Abstract
Background
Since the time of publication of the Women’s Health Initiative (WHI) study, menopausal symptom management has become more complex because of increased awareness of the risks associated with hormone replacement therapy (HRT). Currently, a wide range of management options is available. Some women take prescription drugs, and others use self care strategies, including lifestyle modifications, over‐the‐counter preparations and complementary and alternative therapies, such as herbal preparations, exercise programmes and relaxation techniques. Relaxation techniques consist of a group of behavioural interventions. They are considered relatively harmless, but their effectiveness in treating vasomotor symptoms and sleep disturbances remains debatable.
Objectives
To determine the effectiveness of relaxation techniques as treatment for vasomotor symptoms and associated sleep disturbances in perimenopausal and postmenopausal women.
Search methods
Searches of the following electronic bibliographic databases were performed in February 2014 to identify randomised controlled trials (RCTs): the Cochrane Menstrual Disorders and Subfertility Group Specialised Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AMED, PsycINFO, Social Science Citation Index and CINAHL. Handsearches of trial registers, relevant journals and published conference abstracts were also performed.
Selection criteria
RCTs were included if they compared any type of relaxation intervention with no treatment or other treatments (except hormones) for vasomotor symptoms in symptomatic perimenopausal/postmenopausal women.
Data collection and analysis
Two review authors selected studies, assessed quality and extracted data. Included studies were combined, if appropriate, by using a random‐effects model to calculate pooled mean differences and 95% confidence intervals.
Main results
Four studies were eligible for inclusion (281 participants): Two studies compared relaxation with electroacupuncture or superficial needling, one study compared relaxation with paced respiration or placebo control (α‐wave electroencephalographic biofeedback) and one study compared relaxation with no treatment.
No evidence was found of a difference between relaxation and acupuncture or superficial needle insertion in the number of hot flushes per 24 hours (mean difference (MD) 0.05, 95% confidence interval (CI) ‐1.33 to 1.43, two studies, 72 participants, I2 = 0%; very low‐quality evidence). Nor did any evidence suggest a difference between the two interventions in hot flush severity, measured using the Kupperman Index (MD ‐1.32, 95% CI ‐5.06 to 2.43, two studies, 72 participants, I2 = 0%; very low‐quality evidence).
The other two studies found no clear evidence of a difference in hot flush frequency between relaxation and paced respiration, placebo or no treatment. The data for these comparisons were unsuitable for analysis.
None of these studies reported night sweats, sleep disturbances associated with night sweats or adverse effects as an outcome.
The main limitations of identified evidence were lack of data, imprecision and failure to report study methods in adequate detail.
Authors' conclusions
Evidence is insufficient to show the effectiveness of relaxation techniques as treatment for menopausal vasomotor symptoms, or to determine whether this treatment is more effective than no treatment, placebo, acupuncture, superficial needle insertion or paced respiration.
Keywords: Female, Humans, Middle Aged, Perimenopause, Postmenopause, Acupuncture Therapy, Acupuncture Therapy/methods, Electroacupuncture, Hot Flashes, Hot Flashes/therapy, Neurofeedback, Neurofeedback/methods, Randomized Controlled Trials as Topic, Relaxation Therapy, Relaxation Therapy/methods
Plain language summary
Relaxation for perimenopausal and postmenopausal symptoms
Review question: We wanted to discover whether relaxation techniques were better or worse than other interventions, such as acupuncture, in the management of menopausal symptoms. We reviewed the evidence on the effects of these techniques on hot flushes, night sweats and sleep disturbances in menopausal women.
Background: Management of menopausal symptoms, such as hot flushes, depressed mood or sleep disturbances, has become more complicated because of increased awareness of the risks associated with hormone replacement therapy (HRT). Options include prescription drugs and self care strategies such as relaxation techniques. Relaxation techniques are thought to be relatively harmless, but their effectiveness in treating hot flushes and sleep disturbances remains unclear.
Study characteristics: We found four randomised controlled studies, with 281 participants. Relaxation was compared with electroacupuncture, superficial needling, paced respiration, placebo and no treatment. The age range of participants was 30 to 77 years. These trials were conducted in Sweden, the UK and the USA. No study was funded by an agency with commercial interest in the results of the study. The evidence is current to February 2014.
Key results: Evidence is insufficient to show the effectiveness of relaxation techniques as treatment for menopausal vasomotor symptoms, or to determine whether this treatment is more effective than no treatment, placebo, acupuncture, superficial needle insertion or paced respiration. No evidence indicates that relaxation reduces the number of hot flushes per 24 hours or their severity. None of the studies reported night sweats, sleep disturbances associated with night sweats or adverse effects as an outcome.
Quality of the evidence: The quality of the evidence was very low. The main limitations of identified evidence included lack of data, imprecision and failure to report study methods in adequate detail.
Summary of findings
for the main comparison.
| Relaxation compared with acupuncture for hot flushes | |||||
|
Population: perimenopausal and postmenopausal women with vasomotor symptoms Intervention: relaxation Comparison: acupuncture or superficial needling | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Relaxation versus acupuncture or superficial needling | |||||
|
Change in number of hot flushes/24 h (follow‐up: 12 weeks) |
Mean frequency of hot flushes per 24 hours in the relaxation group was 0.05 lower | 1.33 to 1.43 | 72 (2) | ⊕⊝⊝⊝ very low quality1,2 | |
|
Improvement in severity of hot flushes (follow‐up: 12 weeks) |
Mean hot flush severity score in relaxation group was 1.32 points lower on a 1 to 16 scale | ‐5.06 to 2.43 | 72 (2) | ⊕⊝⊝⊝ very low quality1,2 | |
| Adverse effects | This outcome was not reported | ||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Very serious imprecision: small sample size, confidence intervals compatible with substantial benefit from either intervention or with no effect.
2Studies did not report adequate details about methods used.
Background
Description of the condition
Reduced oestrogen levels during the menopause contribute to menopausal symptoms (NIH 2005). The major systems affected are the endocrine, reproductive and central nervous systems (WHO 1996; STRAW 2000). It is estimated that 85% of menopausal women report at least one symptom, such as hot flushes, depressed mood or sleep disturbances (McKinlay 1992). The prevalence of menopausal symptoms varies widely and may be influenced by a range of factors, including climate, diet, lifestyle, women’s roles and attitudes regarding the end of reproductive life and aging (McKinlay 1992; Freeman 2007). Many women seek treatment for vasomotor symptoms (hot flushes, night sweats) and sleep disturbances (McKinlay 1992; Fauci 1997; Guthrie 2003; McMillan 2004). Severe types of hot flushes commonly cause sleep disruption (Speroff 2000) and are strongly or moderately linked to the menopause (NIH 2005). Peak prevalence of hot flushes occurs during the late menopausal transition (the so‐called late perimenopause in several studies) and during early post menopause (Mathews 1990; Holte 1992; Kaufert 1992; Dennerstein 2000; Freeman 2001; Gold 2004; Williams 2008). According to Straw et al, perimenopause is defined as about or around menopause (STRAW 2000). The prevalence of vasomotor symptoms has been reported to be as high as 79% in perimenopausal women and 65% in postmenopausal women. Women with daily vasomotor symptoms had an average of 2.5 very mild, 2.5 mild, 2.6 moderate, 2.5 severe and 1.4 very severe episodes of daytime hot flushes in a typical day. Women with night sweats had an average of 2.4 moderate, 3.2 severe and 2.7 very severe episodes of night sweats in a typical night (Williams 2008).
Several studies have shown that elevated sympathetic activation, acting through central alpha2‐adrenergic receptors, contributes to the initiation of hot flushes (Bruck 1980; Freedman 2005). Hot flushes are triggered by small elevations in core body temperature (Bruck 1980; Freedman 2005), and clonidine was found to consistently and significantly reduce flush frequency (Freedman 2005). Moreover, one study showed that injection of yohimbine, an alpha2‐adrenergic antagonist that raises the levels of brain norepinephrine, provoked hot flushes in symptomatic women; injection of clonidine, an alpha2‐adrenergic agonist that reduces brain norepinephrine, ameliorated them (Goldberg 1994). These data suggest that elevated sympathetic activation, acting through central alpha2‐receptors, plays a role in the initiation of hot flushes. A steep decline in oestrogen levels during the menopause is probably involved because oestrogens can modulate the receptors (El‐Mas 2004). It is generally believed that hot flushes produce arousals and awakenings from sleep. Many epidemiological studies have confirmed the presence of sleep disturbances during the menopausal transition (Speroff 2000; Barton 2001; Celik 2002; Celentano 2003; Freedman 2004).
Description of the intervention
A variety of treatments for menopausal symptoms have been studied in randomised controlled trials (RCTs) (ERTA 2005; NIH 2005). Oestrogen plus progestin is the standard treatment, and oestrogen alone is used if the woman has had a previous hysterectomy. Such therapies, commonly known as hormone replacement therapy (HRT), were widely prescribed for menopausal women for many years. After publication of the Women’s Health Initiative (WHI) study, a marked global decline in the use of HRT occurred, probably as the result of increased awareness of associated risks (NAMS 2004). Combined, continuous HRT significantly increased the risk of venous thromboembolism or a coronary event (after one year’s use), stroke and gallbladder disease (after three years’ use) and breast cancer (after five years’ use) (Marjoribanks 2012). Long‐term oestrogens‐only HRT also significantly increased the risk of stroke and gallbladder disease (Marjoribanks 2012). Therefore, decisions on how to treat menopausal symptoms require a balance between possible risks and benefits (NIH 2005; Mosconi 2009). Progestin only shows mixed results for the amelioration of vasomotor symptoms (Hickey 2005). A few trials reported no significant difference between testosterone plus oestrogens and oestrogen alone in the treatment of severe hot flushes, vaginal dryness or sleep problems (ERTA 2005). Alternative treatments that have been investigated include antidepressants, isoflavone and other phyto‐oestrogens, botanicals, acupuncture and behavioural interventions (ERTA 2005; NIH 2005). Relaxation techniques, a behavioural intervention, appear to reduce sympathetic activity (Hjemdahl 1989; Lee 1989) while lowering blood pressure. This effect may be explained by reduced plasma norepinephrine levels (Hjemdahl 1989).
Cognitive techniques (Payne 2005) include (1) self awareness, a process of awareness of the real self apart from the body, tribulations, emotions and excitement. This technique begins with cessation of body movement and control of the limbs, speech and sense organs; (2) imagery, the creation of a mental picture of a safe, peaceful, restful, beautiful and happy place or event; (3) goal‐directed visualisation, the discovery of one’s emotional and spiritual balance to fulfil goals; (4) autogenic training, involving the production of a profound state of physical relaxation, bodily health and mental peace by creating a feeling of warmth and heaviness throughout the body; and (5) meditation, a process selected to calm down a busy mind. Relaxation techniques (Payne 2005) include (1) Jacobson progressive relaxation, which requires an understanding of neural physiology to calm down the mind and nervous system; (2) a modified version of Jacobson progressive relaxation, known as Bernstein and Borkovec’s version, which involves the number and duration of training sessions, the duration of muscle tension and release and the use of suggestion to facilitate relaxation; (3) Everly and Rosenfeld’s passive relaxation, which focuses on muscle relaxation; (4) Madder release, which employs mainly physical exercise to relax both the mind and the body; (5) Ost’s applied relaxation, which involves the use of rapid relaxation whenever symptoms appear (Ost 1987); (6) Poppen’s behavioural relaxation training, which stresses the use of different self control techniques appropriate for each environmental stress; (7) the Mitchell method, which involves a physiological technique of relieving stress‐induced muscle tension by relaxing the whole or a part of the body; (8) the Alexander technique, which helps a person discover a new balance in the body by re‐educating the mind and body to release unnecessary tension; and (9) Benson’s relaxation response, which involves repetition of a word, sound, prayer or muscular activity to oppose the fight‐or‐flight response. Refocusing on repetition when other thoughts intrude is a determining factor that influences cognitive behaviour.
This review concentrated on the use of relaxation techniques for the treatment of menopausal symptoms. These relaxation techniques were based on physiological principles of somatic or cognitive relaxation, or both. Two well‐known somatic relaxation techniques for vasomotor symptoms are paced respiration (slow, controlled diaphragmatic breathing) and muscle relaxation. The most common forms of muscle relaxation are progressive muscle relaxation and passive muscle relaxation (Benson 2000).
How the intervention might work
It has been suggested that relaxation techniques effectively reduce muscle sympathetic activity and venous norepinephrine concentrations, leading to lower blood pressure (Hjemdahl 1989; Lee 1989). Paced respiration and muscle relaxation reduce the frequency and intensity of hot flushes through a similar mechanism (Germaine 1984; Freedman 2005; Nedstrand 2005), thus minimising discomfort (NAMS 2004) and sleep disturbances. Many clinical trials have suggested that paced respiration, applied relaxation and progressive relaxation are beneficial in treating menopausal symptoms (Germaine 1984; Freedman 1992; Hunter 1996; Irvin 1996; Wijma 1997; NAMS 2004; Freedman 2005; Nedstrand 2005). However, no systematic review with meta‐analysis has been conducted on this topic.
Why it is important to do this review
Since the time of publication of the WHI study, menopausal symptom management has become more complex because of increased awareness of the risks associated with HRT (NAMS 2004). Since 1992, women have used a wide range of alternative options. Some take prescription drugs, and others use self care strategies, such as lifestyle modifications, over‐the‐counter preparations, complementary and alternative therapies including herbal preparations and exercise programmes (McKinlay 1992; Bair 2002). Relaxation is an alternative method of treating vasomotor symptoms, but its effectiveness remains debatable. In this review, we aim to ascertain the effectiveness of relaxation techniques in treating hot flushes, night sweats and sleep disturbances compared with no treatment or other treatments, except hormonal therapy.
Objectives
To determine the effectiveness of relaxation techniques as treatment for vasomotor symptoms and associated sleep disturbances in perimenopausal and postmenopausal women.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and cross‐over RCTs that included assessment of vasomotor symptoms and sleep disturbances. In the case of cross‐over trials, we planned to use only pre‐cross‐over data. Quasi‐ or pseudo‐RCTs were excluded from the review.
Types of participants
Perimenopausal and postmenopausal women and women during or after natural or surgically induced menopause, regardless of ethnicity. They were not to have received any HRT for a minimum of three months before randomisation. We included all studies irrespective of the presence of other menopausal signs and symptoms before randomisation.
Types of interventions
Trials were included if they compared any type of relaxation intervention with no treatment or other treatments, except hormonal therapy. Any dosage or duration of relaxation intervention was included. No restriction was placed on who delivered the intervention (medical doctors, primary health practitioners, physical therapists, health promotion agencies or researchers). Interventions not involving any type of relaxation were excluded. Studies in which both arms received hormonal treatment were eligible.
Types of outcome measures
Primary outcomes
Primary outcomes
Frequency and intensity of:
1. hot flushes;
2. night sweats; and
3. sleep disturbance in association with night sweats.
Outcomes were required to be measured and scored by validated questionnaires.
Secondary outcomes
4. Attrition rate.
5. Adverse effects.
6. Quality of life assessment using a valid questionnaire.
Search methods for identification of studies
We searched for all published and unpublished RCTs of relaxation for perimenopausal and postmenopausal symptoms, without language restriction and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator.
The study flow diagram is summarised in Figure 1.
1.
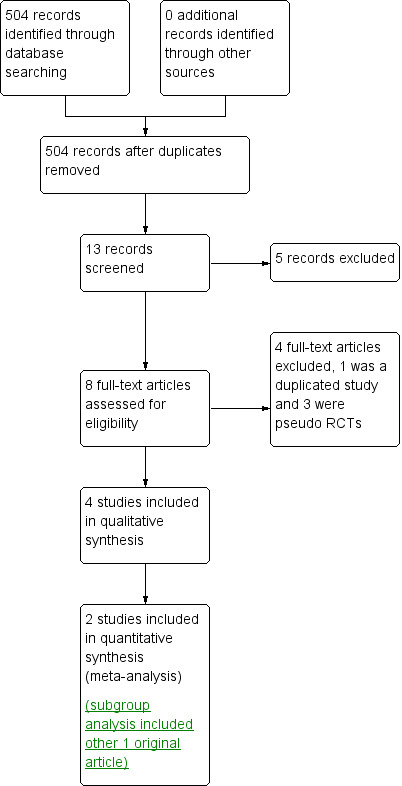
Study flow diagram.
Electronic searches
Searches were based on text words and index terms (Appendix 1). Searches of the following electronic bibliographic databases were performed to identify RCTs: the Cochrane Menstrual Disorders and Subfertility Group Specialised Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL, part of The Cochrane Library) (Wiley Internet interface) (Appendix 2), MEDLINE (Ovid) (Appendix 3), EMBASE (Ovid) (Appendix 4), the Allied and Complementary Medicine Database (AMED) (Appendix 5) and PsycINFO (Ovid) (Appendix 6). Information about ongoing trials and recently completed studies was obtained by searching the National Research Register (NRR), Current Controlled Trials and ClinicalTrials.gov. The last search date was 19 February 2014.
Searching other resources
Handsearches of relevant journals and published conference abstracts were performed; the authors also searched the Latin American and Caribbean Health Science Information Database (LILACS), PubMed and the Open System for Information on Grey Literature in Europe (OpenSIGLE), using the same search terms. Relevant review articles were searched and experts were contacted for information on additional trials. Published reviews of relaxation interventions and menopausal symptoms were used as sources of studies. Reference lists of identified studies were checked for additional citations.
Data collection and analysis
Data collection and analysis were conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (SS and WS) independently selected reports potentially fulfilling the inclusion criteria of this review based on the titles and abstracts. Disagreements were resolved through discussion. Authors of the original reports were contacted to obtain further details if the articles contained insufficient information. A reminder was sent if no response was received after three weeks.
Data extraction and management
Two review authors (SS and WS) independently extracted data by using a data extraction form designed by the review authors. When disagreements were not resolved by consensus, other review authors (TV and MS) were consulted to resolve the discrepancies. Trial ID and names and contact details of authors of the full articles were recorded. In addition, the date and method of query to the study authors (e.g. by phone) and their responses were recorded. This process was undertaken for all trials, regardless of their inclusion or exclusion from the review. For each included trial, information was collected regarding the location of the trial, the quality of the trial and the risk of bias, participant characteristics (age range, eligibility criteria, menopausal status, natural vs surgically induced menopause), baseline characteristics of treatment groups, the nature of the interventions (types of therapies, mode of administration, doses administered and duration of treatment) and data related to outcomes, for example, body mass index, well‐being score, menopausal symptoms and hormonal profiles. Information on reported benefits and adverse effects (if available) was also collected. Data were checked for accuracy and were entered into the Review Manager software (RevMan 2012).
Assessment of risk of bias in included studies
Two review authors (SS and WS) independently used the Cochrane risk of bias assessment tool to evaluate sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data; selective outcome reporting; and other sources of bias. Disagreements were resolved through discussion or by consultation with a third review author (TV). The conclusions were summarised in a risk of bias table (Figure 2; Figure 3).
2.
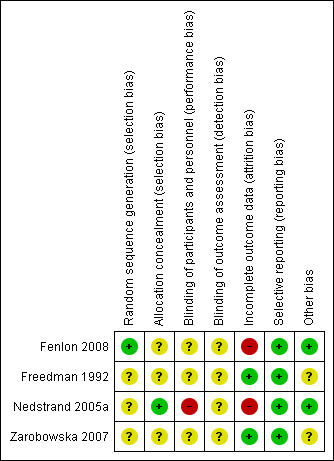
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
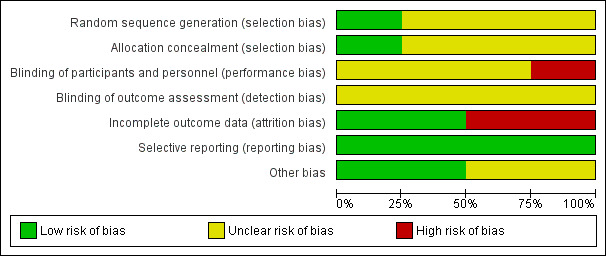
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
For dichotomous outcomes, the numbers of events in control and intervention groups of included studies were used to calculate Peto odds ratios. For continuous outcomes, the mean difference (MD) between control and intervention groups was calculated, if outcomes were measured in the same way across different trials. We planned to use standardised mean difference (SMD) if the outcomes were reported on different scales. Confidence intervals (CIs; 95%) were presented for all outcomes and comparisons.
Unit of analysis issues
The analysis was conducted "per woman randomised."
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by using sensitivity analysis. For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, that is, we were attempting to include in the analyses all participants randomly assigned to each group. The denominators for each outcome in each trial represented the number randomly assigned minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity, that is, above 50%, we planned to explore it by performing a prespecified subgroup analysis.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If 10 or more studies were included in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (i.e. a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2012). We used a fixed‐effect model inverse variance meta‐analysis to combine data when trials examined the same intervention and their populations and methods were judged sufficiently similar. In the event of substantial clinical, methodological or statistical heterogeneity, we did not combine study results by means of metaanalysis but instead summarised them in narrative form.
This review will be updated every two years or whenever new RCTs are published.
Subgroup analysis and investigation of heterogeneity
If sufficient studies were available, we planned to conduct the following subgroup analyses for the primary outcomes.
Perimenopause or postmenopause.
Type of relaxation technique.
Severity of symptoms at baseline.
Studies with hormonal treatment as a co‐intervention versus those without.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of various factors on effect size by (1) repeating the analysis while excluding unpublished studies; (2) repeating the analysis while taking account of study quality, in particular, studies with adequate treatment concealment and double‐blinding; (3) repeating the analysis by excluding very long or large studies to establish how much they dominate the results; and (4) repeating the analysis by excluding studies using the filter of language of publication, source of funding (industry vs other) or country.
However, too few studies were identified to allow meaningful sensitivity analysis.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
A total of 504 potentially relevant references were identified and screened (Figure 1). In all, 491 references were excluded on the basis of titles and abstracts. Only 13 references were retrieved for more detailed evaluation. Five were considered not suitable for inclusion in the review, as they were commentaries, observational studies or reviews. After risk of bias assessment was performed, three more publications were excluded, as they were pseudo‐RCTs.
Included studies
Study design
Four studies met the eligibility criteria (Freedman 1992; Nedstrand 2005a; Zarobowska 2007; Fenlon 2008). Two trials were done in Sweden (Nedstrand 2005a; Zarobowska 2007), one in the UK (Fenlon 2008) and one in the USA (Freedman 1992).
All included trials were RCTs. One trial (Zarobowska 2007) had two RCTs performed in parallel. Intention‐to‐treat analysis was clearly specified in only two trials (Freedman 1992; Fenlon 2008). Two studies stated that power calculations were used to statistically estimate the sample size (Nedstrand 2005a; Fenlon 2008).
Participants
The four studies in this review randomly assigned 281 menopausal women. The age range of participants was 30 to 77 years. Three trials (Freedman 1992; Nedstrand 2005a; Fenlon 2008) reported comparable demographic characteristics of study groups at baseline. This information was unclear in one trial (Zarobowska 2007). Three trials recruited women whose last menstrual bleeding had taken place six or more months ago (Nedstrand 2005a; Zarobowska 2007; Fenlon 2008). Only one trial (Freedman 1992) included women who had amenorrhoea for one year or longer. Two trials (Nedstrand 2005a; Zarobowska 2007) confirmed menopausal status by serum follicle‐stimulating hormone and oestradiol levels. Two studies (Nedstrand 2005a; Fenlon 2008) recruited postmenopausal women with primary breast cancer who were suffering from hot flushes. Two trials (Zarobowska 2007; Fenlon 2008) recruited only women with natural menopause. One trial (Zarobowska 2007) excluded women with severe metabolic, thromboembolic or endocrine diseases; women with uncontrolled hypertension (> 95 mmHg diastolic blood pressure) and those who used sedatives, tranquillisers and antidepressants on a daily basis. Two trials (Freedman 1992; Fenlon 2008) excluded women who had stopped hormone treatment within the previous six months.
Intervention
One RCT compared applied relaxation with electroacupuncture (Nedstrand 2005a). One trial (Zarobowska 2007) compared applied relaxation with superficial needle insertion, electroacupuncture or oral oestrogen. In this meta‐analysis, participants in the superficial needle insertion and electroacupuncture groups were combined as the “acupuncture group (n=30) and compared with those in the applied relaxation group (n=15)."
One trial (Freedman 1992) compared participants given paced respiration or muscle relaxation with controls, who received only α‐wave electroencephalographic biofeedback. One trial (Fenlon 2008) compared relaxation techniques (deep‐breathing techniques, muscle relaxation and guided imagery) with no treatment.
We excluded one RCT, which compared the effects of transdermal oestrogen and placebo. In another RCT, women were randomly assigned to receive oral oestrogens, applied relaxation, electroacupuncture and superficial needle insertion. We included only data from the two groups of women who received applied relaxation or electroacupuncture.
The duration of intervention was 12 weeks in three trials (Nedstrand 2005a; Zarobowska 2007; Fenlon 2008) and four weeks in one trial (Freedman 1992). The number and time of follow‐up evaluation varied considerably, at one week (Zarobowska 2007), four weeks (Freedman 1992; Nedstrand 2005a; Zarobowska 2007), seven weeks (Fenlon 2008), eight weeks (Nedstrand 2005a), 12 weeks (Nedstrand 2005a; Zarobowska 2007), three months (Nedstrand 2005a; Fenlon 2008) and six months after treatment (Nedstrand 2005a). One RCT compared applied relaxation with electroacupuncture (Nedstrand 2005a).
Outcomes
All studies used self report to assess vasomotor symptoms. Three trials (Freedman 1992; Nedstrand 2005a; Zarobowska 2007) recorded the number of hot flushes per 24 hours. Two trials (Nedstrand 2005a; Fenlon 2008) used the Kupperman Index score of climacteric symptoms. No study reported night sweats or sleep disturbances as an outcome.
No studies reported data on adverse effects or quality of life.
Two trials (Nedstrand 2005a; Zarobowska 2007) compared relaxation techniques with acupuncture and reported outcomes as continuous data that could be combined in the meta‐analysis. Data from the final follow‐up visit were pooled, regardless of the time since baseline.
The other two trials (Freedman 1992; Fenlon 2008) compared participants who received relaxation interventions versus controls given paced respiration (Freedman 1992), placebo (α‐wave electroencephalographic biofeedback) (Freedman 1992) or no treatment (Fenlon 2008), but outcomes were reported as mean or median, which could not be analysed because the data had a wide standard deviation (Freedman 1992) and no data on standard deviation were provided (Fenlon 2008).
Excluded studies
Two reports were not RCTs (Irvin 1996; IhnSook 2004). One trial (Nedstrand 2005b) compared a relaxation intervention with hormonal treatment.
Risk of bias in included studies
For details, please refer to the methodological quality summary (Figure 2; Figure 3).
Allocation
Random sequence generation
One study (Fenlon 2008) was rated as having low risk of bias in this domain, and three (Freedman 1992; Nedstrand 2005a; Zarobowska 2007) as having unclear risk.
Allocation concealment
Two studies (Nedstrand 2005a; Fenlon 2008) were rated as having low risk of bias in this domain, and two (Freedman 1992; Zarobowska 2007) as having unclear risk.
Blinding
Blinding of participants and personnel
One study (Nedstrand 2005a) was rated as having high risk in this domain, and three (Freedman 1992; Zarobowska 2007; Fenlon 2008) as having unclear risk.
Blinding of outcome assessment
All four studies (Freedman 1992; Nedstrand 2005a; Zarobowska 2007; Fenlon 2008) were rated as having unclear risk of bias in this domain. All four trials (Freedman 1992; Nedstrand 2005a; Zarobowska 2007; Fenlon 2008) were unblinded.
Incomplete outcome data
Two studies were rated as having low risk of attrition bias (Freedman 1992; Zarobowska 2007), and two studies having high risk of attrition bias (Nedstrand 2005a; Fenlon 2008).
Selective reporting
All included trials (Freedman 1992; Nedstrand 2005a; Zarobowska 2007; Fenlon 2008) were judged to be at low risk of selective outcome reporting.
Other potential sources of bias
Two studies (Zarobowska 2007; Fenlon 2008) were rated as low risk and two (Freedman 1992; Nedstrand 2005a) as unclear risk. We found no potential sources of within‐study bias in any of the four studies.
Effects of interventions
See: Table 1
The nature of the included studies meant that data from only two studies could be pooled (Nedstrand 2005a; Zarobowska 2007). A total of 72 participants were included in the meta‐analysis.
1 Relaxation versus acupuncture
Two studies made this comparison (Nedstrand 2005a; Zarobowska 2007). One of these studies (Zarobowska 2007) included an arm receiving electroacupuncture and an arm receiving superficial needle insertion. For our primary analysis, participants in the superficial needle insertion and electroacupuncture groups were combined as the “acupuncture group (n=30). The effect was examined in a sensitivity analysis."
Primary outcomes
1.1 Hot flushes
Changes in the number of hot flushes per 24 hours
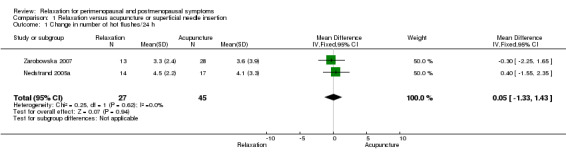
No evidence was found of a difference between relaxation and acupuncture or superficial needle insertion in the change in number of hot flushes per 24 hours (MD 0.05, 95% CI ‐1.33 to 1.43, two studies, 72 women, I2 = 0%) (Figure 4).
4.
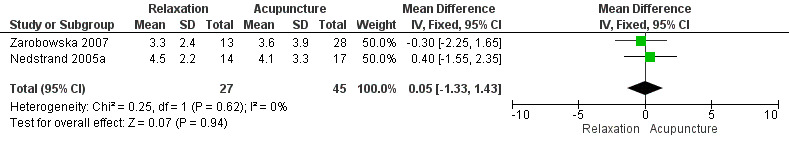
Forest plot of comparison: 1 Relaxation versus acupuncture, outcome: 1.1 Change in number of hot flushes/24 h.
When we excluded from the analysis the group given only superficial needle insertion, no difference between groups was noted (MD 0.16, 95% CI ‐1.35 to 1.68, two studies, 59 participants, I2 = 0%).
Improvement in the severity of hot flushes
No evidence showed a difference between the two groups in hot flush severity, measured using the Kupperman Index (MD ‐1.32, 95% CI ‐5.06 to 2.43, two studies, 72 women, I2 = 0%) (Figure 5).
5.
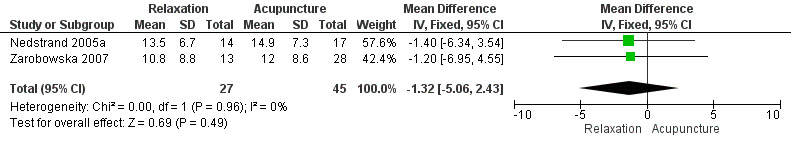
Forest plot of comparison: 1 Relaxation versus acupuncture, outcome: 1.2 Change in severity of hot flushes (Kupperman score).
When we excluded from analysis the group given only superficial needle insertion, no difference between groups was noted (MD ‐0.64, 95% CI ‐4.30 to 3.0, two studies, 59 participants, I2 = 0%).
1.2 Night sweats
This outcome was not reported.
1.3 Sleep disturbance
This outcome was not reported
Secondary outcomes
1.4 Attrition rate
Nedstrand 2005a reported that five of 19 in the relaxation group and two of 19 in the acupuncture group had dropped out at 12 weeks post treatment.
Zarobowska 2007 reported that two of 15 in the relaxation group and two of 30 in the acupuncture plus superficial needle insertion group had dropped out at 12 weeks post treatment.
1.5 Adverse effects
This outcome was not reported
1.6 Quality of life
This outcome was not reported
2 Relaxation versus paced respiration
One study made this comparison (Freedman 1992).
Primary outcomes
2.1 Hot flushes
Change in hot flush frequency per 24 hours
A significant decrease in hot flush frequency was reported in the paced respiration group (P value < 0.02) but not in the muscle relaxation group. However, no significant differences between the two interventions were reported. Pretest and post‐test, mean numbers of hot flushes per 24 hours were as follows: muscle relaxation group: pretest 14.2 (standard deviation (SD) 9.8), post‐test 13.6 (SD 10.6); paced respiration group: pretest 15.7 (SD 8.1), post‐test 9.6 (SD 6.2).
Our other outcomes of interest were not reported in this study.
3 Relaxation versus no treatment or placebo
Two studies made this comparison (Freedman 1992; Fenlon 2008). Data were reported as medians in one (Fenlon 2008) and as means with very large standard deviations in the other (Freedman 1992), so they were unsuitable for analysis.
Primary outcomes
3.1 Hot flushes
Changes in the number of hot flushes per week
One study (Fenlon 2008) reported no evidence of a difference between relaxation and no‐treatment groups at three‐month follow‐up (median difference five flushes per week, P value 0.06).
The other study (Freedman 1992) reported no evidence of a difference between pretreatment and post‐treatment frequency of hot flushes during 24 hours' ambulatory monitoring after the muscle relaxation technique had been used (mean ± SD: 14.2 ± 9.8, 13.6 ± 10.6).
Our other outcomes of interest were not reported in these studies.
Other analyses
No statistical heterogeneity was detected. Too few studies were identified to allow meaningful sensitivity analysis or to assess for publication bias. We will carry out these analyses in future updates if more studies become available.
Discussion
Summary of main results
Evidence is insufficient to show the effectiveness of relaxation techniques as treatment for menopausal vasomotor symptoms, or to reveal whether this treatment is more effective than no treatment, placebo, acupuncture, superficial needle insertion or paced respiration.
Overall completeness and applicability of evidence
Too few data were available for review authors to evaluate the effectiveness of relaxation techniques for vasomotor symptoms. No data on night sweats and sleep disturbances were reported. Although several trials included quality of life as an outcome, investigators typically used invalid measures. The percentage of dropouts in the relaxation group was higher than that in the acupuncture or superficial needle insertion group, but the reasons for this loss to follow‐up were unclear. Adverse events and tolerability of relaxation interventions were not reported. It is important to know the benefits and harms of a relaxation intervention before it can be recommended as a treatment option for menopausal symptoms.
Quality of the evidence
The quality of the evidence was very low. The main limitations of the evidence were lack of data, imprecision and failure to report study methods in adequate detail. No studies reported adverse events, night sweats or sleep disturbances associated with night sweats, and two studies did not provide data that were suitable for analysis.
Potential biases in the review process
Every effort was made to identify all relevant studies. However, additional trials in other database sources may not have been accessible through our search. Our decision to combine the two control groups (electroacupuncture and superficial needling) in one study (Zarobowska 2007) could have influenced our results, but a sensitivity analysis including only the electroacupuncture group did not influence our findings.
Agreements and disagreements with other studies or reviews
With the limited evidence obtained, this review reported no differences or changes in the number and severity of hot flushes per 24 hours between participants given relaxation therapy and those treated with acupuncture or superficial needle insertion. The results were consistent with those of other RCTs (Freedman 1992; Fenlon 2008).
Authors' conclusions
Implications for practice.
Few RCTs were available that had assessed the effectiveness of relaxation intervention for the management of menopausal vasomotor symptoms. We found insufficient evidence to evaluate the effects of a relaxation intervention in comparison with acupuncture or superficial needle insertion in reducing vasomotor symptoms. The quality of the evidence was very low. However, relaxation techniques require long and continuous practice to achieve the treatment effect, and participant compliance can be a problem.
Implications for research.
Good‐quality RCTs of an adequate sample size that compare relaxation intervention with placebo or other types of interventions are urgently needed. Studies should evaluate not only the effect of the intervention on the frequency and intensity of vasomotor symptoms, but also the impact of treatment on women’s daily life and compliance with treatment.
Acknowledgements
We would like to thank the Thai Cochrane Network and the Australasian Cochrane Centre for providing excellent training in writing protocols and other related processes for developing a Cochrane review. We appreciated the kind help of referees who were involved in the review. Last but not least, we would like to thank the Clinical Epidemiology PhD Programme administrative staff and faculty, especially Professor Dr Jayanton Patumanond and Associate Professor Chamaiporn Tawichasri, for their kind support and advice.
Appendices
Appendix 1. Search string
Keywords CONTAINS "menopausal" or "menopausal symptoms" or "menopausal symmtoms" or "Menopause" or "perimenopausal" or "perimenopause" or "perimenpause" or "hot flashes" or "hot flushes" or "hot flushes frequency" or "hot flushes severity" or "climacteric " or "climacteric symptoms" or "climacteric symptoms ‐ vasomotor" or "climacteric symtoms" or "climacteric symptoms‐psychological" or "climacteric depression" or "nocturnal diaphoresis" or "night sweats" or "night time awakenings" or "sleep disturbances" or Title CONTAINS "menopausal" or "menopausal symptoms" or "menopausal symmtoms" or "Menopause" or "perimenopausal" or "perimenopause" or "hot flashes" or "hot flushes" or "hot flushes frequency" or "hot flushes severity" or "climacteric " or "climacteric symptoms" or "climacteric symptoms ‐ vasomotor" or "climacteric symptoms‐psychological" or "climacteric depression"
AND
Keywords CONTAINS "Relaxation Techniques" or "yoga" or "massage therapy" or "coping strategies" or "*Aromatherapy" or Title CONTAINS "Relaxation Techniques" or "yoga" or "massage therapy" or "coping strategies" or "*Aromatherapy"
Appendix 2. CENTRAL search strategy
Source: CENTRAL
Database: EBM Reviews—Cochrane Central Register of Controlled Trials Date of search: December 2012 1 exp menopause/ or exp perimenopause/ or exp postmenopause/ (4991) 2 (menopaus$ or perimenopaus$ or postmenopaus$).tw. (9942) 3 exp Climacteric/ (5209) 4 climacter$.tw. (590) 5 vasomotor.tw. (810) 6 hot flash$.tw. (343) 7 hot flush$.tw. (588) 8 night sweat$.tw. (84) 9 nocturnal sweat$.tw. (8) 10 (sleep adj2 disturb$).tw. (1208) 11 or/1‐10 (12723) 12 exp Relaxation/ or exp Muscle Relaxation/ or exp Relaxation Therapy/ (3269) 13 aromatherapy/ or breathing exercises/ or meditation/ or exp relaxation therapy/ or exp tai ji/ or exp therapeutic touch/ or exp yoga/ (1923) 14 relax$.tw. (5895) 15 breathing.tw. (4081) 16 meditation.tw. (379) 17 massag$.tw. (1035) 18 yoga.tw. (332) 19 (pac$ adj2 respiration$).tw. (12) 20 (cop$ adj2 technique$).tw. (184) 21 or/12‐20 (13578) 22 11 and 21 (210) 23 limit 22 to yr="2012 ‐Current" (7)
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Searched from 1946 to present
1 exp menopause/ or exp perimenopause/ or exp postmenopause/ (42642) 2 (menopaus$ or perimenopaus$ or postmenopaus$).tw. (62709) 3 exp Climacteric/ (46017) 4 climacter$.tw. (3832) 5 vasomotor.tw. (9956) 6 hot flash$.tw. (1570) 7 hot flush$.tw. (1669) 8 night sweat$.tw. (1284) 9 nocturnal sweat$.tw. (79) 10 (sleep adj2 disturb$).tw. (9252) 11 or/1‐10 (96466) 12 exp Relaxation/ or exp Muscle Relaxation/ or exp Relaxation Therapy/ (44392) 13 aromatherapy/ or breathing exercises/ or meditation/ or exp relaxation therapy/ or exp tai ji/ or exp therapeutic touch/ or exp yoga/ (11451) 14 relax$.tw. (114042) 15 breathing.tw. (47244) 16 meditation.tw. (2047) 17 massag$.tw. (6835) 18 yoga.tw. (1539) 19 (pac$ adj2 respiration$).tw. (75) 20 (cop$ adj2 technique$).tw. (2419) 21 or/12‐20 (202595) 22 11 and 21 (2218) 23 randomized controlled trial.pt. (338451) 24 controlled clinical trial.pt. (85028) 25 randomized.ab. (255606) 26 placebo.tw. (143862) 27 clinical trials as topic.sh. (161921) 28 randomly.ab. (187126) 29 trial.ti. (109055) 30 (crossover or cross‐over or cross over).tw. (55168) 31 or/23‐30 (831347) 32 (animals not (humans and animals)).sh. (3659106) 33 31 not 32 (766620) 34 22 and 33 (297) 35 (2012$ or 2013$).ed. (1088248) 36 34 and 35 (32)
Appendix 4. EMBASE search strategy
Source: EMBASE
Search from 1980 to 2013 Week 3 1 exp MENOPAUSE/ or exp MENOPAUSE RELATED DISORDER/ or exp "MENOPAUSE AND CLIMACTERIUM"/ (91019) 2 (menopaus$ or perimenopaus$).tw. (46809) 3 climacter$.tw. (4599) 4 vasomotor.tw. (11683) 5 hot flash$.tw. (2165) 6 hot flush$.tw. (2334) 7 night sweat$.tw. (2091) 8 nocturnal sweat$.tw. (124) 9 (sleep adj2 disturb$).tw. (13386) 10 or/1‐9 (129829) 11 exp leisure/ (17018) 12 (relax$ or leisure).tw. (134118) 13 breathing.tw. (57337) 14 meditation.tw. (2684) 15 massag$.tw. (8572) 16 yoga.tw. (2046) 17 (pac$ adj2 respiration$).tw. (86) 18 (cop$ adj2 technique$).tw. (2082) 19 or/11‐18 (211854) 20 10 and 19 (2939) 21 Clinical Trial/ (876008) 22 Randomized Controlled Trial/ (335805) 23 exp randomization/ (60515) 24 Single Blind Procedure/ (16894) 25 Double Blind Procedure/ (112757) 26 Crossover Procedure/ (36012) 27 Placebo/ (211904) 28 Randomi?ed controlled trial$.tw. (82815) 29 Rct.tw. (10760) 30 random allocation.tw. (1201) 31 randomly allocated.tw. (18191) 32 allocated randomly.tw. (1858) 33 (allocated adj2 random).tw. (716) 34 Single blind$.tw. (12962) 35 Double blind$.tw. (133481) 36 ((treble or triple) adj blind$).tw. (297) 37 placebo$.tw. (183892) 38 prospective study/ (223387) 39 or/21‐38 (1302348) 40 case study/ (18321) 41 case report.tw. (237295) 42 abstract report/ or letter/ (855879) 43 or/40‐42 (1106595) 44 39 not 43 (1266512) 45 20 and 44 (463) 46 (2012$ or 2013$).em. (1356428) 47 45 and 46 (62)
Appendix 5. AMED search strategy
Source: AMED (Allied and Complementary Medicine Database)
Searched from 1985 to January 2013 1 exp climacteric/ or exp menopause/ (504) 2 (menopaus$ or perimenopaus$).tw. (671) 3 climacter$.tw. (50) 4 vasomotor.tw. (86) 5 hot flash$.tw. (48) 6 hot flush$.tw. (33) 7 night sweat$.tw. (19) 8 nocturnal sweat$.tw. (0) 9 (sleep adj2 disturb$).tw. (369) 10 or/1‐9 (1176) 11 exp breathing therapies/ or exp meditation/ or exp relaxation/ or exp reiki/ (1379) 12 relax$.tw. (2739) 13 breathing.tw. (1239) 14 meditation.tw. (524) 15 massag$.tw. (2294) 16 yoga.tw. (472) 17 (pac$ adj2 respiration$).tw. (1) 18 (cop$ adj2 technique$).tw. (21) 19 or/11‐18 (6658) 20 10 and 19 (50) 21 limit 20 to yr="2012 ‐Current" (1)
Appendix 6. PsycINFO search strategy
Source: PsycINFO
Searched from 1806 to January 2013 Week 3 1 exp menopause/ (2731) 2 (menopaus$ or perimenopaus$).tw. (3614) 3 climacter$.tw. (427) 4 vasomotor.tw. (1107) 5 hot flash$.tw. (273) 6 hot flush$.tw. (158) 7 night sweat$.tw. (100) 8 nocturnal sweat$.tw. (7) 9 (sleep adj2 disturb$).tw. (5367) 10 or/1‐9 (10639) 11 exp Relaxation Therapy/ or exp Relaxation/ (5112) 12 relax$.tw. (16801) 13 breathing.tw. (5027) 14 meditation.tw. (4322) 15 massag$.tw. (1083) 16 yoga.tw. (1440) 17 (pac$ adj2 respiration$).tw. (39) 18 (cop$ adj2 technique$).tw. (540) 19 or/11‐18 (27096) 20 10 and 19 (383) 21 limit 20 to "2000treatment outcome/randomized clinical trial" (17) 22 limit 21 to yr="2012 ‐Current" (1)
Data and analyses
Comparison 1. Relaxation versus acupuncture or superficial needle insertion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in number of hot flushes/24 h | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.33, 1.43] |
| 2 Change in severity of hot flushes (Kupperman score) | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐5.06, 2.43] |
1.1. Analysis.
Comparison 1 Relaxation versus acupuncture or superficial needle insertion, Outcome 1 Change in number of hot flushes/24 h.
1.2. Analysis.
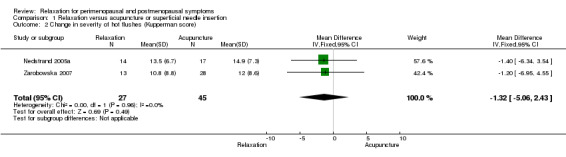
Comparison 1 Relaxation versus acupuncture or superficial needle insertion, Outcome 2 Change in severity of hot flushes (Kupperman score).
Comparison 2. Relaxation versus acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in number of hot flushes/24 h | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐1.35, 1.68] |
| 2 Change in severity of hot flushes (Kupperman score) | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐4.30, 3.03] |
2.1. Analysis.
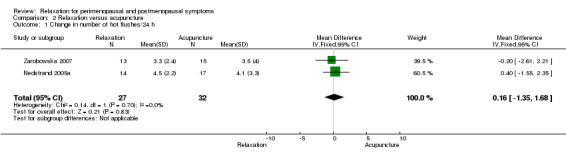
Comparison 2 Relaxation versus acupuncture, Outcome 1 Change in number of hot flushes/24 h.
2.2. Analysis.
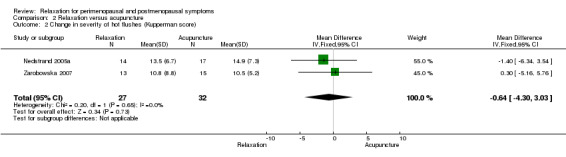
Comparison 2 Relaxation versus acupuncture, Outcome 2 Change in severity of hot flushes (Kupperman score).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fenlon 2008.
| Methods | RCT comparing relaxation with no intervention | |
| Participants | This study randomly assigned 150 women to applied relaxation (n = 74) or control (n = 76). At 1 month after completion of the trial, 50 and 54 participants remained in the relaxation and control groups, respectively. At 3 months, 46 and 51 participants remained in these respective groups. However, data were expressed as median and not mean scores as in the other trials (Freedman 1992; Nedstrand 2005a; Zarobowska 2007). 150 women with natural menopause aged 36 to 77 years. 61 women were in the relaxation group and 64 were in the control group. Post menopause was defined as 6 months without menstruation. Participants had primary breast cancer and were suffering from troublesome hot flushes. Women taking oestrogen, aromatase inhibitors or other hormone therapies, except tamoxifen, were excluded | |
| Interventions | Applied relaxation in the treatment arm. Controls received general discussion on menopause management and advice about lifestyle measures to improve health, such as diet, exercise, vaginal moisturisers and stress reduction | |
| Outcomes | Frequency and severity of hot flushes (reported as median and median difference) Hunter menopause scale to measure distress caused by flashes, quality of life using Functional Assessment of Cancer Therapy with the endocrine subscale (FACT‐ES) and anxiety by the Spielberg State/Trait Anxiety Index (STAI) Outcomes were assessed at 1 and 3 months after treatment |
|
| Notes | 150 randomly assigned; 125 participated at day 0 (start of relaxation treatment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent trials office was responsible for randomisation, using a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | The recruiting nurse did not have access to the randomisation list. Allocation was made by a telephone call to an independent trial office |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 74, 46 women were randomly assigned/analysed to the relaxation group, and of 76, 51 women were randomly assigned/analysed to the control group. Reasons given for dropping out were “not like the diary,” flushes stopped, reminder of cancer, illness, no time for relaxation practice, family reasons and no reason given |
| Selective reporting (reporting bias) | Low risk | Outcomes were prospectively measured, using appropriate measuring instruments. All outcomes were reported |
| Other bias | Low risk | Appeared to be free from other sources of bias |
Freedman 1992.
| Methods | RCT, 3 parallel groups in equal numbers: paced respiration, muscle relaxation and α‐wave biofeedback (control) | |
| Participants | This study randomly assigned 33 participants to muscle relaxation, paced respiration or placebo control (n = 11 per group). 11 participants in paced respiration, in muscle relaxation and in α‐wave feedback group. All postmenopausal women experienced at least 5 hot flushes per day and had been amenorrhoeic ≥ 1 year | |
| Interventions | Participants in paced respiration group were instructed to breathe 6 to 8 cycles/min and to increase the amplitude of the abdominal tracing. Participants in the muscle relaxation group were initially trained to systematically tense and then relax 16 gross muscle groups. Participants in the α‐wave feedback group received visual feedback for the production of 8 to 13 Hz electroencephalographic activity. Training was done in three 10‐minute trials, separated by 5‐minute rest periods | |
| Outcomes | Hot flush frequency during 24‐hour ambulatory monitoring, respiration rate and tidal volume | |
| Notes | Trial received funding from the National Institute on Aging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a judgement of yes or no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 participants randomly assigned to paced respiration, 11 to muscle relaxation and 11 to α‐wave feedback group. All participants were included in the final analysis |
| Selective reporting (reporting bias) | Low risk | Number of flushes was measured over a 24‐hour period, and other outcomes were objectively measured by using appropriate equipment, thus less prone to bias. All outcomes were reported |
| Other bias | Unclear risk | Duration of the 3 interventions was not specified. Unclear information regarding the exact time when outcome assessment was performed |
Nedstrand 2005a.
| Methods | RCT comparing applied relaxation with electroacupuncture in equal numbers 38 participants were randomly assigned to applied relaxation or electroacupuncture (n = 19 per group). Attrition rate at 12 weeks after treatment was 26.3% (5/19) in the relaxation group and 10.5% (2/19) in the acupuncture group |
|
| Participants | Of 19, 14 women were randomly assigned/analysed to the applied relaxation group and of 19, 17 women were randomly assigned/analysed to the electroacupuncture group. All women were naturally or surgically postmenopausal with breast cancer and at least 2 hot flushes/24 h | |
| Interventions | The study group received 12 weekly sessions of applied relaxation, each lasting 60 minutes. The control group received 30 minutes of electroacupuncture treatment twice a week for the first 2 weeks and once a week for 10 weeks Participants were followed at 3 and 6 months after treatment |
|
| Outcomes | Changes in number of flushes/24 h and in sum score of Kupperman Index | |
| Notes | Trial received funding from Swedish Foundation for Heath Care Science and Allergy Research, Cancer and Trafikskadades Forbund and Lion Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Low risk | Labels in sealed, opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Possibly an unblinded study, as mentioned in the discussion |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a judgement of yes or no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 19, 14 women were randomly assigned/analysed to the applied relaxation group and of 19, 17 women were randomly assigned/analysed to the electroacupuncture group. In applied relaxation group, 5 participants dropped out during ot before the 12 weeks of treatments with various social or personal reasons. 2 dropped out from the electroacupuncture group (1 moved away and the other dropped out for an unknown reason) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Appeared to be free of other sources of bias |
Zarobowska 2007.
| Methods | 2 RCTs performed in parallel at the same outpatient clinic. In the first RCT, 60 women were randomly assigned in equal numbers to therapy with applied relaxation, electroacupuncture, superficial needle insertion or oral oestradiol. In the second RCT, 42 women were randomly assigned to transdermal oestrogen therapy or placebo. Only data from the first RCT were included in this meta‐analysis, as the second RCT did not meet eligibility criteria | |
| Participants | This study randomly assigned 60 participants to applied relaxation, superficial needle insertion, electroacupuncture or oral oestrogen (n = 15 per group). At 3 months' follow‐up, 4 participants were lost (2 in the applied relaxation group, and 2 in the acupuncture group). Only 41 participants completed the study and were included in the analyses. Attrition rate was 13.3% (2/15) in the relaxation group and 6.7% (2/30) in the acupuncture group. 15 participants were randomly assigned to applied relaxation, 15 to electroacupuncture and 15 to superficial needle insertion. Naturally or surgically postmenopausal women with at least 6 months of amenorrhoea. All suffered from hot flushes severe enough that they requested therapy. Women with severe metabolic, thromboembolic or endocrine disease, with uncontrolled hypertension (> 95 mmHg diastolic blood pressure) and who used sedatives, tranquillisers and antidepressants on a daily basis were excluded | |
| Interventions | The relaxation group received weekly training sessions, lasting 60 minutes each, for a 12‐week period. The electroacupuncture group received 30 minutes of treatment twice a week for the first 2 weeks and once a week for another 10 weeks. No information was given for the superficial needle insertion group. The hormonal treatment group received 17beta‐oestradiol 2 mg for 12 weeks and 10 mg medroxyprogesterone acetate daily for another 2 weeks to shed the endometrium | |
| Outcomes | Mean number of hot flushes/24 h and change in number of hot flushes/24 h Kupperman Index used to assess 11 different menopausal symptoms subjectively | |
| Notes | In this meta‐analysis, participants in the superficial needle insertion group and in the electroacupuncture group were combined as the “acupuncture group (n=30) and compared with those in the applied relaxation group (n=15)" Trial received funding from the Swedish Medical Research Council. Study authors stated that parts of the first RCT had been previously published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information given on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit a judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 15, 13 women were randomly assigned/analysed to applied relaxation group. Of 15, 15 women were randomly assigned/analysed to electroacupuncture group and fo 15, 13 women were randomly assigned/analysed to superficial needle insertion group. 2 participants dropped out from the applied relaxation group because they considered the training program to be too time‐consuming. All women in the electroacupuncture and oral oestradiol group completed 12 weeks of treatment. 2 women were excluded from the superficial needle insertion group because 1 did not start treatment because of severe migraine, and the other was repeatedly absent from therapy |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | Insufficient information for evaluation of equality of baseline demographic data Part of the first RCT had been previously published elsewhere. Unclear whether some patients might not be represented in Nedstrand 2005a and Nedstrand 2006 |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| IhnSook 2004 | Not an RCT |
| Irvin 1996 | Pseudo‐RCT |
| Nedstrand 2005b | Comparison of applied relaxation with oral oestradiol treatment |
Differences between protocol and review
Because of the limited number of studies included, we could not carry out subgroup analysis and investigation of heterogeneity.
Contributions of authors
Suprawita Saensak: search, selection of studies, data extraction, drafting of protocol and review, data analysis, data presentation, result interpretation, publication.
Teraporn Vutyavanich: methods of the review, resolution of discrepancies, result interpretation, editing of the manuscript.
Woraluk Somboonporn: search, selection of studies, appraisal of quality of articles, data extraction, co‐drafting of the protocol/review, assistance with statistics, data analysis.
Manit Srisurapanont: methods of the review, resolution of discrepancies, editing of the manuscript.
Sources of support
Internal sources
-
PhD in Clinical Epidemiology programme, Thailand.
- Supported consultation by PhD in Clinical Epidemiology Administrators (Associate Professor Dr Jayanton Patumanond and Associate Professor Chamaiporn Tawichasri), Department of Community Medicine, Faculty of Medicine, Chiang Mai University.
External sources
-
MDSG, New Zealand.
- Information and helpful comments for developing protocol.
Declarations of interest
None known.
Position: PhD candidate (Clinical Epidemiology), Faculty of Medicine, Chiang Mai University, Muang, Chiang Mai, Thailand
New
References
References to studies included in this review
Fenlon 2008 {published data only}
- Fenlon D, Corner J, Haviland JS. A randomized controlled trial of relaxation training to reduce hot flashes in women with primary breast cancer. Journal of Pain and Symptom Management 2008;35(4):397‐405. [DOI] [PubMed] [Google Scholar]
Freedman 1992 {published data only}
- Freedman R, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. American Journal of Obstetrics and Gynecology 1992;167:436‐9. [DOI] [PubMed] [Google Scholar]
Nedstrand 2005a {published data only}
- Nedstrand E, Wijma K, Wyon Y, Hammar M. Vasomotor symptoms decrease in women with breast cancer randomised to treatment with applied relaxation or electro‐acupuncture: a preliminary study. Climaceric 2005;8:243‐50. [DOI] [PubMed] [Google Scholar]
Zarobowska 2007 {published data only}
- Wyon Y, Wijma K, Nedstrand E, Hammar M. A comparison of acupuncture and oral estradiol treatment of vasomotor symptoms in postmenopausal women. Climacteric 2004;7:153–64. [DOI] [PubMed] [Google Scholar]
- Zaborowska E, Brynhildsen J, Danberg S, Fredriksson M, Lindh‐Astrand L, Nedstrand E, et al. Effects of acupuncture, applied relaxation, estrogens and placebo on hot flushes in postmenopausal women: an analysis of two prospective, parallel, randomised studies. Climacteric 2007 2007;10(1):38‐45. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
IhnSook 2004 {published data only}
- Ihn‐Sook J. Effect of progressive muscle relaxation using biofeedback on perceived stress, stress response, immune response and climacteric symptoms of middle‐aged women. Daehan Ganho Haghoeji 2004;34(2):213‐24. [DOI] [PubMed] [Google Scholar]
Irvin 1996 {published data only}
- Irvin JH, Domar AD, Clark C, Zuttermeister PC, Friedman R. The effects of relaxation response training on menopausal symptoms. Journal of Psychosomatic in Obstetrics and Gynecology 1996;17:202‐7. [DOI] [PubMed] [Google Scholar]
Nedstrand 2005b {published data only}
- Nedstrand E, Wijma K, Wyon Y, Hammar M. Applied relaxation and oral estradiol treatment of vasomotor symptoms in postmenopausal women. Maturitas 2005;51:154–62. [DOI] [PubMed] [Google Scholar]
Additional references
Bair 2002
- Bair YA, Gold EB, Greendale GA, Sternfeld B, Adler SR, Azari R, et al. Ethnic differences in use of complementary and alternative medicine at midlife: longitudinal results from SWAN participants. American Journal of Public Health 2002;92(11):1832‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barton 2001
- Barton D, Loprinzi C, Wahner‐Roedler D. Hot flashes: aetiology and management. Drugs & Aging 2001;18(8):597‐606. [DOI] [PubMed] [Google Scholar]
Benson 2000
- Benson H, Klipper MZ. The Relaxation Response. New York: HarperCollins Publishers, November 2000. [Google Scholar]
Bruck 1980
- Bruck K, Hinckel P. Thermoregulatory noradrenergic and serotonergic pathways to hypothalamic units. Journal of Physiology 1980;304:193‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celentano 2003
- Celentano E, Galasso R, Berrino F. Correlates of age at natural menopause in the cohorts of EPIC‐Italy. Tumori 2003;89(6):608‐14. [DOI] [PubMed] [Google Scholar]
Celik 2002
- Celik H, Ayar A, Tug N, Cikim G, Kilic N, Parmaksiz C. Effects of tibolone on plasma homocysteine levels in postmenopausal women. Fertility and Sterility 2002;78(2):247‐50. [DOI] [PubMed] [Google Scholar]
Dennerstein 2000
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population‐based study of menopausal symptoms. Obstetrics and Gynecology 2000;96:351‐8. [DOI] [PubMed] [Google Scholar]
El‐Mas 2004
- El‐Mas MM, Abdel‐Rahman AA. Differential modulation by oestrogen of 2‐adrenergic and I1‐imidazoline receptor‐mediated hypotension in female rats. Journal of Applied Physiology 2004;97:1237‐44. [DOI] [PubMed] [Google Scholar]
ERTA 2005
- Evidence Report/Technology Assessment. Menopause‐Related Symptoms. Management of Evidence Report/Technology Assessment Number 120. AHRQ Publication No. 05‐E016‐2, March 2005. [Google Scholar]
Fauci 1997
- Fauci AS, Braunwald E, Isselbacker KJ. Harrison’s Principles of Internal Medicine. 14th Edition. New York: McGraw‐Hill, 1997. [Google Scholar]
Freedman 2004
- Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertility and Sterility 2004;82(1):138‐44. [DOI] [PubMed] [Google Scholar]
Freedman 2005
- Freedman EW, Sammel MD, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause 2005;12:258‐66. [DOI] [PubMed] [Google Scholar]
Freeman 2001
- Freeman RR. Physiology of hot flashes. American Journal of Human Biology 2001;13:453‐64. [DOI] [PubMed] [Google Scholar]
Freeman 2007
- Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstetrics and Gynecology 2007;110:230‐40. [DOI] [PubMed] [Google Scholar]
Germaine 1984
- Germaine LM, Freedman RR. Behavioral treatment of menopausal hot flashes: evaluation by objective methods. Journal of Consulting and Clinical Psychology 1984;52:1072‐9. [DOI] [PubMed] [Google Scholar]
Gold 2004
- Gold EB, Block G, Crawford S, Lachance L, FitzGerald G, Miracle H, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. American Journal of Epidemiology 2004;159:1189–99. [DOI] [PubMed] [Google Scholar]
Goldberg 1994
- Goldberg RM, Loprinzi CL, O'Fallon JR, Veeder MH, Miser AW, Mailliard JA, et al. Transdermal clonidine for ameliorating tamoxifen‐induced hot flashes. Journal of Clinical Oncology 1994;12:155‐8. [DOI] [PubMed] [Google Scholar]
Guthrie 2003
- Guthrie JR, Dennerstein L, Taffe JR, Donnelly V. Health care‐seeking for menopausal problems. Climacteric 2003 Jun;6(2):112‐7. [PubMed] [Google Scholar]
Hickey 2005
- Hickey M, Davis SR, Sturdee DW. Treatment of menopausal symptoms: what shall we do now?. Lancet 2005;366(9483):409‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
Hjemdahl 1989
- Hjemdahl P, Fagius J, Freyschusss U. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. American Journal of Physiology 1989;257:341‐7. [DOI] [PubMed] [Google Scholar]
Holte 1992
- Holte A. Influences of natural menopause on health complaints: a prospective study of healthy Norwegian women. Maturitas 1992;14:127‐41. [DOI] [PubMed] [Google Scholar]
Hunter 1996
- Hunter M, Liao KL. Evaluation of a four‐session cognitive behavioural intervention for menopausal hot flushes. British Journal of Health Psychology 1996;1:113‐25. [Google Scholar]
Kaufert 1992
- Kaufert PA, Gilbert P, Tate R. The Manitoba project: a re‐examination of the link between menopause and depression. Maturitas 1992;14:143‐55. [DOI] [PubMed] [Google Scholar]
Lee 1989
- Lee DD, Kimura S, DeQuattro V, Davison G. Relaxation therapy lowers blood pressure in hypertensive with raised plasma norepinephrine and blunts pressor response to anger. Clinical and Experimental Hypertension. Part A, Theoretical Practice 1989;11 Suppl 1:191‐8. [DOI] [PubMed] [Google Scholar]
Marjoribanks 2012
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004143.pub4] [DOI] [PubMed] [Google Scholar]
Mathews 1990
- Mathews KA, Wing RR, Kuller LH, Meilahn EN, Kelsey SF. Influences of natural menopause on psychological characteristics and symptoms of middle‐aged healthy women. Journal of Consulting and Clinical Psychology 1990;58:345‐51. [DOI] [PubMed] [Google Scholar]
McKinlay 1992
- McKinlay SM, Brambilla DJ, Posner J. The normal menopause transition. Maturitas 1992;14:103‐15. [DOI] [PubMed] [Google Scholar]
McMillan 2004
- McMillan TL, Mark S. Complementary and alternative medicine and physical activity for menopausal symptoms women’s health. Journal of the American Medical Women's Association 2004;59:270‐7. [PubMed] [Google Scholar]
Mosconi 2009
- Mosconi P, Donati S, Colombo C, Mele A, Liberati A, Satolli R, Consensus Conference Working Group. Informing women about hormone replacement therapy: the consensus conference statement. BMC Women's Health 2009;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
NAMS 2004
- The North American Menopause Society. Treatment of menopause‐associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause 2004;11:11‐33. [DOI] [PubMed] [Google Scholar]
Nedstrand 2005
- Nedstrand E, Wijma K, Wyon Y, Hamma M. Applied relaxation and oral estradiol treatment of vasomotor symptoms in postmenopausal women. Maturitas 2005;51:154‐62. [DOI] [PubMed] [Google Scholar]
NIH 2005
- National Institute of Health. NIH State‐of‐the‐Science Conference Statement on Management of Menopause‐Related Symptoms. NIH Consensus and State‐of‐the‐Science Statements March 21–23, 2005:5‐13. [PubMed]
Ost 1987
- Ost LG. Applied relaxation: description of a coping technique and review of controlled studies. Behaviour Research and Therapy 1987;25(5):397‐409. [DOI] [PubMed] [Google Scholar]
Payne 2005
- Payne RA. Relaxation Techniques: A Practical Handbook for the Health Care Professional. Third Edition. China: Elsevier Limited, 2005. [Google Scholar]
RevMan 2012 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan) Version 5.2. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2012.
Speroff 2000
- Speroff L, Symons J, Kempfert N, Rowan J. The effect of varying low‐dose combinations of norethindrone acetate and ethinyl estradiol on the frequency and intensity of vasomotor symptoms. Menopause 2000;7(6):383‐90. [DOI] [PubMed] [Google Scholar]
STRAW 2000
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW), Park City, Utah, July 2001. Menopause 2001;8:402‐7. [DOI] [PubMed] [Google Scholar]
WHO 1996
- World Health Organization. WHO Scientific Group on Research on the Menopause in the 1990s. WHO Technical Report Series. Geneva, Switzerland: WHO, 1996. WHO Scientific Group on Research on the Menopause in the 1990s. WHO Technical Report Series. Geneva, Switzerland: WHO, 1996. [PubMed] [Google Scholar]
Wijma 1997
- Wijma K, Melin A, Nedstrand E, Hammar M. Treatment of menopausal symptoms with applied relaxation: a pilot study. Journal of Behavior Therapy and Experimental Psychiatry 1997;28:251‐64. [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams E, Kalilani L, DiBenedetti B, Zhou X, Granger AL, Fehnel SE, et al. Frequency and severity of vasomotor symptoms among perimenopausal and postmenopausal women in the United States. Climacteric 2008;11(1):32‐43. [DOI] [PubMed] [Google Scholar]


