Abstract
Ethnopharmacological relevance:
The two Tinospora species, T. crispa and T. sinensis, native to Southeast Asia, are integral components of various traditional preparations with structure-function claims to treat various disorders, including diabetes and inflammation.
Aim of the study:
To assure the safety of the botanicals finished products, herb-drug interaction potential of T. crispa and T. sinensis was investigated by testing their extracts and compounds for in vitro activation of the pregnane X-receptor (PXR) and the modulation of CYP3A4 isozyme, selectively.
Materials and methods:
A total of sixteen fully characterized phytochemicals from T. crispa and T. sinensis were evaluated for PXR activation by luciferase reporter gene assay. CYP3A4 inhibition studies were carried out for eleven compounds. In addition, docking studies were performed to elucidate the possible binding modes to the PXR by the compounds using computational methods.
Results:
Significant activation of PXR (2-fold) was observed for both extracts and non-polar fractions of T. crispa. Among the pure compounds, columbin showed highest activation of PXR (3-fold), which was comparable with the positive control, rifampicin. Vital interactions were predicted with docking simulation of PXR-columbin complex with critical amino acid residues (Trp-299) that are known for the activation of PXR. The methanolic extracts of T. crispa and T. sinensis also showed considerable CYP3A4 inhibition.
Conclusion:
T. crispa and T. sinensis, both demonstrated the potential to mediate herb-drug interaction through PXR activation and inhibition of CYP3A4 isozyme. Moreover, the elucidation of the potential to induce herb-drug interaction, by the phytochemicals of these Tinospora plants, thereby supports the need for further investigation to establish the clinical relevancy of these constituents for possible adverse interactions with pharmaceutical drugs.
Keywords: Herb-drug interaction, Tinospora crispa, Tinospora sinensis, Columbin, Furanoditerpenoids
1. Introduction
The medicinal plants of the genus Tinospora, including T. crispa, T. sinensis and T. cordifolia in particular, have traditional significance in the therapy of numerous ailments. T. crispa and T. sinensis, native to Southeast Asia, are integral components of various traditional preparations with structure-function claims to treat various disorders, including diabetes and inflammation. These two species are of interest for research for a better understanding of their safety profile and associated health hazard due to their substitution for T. cordifolia. According to Ayurveda, T. cordifolia (aka Guduchi) is an adaptogen with antipyretic, anti-inflammatory, antirheumatic, spasmolytic, hypoglycemic, and hepatoprotective properties. Particularly the stems of T. cordifolia have been traditionally practiced for treating various ailments such as fever, jaundice, diarrhea, bone fractures, leucorrhea, skin diseases, poisonous bites, and eye disorders (Chi et al., 2016). Akin to T. cordifolia, the stems of T. crispa (aka Boraped) are widely used for treating several disorders in Malaysia, Thailand, and other south Asian countries (Ahmad et al., 2016). T. sinensis (aka Kuan Jin Teng), another botanically close mimic, is used in China to treat bone fractures, lumbar disc herniation, and chronic rheumatism (Chi et al., 2016). The possibility of their substitution with T. cordifolia coupled with reports of mislabeling of dietary supplements containing either of the two species with labels of T. cordifolia further highlighted the need to probe the phytoconstituents in T. crispa and T. sinensis. The most likely attributes for the ongoing (un) intentional adulteration or substitution of T. cordifolia with these two species in global markets are overlapping geographical occurrence, demand in traditional medicines, and morphological resemblance (Sereena et al., 2014) in addition to lack of appreciation for the varied phytochemical attributes at species level. An accurate and rapid UHPLC-UV-MS method has been reported to identify and differentiate T. crispa from other closely related species including T. sinensis both qualitatively and quantitatively (Parveen et al., 2020b).
The importance for probing T. crispa and T. sinensis gained significance following reports of hepatotoxicity from their use on the basis of traditional knowledge. T. cordifolia has been reported to be safe and exhibiting less interaction potential with CYP isozymes (Bahadur et al., 2016). T. crispa and T. sinensis, on the contrary, have been reported to cause hepatotoxicity as a result of self-medication by consumers of botanical supplements (Denis et al., 2007; Langrand et al., 2014; Pelclova et al., 2013). The furanoditerpenoid constituents in T. crispa and T. sinensis have been implicated with hepatotoxicity as a result of xenobiotic metabolism (Cachet et al., 2018; Huang et al., 2019). The metabolic activation of the furan ring into a cis-enedione intermediate by CYP 3A isozymes triggers hepatotoxicity (Peterson, 2013). Usually, the toxic conditions are reversed upon cessation of the consumption of herbal medicine (Calitz et al., 2015). It is also likely that herb-drug interaction may be an underlying cause for the occurrence of the reported cases of hepatotoxicity caused by T. crispa or T. sinensis.
Pregnane X receptor (PXR) and Cytochrome P450 enzymes (CYPs) are two of the three main modulators of drug-drug interactions that may lead to adverse drug effects as a result of alteration in their pharmacokinetics (Chauncey Leake, 1975; Sionneau P, 1995). PXR, being a nuclear receptor that regulates the expression of drug metabolizing enzymes and transporters, is known to regulate xenobiotic metabolism. There has been a recent surge in interest in this target as the activation of PXR by herbal constituents has been identified to be responsible for herb-drug interactions that could render clinical drugs less effective due to enhanced clearance. St John’s wort is a well-studied example of causing herb-drug interaction through PXR-mediated mechanism (Moore et al., 2000). PXR has a vast control upon many metabolic enzymes and transporters. So far, it has been shown to control the target genes of phase I CYP450 (CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A1, CYP3A4, CYP3A5, CYP3A7, CYP4F12, CYP24, and CYP27A1), phase II uridine diphosphate (UDP)-glucuronosyltransferases (UGT1A1, UGT1A3, UGT1A4, UGT1A6, and UGT1A9), sulfotransferases (Sult2a1), and glutathione S-transferases (Gsta2, GSTA4). In addition to the carboxylesterases and phase III P-glycoprotein (MDR1/ABCB1), multidrug resistance-associated protein 1 (Abcc1), multidrug resistance-associated protein 2 (Abcc2), multidrug resistance-associated protein 3 (Abcc3) and organic anion transporting polypeptide 2 (OATP2) (Hogle et al., 2018). Parallel to the other nuclear receptors, it functions as a ligand-induced transcriptional factor. The binding of the ligand elicits a conformational change in the protein that initiates a cascade of events leading to a controlled and specific genetic induction. As a result, PXR regulates the expression of many metabolic enzymes and transporters responsible for xenobiotics’ excretion. In addition, it has a vital role in endobiotic synthesis, metabolism and homeostasis including bile acids, lipids, glucose, bilirubin, vitamins and other steroidal hormones (Tirona and Kim, 2005).
The availability of Tinospora dietary supplements and the possibility of their concomitant use with the clinical drugs highlight the need to investigate the potential of these two Tinospora species and their phytochemicals to affect drug-metabolizing enzymes and transporters. Several recent studies have shown that dietary phytochemicals can exhibit dual-functional behavior. They function as agonists for nuclear receptors and increase the expression of metabolically active CYP proteins. At the same time, they may act as catalytic inhibitors for post-translationally matured proteins that are actively involved in phase-I drug metabolism (e.g. CYPs and P-gp) (Husain et al., 2021a). Cytochrome P450s (CYPs) is a superfamily of membrane-bound, heme-containing mixed-function oxygenases which play a vital role in phase-I metabolism of drugs and xenobiotics as well as biosynthesis of endobiotics such as steroid hormones, cholesterol, and bile acids (Husain et al., 2021b). PXR is a key regulator of xenobiotic-inducible CYP3A4 which constitutes about 46% of total cytochrome P450 enzymes and is involved in the metabolism of >50% of the medicines marketed (Kamel and Harriman, 2013). Activation of the PXR mediates CYP3A4 induction as a mechanism to metabolize and eliminate xenobiotics from the body (Sinz, 2013). Several phytoconstituents have been frequently reported to interact with CYP3A4 (such as hyperforin from St John’s wort and bergamottin from grapefruit), predisposing the patients to unpleasant episodes of herb-drug interactions.
In our continued quest to probe the safety of botanicals, the methanolic extracts and phytochemicals of T. crispa and T. sinensis were evaluated in vitro to gauge their potential to activate the PXR which may result in inducing the expression of CYP3A4 enzyme. To date, no PXR activation studies have been reported for T. crispa and T. sinensis to the best of our knowledge. The methanolic extract of T. crispa has been reported to inhibit the drug metabolism mediated by CYP3A4 and CYP2D6 isozymes (Subehan Usia et al., 2006; Usia et al., 2006). However, the active phytoconstituents responsible for the inhibition were not identified. In case of T. sinensis, CYP3A4 inhibitory activity has not been reported for either the extract or its phytochemicals.
Hence, the study of PXR mediated drug metabolism is extensively important. We kept these findings in mind, and investigated the effect of Tinospora species on PXR mediated CYP3A4 induction. In vitro PXR and CYP3A4 assays are, thus, useful in predicting the risk of herb-drug interactions resulting from the concomitant use of herbal supplements and pharmaceutical drugs.
2. Material and methods
2.1. Preparation of plant extracts/fractions/compounds
Whole stems of T. crispa (NCNPR# 17091) and T. sinensis (NCNPR# 17003) were obtained from commercial sources. These were authenticated by co-TLC with voucher samples of T. crispa (NCNPR# 17320) and T. sinensis (NCNPR# 17322) respectively, deposited at the Botanical Repository of the National Center for Natural Products Research (NCNPR), University of Mississippi, Mississippi, United States. The methanolic extracts were prepared by the method of percolation followed by evaporation of solvent under reduced pressure in the rotary evaporator. The extracts were freeze dried for the complete removal of traces of solvents. The methanolic extract of T. crispa was fractionated with hexanes, chloroform, ethyl acetate and n-butanol successively and were available in sufficient quantities to be used in this study. However, fractionation and isolation procedures for T. sinensis extract ended in amounts that were insignificant due to which it was not considered for the study. The extracts/fractions of T. crispa and T. sinensis were subjected to repeated chromatographic procedures for isolation of phytochemicals. The characterization of the phytochemicals used in this study has been reported earlier (Parveen et al., 2020a, 2021). The structures of the isolated compounds were elucidated by 1D and 2D NMR and further confirmed by HRESIMS. The purity of compounds was confirmed by injecting concentrated standards (1 mg/mL) with detection by PDA and MS. Sixteen phytochemicals with purity higher than 95% were included in this study and their chemical structures are given in Fig. 1. These included tinosineside A, tinosinen, tinosposinoside, and cordifoliside C isolated from T. sinensis and borapetosides B, C and F, (2R,5R,6R,8R,9S, 10S,12S)-15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl)-cleroda-3, 13(16),14-trien-17,12-olid-18-oicacidmethyl ester, baenzigeride A, columbin, cycloeucalenol and N-trans-feruloyl tyramine isolated from T. crispa. Jatrorrhizine and palmatine were purchased from Wuhan ChemFaces Biochemical Co., Ltd (China). Magnoflorine and berberine were obtained from the NCNPR natural product repository.
Fig. 1.
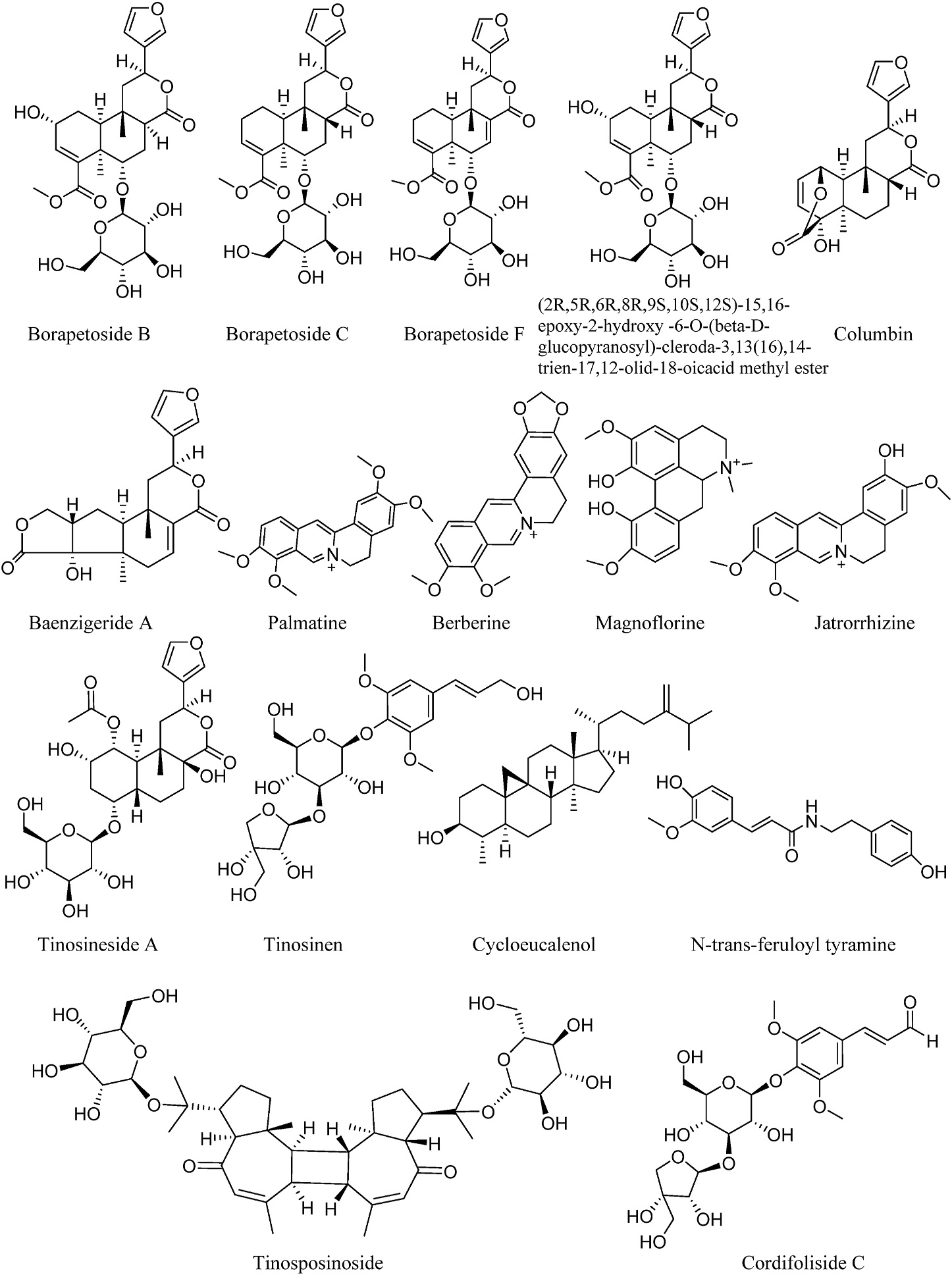
Structures of compounds employed in the study.
2.2. Pregnane X receptor activation by luciferase reporter gene assay
The methanolic extracts and the compounds of T. crispa and T. sinensis were tested for PXR activation by the method described earlier (Fantoukh et al., 2019b; Husain et al., 2021c). Briefly, transiently transfected HepG2 cells (ATCC) with pSG5-hPXR (25 μg) and PCR5 plasmid DNA (25 μg), were seeded in 96-well plates at a density of 50, 000 cells per well and incubated for 24 h for confluency. Test samples of extracts and compounds were added at desired concentrations (100, 50, and 25 μg/mL for extracts and 50, 25, and 12.5 μg/mL for compounds) followed by an additional incubation of 24 h. The media was aspirated, and luminescence was measured on the Spectramax M5 plate reader (Molecular devices) after the addition of 40 μL luciferase reagent (Promega Corporation). The results for PXR activation were calculated as fold increase in the luminescence of sample-treated cells compared to the vehicle-treated cells. Rifampicin (10, 5 and 2.5 μM) was used as the positive control.
2.3. Cytochrome P450 inhibition assay
CYP 3A4 inhibition assay was conducted as reported earlier (Crespi et al., 1997; Fantoukh et al., 2019) in a total volume of 200 μL in 96-well microplates. Test samples or positive controls were serially diluted in a solution (100 μL) of cofactors mix, control protein (0.05 mg of protein/mL), and G- 6-PDH to achieve six desired test concentrations (200, 66.67, 22.22, 7.41, 2.47, 0.82 μg/mL for extracts and 100, 33.33, 11.11, 3.70, 1.23, 0.41 μg/mL for compounds). The plates were incubated at 37 °C for 10 min. Initiation of the reaction was achieved by adding an enzyme-substrate mixture (100 μL), followed by incubation for 15 min. Termination of reaction was achieved by addition of 75 μL of ice-cold acetonitrile/0.5M Tris base (80:20). Fluorescence was measured on a Spectramax M5 plate reader (Molecular Devices) at specified excitation and emission wavelengths. IC50 values were obtained from concentration-response curves generated by plotting concentration versus % inhibition. Ketoconazole (1.0–0.004 μM) was used as the positive control. Only eleven compounds were tested for CYP3A4 inhibition activity while the remaining five could not be tested due to insufficient amounts.
2.4. PXR docking study
The X-ray crystal structure 1NRL (resolution: 2 Å) in a complex with SR12813 and the SRC-1 coactivator peptide (Watkins et al., 2003) was downloaded from the protein data bank (https://www.rcsb.org/). For docking simulation, the protein was loaded into Maestro (Schrödinger software). Chains A and C were deleted, whereas chains B and D were kept and processed with the protein preparation wizard. Default optimization parameters at pH 7.4 were applied. Further investigation for the protonation and sidechain orientation of the binding site residues was carried out using an interactive optimizer. Only water molecules with 2 H-bonds to non-waters were kept within 5 Å of the ligand. Geometry refinement of the protein-ligand complex was done. The grid box was generated without constraints. In this study, the PXR analyzed phytochemicals (16 compounds) were prepared in Maestro using the Lig-prep module. The prepared ligands were confined to the absolute configuration of the compounds reported in the native plant. The glycosides tested were prepared twice with and without their sugar units for comparison. The prepared ligands were docked into the generated grid using XP docking protocol, and the best docking pose was kept for each ligand. The estimated energy differences of the optimized ligand-protein complex were calculated using the prime MM-GBSA model in kcal/mol.
3. Results and discussion
The results of the in vitro testing of the extracts and phytochemicals of T. crispa and T. sinensis for PXR activation (Tables 1 and 2) and CYP3A4 inhibition (Table 3) are represented as mean ± SD of three independent experiments.
Table 1.
PXR activation by Tinospora species methanolic extracts/fractions.
| Test extracts and fractions | Fold induction in PXR activity at |
||
|---|---|---|---|
| 100 μg/mL | 50 μg/mL | 25 μg/mL | |
|
| |||
| T. sinensis crude methanolic | 2.24 ± 0.04 | 1.73 ± 0.07 | 1.51 ± 0.10 |
| T. crispa crude methanolic | 2.16 ± 0.004 | 2.23 ± 0.16 | 2.00 ± 0.38 |
| T. crispa hexane Fraction | 0.90 ± 0.19 | 2.50 ± 0.12 | 2.13 ± 0.44 |
| T. crispa chloroform Fraction | 1.81 ± 0.28 | 2.00 ± 0.48 | 1.78 ± 0.33 |
| T. crispa ethyl acetate Fraction | 1.68 ± 0.28 | 1.23 ± 0.12 | 1.12 ± 0.06 |
| T. crispa butanolic Fraction | 1.05 ± 0.03 | 1.01 ± 0.07 | 0.94 ± 0.14 |
| Rifampicina | 3.25 ± 0.08 | 3.24 ± 0.15 | 2.62 ± 0.33 |
Positive control; Test concentrations: 10, 5 and 2.5 μM.
Table 2.
PXR activation by phytochemicals of T. crispa and T. sinensis.
| Plant source | Test sample | Fold induction in PXR activity at |
||
|---|---|---|---|---|
| 50 μg/mL | 25 μg/mL | 12.5 μg/mL | ||
|
| ||||
| T.sinensis | Tinosineside A | 1.53 ± 0.11 | 1.50 ± 0.00 | 1.42 ± 0.06 |
| T. sinensis | Tinosinen | 1.42 ± 0.06 | 1.67 ± 0.02 | 1.48 ± 0.06 |
| T. sinensis | Tinosposinoside | 1.52 ± 0.15 | 1.55 ± 0.08 | 1.30 ± 0.08 |
| T. sinensis | Cordifoliside C | 1.95 ± 0.04 | 1.47 ± 0.03 | 1.22 ± 0.06 |
| T. crispa | Borapetoside B | NA | NA | NA |
| T. crispa | Borapetoside C | NA | NA | NA |
| T. crispa | Borapetoside F | NA | NA | NA |
| T. crispa | (2R, 5R,6R,8R,9S, 10S, 12S) –15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl) -cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester | 1.39 ± 0.11 | 1.01 ± 0.13 | 0.93 ± 0.04 |
| T. crispa | Baenzigeride A | 1.96 ± 0.16 | 1.54 ± 0.16 | 1.18 ± 0.09 |
| T. crispa | Columbin | 3.58 ± 0.61 | 2.89 ± 0.62 | 2.02 ± 0.02 |
| T .crispa | Cycloeucalenol | 2.42 ± 0.30 | 1.91 ± 0.23 | 1.42 ± 0.06 |
| T. crispa | N trans-feruloyl tyramine | 2.29 ± 0.41 | 2.16 ± 0.27 | 1.59 ± 0.07 |
| T. crispa | Palmatine | 1.43 ± 0.00 | 1.30 ± 0.00 | 1.08 ± 0.00 |
| Jatrorrhizine | 2.44 ± 0.05 | 1.94 ± 0.04 | 1.48 ± 0.01 | |
| Magnoflorine | 1.41 ± 0.12 | 1.16 ± 0.15 | 1.01 ± 0.15 | |
| Berberine | NA | NA | NA | |
NA-No activation.
Table 3.
In vitro CYP3A4 inhibition by phytochemicals of T. crispa and T. sinensis.
| Plant Source | Test sample | CYP 3A4 Inhibition - IC50 (μg/mL) |
|---|---|---|
|
| ||
| T. crispa | Methanolic extract | 5.90 ± 1.22 |
| T. crispa | Borapetoside B | 100.0 ± 0.00 |
| T. crispa | Borapetoside C | 37.50 ± 12.27 |
| T. crispa | Borapetoside F | 13.00 ± 0.82 |
| T. crispa | (2R, 5R,6R,8R,9S,10S, 12S) –15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl) -cleroda-3,13(16), 14-trien-17,12-olid-18-oic acid methyl ester | 29.00 ± 0.82 |
| T. crispa | N-trans-feruloyl tyramine | 2.70 ± 0.08 |
| T.crispa | Palmatine | 34.00 ± 0.00 |
| T. sinensis | Methanolic extract | 8.90 ± 0.73 |
| T. sinensis | Tinosineside A | 45.00 ± 5.72 |
| T. sinensis | Tinosinen | >100 |
| Berberine | 0.72 ± 0.17 | |
| Jatrorrhizine | 9.50 ± 2.29 | |
| Magnoflorine | 36.00 ± 3.27 | |
| Positive control | Ketoconazole | 0.03 ± 0.01 |
Phytochemicals interact with PXR and mediate herb-drug interaction by upregulating phase-I and phase-II metabolic enzymes and transporters due to increased transcriptional activity of PXR (Husain et al., 2021c). The methanolic extracts of both species activated PXR demonstrating activation higher than two fold at 100 μg/mL concentrations (Table 1). T. crispa extract seemed to be effective at lower concentrations as well. Among the fractions of T. crispa tested, hexane and the chloroform fractions caused a 2-fold or more activation of PXR at both 50 and 25 μg/mL. In contrast, the ethyl acetate fraction was not as active (<2-fold activation), and the butanolic fraction showed negligible fold-induction (Table 1). The PXR activation profile of phytochemicals from T. crispa and T. sinensis is shown in Table 2. Among the constituents from T. sinensis, cordifoliside C showed stronger PXR activation potential (1.95-fold at 50 μg/mL) in comparison to tinosineside A, tinosinen, and tinosposinoside (1.4–1.5-fold). Among the compounds isolated from T. crispa, columbin showed the highest PXR fold-activation (3.58-fold at 50 μg/mL) followed by baenzigeride A (1.96-fold), cycloeucalenol (2.42-fold), and N-trans-feruloyl tyramine (2.29-fold) whereas the borapetosides B, C and F did not show any activation of PXR. Among the alkaloids, jatrorrhizine was the most effective in activating PXR with 2.44, 1.94, and 1.48-fold induction at 50, 25, and 12.5 μg/mL, respectively.
Among all phytoconstituents, columbin, a furano-lactone diterpene, was the only compound that activated PXR greater than 2 -fold at the lowest tested concentration of 12.5 μg/mL, which aspired us to elucidate its interactions with the PXR binding site by docking studies (Fig. 2) and compare it with other similar furanoterpenoid glycosides (tinosineside A, borapetosides B, C, and F). The docking simulation results were found to be in accordance with the in vitro results. Columbin had a confound fit in the binding site of PXR along with multiple vital interactions known for PXR induction, including Trp-299 inevitable to the activity (Banerjee et al., 2016; Huber et al., 2021). On the contrary, the furanoditerpenoid glycosides returned with unfavorable docking poses and inferior interactions with critical residues. The furan ring in these furanoditerpenoids appears to play a vital role by occupying the hydrophobic groove through π-π interactions with the aromatic amino acids W299 and Y306. In addition, the docking poses of the glycosides were further contrasted with their respective aglycones, which led us to the conclusion that the presence of the sugar unit connected to the furanoterpenoid will flip the orientation of the furan ring to the opposite side to fit itself in the pocket as shown for borapetoside C (Fig. 3). The positioning of the sugar unit in proximity to the aromatic cage will circumvent the π-π bond necessary to stabilize the C-terminal activation function 2 helix in the inward position. After being stabilized in the inward position by ligand binding, the later C-terminal will interact with different transcriptional factors (Huber et al., 2021). The calculation of the MMGBSA energy, in combination with the pose analysis for the tested compounds and their hypothetical aglycone counterparts, supports our observation (Table 4). Our findings lead us to postulate that the highly polar glycosides could be more soluble in water extract of the herbal preparation. After ingestion of which, their possible hydrolysis with different glycosidases might potentially give rise to multiple furanoditerpenoid aglycones with greater potential for PXR activation.
Fig. 2.
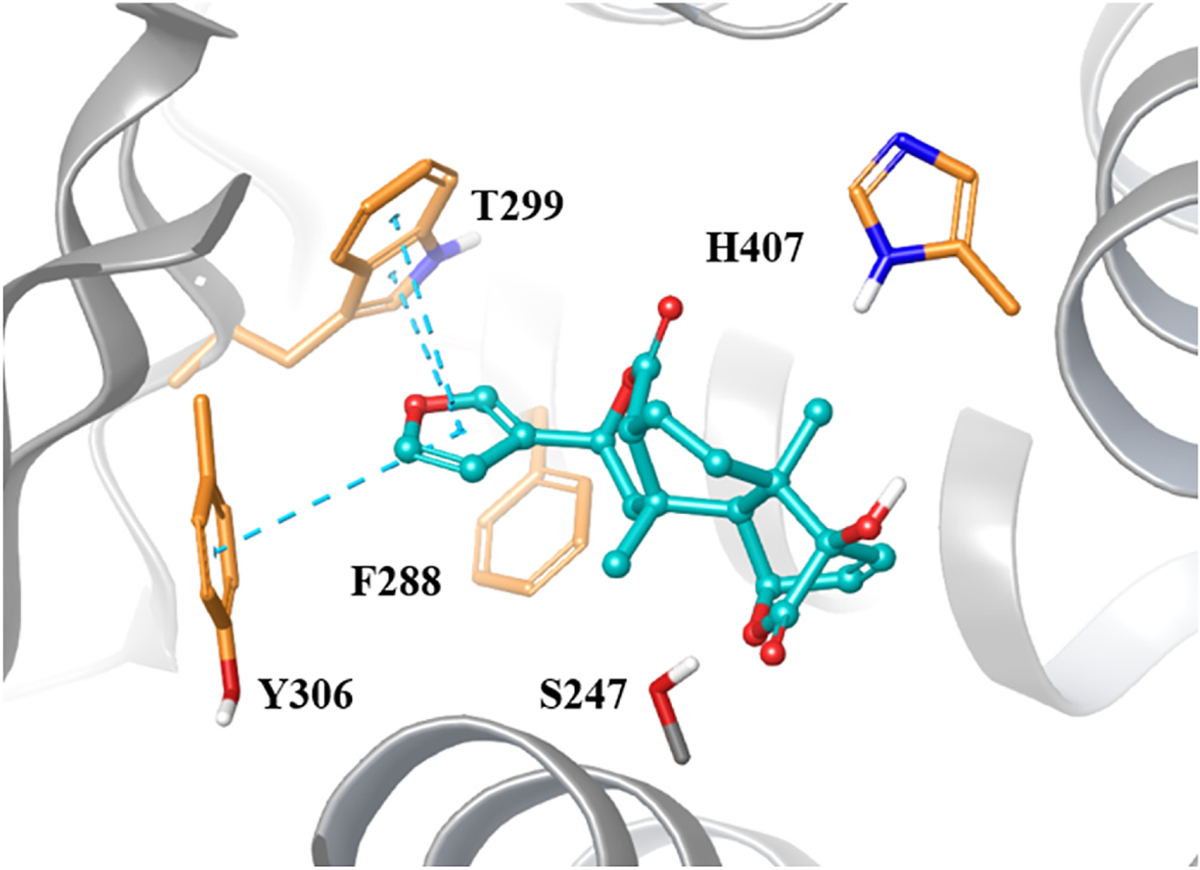
Columbin (represented in teal ball and stick) docked into the active site of PXR (PDB ID: 1NRL). Key amino acids found in the binding pocket are labeled (represented in thick orange tubes). The ligand docking pose emphasizes π-π interaction (Blue dashed lines) with the aromatic amino acids T299 and Y306. The figure was generated using Maestro.
Fig. 3.
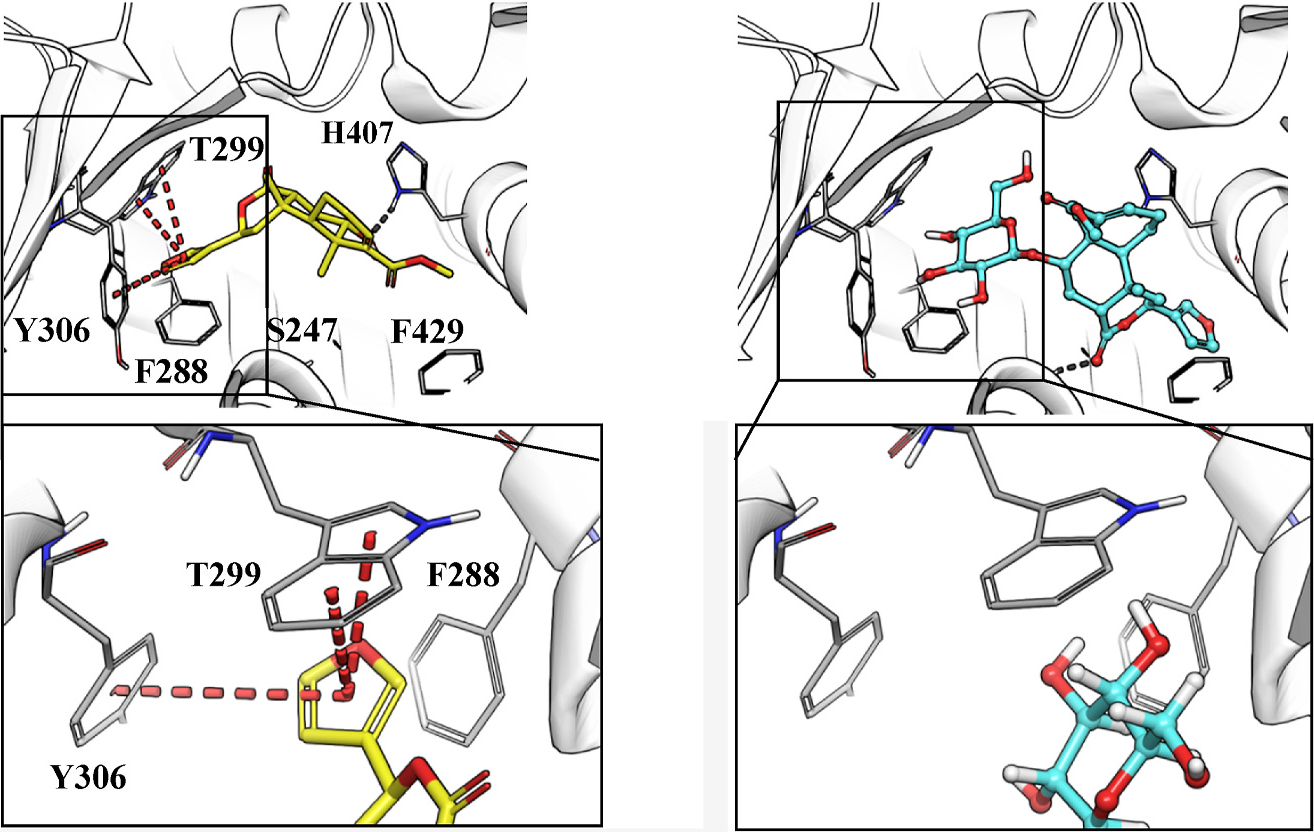
Comparison between the docking poses of glycoside, borapetoside C (upper right) represented in cyan ball and stick and its aglycone (upper left) represented in thick yellow tubes in the binding site of PXR (PDB ID:1NRL). π-π stacking is shown in red dashed lines, and hydrogen bonds in gray dashed lines. This figure is generated using Maestro.
Table 4.
MMGBSA energy calculated for the test compounds and their hypothetical aglycone.
| Ligand | MMGBSA energya |
|---|---|
|
| |
| Palmatine | −64.08 |
| Cordifoliside C | −62.96 |
| Tinosinen | −61.23 |
| N-trans-feruloyl tyramine | −60.20 |
| Borapetoside B aglycone | −59.98 |
| Magnoflorine | −59.05 |
| Borapetoside F aglycone | −57.32 |
| Borapetoside C | −53.74 |
| Borapetoside C aglycone | −53.57 |
| Columbin | −52.16 |
| Tinosineside A | −48.92 |
| Jatrorrhizine | −47.59 |
| (2R, 5R,6R,8R,9S,10S, 12S) -15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl) -cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester-aglycone | −47.20 |
| Borapetoside B | −45.72 |
| Tinosineside A aglycone | −42.73 |
| Borapetoside F | −37.79 |
| Cycloeucalenol | −37.47 |
| Baenzigeride A | −37.24 |
The relative energy is calculated in kcal/mol.
The results presented in Table 3, clearly showed, the methanolic extracts derived from T. crispa and T. sinensis significantly inhibited the catalytic activity of CYP3A4, and the concentrations responsible for 50% inhibition (IC50) were identified as 5.9 μg/mL and 8.9 μg/mL, respectively. Among the four major furanoditerpenoids, borapetoside B showed the least activity (IC50 100 μg/mL), whereas borapetoside F showed the highest inhibitory activity with an IC50 of 13 μg/mL. On the other hand, N-trans-feruloyl tyramine, a phenyl amide, showed stronger inhibitory activity (IC50 2.7 μg/mL) than borapetoside F. Phenylpropanoids are substrates for CYP450 enzymes, which are also responsible for their hydroxylation, as a post-translational modification, in their biosynthesis. Consequently, the substrate-inhibitor inter-relationships of these compounds are comprehensible.
Most plants in Tinospora genus have been reported to contain quaternary alkaloids viz berberine (Bisset and Nwaiwu, 1983), palmatine (Dong et al., 2010), magnoflorine (Iqbal Choudhary et al., 2010; Yusoff et al., 2014), and jatrorrhizine (Maurya et al., 2009). These alkaloids are also widely distributed in nature (Grycová et al., 2007). In our study, berberine and jatrorrhizine, showed stronger CYP3A4 inhibitory activity (IC50 0.7 and 9.5 μg/mL), whereas magnoflorine and palmatine were marginally active (IC50 36 and 34 μg/mL). Protoberberine alkaloids, in general, have been extensively studied for toxicity and safety (Chatuphonprasert et al., 2011; Lo et al., 2013); berberine has been reported to be cytotoxic to a higher degree than palmatine (Ma et al., 2010; Yi et al., 2013). Berberine has been demonstrated to decrease hepatic CYP total content and mediate a concentration dependent inhibition of CYP2E1 and CYP1A2 (Zhao et al., 2008). It has also been reported to inhibit CYP2C9, CYP2D6, CYP3A4 and CYP1A2 (Chatterjee and Franklin, 2003; Zhao et al., 2012). The nature of herbal remedies, however, as a multi-component system should not be overlooked. The presence of multiple phytoconstituents in extracts of T. crispa and T. sinensis might increase the chance of their interaction with CYP3A4 by synergism. Due to the increase in demand for complementary and alternative medicine, the sales of many herbal supplements have outpaced the proper understanding of their safety and efficacy. Mistakenly perceived all-natural as safe, many patients self-medicate their chronic diseases with herbal supplements without consulting their healthcare providers. Occasionally, this will predispose the patients to fatal results, especially when consuming narrow therapeutic index drugs concomitantly. Our studies indicate that the consumption of Tinospora formulated dietary supplements could increase half-life of conventional drugs which are CYP3A4 substrates. Subsequently, the different phytochemical classes of T. crispa and T. sinensis including furanoditerpenoids and alkaloids which have strong potential for CYP3A4 inhibition may significantly be contributing in hepatotoxicity. However, for strengthening this phenomenon, more in depth studies are required.
4. Conclusions
Plant extracts are multi-component systems that confer complexity to a biological response, whether efficacious or toxicological. The investigation undertaken constitutes the first report on the PXR activation studies for T. crispa and T. sinensis and their phytochemicals. To conclude, among the furanoditerpenoid constituents of T. crispa, columbin showed strong PXR activation potential, whereas borapetoside F caused strong CYP3A4 inhibition. N-trans feruloyl tyramine, an amide, and jatrorrhizine, an isoquinoline alkaloid, showed CYP3A4 inhibition and PXR activation potential. Based on these findings, we may infer that T. crispa and T. sinensis contain chemical constituents that could modulate the activity of the CYP 3A4 enzyme leading to a possibility of pharmacokinetic herb-drug interaction. Further investigation is needed to establish the role of these constituents in mediating herb-drug interactions clinically.
Acknowledgments
Research supported by “Science-Based Authentication of Dietary Supplements” funded by the United States Food and Drug Administration [grant number 2U01FD004246–06]. Ms. Olivia Dale for her excellent technical support in bioassays. Researchers Supporting Project number (RSP2022R430), King Saud University, Riyadh, Saudi Arabia.
Abbreviations
- PXR
Pregnane X Receptor
- MMGBSA
Molecular Mechanics-Generalized Born Surface Area
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmad W, Jantan I, Bukhari SNA, 2016. Tinospora crispa (L.) Hook. f. & Thomson: a review of its ethnobotanical, phytochemical, and pharmacological aspects. Front. Pharmacol. 7, 59. 10.3389/fphar.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadur S, Mukherjee P, Milan Ahmmed S, Kar A, Harwansh R, Pandit S, 2016. Metabolism-mediated interaction potential of standardized extract of Tinospora cordifolia through rat and human liver microsomes. Indian J. Pharmacol. 48, 576–581. 10.4103/0253-7613.190758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Chai SC, Wu J, Robbins D, Chen T, 2016. Tryptophan 299 is a conserved residue of human pregnane X receptor critical for the functional consequence of ligand binding. Biochem. Pharmacol. 104, 131–138. 10.1016/j.bcp.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset N, Nwaiwu J, 1983. Quaternary alkaloids of Tinospora species. Planta Med. 48, 275–279. 10.1055/s-2007-969933. [DOI] [PubMed] [Google Scholar]
- Cachet X, Langrand J, Riffault-Valois L, Bouzidi C, Colas C, Dugay A, Michel S, Boucaud-Maitre D, 2018. Clerodane furanoditerpenoids as the probable cause of toxic hepatitis induced by Tinospora crispa. Sci. Rep. 8, 13520. 10.1038/s41598-018-31815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calitz C, du Plessis L, Gouws C, Steyn D, Steenekamp J, Muller C, Hamman S, 2015. Herbal hepatotoxicity: current status, examples, and challenges. Expet Opin. Drug Metabol. Toxicol. 11, 1551–1565. 10.1517/17425255.2015.1064110. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Franklin MR, 2003. Human cytochrome P450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab. Dispos. 31, 1391–1397. 10.1124/dmd.31.11.1391. [DOI] [PubMed] [Google Scholar]
- Chatuphonprasert W, Sangkawat T, Nemoto N, Jarukamjorn K, 2011. Suppression of beta-naphthoflavone induced CYP1A expression and lipid-peroxidation by berberine. Fitoterapia 82, 889–895. 10.1016/j.fitote.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Chi S, She G, Han D, Wang W, Liu Z, Liu B, 2016. Genus Tinospora: ethnopharmacology, phytochemistry, and pharmacology. Evid.-based Complement. Altern. Med 10.1155/2016/9232593, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi CL, Miller VP, Penman BW, 1997. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal. Biochem. 248, 188–190. 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- Denis G, Gérard Y, Sahpaz S, Laporte R, Viget N, Ajana F, Riff B, Mouton Y, Bailleul F, Yazdanpanah Y, 2007. Prophylaxie antipaludéenne par plantes médicinales : hépatite toxique à Tinospora crispa. Therapie 62, 271–272. 10.2515/therapie:2007036. [DOI] [PubMed] [Google Scholar]
- Dong LP, Chen CX, Ni W, Xie BB, Li JZ, Liu HY, 2010. A new dinorclerone diterpenoid glycoside from Tinospora sinensis. Nat. Prod. Res. 24, 13–17. 10.1080/14786410802253197. [DOI] [PubMed] [Google Scholar]
- Fantoukh OI, Albadry MA, Parveen A, Hawwal MF, Majrashi T, Ali Z, Khan SI, Chittiboyina AG, Khan IA, 2019a. Isolation, synthesis, and drug interaction potential of secondary metabolites derived from the leaves of miracle tree (Moringa oleifera) against CYP3A4 and CYP2D6 isozymes. Phytomedicine 60, 1–9. 10.1016/j.phymed.2019.153010. [DOI] [PubMed] [Google Scholar]
- Fantoukh OI, Dale OR, Parveen A, Hawwal MF, Ali Z, Manda VK, Khan SI, Chittiboyina AG, Viljoen A, Khan IA, 2019b. Safety assessment of phytochemicals derived from the globalized South African Rooibos Tea (Aspalathus linearis) through Interaction with CYP, PXR, and P-gp. J. Agric. Food Chem. 67, 4967–4975. 10.1021/acs.jafc.9b00846. [DOI] [PubMed] [Google Scholar]
- Grycová L, Dostál J, Marek R, 2007. Quaternary protoberberine alkaloids. Phytochemistry 68, 150–175. 10.1016/j.phytochem.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Hogle BC, Guan X, Folan MM, Xie W, 2018. PXR as a mediator of herb–drug interaction. J. Food Drug Anal. 26, S26–S31. 10.1016/j.jfda.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Te, Tu CY, Wang FY, Huang ST, 2019. Literature review of liver injury induced by Tinospora crispa associated with two cases of acute fulminant hepatitis. Compl. Ther. Med. 42, 286–291. 10.1016/j.ctim.2018.11.028. [DOI] [PubMed] [Google Scholar]
- Huber AD, Wright WC, Lin W, Majumder K, Low JA, Wu J, Buchman CD, Pintel DJ, Chen T, 2021. Mutation of a single amino acid of pregnane X receptor switches an antagonist to agonist by altering AF-2 helix positioning. Cell. Mol. Life Sci. 78, 317–335. 10.1007/s00018-020-03505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I, Bala K, Khan IA, Khan SI, 2021a. A review on phytochemicals, pharmacological activities, drug interactions, and associated toxicities of licorice (Glycyrrhiza sp.). Food Front. 10.1002/fft2.110. [DOI] [Google Scholar]
- Husain I, Dale OR, Manda V, Ali Z, Gurley BJ, Chittiboyina AG, Khan IA, Khan SI, 2021b. Bulbine natalensis (currently Bulbine latifolia) and select bulbine knipholones modulate the activity of AhR, CYP1A2, CYP2B6, and P-gp. Planta Med. 10.1055/a-1557-2113. [DOI] [PubMed] [Google Scholar]
- Husain I, Manda V, Alhusban M, Dale OR, Bae J-Y, Avula B, Gurley BJ, Chittiboyina AG, Khan IA, Khan SI, 2021c. Modulation of CYP3A4 and CYP2C9 activity by Bulbine natalensis and its constituents: an assessment of HDI risk of B. natalensis containing supplements. Phytomedicine 81, 153416. 10.1016/j.phymed.2020.153416. [DOI] [PubMed] [Google Scholar]
- Iqbal Choudhary, Ismail M, Muhammad Ali, Zulfiqar Shaari, Khozirah Lajis, Nordin H, Atta-ur-Rahman, 2010. Alkaloidal constitutents of Tinospora crispa. Nat. Prod. Commun. 5, 1747–1750. [PubMed] [Google Scholar]
- Kamel A, Harriman S, 2013. Inhibition of cytochrome P450 enzymes and biochemical aspects of mechanism-based inactivation (MBI). Drug Discov. Today Technol. 10, e177–e189. 10.1016/j.ddtec.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Langrand J, Regnault H, Cachet X, Bouzidi C, Villa AF, Serfaty L, Garnier R, Michel S, 2014. Toxic hepatitis induced by a herbal medicine: Tinospora crispa. Phytomedicine 21, 1120–1123. 10.1016/j.phymed.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Leake Chauncey, 1975. An Historical Account of Pharmacology to the Twentieth Century. 10.1086/351737. Springfield, IL. [DOI] [Google Scholar]
- Lo SN, Chang YP, Tsai KC, Chang CY, Wu TS, Ueng YF, 2013. Inhibition of CYP1 by berberine, palmatine, and jatrorrhizine: selectivity, kinetic characterization, and molecular modeling. Toxicol. Appl. Pharmacol. 272, 671–680. 10.1016/j.taap.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Ma B-L, Ma Y-M, Shi R, Wang T-M, Zhang N, Wang C-H, Yang Y, 2010. Identification of the toxic constituents in Rhizoma Coptidis. J. Ethnopharmacol. 128, 357–364. 10.1016/j.jep.2010.01.047. [DOI] [PubMed] [Google Scholar]
- Maurya R, Gupta P, Chand K, Kumar M, Dixit P, Singh N, Dube A, 2009. Constituents of Tinospora sinensis and their antileishmanial activity against Leishmania donovani. Nat. Prod. Res. 23, 1134–1143. 10.1080/14786410802682239. [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA, 2000. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. Unit. States Am 97, 7500–7502. 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen A, Ali Z, Fantoukh O, Alhusban M, Wang W, Chittiboyina AG, Khan IA, 2020a. Undescribed phenylpropanoid and a dimeric sesquiterpenoid possessing a rare cyclobutane ring from Tinospora sinensis. Nat. Prod. Res. 10.1080/14786419.2020.1752207. [DOI] [PubMed] [Google Scholar]
- Parveen A, Wang Y-H, Fantoukh O, Alhusban M, Raman V, Ali Z, Khan IA, 2020b. Development of a chemical fingerprint as a tool to distinguish closely related Tinospora species and quantitation of marker compounds. J. Pharm. Biomed. Anal. 178, 112894 10.1016/j.jpba.2019.112894. [DOI] [PubMed] [Google Scholar]
- Parveen A, Huang Y, Fantoukh O, Alhusban M, Raman V, Wang Y-H, Wang W, Ali Z, Khan IA, 2021. Rearranged clerodane diterpenoid from Tinospora crispa. Nat. Prod. Res. 35, 369–376. 10.1080/14786419.2019.1633648. [DOI] [PubMed] [Google Scholar]
- Pelclova D, Zakharov S, Navratil T, Hovda KE, Centre I, Republic C, 2013. Acute hepatitis and jaundice due to Tinospora sinensis or Tinospora crispa. Clin. Toxicol. 51, 252–378. 10.3109/15563650.2013.785188. [DOI] [Google Scholar]
- Peterson LA, 2013. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem. Res. Toxicol. 26, 6–25. 10.1021/tx3003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereena K, Remashree AB, Vemballur P, 2014. Histological, histochemical and phytochemical studies of the raw drug amrita from different raw drug markets of Kerala. Int. J. Interdiscip. Multidiscip. Stud. 1, 182–191. [Google Scholar]
- Sinz MW, 2013. Evaluation of pregnane X receptor (PXR) - mediated CYP3A4 drug-drug interactions in drug development. Drug Metab. Rev. 45, 3–14. 10.3109/03602532.2012.743560. [DOI] [PubMed] [Google Scholar]
- Sionneau P PZ, 1995. An Introduction to the Use of Processed Chinese Medicinals. Blue Poppy Press, Boulder, CO. [Google Scholar]
- Subehan Usia, T., Iwata H, Kadota S, Tezuka Y, 2006. Mechanism-based inhibition of CYP3A4 and CYP2D6 by Indonesian medicinal plants. J. Ethnopharmacol. 105, 449–455. 10.1016/j.jep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Kim RB, 2005. Nuclear receptors and drug disposition gene regulation. J. Pharm. Sci. 94, 1169–1186. 10.1002/jps.20324. [DOI] [PubMed] [Google Scholar]
- Usia T, Iwata H, Hiratsuka A, Watabe T, Kadota S, Tezuka Y, 2006. CYP3A4 and CYP2D6 inhibitory activities of Indonesian medicinal plants. Phytomedicine 13, 67–73. 10.1016/j.phymed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Davis-Searles PR, Lambert MH, Redinbo MR, 2003. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J. Mol. Biol. 331, 815–828. 10.1016/S0022-2836(03)00795-2. [DOI] [PubMed] [Google Scholar]
- Yi J, Ye X, Wang D, He K, Yang Y, Liu X, Li X, 2013. Safety evaluation of main alkaloids from Rhizoma Coptidis. J. Ethnopharmacol. 145, 303–310. 10.1016/j.jep.2012.10.062. [DOI] [PubMed] [Google Scholar]
- Yusoff M, Hamid H, Houghton P, 2014. Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules 19, 1201–1211. 10.3390/molecules19011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang J-J, Wang X, Bu X-Y, Lou Y-Q, Zhang G-L, 2008. Effect of berberine on hepatocyte proliferation, inducible nitric oxide synthase expression, cytochrome P450 2E1 and 1A2 activities in diethylnitrosamine- and phenobarbital-treated rats. Biomed. Pharmacother. 62, 567–572. 10.1016/j.biopha.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hellum BH, Liang A, Nilsen OG, 2012. The in vitro inhibition of human CYP1A2, CYP2D6 and CYP3A4 by tetrahydropalmatine, neferine and berberine. Phytother Res. 26, 277–283. 10.1002/ptr.3554. [DOI] [PubMed] [Google Scholar]


