Abstract
The murine embryonal stem (ES) cell virus (MESV) can express transgenes from the long terminal repeat (LTR) promoter/enhancer in undifferentiated ES cells, but expression is turned off upon differentiation to embryoid bodies (EBs) and hematopoietic cells in vitro. We examined whether a human immunodeficiency virus type 1-based lentivirus vector pseudotyped with the vesicular stomatitis virus G protein (VSV-G) could transduce ES cells efficiently and express the green fluorescent protein (GFP) transgene from an internal phosphoglycerate kinase (PGK) promoter throughout development to hematopoietic cells in vitro. An oncoretrovirus vector containing the MESV LTR and the GFP gene was used for comparison. Fluorescence-activated cell sorting analysis of transduced CCE ES cells showed 99.8 and 86.7% GPF-expressing ES cells in the VSV-G-pseudotyped lentivirus (multiplicity of infection [MOI] = 59)- and oncoretrovirus (MOI = 590)-transduced cells, respectively. Therefore, VSV-G pseudotyping of lentiviral and oncoretrovirus vectors leads to efficient transduction of ES cells. Lentivirus vector integration was verified in the ES cell colonies by Southern blot analysis. When the transduced ES cells were differentiated in vitro, expression from the oncoretrovirus LTR was severely reduced or extinct in day 6 EBs and ES cell-derived hematopoietic colonies. In contrast, many lentivirus-transduced colonies, expressing the GFP gene in the undifferentiated state, continued to express the transgene throughout in vitro development to EBs at day 6, and many continued to express in cells derived from hematopoietic colonies. This experimental system can be used to analyze lentivirus vector design for optimal expression in hematopoietic cells and for gain-of-function experiments during ES cell development in vitro.
Gene transfer into hematopoietic stem cells (HSCs) of humans and large animals has been inefficient using oncoretrovirus vectors (18, 19, 25). This is to a large extent due to the quiescent nature of HSCs since Moloney murine leukemia virus (MMLV)-based vectors require dividing target cells for successful transduction (28). This has led to a recent interest in lentivirus vectors as potential gene delivery vehicles to human HSCs (6, 22, 33), since these have the ability to transduce quiescent cells (1, 5, 15, 23, 24, 31). To study gene expression characteristics of lentivirus vectors in hematopoietic cells, we transduced embryonic stem (ES) cells with lentivirus vectors and developed lentivirus-transduced ES cell clones that subsequently differentiated into hematopoietic cells. In this fashion, we could examine whether persistence of gene expression can be maintained during development of lentivirus-transduced ES cells from the undifferentiated state to hematopoietic colonies in vitro. Similarly, we were able to investigate whether the level of expression is copy number related or whether it is subjected to position effects (integration site dependent).
MMLV vectors can transduce ES cells efficiently, but expression from the viral long terminal repeat (LTR) is not active due to transcriptional silencing immediately following infection which is attributable to trans-acting factors (12, 21, 30). A recombinant viral LTR in a virus called the murine ES cell virus (MESV) allows effective expression in undifferentiated ES cells (12). This virus has a high-affinity binding site for the Sp1 transcription factor (13, 27), elimination of a binding site in the LTR for a transcriptional repressor (4, 12, 13, 34), and elimination of the negative regulatory element in the primer binding site (2, 12, 21, 35). These changes in and around the LTR do not allow expression in transduced ES cells that are differentiated in vitro, including cells that are differentiated into hematopoietic cells. During the differentiation process, expression is turned off by a cis-acting mechanism (20). We decided to examine whether a human immunodeficiency virus type 1 (HIV-1)-based vector that lacks the tat gene and uses an internal phosphoglycerate kinase (PGK) promoter to drive the enhanced green fluorescent protein (GFP) gene would be able to express the gene throughout differentiation from undifferentiated ES cells through embryonic bodies (EBs) and to hematopoietic colonies grown in methylcellulose (16, 17, 36). Our hypothesis was that the internal promoter would be active and this experimental model system could be used to study lentivirus gene expression in hematopoietic cells in vitro following transduction of ES cells. The basis for our hypothesis was that early work using MMLV vectors with internal promoters in embryonic carcinoma cells or after infection of mouse embryos showed expression from internal promoters (29, 34). Also, there is no need for active transcriptional repression of the HIV-1 LTR by the cells since without the Tat protein, the HIV-1 LTR is transcriptionally inactive, although this does not exclude an additional inactivation mechanism by the cell (8, 9).
Lentivirus vector gene transfer efficiency of ES cells.
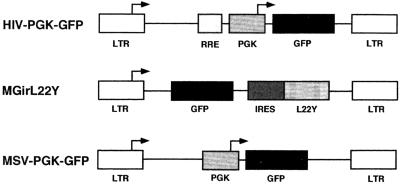
We first examined whether it was possible to transduce ES cells by using lentivirus vectors and, if so, how the transduction efficiency compared that of to standard oncoretrovirus vectors. The feeder-independent CCE ES cells were transduced for 48 h by the lentivirus vector HIV-PGK-GFP packaged in 293T cells with vesicular stomatitis virus G protein (VSV-G) and the oncoretrovirus vector MGirL22Y packaged in the ecotropic packaging cell line GP+E86. The HIV-PGK-GFP vector has the same backbone structure as HR′CMV-GFP (38), where the PGK promoter replaces the cytomegalovirus promoter. The structures of these vectors are shown in Fig. 1. The lentivirus vector HIV-PGK-GFP was produced by transient transfection into 293T cells as previously described (3, 7, 24). A total of 40 μg of plasmid DNA was used for transfection of one 10-cm-diameter dish: 10 μg of the envelope plasmid pMD.G encoding VSV-G, 10 μg of packaging plasmid pCMVΔR 8.91 (37) expressing Gag, Pol, Tat, and Rev, and 20 μg of vector plasmid HIV-PGK-GFP, which contained the enhanced GFP gene under the control of the murine PGK promoter (Fig. 1). The conditioned medium was collected after 96 h, filtered through 0.45-μm-pore-size cellulose acetate filters (Nalge Company, Rochester, N.Y.), and ultracentrifuged with a Beckman T28W rotor at 50,000 × g for 1.5 h. The oncoretrovirus vector MGirL22Y (Fig. 1) was harvested from the conditioned medium of MGirL22Y producer cells (kindly provided by D. A. Persons and A. W. Nienhuis, St. Jude Children's Hospital, Memphis, Tenn.) (26). The MGirL22Y vector contains the MESV LTR (12) to drive the GFP gene. One million MGirL22Y cells were seeded on a 10-cm-diameter dish; the conditioned medium was collected after 96 h, filtered through 0.45-μm-pore-size cellulose acetate filters, and concentrated in a Centricon (Amicon, Beverly, Mass.). The titer of the vectors was determined on NIH 3T3 cells. NIH 3T3 cells (105) were plated in 12-well plates and transduced in the presence of protamine sulfate (Sigma Chemical Co., St. Louis, Mo.). GFP expression was evaluated by fluorescence-activated cell sorting (FACS) analysis in a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) after 48 h, and virus titers were estimated. The lentivirus and oncoretrovirus vector titers on NIH 3T3 cells were 1.18 × 107 and 1.70 × 107 TU/ml, respectively. CCE ES cells were maintained in Dulbecco's modified Eagle's medium with 15% fetal calf serum (FCS; GIBCO Life Technologies, Gaithersburg, Md.), 1 mM glutamine (GIBCO), 1 mM sodium pyruvate (GIBCO), 150 mM α-monothioglycerol (Sigma), and 1,000 U of leukemic inhibitory factor (LIF; GIBCO) on gelatinized plates. CCE cells (104) were transduced with the viral vectors in the gelatinized T25 bottles in the presence of protamine sulfate. The efficiency of viral transfer in the bulk population was estimated by determining the ratio of GFP-expressing cells with a FACSCalibur. As shown in Table 1, the efficiency of lentivirus transduction was 99.8% ± 0.1% at a multiplicity of infection (MOI) of 59, and the efficiency increased in proportion to the MOI. A similar correlation was seen between MOI and transduction efficiency for oncoretrovirus transduction, but the latter was lower than that of lentivirus vectors at the same MOI (5.9 to 59). The efficiency of oncoretrovirus transduction increased to 56.2% ± 2.8% at an MOI of 590 (Table 1). After transduction, part of the ES cell bulk population was washed and then replated in 96-well plates at 0.5 to 2 cells per well by the limiting dilution method to create clones from single, transduced ES cells. Undifferentiated ES colonies were formed after 7 days of culture in 96-well plates in the presence of LIF. GFP-expressing colonies were scored by fluorescence microscopy. Almost all of the ES colonies were transduced by the lentivirus vector at an MOI of 59 (94.8% ± 1.5% positive for GFP expression [Table 1]). This number was almost identical to the number of GFP-positive ES cells from the bulk population as estimated by FACS (99.8% ± 0.1%, MOI = 59). There was a good correlation between the proportion of GFP-positive ES cells from the bulk population and the number of positive ES cell colonies that scored visually positive in the microscope at all MOIs used (5.9 to 59 [Table 1]). These results suggest that the lentivirus and oncoretrovirus transgenes are integrated stably into the genomes of their ES target cells (see below). These results demonstrate that transduction of ES cells with VSV-G-pseudotyped lentivirus vectors is very efficient. The transduction efficiency was close to 100% at an MOI of 59. Ecotropic oncoretrovirus vectors transduce ES cells less efficiently, requiring very high MOIs to transduce approximately 50% of the cells.
FIG. 1.
Vectors. The lentivirus vector (HIV-PGK-GFP) uses the internal PGK promoter to drive expression of GFP gene. The Rev-responsive element (RRE) is indicated. The oncoretrovirus vectors (MGirL22Y [28]) and MSV-PGK-GFP) contain the same vector backbone using the MESV LTR, the same primer binding site, and the same length of the gag region (the figure is not drawn to scale) (14, 28). MGirL22Y contains the GFP gene followed by an internal ribosomal entry site (IRES) from the encephalomyocarditis virus linked to a mutant dihydrofolate reductase gene (L22Y). MSV-PGK-GFP contains the MESV LTR and the internal PGK promoter followed by the GFP gene.
TABLE 1.
VSV-G pseudotyping of oncoretrovirus vectors improves transduction efficiency of ES cells
| Virus | MOI | GFP+ ES cells (%)a | GFP+ ES colonies (%) |
|---|---|---|---|
| HIV-PGK-GFP | 5.9 | 46.0 ± 2.0 | 53.4 ± 0.6 |
| 11.8 | 70.7 ± 2.6 | 66.4 ± 3.0 | |
| 23.6 | 84.0 ± 2.7 | 82.7 ± 4.0 | |
| 59 | 99.8 ± 0.1 | 94.8 ± 1.5 | |
| MGirL22Y | |||
| Ecotropic | 5.9 | 6.7 ± 0.5 | 6.8 ± 0.6 |
| 11.8 | 10.9 ± 0.2 | 14.5 ± 0.3 | |
| 23.6 | 19.6 ± 1.5 | 23.9 ± 2.3 | |
| 59 | 39.8 ± 1.8 | 38.8 ± 3.6 | |
| 590 | 56.2 ± 2.8 | 57.2 ± 0.2 | |
| VSV-G pseudotyped | 59 | 86.7 | NDb |
| 590 | 99.4 | ND | |
| MSV-PGK-GFP | |||
| Ecotropic | 59 | 7.9 | ND |
| 590 | 22.6 | ND | |
| VSV-G pseudotyped | 59 | 55.2 | ND |
| 590 | 98.3 | ND |
Scored by FACS.
ND, not done.
ES cell transduction with VSV-G-pseudotyped oncoretrovirus vectors.
To determine whether the difference in lentivirus and oncoretrovirus transduction efficiency of ES cells was due to the differences in the envelope protein used, we pseudotyped two oncoretrovirus vectors, MGirL22Y and MSV-PGK-GFP (Fig. 1), with VSV-G. MSV-PGK-GFP (provided by Keith Humphries, Vancouver, British Columbia, Canada) was derived from the MSCV vector that contains the internal PGK promoter driving the neomycin resistance (neo) gene (14) where the GFP gene replaces the neo gene. It has the same vector backbone as MGirL22Y and contains the MESV LTR. Amphotropic vector MSV-PGK-GFP was produced by transient transfection into Phoenix amphotropic cells (provided by G. Nolan, Stanford University, Palo Alto, Calif.). Ecotropic MSV-PGK-GFP vector producer cell lines were established by transduction of GP+E86 cells with the amphotropic MSV-PGK-GFP vector supernatant. VSV-G-pseudotyped MGirL22Y and MSV-PGK-GFP producer cell lines were established by transduction of 293GPG cells with each amphotropic vector. Table 1 shows that VSV-G-pseudotyped oncoretrovirus vectors transduce ES cells with higher efficiency than the same vectors packaged in ecotropic packaging cells. To transduce practically 100% of the cells, an MOI of 59 was needed for the HIV-1-based lentivirus vector. VSV-G pseudotyping of oncoretrovirus vectors improved their transduction efficiency of ES cells substantially. These results show that VSV-G pseudotyping of both lentivirus and oncoretrovirus vectors leads to very efficient transduction of ES cells, although a very high MOI (590) is needed to reach the oncoretrovirus maximum transduction level of approximately 100% of the cells.
Integration of viral vectors in ES cells.
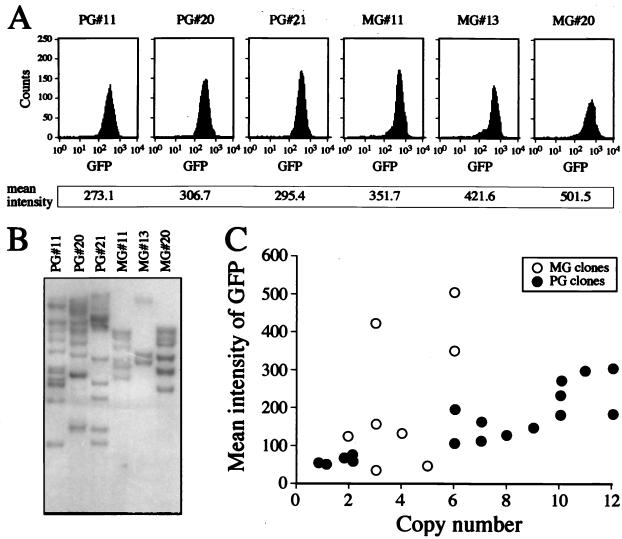
As shown above, the lentivirus-transduced clones seemed to be stably transduced, since they were expressing the transgene for more than 10 days after transduction. To determine whether the lentivirus genome had integrated into the chromosomal DNA of the target cell, genomic DNA from the transduced ES cell clones was subjected to Southern blot analysis. Genomic DNAs of the lentivirus- and oncoretrovirus-transduced clones were digested with BamHI and EcoRI, respectively, and then hybridized with a 32P-labeled GFP probe (Fig. 2). Lentivirus-transduced clones that expressed the GFP transgene 11 days after transduction, as detected visually in a fluorescence microscope, proved to have one or more integrated copies of the HIV-PGK-GFP provirus when analyzed by Southern blot analysis. As shown in Fig. 2, the lentivirus-transduced clones contained approximately 10 integrated copies in the ES genome. The lentivirus-transduced clones expressing the transgene at day 11 following transduction were all found to harbor an integrated provirus. Since the ES cells were rapidly dividing during transduction and after plating into the microtiter plates, the data suggest that integration takes place relatively rapidly following transduction.
FIG. 2.
Expression level of GFP and number of integrated proviral copies in transduced ES clones. (A) Three lentivirus (HIV-PGK-GFP vector)- and three oncoretrovirus (MGirL22Y vector)-transduced ES clones (PG and MS clones, respectively) which showed high GFP expression levels. (B) Southern blot analysis of the clones. Genomic DNAs of the PG and MG clones were digested with BamHI and EcoRI, respectively, and hybridized with GFP cDNA. (C) Relationship between the integrated copy number and expression level of GFP.
The oncoretrovirus-transduced clones all had fewer than six copies despite a GFP expression level equal to or higher than that seen in the lentivirus-transduced clones (Fig. 2). These results indicate that the oncoretrovirus transgene may be expressed more effectively in ES cells by the MESV LTR than the lentivirus transgene is by the internal PGK promoter on a proviral copy basis. This is a concern because we cannot expect to have multiple proviral copies in transduced human hematopoietic cells when developing gene therapy for hematological disorders. Therefore, it is important to investigate gene-regulatory elements that will allow a higher level of expression per proviral copy of the lentivirus vector. Several promoters need to be tested, and it is also important to see whether deletions in the 3′ HIV LTR to form safer self-inactivating vectors will affect expression levels positively or negatively (39). Recently, the posttranscriptional regulatory element from the woodchuck hepatitis virus was reported to increase expression levels from HIV-1-based lentivirus vectors (38). The experimental system described here can be used to test various vector constructs to evaluate the most desirable lentivirus vector design for transfer and expression in hematopoietic cells.
Figure 2C shows the relationship between the number of integrated copies and the mean intensity of GFP expression in the transduced ES clones. In the lentivirus-transduced clones, the GFP expression level was largely proportional to the number of proviral vector copies, suggesting that the expression level from the lentivirus vector is relatively independent of the position where the provirus is integrated and is primarily dependent on the number of proviral copies. In contrast, the same correlation was not seen in the oncoretrovirus-transduced clones, suggesting that MESV oncoretrovirus transgene expression is more position dependent than expression from the PGK-GFP lentivirus transgene. It is not clear whether all lentivirus vectors of this type will express their transgenes relatively independently of the site of integration or whether this phenomenon is observed here due to the nature of the PGK promoter, indicating that the PGK promoter is relatively position independent within the context of an HIV-1-based lentivirus vector.
Transgene expression in ES cells during development in vitro.
The transduced ES cell clones were used to monitor vector expression during hematopoietic development in culture to examine whether gene expression persists as differentiation to hematopoietic lineages proceeds. Expression of the vector transgene was monitored in undifferentiated ES cells, in ES cell-derived day 6 EBs, and in hematopoietic colonies. Two days before the initiation of differentiation, ES cells, maintained in the complete medium mentioned above, were transferred to Iscove's modified Dulbecco's medium (IMDM) containing 15% FCS, 1 mM glutamine, 150 mM α-monothioglycerol, and 1,000 U of LIF. After 2 days, ES cells were plated at 900 cells/ml into differentiation medium containing IMDM supplemented with 15% FCS, 2 mM glutamine, 0.5 mM ascorbic acid (Sigma), and 450 mM α-monothioglycerol. The differentiation cultures were maintained in petri dishes for 6 days. Day 6 EBs were trypsinized into single-cell suspensions and plated at 5 × 104 cells per 3.5-cm-diameter plate in IMDM containing 1.0% methylcellulose, 10% plasma-derived serum (Animal Technologies, Tyler, Tex.), 2 mM glutamine, transferrin (300 μg/ml; Boehringer Mannheim, Mannheim, Germany), interleukin-3 (20 ng/ml; Immunex, Seattle, Wash.), c-Kit ligand (50 ng/ml; Amgen, Thousand Oaks, Calif.), erythropoietin (2 U/ml; Janssen-Cilag, Sollentuna, Sweden), and 5% protein-free hybridoma medium (GIBCO). The cells were cultured for 10 days.
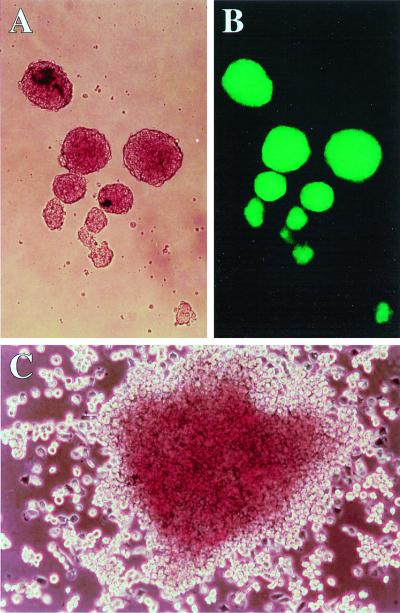
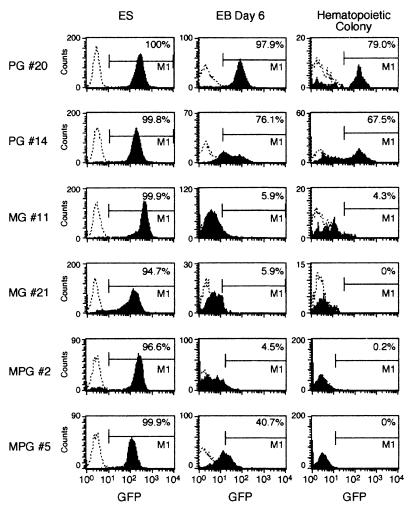
We compared the GFP expression levels in lentivirus (MOI of 59)- and oncoretrovirus (MOI of 590)-transduced colonies. Figure 3 shows a picture of EBs and an ES cell-derived hematopoietic colony. Table 2 shows the percentage of cells expressing the GFP transgene during differentiation in vitro. In all clones transduced with all three vectors, the transgene was expressed in 100% of the cells when they were in the undifferentiated state (Fig. 4 and Table 2). After differentiation to day 6 EBs, a large fraction of the cells in most of the lentivirus-transduced clones expressed the transgene, but a very low fraction of the MGirL22Y-transduced cells did so at this stage. However, the MSV-PGK-GFP vector-derived clones expressed the transgene in 4.5 to 40.7% of the cells in day 6 EBs. The GFP expression level of oncoretrovirus-transduced cells was severely reduced and almost extinct in day 6 EBs when the MESV LTR enhancer/promoter was used to drive expression of the transgene. The lentivirus and oncoretrovirus vectors that use the internal PGK promoter to drive the transgene allowed substantial expression following differentiation into day 6 EBs. The same trend was seen in hematopoietic colonies derived from the vector-transduced clones. Expression was further reduced with this additional differentiation step; however, a substantial fraction of the hematopoietic cells in the lentivirus-transduced clones but none in the oncoretrovirus-transduced clones demonstrated expression. All clones transduced with the MSV-PGK-GFP oncoretrovirus vector had one proviral copy (it was difficult to generate clones with more than one), but most lentivirus-transduced clones had many proviral copies with the transduction method used. Therefore, comparison between clones represent comparison between total expression levels, not expression per proviral copy number. Figure 4 shows representative FACS analysis of ES cell clones transduced with the three different vectors during in vitro development from undifferentiated cells to hematopoietic colonies. We could not find oncoretrovirus-transduced clones with either MGirL22Y or MSV-PGK-GFP that expressed the transgene to any extent in hematopoietic colonies. In contrast, approximately 50% of the lentivirus-transduced clones expressed the transgene well in hematopoietic colonies derived from ES cells. When we compared the levels of expression in cells derived from hematopoietic colonies, the mean fluorescence intensities in the three lentivirus- three oncoretrovirus-transduced clones were 48 (n = 6) and 5 (n = 4), respectively. These results indicate that the lentivirus transgene continued to be expressed, albeit at a reduced level, following hematopoietic differentiation, in contrast to the oncoretrovirus transgene, which showed a dramatic suppression of expression as has been described before (20), even in clones that have high expression levels at the undifferentiated stage.
FIG. 3.
ES-derived differentiated cells. (A and B) Day 6 EBs derived from the lentivirus-transduced clone PG20 (magnification, ×100). Panel B is visualized by a fluorescence microscope. (C) Day 10 hematopoietic colony derived from ES cells (×150).
TABLE 2.
Expression of the GFP gene during development of transduced ES cell clones in vitro
| Virus | Clone no. | Copy no. | GFP+ cellsa in:
|
|
|---|---|---|---|---|
| Day 6 EBs | Hematopoietic colonies | |||
| HIV-PGK-GFP | 20 | 12 | 97.9 | 79.0 |
| 14 | 12 | 76.1 | 67.5 | |
| 2 | 2 | 58.8 | 14.2 | |
| 11 | 10 | 93.2 | 31.9 | |
| 13 | 8 | 80.7 | 3.5 | |
| 15 | 7 | 91.2 | 17.4 | |
| MGirL22Y | 11 | 6 | 5.9 | 4.3 |
| 21 | 3 | 5.9 | 0 | |
| 32 | 3 | 2.4 | 0 | |
| 37 | 5 | 1.9 | 0 | |
| MSV-PGK-GFP | 2 | 1 | 4.5 | 0.2 |
| 5 | 1 | 40.7 | 0 | |
| 10 | 1 | 23.5 | 0 | |
| 12 | 1 | 14.0 | 0 | |
| 14 | 1 | 6.8 | 0 | |
Scored by FACS. In the undifferentiated state, 100% of the cells in each clone expressed the transgene.
FIG. 4.
GFP expression in lentivirus- and oncoretrovirus-transduced clones during hematopoietic differentiation. FACS analysis shows the number of GFP-positive cells in undifferentiated ES cells, in day 6 EBs, and in hematopoietic colonies from methylcellulose cultures. Cells from day 6 EBs and hematopoietic colonies were analyzed by gating away dead cells stained with propidium iodide. PG, MG, and MPG indicate clones transduced with HIV-PGK-GFP, MGirL22Y, and MSV-PGK-GFP, respectively.
Our initial hypothesis was that a lentivirus vector with an internal PGK promoter would be able to express the transgene throughout ES cell development to hematopoietic cells in vitro. The hypothesis turned out to be partially correct. Most of the ES clones were still expressing the transgene in day 6 EBs, and a substantial fraction of clones expressed the transgene in cells derived from hematopoietic colonies. We also examined whether an oncoretrovirus vector similar to MGirL22Y with an internal PGK promoter would also be able to express transgenes throughout development. This vector, MSV-PGK-GFP, expresses the transgene in a fraction of the cells derived from day 6 EBs but at a level 10- to 20-fold lower than that in undifferentiated ES cells. Expression was extinct in cells derived from hematopoietic colonies in all clones tested. Therefore, the internal PGK promoter within the context of an oncoretrovirus vector was still active in differentiated EBs but less so than in the lentivirus-transduced clones, and the percentage reduction from undifferentiated cells was also higher than in the lentivirus-transduced clones. These data are consistent with earlier results of studies using internal promoters in oncoretrovirus vectors to transduce embryos or embryonic carcinoma cells, both of which show some expression in the differentiated progeny cells (34, 29). Our data show that the internal PGK promoter within the HIV-1-based lentivirus vector undergoes less silencing than the same promoter within an oncoretrovirus vector.
Transcriptional control of the GFP transgene in ES cells.
To determine whether the lentivirus transgene in the ES clones is expressed from the internal PGK promoter, Northern blot analysis was performed. Total RNA was prepared from four ES clones, from 293T cells transiently transfected with the lentivirus vector construct, and from the oncoretrovirus (MGirL22Y) producing cells. As shown in Fig. 5A, the MGirL22Y-transduced clone contained messages of 2.5 and 3.2 kb, derived from the oncoretrovirus LTR. In contrast, a 1.4-kb mRNA was detected in all of the lentivirus-transduced clones, suggesting that the internal PGK promoter controls the transgene. No genomic lentivirus mRNA was detected in the ES clones, indicating that the lentivirus LTR is silent in ES cells. The lentivirus GFP is expressed during hematopoietic differentiation in PG clones 20 and 14 (Fig. 4), due to the activity of the lentivirus internal PGK promoter throughout differentiation as shown in Fig. 5A. There is no major reduction in RNA levels in PG clone 20 following differentiation, but the reduction is substantial in PG clone 14. Similarly, the internal PGK promoter is the main generator of GFP containing RNA in the MSV-PGK-GFP-transduced ES clones. Northern blot analysis shows that the main RNA species in the undifferentiated clones was generated by the internal promoter (Fig. 5B). Following differentiation to day 6 EBs, levels of the LTR-generated RNA species and the species derived from the internal promoter were both reduced. Therefore, the internal PGK promoter seems to function well within the context of an oncoretrovirus genome in undifferentiated ES cells, but the expression level is dramatically reduced in their differentiated progeny cells.
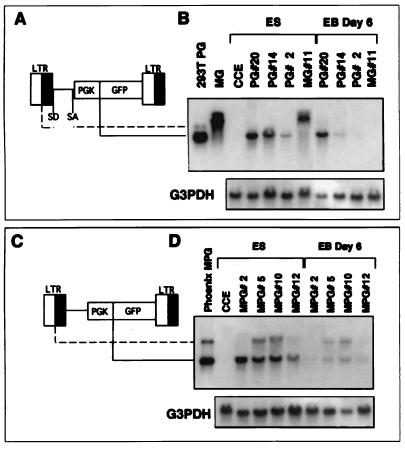
FIG. 5.
Northern blot analysis of GFP expression in ES clones transduced with lentivirus and oncoretrovirus vectors. Schematic drawings of the lentivirus vector, HIV-PGK-GFP (A), and the retrovirus vector, MSV-PGK-GFP (C), depict the species of RNA generated by the internal promoter (solid line, shorter transcript) and the viral LTR (broken line, longer transcript). The splice donor and acceptor sites (SD and SA) are indicated. (B and D) RNA was extracted from virus-transduced ES cell clones in undifferentiated (ES) and differentiated (EB Day 6) cells. Twenty micrograms of RNA was transferred onto a nylon membrane and hybridized with a radioactively labeled DNA fragment specific for GFP and glyceraldehyde 3-phosphate dehydrogenase (G3PDH). RNA from 293T cells producing HIV-PGK-GFP, the MGirL22Y producer cells, and ecotropic Phoenix cells transfected with the MSV-PGK-GFP vector are shown for comparison. The MG lane in panel B shows two bands of 3.2 kb (unspliced) and 2.5 kb (spliced). In the lentivirus vector clones, a 1.4-kb mRNA species is generated by the PGK promoter. PG, MG, and MPG indicate clones transduced with HIV-PGK-GFP, MGirL22Y, and MSV-PGK-GFP, respectively. CCE, untransduced ES cells.
In conclusion, we have shown that lentivirus vectors can be used to transduce ES cells efficiently. These clones can be characterized carefully with respect to copy number and expression level and then induced to differentiate to hematopoietic colonies. A sizable fraction of ES cell clones generate hematopoietic colonies which express the lentivirus transgene, in contrast to oncoretrovirus transgenes, which tend to be silenced during differentiation to hematopoietic colonies. This system can be used to determine a suitable lentivirus vector design for efficient expression in hematopoietic cells under well-defined conditions and also to determine biological effects of transgene overexpression (gain of function) during ES cell-derived hematopoiesis in vitro.
Acknowledgments
We thank A. W. Nienhuis and D. A. Persons for supplying the GP+E86/MGirL22Y vector producer cells and R. K. Humphries for supplying the MSV-PGK-GFP vector construct. We also thank Karin Olsson for expert technical assistance.
This work was supported by grants from the Swedish Cancer Society, Swedish Children's Cancer Foundation, Swedish Medical Research Council, Swedish Gene Therapy Program, and John and Augusta Persson Foundation to S.K. and from the Swiss National Foundation and Gabriella Giorgi-Cavaglieri Foundation to D.T. E.A. was supported by a postdoctoral position, and I.H. was supported by a postdoctoral grant from the Swedish Cancer Society. H.M. was supported by a postdoctoral grant from the Wennergren Foundation.
REFERENCES
- 1.Akkina R K, Walton R M, Chen M L, Li Q X, Planelles V, Chen I S. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular somatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barklis E, Mulligan R C, Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986;47:391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- 3.Bartz S R, Vodicka M A. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular somatitis virus glycoprotein. Methods. 1997;12:337–342. doi: 10.1006/meth.1997.0487. [DOI] [PubMed] [Google Scholar]
- 4.Becker K G, Jedlicka P, Templeton N S, Loitta L, Ozato K. Characterization of hUCRBP(YY1,NF-E1,δ): a transcription factor that binds to the regulatory regions of many viral and cellular genes. Gene. 1994;150:259–266. doi: 10.1016/0378-1119(94)90435-9. [DOI] [PubMed] [Google Scholar]
- 5.Blömer U, Naldini L, Kafri T, Trono D, Verma I M, Gage F H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Case S S, Price M A, Jordan C T, Yu X J, Wang L, Bauer G, Haas D L, Xu D, Stripecke R, Naldini L, Kohn D B, Crooks G M. Stable transduction of quiescent CD34+38− human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayton A I, Sodroski J G, Rosen C A, Goh W C, Haseltine W A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 9.Fisher A G, Feinberg M B, Josephs S F, Harper M E, Marselle L M, Reyes G, Gonda M A, Aldovini A, Debouk C, Gallo R C, Wong-Staal F. The trans-activator gene of HTLV-III is essential for virus replication. Nature. 1986;320:367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan J R, Becker K G, Ennist D L, Gleason S L, Driggers P H, Levi B-Z, Appella E, Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992;12:38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan J R, Krieg A M, Max E E, Kahn A S. Negative control region at the 5′ end of murine leukemia virus long terminal repeats. Mol Cell Biol. 1989;9:739–746. doi: 10.1128/mcb.9.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grez M, Akgün E, Hillberg F, Ostertag W. Embryonic stem cell virus, a recombinant murine retrovirus with expression in embryonic stem cells. Proc Natl Acad Sci USA. 1990;87:9202–9206. doi: 10.1073/pnas.87.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grez M, Zönig M, Nowock J, Ziegler M. A single point mutation activates the Moloney murine leukemia virus long terminal repeat in embryonal stem cells. J Virol. 1991;65:4691–4698. doi: 10.1128/jvi.65.9.4691-4698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley R G, Fong A Z C, Burns B F, Hawley T S. Transplantable myeloproliferative disease induced in mice by an interleukin 6 retrovirus. J Exp Med. 1992;176:1149–1163. doi: 10.1084/jem.176.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kafri T, Blömer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 16.Keller G M. In vitro differentiation of embryonic stem cells. Curr Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 17.Keller G, Kennedy M, Papayannopoulou T, Wiles M V. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiem H-P, Andrews R G, Morris J, Peterson L, Heyward S, Allen J M, Rasko J E J, Potter J, Miller A D. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 19.Kohn D B. Gene therapy for hematopoietic and immune disorders. Bone Marrow Transplant. 1996;18(Suppl. 3):S55–S58. [PubMed] [Google Scholar]
- 20.Laker C, Meyer J, Schopen A, Friel J, Heberlein C, Ostertag W, Stocking C. Host cis-mediated extinction of retrovirus permissive for expression in embryonal stem cells during differentiation. J Virol. 1998;72:339–348. doi: 10.1128/jvi.72.1.339-348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh T P, Sievert L L, Scott R W. Evidence for a stem cell-specific repressor of Moloney murine leukemia virus expression in embryonal carcinoma cells. Mol Cell Biol. 1990;10:4045–4057. doi: 10.1128/mcb.10.8.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyoshi H, Smith K A, Mosier D E, Verma I M, Torbett B E. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi H, Takahashi M, Gage F H, Verma I M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 25.Nienhuis A W, Bertran J, Hargrove P, Vanin E, Yang Y. Gene transfer into hematopoietic cells. Stem Cells. 1997;15(Suppl. 1):123–134. doi: 10.1002/stem.5530150816. [DOI] [PubMed] [Google Scholar]
- 26.Persons D A, Allay J A, Allay E R, Smeyne R J, Ashmun R A, Sorrentino B P, Nienhuis A W. Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates sorting and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood. 1997;90:1777–1786. [PubMed] [Google Scholar]
- 27.Prince V E, Rigby P W J. Derivatives of Moloney murine sarcoma virus capable of being transcribed in embryonal carcinoma stem cells have gained a functional Sp1 binding site. J Virol. 1991;65:1803–1811. doi: 10.1128/jvi.65.4.1803-1811.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roe T, Reynolds T C, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano P, Cone R D, Mulligan R C, Jaenisch R. Tissue-specific and ectopic expression of genes introduced into transgenic mice by retroviruses. Science. 1986;234:1409–1413. doi: 10.1126/science.3024318. [DOI] [PubMed] [Google Scholar]
- 30.Speck N A, Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Molony murine leukemia virus enhancer. Mol Cell Biol. 1987;7:1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton R E, Wu H T M, Rigg R, Böhnlein E, Brown P O. Human immunodeficiency virus type 1 vectors efficiently transduce human hematopoietic stem cells. J Virol. 1998;72:5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsukiyama T, Niwa O, Yokoro K. Mechanism of suppression of the long terminal repeat of Moloney leukemia virus in mouse embryonal carcinoma cells. Mol Cell Biol. 1989;9:4670–4676. doi: 10.1128/mcb.9.11.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida N, Sutton R E, Friera A M, He D, Reitsma M J, Chang W C, Veres G, Scollay R, Weissman I L. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95:11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner E F, Vanek M, Vennstrom B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985;4:663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiher H, Barklis E, Ostertag W, Jaenisch R. Two distinct sequence elements mediate retroviral gene expression in embryonal carcinoma cells. J Virol. 1987;61:2742–2746. doi: 10.1128/jvi.61.9.2742-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiles M V, Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- 37.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 38.Zufferey R, Donello J E, Trono D, Hope T J. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]