Abstract
Context
Traditional Chinese medicines (TCMs) have emerged as potential adjuvant therapies to treat non-small cell lung cancer. More direct comparative studies must be conducted among various oral TCMs.
Objective
This network meta-analysis evaluates the efficacy and safety of seven oral TCMs combined with chemotherapy in treating NSCLC.
Methods
The analysis included Zilongjin, Banmao, Hongdoushan, Huachansu, Kanglaite, Xihuang, and Pingxiao TCMs. Randomized-controlled trials (RCTs) were identified from the following databases: China National Infrastructure, Wanfang, PubMed, Embase, and the Cochrane Library up to April 2023. Two researchers independently extracted data.
Results
Sixty-eight RCTs (5,099 patients) were included. Compared to chemotherapy, Banmao capsules [odds ratio (OR) = 2.69, 95% confidence interval (CI) 1.96–3.69)] and Huachansu tablets [OR = 2.35, 95%CI (1.81, 3.05)] ranked in the top two in terms of increasing disease control rate. The two main TCMs to improve the objective response rate were Banmao capsules [OR = 3.49, 95%CI (2.17, 5.60)] and Zilongjin tablets [OR = 2.62, 95%CI (1.92, 3.57)]. Zilongjin tablets [OR = 3.47, 95%CI (2.14, 5.63)] and Huachansu tablets [OR = 3.30, 95%CI (1.65, 6.60)] were ranked as the top two in improving Karnofsky performance status. Hongdoushan capsules (SUCRA = 18.8%) and Banmao capsules (SUCRA = 19.8%) were the top two in reducing gastrointestinal toxicity. Zilongjin tablets (SUCRA = 18.9%) and Banmao capsules (SUCRA = 26.6%) were the top two to reduce liver and kidney toxicity. Hongdoushan capsules (SUCRA = 15.7%) and Huachansu tablets (SUCRA = 16.8%) ranked the top two in reducing thrombocytopenia. Banmao capsules (SUCRA = 14.3%) and Zilongjin tablets (SUCRA = 26.3%) were the top two decreasing leukopenia.
Conclusions
Combining oral TCMs with platinum-based chemotherapy has shown superior efficacy compared to platinum-based chemotherapy alone in treating NSCLC.
Keywords: Disease control rate, objective control rate, Karnofsky performance status, adverse reaction, efficacy
Introduction
Lung cancer, which accounts for 11.4% of all cancer diagnoses, is one of the most common cancers and the leading cause of cancer-related deaths at 18.0% (Mukherjee et al. 2020; Sung et al. 2021; Nie et al. 2021). Non-small cell lung cancer (NSCLC) represents up to 85% of lung cancer cases (Amatu et al. 2019; Duma et al. 2019). Many NSCLC patients are diagnosed at an advanced stage due to the subtle onset and rapid progression of the disease, often missing the opportunity for surgical intervention and facing a generally unfavorable prognosis. In China, the 5-year survival rate for lung cancer patients from 2012 to 2015 was approximately 19.7% (Zeng et al. 2018), compared to around 26.0% in the United States in 2022 (Miller et al. 2022). Standard treatment for NSCLC typically involves chemotherapy, which can lead to significant side effects such as gastrointestinal discomfort, blood toxicity, and neurotoxicity, adversely affecting the patients’ quality of life (Li et al. 2022). Therefore, it is urgent to explore and develop treatment strategies that improve efficacy, minimize adverse effects, and improve the overall well-being of NSCLC patients.
Traditional Chinese medicine (TCM) as adjuvant therapy in treating NSCLC has recently gained considerable attention. When combined with chemotherapy, TCM has shown promise in improving treatment efficacy and increasing the body’s immune response. Research suggests the potential of TCM to inhibit tumor recurrence and metastasis in NSCLC patients (Li et al. 2022). However, more direct comparative studies must be conducted among various oral TCMs. This study aims to systematically assess the efficacy and safety of seven commonly used oral TCMs in conjunction with chemotherapy drugs in treating NSCLC. The results will provide valuable information for clinicians in selecting appropriate oral TCMs for NSCLC patients undergoing chemotherapy.
Methods
Design
This systematic review adhered to the guidelines outlined in the Cochrane Handbook for Systematic Reviews (Cumpston et al. 2019) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al. 2021). The protocol for this review was registered with the Open Science Framework (OSF, registration DOI 10.37766/inplasy2022.5.0129).
Search strategy
Randomized-controlled trials (RCTs) were identified through searches in the China National Knowledge Infrastructure Database (CNKI), the WanFang Database, PubMed, Embase, and the Cochrane Library. The most recent search was conducted in April 2023. The retrieval strategy involved using the following subject terms, which included a combination of Chinese and English search terms: ‘non-small cell lung cancer,’ ‘NSCLC,’ ‘lung cancer,’ ‘kanglaite,’ ‘zijinlong,’ ‘hongdoushan,’ ‘banmao,’ ‘xihuang,’ ‘huachansu,’ and ‘pingxiao.’ The searches were limited to articles published in English and Chinese.
Inclusion and exclusion criteria
The study included patients aged 18 years and older diagnosed with NSCLC and a Karnofsky Performance Status (KPS) ≥ 60. Patients who did not receive one of the seven oral TCMs were excluded. Reviews, meta-analyses, commentaries, case series, editorials, and letters were excluded. Studies for which the full text was not available were also excluded.
Interventions and control
The RCTs used the following design: the experimental group received a combination of Zilongjin tablets, Banmao capsules, Hongdoushan capsules, Huachansu tablets or capsules, Kanglaite capsules, Xihuang pills or capsules, or Pingxiao capsules, in addition to platinum-based chemotherapy for NSCLC. The control group received only platinum-based chemotherapy.
Outcomes
The following outcomes were assessed: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), objective response rate (ORR), disease control rate (DCR), KPS, gastrointestinal adverse effects, hepatotoxicity, nephrotoxicity, decreased red blood cell count (RBC), decreased platelet count (PLT), and decreased white blood cell count (WBC).
Data extraction
Two authors independently selected eligible studies based on prespecified selection criteria. The discrepancies were resolved by discussion with a third author. The data extracted included the first author’s name, publication year, participant characteristics (sex, age, tumor classification, tumor grading, and sample size), intervention characteristics (medication dose, medication time and frequency, and treatment course), outcomes, and adverse drug reactions.
Quality assessment
The risk of bias (ROB) in RCTs was assessed using the Cochrane Collaboration tool. This tool evaluates ROB in seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias (Schakel et al. 2019). The ROB plot was created using Review Manager (RevMan, version 5.4, Cochrane, London, UK).
Statistical analyses
All statistical analyses were performed with Stata (version 15.1, Stata Corp LP, College Station, TX, USA). The weighted mean difference (WMD) was calculated for continuous variables to summarize their effects, while odds ratios (OR) were applied to dichotomous variables. Initially, a pairwise meta-analysis was performed using the DerSimonian and Laird method. This was followed by a network meta-analysis (NMA) conducted using the Stata mvmeta package (Chaimani et al. 2013). The REML Wald test was used to assess the presence of inconsistency (Veroniki et al. 2013). Treatment rankings were inferred using the surface under the cumulative ranking curve (SUCRA) method. Funnel plots were generated to assess the potential impact of small sample sizes. The results are reported with 95% confidence intervals (CIs), and statistical significance was established at p < 0.05.
Results
Study selection and characteristics
In the preliminary analysis, the search strategy identified 2,663 studies. After removing duplicates, 2,001 studies were screened based on titles and abstracts. During this phase, 1,895 irrelevant citations were excluded, resulting in 106 studies being selected for full-text review. Following the exclusion of reviews, meta-analyses, and studies that did not report outcome data, 68 studies were included (Figure 1).
Figure 1.
Flow chart of the literature review.
The 68 RCTs (Zhang et al. 2000; Li 2011; Wang 2019; Zhang 2015; Cao et al. 2016; Li et al. 2017; Ren et al. 2018; Wang and Cheng 2009; Guo et al. 2008; Du and Min 2018; Liu et al. 2018; Zhang and Niu 2019; Chen 2020; He et al. 2020; Long et al. 2013; Liang et al. 2016; Yan and Ye 2020; Wang 2018; Chi et al. 2021; Song et al. 2019; Fang 2022; Cai 2019; Duan 2016; Wu 2019; Wang and Wang 1999; Liu et al. 2000; Yang and Yi 2002; Shao et al. 2002; Tan and Li 2005; Zhao et al. 2007; Wan et al. 2007; Geng et al. 2009; Ming et al. 2010; Lan et al. 2011; Zhao et al. 2011; Wang and Li 2015; Wei and Xu 2017; Huang 2019; Wu et al. 2020; Li 2019; Chen et al. 2016; Pu et al. 2017; Guan and Li 2021; Miu et al. 2014; Li 2020; Shi 2017; Yu et al. 2018; Chen et al. 2018; Zhou et al. 2016; Liu 2014; Li et al. 2015; Li et al. 2015; Yi and Chen 2014; Chen et al. 2001; Guo et al. 2002; Wu and Zhang 2006; Shu et al. 2002; Yu and Jiang 2018; Zhang and Wei 2012; Yang et al. 2017; Shen et al. 2017; Li and Ma 2015; Chen et al. 2011; Shang 2016; Ma et al. 2017; Sun et al. 2015; Wang and Yan 2008; Wang 2009;) were published between 1999 and 2021. These studies had 5,099 patients, 2,588 in the experimental group and 2,511 in the control group. All participants were Chinese. Details of the included studies are presented in Table 1.
Table 1.
Characteristics of the included studies.
| Study | Total sample | Classification | Grading | Experimental group |
|||
|---|---|---|---|---|---|---|---|
| Gender (M/F) | Age (year) | Sample | Therapeutic schedule | ||||
| Zhang XP(2000) | 60 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 17/13 | 31 ∼ 72 | 30 | Kanglaite 2.7g,QID,two 21day-cycles |
| Li(2011) | 70 | Adenocarcinoma, squamous cell carcinoma | III-IV | 22/13 | 52.5 ± 9.8 | 35 | Kanglaite 1.8g,TID,two 21day-cycles |
| Wang(2019) | 78 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 25/14 | 62.7 ± 7.3 | 39 | Kanglaite 2.7g,TID,two 21day-cycles |
| Zhang(2015) | 60 | — | II-IV | 18/12 | 27 ∼ 67 | 30 | Hongdoushan 0.8g,TID,two 21day-cycles |
| Cao J(2016) | 58 | — | — | 15/14 | 54.1 ± 3.1 | 29 | Hongdoushan 1.2g,TID,two 21day-cycles |
| Li J(2017) | 84 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 25/17 | 67.3 ± 7.4 | 42 | Hongdoushan 0.8g,TID,Four 21day-cycles |
| Ren F(2018) | 76 | Adenocarcinoma, squamous cell carcinoma | III-IV | 26/12 | 53.6 ± 4.8 | 38 | Hongdoushan 0.8g,TID,two 21day-cycles |
| Wang X(2009) | 28 | Adenocarcinoma, squamous cell carcinoma | IV | 11/5 | 36 ∼ 72 | 16 | Xihuang 3.0g,TID,three 21day-cycles |
| Guo H(2009) | 60 | Adenocarcinoma, squamous cell carcinoma | III-IV | — | 42 ∼ 73 | 30 | Xihuang 3.0g,TID,two 28day-cycles |
| Du FH(2018) | 62 | Adenocarcinoma | IIIb-IV | 17/15 | — | 32 | Xihuang 1.0g,TID,four 28day-cycles |
| Liu TF(2018) | 50 | — | III-IV | 16/9 | 65.2 ± 7.3 | 25 | Xihuang 2.0g,TID,two 21day-cycles |
| Zhang JX(2019) | 60 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 24 ∼ 72 | 30 | Xihuang 2.0g,TID,five 28day-cycles | |
| Chen(2020) | 58 | Adenocarcinoma | IV | 18/11 | 71.3 ± 5.1 | 29 | Xihuang 1.0g,TID,six 21day-cycles |
| He SL(2020) | 80 | Squamous cell carcinoma | III-IV | 23/17 | 59.5 ± 8.2 | 40 | Xihuang 3.0g,TID,three 21day-cycles |
| Long Q(2013) | 84 | — | — | 38 ∼ 72 | 40 | Banmao 0.75g,BID,four 28day-cycles | |
| Liang YH(2016) | 100 | IV | 28/22 | 76.0 ± 4.4 | 50 | Banmao 0.75g,BID,two 21day-cycles | |
| Yan MY(2020) | 60 | Adenocarcinoma, squamous cell carcinoma | III | 17/13 | 63.8 ± 9.9 | 30 | Banmao 0.75g,BID,two 28day-cycles |
| Wang(2018) | 90 | Adenocarcinoma, squamous cell carcinoma | III-IV | 32/13 | 56.9 ± 13.6 | 45 | Banmao 0.75g,BID,two 21day-cycles |
| Chi X(2021) | 82 | Adenocarcinoma, squamous cell carcinoma | III-IV | 29/12 | 62.0 ± 4.9 | 41 | Banmao 0.75g,BID,six 21day-cycles |
| Song Z(2019) | 80 | Adenocarcinoma, squamous cell carcinoma | I-IV | 22/22 | 62.5 ± 11.4 | 44 | Banmao 0.75g,BID,six 21day-cycles |
| Fang(2022) | 86 | Adenocarcinoma, squamous cell carcinoma | — | 25/18 | 60.6 ± 8.8 | 43 | Banmao 0.75g,BID,three 28day-cycles |
| Cai(2019) | 100 | — | IV | 25/25 | 57.3 ± 2.6 | 50 | Banmao 0.75g,BID,four 21day-cycles |
| Duan(2016) | 80 | Adenocarcinoma, squamous cell carcinoma | III-IV | 30/10 | 65.6 ± 4.5 | 40 | Banmao 0.75g,BID,four 28day-cycles |
| Wu(2019) | 80 | Adenocarcinoma, squamous cell carcinoma | — | 23/17 | 45 ∼ 78 | 40 | Banmao 0.75g,BID,three 21day-cycles |
| Wang GL(1999) | 68 | Adenocarcinoma, squamous cell carcinoma | III-IV | — | — | 34 | Pingxiao 1.68g,TID,three 28day-cycles |
| Liu ZH(2000) | 67 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 25/9 | 38-72 | 34 | Pingxiao 2.24g,TID,three 28day-cycles |
| Yang HP(2002) | 89 | Adenocarcinoma, squamous cell carcinoma | II-IV | 32/14 | — | 46 | Pingxiao 1.40g,TID,three 14day-cycles |
| Shao JH(2002) | 48 | Adenocarcinoma, squamous cell carcinoma, large cell | IIIb-IV | 19/6 | 32-73 | 25 | Pingxiao 1.40g,TID,two 28day-cycles |
| Tan XY(2005) | 63 | Adenocarcinoma, squamous cell carcinoma, large cell | IIIb-IV | — | — | 31 | Pingxiao 1.68g,TID,two 28day-cycles |
| Zhao YJ(2007) | 73 | Adenocarcinoma, squamous cell carcinoma, large cell | III-IV | — | — | 37 | Pingxiao 2.24g,TID,two 28day-cycles |
| Wang LX(2007) | 118 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | — | — | 63 | Pingxiao 1.40g,TID,two 21day-cycles |
| Geng CX(2009) | 86 | Adenocarcinoma, squamous cell carcinoma, large cell | IIIb-IV | 26/20 | 41-71 | 46 | Pingxiao 2.24g,TID,two 21day-cycles |
| Ming J(2010) | 80 | Adenocarcinoma, squamous cell carcinoma, large cell | III-IV | 28/12 | 33-76 | 40 | Pingxiao 2.24g,TID,two 21day-cycles |
| Lan SL(2011) | 84 | Adenocarcinoma, squamous cell carcinoma | I-IV | 29/13 | 48-72 | 42 | Pingxiao 2.24g,TID,two 21day-cycles |
| Zhao J(2011) | 61 | — | III-IV | — | — | 31 | Pingxiao 1.68g,TID,two 21day-cycles |
| Wang SM(2015) | 88 | Adenocarcinoma, squamous cell carcinoma | IV | 21/23 | 54.7 ± 12.6 | 44 | Pingxiao 1.40g,TID,two 21day-cycles |
| Wei GH(2017) | 68 | Adenocarcinoma, squamous cell carcinoma | III-IV | 19/15 | 57.4 ± 4.3 | 34 | Huachansu 0.5g,TID,two 21day-cycles |
| Huang(2019) | 86 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 24/19 | 61.5 ± 7.3 | 43 | Huachansu 0.5g,TID,two 21day-cycles |
| Wu LM(2020) | 74 | Adenocarcinoma, squamous cell carcinoma | IIIa-IIIb | 20/17 | 74.3 ± 7.9 | 37 | Huachansu 0.5g,BID,four 21day-cycles |
| Li(2019) | 75 | — | III-IV | 28/10 | 58.9 ± 4.6 | 38 | Huachansu 0.5g,TID,two 21day-cycles |
| Chen JY(2016) | 80 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 22/18 | 59.3 ± 7.9 | 40 | Huachansu 0.5g,BID,three 21day-cycles |
| Pu JZ(2017) | 80 | Adenocarcinoma, squamous cell carcinoma | III-IV | 22/20 | 45.6 ± 9.4 | 42 | Huachansu 0.5g,BID,two 28day-cycles |
| Guan W(2021) | 71 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 22/14 | 55.3 ± 13.2 | 36 | Huachansu 0.5g,TID,six 21day-cycles |
| Miu XD(2014) | 60 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 16/14 | 58.0 ± 7.0 | 30 | Huachansu 0.5g,TID,four 21day-cycles |
| Li(2020) | 30 | Adenocarcinoma, squamous cell carcinoma | III-IV | 10/5 | 55.6 ± 6.3 | 15 | Huachansu 0.5g,BID,two 21day-cycles |
| Shi(2017) | 102 | — | III-IV | — | 48 ∼ 84 | 51 | Huachansu 0.5g,TID,two 21day-cycles |
| Yu TT(2018) | 63 | Adenocarcinoma, squamous cell carcinoma | III-IV | 23/10 | — | 33 | Huachansu 0.5g,TID,two 21day-cycles |
| Chen Q(2018) | 63 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 20/11 | 55.8 ± 6.6 | 31 | Huachansu 0.5g,TID,two 21day-cycles |
| Zhou XY(2016) | 100 | Adenocarcinoma, squamous cell carcinoma | III-IV | 45/5 | 52.0 ± 4.0 | 50 | Huachansu 0.5g,TID,two 21day-cycles |
| Liu(2014) | 85 | Adenocarcinoma, squamous cell carcinoma | III-IV | 26/29 | 33 ∼ 72 | 45 | Huachansu 0.5g,BID,two 21day-cycles |
| Li WG(2015) | 126 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 45/18 | 58.7 ± 9.5 | 63 | Huachansu 0.5g,BID,five 21day-cycles |
| Yi HH(2014) | 120 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 36/24 | 52.14 ± 2.18 | 60 | Zijinlong 2.6g,TID,two 21day-cycles |
| Chen PF(2001) | 45 | Adenocarcinoma, squamous cell carcinoma | II-IV | 22/8 | 58.06 | 30 | Zijinlong 2.6g,TID,two 28day-cycles |
| Guo YH(2002) | 45 | Adenocarcinoma, squamous cell carcinoma | II-IV | 25/5 | 59 ∼ 68 | 30 | Zijinlong 2.6g,TID,two 21day-cycles |
| Wu HB(2006) | 60 | – | – | 22/8 | 63.8 | 30 | Zijinlong 2.6g,TID,two 21day-cycles |
| Shu JH(2002) | 61 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 24/7 | 46 ∼ 75 | 31 | Zijinlong 2.6g,TID,two 21day-cycles |
| Yu FM(2018) | 80 | Adenocarcinoma, squamous cell carcinoma | III-IV | 23/17 | 61.8 ± 7.7 | 40 | Zijinlong 2.6g,TID,two 21day-cycles |
| Zhang XF(2012) | 85 | – | IIIb-IV | – | – | 41 | Zijinlong 2.6g,TID,two 21day-cycles |
| Yang XC(2017) | 49 | Adenocarcinoma, squamous cell carcinoma | II-III | 14/11 | 48.65 ± 16. 12 | 25 | Zijinlong 2.6g,TID,two 21day-cycles |
| Shen T(2017) | 86 | Adenocarcinoma, squamous cell carcinoma | III | 22/21 | 57.2 ± 7.3 | 43 | Zijinlong 2.6g,TID,four 21day-cycles |
| Li GS(2015) | 78 | Adenocarcinoma, squamous cell carcinoma,large cell | III-IV | 18/21 | 63 ± 8 | 39 | Zijinlong 2.6g,TID,two 21day-cycles |
| Chen CR(2011) | 48 | Adenocarcinoma, squamous cell carcinoma | III-IV | 14/11 | 35 ∼ 74 | 25 | Zijinlong 2.6g,TID,two 21day-cycles |
| Shang RG(2016) | 60 | – | – | 18/12 | 66.8 ± 1.2 | 30 | Zijinlong 2.6g,TID,four 21day-cycles |
| Ma HW(2017) | 78 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | — | — | 39 | Zijinlong 2.6g,TID,two 21day-cycles |
| Sun CP(2015) | 128 | Adenocarcinoma, squamous cell carcinoma | IIIb-IV | 43/21 | 56.5 ± 5.6 | 64 | Zijinlong 2.6g,TID,two 21day-cycles |
| Wang J(2008) | 63 | Adenocarcinoma, squamous cell carcinoma | II-IV | 18/14 | 40 ∼ 80 | 32 | Zijinlong 2.6g,TID,two 28day-cycles |
| Wang(2009) | 120 | Adenocarcinoma, squamous cell carcinoma | III-IV | 40/20 | 60 ∼ 75 | 60 | Zijinlong 2.6g,TID,two 28day-cycles |
| Li HF(2015) | 80 | Adenocarcinoma, squamous cell carcinoma | II-IV | — | 39 ∼ 75 | 40 | Zijinlong 2.6g,TID,two 21day-cycles |
| Control group |
Outcomes | |||
|---|---|---|---|---|
| Gender (M/F) | Age | Sample | therapeutic schedule | |
| 19/11 | 33 ∼ 71 | 30 | MVP: Mitomycin 6mg/m2, Vindesine 3mg/m2, Cisplatin 30mg/m2 | ①②③④⑤⑥ |
| 23/12 | 51.9 ± 10.6 | 35 | TP: Taxol 135∼175mg/m2, Carboplatin 300mg/m2 | ①②③④⑤⑥ |
| 24/15 | 62.5 ± 7.1 | 39 | GP: Gemcitabine 1000 mg/m2, Cisplatin 30mg/m2 | ①②③④⑤⑥ |
| 19/11 | 27 ∼ 67 | 30 | TP: Taxol 135∼175mg/m2, Carboplatin 300mg/m2 | ①②③④⑤⑥ |
| 16/13 | 55.2 ± 3.9 | 29 | DP: Docetaxel 60mg/m2, Cisplatin 30mg/m2 | ①②③④⑤⑥ |
| 24/18 | 67.8 ± 7.6 | 42 | GP: Gemcitabine 500mg/m2, Cisplatin 15mg/m2 | ①②③④⑥ |
| 27/11 | 54.1 ± 4.6 | 38 | GP: Gemcitabine 1000mg/m2, Cisplatin 75mg/m2 | ①②③④⑤⑥ |
| 7/5 | 42 ∼ 77 | 12 | GP: Gemcitabine 1000mg/m2, Cisplatin 80mg/m2 | ①②③④⑤ |
| — | 42 ∼ 73 | 30 | NP: Vinorelbine 25mg/m2, Cisplatin 80mg/m2 | ①②③④⑤⑥ |
| 14/16 | — | 30 | PP: Pemetrexed 500mg/m2, Cisplatin 75mg/m2 | ①②③④⑥ |
| 15/10 | 66.8 ± 7.9 | 25 | GP: Gemcitabine 1250mg/m2, Cisplatin 75mg/m2 | ①②③④⑥ |
| 24 ∼ 72 | 30 | DP: Docetaxel 75mg/m2, Cisplatin 25mg/m2 | ①②③④⑤⑥ | |
| 19/10 | 70.4 ± 5.1 | 29 | PP: Pemetrexed 500mg/m2, Cisplatin 75mg/m2 | ①②③④⑥ |
| 25/15 | 60.5 ± 7.7 | 40 | NP: Vinorelbine 25mg/m2, Cisplatin 80mg/m2 | ①②③④⑤ |
| 38 ∼ 72 | 44 | GP: Gemcitabine 1000 mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ | |
| 32/18 | 74.0 ± 5.6 | 50 | DO: Docetaxel 75mg/m2, Oxaliplatin 130mg/m2 | ①②③④⑤⑥ |
| 19/11 | 64.0 ± 8.3 | 30 | TP: Taxol 135∼175mg/m2, Cisplatin 30mg/m2 | ①②③④ |
| 31/14 | 55.7 ± 11.5 | 45 | GP: Gemcitabine 1000mg/m2, Cisplatin 80mg/m2 | ①②③④⑥ |
| 28/13 | 61.8 ± 4.4 | 41 | GP: Gemcitabine 1000mg/m2, Cisplatin 25mg/m2 | ①②③④⑥ |
| 20/16 | 62.6 ± 11.5 | 36 | OX: Oxaliplatin 130mg/m2 | ①②③④⑤⑥ |
| 28/15 | 61.2 ± 7.4 | 43 | GP: Gemcitabine 1000mg/m2, Cisplatin 75mg/m2 | ①②③④⑥ |
| 27/23 | 55.8 ± 1.9 | 50 | TP: Taxol 150mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ |
| 31/9 | 64.5 ± 3.8 | 40 | GP: Gemcitabine 1250mg/m2, Cisplatin 75mg/m2 | ①②③④⑤⑥ |
| 21/19 | 45 ∼ 75 | 40 | GP: Gemcitabine 1250mg/m2, Cisplatin 75mg/m2 | ①②③④⑥ |
| — | — | 34 | MVP: Mitomycin 6mg/m2, Vindesine 3mg/m2, Cisplatin 40mg/m2 | ①②③④⑤⑥ |
| 25/8 | 37-76 | 33 | MVP: Mitomycin 8mg/m2, Vindesine 4mg/m2, Cisplatin 40mg/m2 | ①②③④⑥ |
| 30/13 | — | 43 | ICE: Ifosfamide 1200mg/m2, Mesuna 1680mg/m2, Carboplatin 300mg/m2 | ①②③④⑥ |
| 18/5 | 29-71 | 23 | DP: Docetaxel 75mg/m2, Cisplatin 80mg/m2 | ①②③④⑥ |
| — | — | 32 | GP: Gemcitabine 1250mg/m2, Cisplatin 70mg/m2 | ①②③④⑤⑥ |
| — | — | 36 | NP: Vinorelbine 25mg/m2, Cisplatin 80mg/m2 | ①②③④⑤⑥ |
| — | — | 55 | TP: Taxol 150mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ |
| 24/16 | 42-72 | 40 | NP: Vinorelbine 25mg/m2, Cisplatin 80mg/m2 | ①②③④⑤⑥ |
| 26/14 | 32-77 | 40 | GP: Gemcitabine 1000 mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ |
| 32/10 | 46-71 | 42 | GP: Gemcitabine 1000 mg/m2, Cisplatin 80mg/m2 | ①②③④⑥ |
| — | — | 30 | NP: Vinorelbine 25mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ |
| 20/24 | 49.0 ± 13.9 | 44 | PC: Pemetrexed 500 mg/m2, Cisplatin 25mg/m2 | ①②③④⑤⑥ |
| 17/17 | 58.3 ± 2.6 | 34 | DP: Docetaxel 75mg/m2, Cisplatin 25mg/m2 | ①②③④⑤⑥ |
| 23/20 | 61.2 ± 7.1 | 43 | GP: Gemcitabine 1250mg/m2, Cisplatin 25mg/m2 | ①②③④⑥ |
| 21/16 | 71.2 ± 8.5 | 37 | EP: Etoposide 80mg/m2, Cisplatin 80mg/m2 | ①②③④ |
| 26/11 | 60.1 ± 4.8 | 37 | DP: Docetaxel 37.5mg/m2, Cisplatin 25mg/m2 | ①②③④⑥ |
| 23/17 | 59.5 ± 7.5 | 40 | GP: Gemcitabine 1000 mg/m2, Cisplatin 20mg/m2 | ①②③④⑥ |
| 24/14 | 57.8 ± 12.4 | 38 | NP: Vinorelbine 25mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ |
| 20/15 | 55.2 ± 13.2 | 35 | TP: Taxol 135mg/m2, Cisplatin 25mg/m2 | ①②③④⑥ |
| 15/15 | 57.0 ± 6.5 | 30 | TP: Taxol 135mg/m2, Cisplatin 75mg/m2 | ①②③④⑥ |
| 9/6 | 54.6 ± 5.6 | 15 | PC: Pemetrexed 500 mg/m2, Cisplatin 25mg/m2 | ①②③④ |
| — | 48 ∼ 84 | 51 | TP: Taxol 135mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ |
| 18/12 | — | 30 | GP: Gemcitabine 1250mg/m2, Cisplatin 75mg/m2 | ①②③④⑥ |
| 18/13 | 56.3 ± 6.5 | 31 | GP: Gemcitabine 1000mg/m2, Cisplatin 25mg/m2 | ①②③④⑥ |
| 46/4 | 51.0 ± 3.0 | 50 | NP: Vinorelbine, Cisplatin | ①②③④ |
| 23/17 | 33 ∼ 72 | 40 | NP: Vinorelbine 25mg/m2, Cisplatin 20mg/m2 | ①②③④⑥ |
| 35/28 | 57.9 ± 9.8 | 63 | DP: Docetaxel 75mg/m2, Cisplatin 75mg/m2 | ①②③④⑤⑥ |
| 34/26 | 52.33 ± 2.27 | 60 | DP: Docetaxel 75mg/m2, Cisplatin 60mg/m2 | ①②③④⑤⑥ |
| 9/6 | 58.6 | 15 | MVP: Mitomycin 6 ∼ 8mg/m2, Vindesine 4mg/m2, Cisplatin 70 ∼ 80mg/m2 | ①②③④⑤⑥ |
| 14/1 | 56 ∼ 70 | 15 | MVP: Mitomycin 6 ∼ 8mg/m2, Vindesine 4mg/m2, Cisplatin 70 ∼ 80mg/m2 | ①②③④⑤⑥ |
| 23/7 | 62 | 30 | MVP: Mitomycin 6 ∼ 8mg/m2, Vindesine 4mg/m2, Cisplatin 70 ∼ 80mg/m2 | ⑤⑥ |
| 21/9 | 41 ∼ 72 | 30 | MVP: Mitomycin 8mg/m2, Vindesine 4mg/m2, Cisplatin 60mg/m2 | ①②③④⑤⑥ |
| 21/19 | 60.4 ± 7.9 | 40 | GP: Gemcitabine 1000mg/m2, Cisplatin 60mg/m2 | ①②③④⑤⑥ |
| – | – | 44 | GP: Gemcitabine 1000mg/m2, Cisplatin 75mg/m2 | ①②③④⑤⑥ |
| 15/9 | 48.30 ± 16.24 | 24 | TP: Taxol 135mg/m2, Cisplatin 75mg/m2 | ⑤⑥ |
| 23/20 | 56.8 ± 7.5 | 43 | TP: Taxol 135mg/m2, Cisplatin 30mg/m2 | ①②③④⑥ |
| 19/20 | 60 ± 5 | 39 | DP: Docetaxel 75mg/m2, Cisplatin 60mg/m2 | ①②③④⑥ |
| 10/13 | 37 ∼ 70 | 23 | DP: Docetaxel 75mg/m2, Cisplatin 20mg/m2 | ①②③④⑤⑥ |
| 17/13 | 65.8 ± 1.8 | 30 | DP: Docetaxel 75mg/m2, Cisplatin 25mg/m2 | ①②③④⑥ |
| — | — | 39 | GP: Gemcitabine 1000mg/m2, Cisplatin 25mg/m2 | ①②③④⑤⑥ |
| 42/22 | 55.3 ± 4.9 | 64 | TP: Taxol, Cisplatin | ①②③④⑤ |
| 19/12 | 37 ∼ 76 | 31 | NP: Vinorelbine 25mg/m2, Cisplatin 25mg/m2 | ①②③④⑤⑥ |
| 39/21 | 61 ∼ 78 | 60 | NP: Vinorelbine 25mg/m2, Cisplatin 25mg/m2 | ⑤⑥ |
| — | 39 ∼ 75 | 40 | GP: Gemcitabine 1000mg/m2, Cisplatin 75mg/m2 | ⑤⑥ |
Note: ‘-’ Not report; ①CR; ②PR; ③SD; ④PD; ⑤KPS; ⑥Adverse effects. MVP: Mitomycin + Vindesine + Cisplatin; TP: Taxol + Cisplatin/Carboplatin/Nedaplatin; GP: Gemcitabine + Cisplatin/Carboplatin; DCF: Docetaxel + Cisplatin + Fluorouracil; NP: Vinorelbine + Cisplatin; DP: Docetaxel + Cisplatin/Nedaplatin; PP: Pemetrexed + Cisplatin; PC: Pemetrexed + Cisplatin; DO: Docetaxel + Oxaliplatin; ICE: Ifosfamide + Mesuna + Cisplatin; EP: etoposide + cisplatin; OX: Oxaliplatin.
Quality assessment
Among the 68 RCTs analyzed, 26 reported their randomization methods. However, 38 studies had unclear or inadequately reported methods of random sequence generation. Three studies categorized participants based on therapeutic schedules, while one group grouped them according to admission order. In particular, none of the studies reported allocation concealment or double-blinding. Despite this, all studies were assessed to have a low risk of incomplete outcome data and selective reporting. Fifteen studies were evaluated as having a low risk of other biases, but the remaining studies did not provide sufficient information. A detailed quality assessment can be found in Table 2.
Table 2.
The result of quality assessment.
| Study | Random sequence generation | Allocation concealment | Blinding of participants and researchers | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Zhang XP(2000) | U | U | U | U | L | L | U |
| Li(2011) | U | U | U | U | L | L | U |
| Wang(2019) | L | U | U | U | L | L | U |
| Zhang(2015) | U | U | U | U | L | L | U |
| Cao J(2016) | U | U | U | U | L | L | U |
| Li J(2017) | L | U | U | U | L | L | U |
| Ren F(2018) | L | U | U | U | L | L | L |
| Wang X(2009) | U | U | U | U | L | L | U |
| Guo H(2009) | U | U | U | U | L | L | U |
| Du FH(2018) | L | U | U | U | L | L | L |
| Liu TF(2018) | U | U | U | U | L | L | U |
| Zhang JX(2019) | L | U | U | U | L | L | L |
| Chen(2020) | U | U | U | U | L | L | U |
| He SL(2020) | L | U | U | U | L | L | L |
| Long Q(2013) | U | U | U | U | L | L | U |
| Liang YH(2016) | H | U | U | U | L | L | U |
| Yan MY(2020) | L | U | U | U | L | L | L |
| Wang(2018) | U | U | U | U | L | L | U |
| Chi X(2021) | L | U | U | U | L | L | U |
| Song Z(2019) | U | U | U | U | L | L | U |
| Fang(2022) | L | U | U | U | L | L | U |
| Cai(2019) | L | U | U | U | L | L | L |
| Duan(2016) | L | U | U | U | L | L | L |
| Wu(2019) | U | U | U | U | L | L | U |
| Wang GL(1999) | U | U | U | U | L | L | U |
| Liu ZH(2000) | U | U | U | U | L | L | U |
| Yang HP(2002) | U | U | U | U | L | L | U |
| Shao JH(2002) | U | U | U | U | L | L | U |
| Tan XY(2005) | U | U | U | U | L | L | U |
| Zhao YJ(2007) | U | U | U | U | L | L | U |
| Wang LX(2007) | U | U | U | U | L | L | U |
| Geng CX(2009) | U | U | U | U | L | L | U |
| Ming J(2010) | H | U | U | U | L | L | U |
| Lan SL(2011) | U | U | U | U | L | L | U |
| Zhao J(2011) | U | U | U | U | L | L | U |
| Wang SM(2015) | U | U | U | U | L | L | U |
| Wei GH(2017) | L | U | U | U | L | L | L |
| Huang(2019) | L | U | U | U | L | L | L |
| Wu LM(2020) | L | U | U | U | L | L | L |
| Li(2019) | L | U | U | U | L | L | U |
| Chen JY(2016) | H | U | U | U | L | L | U |
| Pu JZ(2017) | L | U | U | U | L | L | U |
| Guan W(2021) | U | U | U | U | L | L | U |
| Miu XD(2014) | L | U | U | U | L | L | U |
| Li(2020) | L | U | U | U | L | L | U |
| Shi(2017) | U | U | U | U | L | L | U |
| Yu TT(2018) | U | U | U | U | L | L | U |
| Chen Q(2018) | L | U | U | U | L | L | U |
| Zhou XY(2016) | L | U | U | U | L | L | U |
| Liu(2014) | L | U | U | U | L | L | U |
| Liu BD(2015) | L | U | U | U | L | L | L |
| Yi HH(2014) | U | U | U | U | L | L | U |
| Chen PF(2001) | U | U | U | U | L | L | U |
| Guo YH(2002) | U | U | U | U | L | L | U |
| Wu HB(2006) | U | U | U | U | L | L | U |
| Shu JH(2002) | U | U | U | U | L | L | U |
| Yu FM(2018) | U | U | U | U | L | L | U |
| Zhang XF(2012) | H | U | U | U | L | L | U |
| Yang XC(2017) | U | U | U | U | L | L | U |
| Shen T(2017) | U | U | U | U | L | L | U |
| Li GS(2015) | L | U | U | U | L | L | L |
| Chen CR(2011) | L | U | U | U | L | L | L |
| Shang RG(2016) | U | U | U | U | L | L | U |
| Ma HW(2017) | L | U | U | U | L | L | L |
| Sun CP(2015) | L | U | U | U | L | L | L |
| Wang J(2008) | U | U | U | U | L | L | U |
| Wang(2009) | U | U | U | U | L | L | U |
| Li HF(2015) | U | U | U | U | L | L | U |
Note: Cochrane Collaboration’s tool for assessing risk of bias for randomized trials, which assesses the risk of bias (L = low, H = high, U = unclear) across 7 domains: random sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias.
The results of the network meta-analysis of the disease control rate
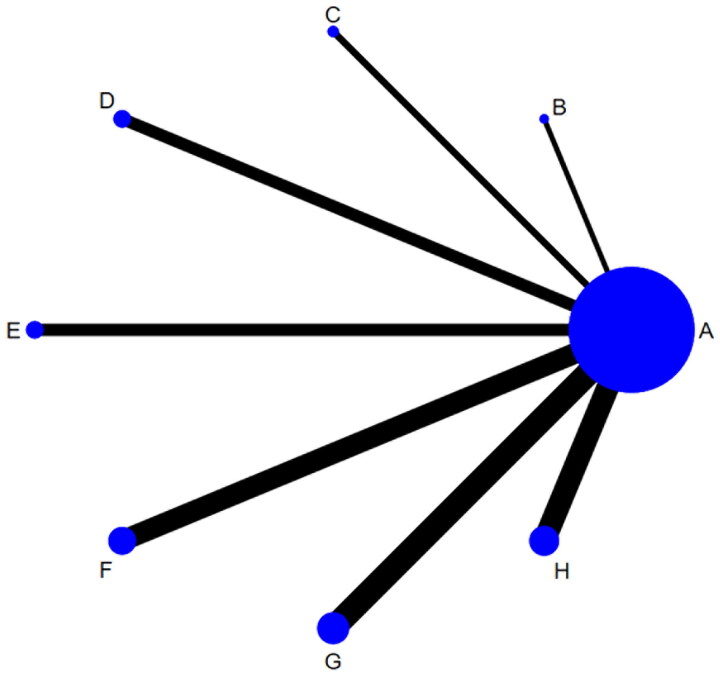
DCR was reported in 61 studies, including eight different interventions and 4,550 patients. The network graph, as illustrated in Figure 2, shows the relative amount of evidence available for these interventions, which include Zilongjin tablets, Banmao capsules, Hongdoushan capsules, Huachansu tablets, Kanglaite capsules, Xihuang pills, Pingxiao capsules, and chemotherapy. This graph highlights 61 direct comparisons among these treatments concerning their impact on DCR.
Figure 2.
Network graph for disease control rate.
Relative effectiveness and uncertainty for all possible pairs of interventions can be presented using a league table. Compared to chemotherapy alone, the OR values of NMA for various treatments were as follows: Banmao capsules [OR = 2.69, 95% CI (1.96, 3.69)], Huachansu tablets [OR = 2.35, 95% CI (1.81, 3.05)], Kanglaite capsules [OR = 2.37, 95% CI (1.31, 4.28)], Hongdoushan capsules [OR = 2.33, 95% CI (1.39, 3.91)], Zilongjin tablets [OR = 1.95, 95% CI (1.45, 2.62)], Pingxiao capsules [OR = 1.80, 95% CI (1.38, 2.34)], and Xihuang pills [OR = 1.65, 95% CI (1.10, 2.49)]. According to the first column of the league table, all active interventions significantly increased DCR compared to chemotherapy alone (Table 3). It should be noted that a single league table can show results for up to two outcomes (Figure 3).
Table 3.
Results of the network meta-analysis of disease control rate (DCR).
| E | |||||||
|---|---|---|---|---|---|---|---|
| 1.14 (0.76,1.72) | G | ||||||
| 1.14 (0.58,2.22) | 0.99 (0.52,1.90) | B | |||||
| 1.15 (0.63,2.11) | 1.01 (0.57,1.80) | 1.02 (0.46,2.23) | C | ||||
| 1.38 (0.89,2.13) | 1.21 (0.81,1.79) | 1.22 (0.63,2.36) | 1.20 (0.66,2.17) | H | |||
| 1.49 (0.99,2.25) | 1.31 (0.90,1.89) | 1.31 (0.69,2.51) | 1.29 (0.73,2.31) | 1.08 (0.73,1.61) | F | ||
| 1.62 (0.97,2.73) | 1.42 (0.88,2.31) | 1.43 (0.70,2.94) | 1.41 (0.73,2.72) | 1.18 (0.71,1.95) | 1.09 (0.67,1.77) | D | |
| 2.69 (1.96,3.69) | 2.35 (1.81,3.05) | 2.37 (1.31,4.28) | 2.33 (1.39,3.91) | 1.95 (1.45,2.62) | 1.80 (1.38,2.34) | 1.65 (1.10,2.49) | A |
A: chemotherapy, B: Kanglaite capsules, C: Hongdoushan capsules, D: Xihuang pills, E: Banmao capsules, F: Pingxiao capsules, G: Huachansu tablets, H: Zilongjin tablets.
Figure 3.
Interval plot for the disease control rate in the network meta-analysis.
According to the NMA results, Banmao capsules were identified to have the highest probability of being the most effective intervention to increase DCR, with a SUCRA value of 84.5%. This was followed by Huachansu tablets, with a SUCRA value of 70.7% (Figure 4).
Figure 4.
Cumulative ranking probabilities for disease control rate in the network meta-analysis.
A comparison-adjusted funnel plot was generated to evaluate possible publication bias among included studies. The number of points of the same color in the plot represents pairwise comparisons of each original study. The symmetrical distribution of these points on both sides of the funnel plot suggests the absence of notable small-sample effects or publication bias. This symmetry in the funnel plot suggests the lack of significant sample size effects or publication bias (Figure 5)).
Figure 5.
Comparison-adjusted Funnel plot of intervention measures for disease control rate.
The results of the network meta-analysis of the objective response rate
ORR was reported in 60 studies that involved eight different interventions and 4,464 patients. The network graph, as shown in Figure 6, illustrates the relative amount of evidence available for these eight interventions: Zilongjin tablets, Banmao capsules, Hongdoushan capsules, Huachansu tablets, Kanglaite capsules, Xihuang pills, Pingxiao capsules, and chemotherapy, about ORR. This graph includes 60 direct comparisons between these treatments.
Figure 6.
Network graph for objective response rate.
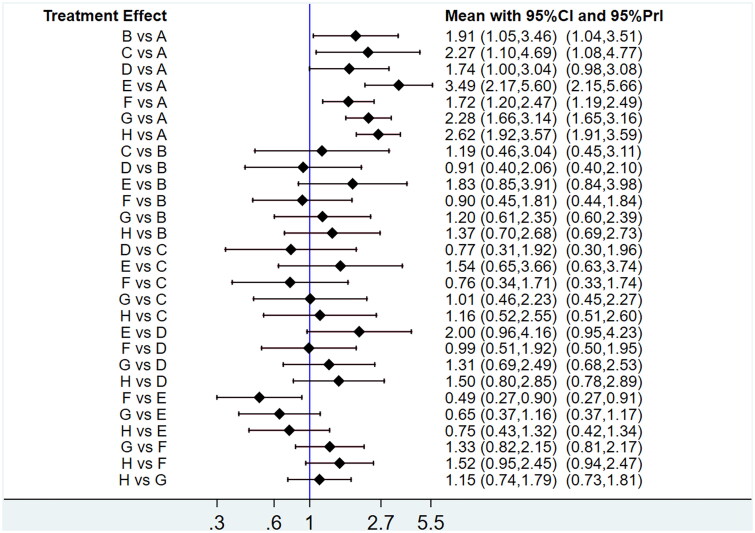
Compared to chemotherapy alone, the OR values obtained from the NMA were as follows: Banmao capsules [OR = 3.49, 95% CI (2.17, 5.60)], Zilongjin tablets [OR = 2.62, 95% CI (1.92, 3.57)], Huachansu tablets [OR = 2.28, 95% CI (1.66, 3.14)], Hongdoushan capsules [OR = 2.27, 95% CI (1.10, 4.69)], Kanglaite capsules [OR = 1.91, 95% CI (1.05, 3.46)], Xihuang pills [OR = 1.74, 95% CI (1.00, 3.04)], and Pingxiao capsules [OR = 1.72, 95% CI (1.20, 2.47)]. The first column of the league table indicates that all active interventions significantly exceed chemotherapy to improve ORR (Table 4). Figure 7 presents a single league table that displays results for up to two outcomes.
Table 4.
Results of network meta-analysis for objective response rate.
| E | |||||||
|---|---|---|---|---|---|---|---|
| 1.33 (0.76,2.34) | H | ||||||
| 1.53 (0.86,2.70) | 1.15 (0.74,1.79) | G | |||||
| 1.54 (0.65,3.66) | 1.16 (0.52,2.55) | 1.01 (0.46,2.23) | C | ||||
| 1.83 (0.85,3.91) | 1.37 (0.70,2.68) | 1.20 (0.61,2.35) | 1.19 (0.46,3.04) | B | |||
| 2.00 (0.96,4.16) | 1.50 (0.80,2.85) | 1.31 (0.69,2.49) | 1.30 (0.52,3.25) | 1.10 (0.48,2.48) | D | ||
| 2.03 (1.12,3.67) | 1.52 (0.95,2.45) | 1.33 (0.82,2.15) | 1.32 (0.58,2.97) | 1.11 (0.55,2.22) | 1.01 (0.52,1.96) | F | |
| 3.49 (2.17,5.60) | 2.62 (1.92,3.57) | 2.28 (1.66,3.14) | 2.27 (1.10,4.69) | 1.91 (1.05,3.46) | 1.74 (1.00,3.04) | 1.72 (1.20,2.47) | A |
A: chemotherapy, B: Kanglaite capsules, C: Hongdoushan capsules, D: Xihuang pills, E: Banmao capsules, F: Pingxiao capsules, G: Huachansu tablets, H: Zilongjin tablets.
Figure 7.
Interval plot for the objective response rate in the network meta-analysis.
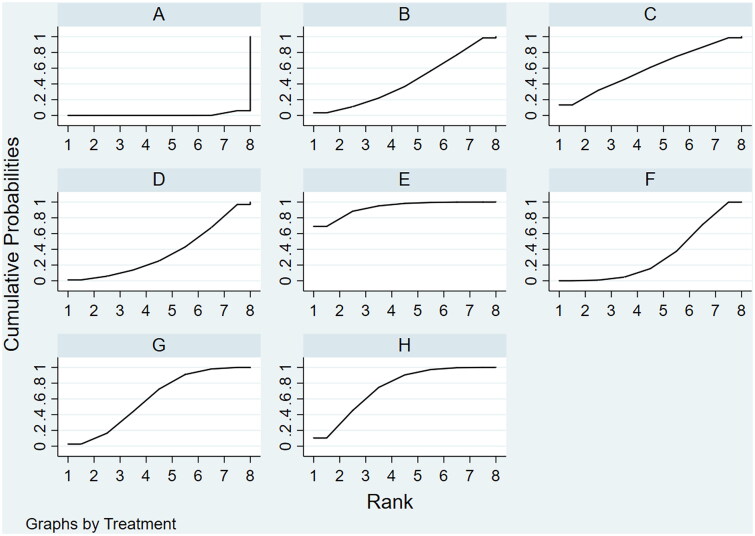
Based on the NMA results, the treatments were ranked in the following order according to their effectiveness: Banmao capsules, Zilongjin tablets, Huachansu tablets, Hongdoushan capsules, Kanglaite capsules, Xihuang pills, Pingxiao capsules, and chemotherapy. Their SUCRA values were 92.9, 74.0, 60.7, 58.9, 43.6, 36.2, 32.8, and 0.9, respectively (Figure 8). The funnel plot analysis for DCR revealed a largely symmetrical distribution, suggesting the absence of significant sample size effects or publication bias (Figure 9).
Figure 8.
Cumulative ranking probabilities for objective response rate in the network meta-analysis.
Figure 9.
Comparison-adjusted Funnel plot of intervention measures for the objective response rate.
Results of the network meta-analysis of the Karnofsky performance status
KPS was reported in 29 studies involving 2,170 patients and investigated seven different interventions. A network graph was created to visually represent the relative amount of evidence available for each of the seven interventions: Zilongjin tablets, Banmao capsules, Huachansu tablets, Kanglaite capsules, Xihuang pills, Pingxiao capsules, and chemotherapy. Furthermore, this graph shows the 29 direct comparisons made about KPS (Figure 10).
Figure 10.
Network graph for karnofsky performance status.
Compared to chemotherapy alone, the OR values obtained from the NMA for various treatments were as follows: Zilongjin tablets [OR = 3.47, 95% CI (2.14, 5.63)], Huachansu tablets [OR = 3.30, 95% CI (1.65, 6.60)], Banmao capsules [OR = 3.10, 95% CI (1.36, 7.05)], Kanglaite capsules [OR = 2.78, 95% CI (1.22, 6.37)], Xihuang pills [OR = 2.58, 95% CI (1.33, 5.01)], and Pingxiao capsules [OR = 2.18, 95% CI (1.29, 3.69)] (Table 5). Figure 11 illustrates a single league table that can display results for up to two different outcomes.
Table 5.
Results of network meta-anaysis for Karnofsky performance status.
| H | ||||||
|---|---|---|---|---|---|---|
| 1.05 (0.45,2.45) | G | |||||
| 1.12 (0.43,2.90) | 1.07 (0.36,3.12) | E | ||||
| 1.25 (0.48,3.25) | 1.19 (0.40,3.49) | 1.11 (0.35,3.57) | B | |||
| 1.34 (0.61,2.97) | 1.28 (0.49,3.34) | 1.20 (0.42,3.45) | 1.08 (0.37,3.11) | D | ||
| 1.59 (0.78,3.22) | 1.51 (0.63,3.60) | 1.42 (0.54,3.76) | 1.27 (0.48,3.40) | 1.18 (0.51,2.75) | F | |
| 3.47 (2.14,5.63) | 3.30 (1.65,6.60) | 3.10 (1.36,7.05) | 2.78 (1.22,6.37) | 2.58 (1.33,5.01) | 2.18 (1.29,3.69) | A |
A: chemotherapy, B: Kanglaite capsules, C: Hongdoushan capsules, D: Xihuang pills, E: Banmao capsules, F: Pingxiao capsules, G: Huachansu tablets, H: Zilongjin tablets.
Figure 11.
Intervals plot for the Karnofsky performance status in the network meta-analysis.
Based on the NMA results, the interventions were ranked according to the SUCRA values as follows: Zilongjin tablets (74.1%), Huachansu tablets (69.3%), Banmao capsules (63.3%), Kanglaite capsules (56.5%), Xihuang pills (50.1%), Pingxiao capsules (36.5%), and chemotherapy (0.3%). Further details can be found in Figure 12.
Figure 12.
Cumulative ranking probabilities for Karnofsky performance status in the network meta-analysis.
The KPS analysis indicated that the funnel plot exhibited a predominantly symmetric pattern. Furthermore, no significant evidence of sample effect or publication bias was observed (Figure 13).
Figure 13.
Comparison-adjusted Funnel plot of intervention measures for Karnofsky performance status.
Subgroup analyses
Ranking probabilities of CR, PR, SD, and PD
For the complete response outcome, the efficacy of treatments was ranked as follows: Banmao capsules, Kanglaite capsules, Huachansu tablets, Pingxiao capsules, Hongdoushan capsules, Zilongjin tablets, Xihuang pills, and chemotherapy. Their SUCRA values were 81.9, 73.4, 61.2, 54.4, 48.3, 41.2, 35.1, and 4.5%, respectively (Figure 14a).
Figure 14.
Cumulative ranking probabilities for CR, PR, SD, and PD in the network meta-analysis.
The efficacy ranking for the partial response outcome was as follows: Zilongjin tablets, Kanglaite capsules, Huachansu tablets, Hongdoushan capsules, Xihuang pills, Pingxiao capsules, Banmao capsules, and chemotherapy. The SUCRA values for these treatments were 76.3, 73.8, 69.2, 61.7, 46.4, 44.8, 25.6, and 2.2%, respectively (Figure 14b).
Regarding stable disease outcomes, the efficacy of treatments was ranked as follows: Zilongjin tablets, chemotherapy, Xihuang pills, Kanglaite capsules, Banmao capsules, Huachansu tablets, Pingxiao capsules, and Hongdoushan capsules. The SUCRA values were 92.6, 78.4, 55.4, 47.2, 45.3, 32.7, 26.3, and 22.0%, respectively (Figure 14c).
The efficacy ranking for the progressive disease outcome was: chemotherapy, Pingxiao capsules, Xihuang pills, Kanglaite capsules, Huachansu tablets, Hongdoushan capsules, Banmao capsules, and Zilongjin tablets. The SUCRA values were 99.2, 69.2, 68.3, 49.7, 46.2, 33.3, 20.6, and 13.6%, respectively (Figure 14d).
The funnel plot analysis for the CR, PR, SD, and PD outcomes suggests a largely symmetrical distribution, indicating no significant presence of sample size effect or publication bias (Figure 15).
Figure 15.
Comparison-adjusted Funnel plot of intervention measures for CR, PR, SD, and PD.
Adverse drug reactions
Chemotherapy-related adverse reactions primarily affected the blood system (thrombocytopenia, leukopenia, erythrocytopenia) and the gastrointestinal, liver, and kidney systems. According to the NMA results, the two interventions with the lowest incidence of leukopenia were Banmao capsules (SUCRA = 14.3%) and Zilongjin tablets (SUCRA = 26.3%) (Figure 16a). In terms of liver/renal toxicity, the interventions that showed the lowest incidence were Zilongjin tablets (SUCRA = 18.9%) and Banmao capsules (SUCRA = 26.6%) (Figure 16b). For erythrocytopenia, Banmao capsules (SUCRA = 0.6%) and Zilongjin tablets (SUCRA = 19.8%) had the lowest incidence rates (Figure 16c). Regarding gastrointestinal toxicity, Hongdoushan capsules (SUCRA = 18.8%) and Banmao capsules (SUCRA = 19.8%) were the least associated interventions (Figure 16d). Lastly, for thrombocytopenia, Hongdoushan capsules (SUCRA = 15.7%) and Huachansu tablets (SUCRA = 16.8%) exhibited the lowest incidence (Figure 16e).
Figure 16.
Cumulative ranking probabilities for adverse reactions in the network meta-analysis.
The results of the adverse reaction analysis revealed that the funnel plot displayed a predominantly symmetric pattern. Furthermore, there was no significant evidence of a sample size effect or publication bias, as indicated by the funnel plot (Figure 17).
Figure 17.
Comparison-adjusted Funnel plot of intervention measures for adverse reaction.
Clustered ranking plot
Based on the efficacy index of DCR from SUCRA values and the safety index, the percentage of gastrointestinal adverse reactions, a network cluster ranking chart was generated for different interventions. The results indicated that the Banmao capsules exhibited the best efficacy and the least gastrointestinal adverse reactions, as illustrated in Figure 18.
Figure 18.
Clustered ranking plot of different intervention measures.
Heterogeneity
There was some heterogeneity in this study, particularly from a clinical perspective. We included patients diagnosed with various types of NSCLC, such as adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and adenosquamous carcinoma. Furthermore, including disease stages ranging from I to IV and variations in patient age and disease duration contributed to this heterogeneity. However, the lack of detailed segmentation in the original literature limited our ability to conduct subgroup analyses.
Discussion
This study represents the first network meta-analysis to evaluate the efficacy of seven oral TCMs in conjunction with chemotherapy to treat NSCLC. The findings reveal that these seven oral TCMs can improve DCR and ORR. Furthermore, they alleviated clinical symptoms and improved the quality of life of NSCLC patients. These interventions show significant benefits in reducing liver and kidney toxicity, improving gastrointestinal toxicity, and reducing hematologic toxicity typically associated with chemotherapy.
Potential mechanisms
TCM has received significant recognition for its role in treating lung cancer. Its benefits go beyond merely reducing adverse reactions and enhancing the success rate of chemotherapy. TCM also has the potential to regulate immune function, induce apoptosis in tumor cells, reduce the risk of recurrence and metastasis, and ultimately prolong patient survival.
Kanglaite capsules, mainly made from Coix seed, contain active components such as Coix seed ester and alcohol extracts. These components are known for their anticancer effects, such as disrupting cancer cell DNA, inducing apoptosis, inhibiting cell proliferation, and impeding the formation of blood vessels that support tumors (Li 2011). Hongdoushan capsules, which contain active ingredients such as taxol and ginsenoside, effectively enhance the immune function of cancer patients, aiding in disease recovery. Furthermore, compounds such as glycyrrhizin in these capsules contribute to detoxification and liver protection, thus reducing the toxic side effects of drugs (Li et al. 2011). Xihuang capsules have been noted for their diverse therapeutic properties. These include inhibiting angiogenesis, reducing chemotherapy toxicity, preventing tumor metastasis, improving the hypercoagulable state associated with tumors, enhancing natural killer cell activity, and alleviating inflammation and pain (Du and Min 2018).
Banmao capsules show a specific affinity for lung cancer cells, inhibiting DNA and RNA synthesis. They are also known for increasing white blood cell counts and exhibiting antiviral and anti-inflammatory effects, all without myelosuppressive effects (Yan and Ye 2020). Pingxiao capsules enhance the body’s antibacterial capabilities, effectively inhibiting and eliminating the excessive proliferation of mutant cells. They strengthen white blood cells by enhancing phagocytosis and inhibiting tumor cell growth. Furthermore, they improve cellular and humoral immune functions, thus reducing the risk of recurrence and metastasis (Geng et al. 2009).
Huachansu capsules induce differentiation, proliferation, and apoptosis in tumor cells, exerting their anticancer effects by influencing tumor cell gene expression (Shi 2017). Zilongjin tablets block DNA synthesis and induce apoptosis in tumor cells, eliminating them. They also regulate cell dynamics to prevent tumor metastasis and modulate immune function by activating natural killer cells, T lymphocytes, and phagocytic cells (Zhang and Wei 2012).
Compared with other studies
In 2021, a network meta-analysis evaluated six commonly used oral Chinese patent medicines combined with platinum-based chemotherapy to treat NSCLC (Kong et al. 2021). Compared to their study, ours included a more comprehensive range of literature and more outcome indicators. Although there are similarities in our findings, particularly in the indicators of KPS and DCR, where Zilongjin tablets and cantharides showed the best effects, respectively, our study revealed inconsistencies in adverse reactions. For example, our analysis indicated that cantharides had the least gastrointestinal adverse reactions. Furthermore, we expanded our study to include additional efficacy indicators such as ORR, CR, PR, SD, and PD and the adverse effects on liver and renal toxicity.
Our study also differs from a previous meta-analysis published in 2020 (Yang et al. 2020). We included a wider variety of oral Chinese patent medicines, making our study more comprehensive and rich in content. Furthermore, as a network meta-analysis, our results offer more clinically relevant insights compared to that traditional meta-analysis.
Clinical implications and limitations
Adjuvant treatment of NSCLC includes a variety of TCM interventions. The insights gained from this study provide an evidence-based foundation for clinicians, helping them make informed decisions about selecting safe and appropriate TCM treatments. Furthermore, it is essential to acknowledge that patients may simultaneously present with multiple abnormal indicators in clinical practice. Therefore, choosing one drug to address one indicator and another to address a different indicator is insufficient. Instead, a comprehensive evaluation is necessary to devise an appropriate treatment plan that considers the multifaceted nature of the patient’s condition.
Our study has several limitations. First, the overall quality of the included studies was suboptimal. Many articles lacked detailed information on blinding and allocation concealment and don’t mention how patients were randomized, which could lead to implementation and measurement biases. Second, there was a disparity in the number of original studies available for each of the seven TCMs, which might introduce uncertainty in the ranking results. Third, the included patients varied in terms of disease progression (tumor classifications: adenocarcinoma, squamous cell carcinoma, large cell; tumor gradings: I-IV), treatment duration (ranging from 42 to 140 days), and medication regimens (differences in herb dosage and chemotherapy regimen). These variations could have influenced the outcomes and introduced potential biases. Fourth, our study was limited to articles in Chinese and English, raising the possibility of missing relevant studies in other languages, thus restricting the comprehensiveness of our findings. Future research should focus on conducting more high-quality studies to enhance our understanding of TCM in clinical practice. These studies should strive for rigorous design elements, such as large sample sizes, multi-center collaborations, randomized group assignments, allocation concealment, and blinding methods, to improve the robustness of the research.
Conclusions
Combining oral Chinese patent medicines with platinum-based chemotherapy has shown superior efficacy compared to platinum chemotherapy alone in treating NSCLC. The Zilongjin tablet has been particularly effective in improving patients’ quality of life. To improve DCR and ORR, the Banmao capsule and the Zilongjin tablet have shown better outcomes. The Zilongjin tablet has been effective in reducing gastrointestinal toxicity. Hongdoushan capsule and Banmao capsule have shown better results in mitigating liver and kidney toxicity. Zilongjin tablets and Banmao capsules have demonstrated significant advantages in reducing blood toxicity, highlighting their potential as effective adjunct treatments in NSCLC therapy.
Funding Statement
This work was supported by the Henan Key Research and Development and Promotion Project (grant number 232102310245), the Clinical Pharmacy Research Fund of the Chinese Society of Clinical Pharmacy in 2022 (grant numbers Z-2021-46-2101), and the Henan Medical Science and Technology Joint Construction Project (grant number LHGJ20220390)
Disclosure statement
Shusen Sun is an Associate Editor of this journal but was not involved in the peer review process, in line with journal protocol, COPE guidelines and current best practice. No other authors reported a conflict of interest.
References
- Amatu A, Sartore-Bianchi A, Bencardino K, Pizzutilo EG, Tosi F, Siena S.. 2019. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol. 30(Suppl_8):viii5–viii15. doi: 10.1093/annonc/mdz383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. 2019. Clinical study of compound cantharides capsule combined with chemotherapy in the treatment of advanced non-small cell lung cancer. World Latest Med Info. 74(19):163,226. Chinese. [Google Scholar]
- Cao J, Zhou J, Yang D, Chu J.. 2016. Compound taxane combined with chemotherapy and chemotherapy alone A controlled study of advanced non-small cell lung cancer. The Worl Clin Med. 16:100–100,103. Chinese. [Google Scholar]
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G.. 2013. Graphical tools for network meta-analysis in STATA. PLoS One. 8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, Zhang CM, Wang JY, Li CX, Yu G.. 2011. Clinical efficacy of Zilongjin tablets in combined with docetaxel plus cisplatin in the treatment of intermediate to advanced non-small cell lung cancer. China Prac Med. 6:139–140. Chinese. [Google Scholar]
- Chen JY, Hu XQ, Huang SX, Qin LJ, Wang X.. 2016. Effects of cinobufagin capsule combined with GP protocol on immune function in patients with advanced non-small cell lung cancer. Chin Mod Doc. 54:12–15. Chinese. [Google Scholar]
- Chen PF, Wu LC, Shu QJ, et al. 2001. Observation of curative effect of BaiLong tablets combined with chemotherapy in treating non small cell lung cancer (NSCLC). Chin Tradit Pat Med. 03:37–39. Chinese. [Google Scholar]
- Chen Q, Zhang MK, Li XQ, Zhang BB, Wang XL, Song JW.. 2018. Huachansu capsules combined with chemotherapy for advanced non-small cell lung cancer observation of curative effect on lung cancer. Mod J Int Tradi Chin Wes Med. 27:984–986. Chinese. [Google Scholar]
- Chen Y. 2020. To investigate the clinical efficacy and safety of Xihuang capsule combined with chemotherapy in the treatment of advanced lung adenocarcinoma. Med Die Heal. 18:12–13. Chinese. [Google Scholar]
- Chi X, Liu LJ, Wang Y, Wang Y.. 2021. Effect of compound cantharidin capsules combined with GP chemotherapy in the treatment of advanced non-small cell lung cancer. China Med Her. 18:97–100,103. Chinese. [Google Scholar]
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J.. 2019. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 10(10):ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du FH, Min XH.. 2018. Clinical effect of chemotherapy alone or in combination with Xihuang capsule in treatment of advanced lung adenocarcinoma. J Anhui Univ Chin Med. 37:34–36. Chinese. [Google Scholar]
- Duan QL. 2016. Efficacy of compound cantharides capsule in the treatment of non-small cell lung cancer. Pharmacol Clin Chin Mater Med. 32:191–192. Chinese. [Google Scholar]
- Duma N, Santana-Davila R, Molina JR.. 2019. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Fang YY. 2022. To investigate the efficacy of compound cantharides capsule combined with chemotherapy in the treatment of non-small cell lung cancer and its influence on immune function and adverse reactions of chemotherapy. Zhejiang J Trad Chin Med. 57:488–489. Chinese. [Google Scholar]
- Geng CX, Yao J, Wang XL.. 2009. Clinical evaluation of Pingxiao capsule combined with chemotherapy for advanced non-small cell lung cancer. China Pharmaceuticals. 21:68–69. Chinese. [Google Scholar]
- Guan W, Li B.. 2021. To investigate the effect of Huachansu capsule combined with TP regimen on immune function and quality of life in patients with advanced non-small cell lung cancer. Guizhou Med J. 45:1865–1867. Chinese. [Google Scholar]
- Guo H, Tian F, Jia WJ, Xing XL.. 2008. Xihuang pill combined with chemotherapy in the treatment of advanced non-small cell lung cancer clinical observation. J Emer Trad Chin. 17:22–23. Chinese. [Google Scholar]
- Guo YH, Shi HZ, Huang HY, Ma J, Lin QJ.. 2002. Clinical observation on the treatment of primary non-small cell lung cancer with Bailong Tablet. Shanghai Med Pharm J. 04:169–170. Chinese. [Google Scholar]
- He SL, Gao YY, Jin XX, Wang Y, Zhang FH, Liu B.. 2020. Vinorelbine + cisplatin chemotherapy combined with Xihuang pill observation of curative effect in patients with lung squamous cell carcinoma. Clin J Med Offic. 48:305–306. Chinese. [Google Scholar]
- Huang ML. 2019. To observe the clinical efficacy of cinobufagin combined with GP chemotherapy in the treatment of advanced non-small cell lung cancer. Prac Clin J Int Tradi Chin Wes Med. 19:51–52, 127. Chinese. [Google Scholar]
- Kong YN, Li Y, Xu ZL, Guo Y. 2021. Six common oral Chinese patent medicine combined with platinum-based chemotherapy for NSCLC: Network Meta-analysis. Chin Tradit Herb Drugs. 52(02):507–518. Chinese. [Google Scholar]
- Lan SL, Wu SF, Gao LW, Zuo YF.. 2011. 42 cases of non-small cell lung cancer were treated with chemotherapy combined with Pingxiao capsule clinical observation of lung cancer. J Chin Oncol. 17:154–155. Chinese. [Google Scholar]
- Li B, Shao H, Gao L, Li H, Sheng H, Zhu L.. 2022. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022 29(1):2130–2161. doi: 10.1080/10717544.2022.2094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GS, Ma SP.. 2015. Clinical observation of Zilongjin tablets combined with docetaxel and nedpltin in treatment of locally advanced non-small cell lung cancer. Drugs Clinic. 30:1506–1510. Chinese. [Google Scholar]
- Li HF, Wang HB, Dai Y, Yu Q.. 2015. Clinical experience of Zilongjin tablet in the treatment of advanced non-small cell lung cancer. Wom Heal Res. 8:30. Chinese. [Google Scholar]
- Li J, Zhu GH, Liu TT, Xu BW, Li J.. 2020. Comparative efficacy of Chinese herbal injections combined with GP regimen chemotherapy for patients with advanced NSCLC: a protocol for systematic review and network meta-analysis. Medicine (Baltimore).). 99(28):e21041. doi: 10.1097/MD.0000000000021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang SY, Jin E, Wang XN, Gao Y.. 2017. Clinical efficacy of Hongdoushan capsule combined with chemotherapy on the levels of TSGF, Cyfra21-1,CA125 and CEA for elderly patients with non-small cell lung cancer. Chin J Gene Prac. 15:1524–1526. Chinese. [Google Scholar]
- Li WG, Cui J, Wang JJ, Wang JY.. 2015. Clinical observation of bronchial artery infusion chemotherapy combined with Huachansu capsule in the treatment of advanced non-small cell lung cancer. China Pharm. 26:3703–3706. Chinese. [Google Scholar]
- Li XZ. 2020. To investigate the efficacy of Huachansu capsule combined with chemotherapy in patients with advanced non-small cell lung cancer. Prac Clin J Int Tradi Chin Wes Med. 20:47–48. Chinese. [Google Scholar]
- Li YM. 2011. Clinical observation of treating 70 cases of non-small cell lung cancer by KLT capsules in combination with chemotherapy. Clin J Chin Med. 21:8–9. Chinese. [Google Scholar]
- Li ZS. 2019. Huachansu capsule combined with DP chemotherapy in the treatment of advanced non-small cell lung cancer. Henan Med Res. 28:2244–2246. Chinese. [Google Scholar]
- Liang YH, Liu ML, Zhou JL.. 2016. To observe the curative effect of compound banao capsule combined with chemotherapy in advanced lung cancer. Shenzhen J Int Trad Chin Wes Med. 26:29–30. Chinese. [Google Scholar]
- Liu BD. 2014. Observe the clinical effect of Huachansu capsules in the treatment of small cell lung cancer. . J Clin Exp Med. 13:1263–1265. Chinese. [Google Scholar]
- Liu TF, Chen XM, Qin XB.. 2018. Effect of Xihuang capsules combined with chemotherapy on the untoward and side effect and immune function of patients with non-small cell lung cancer. Sys Med. 3:1–3,6. Chinese. [Google Scholar]
- Liu ZH, Ning TL, Yu RB.. 2000. Comparative study of three different combined schemes for advanced non-small cell lung cancer. J Bas Clin Onco. 13:179–180. Chinese. [Google Scholar]
- Long Q, Liu D, Tang WJ.. 2013. Clinical observation of compound cantharides capsule combined with chemotherapy after lung cancer surgery. Inn Mong J Tradi Chin Med. 32:10–11. Chinese. [Google Scholar]
- Ma HW, Fang J, Wang SX, Wang DL, Wang T, Wang EW.. 2017. Clinical efficacy of Zilongjin tablets combined with chemotherapy in advanced lung cancer. Lab Med Clin. 14:3318–3320. Chinese. [Google Scholar]
- Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL.. 2022. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 72(5):409–436. doi: 10.3322/caac.21731. [DOI] [PubMed] [Google Scholar]
- Ming J, Jiang XJ, Li JR.. 2010. Administration of pingxiao tablets before and after GP chemotherapy in patients with intermediate stage or advanced non-small cell lung cancer. J Clin Exp Med. 9:1055–1057. Chinese. [Google Scholar]
- Miu XD, Cao HF, Wang WX.. 2014. To observe the short-term efficacy of Huachansu capsule combined with TP regimen in the treatment of advanced non-small cell carcinoma. Chin Fore Med Res. 12:131–133. Chinese. [Google Scholar]
- Mukherjee P, Zhou M, Lee E, Schicht A, Balagurunathan Y, Napel S, Gillies R, Wong S, Thieme A, Leung A, et al. 2020. A shallow convolutional neural network predicts prognosis of lung cancer patients in multi-institutional CT-image data. Nat Mach Intell. 2(5):274–282. doi: 10.1038/s42256-020-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie M, Yao K, Zhu X, Chen N, Xiao N, Wang Y, Peng B, Yao L, Li P, Zhang P, et al. 2021. Evolutionary metabolic landscape from preneoplasia to invasive lung adenocarcinoma. Nat Commun. 12(1):6479. doi: 10.1038/s41467-021-26685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu JZ, Lu P, Pan Y.. 2017. Effects of Huachansu capsule combined with NP chemotherapy on serum CYFRA21-1, NSE levels and ummune function in advanced NSCLC patients. J Hubei Uni Chin Med. 19:26–29. Chinese. [Google Scholar]
- Ren F, Zhang Y, Wang HP, Cui YX, Wang MY, Chen GG.. 2018. The clinical observation on the treatment of mon-small cell lung cancer by compound Taxus capsule combined with cisplatin. Worl Chin Med. 13:1108–1110. Chinese. [Google Scholar]
- Schakel L, Veldhuijzen DS, Crompvoets PI, Bosch JA, Cohen S, van Middendorp H, Joosten SA, Ottenhoff THM, Visser LG, Evers AWM.. 2019. Effectiveness of stress-reducing interventions on the response to challenges to the immune system: a meta-analytic review. Psychother Psychosom. 88(5):274–286. doi: 10.1159/000501645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang RG. 2016. Clinical effects of the Zilongjin tablet plus docetaxel and cisplatin on mid-term or late stage non-small cell lung cancer. Chin J Clin Res. 8:45–46. Chinese. [Google Scholar]
- Shao JH, Huang FM, Fu JW.. 2002. Clinical observation of Pingxiao capsule combined with topotecan combined with cisplatin in the treatment of non-small cell lung cancer. J Mod Oncol. 10:229–230. Chinese. [Google Scholar]
- Shen T, Wei YS, Zhang HZ.. 2017. Clinical efficacy of Zilongjin tablets in combination with TP regimen for locally advanced non-small cell lung cancer and its effect on T-lymphocyte subsets. Hebei J TCM. 39:1539–1542. Chinese. [Google Scholar]
- Shi W. 2017. To observe the curative effect of Huachansu capsule combined with chemotherapy on advanced non-small cell lung cancer. Guid Chin Med. 15:179–180. Chinese. [Google Scholar]
- Shu JH, Wu LY, Zhou RY.. 2002. Clinical observation on the effect of Zilongjin tablets on the reduction of toxicity and efficacy of chemotherapy for middle and late stage lung cancer. Chin J Clin Oncol. 08:65–66. Chinese. [Google Scholar]
- Song Z, Zhou LR, Wang XC, Wang FL.. 2019. Clinical observation on compound Banmao capsules combined with oxaliplatin in treatment of non-small cell lung cancer. Drugs Clin. 34:1525–1528. Chinese. [Google Scholar]
- Sun CP, Wang JF, Chen XL, Ye WL, Zhu XL, Liu QX.. 2015. Study of Zilongjin tablets combined with chemotherapy on advanced non small cell lung cancer. Chin Arch Tradit Chin Med. 33:1145–1147. Chinese. [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F.. 2021. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tan XY, Li JC.. 2005. Clinical observation of Pingxiao capsule combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Hubei J Tradi Chin Med. 27:29–30. Chinese. [Google Scholar]
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G.. 2013. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 42(1):332–345. doi: 10.1093/ije/dys222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan LX, Wang Y, Wang WL.. 2007. Clinical research of Canelim capsule with chemotherapy for advanced non-small cell lung cancer. J Mod Oncol. 15:534–536. Chinese. [Google Scholar]
- Wang GL, Wang JH.. 1999. Pingxiao capsule combined with chemotherapy in the treatment of non-small cell lung cancer. Hebei Med. 09:8–10. Chinese. [Google Scholar]
- Wang J, Yan WR.. 2008. Study of Zilongjin tablets combined with chemotherapy on non small cell lung cancer in middle or advanced stage. Mod J Integr Tradit Chin West Med. 01:3–4. +7.Chinese. [Google Scholar]
- Wang SM, Li J.. 2015. Comparison of the efficacy of Pingxiao capsule and shendan sanjie capsule combined with CP program in the treatment of mon-small cell lung cancer in stage IV. China Pharm. 26:4200–4202. Chinese. [Google Scholar]
- Wang T. 2018. Clinical study on compound Banmao capsules combined with GP regiment in treatment of advanced non-small cell lung cancer. Drugs Clin. 33:3264–3269. Chinese. [Google Scholar]
- Wang X, Cheng ZQ.. 2009. Clinical observation of Xihuang pill combined with GP regimen in the treatment of 16 cases of advanced non-small cell lung cancer. China J Tradit Chin Med Pharm. 2:47–48. Chinese. [Google Scholar]
- Wang Y. 2019. Efficacy and safety of Kanglaite capsule combined with GP chemotherapy in patients with advanced non-small cell lung cancer. Chin Fore Med Trea. 33:23–25. Chinese. [Google Scholar]
- Wang ZH. 2009. Analysis of 60 cases of lung cancer in the elderly treated with Zilongjin tablets. Hebei Med J. 31:2156–2157. Chinese. [Google Scholar]
- Wei GH, Xu CM.. 2017. Efficacy of different chemotherapy regimens for advanced non-small cell lung cancer. Chin J Clin Oncol. 24:167–169. Chinese. [Google Scholar]
- Wu HB, Zhang J.. 2006. Clinical observation on the efficacy and toxicity reduction of Zilongjin tablets in chemotherapy for primary lung cancer. Tianjin Pharm. 06:29–30. Chinese. [Google Scholar]
- Wu LM, Jin L Ma JM, Song CF.. 2020. Effect of Huachansu capsule plus etoposide combined with cisplatin on inflammatory factors and serum tumor markers in elderly patients with non-small-cell lung cancer. Hainan Med J. 31:20–22. Chinese. [Google Scholar]
- Wu XF. 2019. Clinical observation on compound cantharidin capsule in the treatment of mon-small cell lung cancer. Chin Med Mod Dis Edu Chin. 17:62–63. [Google Scholar]
- Yan MY, Ye ZR.. 2020. Clinical study on compound Banmao capsules assisting chemotherapy for advanced non-small cell lung cancer in senile patients. New J Tradit Chin Med. 52:112–114. Chinese. [Google Scholar]
- Yang HP, Yi B.. 2002. To observe the clinical effect of Pingxiao capsule combined with chemotherapy on advanced non-small cell lung cancer. J Mod Oncol. 10:60–61. Chinese. [Google Scholar]
- Yang XC, Tian F, Yu JC.. 2017. Clinical study on treatment of primary non-small cell lung cancer with Zilongjin tablets combined with PC chemotherapy. Acta Chin Med. 32:715–717. Chinese. [Google Scholar]
- Yi HH, Chen JH.. 2014. Efficacy and economic evaluation of three Chinese patent medicines added to chemotherapy for non-small cell lung cancer. Eval Ana Dru Hos Chin. 14:589–591. Chinese. [Google Scholar]
- Yang J, Zhu X, Yuan P, Liu J, Wang B, Wang G.. 2020. Efficacy of traditional Chinese Medicine combined with chemotherapy in patients with non-small cell lung cancer (NSCLC): a meta-analysis of randomized clinical trials. Support Care Cancer. 28(8):3571–3579. doi: 10.1007/s00520-020-05433-w. [DOI] [PubMed] [Google Scholar]
- Yu FM, Jiang DL.. 2018. Clinical study on Zilongjin Tablets combined with GP chemotherapy in treatment of non-small cell lung cancer. Drugs Clinic. 33:1184–1188. Chinese. [Google Scholar]
- Yu TT, Chen F, Chen D, Li J.. 2018. Clinical effect and safety of Huachansu capsules combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin Mod Med. 25:53–55. Chinese. [Google Scholar]
- Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, et al. 2018. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Niu YN.. 2019. Analysis on the curative effect of Xihuang capsule combined with DP regimen chemotherapy on patients with advanced Non-small cell lung cancer. Chin Med Mod Dis Edu Chin. 17:111–113. Chinese. [Google Scholar]
- Zhang QP, Lou GY, Xu N, Fan Y, Zhong HJ, Lou CJ.. 2000. Clinical study of Kanglaite capsules combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin J Clin Oncol. 27:77–79. Chinese. [Google Scholar]
- Zhang XF, Wei YQ.. 2012. 41 cases of advanced non-small cell lung cancer treated with Zilongjin tablets combined with GP regimen. Shaanxi Med J. 41:875–877. Chinese. [Google Scholar]
- Zhang XW. 2015. To observe the effect of compound taxus capsule combined with TP regimen in the treatment of advanced non-small cell lung cancer. Shaanxi J Tradit Chin Med. 36:833–835. Chinese. [Google Scholar]
- Zhao J, Yuan FH, Zhao ZX.. 2011. Pingxiao capsule oral adjuvant chemotherapy in the treatment of middle and late stage observation of curative effect on Lung cancer. Shandong Med J. 51:61. Chinese. [Google Scholar]
- Zhao YJ, Jian GQ, Lv SL.. 2007. Effect of pinxiao capsule with chemotherapy in treating non- small cell lung cancer. J Mod Oncol. 09:1323–1324. Chinese. [Google Scholar]
- Zhou XY, Wang PL, Liu XH.. 2016. Huachansu capsule and chemotherapy in treatment of patients with advanced lung cancer: a clinical study. Chin Rem Clin. 16:1406–1408. Chinese. [Google Scholar]