Abstract
Anomaluromorpha is a particularly puzzling suborder of Rodentia. Endemic to Africa, this clade includes the extant genera Idiurus, Anomalurus, Zenkerella, and Pedetes. These rodents present an hystricomorphous condition of the skull, characterized by a large infraorbital foramen, which evolved independently within the mouse‐related clade over a span of approximately 57 million years. They exhibit a high disparity in craniomandibular and dental morphology that has kept their phylogenetic affinities disputed for a long time. Given the past significance of masticatory morphotypes in establishing the classification of Rodentia, we propose to explore variations in the masticatory apparatus of Anomaluromorpha in order to evaluate whether its related features can offer additional data for systematics and contribute to our understanding of the complexity of hystricomorphy. In order to do so, we used traditional dissection and diffusible iodine‐based contrast‐enhanced computed tomography (diceCT) to accurately describe and compare the anatomy of the specimens. We found that the muscle morphology displays clear differentiation among each anomaluromorph taxonomic unit. Specifically, the masseteric complex of Anomaluromorpha exhibits distinctive synapomorphies such as the infraorbital part of the zygomaticomandibularis muscle being separated into a rostral and orbital part and an absence of a posterior part of the zygomaticomandibularis. Additionally, the orbital portion of the infraorbital part originates from a well‐marked ridge and fossa at the level of its area of origin on the anteromedial wall of the orbital cavity, a feature that is absent in other members of the mouse‐related clade. This evident bony feature, among others, is strongly associated with muscular anatomy and can contribute to ascertaining the taxonomic status of extinct representatives of the clade. Finally, we showed that the hystricomorphy of Anomaluromorpha largely differs from those of Ctenohystrica and Dipodoidea and that the definition of this morphotype is complex and cannot be reduced simply to the size of the opening of the infraorbital foramen.
Keywords: Anomaluridae, function, masticatory apparatus, morphology, Pedetidae, systematics, Zenkerellidae
The digital dissection of the masticatory musculature of (a) Anomalurus, (b) Idiurus, (c) Zenkerella, and (d) Pedetes reveals a great degree of diversity in morphology. These taxa present a hystricomorphous condition of the skull that largely differs from those of other rodents with an enlarged foramen and reaffirms the significance of the descriptive anatomical features of the masticatory apparatus as a valuable source of information for understanding the evolutionary relationships among closely related taxa.

1. INTRODUCTION
Anomaluromorpha is a rodent suborder of the mouse‐related clade comprising three different families, four genera, and nine extant species restricted to Africa. Anomaluroidea are commonly named “scaly‐tailed squirrels” for showing a unique keratinous structure at the ventral base of their tail, which is hypothesized to help with tree‐climbing or supporting the body while resting in the tree (e.g. Heritage et al., 2016; Kingdon, 2013; Rosevear, 1969). They comprise seven species belonging to the family Anomaluridae with the genera Anomalurus and Idiurus, and Zenkerellidae with the genus Zenkerella. All these species are endemic to forests of western and central Africa (Julliot et al., 1998; Kingdon, 2013). The Anomaluridae are commonly named “flying scaly‐tailed squirrels” due to their remarkable adaptation to gliding (Julliot et al., 1998). Zenkerellidae is an elusive monotypic family, with little knowledge available about its ecological, behavioral, and dietary habits. Biologists encountered Zenkerella only once in its natural environment (Dinets, 2017) and the fact that only 11 specimens are stored in museum collections further contributes to its mysterious nature (Pérez del Val et al., 1995). Finally, the family Pedetidae is monogeneric with its sole representative Pedetes, commonly referred to as “springhares”, distributed in open dry habitats in southern and eastern Africa (Matthee & Robinson, 1997). They are large, nocturnal, bipedal, saltatorial rodents that shelter in burrows during the day.
The monophyly of Anomaluromorpha is strongly supported by molecular data (Fabre et al., 2012; Huchon et al., 2002; Montgelard et al., 2002; Swanson et al., 2019). In contrast, previous attempts to uncover anomaluromorph phylogenetic affinities based solely on morphological data resulted in conflicting hypotheses. For instance, establishing the sister relationship between Anomaluroidea and Pedetidae has proven to be problematic, particularly when dealing with dental features. This is evidenced by the highly derived bilophed cheek tooth pattern of Pedetidae, which limits comparisons with other rodents (Luckett & Hartenberger, 1985). Moreover, the fossil record of Pedetidae is scarce and limited to early Miocene forms discovered in eastern Africa (Lavocat, 1973; Pickford & Mein, 2011), while stem Anomaluroidea are known from the late middle Eocene of South East Asia (Marivaux et al., 2005) and the late Eocene of North Africa (Jaeger et al., 1985). Anomaluromorpha were hypothesized to be related to Theridomyoidea (Lavocat, 1951; Wood, 1955, 1965), an extinct clade of hystricomorphous rodents from the Eocene of Europe (Vianey‐Liaud & Marivaux, 2021), or northern American and Eurasian Eomyidae from the same period (Stehlin & Schaub, 1951). However, paleontological data never ascertained a close connection between Anomaluroidea and Pedetidae due to the limited nature of their fossil record (Jaeger, 1988; Marivaux et al., 2005). Nevertheless, various studies examining the comparative anatomy of cranial structures have consistently found that pedetids and anomalurids exhibit greater similarity to each other than to other rodents, particularly when considering soft tissues. For instance, the morphology of the circulatory system of Anomaluromorpha displays a unique anastomosis between the distal end of the internal carotid artery and the infraorbital branch, which led Bugge (1974, 1985) to group them into the suborder Anomaluromorpha. These similarities are also present in blood vascular patterns, including the presence of a cricetid pattern of the arterial arches and the absence of the azygos vein (George, 1981). Osteologically, the morphology of the auditory region also suggests close relationships between anomaluromorphs (Lavocat & Parent, 1985; Meng, 1990; Ruf et al., 2009).
The scarcity of anatomical studies on Anomaluroidea (de Winton, 1898; Ellerman et al., 1940; Fabre et al., 2018; Panyutina et al., 2020; Parsons, 1899; Potapova, 2018; Tullberg, 1899) led to a lack of comprehensive classifications. Recent studies shed light on the relationships within Anomaluroidea (Anomaluridae + Zenkerellidae) through field acquisition of specimens of Zenkerella insignis (Heritage et al., 2016) and mitogenomic sequencing of museum specimens (Fabre et al., 2018). The superfamily has long been considered at a lower taxonomic level, with the recognition of the family “Anomaluridae” commonly separated into two subfamilies: Anomalurinae with the genus Anomalurus and sometimes the deprecated Anomalurops (see Fabre et al., 2018) and Zenkerellinae including the genera Idiurus and Zenkerella (Ellerman et al., 1940; Wilson et al., 2016). This assumption was established on the basis of superficial craniodental characters (Ellerman et al., 1940; Fabre et al., 2018). Interestingly, a close relationship between Anomalurus and Idiurus, rather than Idiurus and Zenkerella, was only recently proposed based on dental features of living and fossil anomaluroids (Coster et al., 2015). Since then, multiple new autapomorphic features characterizing Zenkerella and shared skeletal features between Anomalurus and Idiurus were also described (Fabre et al., 2018). The origin of Anomaluroidea can be traced back to the Eocene, with an emergence from either the extinct clade Zegdoumyidae (Vianey‐Liaud, 1994; Marivaux et al., 2011, 2015, 2017) or from primitive Myodonta, Castoroidea, and Geomyoidea (Coster et al., 2015). The fossil record of Anomaluroidea is largely limited to a few Miocene localities in eastern Africa (Lavocat, 1973; Pickford & Mein, 2006, 2011), but the earliest occurrences of modern families Anomaluridae and Zenkerellidae were found in late Eocene deposits of North Africa and early Oligocene deposits of northern and eastern Africa (Coster et al., 2015; Marivaux et al., 2017; Sallam et al., 2010). These fossils suggest that anomalurids and zenkerellids diverged in the late Eocene, which is consistent both with fossil‐tip dating and calibrated molecular divergence dates (Fabre et al., 2018; Heritage et al., 2016).
Documenting additional sources of morphological information can hold the key to provide a comprehensive understanding of the evolutionary history of Anomaluromorpha. The general masticatory and skull morphology of rodents served as the fundamental criteria for the establishment of suborder nomenclature in Rodentia by Brandt (1855), following Waterhouse (1839). This typology was maintained for nearly a century (Simpson, 1945) and as such was largely used in systematic works (e.g. Ellerman et al., 1940; Parsons, 1894, 1899; Thomas, 1896; Tullberg, 1899; Winge, 1887). This categorization into three morphotypes was divided into the sciuromorphy, the myomorphy, and the hystricomorphy. Protrogomorphy was later added by Wood (1965) without properly referring to a taxonomic unit. All Anomaluromorpha share an hystricomorphous condition of their skull, exhibiting an enlarged infraorbital foramen facilitating the passage of the anterior‐most portion of the zygomaticomandibularis muscle on the rostrum and lacking a zygomatic plate, which makes the origin of the anterior part of the deep masseter ventral to the zygomatic arch (Figure 1). Within hystricomorphous rodents, Anomaluromorpha are particularly intriguing as their hystricomorphy independently evolved from that of Ctenohystrica, Dipodoidea, and Gliridae (Hautier et al., 2015; Swanson et al., 2019; Wood, 1965) and because the origin of this cranial condition still remains unknown. Consequently, the investigation of their masticatory apparatus holds particular interest, especially due to the limited number of studies that described its anatomy within the clade (Offermans & De Vree, 1989; Parsons, 1898, 1899; Potapova, 2017; Tullberg, 1899), and even fewer that examined it from a morphofunctional perspective (Cox, 2017; Offermans & De Vree, 1993). In this study, we describe and compare the masticatory morphology of the genera Anomalurus, Idiurus, Pedetes, and Zenkerella with both traditional and digital dissections using diffusible iodine‐based contrast‐enhanced computed tomography (diceCT; Gignac et al., 2016) to visualize the spatial arrangement and complexity of the musculature in this group. We then assess the relevance of several potential characters linked to the masticatory morphology for systematics and further discuss the concept of the hystricomorph morphotype and its evolution based on our findings.
FIGURE 1.

Left lateral view of the 3D reconstruction of the skull, mandible, and masticatory musculature of (a) Idiurus macrotis; (b) Anomalurus derbianus; (c) Zenkerella insignis and (d) Pedetes capensis. aDM, anterior deep masseter; apo, aponeurosis; DIG, digastric; ePT, external pterygoid; iPT, internal pterygoid; lT, lateral part of the temporalis; mT, medial part of the temporalis; oioZM, orbital portion of the infraorbital part of the zygomaticomandibularis; oT, orbital part of the temporalis; pDM, posterior deep masseter; ppDM, posterior portion of the posterior deep masseter; rioZM, rostral portion of the infraorbital part of the zygomaticomandibularis; SM, superficial masseter; TM, transverse mandibular; ZM, anterior part of the zygomaticomandibularis. Scale bars are 5 mm.
2. MATERIALS AND METHODS
2.1. Nomenclature and anatomical descriptions
Due to the great diversity of the craniomandibular morphology and methods used to define muscular anatomy in rodents and mammals in general, great differences in the nomenclature of the masticatory muscles are found in the literature (Druzinsky et al., 2011). In this study, the basis of the nomenclature used was provided by Druzinsky et al. (2011), with a notable distinction concerning the location of the insertion of the posterior‐most portion of the pDM, the ppDM. In our study, we recognize this insertion on the lateral surface of the condylar process, deviating from the placement on the lateral surface of the mandible ventral to the condyle defined by the authors (Table 1). Muscle portions were distinguished based on their origin and insertion area on the skull and mandible (Figure 2) as well as on the location of the passage of the masseteric nerve for the deep masseter and zygomaticomandibularis muscles (see Figures S1–S12 for further details). Considering the low specific diversity of Anomaluromorpha and the high disparity in skull morphology, we decided to perform an extensive anatomical description of the masticatory musculature for each taxon, respectively, and a table summarizing the muscles origin, insertion, and orientation of the fibers was made to ease the anatomical comparisons of the discussion (Table 2).
TABLE 1.
Table of the nomenclature and topology of the masticatory musculature adapted from Druzinsky et al. (2011).
| Muscular complex | Name | Abbreviation | Origin | Insertion |
|---|---|---|---|---|
| Masseter complex | M. masseter superficialis | SM | Ventral or ventrolateral surface of the zygomatic arch, anterior root of the zygomatic arch, or maxillary bone | Ventral edge of the mandible |
| M. masseter superficialis, pars reflexa | pr | NA | Fibers of the superficial masseter that attach distally onto the medial surface of the mandible | |
| M. masseter profundus, anterior part | aDM | Anteroventral surface of the zygomatic arch | Lateral surface of the mandible, along the masseteric crest | |
| M. masseter profundus, posterior part | pDM | Posteroventral surface of the zygomatic arch | Lateral surface of the mandible, along the masseteric crest | |
| M. masseter profundus, posterior‐most part | ppDM | Posteroventral‐most surface of the zygomatic arch | Lateral surface of the condyle | |
| M. zygomaticomandibularis, rostral part | rioZM | Lateral surface of the rostrum and medial wall of the infraorbital foramen | Lateral surface of the mandible, via a tendon | |
| M. zygomaticomandibularis, orbital part | oioZM | Lateral surface of the medial wall of the orbit posterior to the infraorbital foramen | Lateral surface of the mandible, via a tendon | |
| M. zygomaticomandibularis | ZM | Medial surface of the zygomatic arch | Lateral surface of the mandible | |
| M. zygomaticomandibularis, posterior part | pZM | Medial surface of the zygomatic arch | Lateral surface of the mandible | |
| Temporal complex | M. temporalis lateralis | lT | Temporal crest via an aponeurosis | Coronoid process of the mandible |
| M. temporalis medialis | mT | Lateral surface of the temporal fossa | Coronoid process of the mandible | |
| M. temporalis orbitalis | oT | Lateral surface of the wall of the orbit ventral to the temporal fossa | Anterior and medial surfaces of the coronoid process | |
| Pterygoid complex | M. pterygoideus internus | iPT | Lateral surface of the alisphenoid | Medial surface of the condyle |
| M. pterygoideus externus | ePT | Lateral surface of the alisphenoid | Medial surface of the angular | |
| Other muscles | M. digastricus | Dig | Ventral surface of the skull, paraoccipital process | Ventral surface of the mandible, mandibular symphysis |
| M. transversus mandibulae | TM | NA | Singular muscle ventral to the digastric attaching to both hemimandibules near the mandibular symphysis |
FIGURE 2.

Skull and mandible of Idiurus macrotis (RMCA 29335) in (a) lateral view, (b) medial view, and (c) ventral view. acm, accoustic meatus; af, accessory foramen; ah, articular head; air, anterior infraorbital ridge; ali, alisphenoid; ann, angular notch; ap, angular process; aub, auditory bulla; bao, basioccipital; bsp, basispenoid; cf, condylar fossa; con, condylar process; cor, coronoid process; drim, dorsal ramus of the zygomatic process of the maxilla; eo, exoccipital; fm, foramen magnum; fo, foramen ovale; fr, frontal; glf, glenoid fossa; hpp, hamular process of the pterygoid; ial, incisor alveolus; if, incisive foramen; inc, incisor; jss, jugal‐squamosal suture; jug, jugal; lac, lacrimal; lfs, lacrimal‐frontal suture; lpr, lower premolar; man, mandibular notch; max, maxilla‐frontal suture; mc, masseteric crest; mfs, maxilla‐frontal suture; mjs, maxilla‐jugal suture; mls, maxilla‐lacrimal suture; msy, mandibular symphysis; nas, nasal; nc, nuchal crest; nms, nasal‐maxilla suture; oc, occipital; occ, occipital condyle; otc, orbital part of the temporal crest; pal, palatine; par, parietal; pms, premaxilla‐maxilla suture; pp, paroccipital process; prem, premaxilla; ptc, posterior part of the temporal crest; ptf, pterygoid fossa; rfo, retromolar fossa; sin, sigmoid notch; smc, secondary masseteric crest; sos, squamosal‐occipital suture; spf, sphenopterygoid fossa; sps, squamosal‐parietal suture; sq, squamosal; vrzm, ventral ramus of the zygomatic process of the maxilla; zmr, zygomasseteric ridge. The anterior infraorbital ridge forms a depression in Anomalurus, Zenkerella, and Pedetes and the lateral crest of the mandibule present in Anomalurus, Zenkerella, and Pedetes is absent in Idiurus. Scale bars are 5 mm.
TABLE 2.
Comparison table summarizing the origin, insertion, and orientation of the masticatory muscles of anomaluromorph rodents.
| Muscles | Disposition | Idiurus macrotis | Anomalurus derbianus | Zenkerella insignis | Pedetes capensis |
|---|---|---|---|---|---|
| SM | Origin | Ventrolaterally on the ventral ramus of the infraorbital process of the maxilla and the zygomatic arch | Ventrolaterally on the ventral ramus of the infraorbital process of the maxilla and the zygomatic arch | Laterally on the anterior infraorbital depression of the ventral ramus of the infraorbital process of the maxilla | Laterally on the anterior infraorbital depression of the ventral ramus of the infraorbital process of the maxilla |
| Insertion | Ventrally on the posterior half of the mandible and ventrolateral surface and tip of the angular process | Ventrally on the posterior half of the mandible and ventrolateral surface and tip of the angular process | Ventrally on the posterior half of the mandible and ventrolateral surface and tip of the angular process | Ventrally on the posterior half of the mandible and ventrolateral part of the angular | |
| Orientation | Mainly posteroventral | Mainly posteroventral | Mainly posteroventral | Mainly posteroventral | |
| pars reflexa | Insertion | Posteriorly on the medial side of the mandible and contact with the iPT | Posteriorly on the medial side of the mandible and contact with the iPT | Posteriorly on the medial side of the mandible | NA |
| Orientation | Mainly posterodorsal | Mainly posterior and slightly ventral | Mainly posterodorsal | NA | |
| aDM | Origin | No attachment on the skull but on the dorsolateral side of the ZM instead | Ventrolaterally on the anterior infraorbital depression | Laterally on the anterior infraorbital depression | Laterally and ventrally on the anterior infraorbital depression |
| Insertion | Laterally on the anterior half of the secondary masseteric crest | Laterally on the anterior half of the masseteric crest | Laterally on the anterior half of the masseteric crest | Laterally on the anterior half of the masseteric crest | |
| Orientation | Mainly posteroventral | Mainly posteroventral | Mainly posterior and slightly ventral | Mainly posterior and slightly ventral | |
| pDM | Origin | Ventrolateral surface of the posterior end of the jugal via a thin aponeurosis | Ventrolateral surface of the posterior end of the jugal via a thin aponeurosis | Laterally and ventrally on the depression of the jugal | Laterally and ventrally on the anterior depression of the jugal |
| Insertion | Laterally on the surface of the angular process | Laterally on the posterior half of the masseteric crest and the lateral surface of the angular process | Laterally on the posterior half of the masseteric crest and the lateral surface of the angular process | Laterally on the posterior half of the masseteric crest and the lateral surface of the angular process | |
| Orientation | Mainly posteroventral | Mainly posteroventral | Mainly posterior and slightly ventral | Mainly posteroventral | |
| ppDM | Origin | NA | NA | NA | Ventrolaterally on the posterior depression of the jugal |
| Insertion | NA | NA | NA | Lateral surface of the condylar process | |
| Orientation | NA | NA | NA | Anteroposterior | |
| rioZM | Origin | Dorsolateral surface of the premaxilla and maxilla and medial side and posterior edge of the dorsal ramus of the infraorbital process of the maxilla | Dorsolateral surface of the rostrum around the premaxilla‐maxilla suture and medial side and posterior edge of the dorsal ramus of the infraorbital process of the maxilla | Dorsolateral surface of the premaxilla and maxilla, medially on the dorsal ramus of the infraorbital process of the maxilla and jugal, and anterior part of the medial side of the jugal | Dorsolateral surface of the premaxilla and maxilla, medially on the dorsal ramus of the infraorbital process of the maxilla and jugal, and anterior part of the medial side of the jugal |
| Insertion | Anterior to the premolar, on the dorsolateral surface of the ascending edge of the diastema | Posterior to the premolar, on the anterior protuberance of the masseteric crest | Anterior to the premolar, on the dorsolateral surface of the ascending edge of the diastema | Anterior to the premolar, on the lateral crest of the mandible along the ascending edge of the diastema | |
| Orientation | Posteroventral, then dorsoventral | Posteroventral, then dorsoventral | Posteroventral, then dorsoventral | Posteroventral, then dorsoventral | |
| oioZM | Origin | Fossa and ridge on the anteromedial surface of the wall of the orbital cavity | Fossa and ridge on the anteromedial surface of the wall of the orbital cavity | Fossa and ridge on the anteromedial surface of the wall of the orbital cavity | Ridge on the anteromedial surface of the wall of the orbital cavity |
| Insertion | Medial side of the tendon of the rioZM | Medial side of the tendon of the rioZM | Medial side of the tendon of the rioZM | Medial side of the tendon of the rioZM | |
| Orientation | Mainly posteroventral | Mainly posteroventral | Mainly posteroventral | Mainly posteroventral | |
| ZM | Origin | Medial side of the jugal and zygomatic process of the squamosal | Medial side of the jugal and zygomatic process of the squamosal | Medial side of the jugal and zygomatic process of the squamosal | Medial side of the jugal and zygomatic process of the squamosal |
| Insertion | Lateral surface and lateral crest of the mandible | Lateral surface and lateral crest of the mandible | Lateral surface of the mandible | Laterally on the lateral crest of the mandible | |
| Orientation | Dorsoventral | Dorsoventral | Dorsoventral | Dorsoventral | |
| pZM | NA | NA | NA | NA | NA |
| lT | Origin | Laterally on the anterior half of the temporal crest, the most dorsal fibers fuse with the mT | Laterally on the two‐thirds of the temporal crest | Laterally on the anterior half of the temporal crest | NA |
| Insertion | Tip and dorsal edge of the coronoid process | Dorsomedial surface of the coronoid process | Tip and dorsal edge of the coronoid process | NA | |
| Orientation | Mainly anteroventral then dorsoventral | Mainly anteroventral then dorsoventral | Mainly anteroventral then dorsoventral | NA | |
| mT | Origin | Lateral surface of the temporal fossa of the squamosal and temporal crest | Lateral surface of the temporal fossa of the squamosal and temporal crest | Lateral surface of the temporal fossa of the squamosal and temporal crest | Lateral surface of the temporal fossa of the squamosal and posterior end of the zygomatic process of the squamosal |
| Insertion | Medially on the dorsal half of the coronoid process | Medially on the dorsal half of the coronoid process | Medially on the dorsal half of the coronoid process | Medially on the dorsal half of the coronoid process | |
| Orientation | Mainly anteroventral then dorsoventral | Mainly anteroventral then dorsoventral | Mainly anteroventral then dorsoventral | Anteroventral | |
| oT | Origin | Medioventral surface of the posterior wall of the orbital cavity | Medioventral surface of the posterior wall of the orbital cavity | Medioventral surface of the posterior wall of the orbital cavity | Medioventral surface of the posterior wall of the orbital cavity |
| Insertion | Medially on the ventral half of the coronoid process and retromolar fossa | Medially on the ventral half of the coronoid process and retromolar fossa | Medially on the ventral half of the coronoid process and retromolar fossa | Medially on the ventral half of the coronoid process and retromolar fossa | |
| Orientation | Mainly dorsoventral | Mainly dorsoventral | Mainly dorsoventral | Anteroventral | |
| iPT | Origin | Lateral surface at the root of the hamular process of the pterygoid | Lateral surface at the root of the hamular process of the pterygoid | Lateral surface at the root of the hamular process of the pterygoid | Lateral and posterior surface at the root of the hamular process of the pterygoid |
| Insertion | Medially in the pterygoid fossa of the mandible | Medially in the pterygoid fossa of the mandible | Medially in the pterygoid fossa of the mandible | Medially in the pterygoid fossa of the mandible | |
| Orientation | Posteroventral and mediolateral | Posteroventral and mediolateral | Posteroventral and mediolateral | Dorsoventral and mediolateral | |
| ePT | Origin | Laterally on the parapterygoid fossa and surface of the alisphenoid | Laterally on the parapterygoid fossa and surface of the alisphenoid | Laterally on the parapterygoid fossa and surface of the alisphenoid | Laterally to the parapterygoid fossa |
| Insertion | Dorsally on the medial side of the condylar process | Dorsally on the medial side of the condylar process | Dorsally on the medial side of the condylar process | Dorsally on the medial side of the condylar process | |
| Orientation | Posteroanterior and mediolateral | Posteroanterior and mediolateral | Posteroanterior and mediolateral | Posteroanterior and mediolateral | |
| Dig | Origin | Encompassing the paraoccipital process | Encompassing the paraoccipital process | Encompassing the paraoccipital process | Encompassing the paraoccipital process |
| Insertion | Posteroventral to the mandibular symphysis with a contact between the anterior bellies | Posteroventral to the mandibular symphysis with a contact between the anterior bellies | Posteroventral to the mandibular symphysis with a contact between the anterior bellies | Posteroventral to the mandibular symphysis with a contact between the anterior bellies | |
| Orientation | Anteromedial then posteroanterior | Anteromedial then posteroanterior | Anteromedial then posteroanterior | Anteromedial then posteroanterior | |
| TM | Insertion | Posteroventral edge of the anterior part of the corpus of the two hemimandibles | Posteroventral edge of the anterior part of the corpus of the two hemimandibles | Posteroventral edge of the anterior part of the corpus of the two hemimandibles | – |
| Orientation | Mediolateral | Mediolateral | Mediolateral | NA |
2.2. Data acquisition, dissection and processing
A total of eight adult specimens belonging to five species of the suborder Anomaluromorpha were studied. Five of them are spirit anomalurid specimens loaned from the Royal Museum for Central Africa, Tervuren, Belgium, and the National History Museum, London, UK. They correspond respectively to the species Anomalurus derbianus (RMCA 325 and RMCA 21804), Idiurus macrotis (RMCA 38520 and RMCA 29335), and Idiurus zenkeri (NHMUK 32439). Two other specimens belong to the species Pedetes capensis: one specimen (personal collection from A. Herrel) came from the Museum National d'Histoire Naturelle, Paris, France, and the other one (unregistered specimen from the University of South Bohemia, České Budějovice) was collected frozen from the Plzeň Zoo, Plzeň, Czech Republic and stored at the Institut des Sciences de l'Evolution, University of Montpellier, Montpellier, France. The frozen specimen from the Plzeň Zoo was fixed in phosphate‐buffered formal saline solution (paraformaldehyde dissolved at 5% solution with phosphate‐buffered saline) for long‐term preservation with limited tissue shrinkage and then stored in 70% ethanol. The remaining specimen (NHMUK 552327) corresponds to Zenkerella insignis and comes from the National History Museum, London, UK.
Careful traditional dissection of the masticatory muscles was carried out using a Leica S9i stereomicroscope with ×10 eyepieces in order to document our observations on muscle origin and insertion areas, as well as muscle fiber orientation. The specimen of Zenkerella was excluded from the traditional dissections due to its great rarity both in museum collections and in the wild. All major steps of dissections were photographed using the integrated 10MP digital camera of the stereomicroscope and a Nikon D750 with a AF‐S Micro Nikkor 105 mm 1:2.8G ED lens fixed on a copy‐stand to visualize the relative muscle configuration on the complete specimens. The separation of individual muscles was done primarily according to their area of origin and insertion on the skull and the mandible. The relative position, orientation of muscle fibers, and the innervation pattern of the masseteric branch of the trigeminal nerve were used to separate parts of the different muscle groups from each other. For instance, the passage of the masseteric nerve serves as a demarcation between the aDM and pDM (Figures S2, S5, S8 and S11). The ioZM was differentiated from the ZM by the presence of the insertion tendon and the specific location where the zygomatic branch of the masseteric nerve entered the muscular fibers (Figures S3, S6, S9 and S12). Given that the masseteric nerve lies posterior to the posterior end of the ZM and the lack of noticeable differences in fiber orientation, we consider that the pZM is not individualized in anomaluromorph rodents (Figure 3 and Figures S6, S9 and S12). The dissection of both species of Idiurus revealed an identical muscular configuration.
FIGURE 3.

Comparative digital dissection of the superficial and deep masseter muscles of anomaluromorph rodents. Left lateral view of the 3D reconstruction of: (a, b) Idiurus macrotis; (c, d) Anomalurus derbianus; (e, f) Zenkerella insignis and (g, h) Pedetes capensis. Definition of the abbreviations can be found in the legend of Figure 1. Scale bars are 10 mm.
2.3. Three‐dimensional (3D) imaging and reconstruction
The best‐preserved individual of each species was chosen to perform diceCT, a method which consists in performing a contrast‐enhanced micro‐computed tomography (microCT) using Lugol iodine (I2KI) for visualizing muscle and bone configuration non‐destructively, as well as comparing the results with observations made during traditional dissections. Lugol iodine was chosen as a staining agent as it is both cost‐effective, offers excellent resolution of muscle fibers, and works effectively on formalin‐fixed specimens (Gignac et al., 2016). The specimens were put in sealed containers filled with 5% I2KI and with a large volume of solution to ease the diffusion process. The staining time depended on the size of the specimen: A. derbianus and I. macrotis took 3 weeks to stain and P. capensis and Z. insignis 4 weeks. All three specimens were scanned twice, before and after the contrast‐enhancement process, with image parameters optimized for each specimen using an EasyTom 150 X‐Ray microtomograph hosted at the Institut des Sciences de l'Évolution de Montpellier, University of Montpelier (MRI; ISE‐M, Montpellier, France). The independent imaging of the bone and muscles eased the segmentation process of the skull and enabled us to segment the skull from the bone‐optimized scans and align it back with the muscles‐optimized scans automatically with the use of the “Magic Wand” tool and the “Register Images” module respectively in the Avizo software. In turn, the muscles were segmented manually due to the extensive interconnection of fibers and the low to non‐existent variation in grayscale values between the different muscle bundles. When well‐developed and distinguishable in the diceCT scans, the aponeuroses and tendons were differentiated from muscle fibers. The incremental segmentation of each muscle was done every 10 slices for Anomalurus and Idiurus, and every 20 slices for Pedetes and Zenkerella. The image data and pre‐segmented slices of the muscles were then imported to Biomedisa (Lösel et al., 2020) with the “all axes” parameter selected in the label field. The results were retrieved after selecting the cleaned and smoothed parameters, and then, minor adjustments were made to correct for errors that happened in the semi‐automatic image segmentation back in our segmentation software. Three‐dimensional surface models of the bone and muscles were created, then down‐sampled, to minimize file size and ease the visualization of the structures. They are available on MorphoMuseuM.
2.4. Mapping of potentially phylogenetic characters
The structure of the phylogenetic trees presented by Heritage et al. (2016) and Fabre et al. (2018) served as the basis for illustrating the phylogenetic relationships within Anomaluromorpha (Figure 8). Due to the limited generic diversity of the clade, these tree structures were simplified at the genus level. We chose to hand draw them directly, as branch lengths were irrelevant for our objective. Then, some notable craniomandibular and muscular traits were identified from both the results section and Table 2 and were directly used by superimposing them onto the pruned phylogeny of Anomaluromorpha. This process aimed to depict the evolution of some characters and evaluate their potential phylogenetic significance.
FIGURE 8.
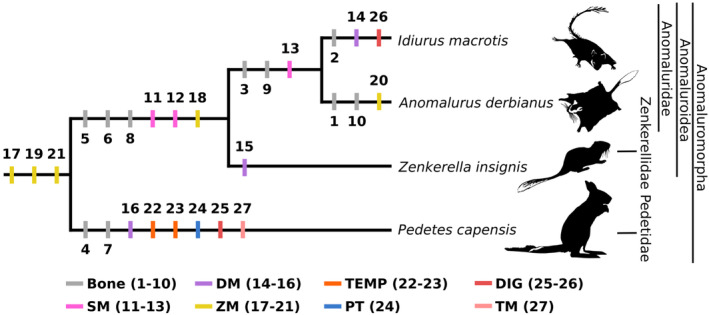
Mapping of the cranial and muscular characters of the masticatory morphology in the simplified phylogeny of Anomaluromorpha. (1) no anterior shift of the ventral ramus of the zygomatic process of the maxilla; (2) absence of an anterior infraorbital depression; (3) absence of an anterior jugal depression; (4) presence of an anterior and posterior jugal depression; (5) presence of a ridge or fossa on the anteromedial surface of the wall of the orbit; (6) developed angular process; (7) reduced coronoid process; (8) presence of a mandibular notch; (9) absence of contact between the lacrimal and jugal; (10) decreased size of the infraorbital foramen; (11) origin on the whole zygomatic arch; (12) pars reflexa present; (13) pars reflexa in contact with the medioventral surface of the iPT; (14) DM reduced; (15) DM developed; (16) ppDM present; (17) rioZM passing through the infraorbital foramen; (18) rioZM reduced; (19) oioZM present; (20) oioZM developed; (21) pZM not individualized; (22) lT absent; (23) mT reduced; (24) iPT developed; (25) interdigastic tendon developed; (26) interdigastic tendon reduced; (27) TM absent. Definition of the abbreviations can be found in the legend of Figure 1.
3. RESULTS
3.1. Idiurus macrotis and Idiurus zenkeri
3.1.1. Masseter complex
M. Masseter superficialis
The origin of the SM is on the ventrolateral surface of the zygomatic arch via an aponeurosis expanding from the anterior edge of the ventral ramus of the zygomatic process of the maxilla, at the level of the anterior infraorbital ridge, to the mid‐length of the jugal, where it fuses with the fibers of the muscle. The muscle fibers then attach to around the mid‐length jugal‐squamosal suture. Its insertion takes place on the ventrolateral side of the mandible and ventrally to the masseteric crest from the anterior end of the masseteric crest to the posterior tip of the angular process (Figure 3 and Figures S1, S4). On the medial side of the mandible, the pars reflexa is well‐developed and in contact with the ventral half of the iPT. The aponeurosis and muscle fibers is oriented posteroventrally in lateral view and posterodorsally for the pars reflexa (Figure 7).
FIGURE 7.

Comparative digital dissection of the muscles inserting on the medial side of the mandible of anomaluromorph rodents. The 3D reconstruction is in medial view. (a) Idiurus macrotis; (b) Anomalurus derbianus; (c) Zenkerella insignis and (d) Pedetes capensis. Definition of the abbreviations can be found in the legend of Figure 1. Scale bars are 10 mm.
M. Masseter profundus
The aDM does originate on the zygomatic arch via a very thin aponeurosis and forms an imprint on the dorsolateral part of the zygomaticomandibularis muscle. The insertion of the muscle expands posterodorsally from the slight protuberance located at the anterior end of the masseteric crest to the posterior end of the secondary masseteric crest. The orientation of the fibers is posteroventral (Figure 3 and Figures S2 and S5).
The pDM originates from the lateral surface of the jugal by an aponeurosis, with no muscle fibers underneath. The area of origin expands from the mid‐length of the jugal bone to the posterior end of the zygomatic process of the squamosal. The anterior‐most part of the muscle attaches on the ZM. As for its insertion, the muscle follows the posterior half of the masseteric crest before ending laterally and on the tip of the well‐developed angular process. The fibers are oriented posteroventrally (Figure 3 and Figures S2 and S5).
M. Zygomaticomandibularis
The rioZM is located in the anterior part of the skull. It is in contact with the premaxilla and maxilla, on the upper half of the surface immediately anterior to the infraorbital foramen as well as on the anterior edge and medial side of the dorsal ramus of the zygomatic process of the maxilla. The muscle expands from around the level of the posterior upper incisor root and follows the nasal‐maxilla suture until it reaches the anterior edge of the dorsal ramus of the zygomatic process of the maxilla. Posterior to the infraorbital foramen, the muscle also originates on the postero‐medial edge of the dorsal ramus of the zygomatic process of the maxilla until it reaches the maxilla‐jugal suture. This also corresponds to the area where the zygomatic branch of the masseteric nerve reenters the muscle. It inserts on the mandible via a strong tendon on a rugosity located anterior to the lower premolar; anterior and lateral to the surface of the ascending edge of the diastema. The muscle fibers originating on the rostrum appear to be bipennate: they are posteroventral and anteroventral, respectively posteriorly and anteriorly to the aponeurosis expanding from the tendon of insertion, which is itself oriented dorsoventrally (Figure 4 and Figures S3 and S6).
FIGURE 4.

Comparative digital dissection of the zygomaticomandibularis muscle of anomaluromorph rodents. Left lateral and dorsolateral view of the 3D reconstruction of: (a, b) Idiurus macrotis; (c, d) Anomalurus derbianus; (e, f) Zenkerella insignis and (g, h) Pedetes capensis. Definition of the abbreviations can be found in the legend of Figure 1. Scale bars are 10 mm.
The oioZM takes its origin on the surface of the anteromedial side of the wall of the orbit; on the ridge intersecting the maxilla‐lacrimal and lacrimal‐frontal sutures. It attaches to the surface of the maxilla, lacrimal, and frontal bones and expands well dorsally, nearly reaching the antero‐dorsal edge of the orbit. Its insertion is on the medial side of the rioZM insertion tendon. The muscle fibers and the tendon are oriented posteroventrally and slightly ventrolaterally (Figure 4 and Figures S3 and S6).
The ZM originates from the medial side of the jugal and expands posteriorly on the zygomatic arch from the maxilla‐jugal suture to the dorsal surface of the zygomatic process of the squamosal, above the glenoid fossa. The muscle inserts laterally on the tendon of the rioZM and the mandible, from the surface anteroventral to the lower premolar to the dorsolateral surface of the condylar process, ventral to the anterior end of the articular head. The orientation of the fibers is dorsoventral anteriorly, then shifts slightly anteroventrally posteriorly (Figure 4 and Figures S3 and S6).
3.1.2. Temporalis complex
M. Temporalis lateralis
The lT partially anastomoses with the dorsal fibers of the medial part of the muscle. This configuration does not allow for the complete separation of the lT both in traditional and digital dissection. Thus, the muscle originates from the anterodorsal part of the mT, from around the level of the posterior edge of the jugal‐squamosal suture, and it inserts on the tip and dorsal edge of the coronoid process. The orientation of the muscle fibers is anterolateral anteriorly and slightly anteroventral at the level of the posterior part of the temporal fossa and then shifts dorsoventrally more posteriorly at the junction between the wall of the orbit and the orbital part of the temporal fossa (Figures 5 and 7).
FIGURE 5.

Comparative digital dissection of the temporalis muscle of anomaluromorph rodents. Left lateral and anterolateral view of the 3D reconstruction of: (a, b) Idiurus macrotis; (c, d) Anomalurus derbianus; (e, f) Zenkerella insignis and (g, h) Pedetes capensis. Definition of the abbreviations can be found in the legend of Figure 1. Scale bars are 10 mm.
M. Temporalis medialis
The mT muscle originates in the temporal fossa. It attaches along the temporal crest via an aponeurosis and on the two‐thirds of the squamosal from the squamosal‐parietal and squamosal‐occipital sutures to the anterior end of the orbital part of the temporal crest. It inserts medially on the posterior and dorsal half of the coronoid process via a tendon. The muscle fibers lying in the temporal portion of the muscle are anterolateral and slightly ventral close to the origin and progressively become more anteroposterior as they draw near to the ventral edge of the muscle. The fibers in contact with the posterior wall of the orbit are oriented dorsoventrally and slightly anteriorly. Laterally, the muscle slightly covers the root of the zygomatic process of the squamosal dorsally to the glenoid fossa (Figures 5 and 7).
M. Temporalis orbitalis
The oT muscle originates from the medial surface of the posterior wall of the orbit cavity. The fibers of the muscle insert on the medioventral surface of the coronoid process and the retromolar fossa, laterally to the third upper molar. The orientation of the fibers is dorsoventral and slightly anteroventral (Figures 5 and 7).
3.1.3. Pterygoid complex
M. Pterygoideus internus
The origin of the iPT is close to the sphenopterygoid fossa located on the medial part of the alisphenoid. It attaches laterally on a rugose surface at the root of the hamular process of the pterygoid and inserts in the pterygoid fossa of the mandible. The orientation of the fibers is posteroventral and mediolateral (Figure 6).
FIGURE 6.

Comparative digital dissection of the digastric muscle and transverse mandibular muscle (left) and pterygoid muscle (right) of anomaluromorph rodents. The 3D reconstruction of the skull is in ventral view and the mandible in medial view. (a, b) Idiurus macrotis; (c, d) Anomalurus derbianus; (e, f) Zenkerella insignis and (g, h) Pedetes capensis. The transverse mandibular (TM) of Idiurus and Anomalurus was removed during the dissection and is not illustrated here (see Figure S4 for dissection photos). Definition of the abbreviations can be found in the legend of Figure 1. Scale bars are 10 mm.
M. Pterygoideus externus
The ePT takes its origin in the sphenopterygoid fossa and on the lateral surface of the alisphenoid. It expands posteriorly to the third upper molar, where it is in contact with the iPT, to anterodorsal to the external auditory meatus of the auditory bulla in lateral view. It inserts on the medial side of the mandible, on the dorsal surface of the medial side of the condylar process, extending from the mandibular notch to posterior to the articular surface of the condylar process. The orientation of the fiber is posteroanterior and mediolateral (Figure 6).
3.1.4. Other muscles of the masticatory apparatus
M. Digastricus
The origin of the posterior belly is on the surface of the paraoccipital process and covers its medial and lateral side with a strong aponeurosis. The anterior belly inserts on the anteroventral surface of the mandible, on the posterior end of the unfused mandibular symphysis. The orientation of the fibers is anteroposterior and slightly medial for the posterior belly and anteroposterior for the anterior belly. The posterior belly is larger than the anterior belly and the left and right anterior bellies are in contact with their medial margins (Figure 6 and Figure S13).
M. Transversus mandibulae
The TM is well‐developed and attaches on the anteroventral part of the mandible, at the level of the mandibular symphysis, and follows the ventral edge of the bone posteriorly until it reaches the anterior part of the hyoid. The morphology of the muscle resembles an inverted “V” and as such seems to be separated in two parts posteriorly. The muscle fibers are mediolaterally oriented (Figure S13).
3.2. Anomalurus derbianus
3.2.1. Masseter complex
M. Masseter superficialis
The muscle originates anteriorly and laterally to the depression located ventrally on the ventral ramus of the zygomatic process of the maxilla, the anterior infraorbital depression, via a flat tendon, and extends posteriorly along the lateral side of the zygomatic arch until it reaches approximately the mid‐length of the jugal‐squamosal suture. Its insertion is located on the ventral edge of the mandible to the posterior tip of the angular process (Figure 3 and Figures S7). The pars reflexa is developed on the medial side of the angular process of the mandible and it overlaps medially with the most ventral part of the iPT. The orientation of the muscle fibers is first posteroventral laterally, then shifts anteroposteriorly close to the ventral side of the angular process. The fibers of the pars reflexa are oriented posterodorsally (Figure 7).
M. Masseter profundus
The aDM originates ventrolaterally to the zygomatic arch via an aponeurosis. The origin of the aDM is medial to the tendon of origin of the SM, on the medial side of the anterior infraorbital depression, as well as the ventrolateral surface of the anterior half of the jugal bone. It inserts on the anterior half of the masseteric crest. The fibers are oriented posteroventrally (Figure 3 and Figure S8).
The pDM originates from an aponeurosis located on the ventrolateral surface of the posterior end of the maxilla‐jugal suture, which expands posteriorly until it reaches the level of the mid‐length of the jugal‐squamosal suture. Its insertion on the posterior half of the masseteric crest as well as the lateral surface and dorsal tip of the angular process. The fibers are oriented posteroventrally near the origin then curve anteriorly around the mid‐height of the muscle. They become almost completely dorsoventral near the insertion area (Figure 3 and Figure S8).
M. Zygomaticomandibularis
Anterodorsally to the infraorbital foramen, the rioZM is reduced and it originates from the dorsal half of the surface immediately anterior to the infraorbital foramen, at the level of the suture between the premaxilla and the maxilla. Posterior to the infraorbital foramen, the muscle takes its origin on the posteromedial edge of the dorsal ramus of the zygomatic process of the maxilla. The fibers converge towards a strong tendon that inserts on the rugose surface anterior to the lateral crest and masseteric crest of the mandible. The muscle is unipennate and the orientation of the fibers is posteroventral and slightly lateromedial (Figure 4 and Figure S9).
The oioZM is well‐developed; it is laterally in contact with the rioZM. It originates from the fossa on the anteromedial surface of the wall of the orbital cavity and on a well‐developed ridge dorsal to the lacrimal‐maxilla and maxilla‐frontal sutures. It attaches to the lacrimal, maxilla, and frontal bones. It inserts on the medial side of the rioZM tendon of insertion. The orientation of the fibers is posteroventral and slightly ventrolateral as they converge towards the tendon of insertion of the rioZM (Figure 4 and Figure S9).
The ZM originates anteriorly from the maxilla‐jugal suture, on the medial surface of the jugal, and extends posteriorly until it reaches the zygomatic process of the squamosal, immediately anterior to the glenoid fossa dorsally and at the posterior end of the jugal more laterally. It inserts partially on the rioZM tendon of insertion as well as on the lateral surface of the mandible, from the anterior edge of the first lower molar to the posterior end of the glenoid fossa, on the condylar process and ventral to the coronoid process. The orientation of the fibers is dorsoventral (Figure 4 and Figure S9).
3.2.2. Temporalis complex
M. Temporalis lateralis
The lT is well‐developed and originates from a large aponeurosis expanding along the orbital and posterior part of the temporal crest from the level of the center of the external acoustic meatus to the posterior end of the jugal‐squamosal suture. It inserts on the tip and slightly on the dorsomedial surface of the coronoid process. The orientation of the fibers of the posterior part of the muscle is oblique, essentially anteroventral, and slightly lateral at the level of the temporal fossa, before shifting dorsoventrally and slightly medially from the junction of the temporal fossa with the posterior wall of the orbit for the more anterior fibers. The muscle partially covers the mT (Figures 5 and 7).
M. Temporalis medialis
The mT takes its origin on the surface of the temporal fossa, and its fibers are attached to the temporal and nuchal crests from the squamosal‐parietal and squamosal‐occipital sutures to the anterior end of the orbital part of the temporal crest. Its insertion is located on the dorsal half of the medial side of the coronoid process. The part of the muscle attached to the temporal fossa has fibers oriented anterolaterally and slightly ventrally along its origin before becoming anteroposterior in the ventral part of the muscle, along its lower limit. The orientation of the fibers in contact with the posterior wall of the orbit is dorsoventral and slightly anterior (Figures 5 and 7).
M. Temporalis orbitalis
The oT originates from the medial surface of the posterior wall of the orbital cavity and inserts on the medioventral surface of the coronoid process and in the retromolar fossa. The fibers are oriented dorsoventrally and slightly anteriorly. The muscle partially merges with the medial border of the mT (Figures 5 and 7).
3.2.3. Pterygoid complex
M. Pterygoideus internus
The origin of the iPT is located on the rugose surface at the root of the hamular process of the pterygoid process, located medially to the sphenopterygoid fossa. It inserts in the pterygoid fossa of the mandible. The orientation of the iPT fibers is posteroventral and mediolateral (Figure 6).
M. Pterygoideus externus
The ePT originates from the lateral surface of the sphenopterygoid fossa and the alisphenoid. It expands from an area posterior to the third upper molar to an area anterior to the dorsal edge of the external meatus of the auditory bulla. Its insertion is located on the medial side of the mandible, on the dorsal surface of the medial side of the condylar process, and the muscle extends slightly posteriorly from the articular surface of the condylar process. The orientation of the fibers is anteroposterior and mediolateral (Figure 6).
3.2.4. Other muscles of the masticatory apparatus
M. Digastricus
The posterior belly is slightly larger than the anterior belly; it originates from a well‐developed paraoccipital process of the skull and encompasses it on its medial, lateral, and dorsal sides. The muscle is cone‐like in shape, larger at its origin than its insertion area on the interdigastric tendon. The anterior belly is attached to the anteroventral part of the mandible, posteroventral to the unfused mandibular symphysis. Unlike the posterior belly, the anterior belly is flatter and more rectangular. The fibers are oriented anteroposteriorly and slightly medially for the posterior belly and anteroposteriorly for the anterior belly. The left and right anterior bellies are in contact on their medial margins (Figure 6 and Figure S13).
M. Transversus mandibulae (TM)
The TM is developed and attaches to the two hemimandibles, from the posteroventral edge of the mandibular symphysis to the mid‐length between the posterior edge of the mandibular symphysis and the angular process. The orientation of the fibers is mediolateral.
3.3. Zenkerella insignis
3.3.1. Masseter complex
M. Masseter superficialis
The muscle takes its origin anteriorly and laterally on the anterior infraorbital depression via a strong tendon that becomes progressively thinner posteriorly as it reaches the fibers of the muscle located more ventrally. The aponeurosis of origin likely extends posteriorly on the lateral surface of the zygomatic arch due to the position of the posterior fibers of the muscle and the similarity in the morphology of the zygomatic arch with Idiurus, but the retention of the animal skin and facial muscles did not allow to distinguish the latter during the digital dissection. The SM inserts ventrally to the masseteric crest, along the ventral edge of the mandible from the level of the first lower molar to the posterior end of the angular process (Figure 3). Medially, the pars reflexa isn't well‐developed posterodorsally on the mandible; it has a small overlapping area with the anteroventral part of the iPT. The fibers on the lateral side of the muscle are mainly posteroventrally oriented. The fibers of the pars reflexa are posterodorsally oriented (Figure 7).
M. Masseter profundus
The aDM is well‐developed and originates from the anterior infraorbital depression, medial to the SM, and ends at the level of the anteriormost margin of the depression located on the lateral surface of the posterior half of the jugal. It inserts laterally on the anterior half of the masseteric crest on the mandible. The orientation of the fibers is slightly posteroventral (Figure 3).
The pDM is well‐developed and originates from the well‐marked posterior jugal depression located on the lateral surface of the posterior half of the jugal bone, from its anterior margin to the level of the mid‐length of the jugal‐squamosal suture. It inserts on the posterior half of the masseteric crest and on the posterior tip of the angular process. The fibers are oriented posteroventrally (Figure 3).
M. Zygomaticomandibularis
The rioZM is extremely developed on the rostrum. Anterior to the infraorbital foramen, it originates close to the anterior edge of the nasal‐premaxilla suture and extends posteriorly on the surface of the premaxilla and maxilla until it attaches on the medial side of the dorsal ramus of the zygomatic process of the maxilla. Posterior to the infraorbital foramen, the muscle attaches on the anterior part of the medial side of the jugal bone to the level of the posterior edge of the upper premolar. The muscle inserts via a wide tendon on the dorsolateral surface of the ascending edge of the diastema. The muscle appears to be unipennate and the orientation of the fibers is slightly posteroventral (Figure 4).
The oioZM is well‐developed and originates from the anteromedial surface of the orbital cavity, immediately posterior to the infraorbital foramen. It follows a shallow ridge ventral to the maxilla‐lacrimal suture and along the maxilla‐frontal suture. It attaches to the surface of the maxilla and frontal bones. It inserts on the medial side of the tendon of insertion of the rioZM. The orientation of the fibers is primarily dorsoventral with a slight posterolateral component (Figure 4).
The ZM takes its origin on the medial side of the jugal. It extends from the mid‐length between the posterior end of maxilla‐jugal suture and the anterior end of the jugal‐squamosal suture to the medial side of the zygomatic process of the squamosal, immediately anterior to the glenoid fossa dorsally and near the posterior end of the jugal laterally. It partially inserts on the lateral side of the ioZM insertion tendon as well as the lateral surface of the mandible; which extends from the level of the anterior end of the first lower premolar to the posterodorsal side of the condylar process. The orientation of the fibers is dorsoventral (Figure 4).
3.3.2. Temporalis complex
M. Temporalis lateralis
The lT is developed and takes its origin along the orbital part of the temporal crest, from anterior to the frontal–parietal suture to the mid‐length of the temporal crest, at the level of the posterior edge of the jugal‐squamosal suture. It inserts on the tip and dorsal edge of the coronoid process. The orientation of the fibers is anterolateral at the level of the temporal fossa, then ventral and slightly medial as it reaches the junction with the temporal fossa and the posterior wall of the orbit. The muscle partially covers the mT and anastomoses with it medially (Figures 5 and 7).
M. Temporalis medialis
The mT originates from the surface of the temporal fossa of the squamosal and the temporal crest. It extends from the squamosal‐parietal and squamosal‐occipital sutures to the level of the mid‐length of the anterior end of the orbital part of the temporal crest. It inserts on the posteromedial side of the dorsal half of the coronoid process. The orientation of the fibers located on the surface of the temporal fossa shifts from anterolateral near the temporal crest to anteroposterior close to the zygomatic process of the squamosal. The orientation of the fibers in contact with the wall of the orbit is dorsoventral (Figures 5 and 7).
M. Temporalis orbitalis
The oT is reduced and is in contact with the medial edge of the mT. It originates from the posteromedial surface of the posterior wall of the orbital cavity and inserts on the medioventral surface of the coronoid process as well as in the retromolar fossa. The fibers are oriented dorsoventrally and slightly anteroventrally (Figures 5 and 7).
3.3.3. Pterygoid complex
M. Pterygoideus internus
The origin of the iPT is located medially to the sphenopterygoid fossa, on the rugose surface at the root of the hamular process of the pterygoid and it inserts medially on the pterygoid fossa of the mandible. The orientation of the iPT fibers is posteroventral and mediolateral (Figure 6).
M. Pterygoideus externus
The ePT originates from the sphenopterygoid fossa and the lateral surface of the alisphenoid and covers the alisphenoid‐squamosal suture. It extends antero‐posteriorly from the posteriormost edge of the palatine‐maxilla suture to the anterodorsal edge of the external acoustic meatus. The insertion area is medial on the surface of the condylar process of the mandible, immediately ventral to the articular head. Some fibers are attached to the dorsal and posterior edges of the mandibular and angular notches. The orientation of the fibers is posteroanterior with a slight dorsal component and mediolateral (Figure 6).
3.3.4. Other muscles of the masticatory apparatus
M. Digastricus
The posterior belly is cone‐like in shape, wider posteriorly than anteriorly. It takes its origin on the paraoccipital process, completely covering it dorsoventrally and mediolaterally, and is connected by a medium‐sized interdigastric tendon to the anterior belly. The anterior belly is flatter and inserts on the anteroventral rugose surface on the mandible, immediately posteroventral to the unfused mandibular symphysis. The orientation of the fibers is anteromedial for the posterior belly and anteroposterior for the anterior belly. The left and right anterior bellies are in contact on their medial margins (Figure 6).
M. Transversus mandibulae
The muscle is well‐developed and attaches on the anterior part of the two hemimandibles, from the posteroventral edge of the mandibular symphysis to the mid‐length between the posterior edge of the mandibular symphysis and the angular process. The fibers are mediolaterally oriented (Figure 6).
3.4. Pedetes capensis
3.4.1. Masseter complex
M. Masseter superficialis
As a whole, the muscle originates from a wide tendon attached on the anterior infraorbital depression, from the level of the mid‐point of the upper diastema to the anteroventral edge of the maxilla‐jugal suture. The muscle fibers expand posteroventrally from the tendinous origin, before ending up almost parallel to the ventral part of the mandible posteriorly. The ventral‐most fibers of the muscle insert on the ventral surface of the mandible, ending with a small insertion area on the ventrolateral surface and the posterior tip of the angular process (Figure 3 and Figure S10). The pars reflexa is absent (Figure 7).
M. Masseter profundus
The aDM takes its origin via two distinct aponeuroses observed during the dissection, laterally and ventrally on the anterior infraorbital depression. The first aponeurosis expands from the anteroventral edge of the anterior infraorbital depression to immediately posterior to the maxilla‐jugal suture on the lateral surface of the jugal. The second aponeurosis expands ventrally from the anterior edge to the posterior edge of the anterior infraorbital depression. The fibers attached to these aponeuroses fully merge close to their origin and insert laterally on the mandible, from the surface located just posterior to the anterior edge of the masseteric crest to a well‐developed rugosity located at the mandibular mid‐length on the crest. The dorsal half of the fibers are oriented posteroventrally and the ventral half dorsoventrally (Figure 3 and Figure S11).
Due to the presence of darkened muscle fibers and conjunctive tissues posteroventral to the orbit, the pDM appeared as a single muscular bundle during the dissection. But according to the digital dissection, the posterior part of the deep masseter revealed an additional part located posteriorly, the ppDM. The pDM takes its origin laterally on the lateral surface of the anterior depression of the jugal, from the maxilla‐jugal suture to the junction between the anterior and posterior jugal depressions. It inserts laterally on the mandible from a tubercle located at the mid‐length of the masseteric crest to two different surfaces. The two areas of insertions are on the posterior half of the masseteric crest and, posterodorsally to it, on a well‐developed ridge with muscle fibers which forms a bulge covered by a strong, superficial aponeurosis. The fibers of the pDM have a fan‐shaped appearance; shifting orientation anteroposteriorly from posteroventral to dorsoventral (Figure 3 and Figure S11).
The ppDM takes its origin on the posterior jugal depression located posteroventrally on the jugal and inserts on the lateral surface of the condylar process of the mandible. The fibers are anteroposteriorly oriented (Figure 3 and Figure S11).
M. Zygomaticomandibularis
The rioZM takes its origin on the dorsal half of the well‐developed rostral fossa located on the premaxilla and maxilla bones. It expands from the anterior edge of the rostral crest located along the premaxilla and maxilla, ventrolaterally to the nasal‐premaxilla suture, to the anterior edge and ventral surface of the dorsal ramus of the infraorbital process of the maxilla. Posterior to the infraorbital foramen, the muscle attaches on the medial side of the jugal until it reaches the level of the anterior end of the first molar. It inserts dorsally on the lateral surface of the mandible, from the anterior end of the lateral crest to its posterior end. The orientation of the fibers is slightly posteroventral, then becomes dorsoventral at the level of the tendon (Figure 4 and Figure S12).
The oioZM is extremely reduced and originates on the maxilla from the anteromedial surface inside the orbit cavity, ventrally to the maxilla‐lacrimal suture. It inserts medially to the tendon of the rioZM. The fibers are oriented dorsoventrally with a slight mediolateral component (Figure 4 and Figure S12).
The ZM is closely connected to the ioZM and as such is hard to differentiate from it. Its origin is on the dorsal half of the medial side of the zygomatic arch, posterior to the maxilla‐jugal suture and at the level of the posterior end of the first upper molar. It inserts on the lateral crest, via a strong aponeurosis, from the posterior end of the first lower molar to the dorsolateral side of the reduced coronoid process. The orientation of the fibers is dorsoventral (Figure 4 and Figure S12).
3.4.2. Temporalis complex
M. Temporalis lateralis
M. Temporalis medialis
The mT is extremely reduced. It takes its origin in the temporal fossa, on the posterior end of the zygomatic process of the squamosal along a dorsal ridge extending ventrally and anteriorly from the highly inflated auditory region and mastoids. The mT inserts on the posteromedial surface of the dorsal half of the coronoid process. Its fibers are anteroventrally and slightly medially oriented. The anterior end of the fibers is in contact with the posterior part of the ZM (Figures 5 and 7).
M. Temporalis orbitalis
The oT is separated from the mT by a thin but strong tendon. It takes its origin on the anterior end of the squamosal and on the posterior wall of the orbital cavity and inserts ventromedially on the coronoid process and the retromolar fossa. The fibers are oriented anteroventrally (Figures 5 and 7).
3.4.3. Pterygoid complex
M. Pterygoideus internus
The iPT is very large and takes its origin on the lateral and posterior edge of the deep sphenopterygoid fossa located laterally to the hamular process of the pterygoid. It inserts on the pterygoid fossa of the mandible. The orientation of the fibers is dorsoventral and mediolateral (Figure 6).
M. Pterygoideus externus
The ePT takes its origin laterally to the sphenopterygoid fossa, on a small fossa located immediately ventral to the squamosal‐alisphenoid suture. Its insertion is located on the medial side of the condylar process of the mandible, and its fibers are oriented posteroanteriorly and mediolaterally (Figure 6).
3.4.4. Other muscles of the masticatory apparatus
M. Digastricus
The two bellies are well‐separated by a strong interdigastric tendon and the posterior belly is smaller than the anterior belly. The muscle takes its origin with the posterior belly on the lateral and medial sides of the paroccipital process located ventrally to the tympanic bulla. The anterior belly inserts on a small tubercle located on the anteroventral part of the mandible, posteroventrally to the mandibular symphysis, which is unfused but immovable. The orientation of the fibers of the posterior belly is anteromedial in ventral view and those of the anterior belly are anteroposterior. The left and right anterior bellies are in contact on their medial margin (Figure 6 and Figure S13).
M. Transversus mandibulae
The TM is absent (Figure 6).
3.5. Comparative description and identification of diagnostic traits of Anomaluromorpha
In Anomaluromorpha, the ventral ramus of the zygomatic process of the maxilla presents an anterior depression hosting the origin of the SM and aDM muscles. This structure is accompanied by an aDM originating from a well‐marked depression on the lateral surface of the zygomatic process of the maxilla and jugal bone. This holds true except for Idiurus, as the taxon shows a reduced anterior infraorbital ridge (Figure 8: character 2) and an aDM reduced dorsoventrally in the middle of the muscle and forming a deep imprint on the ZM (Figure 8: character 14). The Anomaluromorpha also shows a tendency towards a relative reduction of the aDM, except for Zenkerella (Figures 3b,d,f,g and 8: character 15). The ZM is characterized by an ioZM separated into two portions, which are anterior and posteromedial to the infraorbital foramen in all taxa (Figure 4). Their infraorbital foramen shows an anterior shift of the ventral ramus of the maxilla that allows for a large origin of the rioZM on the rostrum (Figure 8: character 17). Only Anomalurus does not display such an anterior shift of the ventral ramus of the zygomatic process of the maxilla (Figure 2b). Therefore, both the zygomatic process and the rioZM are reduced in size. (Figures 4c,d and 8: characters 1, 10 and 18). As for the oioZM, the muscle is very developed in Anomalurus, Idiurus, and Zenkerella and accompanied by a well‐marked ridge on the medial wall of the orbit. In Pedetes the muscle is still present, albeit extremely reduced, with no distinctive origin on the medial wall of the orbit. This part is likely a synapomorphy of the clade (Figure 8: character 19), like the non‐individualized pZM (Figure 8: character 21). All anomaluromorph rodents show a digastric separated into an anterior and posterior belly by a strong interdigastric tendon. The tendon shows a tendency to a shortening: it is long in Pedetes, of medium size in Zenkerella, of medium to short size in Anomalurus, and short in Idiurus (Figures 6a,c,e,g and 8: characters 25 and 26). The morphology of the temporal, pterygoid, and transverse mandibular muscles are more distinctive of the clades Anomaluroidea and Pedetidae.
Anomaluroidea exhibits distinctive anatomical features compared to Pedetidae, such as a developed angular process (Figure 8: character 6) and a SM that takes its origin on the whole zygomatic arch (Figure 8: character 11). The overall morphology of the SM muscle is more similar between Idiurus and Zenkerella because muscle fibers are very reduced anteriorly (Figure 3a,e). The mandible is characterized by the presence of a mandibular notch on its ventral margin, allowing for the passage of the pars reflexa medially (Figure 8: characters 8 and 12). Interestingly, the pars reflexa exhibits an increasing degree of posterodorsal elongation from Zenkerella, where the pars reflexa is poorly developed, to Anomalurus where the muscle inserts on the most medioventral surface of the iPT, and finally Idiurus where it is in contact with the ventral half of the iPT. Contrary to Pedetidae, the DM is separated into two parts and is extremely well‐developed in Zenkerella. At the level of its origin area, the development of the oioZM of anomaluroid rodents is accompanied by the presence of a fossa delimited by a ridge on the anteromedial surface of the orbit cavity (Figures 4b,d,f,g and 8: characters 5 and 20). The ZM is not distinctive of the clade but Zenkerella shows an especially important dorsoventral development compared to anomalurids (Figure 4e). The morphology of the transverse mandibular is identical in Zenkerella and Anomalurus, with a triangular aspect (Figure 6e), whereas Idiurus shows a surprising morphology resembling an inverted “V.” The morphology of the temporal and pterygoid muscles are very similar between Anomalurus, Idiurus, and Zenkerella (Figures 5 and 6b,d,f) and the morphology of the digastric is quite similar between the taxa and can't be used to assess the monophyly of the clade.
Anomaluridae is characterized by an absence of depression on the jugal bone (Figure 8: character 3). The lacrimal and jugal bone, which participate in the structure of the infraorbital foramen, are also in contact (Figure 8 character 9). The pars reflexa is most similar between Anomalurus and Idiurus as it does not attach on the medial surface of the mandible and is in contact with the medioventral surface of the iPT instead (Figure 8: character 13). Concerning the ZM, its relative proportion and morphology are extremely similar between the two taxa (Figure 4a,c). The morphology of the digastric muscle is very similar between Anomalurus and Idiurus in terms of relative proportion of the anterior and posterior bellies (Figure 6a,c,e,g). The morphology of the temporal and pterygoid muscles is very similar whereas the morphology of the DM, ioZM, and TM are very different between the taxa and cannot be used to assess the monophyly of this clade. Zenkerellidae are characterized by an aDM and pDM that are extremely developed, with well‐marked origins on the ventral ramus of the zygomatic process of the maxilla and the depression of the jugal, respectively.
Finally, Pedetidae displays a very different muscular architecture as compared to that of Anomaluroidea. The SM of Pedetidae does not attach on the whole lateral surface of the zygomatic arch, resulting in a notable reduction of the relative size of the muscle compared to the other taxa (Figure 3g) and the pars reflexa is absent (Figure 7d). The group is also characterized by the presence of several depressions on the maxilla and jugal bone and it is the only clade that exhibits both an anterior and posterior jugal depression (Figures 4g, 5g and 8: character 4). The latter is located close to the origin area of the additional part of the pDM, the ppDM (Figure 8: character 16). This muscle part is oriented anteroposteriorly and inserts on the condylar process. The coronoid process is reduced, the lT absent and the mT extremely reduced (Figures 5g,h and 8: characters 7, 22, and 23). The iPT of Pedetes is also extremely developed relative to the ePT and is oriented dorsoventrally instead of medioventrally (Figures 7d and 8: character 24). This orientation might be related to the heavy reduction of the angular process in this taxa (Figure 6h). Finally, the clade shows a greatly elongated interdigastric tendon (Figure 8: character 25) and the TM is absent (Figure 8: character 27).
4. DISCUSSION
4.1. Relevance of the masticatory morphology of anomaluromorph rodents for systematics
Throughout the years, the study of masticatory muscles anatomy has played a crucial role in the classification of extant rodents (Brandt, 1855; Parsons, 1894, 1899; Winge, 1887; Tullberg, 1899; Rinker, 1954; Klingener, 1964; Hautier, 2010). However, it is now widely acknowledged that the morphology of the masticatory apparatus does not provide a conclusive insight into the subordinal relationships of Rodentia. This is substantiated by both morphological (Hautier et al., 2008; Potapova, 2020; Wood, 1965) and molecular data (e.g. Blanga‐Kanfi et al., 2009; D'Elía et al., 2019; Fabre et al., 2012; Huchon et al., 2002). By considering detailed character description, we show that anatomical features of the masticatory apparatus can still be relevant for retracing evolutionary relationships of closely related taxa. The unique combination of the masticatory muscles of Anomaluromorpha (“anomaluromorphic zygomasseteric structure”; Potapova, 2020) is highly distinctive and allows recognition of different taxonomic units. Indeed, different osteological and myological traits are interpreted as potentially autapomorphic or synapomorphic characters (Figure 8), and could prove to be relevant for future cladistic studies or in assessing the affinities of ambiguous fossils.
Among the most noticeable characters of the craniomandibular complex of Anomaluromorpha, the presence of an oioZM was most surprising as this muscle was considered to be absent in rodents (Druzinsky et al., 2011). Such an orbital origin of the ioZM has recently been documented for bathyergids (Cox & Faulkes, 2014; Cox et al., 2020), but without the double origin of the ioZM on the rostrum as well as the medial wall of the orbit cavity described here in Anomaluromorpha. This part of the ioZM was first described in Anomalurus by Tullberg (1899), then recognized in both Anomalurus and Idiurus by Potapova (2017) and may constitute a synapomorphy of Anomaluromorpha as it is also present in Pedetes and Zenkerella (Figure 8: character 19). The presence of this part of the zygomaticomandibularis complex is also coupled with a non‐individualized pZM in all genera (Figure 8: character 21). Some interesting characters of Anomaluroidea consist in the great development of the origin of the SM posteriorly that is accompanied by the presence of a pars reflexa (Figure 8). Even if those characters are present in Zenkerella, the taxon shows a unique combination of pedetid and anomalurid characters. For instance, the relative length of the interdigastric tendon is elongated in Pedetes and Zenkerella, but more reduced in Anomalurus and Idiurus. Unlike Pedetes, Zenkerella has a pars reflexa, although more reduced than in its Anomaluridae close relatives (Figures 6, 7 and 8: characters 13, 25 and 26). Also of interest is the lack of contact between the jugal and lacrimal bone on the dorsal ramus of the zygomatic process of maxilla in Zenkerella that is shared with Pedetes but not Anomaluridae. Given the involvement of both the lacrimal and jugal in the formation and morphology of the infraorbital foramen, this aspect holds particular relevance to the morphology of its associated musculature. Our finding seems to contradict the recognition of the presence of a contact between the bones as an argument for bringing Zenkerella closer to Idiurus (Ellerman et al., 1940). The great development and relative proportions of the aDM and pDM in Zenkerella are also distinctive within the Anomaluromorpha. The DM is usually reduced and the aDM less developed than the pDM in other taxa, and may correspond to an autapomorphy of the genus, or a plesiomorphic condition in the mouse‐related clade (Figure 8: character 15). Indeed, this morphology of the DM is somewhat similar to what is observed in the members of the mouse‐related clade (e.g. Baverstock et al., 2013; Fabre et al., 2017; Rinker, 1954; Satoh & Iwaku, 2004, 2006, 2009; Voss, 1988), but the fact that the ventral ramus of the zygomatic process of Zenkerella is more reduced and without a zygomatic plate, completely sets the taxon apart from non‐anomaluromorph rodents. The association of a reduced rioZM and an absence of the anterior shift of the ventral ramus of the zygomatic process in Anomalurus may also be interpreted as an autapomorphy of the genus within the clade as Idiurus, Zenkerella, and Pedetes all exhibit a large portion of the muscle on the rostrum and an anterior shift of the ventral ramus (Figure 8: characters 1, 10 and 18). Most of the characters identified as informative for differentiating Pedetidae from Anomaluroidea were also described by Offermans and De Vree (1989), such as the lack of a pars reflexa or the presence of an additional pars posterior to the pDM. The morphology of the latter appears to be more similar to the condition observed in Caviomorpha (Woods, 1972; Woods & Howland, 1979) than that described in Muroidea (e.g. Rinker, 1954; Voss, 1988) due to the anteroposterior orientation of the muscle fibers and the insertion of the muscle on the lateral surface of the condylar process. Finally, the reduction of the temporal complex observed in Pedetes is most similar to what is observed in bipedal saltatorial rodents with a very developed auditory bulla (Klingener, 1964), and therefore completely different from the condition observed in all Anomaluroidea, without exception, based on our sampling (Figure 8: characters 22 and 23).
4.2. Anomaluromorpha is an elusive lineage within the mouse‐related clade
Unraveling relationships among the mouse‐related clade has proven challenging due to the rapid early divergence that occurred during the Paleogene (Blanga‐Kanfi et al., 2009; Fabre et al., 2015; Swanson et al., 2019). Anomaluromorpha, Castorimorpha, and Myomorpha relationships are still poorly resolved, with each of these lineages having accumulated many morphological and molecular differences over time (Fabre et al., 2015; Swanson et al., 2019). Indeed, the mouse‐related clade holds a unique position among Rodentia for being the only group with extant taxa that exhibit all three cranial morphotypes (Swanson et al., 2019). The clade Castorimorpha is strictly sciuromorphous, characterized by the presence of a well‐developed ventral zygomatic plate where the deep masseter muscle originates and the absence of a rostral expansion of the zygomaticomandibularis muscle. Myomorpha encompasses two distinct morphotypes: the hystricomorphous Dipodoidea and the myomorphous Muroidea. The former exhibits an enlarged infraorbital foramen, which allows the anterior portion of the zygomaticomandibularis to pass through on the rostrum, and an absence of a zygomatic plate. The latter combines elements from both previous morphotypes, with a portion of the zygomaticomandibularis passing through the infraorbital foramen and a deep masseter extending anteriorly onto the zygomatic plate. The masticatory musculature of Anomaluromorpha stands out among the mouse‐related clade due to its distinctive hystricomorphous craniomandibular condition, which they evolved convergently with Dipodoidea (Hautier et al., 2015; Swanson et al., 2019; Wood, 1965). Based on such association of morphological characters, Anomaluromorpha have often been placed outside the mouse‐related clade in past classifications (e.g. Thomas, 1896; Winge, 1924) or brought closer to dipodoid rodents (Miller & Gidley, 1918). However, we revealed noticeable similarities and differences in muscle morphology with the known members of the mouse‐related clade and showed that Anomaluromorpha exhibits unique features, such as a pars reflexa that partially surrounds the iPT instead of inserting on the mandible for Anomaluroidea, and the presence of an orbital portion of the ioZM for both Pedetidae and Anomaluroidea that has never been described in other members of the mouse‐related clade. The latter is accompanied by a marked zygomasseteric ridge on the medial wall of the orbital cavity (Figure 2). Interestingly, Anomaluroidea displays a remarkable degree of morphological conservatism, especially in terms of dental patterns (Marivaux et al., 2017). Bony characters such as the presence of a zygomasseteric ridge or the presence, number, and position of depressions of the maxilla and jugal bone, appear to be closely related to masticatory muscular morphology. Taking them into consideration in future paleontological investigations and within the framework of rodent systematics would contribute to further advancements in the field.
Within Castorimorpha, Castoroidea are similar to Anomaluroidea due to their SM originating from the whole zygomatic arch and the lack of a ppDM (Cox & Baverstock, 2016). Castoroidea distinctly display a split tendon of origin of the SM with two attachment areas: one on the anterior edge of the masseteric fossa and the other on the ventral surface of the zygomatic arch. The pars reflexa is also absent (Cox & Baverstock, 2016). The aDM is well‐developed and takes its origin more dorsally, on the zygomatic plate of the maxilla (Cox & Baverstock, 2016). As a whole, the DM is also more developed than that of Anomaluromorpha. This clade is extremely different from both anonomaluroids and pedetids due to the extreme reduction of the ZM. Castoroidea displays a sciuromorphous skull characterized by the absence of enlargement of the infraorbital foramen. Therefore, attachment of the anteriormost part of the ZM does not attach on the rostrum. Interestingly, even if the ZM is separated into two parts in Castor, its anteriormost portion expands anterodorsally and can be likened to an incipient ioZM (Cox & Baverstock, 2016). In Geomyoidea, the SM originates from a small tendon located ventral to the infraorbital foramen, like in Castoroidea, with no posterior attachment on the zygomatic arch and a reduced pars reflexa (Merriam, 1885; Orcutt, 1940; Ryan, 1989) similar to what can be observed in Pedetes. In Castoroidea, the aDM is relatively larger than the pDM in most taxa, and therefore completely different from what is observed in Anomaluromorpha. The ZM is more reduced than in Castor but also separated into two parts, with no budding ioZM (Cox & Baverstock, 2016; Ryan, 1989).
Myomorpha is separated into the superfamilies Muroidea and Dipodoidea. The former presents a SM different from Anomaluroidea with a tendon of origin ventral to the infraorbital foramen with no posterior expansion on the zygomatic arch, most similar to what is observed in Geomyoidea (Orcutt, 1940; Ryan, 1989). The pars reflexa is small to medium‐sized and inserts on the ventral margin of the medioventral surface of the mandible (e.g. Baverstock et al., 2013; Fabre et al., 2017; Rinker, 1954; Satoh & Iwaku, 2004, 2006, 2009; Voss, 1988). The aDM takes its origin on the zygomatic plate and the DM shows an additional part of the pDM. This posterior part is well‐defined and originates from the posterior half of the jugal to the lateral surface of the squamosal and inserts on the posterodorsal end of the angular process (“posterior portion of the m. masseter lateralis pars posterior” in Rinker & Hooper, 1950; Rinker, 1954; “deep masseter medial horizontal part” in Satoh & Iwaku, 2004, 2006, 2009; “pDM2” in Fabre et al., 2017; Voss, 1988). It is unlikely functionally homologous with the ppDM observed in Pedetes, as evidenced by the great differences in insertion area and orientation of the fibers, but topologically the muscle remains posterior to the pDM and originates from the posterior end of the jugal on the zygomatic arch (Figure 3h; Table 2). Muroidea have a smaller enlarged infraorbital foramen than Anomaluromorpha that does not allow the ioZM to attach anteriorly on the rostrum. The ZM can be separated into three parts with an ioZM forming a unique bundle and the presence of an individualized pZM separated from the aZM by the masseteric nerve (e.g. Baverstock et al., 2013; Fabre et al., 2017; Voss, 1988), or in two parts where the ioZM and aZm are considered as single part separated from the pZM (Rinker, 1954; Rinker & Hooper, 1950). In both cases, this condition is completely different from what is observed in Anomaluromorpha, where the pZM is not individualized.
Dipodoidea warrants special attention due to the hystricomorphous state of the skull. The origin of the SM is highly variable in this clade, with genera like Cardiocranius, Jaculus, Stilodipus, and Salpingotus showing an origin solely from the ventrolateral surface of the ventral ramus of the zygomatic process, similar to Pedetes, while genera like Dipus, Euchoreutes, Sicista and Zapus extend it to the ventrolateral surface of the zygomatic arch, resembling Anomaluroidea (Gambaryan et al., 1980; Klingener, 1964; Potapova, 2020; Tullberg, 1899). However, unlike Anomaluromorpha, Dipodoidea are considered to have a very reduced pars reflexa inserting medially on the angular below the root of the incisors (Klingener, 1964). Concerning the DM, previous studies found no anteroposterior separation of the muscle (Klingener, 1964; Tullberg, 1899). The configuration of the ZM resembles that of Anomaluromorpha, primarily characterized by the anterior insertion of the ioZM on the rostrum. Nevertheless there are distinct variations, such as the ioZM forming a whole bundle with no origin on the medial wall of the orbit and the presence of an individualized pZM posteriorly (Gambaryan et al., 1980; Klingener, 1964).
4.3. Implications for the complexity of hystricomorphy
The definition of hystricomorphy, like every rodent cranial morphotype, is primarily based on the morphology of the anterior root of the zygomatic arch and opening of the infraorbital foramen (Brandt, 1855; Landry, 1999). Therefore, this definition solely relies on the relative position of the associated musculature with a strong focus on the deep masseter and the zygomaticomandibularis muscle (Wood, 1965). However, this overly simplistic view does not take into account the extent of the phenotypic variation observed in the masticatory apparatus as a whole, nor does it consider the potential differences in its morphofunctional aspects. Here, we contend that the hystricomorphy of Anomaluromorpha substantially diverges from that of Ctenohystrica and Dipodoidea in terms of anatomy and morphofunction, and suggest that it also diverges between members of the clade depending on their masticatory morphology. Distinctive features observed in this group include a pars reflexa covering the internal pterygoid, the presence of an orbital portion of the infraorbital part of the zygomaticomandibularis and the absence of its posterior part, among other characteristics. These traits have the potential to influence masticatory movements and alter mechanical advantages during biting, and only an exhaustive description of the masticatory apparatus can adequately capture these differences and similarities.
We established that Anomaluromorpha exhibits a well‐developed ioZM that passes through the infraorbital foramen, the condition of the zygomaticomandibularis defining an hystricomorphous zygomasseteric construction (Wood, 1965). However, the clade is also characterized by the separation of the muscle into a rostral (rioZM) and orbital part (oioZM) that was first described by Tullberg (1899) in Anomalurus peli, and then by Potapova (2017) in several unspecified species of Idiurus and Anomalurus. This part has never been observed in any other hystricomorphous rodents. While no functional studies report on the effect of the presence of an oioZM on masticatory movements and bite force, we hypothesize that its anterior and medial position, along with its insertion on the medial side of the infraorbital tendon, potentially contributes to the elevation of the jaws and, to a lesser extent, mediolateral movements during chewing. Interestingly, there seems to be no direct link between the size of the orbital and rostral part of the ioZM. Pedetes displays an extremely developed rioZM but a relatively smaller oioZM, whereas Anomalurus exhibits a relatively much larger oioZM despite having a smaller rioZM (Figure 4). However, the relative size of the rioZM tendon appears to be linked to the position of insertion of the infraorbital tendon. In Idiurus, Zenkerella, and Pedetes, the rioZM is well‐developed and expands very anteriorly on the rostrum, reaching the posterior edge of the superior incisors, and the tendon inserts anterior to the first cheek tooth on the dorsolateral surface of the mandible (Figure 4a,c,g). This morphology is accompanied by a significant anterior displacement of the ventral ramus of the zygomatic process, resulting in the enlargement of the infraorbital foramen. Such a configuration likely facilitates the forward shift of a larger part of the zygomaticomandibularis. In contrast, the rioZM of Anomalurus is less developed and the infraorbital tendon inserts posterior to the first cheek tooth and more ventrally on the lateral surface of the mandible. The conformation of the infraorbital foramen is also different, with the ventral ramus of the zygomatic process being positioned at the same level as the dorsal ramus, resulting in a relatively smaller infraorbital foramen. In this case, the morphology of the foramen is more adequate for a decreased size of the rioZM, which originates less anteriorly on the rostrum. In rodents, the ioZM is the main muscle contributing to operating as a second‐class lever for the jaws (Becht, 1953; Cox, 2017). The anterior shift of the origin and insertion of the rioZM as well as the relative size increase of the muscle observed in Idiurus, Zenkerella, and Pedetes may lead to the transition of the masticatory system from a third‐class lever to a second‐class lever (Turnbull, 1970). Such a modification entails an increase in mechanical advantage, increase in bite force, a decrease in gap amplitude, and a decrease in tensile force at the temporomandibular joint which increases the chance of dislocation of the jaws (Greaves, 2012). This shift in lever biomechanics has been previously demonstrated in Pedetes (Cox, 2017) and given the aforementioned similarities between Pedetes, Idiurus, and Zenkerella, it is reasonable to anticipate a somewhat similar mechanism in the latter two taxa. In contrast, the morphology of Anomalurus appears to be more adapted towards a third‐class lever because the rioZM is positioned posteriorly to the first cheek tooth. This configuration allows for wider gap and high tensile forces at the temporomandibular joint, but the trade‐off is that bite force and mechanical advantage are reduced (Greaves, 2012).
The deep masseter of rodents is usually separated into two or three parts (Druzinsky et al., 2011). In hystricomorphous rodents, this muscle is considered to be positioned ventral to the infraorbital foramen (Wood, 1965), due to the absence of a zygomatic plate and the enlarged foramen. In Anomaluromorpha, the DM is divided into an aDM and pDM in Anomalurus, Idiurus, and Zenkerella, but Pedetes displays an additional part, the ppDM, which was also observed by Offermans and De Vree (1989). The origin and insertion of the ppDM in Pedetes are more similar to the condition observed in caviomorph rodents (“masseter lateralis” in Tullberg, 1899; “masseter lateralis profundus pars posterior, deep division” in Woods, 1972; “masseter posterior” in Woods & Hermanson, 1985; Woods & Howland, 1979), with an origin on the posterior depression of the jugal and an insertion on the lateral surface of the condylar process. It contrasts with the posterior portion of the deep masseter of most myomorph rodents that inserts on the dorsal end of the angular process (e.g. Fabre et al., 2017; Rinker & Hooper, 1950; Rinker, 1954, 1963; Satoh & Iwaku, 2004, 2006, 2009). As such, the ppDM is most likely derived in Pedetes with regards to Anomaluroidea and convergent functionally and structurally with Caviomorpha. The anteroposterior orientation of the fibers and the position of the muscle may contribute to propalinal movements of the jaws and stabilization of the temporomandibular joint in order to avoid dislocation of the mandible (Cox, 2017; Greaves, 2012). The presence of a ppDM in Pedetes may be linked to the extreme reduction of its ZM and the increased size of its ioZM: additional muscle fibers near the joint may be necessary to compensate for increased biting force at the incisors (Cox, 2017). Moreover, the fact that all Anomaluroidea lack a ppDM is consistent with the presence of a developed ZM in those species, especially Zenkerella (Figure 4e), which is elongated posteriorly, probably to offset the lack of an individualized pZM. These observations may imply an important covariation of the posterior‐most musculature of the DM and ZM at the temporomandibular joint.
The presence of a pars reflexa of the superficial masseter in Rodentia has long been used for systematic purposes. Tullberg (1899) considered that the development and insertion of a pars reflexa of the SM in the groove on the medial side of the mandible up to the condyle to be a characteristic of the tribe “Hystricognathi,” including all extant hystricomorphous rodents with an hystricognathous jaw. This view persisted until Woods (1972) suggested that the passage of the pars reflexa on the medial side of the mandible caused the formation of the groove in hystricognathous jaws. However, multiple studies showed since then that the presence of a pars reflexa was not dependent on the sciurognathous or hystricognathous condition of the jaw, as evidenced by Diatomyidae (Laonastes aenigmamus: Hautier & Saksiri, 2009), Ctenodactylidae (Ctenodactylus vali: Hautier, 2010, Ctenodactylus gundi: Zherebtsova & Potapova, 2019), Myomorpha (Dipodoidea: Klingener, 1964, Heteromyidae: Howell, 1932, Calomyscidae and Arvicolidae: Vorontsov, 1982, and Muridae: Baverstock et al., 2013; Fabre et al., 2017; Rinker, 1954), and as a short posteromedial insertion in Sciuromorpha (Aplodontia rufa: Kunstler, 1887 and Sciuridae: Ball & Roth, 1995; Hill, 1937; Thorington & Darrow, 1996). Therefore, the presence of a pars reflexa cannot be viewed as a typical feature of hystricognath rodents. Yet, while the presence or absence of a pars reflexa is well‐documented, few studies investigated the anatomy of this muscle part and its effect on systematics and function, most probably due to the difficulty to retrieve it using traditional dissection methods due to its medial positioning on the mandible. The peculiar configuration of the pars reflexa in anomalurids and perhaps even anomaluroids, is different from that observed in most Ctenohystrica. In the Ctenodactylidae, Ctenodactylus shows an extremely short pars reflexa that inserts medially on the mandible, anteriorly to the iPT (Hautier, 2010; Tullberg, 1899; Zherebtsova & Potapova, 2019). Laonastes exhibits an elongated pars reflexa (Hautier & Saksiri, 2009) most similar to the one of Caviomorpha (with the exception of a lack of a medial groove), where the muscle is extremely developed dorsally, following the groove of the mandible located laterally to the iPT, and attaches almost to the medial side of the condyle (Álvarez et al., 2023; Müller, 1933; Tullberg, 1899; Woods, 1972; Woods & Hermanson, 1985; Woods & Howland, 1979). This morphology is identical to the ones of Hystricidae and Bathyergidae (Boller, 1970; Tullberg, 1899). In Anomalurus and Idiurus, the pars reflexa occupies the ventral half of the angular process and covers the medial side of the iPT (Figure 7a,b). It is in an intermediate state in Zenkerella, not as elongated posterodorsally as the other two taxa (Figure 7c,d). Such a morphology appears to be unique in hystricomorphous rodents and Rodentia as a whole. The lack of an attachment area on the mandible and the direct contact with the iPT could be linked to a different muscular function in Anomaluromorpha compared to other hystricomorphous rodents. In Caviomorpha, the deep extension of the pars reflexa onto the medial side of the mandible is hypothesized to contribute significantly to anteroposterior movements of the jaw (Woods, 1972), whereas the entire SM is responsible for elevation and protraction of the jaw (Hiiemae, 1967) and contributes to power stroke during chewing in Cavia (Byrd, 1981). Unlike caviomorphs, anomaluroid rodents have a mobile mandibular symphysis, which enables more complex lateral movements of the jaws. Kunstler (1887) also concluded that the SM of Aplodontia acted as an elevator and protractor of the jaw, but also brought the incisors closer to each other. The presence of an unfused mandibular symphysis as well as the posteromedial position of the pars reflexa in direct contact with the ventral half of the iPT likely induce the tightening of the incisors in a scissor‐like movement during contraction of the SM; all the more so because they possess a developed TM muscle which is partly responsible for spreading the incisors (Kunstler, 1887; Wood & White, 1950) that is heavily reduced in hystricognathous rodents (Woods, 1972). Therefore, the differences in the morphology of the pars reflexa in Caviomorpha and Anomaluroidea could be interpreted as differences in the muscle role in the production of anteroposterior movements during chewing in the former and mediolateral scissor‐like movements at the incisors in the latter. It is also highly probable that the morphology and development of the pars reflexa is linked to the morphology of the mandible. Indeed, a relative increase in the surface area of the angular process and concavity of the ventral margin of the mandible is observed in rodents with a large pars reflexa (Hautier et al., 2011). It may have co‐occurred with the backward pull of the angular process and the great development of the masseteric crest that naturally enlarged the insertion area of the SM and at the same time the pars reflexa, at the expense of a deep pterygoid fossa. Such a condition is present in Hystricognathi (Álvarez et al., 2023; Müller, 1933; Tullberg, 1899; Woods, 1972; Woods & Hermanson, 1985; Woods & Howland, 1979) as well as in Laonastes aenigmatus, a sciurognathous rodent (Hautier et al., 2011; Hautier & Saksiri, 2009; Zherebtsova & Potapova, 2019) but not in Ctenodactylus (Hautier, 2010; Zherebtsova & Potapova, 2019). These observations may be related to the posterodorsal development and deep insertion of the pars reflexa on the medial side of the mandible. This is not the case in Anomaluroidea, where the masseteric crest is not as well‐developed, and where the angular process is less projected posteriorly and more developed dorsoventrally rather than anteroposteriorly than in most Ctenohystrica (Figure 2b). Instead, anomaluroids show an inflexion of the ventral margin of the mandible that forms a mandibular notch (Figure 2b) which seems to allow for the passage of a substantial portion of the pars reflexa medially (Figure 7a–c). This morphology is accompanied by a well‐developed and deep pterygoid fossa, the reduction of the tendon of origin of the SM, and the subsequent deep insertion of the iPT inside the pterygoid fossa that could explain the lack of space for the posterodorsal elongation of the pars reflexa laterally to the muscle, and the insertion of the pars reflexa of anomaluroid rodents on the medial side of the muscle instead (Figure 7). Nevertheless, the great diversity in angular morphology of these taxa, coupled with diverse muscle associations, likely results in distinct jaw movements among them.
The digastric muscles in Rodentia are categorized into sciuromorphine and hystricomorphine types based on the contact—or lack thereof—of the left and right anterior bellies on their medial margins. According to Parsons (1894), sciuromorphine digastrics are in contact anteriorly and joined to the posterior bellies by an interdigastric tendon while hystricomorphine digastrics are well‐separated with no interdigastric tendon. The contact between the two anterior bellies and the presence of the tendon between the anterior and posterior bellies is a common character state in Rodentia; it can be found in Geomyoidea (Orcutt, 1940; Ryan, 1989), Myomorpha (e.g. Klingener, 1964; Rinker, 1954; Satoh & Iwaku, 2004), Sciuromorpha (e.g. Ball & Roth, 1995; Druzinsky, 2010; Kunstler, 1887), Dipodoidea (Klingener, 1964), and Anomaluromorpha (see the above description; Offermans & De Vree, 1989) whereas it is absent in Ctenohystrica except for Laonastes and Ctenodactylus (Hautier, 2010; Hautier & Saksiri, 2009; Zherebtsova & Potapova, 2019), and Erethizon and Coendou in Caviomorpha (Woods, 1972). Hence, the designation of the digastric muscle as sciuromorphine or hystricomorphine in Rodentia is unrelated to the acquisition of a specific cranial morphotype, but rather to its mandibular type (i.e. hystricognathous vs sciurognathous, see Hautier et al., 2011). The contact of the anterior bellies and the presence of the interdigastric tendon in Anomaluromorpha could provide additional arguments in favor of distinct functioning of masticatory movements from Ctenohystrica. Since the digastric is the main muscle responsible for the lowering of the mandible in rodents (Hiiemae, 1967), we can expect its morphology to impact mouth gape or mandible opening speed during feeding.
5. CONCLUSION
This study reaffirmed the significance of the anatomical features of the masticatory apparatus as a valuable source of information for understanding the evolutionary relationships among closely related taxa. The combination of both traditional and digital dissection techniques has proven to be complementary. This integration allowed for a more comprehensive understanding of the intricate myological structures involved in mastication and their relation with craniomandibular morphology in Anomaluromorpha. Significant findings include the identification of unique features of the masticatory apparatus of Anomaluromorpha, allowing for the recognition of different taxonomic units in the clade. Notably, the presence of a well‐developed pars reflexa partially surrounding the internal pterygoid or the presence of the orbital portion of the infraorbital part of the zygomaticomandibularis muscle accompanied by a well‐developed zygomasseteric ridge, among other characteristics, distinguishes them from other members of the mouse‐related clade. These novel bony and muscular characters are particularly interesting because those conditions can potentially be inferred from fossilized cranial remains. Such findings underscore the importance of considering the morphology of the masticatory apparatus in taxonomic and phylogenetic studies. Additionally, our study revealed that the hystricomorphy of anomaluromorph rodents largely differs from that of Ctenohystrica and Dipodoidea. The anatomical differences observed most probably translate into distinct masticatory movements, like scissor‐like movements at the incisors in Anomaluroidea. Morphotypes have traditionally served as a means to classify taxa and better appreciate the complexity in highly diverse clades. The enduring influence of their historical usage significantly biases our appreciation of the morphological diversity of organisms, especially in the case of Rodentia. However, contrary to their intended purpose, the elaboration of such morphotypes actually has proven to be systematically uninformative and their use as self‐sufficient anatomical terms can also lead to limited or misleading information regarding the anatomy of a structure. This emphasizes the need for caution when employing terms such as “hystricomorphy” or any kind of morphotype to describe the entirety of the masticatory anatomy of rodent clades or other groups of organisms, as it oversimplifies both the biological reality of the morphological variation and the potential differences in the functional aspects of the masticatory apparatus. By embracing a more nuanced perspective and by considering these complexities through extensive anatomical descriptions across a large number of taxa, a better understanding of the evolution of the masticatory complex can be achieved. To further advance this understanding, future prospective studies can explore the evolutionary development of masticatory musculature in order to better assess masticatory muscle homology in Rodentia. Additionally, conducting experimental research on masticatory functions and their ecological correlates will provide valuable knowledge to enhance our understanding of the diversification of the masticatory apparatus in rodents.
AUTHOR CONTRIBUTIONS
The study was conceptualized by LDC, P‐HF, and LH. Specimen collection and CT‐scan data acquisition were carried out by P‐HF and LH, while LDC performed the traditional and digital dissections with assistance from LH. The figures were created by LDC, and the manuscript was written by LDC with contributions and feedback from P‐HF and LH.
FUNDING INFORMATION
This research was supported by the French “Agence Nationale de la Recherche” (ANR) in the framework of the DispaRat project (ANR‐20‐CE02‐0022‐01).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
Supporting information
Data S1:
ACKNOWLEDGMENTS
We express our gratitude to R. P. Miguez and E. Gilissen for their generous assistance in providing access to the mammal collections and the fluid specimen at the Natural History Museum of London and the Royal Museum for Central Africa in Tervuren, respectively. We would like to extend our thanks to R. Šumbera, J. Jan Okrouhlík, M. Lövy, and T. Pes for their welcome in the University of South Bohemia, České Budějovice, and for granting us access to the Pedetes specimen from the Plzeň Zoo. Additionally, we want to express our thanks to A. Herrel and V. Nicolas‐Colin for allowing us to acquire the other Pedetes specimen from the collection of the Museum d'Histoire Naturelle, in Paris. We also want to acknowledge the insightful conversations we had with A. Naas, A. Kraus, and F. Vejmělka as well as their assistance to bring back specimens for dissections. Furthermore, we would like to thank R. Lebrun for his help in elaborating the protocol for the dice‐CT scanning of Zenkerella. Thanks to E. Potapova and the other two anonymous reviewers for their constructive feedback. Lastly, we are thankful to the Montpellier RIO Imaging (MRI) and the LabEx CeMEB for access to the μCT‐scanning station EasyTom 150/Rx Solutions (ISE‐M, Montpellier, France). This is ISE‐M publication.
Da Cunha, L. , Fabre, P.‐H. & Hautier, L. (2024) Springhares, flying and flightless scaly‐tailed squirrels (Anomaluromorpha, Rodentia) are the squirrely mouse: comparative anatomy of the masticatory musculature and its implications on the evolution of hystricomorphy in rodents. Journal of Anatomy, 244, 900–928. Available from: 10.1111/joa.14013
DATA AVAILABILITY STATEMENT
The 3D surface models of the masticatory apparatus of the specimens are available on MorphoMuseuM.
REFERENCES
- Álvarez, A. , Ercoli, M.D. , Boivin, M. , Ortiz Tejerina, A.M. & Moyano, S.R. (2023) Head myology of wild cavies (Caviidae, Caviomorpha) and functional implications of hystricomorphous and hystricognathous configurations. Journal of Mammalian Evolution, 30, 1–25. [Google Scholar]
- Ball, S.S. & Roth, V.L. (1995) Jaw muscles of new world squirrels. Journal of Morphology, 224, 265–291. Available from: 10.1002/jmor.1052240303 [DOI] [PubMed] [Google Scholar]
- Baverstock, H. , Jeffery, N.S. & Cobb, S.N. (2013) The morphology of the mouse masticatory musculature. Journal of Anatomy, 223, 46–60. Available from: 10.1111/joa.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht, G. (1953) Comparative biologic‐anatomical researches on mastication in some mammals I and II. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, 56, 508–527. [Google Scholar]
- Blanga‐Kanfi, S. , Miranda, H. , Penn, O. , Pupko, T. , DeBry, R.W. & Huchon, D. (2009) Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evolutionary Biology, 9, 71. Available from: 10.1186/1471-2148-9-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, N. (1970) Untersuchungen an Schädel, Kaumuskulatur und äußerer Hirnform von Cryptomys hottentotus (Rodentia, Bathyergidae). Zeitschrift für Wissenschaftlische Zoologie, 181, 7–65. [Google Scholar]
- Brandt, J.F. (1855) Beiträge zur nähern Kenntniss der Säugethiere Russlands. Mémoires de l'Académie Impériale Des Sciences de St. Petersbourg, 69, 1–365. [Google Scholar]
- Bugge, J. (1974) The cephalic arterial system in insectivores, primates, rodents and lagomorphs, with special reference to the systematic classification. Acta Anatomica, 87, 1–159. [PubMed] [Google Scholar]
- Bugge, J. (1985) Systematic value of the carotid arterial pattern in rodents. In: Luckett, W.P. & Hartenberger, J.‐L. (Eds.) Evolutionary relationships among rodents. Boston, MA: Springer US, pp. 355–379. [Google Scholar]
- Byrd, K.E. (1981) Mandibular movement and muscle activity during mastication in the Guinea pig (Cavia porcellus). Journal of Morphology, 170, 147–169. Available from: 10.1002/jmor.1051700203 [DOI] [PubMed] [Google Scholar]
- Coster, P.M.C. , Beard, K.C. , Salem, M.J. , Chaimanee, Y. & Jaeger, J.J. (2015) New fossils from the Paleogene of central Libya illuminate the evolutionary history of endemic African anomaluroid rodents. Frontiers in Earth Science, 3, 56. Available from: 10.3389/feart.2015.00056 [DOI] [Google Scholar]
- Cox, P.G. (2017) The jaw is a second‐class lever in Pedetes capensis (Rodentia: Pedetidae). PeerJ, 5, e3741. Available from: 10.7717/peerj.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, P.G. & Baverstock, H. (2016) Masticatory muscle anatomy and feeding efficiency of the American beaver, Castor canadensis (Rodentia, Castoridae). Journal of Mammalian Evolution, 23, 191–200. Available from: 10.1007/s10914-015-9306-9 [DOI] [Google Scholar]
- Cox, P.G. & Faulkes, C.G. (2014) Digital dissection of the masticatory muscles of the naked mole‐rat, Heterocephalus glaber (Mammalia, Rodentia). PeerJ, 2, e448. Available from: 10.7717/peerj.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, P.G. , Faulkes, C.G. & Bennett, N.C. (2020) Masticatory musculature of the African mole‐rats (Rodentia: Bathyergidae). PeerJ, 8, e8847. Available from: 10.7717/peerj.8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elía, G. , Fabre, P.‐H. & Lessa, E.P. (2019) Rodent systematics in an age of discovery: recent advances and prospects. Journal of Mammalogy, 100(3), 852–871. Available from: 10.1093/jmammal/gyy179 [DOI] [Google Scholar]
- de Winton, W.E. (1898) On a new genus and species of rodents of the family Anomaluridae, from West Africa. Proceedings of the Zoological Society of London, 66, 450–454. [Google Scholar]
- Dinets, V. (2017) First observations on the behavior of the flightless anomalure (Zenkerella insignis). Zoology, 123, 121–123. Available from: 10.1016/j.zool.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Druzinsky, R.E. (2010) Functional anatomy of incisal biting in Aplodontia rufa and Sciuromorph Rodents – part 1: masticatory muscles, skull shape and digging. Cells, Tissues, Organs, 191, 510–522. Available from: 10.1159/000284931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzinsky, R.E. , Doherty, A.H. & De Vree, F.L. (2011) Mammalian masticatory muscles: homology, nomenclature, and diversification. Integrative and Comparative Biology, 51, 224–234. Available from: 10.1093/icb/icr067 [DOI] [PubMed] [Google Scholar]
- Ellerman, J.R. , Hayman, R.W. & Holt, G.W.C. (1940) The families and genera of living rodents. London: British Museum. Available from: 10.5962/bhl.title.8332 [DOI] [Google Scholar]
- Fabre, P.‐H. , Hautier, L. , Dimitrov, D. & P Douzery, E.J. (2012) A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evolutionary Biology, 12, 88. Available from: 10.1186/1471-2148-12-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre, P.‐H. , Hautier, L. & Douzery, E.J.P. (2015) A synopsis of rodent molecular phylogenetics, systematics and biogeography. In: Hautier, L. & Cox, P.G. (Eds.) Evolution of the rodents: advances in phylogeny, functional morphology and development. Cambridge studies in morphology and molecules: new paradigms in evolutionary bio. Cambridge: Cambridge University Press, pp. 19–69. Available from: 10.1017/CBO9781107360150.003 [DOI] [Google Scholar]
- Fabre, P.‐H. , Herrel, A. , Fitriana, Y. , Meslin, L. & Hautier, L. (2017) Masticatory muscle architecture in a water‐rat from Australasia (Murinae, Hydromys) and its implication for the evolution of carnivory in rodents. Journal of Anatomy, 231, 380–397. Available from: 10.1111/joa.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre, P.‐H. , Tilak, M.‐K. , Denys, C. , Gaubert, P. , Nicolas, V. , Douzery, E.J.P. et al. (2018) Flightless scaly‐tailed squirrels never learned how to fly: a reappraisal of Anomaluridae phylogeny. Zoologica Scripta, 47, 404–417. Available from: 10.1111/zsc.12286 [DOI] [Google Scholar]
- Gambaryan, P.P. , Potapova, E.G. & Fokin, I.M. (1980) Morphofunctional features of the muscular apparatus of the head in the jerboas (to the justification of the natural system of Dipodoidea, Rodentia, Mammalia). Trudy Zoologicheskogo Instituta AN SSSR, 91, 3–51. [Google Scholar]
- George, W. (1981) Blood vascular patterns in rodents: contributions to an analysis of rodent family relationships. Zoological Journal of the Linnean Society, 73, 287–306. Available from: 10.1111/j.1096-3642.1981.tb01597.x [DOI] [Google Scholar]
- Gignac, P.M. , Kley, N.J. , Clarke, J.A. , Colbert, M.W. , Morhardt, A.C. , Cerio, D. et al. (2016) Diffusible iodine‐based contrast‐enhanced computed tomography (diceCT): an emerging tool for rapid, high‐resolution, 3‐D imaging of metazoan soft tissues. Journal of Anatomy, 228, 889–909. Available from: 10.1111/joa.12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves, W.S. (2012) The mammalian jaw: a mechanical analysis. Cambridge: Cambridge University Press. Available from: 10.1017/CBO9781139060851 [DOI] [Google Scholar]
- Hautier, L. (2010) Masticatory muscle architecture in the gundi Ctenodactylus vali (Mammalia, Rodentia). Mammalia, 74, 153–162. Available from: 10.1515/mamm.2010.025 [DOI] [Google Scholar]
- Hautier, L. , Lebrun, R. , Saksiri, S. , Michaux, J. , Vianey‐Liaud, M. & Marivaux, L. (2011) Hystricognathy vs sciurognathy in the rodent jaw: a new morphometric assessment of hystricognathy applied to the living fossil Laonastes (Diatomyidae). PLoS ONE, 6, e18698. Available from: 10.1371/journal.pone.0018698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautier, L. & Saksiri, S. (2009) Masticatory muscle architecture in the Laotian rock rat Laonastes aenigmamus (Mammalia, Rodentia): new insights into the evolution of hystricognathy. Journal of Anatomy, 215, 401–410. Available from: 10.1111/j.1469-7580.2009.01130.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautier, L. , Cox, P.G. & Lebrun, R. (2015) Grades and clades among rodents: the promise of geometric morphometrics. In: Cox, P.G. & Hautier, L. (Eds.) Evolution of the Rodents. Cambridge: Cambridge University Press, pp. 277–299. Available from: 10.1017/cbo9781107360150.011 [DOI] [Google Scholar]
- Hautier, L. , Michaux, J. , Marivaux, L. & Vianey‐Liaud, M. (2008) Evolution of the zygomasseteric construction in Rodentia, as revealed by a geometric morphometric analysis of the mandible of Graphiurus (Rodentia, Gliridae). Zoological Journal of the Linnean Society, 154, 807–821. Available from: 10.1111/j.1096-3642.2008.00453.x [DOI] [Google Scholar]
- Heritage, S. , Fernández, D. , Sallam, H.M. , Cronin, D.T. , Esara Echube, J.M. & Seiffert, E.R. (2016) Ancient phylogenetic divergence of the enigmatic African rodent Zenkerella and the origin of anomalurid gliding. PeerJ, 4, e2320. Available from: 10.7717/peerj.2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiiemae, K.M. (1967) Masticatory function in the mammals. Journal of Dental Research, 46, 883–893. Available from: 10.1177/00220345670460054601 [DOI] [PubMed] [Google Scholar]
- Hill, J.E. (1937) Morphology of the pocket gopher, mammalian genus Thomomys . University of California Publications in Zoology, 42, 81–172. [Google Scholar]
- Howell, A.B. (1932) The Saltatorial rodent Dipodomys: the functional and comparative anatomy of its muscular and osseous systems. Proceedings of the American Academy of Arts and Sciences, 67, 377–536. Available from: 10.2307/20022915 [DOI] [Google Scholar]
- Huchon, D. , Madsen, O. , Sibbald, M.J.J.B. , Ament, K. , Stanhope, M.J. , Catzeflis, F. et al. (2002) Rodent phylogeny and a timescale for the evolution of Glires: evidence from an extensive taxon sampling using three nuclear genes. Molecular Biology and Evolution, 19, 1053–1065. Available from: 10.1093/oxfordjournals.molbev.a004164 [DOI] [PubMed] [Google Scholar]
- Jaeger, J.‐J. (1988) Rodent phylogeny: new data and old problems. In: Benton, M.J. (Ed.) The phylogeny and classification of the Tetrapods. Oxford: Clarendon Press, pp. 177–199. [Google Scholar]
- Jaeger, J.‐J. , Denys, C. & Coiffait, B. (1985) New Phiomorpha and Anomaluridae from the late Eocene of north‐West Africa: phylogenetic implications. In: Luckett, W.P. & Hartenberger, J.‐L. (Eds.) Evolutionary relationships among rodents. Boston, MA: Springer US, pp. 567–588. [Google Scholar]
- Julliot, C. , Cajani, S. & Gautier‐Hion, A. (1998) Anomalures (Rodentia, Anomaluridae) in Central Gabon: species composition, population densities and ecology. Mammalia, 62, 9–22. Available from: 10.1515/mamm.1998.62.1.9 [DOI] [Google Scholar]
- Kingdon, J. (2013) Family Anomaluridae: anomalures. In: Happold, D.C.D. (Ed.) Mammals of Africa: volume III: rodents, hares and rabbits. London: Bloomsbury Publishing, pp. 602–617. [Google Scholar]
- Klingener, D. (1964) The comparative myology of four dipodoid rodents (genera Zapus, Napaeozapus, Sicista, and Jaculus) . Miscellaneous Publications of the Museum of Zoology, University of Michigan, 124.
- Kunstler, M.J. (1887) L’appareil masticateur chez les rongeurs. Annales des sciences Naturelles, Zoologie, 7, 150–166. [Google Scholar]
- Landry, S.O. (1999) A proposal for a new classification and nomenclature for the Glires (Lagomorpha and Rodentia). Zoosystematics and Evolution, 75, 283–316. Available from: 10.1002/mmnz.19990750209 [DOI] [Google Scholar]
- Lavocat, R. (1951) Révision de la faune des mammifères oligocènes d'Auvergne et du Velay. Paris: Sciences et Avenir. [Google Scholar]
- Lavocat, R. (1973) Les Rongeurs du Miocène d’Afrique Orientale. Ecole Pratique des Hautes Etudes, Mémoires et Travaux de l’Institut de Montpellier, 1, 1–284. [Google Scholar]
- Lavocat, R. & Parent, J.‐P. (1985) Phylogenetic analysis of middle ear features in fossil and living rodents. In: Luckett, W.P. & Hartenberger, J.‐L. (Eds.) Evolutionary relationships among Rodents, NATO Advanced Science Institutes (ASI) Series. Boston, MA: Springer US, pp. 333–354. [Google Scholar]
- Lösel, P.D. , van de Kamp, T. , Jayme, A. , Ershov, A. , Faragó, T. , Pichler, O. et al. (2020) Introducing Biomedisa as an open‐source online platform for biomedical image segmentation. Nature Communications, 11, 5577. Available from: 10.1038/s41467-020-19303-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckett, W.P. & Hartenberger, J.‐L. (1985) Evolutionary relationships among rodents: comments and conclusions. In: Luckett, W.P. & Hartenberger, J.‐L. (Eds.) Evolutionary relationships among rodents. Boston, MA: Springer US, pp. 685–712. [Google Scholar]
- Marivaux, L. , Adaci, M. , Bensalah, M. , Rodrigues, H.G. , Hautier, L. , Mahboubi, M. et al. (2011) Zegdoumyidae (Rodentia, Mammalia), stem anomaluroid rodents from the Early to Middle Eocene of Algeria (Gour Lazib, Western Sahara): new dental evidence. Journal of Systematic Palaeontology, 9, 563–588. [Google Scholar]
- Marivaux, L. , Adnet, S. , Benammi, M. , Tabuce, R. & Benammi, M. (2017) Anomaluroid rodents from the earliest Oligocene of Dakhla, Morocco, reveal the long‐lived and morphologically conservative pattern of the Anomaluridae and Nonanomaluridae during the tertiary in Africa. Journal of Systematic Palaeontology, 15, 539–569. Available from: 10.1080/14772019.2016.1206977 [DOI] [Google Scholar]
- Marivaux, L. , Ducrocq, S. , Jaeger, J.‐J. , Marandat, B. , Sudre, J. , Chaimanee, Y. et al. (2005) New remains of Pondaungimys anomaluropsis (Rodentia, Anomaluroidea) from the latest middle Eocene Pondaung formation of Central Myanmar. Journal of Vertebrate Paleontology, 25, 214–227. Available from: 10.1671/0272-4634(2005)025[0214:NROPAR]2.0.CO;2 [DOI] [Google Scholar]
- Marivaux, L. , Essid, E.M. , Marzougui, W. , Khayati Ammar, H. , Merzeraud, G. , Tabuce, R. et al. (2015) The early evolutionary history of anomaluroid rodents in Africa: new dental remains of a zegdoumyid (Zegdoumyidae, Anomaluroidea) from the Eocene of Tunisia. Zoologica Scripta, 44, 117–134. [Google Scholar]
- Matthee, C.A. & Robinson, T.J. (1997) Mitochondrial DNA Phylogeography and comparative cytogenetics of the springhare, Pedetes capensis (Mammalia: Rodentia). Journal of Mammalian Evolution, 4, 53–73. Available from: 10.1023/A:1027331727034 [DOI] [Google Scholar]
- Meng, C. (1990) The auditory region of Reithroparamys delicatissimus (Mammalia, Rodentia) and its systematic implications. American Museum Novitates, 2972, 1–35. [Google Scholar]
- Merriam, C.H. (1885) Monographic revision of the pocket gophers: family Geomyidæ (exclusive of the species of Thomomys). North American Fauna, 8, 1–258. [Google Scholar]
- Miller, G.S. & Gidley, J.W. (1918) Synopsis of the supergeneric groups of rodents. Journal of the Washington Academy of Sciences, 8, 431–448. [Google Scholar]
- Montgelard, C. , Bentz, S. , Tirard, C. , Verneau, O. & Catzeflis, F.M. (2002) Molecular systematics of Sciurognathi (Rodentia): the mitochondrial cytochrome b and 12S rRNA genes support the Anomaluroidea (Pedetidae and Anomaluridae). Molecular Phylogenetics and Evolution, 22, 220–233. Available from: 10.1006/mpev.2001.1056 [DOI] [PubMed] [Google Scholar]
- Müller, A. (1933) Die Kaumuskulatur des Hydrochoerus capybara und ihre Bedeutung für die Formgestaltung des Schädels. Morphologisches Jahrbuch, 72, 1–59. [Google Scholar]
- Offermans, M. & De Vree, F. (1989) Morphology of the masticatory apparatus in the springhare, Pedetes capensis . Journal of Mammalogy, 70, 701–711. Available from: 10.2307/1381705 [DOI] [Google Scholar]
- Offermans, M. & De Vree, F. (1993) Electromyography and mechanics of mastication in the springhare, Pedetes capensis (Rodentia, Pedetidae). Belgian Journal of Zoology, 123, 231–261. [Google Scholar]
- Orcutt, E.E. (1940) Studies on the muscles of the head, neck, and pectoral appendages of Geomys bursarius . Journal of Mammalogy, 21, 37–52. Available from: 10.2307/1374656 [DOI] [Google Scholar]
- Panyutina, A.A. , Chernova, O.F. & Soldatova, I.B. (2020) Morphological peculiarities in the integument of enigmatic anomalurid gliders (Anomaluridae, Rodentia). Journal of Anatomy, 237, 404–426. Available from: 10.1111/joa.13211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, F.G. (1894) On the myology of the sciuromorphine and hystricomorphine rodents. Proceedings of the Zoological Society of London, 1894, 251–296. [Google Scholar]
- Parsons, F.G. (1898) On the anatomy of the African jumping‐hare (Pedetes caffer) compared with that of the Dipodidae. Proceedings of the Zoological Society of London, 1898, 858–890. Available from: 10.1111/j.1096-3642.1898.tb03190.x [DOI] [Google Scholar]
- Parsons, F.G. (1899) The position of Anomalurus as indicated by its myology. Zoological Journal of the Linnean Society, 27, 317–334. Available from: 10.1111/j.1096-3642.1899.tb00250.x [DOI] [Google Scholar]
- Pérez del Val, J. , Juste, J. & Castroviejo, J. (1995) A review of Zenkerella insignis, Matschie, 1898 (Rodentia, Anomaluridae) first records in Bioko Island (Equatorial Guinea). Mammalia, 59, 437–439. Available from: 10.1515/mamm.1995.59.3.437 [DOI] [Google Scholar]
- Pickford, M. & Mein, P. (2006) Early Middle Miocene mammals from Moroto II, Uganda. Beiträge zur Paläontologie, 30, 361–386. [Google Scholar]
- Pickford, M. & Mein, P. (2011) Nuevos Pedetidae (Rodentia: Mammalia) del Mio‐Plioceno de Africa. Estudios Geológicos, 67, 455–469. [Google Scholar]
- Potapova, E.G. (2017) Specificity and directions of morpho‐functional specialisation of the jaw apparatus of Anomaluridae (Rodentia, Mammalia). In: Popovkina, A.B. , Potapova, E.G. & Kryukova, N.V. (Eds.) Proceedings of the all‐Russian conference and School for Young Scientists in memory of Felix Yanovitch Dzerzhinsky. Evolutionary and functional morphology of vertebrates. Moscow: Trudy Zoologicheskogo Instituta Scientific Publishing House, pp. 240–246. [Google Scholar]
- Potapova, E.G. (2018) The tongue structure in scaly‐tailed squirrels (Rodentia, Anomaluridae). Biology Bulletin of the Russian Academy of Sciences, 45, 865–871. Available from: 10.1134/S1062359018080137 [DOI] [Google Scholar]
- Potapova, E.G. (2020) Morphofunctional transformations of the jaw muscles in rodent evolution. Biology Bulletin Reviews, 10, 394–406. Available from: 10.1134/S2079086420050072 [DOI] [Google Scholar]
- Rinker, G.C. (1954) The comparative myology of the mammalian genera Sigmodon, Oryzomys, Neotoma, and Peromyscus (Cricetinae), with remarks on their intergeneric relationships . Miscellaneous Publications of the Museum of Zoology, University of Michigan 83. Available from: https://deepblue.lib.umich.edu/handle/2027.42/56328. [Accessed 6th May 2021].
- Rinker, G.C. (1963) A comparative myological study of three subgenera of Peromyscus . Occasional Papers of the Museum of Zoology, University of Michigan 632. Available from: https://deepblue.lib.umich.edu/handle/2027.42/57068. [Accessed 6th May 2021].
- Rosevear, D.R. (1969) The rodents of West Africa. London: Trustees of the British Museum (National History). [Google Scholar]
- Rinker, G.C. & Hooper, E.T. (1950) Notes on the cranial musculature in two subgenera of Reithrodontomys (harvest mice) . Occasional papers of the Museum of Zoology, University of Michigan, pp. 528.
- Ruf, I. , Frahnert, S. & Maier, W. (2009) The chorda tympani and its significance for rodent phylogeny. Mammalian Biology, 74, 100–113. Available from: 10.1016/j.mambio.2008.01.002 [DOI] [Google Scholar]
- Ryan, J.M. (1989) Comparative myology and phylogenetic systematics of the Heteromyidae (Mammalia, Rodentia) . Miscellaneous Publications of the Museum of Zoology, University of Michigan 176. Available from: https://deepblue.lib.umich.edu/handle/2027.42/56420. [Accessed 6th May 2021].
- Sallam, H.M. , Seiffert, E.R. & Simons, E.L. (2010) A highly derived anomalurid rodent (Mammalia) from the earliest late Eocene of Egypt. Palaeontology, 53, 803–813. Available from: 10.1111/j.1475-4983.2010.00962.x [DOI] [Google Scholar]
- Satoh, K. & Iwaku, F. (2004) Internal architecture, origin‐insertion site, and mass of jaw muscles in Old World hamsters. Journal of Morphology, 260, 101–116. Available from: 10.1002/jmor.10198 [DOI] [PubMed] [Google Scholar]
- Satoh, K. & Iwaku, F. (2006) Jaw muscle functional anatomy in northern grasshopper mouse, Onychomys leucogaster, a carnivorous murid. Journal of Morphology, 267, 987–999. Available from: 10.1002/jmor.10443 [DOI] [PubMed] [Google Scholar]
- Satoh, K. & Iwaku, F. (2009) Structure and direction of jaw adductor muscles as herbivorous adaptations in Neotoma mexicana (Muridae, Rodentia). Zoomorphology, 128, 339–348. Available from: 10.1007/s00435-009-0094-8 [DOI] [Google Scholar]
- Simpson, G.G. (1945) The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History, 85, 1–350. [Google Scholar]
- Stehlin, H.G. & Schaub, S. (1951) Die Trigonodontie der simplicidentaten Nager. Mammalia, 15, 204–210. [Google Scholar]
- Swanson, M.T. , Oliveros, C.H. & Esselstyn, J.A. (2019) A phylogenomic rodent tree reveals the repeated evolution of masseter architectures. Proceedings of the Royal Society B: Biological Sciences, 286, 20190672. Available from: 10.1098/rspb.2019.0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, O. (1896) On the genera of rodents: an attempt to bring up to date the current arrangement of the order. Proceedings of the Zoological Society of London, 64, 1012–1028. Available from: 10.1111/j.1096-3642.1896.tb03097.x [DOI] [Google Scholar]
- Thorington, R.K. & Darrow, K. (1996) Jaw muscles of Old World squirrels. Journal of Morphology, 230, 145–165. Available from: [DOI] [PubMed] [Google Scholar]
- Tullberg, T. (1899) Ueber das System der Nagethiere: eine phylogenetische Studie. Upsala: Druck der Akademischen Buchdruckerei, Edv. Berling. Available from: 10.5962/bhl.title.1733 [DOI] [Google Scholar]
- Turnbull, W.D. (1970) Mammalian masticatory apparatus. Chicago, IL: Field Museum Press. Available from: 10.5962/bhl.title.5442 [DOI] [Google Scholar]
- Vianey‐Liaud, M. (1994) La radiation des Gliridae (Rodentia) à l'Eocène supérieur en Europe Occidentale, et sa descendance Oligocène. Münchner Geowissenschaftliche Abhandlungen Reihe A, Geologie Und Paläontologie, 26, 117–160. [Google Scholar]
- Vianey‐Liaud, M. & Marivaux, L. (2021) The beginning of the adaptive radiation of Theridomorpha (Rodentia) in Western Europe: morphological and phylogenetic analyses of early and middle Eocene taxa; implications for systematics. Palaeovertebrata, 44, 1–105. Available from: 10.18563/pv.44.2.e2 [DOI] [Google Scholar]
- Vorontsov, N.N. (1982) The hamsters (Cricetidae) of the world fauna. Part 1. Morphology and ecology. Fauna of the USSR, New Series Mo 125. Mammals, 3, 1–452. [Google Scholar]
- Voss, R.S. (1988) Systematics and ecology of Ichthyomyine rodents (Muroidea): patterns of morphological evolution in a small adaptive radiation. Bulletin of the American Museum of Natural History, 188, 260–493. [Google Scholar]
- Waterhouse, G.R. (1839) Observations on the Rodentia with a view to point out groups as indicated by the structure of the crania in this order of mammals. Magazine of Natural History, 3, 90–96. [Google Scholar]
- Wilson, D.E. , Lacher, T.E. & Mittermeier, R.A. (2016) Handbook of the mammals of the world: vol. 6: Lagomorphs and rodents I. Barcelona, Spain: Lynx Edicions. [Google Scholar]
- Winge, H. (1887) Jordfundne og nulevende gnavere (Rodentia) fra Lagoa Santa, Minas Geraes, Brasilien Med udsigt over Gnavernes indbyrdes. E Museo Lundii, Kjøbenhavn, 1, 1–178. [Google Scholar]
- Winge, H. (1924) Pattedyr‐slaegter. Vol. 2. Rodentia, Carnivora, Primates. Copenhagen: H. Hagerups Forlag. [Google Scholar]
- Wood, A.E. (1955) A revised classification of the Rodents. Journal of Mammalogy, 36, 165–187. [Google Scholar]
- Wood, A.E. (1965) Grades and clades among rodents. Evolution, 19, 115–130. Available from: 10.1111/j.1558-5646.1965.tb01696.x [DOI] [Google Scholar]
- Wood, A.E. & White, R.R. (1950) The myology of the chinchilla. Journal of Morphology, 86, 547–597. Available from: 10.1002/jmor.1050860304 [DOI] [PubMed] [Google Scholar]
- Woods, C. (1972) Comparative myology of jaw, hyoid, and pectoral appendicular regions of new and Old World hystricomorph rodents. Bulletin of the American Museum of Natural History, 147(3), 117–198. [Google Scholar]
- Woods, C.A. & Hermanson, J.W. (1985) Myology of hystricognath rodents: an analysis of form, function, and phylogeny. In: Luckett, W.P. & Hartenberger, J.‐L. (Eds.) Evolutionary relationships among rodents. Boston, MA: Springer US, pp. 515–548. [Google Scholar]
- Woods, C.A. & Howland, E.B. (1979) Adaptive radiation of capromyid rodents: anatomy of the masticatory apparatus. Journal of Mammalogy, 60, 95–116. Available from: 10.2307/1379762 [DOI] [Google Scholar]
- Zherebtsova, O.V. & Potapova, E.G. (2019) Pathways and level of morphological adaptations in modern Diatomyidae and Ctenodactylidae (Rodentia). Biology Bulletin of the Russian Academy of Sciences, 46, 710–729. Available from: 10.1134/S1062359019070124 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:
Data Availability Statement
The 3D surface models of the masticatory apparatus of the specimens are available on MorphoMuseuM.


