable, premature death.3 Accordingly, obesity is accompanied by $1861 excess annual medical expenditures per adult in the US, already costing the nation $172.74 billion in excess medical costs every year.4 A public health strategy to curb this escalating burden requires effective and scalable behavioral treatment.5 However, efforts to find a “just right” approach that is intensive enough to be effective but not so intensive as to be unscalable have thus far fallen short.6
Resource-intensive obesity treatments that might work for most exceed the needs of many and outstrip the funds, time, and capacity available to implement them as routine health care. Given current inability to predict, before treatment, who needs what obesity intervention,7 an adaptive treatment strategy that modifies treatment to match a patient’s history of response to previous treatment offers an alternative approach.8 This trial tests an adaptive strategy of obesity treatment, offering less resource-intensive followed by more resource-intensive treatments for those who require them in an attempt to identify a sequence of treatment components that produces the most weight loss efficiently within routine constraints.8,9,10,11
Methods
Study Design and Objectives
We used a sequential multiple assignment randomized trial (SMART) design12,13 (eFigure 1 in Supplement 1) to determine, for a population of adults with overweight or obesity, an optimal behavioral weight loss approach among 2 initial treatment and 2 treatment step-up strategies for nonresponders to first-line treatment in a single-site trial conducted at Northwestern University between June 2017 and March 2021.14 The trial protocol is available in Supplement 1.15 Participants provided informed consent for study procedures approved by the institutional review board at Northwestern University.
Our primary aim was to compare a 3-month mobile health intervention, specifically a wireless feedback system (WFS) alone or with human coaching (WFS plus coaching), as first-line obesity treatment to engage participants and maximize weight loss across the treated population. We hypothesized that starting with WFS alone would be noninferior to WFS plus coaching for the primary outcome of 6-month weight loss.
Participant Recruitment and Eligibility
Recruitment strategies and eligibility criteria followed those used in previous similar trials11,16 and occurred through research registries, flyers, and advertisements in public transportation and local newspapers. Interested individuals completed a web-based REDCap screening to determine initial eligibility. Telephone screening confirmed web-based responses, and eligible candidates were scheduled for a group orientation. Eligibility criteria were age 18 to 60 years, body mass index (BMI) of 27 to 45, no weight gain or loss greater than 11.3 kg for 6 months, smartphone access, and plans to reside in the research center area (Chicago) for 12 months. Exclusion criteria included safety (eg, unstable medical condition, pregnancy, condition that interferes with physical activity) and potential confounding variables (eg, use of medications or other treatment affecting weight) (Supplement 1).
Randomization
Participants were randomized in a 1:1 ratio to undergo either WFS alone or WFS plus coaching as initial treatment (Figure 1). Stage 1 randomization (before treatment onset) used a permuted block strategy17,18 stratified by sex (male vs female) and BMI (≤35 or >35). Stage 2 randomization could occur ay time in the initial 2 months of treatment and included nonresponders, in which nonresponse was defined as weight loss less than 0.23 kg per week (ie, <0.46 kg by week 2, <0.92 kg by week 4, or <1.84 kg by week 8; eFigure 1 in Supplement 1), a threshold chosen based on previous data indicating that insufficient weight loss by week 2 of treatment is predictive of losing less than 5% by month 6.19,20 Permuted block randomization was used again, this time stratified by whether nonresponders had vs had not lost any weight. For both stages of randomization, the smallest block size possible (ie, 2) was used to minimize the risk for imbalance in sample size per group in each stratum. Randomization was concealed by keeping the block size and stratification variables confidential; staff could only view a participant’s initial treatment after the study’s custom Excel-based macro randomly assigned the treatment and only saw an assigned step-up treatment after the study server, in response to incoming data from the custom smartphone app, classified the participant as a nonresponder and performed a second randomization. Nonresponders were rerandomized in a 1:1 ratio to receive the addition of supportive messaging via mobile device push notifications (app-based screen alerts), without (modest step-up) or with meal replacement or, if not already provided, coaching (vigorous step-up) (eFigure 1 in Supplement 1). Responders continued with their initial treatment. Responders were reassessed at weeks 4 and 8 and, if reclassified as nonresponders, were rerandomized to a step-up intervention. Rerandomization only occurred once, after which the stepped-up treatment was continued through week 12. Consistent responders continued their initial treatment through week 12. Outcome assessors were kept blinded to treatment assignment by blocking their digital access to the information and reminding participants not to unmask their treatment assignment.
Figure 1. Recruitment, Randomization, and Participant Flow in a Trial of Behavioral Intervention for Weight Loss.
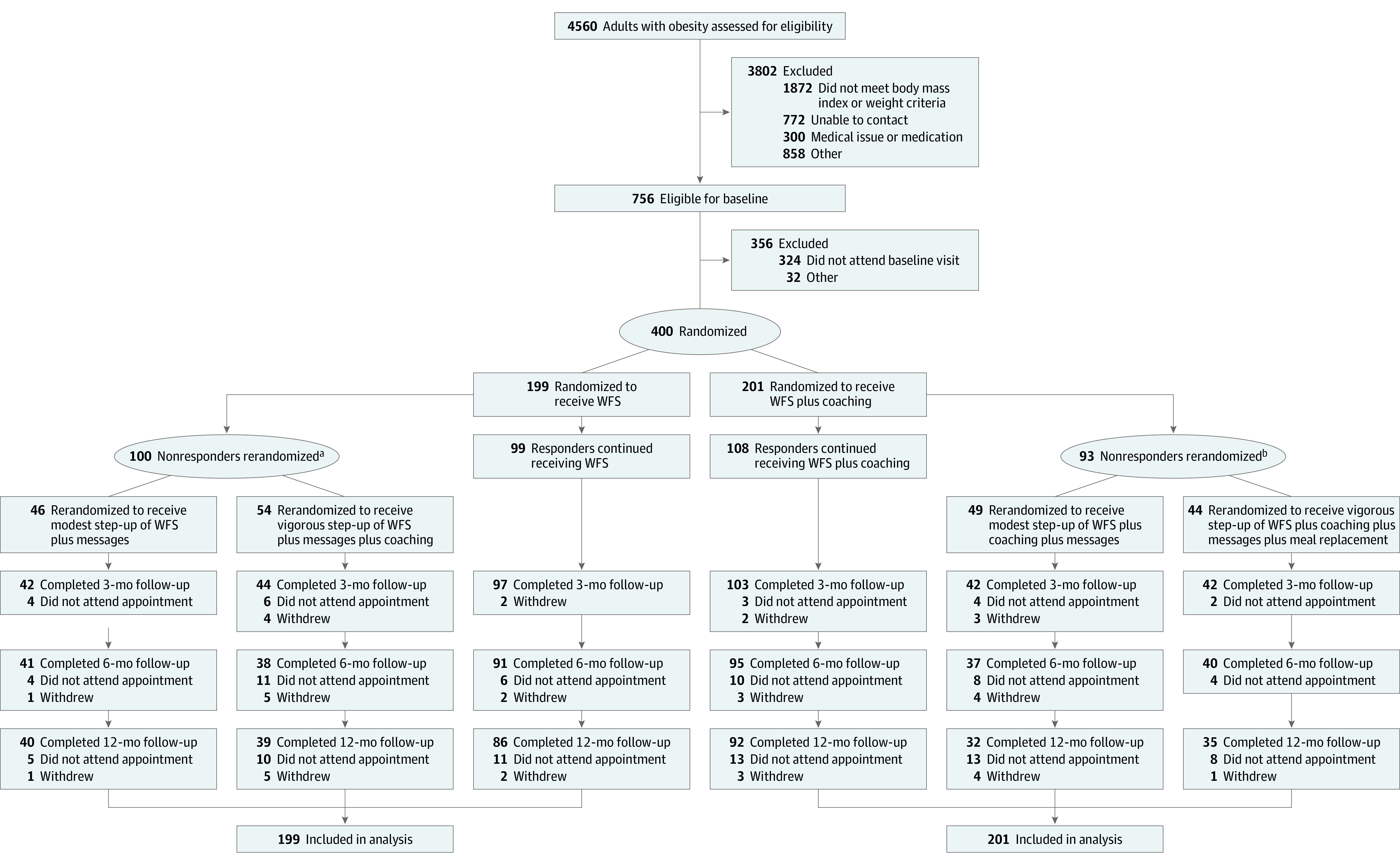
Values for the number of participants who withdrew are cumulative.
aFor those initially randomized to the wireless feedback system (WFS) group, 55 were rerandomized at week 2, 30 at week 4, and 15 at week 8.
bFor those initially randomized to the WFS plus coaching group, 59 were rerandomized at week 2, 18 at week 4, and 16 at week 8.
Interventions
All participants received a core intervention involving behavioral weight loss lessons adapted from the Diabetes Prevention Program11,14,21 and a custom-built wireless feedback system based on self-regulation theory.22 The weight loss treatment mirrored that used in previous trials that demonstrated efficacy of the intervention, particularly a WFS and 12-session telephone coaching protocol that produced 5% weight loss for 57.1% of 237 participants at 6 months.11,16,23 Participants used their own smartphone to download the study’s mobile device app and the study loaned them a wireless activity tracker and scale (Fitbit Zip and Fitbit Aria scale). The WFS used self-reported diet, as well as physical activity and weight data transmitted from the devices, to provide goal attainment feedback to the participant via the app and, for participants randomized to receive coaching, to a “health promotionist” via the coach dashboard (eFigure 2 in Supplement 1). The weight loss goal was 5% at 6 months. Calorie and saturated fat goals were based on participants’ weight and physical activity goals. Physical activity goals started at 60 minutes per week of moderate- to vigorous-intensity activity and increased, based on the performance of the past 2 weeks, to a maximum of 300 minutes per week.
Participants assigned to receive coaching initially or at rerandomization received weekly telephone coaching about diet and physical activity until week 12. Trained bachelor’s degree–level health promotionists, supervised by licensed clinical health psychologists (A.F.P. and B.S.),24 provided coaching guided by participants’ incoming self-monitoring data summarized on the custom dashboard. Health promotionists were trained and supervised to guide health behavior change by telehealth and were available to provide tech support for mobile health tracking tools. Coaching sessions lasted 10 to 15 minutes and involved motivational interviewing, goal setting, problem solving, and technology support. Participants returned the study-provided Wi-Fi activity tracker and scale at week 12.
Modest Step-Up
Participant nonresponders randomized to undergo modest step-up received supportive messages (1-way mobile device push notifications [app-based screen alerts]; maximum of 3 daily) in addition to their initial treatment. The messages, tailored based on self-monitoring adherence, reinforced self-monitoring, self-efficacy, and autonomous motivation. For example, a morning message might read, “Keep it up! Remembering to weigh yourself every morning will help you keep track of your progress.”
Vigorous Step-Up
Participant nonresponders initially treated with only WFS were rerandomized to receive a stepped-up intervention to add both supportive messages and coaching. Nonresponders who were already receiving coaching as part of initial treatment (WFS plus coaching) were stepped up to add supportive messages and a commercially available powdered meal replacement to replace each of 2 meals per day (1 serving per meal) through the end of the intervention period (12 weeks).
Treatment Fidelity
Treatment fidelity was scored using checklists corresponding to the 5 assigned types of treatment delivery: WFS, WFS plus coaching, WFS plus messages, WFS plus coaching and messages, and WFS plus coaching, messages, and meal replacement (Supplement 1). All checklists documented whether rerandomization had occurred and was implemented appropriately. Coaching calls were audio-recorded and scored. Quarterly, 15% of call records were randomly selected and scored. Credit was given for discussing diet and physical activity self-monitoring and assigned treatment components (eg, messages, meal replacements) and was debited for discussing proscribed content (eg, messages or meal replacement with a treatment responder). Total correct responses minus total incorrect responses as a percentage of total possible score for each treatment type reflects the degree to which all assigned treatment aspects were delivered and no contamination occurred with unique features of other treatment types. Each quarter, the study coordinator calculated the mean of a coach’s scores for all reviewed calls and records to calculate their total treatment fidelity score.
Assessments
Outcomes
The prespecified primary outcome was between-group difference in 6-month weight change. Prespecified secondary outcomes were between-group differences for all participants in weight change at 3- and 12-month follow-up and 6-month weight change difference among initial nonresponders who received modest vs vigorous step-up therapy. Prespecified exploratory outcomes were weight change differences at 3 and 12 months among nonresponders who received modest vs vigorous step-up interventions, weight change difference at all follow-up visits by treatment sequence, percentage of participants who achieved at least 5% weight loss at 6 months (the patient treatment goal and a clinically important outcome), maintenance of any end-of-treatment (3-month) weight loss difference at final follow-up (12 months), and weight change difference at all follow-up visits by demographic characteristics. We also examined whether self-efficacy, autonomous motivation, and self-monitoring adherence were mediators of treatment response.
Weight, Height, and BMI
Height and weight were assessed in person at baseline and 3, 6, and 12 months by a trained, certified assessor. Height was measured to the nearest 0.25 cm using a wall-mounted stadiometer. Weight was measured to the nearest 0.10 kg using a calibrated balance beam scale. BMI was calculated. Participants received $20 for the 3-month follow-up visit, $40 for the 6-month visit, and $60 for the 12-month visit.
Sociodemographic Characteristics
Sex assigned at birth and racial and ethnic identity were self-reported as discrete categories at baseline by demographic questionnaire, as required by the US National Institutes of Health funding agency. The same questionnaire assessed socioeconomic status and marital status.
Self-Monitoring Adherence
Diet self-monitoring adherence was measured as the percentage of days within the 3-month intervention that the participant entered an intake of at least 800 kcal.25,26 Adherence to physical activity self-monitoring was the percentage of days that participants wore the physical activity tracker. Weight self-monitoring adherence was the percentage of days the participant either transmitted a Wi-Fi scale weight or manually entered weight into the app. Meal replacement adherence was the percentage of days that those given the meal replacement product consumed it for at least 1 meal.
Self-Efficacy and Autonomous Motivation
We assessed change in perceptions of self-efficacy as a potential mediator of treatment response based on the 11-item DIET Self-Efficacy Scale, which measured confidence about maintaining eating self-control,27 and the analogous 18-item Physical Activity Self-Efficacy Scale, which measured confidence about being physically active28 despite challenges. Both assessments asked participants to self-report confidence about different scenarios using 5-point Likert scales in which higher scores indicated greater self-efficacy. An abbreviated 4-item version of the Treatment Self-Regulation Questionnaire assessed autonomous motivation on 5-point scales, where higher scores indicated greater autonomous motivation.29 All assessments were administered at baseline and 3 months.
Sample Size
Sample size calculations were based on the primary 6-month weight change outcome for participants initially randomized to undergo WFS vs WFS plus coaching. After the trial was funded but before recruitment began, the data and safety monitoring board recommended replacing the planned superiority analysis with a noninferiority analysis testing whether 6-month weight loss with WFS as initial treatment was noninferior to WFS plus coaching. Following guidance30 for a noninferiority margin to be 50% of the difference between a treatment and control condition, noninferiority was considered established if the lower limit of a (1 − 2α) × 100% CI for the mean difference in weight loss between WFS and WFS plus coaching groups was above –δ, where α was the type I error and δ was the noninferiority margin. We used a type I error rate of .05 so that noninferiority was based on the lower limit of a 90% CI. Based on evidence of a 5.1-kg difference between similar treatment groups at 6 months,31 we defined the noninferiority margin as 50% of that difference (ie, 2.5 kg), which aligns with conclusions of a Cochrane systematic review and meta-analysis that the 6-month weight loss difference between face-to-face vs interactive computer-based obesity interventions should exceed 2.1 kg to be clinically important.32
Based on preliminary data, we assumed an overall SD of 13.5 with 0.80 within-person correlation between weight at baseline and month 6. To obtain 90% statistical power to establish noninferiority of WFS vs WFS plus coaching with 1-sided α equal to .05, we estimated the need for 344 participants. Assuming month 6 attrition up to 14%,33 we estimated that 400 total participants would yield 172 participants in each initial group after attrition to provide adequate power for the primary comparison. Assuming a 50% nonresponse rate, we estimated that 86 participants would be randomized to each of the 2 step-up groups, yielding 82% power to detect a small to medium difference (standardized mean difference = 0.30) between step-up interventions.
Statistical Analysis
Analyses were conducted on an intent-to-treat basis, using all available data from all randomized participants. We compared the effects of initial WFS vs WFS plus coaching (regardless of subsequent treatments) on weight loss controlling for sex using 90% CIs. All other analyses were conducted as superiority comparisons using 95% CIs, including comparison of effects of vigorous vs modest step-up interventions on weight loss controlling for sex and weight change since baseline and testing for differences by initial treatment. We used a covariance pattern model (CPM34) via SPSS MIXED to analyze the longitudinal weight data. CPM allowed for incomplete data across time and, under maximum likelihood estimation, accommodated data missing at random.35 CPM included fixed effects for time indicators (with baseline as the reference) and the group × time indicator interactions. An unstructured residual variance-covariance matrix allowed differing variances and covariances across time. We also used the weight and replicate method36,37 to find the optimal sequence of treatment tactics by comparing the effect on weight loss from baseline to month 6 of the 4 treatment sequences embedded in the trial.
We used CPM for prespecified exploratory analyses examining whether end-of-treatment differences in weight loss were maintained through 12-month follow-up and for exploratory moderation analyses examining whether initial treatment effects differed by demographic characteristics. The latter analyses were performed by estimating interaction terms—a 2-way demographic × time term and a 3-way demographic × time × treatment term. Demographic moderators were assessed separately with no correction for multiple comparisons.
We also performed post hoc sensitivity analyses comparing weight change across 12 weeks for 2 groups of 20 pairs matched on age, sex, and baseline BMI from the 2 initial treatment groups that completed follow-up assessments before (n = 20 pairs) vs during (n = 20 pairs) COVID-19 lockdown.
Separate mediation analyses examined whether self-monitoring adherence and changes in self-efficacy and autonomous motivation from baseline to 3 months mediated the initial treatment effect on weight loss during the same period. Using a counterfactual framework,38 the total effect of treatment on 3-month weight loss was decomposed into natural direct and indirect effects. We report the percent mediated: the ratio of the natural indirect effect divided by the total effect. CIs for the percent mediated were obtained using a bias-corrected bootstrap39 with 5000 bootstrap samples.
Results
Participants
Among 756 eligible participants, 400 were enrolled and randomized, with no statistically or demographically significant difference between the groups (mean [SD] age, 40.5 [11.2] years; mean [SD] BMI, 34.4 [4.3]; 305 [76.4%] women; 81 participants were Black and 266 were White) (Table 1; Figure 1). Half of the participants were classified as nonresponders and rerandomized to treatment step-up, with no significant difference in percentages between WFS (n = 100 [50%]) vs WFS plus coaching (n = 93 [46%]) groups. Among participants in the WFS group, 55 (55%) were identified as nonresponders at week 2, 30 (30%) at week 4, and 15 (15%) at week 8; among participants in the WFS plus coaching group, 59 (65%) were identified as nonresponders at week 2, 18 (9%) at week 4, and 16 (17%) at week 8. Retention was 92.8% at 3 months, 85.5% at 6 months, and 81.6% at 12 months, and did not differ between the groups (P = .54). Of 58 participants who did not provide a 6-month follow-up weight, 15 withdrew (8 in the WFS group and 7 in the WFS plus coaching group) and 43 did not attend the appointment (21 in the WFS group and 22 in the WFS plus coaching group).
Table 1. Participant Baseline Characteristics.
| Characteristic | WFS (n = 199) | WFS plus coaching (n = 201) |
|---|---|---|
| Age, mean (SD), y | 40.2 (11.3) | 40.9 (11.1) |
| Weight, mean (SD), kg | 96.8 (16.0) | 97.4 (15.9) |
| BMI, mean (SD) | 34.3 (4.3) | 34.5 (4.4) |
| BMI category, No. (%) | ||
| Obese (30-45) | 168 (84.4) | 166 (82.6) |
| Overweight (27-29.9) | 31 (15.6) | 35 (17.4) |
| Sex, No. (%) | ||
| Female | 152 (76.4) | 153 (76.1) |
| Male | 47 (23.6) | 48 (23.9) |
| Race, No. (%) | n = 186a | n = 196a |
| American Indian or Alaska Native | 0 (0.0) | 2 (1.0) |
| Asian | 7 (3.8) | 10 (5.0) |
| Black or African American | 33 (17.7) | 48 (24.5) |
| Native Hawaiian or Pacific Islander | 0 | 3 (1.5) |
| White | 138 (74.2) | 128 (65.3) |
| More than 1 indicated | 8 (4.3) | 5 (3.0) |
| Hispanic ethnicity, No./total No. (%) | 25/199 (12.6) | 20/201 (10.0) |
| Education level, No. (%) | ||
| College graduate | 171 (85.9) | 178 (88.6) |
| College 1-3 y | 26 (13.1) | 18 (9.0) |
| High school graduate | 2 (1.0) | 5 (2.5) |
| Marital status, No. (%) | ||
| Married | 68 (34.2) | 83 (37.8) |
| Never married | 76 (38.2) | 66 (32.8) |
| Coupled | 32 (16.1) | 31 (15.4) |
| Divorced | 18 (9.0) | 16 (8.0) |
| Separated | 4 (2.0) | 2 (1.0) |
| Widowed | 1 (0.5) | 3 (1.5) |
| Income, No. (%) | ||
| >$75k | 94 (48.7) | 94 (49.5) |
| $50k to $75k | 45 (23.3) | 36 (18.9) |
| $35k to $49.9k | 25 (13.0) | 23 (12.1) |
| $25k to $34.9k | 15 (7.8) | 20 (10.5) |
| ≤$24.9k | 14 (7.3) | 17 (8.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); WFS, wireless feedback system.
The number of participants for the race category represent nonmissing values, which serve as denominator for subsequent percentages.
All baseline assessments and most follow-up assessments were completed before SARS-CoV-2 stay-at-home orders began. The number of follow-up assessments completed remotely using Zoom and the wireless scale was 25 (6.8%) at 3 months (11 in the WFS group and 14 in the WFS plus coaching group), 53 (15.5%) at 6 months (24 in the WFS group and 29 in the WFS plus coaching group), and 97 (29.9%) at 12 months (50 in the WFS group and 47 in the WFS plus coaching group).
Weight Loss Outcomes
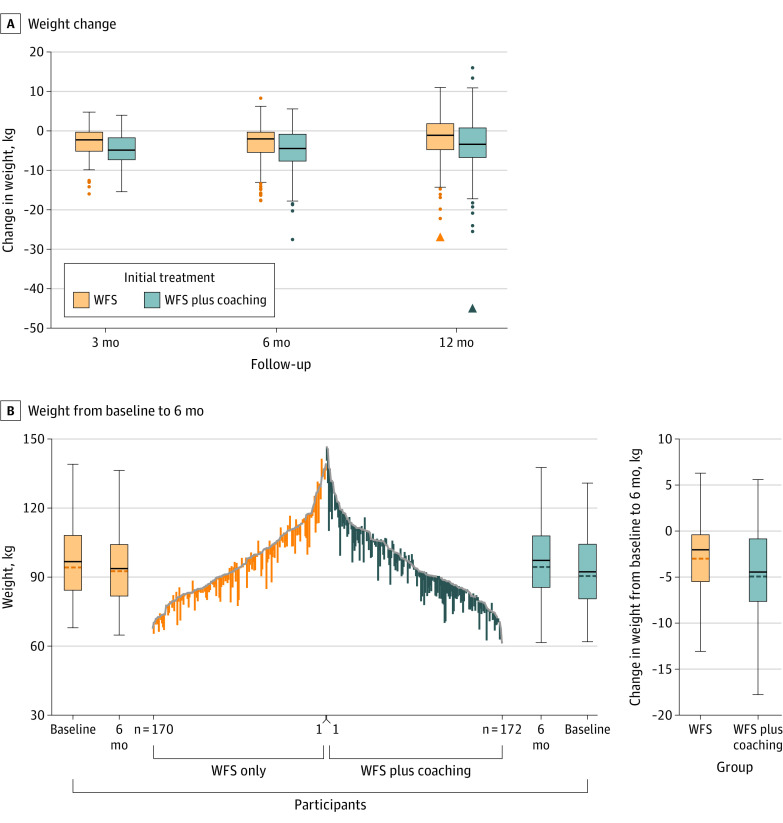
Six-month weight change was −2.8 (95% CI, −3.5 to −2.0) kg among participants in the WFS group and −4.8 (95% CI, −5.5 to −4.1) kg among participants in the WFS plus coaching group, for a between-group difference in weight change of −2.0 (90% CI, −2.9 to −1.1) kg (Table 2). Results were comparable at 3 months (between-group difference in weight change, −1.6 [95% CI, −2.2 to −1.0] kg) and 12 months (between-group difference in weight change, −1.7 [95% CI, −2.9 to −0.5] kg) (Figure 2).
Table 2. Weight Change by Initial Treatment and Between-Treatment Change Difference.
| Follow-up | WFS (n = 199) | WFS plus coaching (n = 201) | Between-treatment difference (90% CI), kgb | ||||
|---|---|---|---|---|---|---|---|
| Baseline weight, mean (SD), kg | Follow-up weight, mean (SD), kg | Weight change (95% CI), kga | Baseline weight, mean (SD), kg | Follow-up weight, mean (SD), kg | Weight change (95% CI), kga | ||
| Respondersc | |||||||
| 3 Months | 97.7 (16.2) | 92.7 (15.7) | −5.0 (−5.7 to −4.4) | 98.0 (16.2) | 91.8 (15.5) | −6.2 (−6.9 to −5.6) | −1.2 (−2.0 to −0.5) |
| 6 Months | 97.7 (16.2) | 92.9 (15.3) | −4.8 (−5.8 to −3.8) | 98.0 (16.2) | 91.4 (15.0) | −6.6 (−7.6 to −5.7) | −1.8 (−3.0 to −0.7) |
| 12 Months | 97.7 (16.2) | 94.3 (15.7) | −3.4 (−4.8 to −1.9) | 98.0 (16.2) | 92.7 (15.6) | −5.3 (−6.7 to −3.9) | −1.9 (−3.6 to −0.2) |
| Nonrespondersc | |||||||
| 3 Months | 95.9 (15.6) | 94.9 (14.6) | −0.9 (−1.5 to −0.3) | 96.8 (15.6) | 94.0 (15.0) | −2.8 (−3.4 to −2.1) | −1.8 (−2.6 to −1.1) |
| 6 Months | 95.9 (15.6) | 95.3 (14.4) | −0.6 (−1.5 to 0.3) | 96.8 (15.6) | 94.2 (14.7) | −2.5 (−3.4 to −1.6) | −2.0 (−3.0 to −0.9) |
| 12 Months | 95.9 (15.6) | 95.9 (14.8) | 0.0 (−1.2 to 1.3) | 96.8 (15.6) | 95.5 (14.1) | −1.3 (−2.6 to −0.0) | −1.3 (−2.8 to 0.2) |
| Full sample | |||||||
| 3 Months | 96.8 (15.9) | 93.7 (15.1) | −3.1 (−3.6 to −2.6) | 97.4 (15.9) | 92.8 (15.2) | −4.6 (−5.2 to −4.2) | −1.6 (−2.2 to −1.0) |
| 6 Months | 96.8 (15.9) | 94.0 (14.8) | −2.8 (−3.5 to −2.0) | 97.4 (15.9) | 92.6 (14.8) | −4.8 (−5.5 to −4.1) | −2.0 (−2.9 to −1.1) |
| 12 Months | 96.8 (15.9) | 95.0 (15.2) | −1.8 (−2.8 to −0.8) | 97.4 (15.9) | 93.9 (14.9) | −3.5 (−4.5 to −2.5) | −1.7 (−2.9 to −0.5) |
Abbreviation: WFS, wireless feedback system.
95% CI for weight change reflects a 2-sided test of treatment phase superiority relative to baseline.
90% CI for between-treatment differences in weight change reflects a 1-sided test of non-inferiority of WFS relative to WFS plus coaching. By study design, the null hypothesis was to be rejected in favor of noninferiority at the .05 level of significance if the lower bound of the 90% CI of the difference in weight change between treatment conditions at 6 months in the full trial sample was greater than the noninferiority margin of −2.5 kg.
There were 99 responders in the WFS group and 108 responders in the WFS plus coaching group and 100 nonresponders in the WFS group and 93 in the WFS plus coaching group.
Figure 2. Weight Change From Baseline Through 3, 6, and 12 Months.
A, The horizonal solid line in each box indicates the median weight change, the upper and lower box borders are 25th and 75th percentiles, and whiskers are 1.5 times the IQR. Individual dots are values beyond the upper or lower whiskers. Triangles are extreme outliers with values greater than 3 IQR above quartile 3 or 3 IQR below quartile 1. B, Vertical lines represent 6-month weight change for each participant randomly assigned to initial treatment with wireless feedback system (WFS) vs WFS plus coaching, where the top of each line is baseline weight and the bottom is 6-month follow-up weight, ordered from highest (center) to lowest baseline values. Boxplots flanking the line graph summarize median (IQR) and mean baseline and 6-month weights. The boxplots to the far right show median and mean weight change from baseline to month 6.
Among nonresponders, differences in weight change between the 2 step-up intervention options were not statistically different at months 3, 6, or 12 (eTable 1 in Supplement 2). Results of the treatment sequence analysis showed that although all 4 embedded treatment sequences produced weight loss at 3, 6, and 12 months and weight loss with initial WFS followed by modest step-up for nonresponders produced slightly less weight loss than other combinations of interventions, differences in weight change across the 4 treatment sequences were not statistically significant, suggesting no optimal treatment sequence (eTable 2 in Supplement 2).
A higher percentage of participants randomized to receive WFS plus coaching achieved weight loss of at least 5% compared with those receiving WFS only at 3, 6, and 12 months, although the statistically significant difference observed at 3 and 6 months was not evident at month 12 (Table 3).
Table 3. Achievement of 5% Weight Loss by Initial Treatment and Difference Between Treatmentsa.
| Follow-up | No. (%) of participants who achieved 5% weight loss | Absolute difference (95% CI) | Unadjusted odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| WFS (n = 199)b | WFS plus coaching (n = 201)b | ||||
| 3 Months | 53 (29.0) | 92 (49.2) | 20.2 (10.48 to 39.63) | 2.38 (1.55 to 3.65) | <.001 |
| 6 Months | 50 (29.4) | 83 (48.3) | 18.9 (8.77 to 29.03) | 2.24 (1.43 to 3.49) | <.001 |
| 12 Months | 45 (27.3) | 58 (36.5) | 9.20 (−0.90 to 19.30) | 1.53 (0.96 to 2.45) | .08 |
Abbreviation: WFS, wireless feedback system.
Prespecified exploratory analysis.
These numbers of participants indicate those who were initially randomized. The numbers of participants in the WFS group who contributed to outcomes were 183 for 3 months, 170 for 6 months, and 165 for 12 months; the numbers of participants in the WFS plus coaching group who contributed to outcomes were 187 at 3 months, 172 at 6 months, and 159 at 12 months.
Subgroup Analyses
Demographics
Mean weight loss at 6 months was less among women than men, Black than White participants, and younger (≤40 y) than older (>40 y) participants (eTable 3 in Supplement 2). There was no evidence that any demographic groups benefited differentially from either initial treatment or step-up tactic.
Post hoc sensitivity analyses comparing weight change across 12 weeks for 2 groups of 20 pairs matched on age, sex, and baseline BMI from the 2 initial treatment groups that completed follow-up assessments before (n = 20 pairs) vs during (n = 20 pairs) COVID-19 lockdown showed no significant difference in between-group weight loss (mean [standard error] weight change in pairs before lockdown vs during, −1.42 kg [0.703] vs −1.46 kg [0.713]; between-group difference, 0.04 kg; P = .97).
Fidelity, Adherence, and Motivation
Treatment Fidelity
Mean [SD] treatment fidelity was 99.3% [5.4%] across all coded sessions (n = 535). Among 6 coaches, none scored lower than 90% or required retraining in any quarter.
Behavioral Adherence
Self-monitoring of dietary intake and weight during the 3-month treatment period was statistically significantly higher for participants initially assigned to receive WFS plus coaching vs WFS only, and greater self-monitoring of diet and weight were significant mediators of the greater weight loss (eTable 4 in Supplement 2). Among the 50% of nonresponders who were adherent to meal replacement (ie, consumed meal replacement for ≥1 meal on 80% of days), weight loss approached that of responders (eFigure 3 in Supplement 2)
Self-Efficacy, Autonomous Motivation
Across the treatment period, measures of diet and exercise self-efficacy and autonomous motivation increased more under initial treatment with WFS plus coaching vs WFS only and statistically significant greater increases in the 3 were mediators of the greater weight loss with WFS plus coaching (eTable 4 in Supplement 2).
Discussion
In this noninferiority trial of adaptive behavioral interventions for weight loss management, 2.0-kg greater weight change was observed at 6 months in participants randomized to the WFS plus coaching group compared with WFS only. Because the 90% CI for the difference between the 2 initial treatments at 6 months included the prespecified noninferiority margin (−2.5 kg), the findings do not support the hypothesis that WFS is noninferior to WFS plus coaching. Weight change results were comparable at 3 and 12 months.
In exploratory analyses, nearly half of participants in the WFS plus coaching group achieved the clinically meaningful threshold of 5% weight loss, and their odds of achieving 5% weight loss were greater compared with those receiving WFS alone.
A rationale for stepped care in obesity management is to offer participants who do not respond to first-line treatment additional interventions to help them catch up to early responders’ weight loss.40 Among the 4 step-up tactics tested, none made up the difference, and there were no weight change differences between modest and vigorous step-up interventions. Nevertheless, at all posttreatment assessments a portion of those initially given only WFS (27%-29%) had achieved 5% weight loss, which is notable because, in addition to initially receiving no ongoing human support from the intervention, budget constraints required removing their wearable activity tracker and Wi-Fi scale after 3 months (for use by new trial participants). Delineation of a feature that preidentifies what individuals are likely to succeed with digital tools alone before launching obesity treatment would support scalable population-level obesity management by helping physicians and policymakers match initial treatment intensity to patient needs.
Despite the failure to demonstrate noninferiority, the trial results are consistent with findings from a meta-analysis showing that app-based feedback systems have a role in obesity treatment, producing a mean weight loss of 2.8 kg in 6 months.41 To date, attainment of greater weight loss has required the addition of human intervention,41 although recent advances in artificial intelligence make it tempting to imagine that chatbot-like interventions could play a role in the future.
Study strengths were that both initial interventions are brief and able to be delivered remotely, increasing access. The WFS was used successfully by older adults—a group thought to lack digital skills,42,43 but whose weight loss exceeded that of younger adults. Initial WFS plus coaching produced clinically significant weight loss in half of those treated, but additional data are needed on long-term maintenance. Barriers to accessing WFS plus coaching were lowered by using telephone rather than video technology to deliver coaching. Affordability and sustainability were enhanced by using bachelor’s degree–level staff rather than advanced practice physicians as coaches.
Limitations
This study has limitations. First, the 12-week intervention was briefer than the 6 to 12 months of standard care, and study-provided digital tools were removed at end of treatment, likely constraining weight loss. Second, the sample was relatively well-educated and mostly female, limiting generalizability. Third, the study entry criterion requiring smartphone access might have biased the sample toward higher technology literacy, although only 17 of 4500 screened potential participants (0.4%) were excluded for lacking a smartphone. Fourth, the effectiveness of the optimized obesity intervention strategy that offers WFS plus coaching initially still needs to be evaluated for comparative effectiveness relative to a control condition in a randomized clinical trial.
Conclusions
A wireless feedback system (Wi-Fi activity tracker and scale with smartphone app to provide daily feedback) was not noninferior to the same system with added coaching. No step-up intervention or, in exploratory analyses, treatment sequence was superior to another for facilitating weight loss among trial participants initially unresponsive to the interventions. Continued efforts are needed to identify strategies for weight loss management and for accurately selecting effective interventions for different individuals to achieve weight loss goals.
Study Protocol
Supplementary Figures and Tables
Fidelity Checklists
Data Sharing Statement
References
- 1.Stierman B, Afful J, Carroll MD, et al. National Health and Nutrition Examination Survey 2017-March 2020 Prepandemic Data Files—Development of Files and Prevalence Estimates for Selected Health Outcomes. Vol 158. National Health Statistics Reports; 2021. [Google Scholar]
- 2.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 3.Ward ZJ, Willett WC, Hu FB, Pacheco LS, Long MW, Gortmaker SL. Excess mortality associated with elevated body weight in the USA by state and demographic subgroup: a modelling study. EClinicalMedicine. 2022;48:101429. doi: 10.1016/j.eclinm.2022.101429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi: 10.1371/journal.pone.0247307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(11):1163-1171. doi: 10.1001/jama.2018.13022 [DOI] [PubMed] [Google Scholar]
- 6.Wing RR. The challenge of defining the optimal lifestyle weight loss intervention for real-world settings. JAMA. 2022;328(22):2213-2214. doi: 10.1001/jama.2022.21908 [DOI] [PubMed] [Google Scholar]
- 7.Yanovski SZ, Yanovski JA. Toward precision approaches for the prevention and treatment of obesity. JAMA. 2018;319(3):223-224. doi: 10.1001/jama.2017.20051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guastaferro K, Collins LM. Achieving the goals of translational science in public health intervention research: the Multiphase Optimization Strategy (MOST). Am J Public Health. 2019;109(S2):S128-S129. doi: 10.2105/AJPH.2018.304874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spring B. Sound health care economics: provide the treatment needed (not less, not more). Health Psychol. 2019;38(8):701-704. doi: 10.1037/hea0000782 [DOI] [PubMed] [Google Scholar]
- 10.Collins LM. Optimization of Behavioral, Biobehavioral, and Biomedical interventions: The Multiphase Optimization Strategy (MOST). Springer; 2018. [Google Scholar]
- 11.Spring B, Pfammatter AF, Marchese SH, et al. A factorial experiment to optimize remotely delivered behavioral treatment for obesity: results of the Opt-IN study. Obesity (Silver Spring). 2020;28(9):1652-1662. doi: 10.1002/oby.22915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavori PW, Dawson R. A design for testing clinical strategies: biased adaptive within-subject randomization. J R Stat Soc Ser A Stat Soc. 2000;163(1):29-38. doi: 10.1111/1467-985X.00154 [DOI] [Google Scholar]
- 13.Kidwell KM, Almirall D. Sequential, multiple assignment, randomized trial designs. JAMA. 2023;329(4):336-337. doi: 10.1001/jama.2022.24324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfammatter AF, Nahum-Shani I, DeZelar M, et al. SMART: Study protocol for a sequential multiple assignment randomized controlled trial to optimize weight loss management. Contemp Clin Trials. 2019;82:36-45. doi: 10.1016/j.cct.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 16.Spring B, Pellegrini CA, Pfammatter A, et al. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obesity (Silver Spring). 2017;25(7):1191-1198. doi: 10.1002/oby.21842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broglio K. Randomization in clinical trials: permuted blocks and stratification. JAMA. 2018;319(21):2223-2224. doi: 10.1001/jama.2018.6360 [DOI] [PubMed] [Google Scholar]
- 18.Sedgwick P. Treatment allocation in trials: block randomisation. BMJ. 2014;348:g2409. doi: 10.1136/bmj.g2409 [DOI] [PubMed] [Google Scholar]
- 19.Waring ME, Schneider KL, Appelhans BM, et al. Early-treatment weight loss predicts 6-month weight loss in women with obesity and depression: implications for stepped care. J Psychosom Res. 2014;76(5):394-399. doi: 10.1016/j.jpsychores.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spring B, Pellegrini C, Nahum-Shani I. How long does it take to identify nonresponders to weight loss treatment. Obesity (Silver Spring). 2014;22S(56). [Google Scholar]
- 21.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carver C, Scheier M. On the Self Regulation of Behavior. Cambridge University Press; 1998. doi: 10.1017/CBO9781139174794 [DOI] [Google Scholar]
- 23.Spring B, Pellegrini C, McFadden HG, et al. Multicomponent mHealth intervention for large, sustained change in multiple diet and activity risk behaviors: the make better choices 2 randomized controlled trial. J Med Internet Res. 2018;20(6):e10528. doi: 10.2196/10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spring B, Stump T, Penedo F, Pfammatter AF, Robinson JK. Toward a health-promoting system for cancer survivors: Patient and provider multiple behavior change. Health Psychol. 2019;38(9):840-850. doi: 10.1037/hea0000760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spring B, Stump TK, Battalio SL, et al. Digitally characterizing the dynamics of multiple health behavior change. Health Psychol. 2021;40(12):897-908. doi: 10.1037/hea0001057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin AW, Morgan N, Ward D, et al. Comparative validity of mostly unprocessed and minimally processed food items differs among popular commercial nutrition apps compared with a research food database. J Acad Nutr Diet. 2022;122(4):825-832.e1. doi: 10.1016/j.jand.2021.10.015 [DOI] [PubMed] [Google Scholar]
- 27.Stich C, Knäuper B, Tint A. A scenario-based dieting self-efficacy scale: the DIET-SE. Assessment. 2009;16(1):16-30. doi: 10.1177/1073191108322000 [DOI] [PubMed] [Google Scholar]
- 28.Hartmann-Boyce J, Jebb SA, Fletcher BR, Aveyard P. Self-help for weight loss in overweight and obese adults: systematic review and meta-analysis. Am J Public Health. 2015;105(3):e43-e57. doi: 10.2105/AJPH.2014.302389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Education Research. 2007;22(5):691-702. doi: 10.1093/her/cyl148 [DOI] [PubMed] [Google Scholar]
- 30.Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ. 1996;313(7048):36-39. doi: 10.1136/bmj.313.7048.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas JG, Bond DS, Raynor HA, Papandonatos GD, Wing RR. Comparison of smartphone-based behavioral obesity treatment with gold standard group treatment and control: a randomized trial. Obesity (Silver Spring). 2019;27(4):572-580. doi: 10.1002/oby.22410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wieland LS, Falzon L, Sciamanna CN, et al. Interactive computer-based interventions for weight loss or weight maintenance in overweight or obese people. Cochrane Database Syst Rev. 2012;8(8):CD007675. doi: 10.1002/14651858.CD007675.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spring B, Duncan JM, Janke EA, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med. 2013;173(2):105-111. doi: 10.1001/jamainternmed.2013.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jennrich RI, Schluchter MD. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42(4):805-820. doi: 10.2307/2530695 [DOI] [PubMed] [Google Scholar]
- 35.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7(1-2):305-315. doi: 10.1002/sim.4780070131 [DOI] [PubMed] [Google Scholar]
- 36.Nahum-Shani I, Qian M, Almirall D, et al. Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods. 2012;17(4):457-477. doi: 10.1037/a0029372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nahum-Shani I, Almirall D, Yap JRT, et al. SMART longitudinal analysis: a tutorial for using repeated outcome measures from SMART studies to compare adaptive interventions. Psychol Methods. 2020;25(1):1-29. doi: 10.1037/met0000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137-150. doi: 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233-239. doi: 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unick JL, Pellegrini CA, Demos KE, Dorfman L. Initial weight loss response as an indicator for providing early rescue efforts to improve long-term treatment outcomes. Curr Diab Rep. 2017;17(9):69-69. doi: 10.1007/s11892-017-0904-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antoun J, Itani H, Alarab N, Elsehmawy A. The effectiveness of combining nonmobile interventions with the use of smartphone apps with various features for weight loss: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2022;10(4):e35479. doi: 10.2196/35479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vercruyssen A, Schirmer W, Geerts N, Mortelmans D. How “basic” is basic digital literacy for older adults? insights from digital skills instructors. Front Educ. Published online September 13, 2023. doi: 10.3389/feduc.2023.1231701 [DOI] [Google Scholar]
- 43.Finkelstein R, Wu Y, Brennan-Ing M. Older adults’ experiences with using information and communication technology and tech support services in New York City: findings and recommendations for post-pandemic digital pedagogy for older adults. Front Psychol. 2023;14:1129512. doi: 10.3389/fpsyg.2023.1129512 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Supplementary Figures and Tables
Fidelity Checklists
Data Sharing Statement