Abstract
XTH genes are key genes that regulate the hydrolysis and recombination of XG components and plays role in the structure and composition of plant cell walls. Therefore, clarifying the changes that occur in XTHs during plant defense against abiotic stresses is informative for the study of the plant stress regulatory mechanism mediated by plant cell wall signals. XTH proteins in Arabidopsis thaliana was selected as the seed sequences in combination with its protein structural domains, 80 members of the BnXTH gene family were jointly identified from the whole genome of the Brassica napus ZS11, and analyzed for their encoded protein physicochemical properties, phylogenetic relationships, covariance relationships, and interoperating miRNAs. Based on the transcriptome data, the expression patterns of BnXTHs were analyzed in response to different abiotic stress treatments. The relative expression levels of some BnXTH genes under Al, alkali, salt, and drought treatments after 0, 6, 12 and 24 h were analyzed by using qRT-PCR to explore their roles in abiotic stress tolerance in B. napus. BnXTHs showed different expression patterns in response to different abiotic stress signals, indicating that the response mechanisms of oilseed rape against different abiotic stresses are also different. This paper provides a theoretical basis for clarifying the function and molecular genetic mechanism of the BnXTH gene family in abiotic stress tolerance in rapeseed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05121-5.
Keywords: Brassica napus, XTH gene family, Abiotic stress tolerance, Expression profiling
Introduction
In complex and changeable natural environments, crops may be exposed to abiotic stresses such as temperature, drought, salt, heavy metals, etc., potentially compromising their yield and quality [1]. As the first barrier, the cell wall plays an important role in the defense of plants against external environmental stresses [2].
The cell wall has a complex structure consisting of pectin embedded microfibrils and non-cellulosic neutral polysaccharides, crosslinked with structural proteins and, depending on the tissue and organ, with lignin [3]. Plant cell walls are divided into the intercellular layer, primary and secondary walls [4]. The main component of the intercellular layer is pectin, which is located between two neighboring cells and is able to adhere to them and buffer intercellular extrusion [5]; the primary wall is mainly composed of cellulose, hemicellulose, and structural proteins, which has a large plasticity, which allows the cell to maintain a certain shape, but also can be extended with the growth of the cell [6]; the secondary wall, which is mainly composed of cellulose and often contains lignin, located between the plasma membrane and the primary wall, is usually thicker and harder, giving the cell wall great mechanical strength [7].
Xyloglucan (XG) is the main chain component of the polysaccharide hemicellulose that accounts for the largest proportion in the primary cell wall of dicotyledonous plants [8]. The glucan backbone of XG is composed of β-1,4-D-Glup, and the O-6 position is connected to α-D-Xylp. These Xyls can be further modified by other glycosyl groups [9]. The structures of XG are diverse, showing variability among plants, organs, tissues and even within the same plant [10]. The basic function of XG is to bind to the outside of cellulose fibrils to form a load-bearing network of the primary cell wall, which determines the tension of the cell wall. The modification and reconstruction of XG can adjust the elasticity and strength of the cell wall skeleton [11].
The recombination of XG requires the participation of Xyloglucan transglycosylase/hydrolase (XTH) of the GH16 family. XTH has dual activities of Xyloglucan endotransglycosylase (XET) and Xyloglucan hydrolase (XEH), allowing it to independently complete the cleavage and recombination process of XG, and can carry out the catalytic reaction of extending the XG chain without the direct involvement of ribose [12]. XTH genes play roles in plant growth and development, especially in response to abiotic stress. The cell wall modification of xth19 could affect freezing tolerance after cold and sub-zero acclimation. Compared with the Col-0 wild type, the cell wall structure and composition of the xth19 mutant of Arabidopsis changed, which resulted in a lower freezing tolerance after cold and sub-zero acclimation [13]. Compared to the control, the expressions of TaXTHs of wheat were significantly altered under drought stress; the drought tolerance of transgenic plants of Arabidopsis overexpressing TaXTH12.5a was improved, with the germination rate, root length, hypocotyl length, and the number of green leaves during the germination stage and nutrient growth stage significantly higher than that of the control lines [14]. Under salt stress, there were 11 differentially expressed PtrXTHs in the roots of poplar, nine differentially expressed PtrXTHs in the stems, and 7 differentially expressed PtrXTHs in the leaves [15]; the water retention capacity of the leaves in transgenic plants of tobacco overexpressing PeXTH of Populus were improved, and the net photosynthetic rate had a significant enhancement, which enhanced the salt tolerance of tobacco, indicating that the XTHs might play roles for plants in the response to salt stress [16]. It was found that many XTHs of soybean responded to ethylene and flooding treatments, The Arabidopsis thaliana AtXTH31 gene is overexpressed in soybean, and transgenic soybean plants with AtXTH31 overexpressing under flooding stress tolerance showed higher germination rate, and longer roots/ hypocotyls compared to the control lines at the seedling and nutrient growth stages, it was confirmed that XTHs played roles in regulating the response of soybean to abiotic stresses [17]. Du et al. found that aluminum (Al) induces ZmXTHs to be up-regulated in the maize root system, especially in the root tips, and this induction was Al3+ specific; ZmXTHs overexpressing plants of Arabidopsis grew more healthily with lower Al content in their roots and root cell walls under Al stress compared to wild-type, suggesting that ZmXTHs could be endowed with aluminum tolerance on transgenic Arabidopsis plants by reducing the accumulation of aluminum in roots and cell walls [18]. XTHs also played important roles in the response of heavy metal stress in plants: most XTHs in ramie (Boehmeria nivea) were responsive to cadmium stress, while heterologous expression of BnXTH3, BnXTH6 and BnXTH15 significantly enhances the cadmium tolerance of transgenic yeast cells [19]; Xuan et al. identified 44 possible MtXTHs in the Medicago truncatula genome, 28 of which were responsive to HgCl2, and copper/mercury stresses would significantly induce the expression of the MtXTH3 both in the roots and shoots. In addition, the expression of MtXTH3 showed an increasing trend with the elevation of Cu and Hg concentrations [20].
While the impact of XTHs on plant cell wall composition and abiotic stress resilience has been thoroughly investigated in Arabidopsis and various crops, the expression profiles of these genes under abiotic stress conditions in Brassica napus remain comparatively underexplored. In this study, we undertook a comprehensive genome-wide identification of the BnXTH gene family members in rapeseed; additionally, we conducted an exhaustive analysis encompassing protein characteristics, gene structure, phylogenetic relationships, and collinearity the expression patterns of BnXTHs in two varieties of rapeseed were compared under alkaline (Na2CO3) stress using RNA-seq analysis. At the same time, by qPCR, the relative expressions of BnXTHs under aluminum (AlCl2), alkali (Na2CO3), salt (NaCl) and drought (PEG 6000) stress at four time points (0, 6 h, 12 h and 24 h) were examined, which provided evidence for the response of BnXTHs to abiotic stress. The findings of our study establish a foundational framework for subsequent investigations into the functional roles of these genes in the abiotic stress response of rapeseed. Furthermore, they offer a theoretical underpinning for the selection and breeding of abiotic stress-tolerant rapeseed germplasms.
Results
Identification and chromosomal localization of the BnXTH gene family
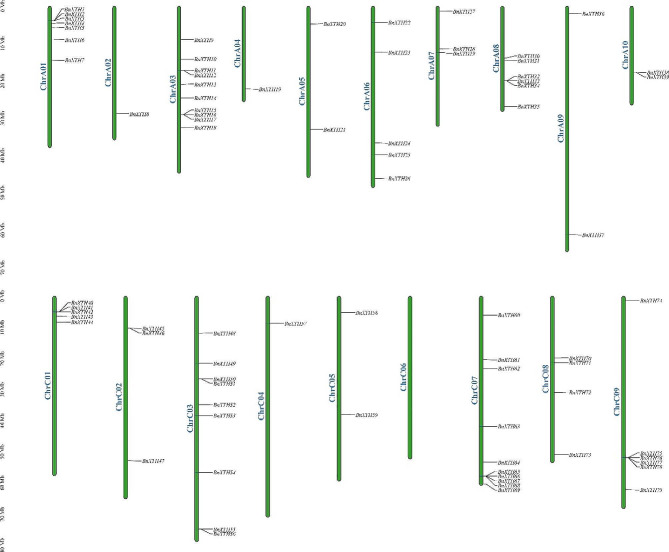
Joint identification was conducted using BLASTP and HMMER, and the NCBI-CDD tool was used to remove the sequences with incomplete structural domains. Finally, 80 BnXTH proteins were obtained, which were named BnXTH1 – BnXTH80 based on the order of the location of their encoding genes on the chromosomes. Among these 80 genes, 79 were mapped to 18 identified chromosomes (Fig. 1), and one was mapped to pseudochromosome Scaffold0027. Of these, 10 BnXTHs were localized on ChrA03 and ChrC07, nine on ChrC09, seven on ChrA01, six on ChrA08 and ChrC09, five on ChrA06 and ChrC01, three on ChrA07, ChrC02, ChrA05, and ChrA09, two were localized on ChrA10 and ChrC05, one was localized on ChrA02, ChrA04, and ChrC04, while no possible BnXTHs were detected on ChrC06. Number of BnXTHs’ exons ranged from one to eight, and the information of their positions in the chromosomes was provided on Table S1.
Fig. 1.
Chromosomal location of the BnXTHs in the B. napus genome
Physicochemical properties and subcellular localization prediction of BnXTH proteins
By predictive analysis, the number of amino acids (AAs) of the BnXTH proteins ranged from 212 to 473, the relative molecular mass (MW) ranged from 24.42 to 55.10, and the theoretical isoelectric point (PI) ranged from 4.77 to 9.96 (Table S1). Subcellular localization prediction results showed that 54 XTH proteins may exist in the cytoplasm, 21 in the cell wall, and 5 in the nucleus.
Phylogenetic analysis of BnXTH proteins
In order to explore the phylogenetic relationship between XTH proteins, 80 XTH proteins of B. napus and 33 XTH proteins of Arabidopsis thaliana were combined to form a phylogenetic tree. Based on the genetic relationship between them and the position of the branch where the AtXTH proteins were located, XTH proteins were divided into four groups: Early diverging group, Group I/II, Group IIIA and Group IIIB (Fig. 2A). According to the phylogenetic tree, it could be seen that the number of proteins contained within each group varies, among which the most proteins were clustered into Group I/II, with 22 AtXTHs and 49 BnXTHs; in Group IIIB, there were 5 AtXTHs and 16 BnXTHs; in the Early diverging group, there were 4 AtXTHs and 8 BnXTHs; Group IIIA had the fewest proteins, with 2 AtXTHs and 7 BnXTHs (Table 1).
Fig. 2.
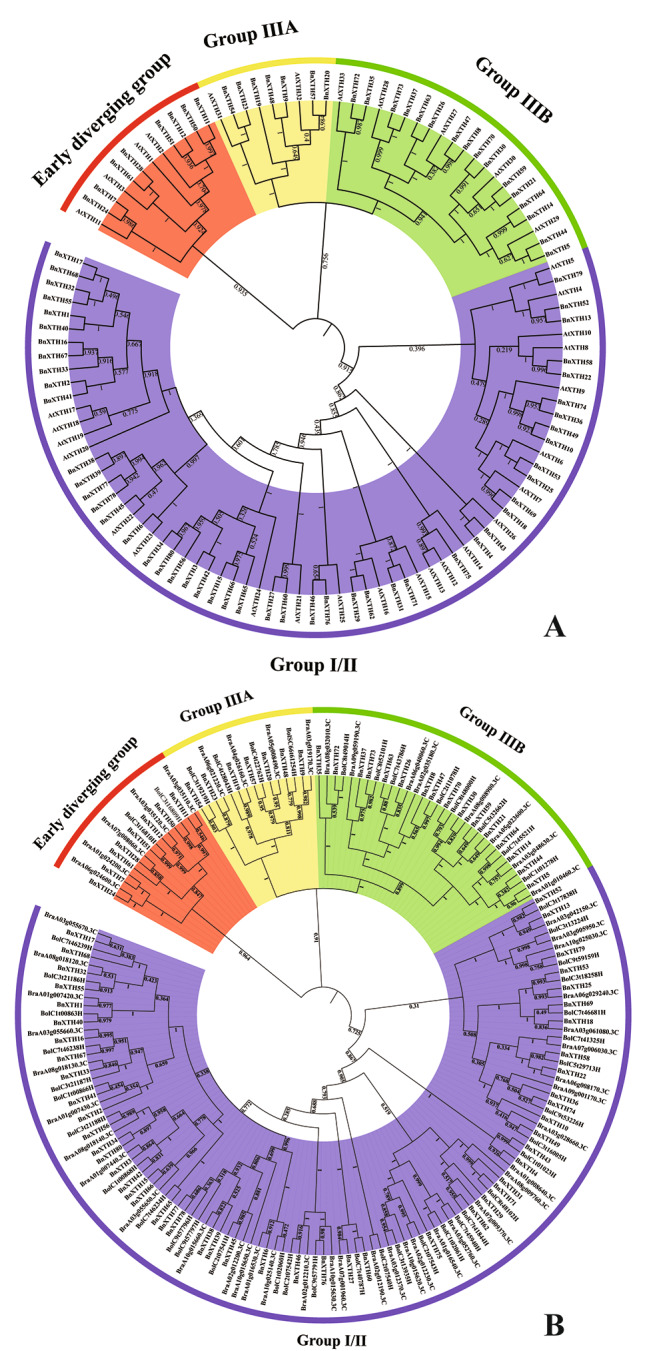
Phylogenetic tree of XTH proteins. (A) Phylogenetic tree of XTH proteins in B. napus and (A) Thaliana. (B) Phylogenetic tree of XTH proteins in (B) napus, B. rapa, and B. oleracea
Table 1.
Number of XTH proteins contained in each group in the phylogenetic tree
| Group | Early diverging group | Group I/II | Group IIIA | Group IIIB |
|---|---|---|---|---|
| AtXTH Number | 4 | 22 | 2 | 5 |
| BnXTH Number | 8 | 49 | 7 | 16 |
| BrXTH Number | 5 | 35 | 4 | 8 |
| BoXTH Number | 2 | 33 | 4 | 8 |
To further confirm the phylogenetic relationships of XTHs within Brassicaceae plants, a total of 52 XTH proteins from Brassica rapa and 47 XTH proteins from Brassica oleracea were identified by using BLASTP and HMMER methodologies for the construction of a phylogenetic tree (Table S2, Fig. 2B). The analysis revealed that these proteins could also be classified into four groups, with the groupings of BnXTHs remaining unchanged, thereby validating the reliability of the phylogenetic tree. Notably, the Group I/II contained the highest number of proteins, with 35 BrXTHs and 33 BoXTHs, followed by Group IIIB, which comprised 8 BrXTHs and 8 BoXTHs. Group IIIA included 4 BrXTHs and 4 BoXTHs, and the Early diverging group consisted of 4 BrXTHs and 4 BoXTHs (Table 1).
Gene structures, conserved motifs and promoter cis-acting elements analysis of BnXTHs
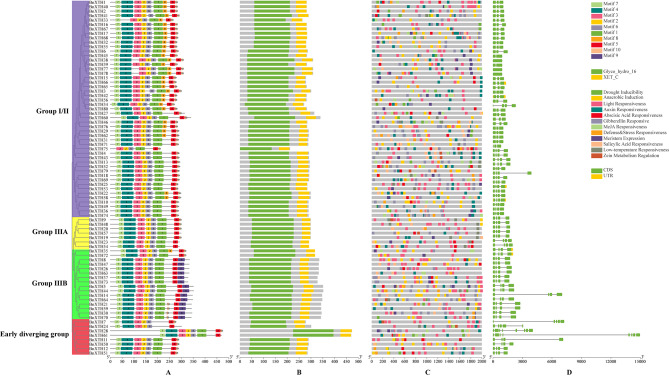
Analysis of motifs in conserved domains is a powerful tool for understanding the function, structure, and evolution of proteins. Conserved motif analysis of BnXTH proteins shows that Motif2, Motif 3, Motif 5 and Motif 6 were common to all BnXTH proteins; Motif7 was merely absent from BnXTH75; Motif4 was absent from BnXTH33 and BnXTH75 of Group I/II; Motif1 was absent in BnXTH7 and BnXTH24 of the Early diverging group; most Group IIIB and a small number of Early diverging group proteins did not contain Motif10; Motif8 existed in some Group I/II and BnXTH35 proteins; Motif9 was present in most of the Group IIIB proteins. (Fig. 3A). The protein structural characteristics of BnXTHs were identified, and all proteins were found to contain a Glyco_hydro_16 and a XET_C domain (Fig. 3B). Analysis of promoter cis-acting elements showed that BnXTHs may have a response mechanism in response to stress and plant hormone signals (Fig. 3C). Among them, there were as many as 910 light responsiveness elements, far more than other cis-acting elements. In addition, BnXTH41 and BnXTH45 contained the most cis-acting elements, amounting to 41, indicating that they may play roles in the growth and development of rapeseed. The study of the mature mRNA structure of BnXTHs revealed that they contained 1–8 CDS (Coding sequence) regions (Fig. 3D), and some BnXTHs also contained 1–2 UTR (Untranslational region) regions.
Fig. 3.
Gene structure analysis of XTH family in B.napus. (A) Conserved motifs of BnXTH family proteins. (B) Pfam structure of BnXTH family proteins. (C) Promoter cis-acting element of BnXTHs. (D) The mRNA structure encoded by the BnXTHs
Collinearity analysis of XTH gene family
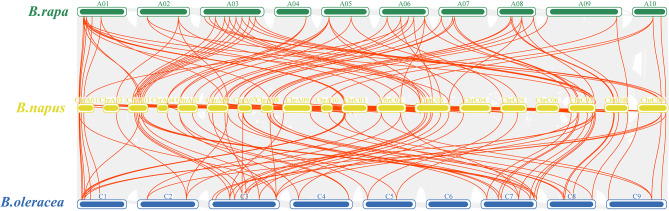
In order to investigate the collinearity of XTH genes, BnXTH80 located on the pseudochromosome was removed and the collinearity maps of rapeseed (B. napus), B. rapa and B. oleracea were drawn (Fig. 4). There were 92 pairs of collinear genes between the XTH genes of B. napus and B. rapa, and 88 pairs of collinear genes between the XTH genes of B. napus and B. oleracea (Table S3). Collinearity analysis of BnXTHs within the B. napus genome showed that there were 83 pairs of collinear genes among BnXTHs (Fig. 5 and Table S4). To understand the genetic relationship of BnXTH collinear gene pairs, KaKs values were calculated to understand their selection pressure relationships (Table S5). The results showed that the Ka/Ks values of these 83 collinear gene pairs were all less than 1, indicating that they were all affected by Purification selection.
Fig. 4.
Collinearity of XTH genes in B. napus, B. rapa, and B. oleracea
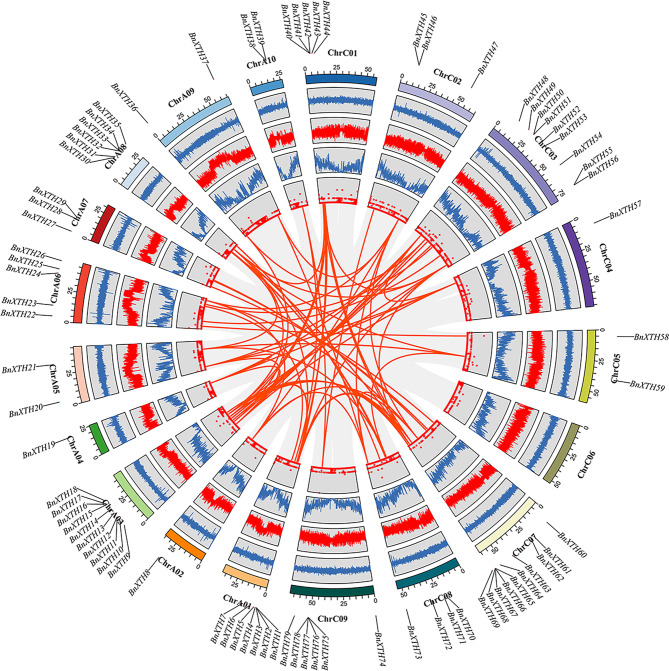
Fig. 5.
Collinearity of BnXTHs. The circles in the figure from inside to outside represent the unknown base N ratio, gene density, GC ratio, GC skew, and chromosome length of the B. napus genome
Prediction of targeting relationship between miRNA and BnXTHs
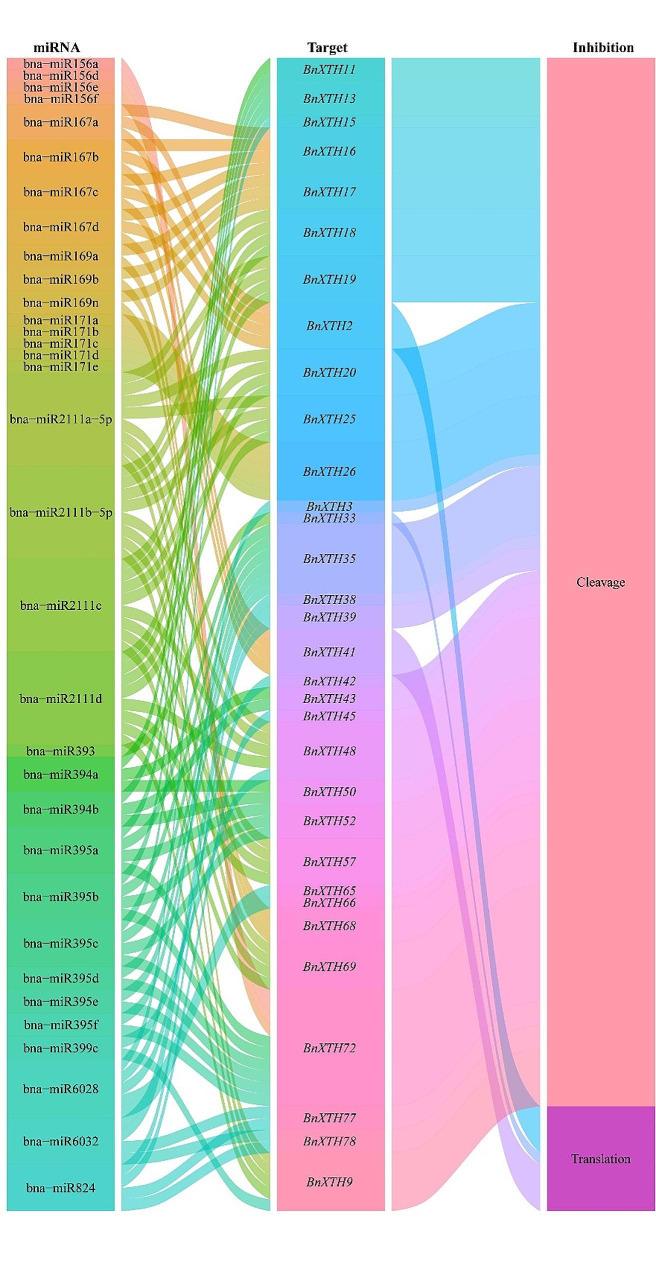
As shown in Fig. 6, a total of 32 miRNAs of B. napus targeted 29 BnXTH genes through cleavage, and 5 miRNAs of B. napus target 3 BnXTH genes through translation. No miRNA was found to have both cleavage and translation inhibition effects on the same BnXTH gene. The results suggest that multiple miRNAs were involved in the post-transcriptional regulation of BnXTHs by targeting them through cleavage and translation.
Fig. 6.
Sankey diagram for the relationships of miRNA targeting to BnXTHs transcripts. The 3 columns represent miRNA, BnXTHs and inhibition effect
Analysis of transcriptome expression patterns of BnXTHs under abiotic stresses
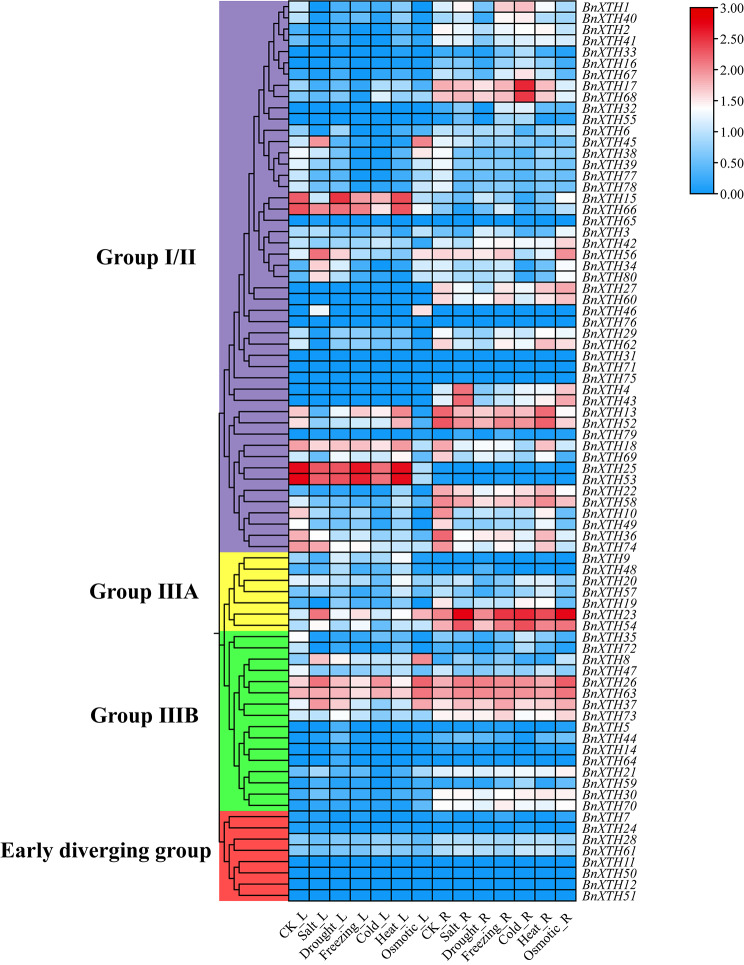
In order to explore the gene expression patterns of BnXTHs under different abiotic stresses, we obtained the transcriptome TPM data of BnXTHs in B. napus ZS11 under CK, salt, drought, freezing, cold, heat and osmotic stress, and expression heat maps were plotted after log10(TPM + 1) treatment (Fig. 7). Some BnXTHs were not expressed or had low expression in leaves and roots under different stresses; however, BnXTH26, BnXTH63 and BnXTH37 showed high expression in leaves and roots under various stresses; BnXTH13 and BnXTH52 had high expression in roots under various stresses and high expression in leaves under some of the stresses; the expression levels of BnXTH15, BnXTH66, BnXTH25, and BnXTH53 were higher in leaves under various stresses; the expression levels of BnXTH17, BnXTH68, BnXTH22, BnXTH58, and BnXTH73 showed higher expression in roots under various stresses.
Fig. 7.
Analysis of expression patterns of BnXTHs under abiotic stress (CK, salt, drought, freezing, cold, heat, and osmotic) treatments. L: leaves; R: Roots
Detection of BnXTHs expression under various abiotic stresses by qPCR technique
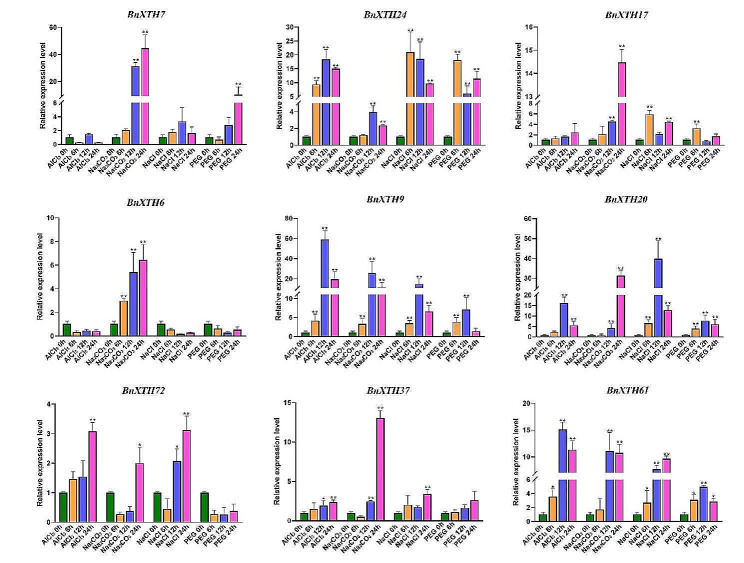
The cell wall is the first barrier to protect plant cells from damage, and the XTH genes plays an important role in plants defense against abiotic stress. Therefore, to investigate the effect of BnXTHs on rapeseed response to abiotic stress, we performed qPCR on leaves of oilseed rape seedling ZS11 treated with 0.5 mmol·L-1 AlCl3, 0.2% (w/v) Na2CO3, 1.2% (w/v) NaCl, and 20% (w/v) PEG 6000 after 0, 6 h, 12 h and 24 h to analyze the relative expression of 9 different BnXTHs (Fig. 8). The results showed that they responded significantly to different abiotic stresses.
Fig. 8.
The relative expression of BnXTH7, BnXTH24, BnXTH17, BnXTH6, BnXTH9, BnXTH20, BnXTH72, BnXTH37, and BnXTH61 in leaves under 0.5 mmol·L-1 AlCl3, 0.2% (w/v) Na2CO3, 1.2% (w/v) NaCl, and 20% (w/v) PEG 6000 after 0, 6 h, 12 h and 24 h. Data represent the mean ± standard error for threebiological experiments. Student’s t-test was used to determine differences. *: significant differences between treatments at p ≤ 0.05. **: significant differences between treatments at p ≤ 0.01
Under AlCl3 treatment, the expression of BnXTH7, BnXTH17, and BnXTH6 did not change significantly at all time points; BnXTH72 and BnXTH37 increased with the extension of the treatment time; BnXTH24, BnXTH9, BnXTH20, and BnXTH61 increased with the extension of the treatment time, reaching the maximum at 12 h, and then decreased at 24 h again.
Under Na2CO3 treatment, the expression of BnXTH7, BnXTH17, BnXTH6, BnXTH20, and BnXTH37 increased with the prolongation of the treatment time; the expression of BnXTH24, BnXTH19, and BnXTH61 increased and then decreased; and the expression of BnXTH72 showed a decreasing tendency first, but it was insignificant, and then increased significantly at 24 h. The expression of BnXTH72 increased significantly at 24 h, but it was not significant.
Under NaCl treatment, the expression of BnXTH7 and BnXTH6 did not change significantly; the expression of BnXTH72, BnXTH37, and BnXTH61 increased with the prolongation of time; and the expression of BnXTH24, BnXTH17, BnXTH9, and BnXTH20 increased and then decreased.
Under PEG 6000-induced drought stress, the expression of BnXTH6, BnXTH72, and BnXTH37 did not change significantly; the expression of BnXTH7 increased with the prolongation of time; the expression of BnXTH17, BnXTH9, BnXTH20, and BnXTH61 increased and then decreased; the expression of BnXTH24 at 6 h and then decreased at 12 h. The expression of BnXTH24 was elevated at 6 h, and then increased again at 24 h. The expression of BnXTH24 increased at 6 h and then decreased at 12 h, and then increased again after 24 h.
Discussion
Gene family analysis is conducive to mining key functional genes in crop genomes and provides a genetic research basis for the development of high-yield and high-quality germplasms. In this study, the Arabidopsis XTH protein sequences were used as the seed sequence, combined with the Pfam domain search results, and finally identified 80 members of the B. napus XTH gene family, distributed on 18 definite chromosomes and 1 pseudochromosome. In this study, 52 BrXTH proteins and 47 BoXTH proteins were identified, the numbers of which differ from previous studies (53 XTHs of B. rapa and 38 XTHs of B. oleracea) [21]. This discrepancy may be due to differences in the sources of genome databases and the selection of thresholds. Compared with the 52 BrXTH proteins and 47 BoXTH proteins, B. napus, as an allotetraploid, was identified with 80 XTH genes, a number smaller than the sum of the former two, which also suggested that recombination or mutation at the gene or chromosome level may have occurred in the B. napus genome during the evolutionary process. Phylogenetic analysis was performed based on the XTH protein sequences of (A) thaliana and (B) napus, and the distribution of XTH proteins in the phylogenetic tree was statistically analyzed. It was found that BnXTH proteins were more uniformly distributed among the four groups of the phylogenetic tree, suggesting that gene duplication events might have occurred in the genome of B. napus at the whole-genome level in these four groups (Fig. 2A). In the construction of phylogenetic trees for three species within the Brassicaceae family, it was observed that the distribution of XTHs within the four groups was broadly consistent (refer to Fig. 2B; Table 1). According to the U-triangle model, B. napus (AACC: 2n = 38), an allopolyploid species, originates from the cross between B. rapa (AA: 2n = 20) and B. oleracea (CC: 2n = 18) [22]. This analogous distribution pattern of XTHs across the species underscores their genetic conservation. Furthermore, the total count of BnXTHs being lesser than the combined total of BrXTHs and BoXTHs highlights the evolutionary distinctiveness of BnXTHs.
Further analysis of the gene structures and conserved domains showed that evolutionary conservation may exist among species including (A) thaliana [23], Osmanthus fragrans [24], Schima superba [25], (B) rapa and B. oleracea [21]. Motif 2, Motif 3, Motif 5, and Motif 6 were identified as ubiquitous across all BnXTH proteins. This uniform presence suggests that these four motifs likely constitute characteristic sequences of BnXTHs, playing pivotal roles in the structural and functional attributes of these proteins. Analysis of promoter cis-acting elements showed that the function of BnXTHs may be related to photosynthesis due to the large number of light responsiveness elements. By modifying the cell wall, XTHs influence leaf thickness, which can affect the internal leaf architecture and, consequently, the efficiency of light capture and the distribution of light within the leaf [16]. XTHs are also involved in the development and functioning of stomata by modulating the flexibility and integrity of the cell walls surrounding guard cells [26]. While the direct relationship between XTH activity and photosynthesis requires further empirical study, it is evident that XTHs, through their role in cell wall modification, indirectly contribute to optimizing the conditions necessary for efficient photosynthesis.
The Ka/Ks analysis is a method used to measure the selective pressure on genes, commonly applied in the study of gene or gene family evolution. In this context, Ka represents the rate of nonsynonymous substitutions, which lead to changes in amino acids, while Ks denotes the rate of synonymous substitutions that do not result in amino acid changes [27]. Ka/Ks analysis serves as a crucial tool for understanding the dynamics of gene evolution and revealing the key mechanisms behind the adaptive evolution of organisms, suitable for discussion in scientific literature. By collinearity analysis between genomes, we found that there were 92 pairs of XTH collinear genes between B. napus and B. rapa, 88 pairs of XTH collinear genes between B. napus and B. oleracea (Fig. 4), and 83 XTH covariance pairs within the B. napus genome (Fig. 5), and their Ka/Ks values were all less than 1 (Table S5). The number of collinear gene pairs of XTHs among species was greater than the number of BnXTHs we identified, indicating that XTHs are evolutionarily correlated and have both inter- and intra-species evolutionary conservation.
miRNAs (microRNA) are small non-coding RNAs that regulate gene expression at the post-transcriptional level by inhibiting the translation of messenger RNAs (mRNAs) or by promoting mRNA degradation [28]. miRNAs are approximately 20–24 nucleotides (nt) in size and are encoded by plants, animals and some viruses [29]. In plants, miRNAs inhibit gene expression mainly by mediating target RNA cleavage or translational repression [30], and they control target genes at the post-transcriptional or translational level by controlling the level of protein synthesis, and have an impact on plant growth, development, and response to environmental stresses [31]. For example, bna-miR159, bna-miR6029, and bna-miR827 negatively regulate target genes related to nitrogen metabolism pathway in B. napus, thereby affecting nitrogen signaling within the B. napus plant and consequently its pod thickness [32]. bna- miR319 overexpressing transgenic lines of B. napus exhibited abnormal development of serrated leaves and stem tip meristematic tissues [33]. Fu et al. analyzed miRNA-mRNA expression of Cd-stressed B. napus seedlings and found that Cd treatment significantly affected the expression of 22 miRNAs belonging to 11 families in roots and 29 miRNAs belonging to 14 families in shoots, and identified 8 miRNA-mRNA interaction pairs in roots and 8 miRNA-mRNA interaction pairs in shoots. 8 miRNA-mRNA interaction pairs in roots and 8 miRNA-mRNA interaction pairs in shoots were identified [34]. In this study, a total of 37 miRNAs of B. napus were targeted to 29 BnXTH genes through cleavage and translational repression (Fig. 6), and it was hypothesized that miRNAs form a miRNA-target gene regulatory network with BnXTHs, which are involved in the process of reorganization and hydrolysis of XG in B. napus cell walls. Interestingly, BnXTHs have similar cis-acting elements (Fig. 3), but could have relatively different expression patterns (Fig. 7). For example, BnXTH2, and BnXTH41 all targetted bna-miR167 and had relatively consistent expression patterns. They were not expressed in leaves under various abiotic stresses but have a certain amount of expression in roots. However, compared with the expression patterns of other BnXTHs, they were different.
Various abiotic stresses may affect the integrity of plant cell walls, but plants are able to repair adverse changes in the cell wall, including changing its composition, structure, and mechanical properties to maintain growth [35]. There has been some research on how the cell wall responds to stress [36]. However, due to the constraints of imaging and biomechanical equipment and the potential crosstalk between cell wall signaling and stress signaling, it is difficult to draw general conclusions about the regulatory mechanisms of the cell wall in response to various abiotic stresses [2]. XTH genes are key genes that regulate the hydrolysis and recombination of XG components and plays an important role in the structure and composition of plant cell walls [25]. Therefore, clarifying the changes that occur in XTHs during plant defense against abiotic stresses is informative for the study of the plant stress regulatory mechanism mediated by plant cell wall signals. In this study, qPCR technology was used to detect the relative expression BnXTH7, BnXTH24, BnXTH17, BnXTH6, BnXTH9, BnXTH20, BnXTH72, BnXTH37, and BnXTH61 in leaves under 4 abiotic stresses treatment after 4 time points: 0, 6 h, 12 h, and 24 h (Fig. 8). It was found that in response to abiotic stresses, the expression of most of the BnXTHs gradually increased or first increased and then decreased with the growth of treatment time, indicating that oilseed rape needs large amounts of recombinant XGs in response to abiotic stress in order to repair the damage that may be caused by the stress to the cell wall. And with the time went, the plant slowly adapted to the stress, and some of the BnXTHs may have received the relevant signals, thus the expression fell back.
Materials and methods
Identification and chromosome mapping of BnXTH family members
The sequence files of ZS11 (a variety of B.napus), cabbage (B. rapa), and kale (B. oleracea) were obtained from the BRAD database (BRAD: http://brassicadb.cn/#/) [37]. 33 AtXTH protein sequences from the A. thaliana genome database (TAIR: https://www.arabidopsis.org/) were downloaded as seed sequences, and searched for possible BnXTH proteins in the whole protein sequences of B.napus (e Value < 1e-10) by BLASTp [38]. The PF06955 and PF00722 conserved structural domain files were downloaded from the Pfam database to search the possible BnXTH proteins by HMMER software (http://www.hmmer.org/) [39, 40], combining the BLAST results to obtain the BnXTH hypothetical proteins. The putative protein sequence was uploaded to the NCBI-CDD website (https://www.ncbi.nlm.nih.gov/cdd/) for further confirmation, and 80 members of the BnXTH gene family were finally identified. The BnXTHs were named according to their chromosomal position sequence, and were named as BnXTH1 ~ BnXTH80. The Protparam function in the ExPASy website was used to predict the physical and chemical properties of BnXTH proteins [41], and the subcellular localization prediction results were implemented by the Plant-mPLoc website [42]. Chromosomal location information of BnXTHs was retrieved using downloaded annotation files and visualized by TBTools [43].
Phylogenetic analysis
The XTH protein sequences of A. thaliana and B.napus were imported into MEGA 11 software, and the parameters were adjusted to neighbor joining (NJ) and 1000 boots-trap repetitions to obtain a phylogenetic tree, and the phylogenetic tree was embellished in the online website iTOL (https://itol.embl.de/) [44, 45].
Prediction of gene structure, protein conserved motifs and promoter cis-acting elements
The CDS/UTR region and gene structure information of BnXTH gene family members were obtained from the NCBI-CDD website and Pfam database. The MEME 5.5.1 website was used to obtain the conserved motif of the BnXTH proteins [46], and the prediction of the cis-acting element 2000 bp upstream of the BnXTHs promoter region was predicted through the PlantCARE website [47]. All data information visualization was completed by TBTools.
Collinearity analysis of XTH genes
MCScanX software was used to analyze the collinear relationship between XTHs within the genomes of B. napus, B. rapa, and B. oleracea, as well as within the genomes of B. napus [48]. Collinearity information visualization was performed using TBTools. Ka/Ks value calculation was implemented using KaKs_calculator 3.0 software [49].
Prediction of targeting relationship between miRNA and BnXTHs
The CDS sequences of BnXTHs were uploaded to the psRNATarget website (https://www.zhaolab.org/psRNATarget/analysis?function=2/) [50], and combined with the miRNA mature sequences information of B. napus collected by the website, the targeting relationships between miRNA and BnXTHs were analyzed. The data were visualized using the Bioinformatics website (https://www.bioinformatics.com.cn/plot_basic_alluvial_plot_017).
Analysis of the expression pattern of BnXTHs under different abiotic stress
To investigate the gene expression patterns of BnXTHs under abiotic stress treatments, the BnXTH gene IDs were uploaded to the BNIR database [51] to obtain the Transcript per Kilobase per Million mapped reads (TPM) of B. napus ZS11 in control (CK), salt (200 mmol·L-1 NaCl treatment), drought (exposure to airflow for 1 h), freezing (recovering to 25 °C after 3 h of stress at -4 °C), cold (4 °C), heat (recovering to 25 °C after 3 h of stress at 38 °C), and osmosis (300 mmol-L-1 mannitol) after 24 h, and the data were visualized using TBTools software after calculating log10(TPM + 1).
Materials and treatment
B. napus ZS11 seeds were germinated on wet gauze (soaked with water) in a plant growth chamber at 20 to 22 °C and 65% humidity under a long-day condition (16-h-light/ 8-h-dark cycle) [52]. The one-week-old seedlings were then transferred into a previously described hydroponic system [53] under the same culture conditions for nearly 20 days until the fourth leaves had extended (plant samples used for transcriptome sequencing were also harvested for root system). For stress treatment research, leaf samples from 4-week-old plants of ZS11 were collected after 0, 6, 12 and 24 h of 0.5 mmol·L-1 AlCl3, 0.2% (w/v) Na2CO3, 1.2% (w/v) NaCl, and 20% (w/v) PEG 6000 treatment. Seedlings without any stress treatment were used as the control. Each treatment includes three biological replications. Leaves were harvested immediately frozen in liquid nitrogen and stored at -80 °C for RNA extraction.
Relative expression of BnXTHs under different abiotic stresses
Total RNA of ZS11 under control and abiotic treatments was extracted using the RNA simple Total RNA kit (Tiangen Biotechnology Co., Ltd., Beijing, China) according to the manufacturer’s protocol. cDNA was synthesized with 1 µg RNA from each sample with HiScript® II Q Select RT SuperMix with gDNA wiper (Vazyme, Nanjing, China). Gene-specific primers used for quantitative real-time PCR (qRT-PCR) listed in Table 2. qRT-PCR was run on the AriaMx real-time PCR system (Agilent Technologies). The following cycling parameters were used: initial denaturation at 95 °C for 5 min; 40 amplification cycles consisting of denaturation at 95 °C for 10s, annealing and extension at 60 °C for 30 s; The melting curve was then tested at 65–95 °C. The internal standard was the B. napus actin gene (BnaA01g27090D). To investigate the expression patterns of BnXTHs under various environmental conditions, 9 BnXTHs were randomly selected from the 4 groups for qRT-PCR experiments. Three biotic replicates were performed for each sample, and each replicate contained three technical replicates. Relative expression levels were calculated according to the 2−ΔΔCt method [54]. Data represent the mean ± standard error for threebiological experiments. Student’s t-test was used to determine differences. *: significant differences between treatments at p ≤ 0.05. **: significant differences between treatments at p ≤ 0.01.
Table 2.
qPCR primer sequence of BnXTH genes
| Gene Name | Forward Primer (5’→3’) | Reverse Primer (3’→5’) | Amplicon Length (bp) |
|---|---|---|---|
| BnXTH6 | AAACGGGAAGTCATCGTGCT | CTGCACCCATCTCATCCGTT | 109 |
| BnXTH7 | GGTGCAGACGATGTTCATGC | AAACTCAGTCGCACACCCAT | 118 |
| BnXTH9 | GGGAAGTGGTGATGGCAGAA | GAAATGTGGCCGCACTCTTC | 178 |
| BnXTH17 | ACTGCATCACATGGAACCCC | GCTCAGCTTCCCAAAGGCTA | 114 |
| BnXTH20 | CTGGCTACACTGCTGGAGTC | GCGAAACTTCATCTCGCGAC | 188 |
| BnXTH24 | ACCGATCCGAGTGTACAGGA | GATGCCTGGAACTCAGCAGT | 135 |
| BnXTH37 | AGGTTCTGAGCTGCCAAGAC | CTAAGCCGCTTAGCCTCCTC | 190 |
| BnXTH61 | GGACTTACGACAGGGAGCAG | AACGTCCAGAGCACCTCAAG | 115 |
| BnXTH72 | TCGCCAAACTCACTCTCGAC | AACCACAACACCAGAGGCAA | 122 |
Conclusions
B. napus genome was identified to contain 80 XTH genes distributed on 18 definite chromosomes and one pseudochromosome. Their phylogenetic relationships, protein physicochemical properties, subcellular localization, gene structures, promoter cis-acting elements, covariance relationships, and reciprocal miRNAs were predicted and analyzed, and their transcriptional expression patterns as well as differences in expression in response to abiotic stress treatments were investigated. The results showed that the expression patterns of BnXTHs under abiotic stress treatments were varied, suggesting that cell wall signaling in B. napus in response to various abiotic stresses changes depending on the type of stress. The analysis of the BnXTH gene family and the study of the response pattern in abiotic stresses provide a good theoretical basis for further research on this family of genes in resistance breeding of B. napus.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Table S1: Physicochemical properties and subcellular localization prediction of BnXTH proteins.
Supplementary Material 2: Table S2: Table S2. XTH IDs of B. rapa and B. oleracea.
Supplementary Material 3: Table S3: Collinearity of XTH genes in B. napus, B. rapa, and B. oleracea.
Supplementary Material 4: Table S5: Collinearity of XTH genes in B. napus.
Supplementary Material 5: Table S4: Collinearity of XTH genes in B. napus.
Acknowledgements
We would like to thank the Hubei Province “515” Action (Cooperative Extension) “Rapeseed New Technology Demonstration and Science and Technology Service Rapeseed Industry Chain Project” and Ministry of Agriculture and Rural Affairs Plantation Management Department “Rapeseed Industry Policy Research and Main Pests and Diseases Prevention and Control Strategies " (15214011), National major biological breeding project of China (2022ZD04010). Thanks for their financial support.
Author contributions
X.Z. and C.Z. designed and managed the project. J.C. and H.W. completed experiments and paper writing. H.Z., X.D., W.W., J.L., J.X. and R.Y. performed experiments, material sampling. Laboratory data measurements. X.Z., C.Z. and B.X. revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the grants from the Hubei Province “515” Action (Cooperative Extension) “Rapeseed New Technology Demonstration and Science and Technology Service Rapeseed Industry Chain Project”, Ministry of Agriculture and Rural Affairs Plantation Management Department “Rapeseed Industry Policy Research and Main Pests and Diseases Prevention and Control Strategies " (15214011), National major biological breeding project of China (2022ZD04010) and Jianghan University scientific research project funding scheme (2022XKZX17).
Data availability
All the data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Study complied with local and national regulations for using plants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Changli Zeng, Email: zengchangli@jhun.edu.cn.
Xuekun Zhang, Email: zhangxuekun@yangtzeu.edu.cn.
References
- 1.Kopecká R, Kameniarová M, Černý M, Brzobohatý B, Novák J. Abiotic stress in Crop Production. Int J Mol Sci 2023, 24(7). [DOI] [PMC free article] [PubMed]
- 2.Rui Y, Dinneny JR. A wall with integrity: surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020;225(4):1428–39. doi: 10.1111/nph.16166. [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan S, Lionetti V, Zabotina OA. Plant Cell Wall Integrity Perturbations and Priming for Defense. Plants (Basel, Switzerland) 2022, 11(24). [DOI] [PMC free article] [PubMed]
- 4.Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C. Cell wall metabolism in response to Abiotic Stress. Plants (Basel Switzerland) 2015;4(1):112–66. doi: 10.3390/plants4010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamil MS, Geitmann A. The middle lamella-more than a glue. Phys Biol. 2017;14(1):015004. doi: 10.1088/1478-3975/aa5ba5. [DOI] [PubMed] [Google Scholar]
- 6.Bidhendi AJ, Chebli Y, Geitmann A. Fluorescence visualization of cellulose and pectin in the primary plant cell wall. J Microsc. 2020;278(3):164–81. doi: 10.1111/jmi.12895. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Giannetti A, Sugiyama Y, Zheng W, Schneider R, Watanabe Y, Oda Y, Persson S. Secondary cell wall patterning-connecting the dots, pits and helices. Open Biology. 2022;12(5):210208. doi: 10.1098/rsob.210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park YB, Cosgrove DJ. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 2015;56(2):180–94. doi: 10.1093/pcp/pcu204. [DOI] [PubMed] [Google Scholar]
- 9.von Schantz L, Gullfot F, Scheer S, Filonova L, Cicortas Gunnarsson L, Flint JE, Daniel G, Nordberg-Karlsson E, Brumer H, Ohlin M. Affinity maturation generates greatly improved xyloglucan-specific carbohydrate binding modules. BMC Biotechnol. 2009;9:92. doi: 10.1186/1472-6750-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh YS, Harris PJ. Xyloglucans of monocotyledons have diverse structures. Mol Plant. 2009;2(5):943–65. doi: 10.1093/mp/ssp061. [DOI] [PubMed] [Google Scholar]
- 11.Park YB, Cosgrove DJ. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012;158(4):1933–43. doi: 10.1104/pp.111.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hrmova M, Stratilová B, Stratilová EJIJMS. Broad specific xyloglucan: xyloglucosyl transferases are formidable players in the re-modelling of plant cell wall structures. 2022, 23(3):1656. [DOI] [PMC free article] [PubMed]
- 13.Takahashi D, Johnson KL, Hao P, Tuong T, Erban A, Sampathkumar A, Bacic A, Livingston DP, Kopka J, Kuroha T, et al. Cell wall modification by the xyloglucan endotransglucosylase/hydrolase XTH19 influences freezing tolerance after cold and sub-zero acclimation. Plant Cell Environ. 2021;44(3):915–30. doi: 10.1111/pce.13953. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Liu Y, Shen Y, Li W. A surprising diversity of Xyloglucan Endotransglucosylase/Hydrolase in wheat: New in Sight to the roles in Drought Tolerance. Int J Mol Sci 2023, 24(12). [DOI] [PMC free article] [PubMed]
- 15.Cheng Z, Zhang X, Yao W, Gao Y, Zhao K, Guo Q, Zhou B, Jiang T. Genome-wide identification and expression analysis of the xyloglucan endotransglucosylase/hydrolase gene family in poplar. BMC Genomics. 2021;22(1):804. doi: 10.1186/s12864-021-08134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, Wang W, Sun J, Ding M, Zhao R, Deng S, Wang F, Hu Y, Wang Y, Lu Y, et al. Populus Euphratica XTH overexpression enhances salinity tolerance by the development of leaf succulence in transgenic tobacco plants. J Exp Bot. 2013;64(14):4225–38. doi: 10.1093/jxb/ert229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, Valliyodan B, Prince S, Wan J, Nguyen HT. Characterization of the XTH Gene Family: New Insight to the roles in soybean flooding tolerance. Int J Mol Sci 2018, 19(9). [DOI] [PMC free article] [PubMed]
- 18.Du H, Hu X, Yang W, Hu W, Yan W, Li Y, He W, Cao M, Zhang X, Luo B, et al. ZmXTH, a xyloglucan endotransglucosylase/hydrolase gene of maize, conferred aluminum tolerance in Arabidopsis. J Plant Physiol. 2021;266:153520. doi: 10.1016/j.jplph.2021.153520. [DOI] [PubMed] [Google Scholar]
- 19.Ma YS, Jie HD, Zhao L, Lv XY, Liu XC, Tang YY, Zhang Y, He PL, Xing HC, Jie YC. Identification of the Xyloglucan Endotransglycosylase/Hydrolase (XTH) gene family members expressed in Boehmeria nivea in response to cadmium stress. Int J Mol Sci 2022, 23(24). [DOI] [PMC free article] [PubMed]
- 20.Xuan Y, Zhou ZS, Li HB, Yang ZM. Identification of a group of XTHs genes responding to heavy metal mercury, salinity and drought stresses in Medicago truncatula. Ecotoxicol Environ Saf. 2016;132:153–63. doi: 10.1016/j.ecoenv.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Liu A, Qu X, Liang J, Song M. Genome-wide identification, and phylogenetic and expression profiling analyses, of XTH gene families in Brassica rapa L. and Brassica oleracea L. BMC Genomics. 2020;21(1):782. doi: 10.1186/s12864-020-07153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yim WC, Swain ML, Ma D, An H, Bird KA, Curdie DD, Wang S, Ham HD, Luzuriaga-Neira A, Kirkwood JS, et al. The final piece of the triangle of U: evolution of the tetraploid Brassica carinata genome. Plant Cell. 2022;34(11):4143–72. doi: 10.1093/plcell/koac249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becnel J, Natarajan M, Kipp A, Braam J. Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Mol Biol. 2006;61(3):451–67. doi: 10.1007/s11103-006-0021-z. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Miao Y, Zhong S, Fang Q, Wang Y, Dong B, Zhao H. Genome-Wide Identification and Expression Analysis of XTH Gene Family during Flower-Opening Stages in Osmanthus fragrans. Plants (Basel, Switzerland) 2022, 11(8). [DOI] [PMC free article] [PubMed]
- 25.Yang Z, Zhang R, Zhou Z. The XTH Gene Family in Schima superba: genome-wide identification, expression profiles, and Functional Interaction Network Analysis. Front Plant Sci. 2022;13:911761. doi: 10.3389/fpls.2022.911761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JY, Seo YS, Kim SJ, Kim WT, Shin JSJP. Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum Cv. Dotaerang) 2011;30:867–77. doi: 10.1007/s00299-010-0989-3. [DOI] [PubMed] [Google Scholar]
- 27.Hurst LDJTiG. The Ka/Ks ratio: diagnosing the form of sequence evolution. 2002, 18(9):486–7. [DOI] [PubMed]
- 28.Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs’ Action through miRNA Editing. Int J Mol Sci 2019, 20(24). [DOI] [PMC free article] [PubMed]
- 29.Kilikevicius A, Meister G, Corey DR. Reexamining assumptions about miRNA-guided gene silencing. Nucleic Acids Res. 2022;50(2):617–34. doi: 10.1093/nar/gkab1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Yu B. Recent advances in the regulation of plant miRNA biogenesis. RNA Biol. 2021;18(12):2087–96. doi: 10.1080/15476286.2021.1899491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai JF, Wang YK, Wang P, Duan WJ, Yuan SH, Sun H, Yuan GL, Ma JX, Wang N, Zhang FT, et al. Uncovering male fertility transition responsive miRNA in a wheat photo-thermosensitive genic male sterile line by deep sequencing and Degradome Analysis. Front Plant Sci. 2017;8:1370. doi: 10.3389/fpls.2017.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Huo Q, Yang H, Jian H, Qu C, Lu K, Li J. Joint RNA-Seq and miRNA profiling analyses to Reveal Molecular mechanisms in regulating thickness of Pod Canopy in Brassica napus. Genes 2019, 10(8). [DOI] [PMC free article] [PubMed]
- 33.Lu H, Chen L, Du M, Lu H, Liu J, Ye S, Tao B, Li R, Zhao L, Wen J, et al. miR319 and its target TCP4 involved in plant architecture regulation in Brassica napus. Plant Science: Int J Experimental Plant Biology. 2023;326:111531. doi: 10.1016/j.plantsci.2022.111531. [DOI] [PubMed] [Google Scholar]
- 34.Fu Y, Mason AS, Zhang Y, Lin B, Xiao M, Fu D, Yu H. MicroRNA-mRNA expression profiles and their potential role in cadmium stress response in Brassica napus. BMC Plant Biol. 2019;19(1):570. doi: 10.1186/s12870-019-2189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novaković L, Guo T, Bacic A, Sampathkumar A, Johnson KL. Hitting the Wall-Sensing and Signaling pathways involved in Plant Cell Wall Remodeling in response to Abiotic Stress. Plants (Basel Switzerland) 2018, 7(4). [DOI] [PMC free article] [PubMed]
- 36.Wang Z, Wang M, Yang C, Zhao L, Qin G, Peng L, Zheng Q, Nie W, Song CP, Shi H, et al. SWO1 modulates cell wall integrity under salt stress by interacting with importin ɑ in Arabidopsis. Stress Biology. 2021;1(1):9. doi: 10.1007/s44154-021-00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Wang T, He X, Cai X, Lin R, Liang J, Wu J, King G, Wang X. BRAD V3.0: an upgraded Brassicaceae database. Nucleic Acids Res. 2022;50(D1):D1432–41. doi: 10.1093/nar/gkab1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2012;40(Database issue):D1202–1210. doi: 10.1093/nar/gkr1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–9. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46(W1):W200–4. doi: 10.1093/nar/gky448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duvaud S, Gabella C, Lisacek F, Stockinger H, Ioannidis V, Durinx C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021;49(W1):W216–27. doi: 10.1093/nar/gkab225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35(Web Server issue):W585–587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative Toolkit developed for interactive analyses of big Biological Data. Mol Plant. 2020;13(8):1194–202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021;38(7):3022–7. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letunic I, Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015;43(W1):W39–49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z. KaKs_Calculator 3.0: calculating selective pressure on Coding and non-coding sequences. Genom Proteom Bioinform. 2022;20(3):536–40. doi: 10.1016/j.gpb.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai X, Zhuang Z, Zhao PX. psRNATarget: a plant small RNA target analysis server (2017 release) Nucleic Acids Res. 2018;46(W1):W49–54. doi: 10.1093/nar/gky316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Z, Wang S, Wei L, Huang Y, Liu D, Jia Y, Luo C, Lin Y, Liang C, Hu Y, et al. BnIR: a multi-omics database with various tools for Brassica napus research and breeding. Mol Plant. 2023;16(4):775–89. doi: 10.1016/j.molp.2023.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Zhang X, Zhao H, Hu J, Wang Z, Yang G, Zhou X, Wan H. Genome-wide identification and expression analysis of phenylalanine ammonia-lyase (PAL) family in rapeseed (Brassica napus L) BMC Plant Biol. 2023;23(1):481. doi: 10.1186/s12870-023-04472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan H, Chen L, Guo J, Li Q, Wen J, Yi B, Ma C, Tu J, Fu T, Shen J. Genome-wide Association Study reveals the Genetic Architecture Underlying Salt Tolerance-related traits in rapeseed (Brassica napus L) Front Plant Sci. 2017;8:593. doi: 10.3389/fpls.2017.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego Calif) 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1: Physicochemical properties and subcellular localization prediction of BnXTH proteins.
Supplementary Material 2: Table S2: Table S2. XTH IDs of B. rapa and B. oleracea.
Supplementary Material 3: Table S3: Collinearity of XTH genes in B. napus, B. rapa, and B. oleracea.
Supplementary Material 4: Table S5: Collinearity of XTH genes in B. napus.
Supplementary Material 5: Table S4: Collinearity of XTH genes in B. napus.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article and its supplementary information files.