Abstract
The etiology of sirenomelia is currently unknown. Data are limited in comparing external and internal abnormalities using modern imaging technologies and molecular genetic analysis. The purpose of the current study was designed to compare external and internal anatomical defects in two cases of sirenomelia and Potter's sequence. Considered rare, Potter's sequence is a fetal disorder with characteristic features of bilateral renal agenesis, obstructive uropathy, atypical facial appearance, and limb malformations. The internal and external malformations of two term fetuses with sirenomelia and Potter's sequence were compared using assessment of external features, radiography and MRI on internal structures, and molecular genetic studies on sex determination. Data reveal that both fetuses were male and manifested with an overlapping but distinct spectrum of abnormalities. Principal differences were noted in the development of the ears, brain, urogenital system, lower limbs, pelvis, and vertebral column. Defects of the axial mesoderm are likely to underlie the abnormalities seen in both fetuses. The first one, which had only caudal defects, was found to have a spectrum of abnormalities most similar to those associated with more severe forms of the small pelvic outlet syndrome, although the structure and orientation of the sacrum and iliae were different from previously reported cases. The other had both caudal and cranial defects, and was most similar to those described in the axial mesodermal dysplasia syndrome. Defects associated with sirenomelia can be evaluated with standard gross anatomy examination, radiology, MRI, and modified PCR techniques to determine anatomical abnormalities and the sex of preserved specimens, respectively. Evidence indicated that sirenomelia could be developed via various etiologies.
Keywords: anatomical abnormalities, birth defect, genetic sex determination, magnetic resonance imaging, radiological analysis, sirenomelia
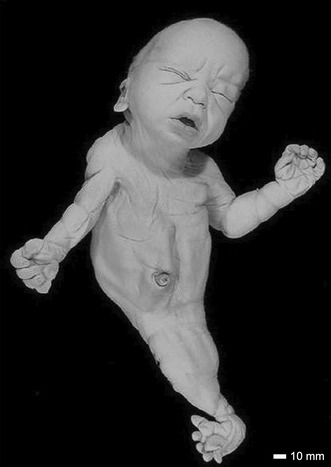
A case of sirenomelia showing lower limbs is of the sympus monopus type.
1. INTRODUCTION
Sirenomelia is a defect of blastogenesis (Opitz et al., 2002) that is only rarely observed in live‐born infants (1.2–4.2/100,000 births) (Banerjee et al., 2003). The underlying defect is believed to be a failure of mesodermal cell proliferation within the caudal region resulting in a deficiency of cells populating the median plane of the embryo. The resulting juxtaposition of the limb buds produces partial to complete fusion of the lower limbs. Associated malformations include hypoplastic lungs, renal agenesis, pelvic abnormalities, absent external genitalia, imperforate anus, and lumbosacral vertebral anomalies. Other anomalies include absent bladder and ureters, blind‐ending gut, incomplete lower limb bones, lateral patellae, double tibiae, single or absent fibulae, and Potter's sequence. Cranial abnormalities, such as hydrocephalus (Onyeije et al., 1998), have been reported in a limited number of cases. There are only believed to be four reported cases of surviving sirenomelic individuals in the literature, all of whom have functional renal systems. The oldest case reported in the literature was 4 years old (Stanton et al., 2003). Four additional cases were also published: two in 2015 (Samal & Rathod, 2015) and two in 2021 (Shojaee et al., 2021).
Given the rarity of the syndrome, understanding the etiology of sirenomelia has been difficult. Predisposing factors include maternal diabetes (Chen et al., 1997), monozygotic twinning (Schinzel et al., 1979) and early maternal age (Kampmeier, 1927), (Resnick, 1945). There is a lack of evidence for a familial occurrence of sirenomelia, thus it is believed to be sporadic. The malformations observed in individuals with sirenomelia are also observed in other syndromes, including VACTER‐L (Onyeije et al., 1998), small pelvic outlet syndrome (Currarino & Weinberg, 1991) and caudal regression syndrome (Duhamel, 1961), suggesting that these disorders represent a continuum of mesodermal defects of both caudal and cranial structures.
In the current investigation, an anatomical assessment and genetic sex determination of two term fetuses with sirenomelia and multiple congenital anomalies that have been preserved for over 60 years was conducted. This was a rare opportunity to study two cases of sirenomelia simultaneously, allowing a comparison of their features with each other and with previously reported cases. Results show that although they share features commonly observed in cases of sirenomelia, they also possess unique features previously not described. This study also demonstrated that it is possible to obtain amplifiable DNA for molecular genetic studies from specimens preserved for several decades in which the initial fixative was unknown.
2. MATERIALS AND METHODS
2.1. Subjects
The Department of Biomedical and Molecular Sciences (formerly Department of Anatomy and Cell Biology) at Queen's University obtained the two term fetuses affected with sirenomelia in the early 1960's. Both were preserved intact and are without evidence of invasive examination. The fixative that was used initially to preserve the specimens is unknown; however, they were transferred to 10% formalin in water once they were received. The specimens are labeled Case 7 and Case 16 in the collection of 40 specimens of various birth defects, and they will continue to be referred to as such in this report. The current location of the family of these cases is unknown. Permission to examine and report the findings on these cases was obtained from the Chief Coroner for the Province of Ontario, Canada. A report on a cyclopic specimen was published in 2002 (Situ et al., 2002) and a report of an asymmetrically conjoined tripus twins was presented in 2012 (Lee et al., 2012) from the collection at Queen's University.
Both fetuses were born in the same remote rural location within 8 months of each other. There was no evidence found in a search of archival newspaper records suggesting that an environmental event played a role in the occurrence of the malformations seen in these two cases. From the medical records obtained at the time, Case 7 was stillborn after a spontaneous breech delivery to a 19‐year‐old prima gravida who had no prenatal care. There was no evidence of maternal diabetes at the time of delivery. Case 16 was also a stillborn to a 20‐year‐old, para 1, gravida 2 female with no history of illness and an uneventful prenatal history.
2.2. Imaging analysis
X‐ray and MRI studies were performed at the Department of Radiology in the Kingston General Hospital. X‐rays were taken of the vertebral column and extremities. For the MRI studies, multiple image sections were obtained using a General Electric Signa 1.5 Telsa magnetic resonance imager and version 5.4.2. Software. Settings for each image were T2 weighted 2D FSE and divided into a slice thickness of 3 mm with a 0.5 mm interslice gap and 0.8 mm gradient recalled echo. A spoiled gradient echo was applied to decrease the horizontal stripping of the images. A second set of MRIs was performed to obtain more detailed images of specific areas of both fetuses. For these, Echo Speed Plus Software version 9.1 was used to analyze the images. Where possible a 512 × 512 matrix was used with a small field view to maximize spatial resolution. The MRI images were converted to digital TIFF images using DicomWorks software version 1.3.5 (Puech P. and Boussel L., http://dicom.online.fr/) (Puech et al., 2007) or MRIcro Software version 1.3.9 (Rorden C., http://www.sph.sc.edu/comd/rorden/mricro.html) (Rorden & Karnath, 2004). Digital photographs of the specimens were taken in the Department of Biomedical and Molecular Sciences at Queen's University.
2.3. Anatomical assessment
An assessment of the external anatomy of both cases was performed. The results of the external and internal assessments of both cases were used to search the OMIM (Online Mendelian Inheritance in Man, www.ncbi.nlm.nih.gov), POSSUM (Pictures of Standard Syndromes and Undiagnosed Malformations) version 5.6 (http://www.possum.net.au) databases to obtain possible diagnoses for these cases.
2.4. Genetic sex determination
To obtain tissue for DNA extraction, punch needle biopsies of lung and thymus tissue were performed, and samples were stored in 10% formalin in water. DNA extractions were performed using 4 M guanidinium isothiocyanate as described by Konomi et al. (2002) and back extracted using phenol: chloroform (1:1) and chloroform to minimize the loss of DNA. Samples were recovered by precipitation with 3 M sodium acetate (pH 5.2) and ethanol, and stored at −20°C in TE (10 mM Tris–HCl, 1 mM EDTA, pH 8.0). The DNA was subjected to nick translation and ligation repair according to the methods of Pusch et al. (1998). The cycled ligation reaction consisted of 60 cycles of 30 s each at 10°C and 30°C to ligate both blunt and cohesive ends. The repaired DNA was extracted with phenol: chloroform (1:1), ethanol precipitated and stored at −20°C.
The X chromosome and Y chromosome loci, WI9327 and SY182, respectively, were amplified in fetal and control DNA samples using the following primer sets:
| WI9327 (117 bp) | WI9237F – 5′TGCTCCCTCCTTAAGGTTATAGG3′ |
| WI9237R – 5′GCTTATTGTTAGCACAACATCACC3′ | |
| SY182F (125 bp) | SY182F – 5′TCAGAAGTGAAACCCTGTATG3′ |
| SY182R – 5′GCATGTGACTCAAAGTATAAGC3′ |
The forward primers were end‐labelled using T4 polynucleotide kinase and γ‐32P ATP (3000 Ci/mM, Perkin Elmer). The amplification conditions for WI9327 were 20 mM Tris–HCl pH 8.4, 50 mM KCl and 2 mM MgCl2, 0.2 pmol of forward primer and 20 pmol of reverse primer, 200 μM dNTP (200 μM each), 2.5 U Taq polymerase (Invitrogen), followed by denaturation at 94°C for 5 min and 30 cycles of 94°C 1 min, 60°C 1 min and 72°C 1 min. The amplification conditions for SY182 were 20 mM Tris–HCl pH 8.4, 50 mM KCl, 3 mM MgCl2, 0.2 pmol of forward primer and 20 pmol of reverse primer, 200 μM dNTP, 2.5 U Taq polymerase (Invitrogen), followed by denaturation at 94°C for 5 min and 30 cycles of 94°C 1 min, 54°C 1 min, and 72°C 1 min. PCR products were electrophoresed on a 6% 35 × 42.5 cm denaturing PAGE gel containing 50% urea in 89 mM Tris‐borate, 89 mM boric acid, 2 mM EDTA, and pH 8.0. Autoradiography was performed using Cronex Ortho‐Vision Film at −70°C for 1–7 days.
3. RESULTS
3.1. Case 7
An exterior view of Case 7 is shown in Figure 1. Features include dolicocephaly, a prominent occiput, a low posterior hairline and redundant nuchal folds. Both ears were low set and posteriorly rotated. The right ear had a squared off and flattened superior helix with a prominent anti‐tragus (Figure 2a). The left ear had a flattened superior helix with a prominent crus (Figure 2b). The midface was flattened and the chin was small. The nose was short with a crease over the nasal root and anteverted nostrils. The oral exam revealed thick lips and gums, with developing teeth, a normal tongue and a hard palate. The brain size and shape were normal and corresponded to a gestational age of approximately 35 weeks.
FIGURE 1.
A full anterior view of Case 7. The sirenomelic lower limbs are of the sympus monopus type. Scale bar = 10 mm.
FIGURE 2.
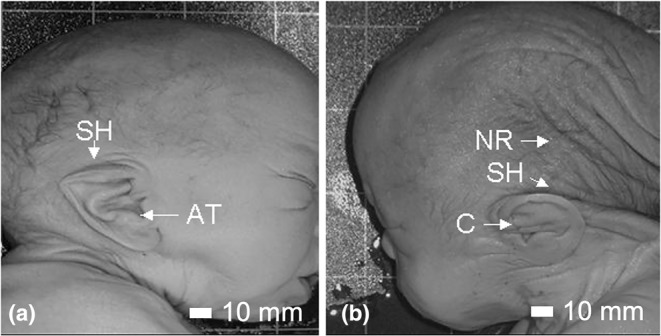
Ear anomalies in Case 7. The right ear of Case 7 is shown in (a) and the left ear in (b). The superior helix (SH) is flattened in both ears and squared off in the right ear. The right ear has a prominent anti‐tragus (AT) and the left has a prominent crus (C). Also visible in (B) is the prominent occiput and nuchal redundancy (NR). Scale bar = 10 mm.
A short sternum and widely spaced nipples were noted. The lungs, trachea and bronchial tubes were structurally normal, but the lungs were hypoplastic (Figure 3). The heart was anatomically normal. Two umbilical arteries and a single umbilical vein are present as were two iliac vessels. The liver, spleen, and esophagus appeared normal. A clearly identifiable stomach was present and lead to a disorganized intestinal mass with a blind ending colon and imperforate anus. Externally, there was hyperpigmentation in the anal area. An intact perineum was not observed. Kidneys, urinary bladder, external genitalia, and gonads were absent, but evidence of an internal penis was found in a centrally located tissue mass inside the abdominal wall.
FIGURE 3.
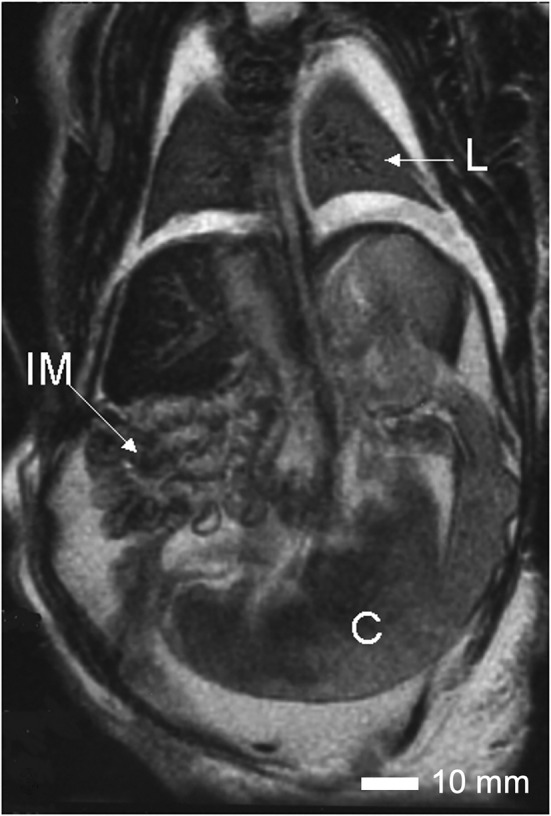
Internal anatomy of Case 7. An MRI image of the thorax and abdomen of Case 7 showing the hypoplastic lungs (L), the disorganized intestinal mass (IM) and the blind ending colon (C). Scale bar = 10 mm.
Both hands were hypoplastic with short thumbs and hyperconvex nails. The left hand had a transverse palmar crease. The right hand had a clinodactyly of the fifth finger. The sirenomelic lower limbs of Case 7 were of the sympus monopus type (Figure 1), whereby one foot is present. There was a single fused femur with two femoral heads and two tibial plateaus of expected size (Figure 4), leading into the right tibia and fibula (Figure 5). The left tibia was hypoplastic and the left fibula was absent. There was a single right calcaneus and multiple phalanges with ten metatarsal bones in the single foot. This fetus most closely resembles the Type IV classification (Lhuaire et al., 2013; Stocker & Heifetz, 1987). The two iliac bones were oriented parallel to the spinal column (Figure 6a). The fused heads of the femurs were located behind the iliac bones, which were not fused with the sacrum. The orientation of the pubic and ischium produced an abnormally small pelvic outlet. The sacrum and coccyx appeared to be intact, however they curved outwards horizontally (Figure 6b) over the top of the fused femurs (Figure 7). The pubis was in the midline. The vertebral column was complete, however this was not confirmed by CT‐scan imaging or by gross examination following dissection.
FIGURE 4.
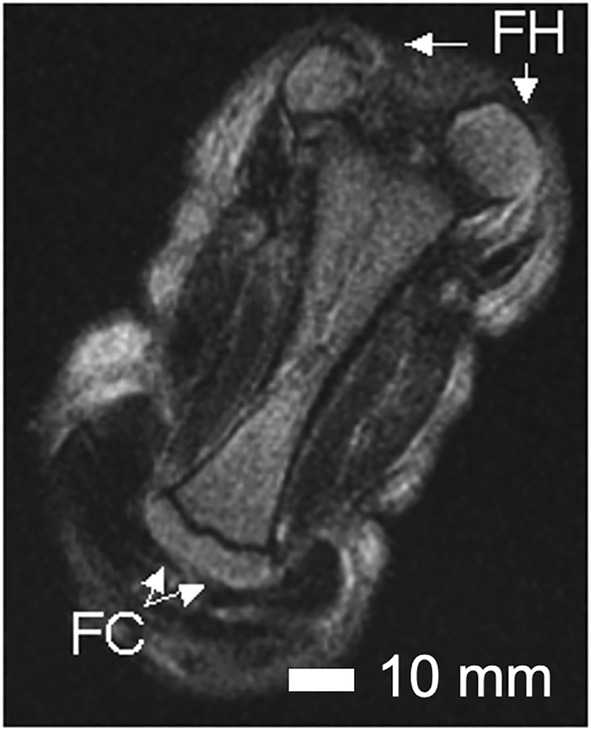
Anomalies of the femur of Case 7. An MRI image of the fused femurs of Case 7 showing the abnormally rotated femoral heads (FH), the bifurcation of the distal end and the two tibial plateaus (TP). The plane of fusion of the two femurs is seen as a darkened line separating the upper portion of the fused femur. Scale bar = 10 mm.
FIGURE 5.
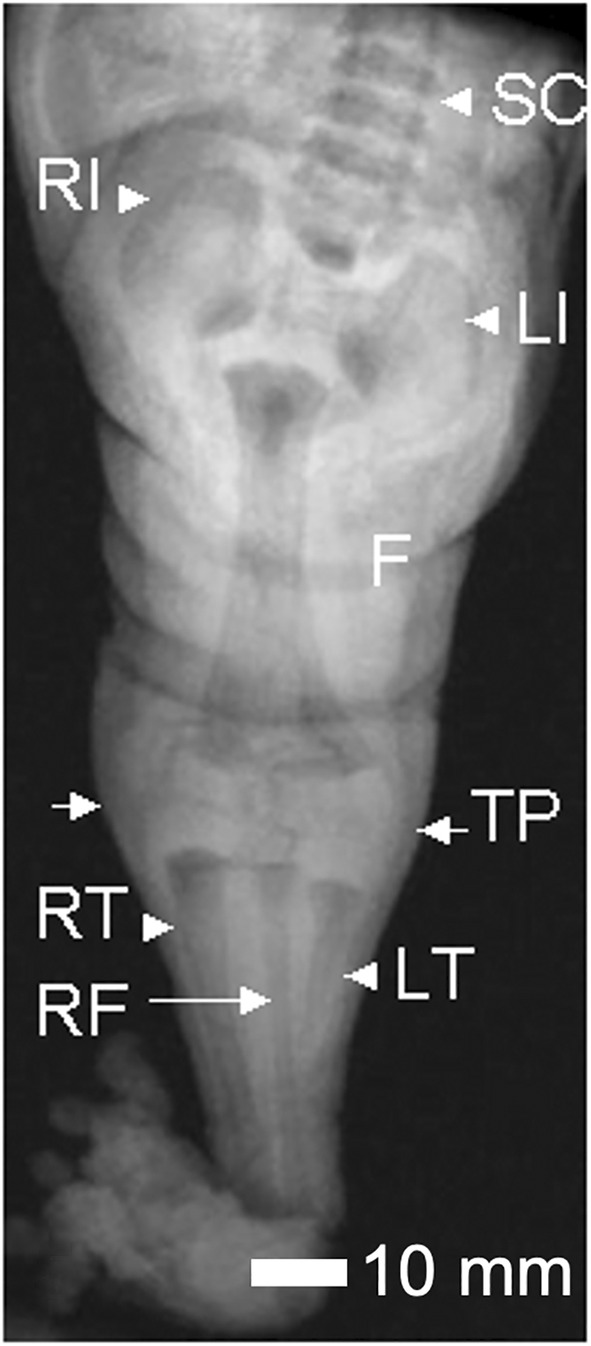
Composite view of the sirenomelic lower limb of Case 7. Coronal MRI images of the lower limb of Case 7 were fused to obtain a composite image. The intact vertebrae of the spinal column (SC) are visible, the right (RI) and left ilia (LI), the fused femur (F), the two tibial plateaus (indicated by small arrows), the right tibia (RT), right fibula (RF), and the hypoplastic left tibia (LT). Scale bar = 10 mm.
FIGURE 6.
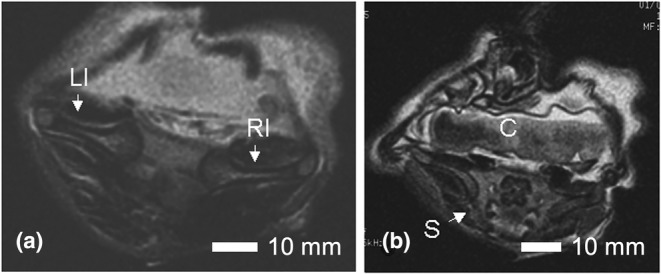
Axial view of the pelvis in Case 7. In (a), the abnormal 180‐degree angle of the left and right ilia (LI, RI) in Case 7 is shown. In (b), the sacrum (S) and coccyx (C) are shown to be intact but oriented in a horizontal plane. Scale bar = 10 mm.
FIGURE 7.
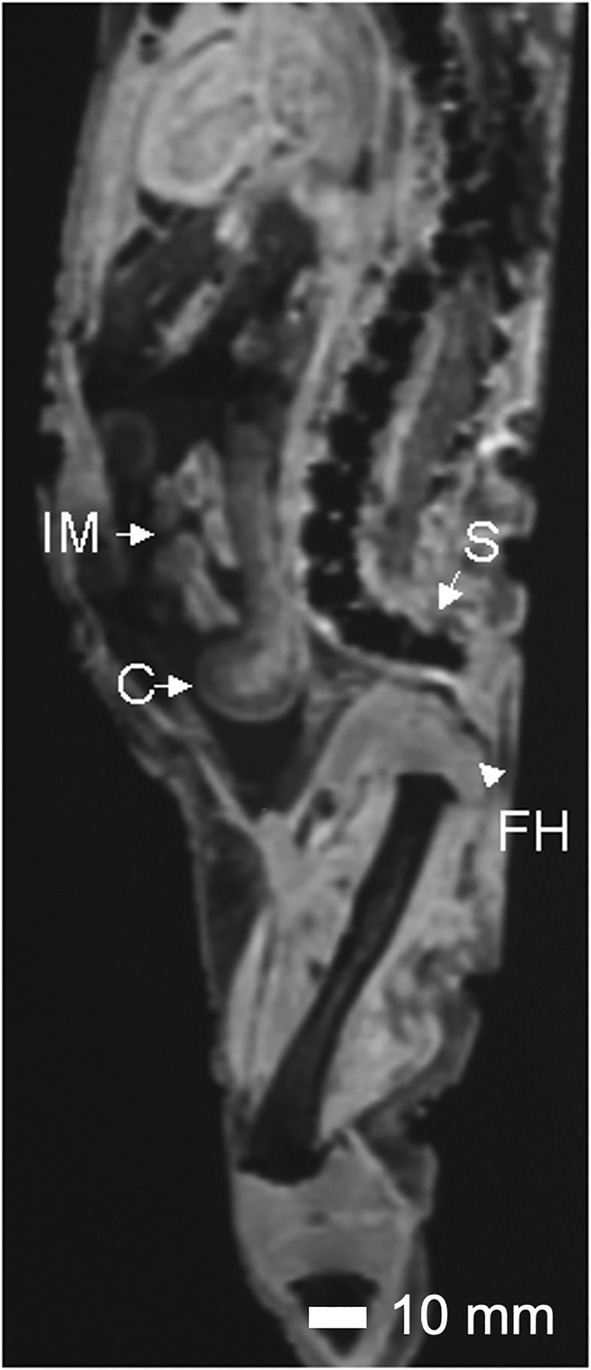
Sagittal view of Case 7. The orientation of the sacrum (S) with respect to the femoral heads (FH) is shown. The femoral heads lie in behind the ilia. The sacrum lies atop the femoral head. Also visible are the disorganized intestinal mass (IM) and blind ending colon (C). Scale bar = 10 mm.
3.2. Case 16
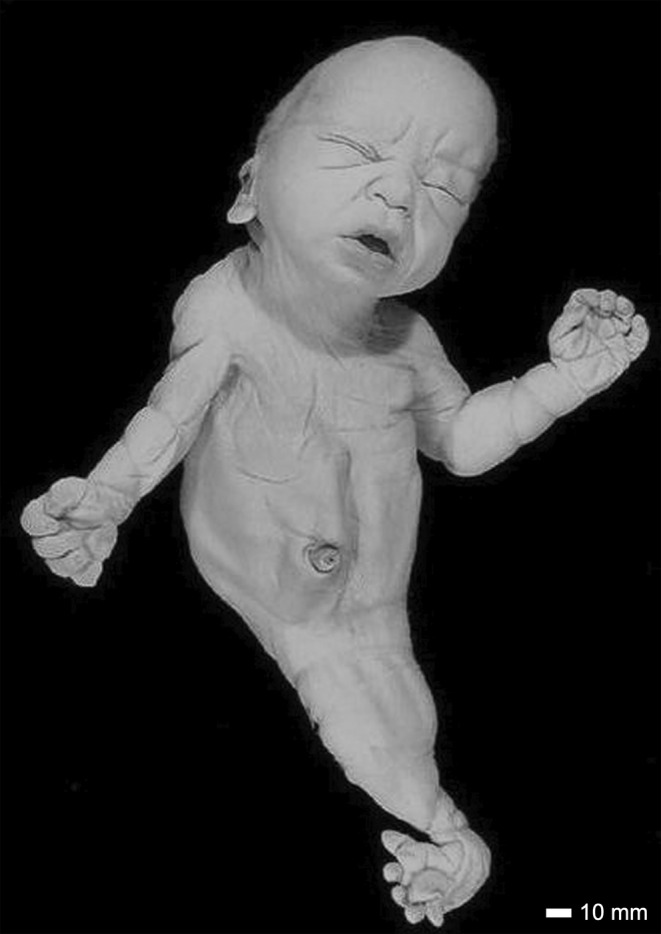
On external examination, Case 16 had a short forehead, with a low‐anterior hairline and nuchal redundancy (Figure 8). Both ears were hypoplastic, posteriorly rotated and low set. The right ear demonstrated remnants of three earbuds with what appeared to be an external otic canal (Figure 9a). The left ear was low set and posteriorly rotated with a flattened helix (Figure 9b). There was a crease over the nasal root. The mouth was normal with an intact hard palate, tongue and developing teeth. MRI studies revealed the presence of severe hydrocephalus with a large posterior cyst (Figure 10a,b). The thalamus was underdeveloped. There was a type 2 Chiari malformation with cerebellar tonsils extending into the foramen magnum and into the spinal canal at C2–C3 (Figure 10c).
FIGURE 8.
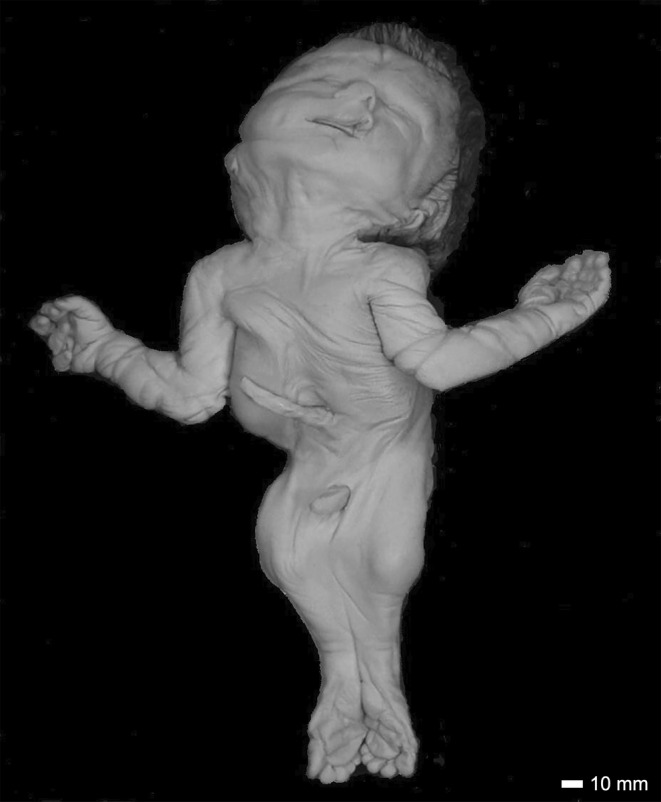
A full anterior view of Case 16. The sirenomelic lower limbs are of the sympus dipus type. The distortion of the mouth and nose is an artifact of more than 60 years of storage. Scale bar = 10 mm.
FIGURE 9.
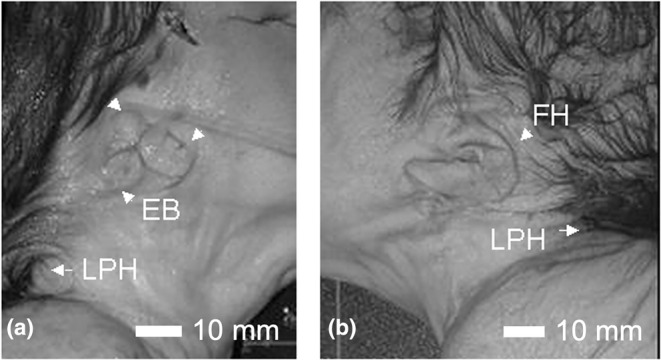
Ear anomalies in Case 16. The right ear of Case 16 showing the remnants of the three earbuds (EB) is seen in (a). The left ear shown in (b) is hypoplastic and malrotated. In both the low posterior hairline (LPH) can be observed. Scale bar = 10 mm.
FIGURE 10.
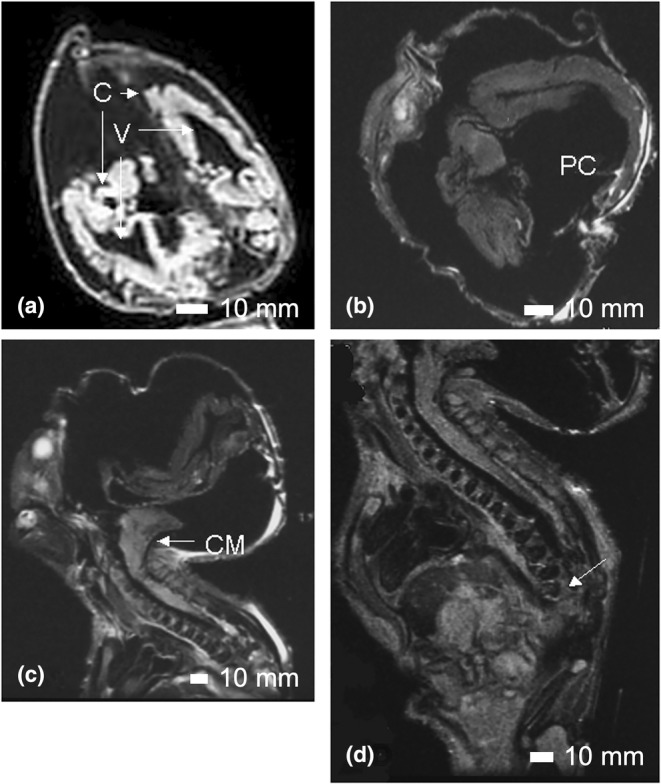
The hydrocephalic brain of Case 16. In (a), a coronal MRI image of the head demonstrates the existence of two cerebral hemispheres (C) and two ventricles (V). In (b), an axial image confirms the existence of two cerebral hemispheres and a posterior cyst (PC). In a sagittal MRI image (c), the Chiari malformation (CM) is visible. In (a), (b), and (c), the poorly ossified skull can be seen as inward or outward folding of the exterior of the head. In (d), the end of the vertebral column at T8 is indicated by the arrow. Scale bar = 10 mm.
The lungs were hypoplastic and, the trachea and bronchial tubes were structurally normal. The heart was anatomically normal. Two umbilical arteries and a single umbilical vein were observed. The sternum was short and the nipples were widely spaced. The kidneys were absent, but a urethra and a urinary bladder were present as well as a penis with the cavernosa and glans. The gonads were observed near the inguinal canal. The liver, spleen, and esophagus were present and appeared normal. A stomach could not be clearly identified. There was a blind ending colon with an absent rectum and imperforate anus. An intact perineum was clearly visible.
The upper limbs were both present. The left hand had a transverse palmar crease, fetal pads on the fingers, hypoplastic nails, and clinodactyly of the baby finger. The right hand was windswept with overlapping fingers and hyperconvex nails. The lower limbs were fused and were of the sympus dipus type, whereby two feet were present. The soft tissue of the lower limbs was fused with two femurs, two medial fibulae, two lateral tibiae and laterally positioned patellae, aligning with the type I classification (Lhuaire et al., 2013; Stocker & Heifetz, 1987). The ankles were fused. The vertebral column was disorganized below T8. Hemivertebrae were present and the sacrum and coccyx were absent (Figure 10d). The pubic rami, ischia and ilia appeared normal in structure, but the pelvic outlet was smaller than expected.
3.3. Sex determination
To confirm the sex of these fetuses, in particular for Case 7 because of the absence of external genitalia, PCR analysis of X and Y linked loci was performed. The extraction of DNA proved to be a challenge given the age of the cases, the unknown original fixative and more than 60 years of storage in formalin. As expected, the DNA was sheared and chemically damaged. Isolation of nuclei from the umbilical cord showed negligible staining with DAPI. From this, the original fixative was suspected to have been an alcohol‐based solution and therefore it was possible the DNA had been leached from the cord. Biopsies of the lung and thymus were obtained, as it was less likely the DNA had leached completely from these sources. The tissues were processed using guanidinium isothiocyanate as a denaturant as it more readily digested the tissue than Proteinase K. The extracted DNA was damaged with a modal size of <100 bp. Thus, the size of the PCR products, which could be amplified from the DNA samples, was expected to be limited. Typically, the loci AMELX and AMELY or ZfX and ZfY are used to determine the sex of an individual. It was expected that the PCR products produced would be too large for the quality of the DNA obtained. Attempts to obtain amplification products were unsuccessful. Instead, two short STS loci WI9327 and SY182 from the X and Y chromosomes, respectively, were used. The DNA was nick translated and ligated to repair nicks and gaps before PCR. Amplification products were obtained from the DNA extracted from the thymus with the result that both Case 7 and Case 16 were identified as male (Figure 11).
FIGURE 11.
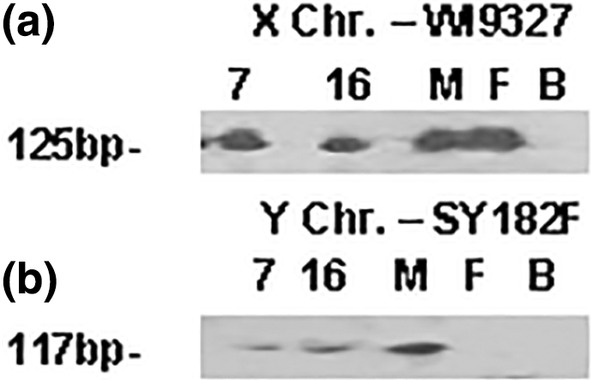
PCR analysis of the X (WI9327) and Y (SY182) chromosomal loci for both Case 7 and Case 16. Amplification products from DNA extracted from the thymus are visible for both loci in each case indicating both are male. M, male control lymphocyte DNA; F, female control lymphocyte DNA; B, PCR blank (no DNA) control.
3.4. Iliac sacral distance and bi‐iliac distance
Hartling et al. (2001) and Kjaer et al. (2003) reported on the finding of a correlation between the iliac sacral distance (ISD) as compared to the crown rump length and the iliac/femoral phenotype in cases of sirenomelia. In their model, a normal ISD would not be observed when complete fusion of the femur was present. As well, a fused femur was associated with decreases in the bi‐iliac distance (BD) of greater than one standard deviation. In this study, the ISD, BD, and crown rump length were measured according to their method. The ISD and BD measurements for Case 16 were consistent with the model proposed by Hartling et al. (2001) (ISD = 56.7 mm, BD = 42.3 mm). However, for Case 7, it was found that the BD was increased to 61.3 mm from the expected normal distance of 43.5 mm, instead of the expected decrease in the presence of a full fusion of the femurs. It was surprising to find that the ISD was normal in Case 7 (39.7 mm) rather than the expected increase (>47.0 mm).
4. DISCUSSION
The external and internal anatomy of two‐term fetuses with sirenomelia and Potter's sequence were evaluated. The findings suggest two different etiologies.
Case 7 has caudal abnormalities with unique features not typically reported in fetuses with sirenomelia. Of particular note is the coincident presence of a complete sacrum and coccyx with a complete fusion of the femurs. In this case, the iliac bones, rather than being oriented at an angle to the spine, are seen to be in parallel, producing a pelvic angle of 180 degrees (Figure 5a). As a result, the acetabulae would be situated together dorsally rather than laterally. The two femoral heads of the single femur were located behind the iliac bones in the position usually occupied by the sacrum and coccyx. The sacrum and coccyx were structurally normal, but curved over the top of the fused femur. The femurs were externally rotated and the patella was oriented laterally rather than anteriorly. Therefore, incorrect orientation of the limb buds with respect to the lower spine may have resulted in the improper orientation and development of distal structures in Case 7.
The other features observed in Case 7 are similar to those documented in other cases of sirenomelia and small pelvic outlet syndrome (SPOS). The shared features of these disorders include imperforate anus, absent or hypoplastic, dysplastic kidneys, absent or dysplastic ureters, bladder and urethra, and malformed or absent external genitalia. The characteristics of sirenomelia and SPOS suggest variable expression of defects of a common developmental field, with the underlying etiology being unknown (Currarino & Weinberg, 1991). Caudal regression syndrome is characterized by spinal malformations ranging from partial or total agenesis of the lumbar and sacral spine; therefore, it is inconsistent with the findings in Case 7.
In contrast to Case 7, Case 16 had both cranial and caudal abnormalities. Both cranial and caudal malformations are reported in the minority of reported cases of sirenomelia and suggest a more pervasive dysplastic phenotype. Russel et al. (1981) have proposed ‘the axial mesodermal dysplasia theory’ to encompass both cranial and caudal anomalies in a single fetus. They described the axial mesodermal dysplasia syndrome as a disturbance during early embryogenesis, which affects mesodermal cell migration during the primitive streak period. They suggested that a disturbance in the cranial migration of cells in the primitive streak would give rise to syndromes affecting cranial structures, and similarly, disturbances in migration to the caudal end would produce defects in caudal structures. They also suggested the presence of both cranial and caudal abnormalities could be attributed to a generalized disturbance in cell migration from the primitive streak with secondary damage or deficiencies to both the cranial and caudal mesoderm regions during early embryonic development. The axial mesodermal dysplasia theory is most consistent with both types of anomalies identified in Case 16.
The vascular steal theory (Stevenson et al., 1986) is unlikely in these cases as both have two umbilical arteries and one umbilical vein. Although both cases have Potter's sequence (Bearn, 1960), this is not believed to cause sirenomelia, but rather, it is a secondary effect of renal agenesis. There was no evidence in the hospital records of either case that parental sub‐fertility, advanced maternal age, or maternal illnesses, such as diabetes, were factors contributing to the etiology. Neither record indicated that these cases were one of twins, thus the monozygotic twinning theory does not appear to apply here.
4.1. Genetic sex determination
Previous attempts at extracting DNA from umbilical cord tissue of these two cases of sirenomelia using protocols that employed proteinase K were unsuccessful. There are many difficulties with the analysis of DNA from archived specimens such as degradation, depolymerization and chemical modification. Analysis of archival DNA is further complicated by the presence of unidentified inhibitors, which can inhibit DNA amplification. From the MRI studies, there was an apparent leaching of the brain of Case 7. The nuclei of the umbilical cords of both cases failed to stain with DAPI during the first attempt with FISH analysis of the X and Y chromosomes. This suggested the initial preservative used was an alcohol‐based solution, and it may have leached DNA out of the more exterior tissues. In this study, samples were harvested from the lung and thymus region because it was hypothesized that the likelihood of leaching from these areas was reduced. By using guanidinium isothiocyanate to digest the tissue and repairing the DNA by nick translation before analysis, it was possible to successfully identify both cases as male. This finding is consistent with the literature that reports an excess of affected males with sirenomelia (Schinzel et al., 1979).
5. CONCLUSIONS
The two fetuses under study were compared based on external and internal anatomy and potential etiologies. Upon comparison of these fetuses, different causative theories that are described in the literature may apply. The characteristics of Case 7 appear more similar to severe forms of the small pelvic outlet syndrome that include sirenomelia, whereas those of Case 16 are more similar to those of the axial mesodermal dysplasia syndrome.
AUTHOR CONTRIBUTIONS
Experimental Design: SLVP, JJM, KJH, CWR, RMLS, SCP, SAMT. Sample Collection and Maintenance: CWR, SCP. Data Collection and Analysis: SLVP, JJM, KJH, CWR, RMLS, LB, SCP, SAMT. Tissue Processing and Genetic Analysis: SLVP, JJM, KJH, SAMT. Manuscript Preparation and Editing: SLVP, JJM, KJH, CWR, RMLS, LB, SCP, SAMT.
FUNDING INFORMATION
This work was supported by grants from the Queen's University Pathology and Molecular Medicine Clinical Trust Fund and the Queen's University Advisory Research Committee.
CONFLICT OF INTEREST STATEMENT
There is no identifiable competing interest in this research project with other organizations.
ETHICS APPROVAL AND CONSENT FOR PUBLICATION
Ethics approval was obtained from the Chief Coroner for the Province of Ontario, Canada to use these specimens for education and research, including permission for publication.
ACKNOWLEDGMENTS
The authors thank Mr. Wayne Lyons and Ms. Donna Situ of the Department of Biomedical and Molecular Sciences for their technical support and assistance on some of the photographic images, and Mr. Lloyd Kennedy of the Clinical Laboratories at the Kingston General Hospital for his assistance in the production some of the digital images used in this manuscript.
Vander Pol, S.L. , MacKenzie, J.J. , Harrison, K.J. , Reifel, C.W. , Smith, R.M.L. , Bale, L. et al. (2024) Sirenomelia: An anatomical assessment and genetic sex determination of two cases. Journal of Anatomy, 244, 1093–1101. Available from: 10.1111/joa.14015
DATA AVAILABILITY STATEMENT
All referenced images are stored in the laboratory of Dr. Stephen Pang at Queen's University and these data can be shared with official requests.
REFERENCES
- Banerjee, A. , Faridi, M.M. , Banerjee, T.K. , Mandal, R.N. & Aggarwal, A. (2003) Sirenomelia. Indian Journal of Pediatrics, 70, 589–591. [DOI] [PubMed] [Google Scholar]
- Bearn, J.G. (1960) The association of sirenomelia with Potter's syndrome. Archives of Disease in Childhood, 35, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.P. , Shih, S.L. , Liu, F.F. & Jan, S.W. (1997) Cebocephaly, alobar holoprosencephaly, spina bifida, and sirenomelia in a stillbirth. Journal of Medical Genetics, 34, 252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currarino, G. & Weinberg, A. (1991) From small pelvic outlet syndrome to sirenomelia. Pediatric Pathology, 11, 195–210. [DOI] [PubMed] [Google Scholar]
- Duhamel, B. (1961) From the mermaid to anal imperforation: the syndrome of caudal regression. Archives of Disease in Childhood, 36, 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartling, U.B. , Fischer Hansen, B. , Skovgaard, L.T. & Kjaer, I. (2001) Bi‐iliac distance and iliac bone position compared to the vertebral column in normal fetal development. American Journal of Medical Genetics, 99, 154–158. [DOI] [PubMed] [Google Scholar]
- Kampmeier, O.F. (1927) On sireniform monsters, with a consideration of the causation and the predominance of the male sex among them. Anatomical Record, 34, 365–389. [Google Scholar]
- Kjaer, K.W. , Keeling, J.W. , Opitz, J.M. , Gilbert‐Barness, E. , Hartling, U. , Hansen, B.F. et al. (2003) Sirenomelia sequence according to the distance between the first sacral vertebra and the ilia. American Journal of Medical Genetics. Part A, 120A, 503–508. [DOI] [PubMed] [Google Scholar]
- Konomi, N. , Lebwohl, E. & Zhang, D. (2002) Comparison of DNA and RNA extraction methods for mummified tissues. Molecular and Cellular Probes, 16, 445–451. [DOI] [PubMed] [Google Scholar]
- Lee, A.W. , Farnquist, B. , Islam, O. , Mackenzie, J. , Taylor, S.A.M. , Pang, S.C. et al. (2012) Noninvasive investigation of asymmetrically conjoined tripus twins with features of rachipagus, parapagus dicephalus, and cephalopagus. Clinical Anatomy, 25, 1023–1029. [DOI] [PubMed] [Google Scholar]
- Lhuaire, M. , Jestin, A. , Boulagnon, C. , Loock, M. , Doco‐Fenzy, M. , Gaillard, D. et al. (2013) Sirenomelia: a new type, showing VACTERL association with Thomas syndrome and a review of literature. Birth Defects Research. Part A, Clinical and Molecular Teratology, 97, 123–132. [DOI] [PubMed] [Google Scholar]
- Onyeije, C.I. , Sherer, D.M. , Handwerker, S. & Shah, L. (1998) Prenatal diagnosis of sirenomelia with bilateral hydrocephalus: report of a previously undocumented form of VACTERL‐H association. American Journal of Perinatology, 15, 193–197. [DOI] [PubMed] [Google Scholar]
- Opitz, J.M. , Zanni, G. , Reynolds, J.F., Jr. & Gilbert‐Barness, E. (2002) Defects of blastogenesis. American Journal of Medical Genetics, 115, 269–286. [DOI] [PubMed] [Google Scholar]
- Puech, P.A. , Boussel, L. , Belfkih, S. , Lemaitre, L. , Douek, P. & Beuscart, R. (2007) DicomWorks: software for reviewing DICOM studies and promoting low‐cost teleradiology. Journal of Digital Imaging, 20, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch, C.M. , Giddings, I. & Scholz, M. (1998) Repair of degraded duplex DNA from prehistoric samples using Escherichia coli DNA polymerase I and T4 DNA ligase. Nucleic Acids Research, 26, 857–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick, L. (1945) Human sirenomelia. Journal of Obstetrics and Gynaecology, 3, 512–515. [DOI] [PubMed] [Google Scholar]
- Rorden, C. & Karnath, H.O. (2004) Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews. Neuroscience, 5, 813–819. [DOI] [PubMed] [Google Scholar]
- Russell, L.J. , Weaver, D.D. & Bull, M.J. (1981) The axial mesodermal dysplasia spectrum. Pediatrics, 67, 176–182. [PubMed] [Google Scholar]
- Samal, S.K. & Rathod, S. (2015) Sirenomelia: the mermaid syndrome: report of two cases. Journal of Natural Science, Biology, and Medicine, 6, 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel, A.A. , Smith, D.W. & Miller, J.R. (1979) Monozygotic twinning and structural defects. The Journal of Pediatrics, 95, 921–930. [DOI] [PubMed] [Google Scholar]
- Shojaee, A. , Ronnasian, F. , Behnam, M. & Salehi, M. (2021) Sirenomelia: two case reports. Journal of Medical Case Reports, 15, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Situ, D. , Reifel, C.W. , Smith, R. , Lyons, G.W. , Temkin, R. , Harper‐Little, C. et al. (2002) Investigation of a cyclopic, human, term fetus by use of magnetic resonance imaging (MRI). Journal of Anatomy, 200, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton, M.P. , Penington, E.C. & Hutson, J.M. (2003) A surviving infant with sirenomelia (mermaid syndrome) associated with absent bladder. Journal of Pediatric Surgery, 38, 1266–1268. [DOI] [PubMed] [Google Scholar]
- Stevenson, R.E. , Jones, K.L. , Phelan, M.C. , Jones, M.C. , Barr, M., Jr. , Clericuzio, C. et al. (1986) Vascular steal: the pathogenetic mechanism producing sirenomelia and associated defects of the viscera and soft tissues. Pediatrics, 78, 451–457. [PubMed] [Google Scholar]
- Stocker, J.T. & Heifetz, S.A. (1987) Sirenomelia. A morphological study of 33 cases and review of the literature. Perspectives in Pediatric Pathology, 10, 7–50. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All referenced images are stored in the laboratory of Dr. Stephen Pang at Queen's University and these data can be shared with official requests.


