HISTORIC BACKGROUND
Myriad theories attempting to explain the underlying causes of cognitive, emotional, and behavioral abnormalities have existed throughout history. In ancient Greek civilization the heart was the organ touted as the seat of the soul, responsible for mental functions. A shift from this belief to one professing that the seat of mental functions was the head dates back to Democritus and Plato (Finger, 1994), but it was not until Galen (ad 129–200) that sensory, motor, and cognitive processes were hypothesized as distinct functions of the nervous system and that the brain was the center of this nervous system (see Zola-Morgan, 1995, for a review).
From the time of Galen to approximately the 18th century, debate ensued as to whether the ventricular system or the brain itself subserved cognition, emotion, and behavior. Nemesius (c. 400 ad), for example, postulated that sensation and perception resided in the anterior ventricles, thinking and reasoning in the third ventricle, and memory in the fourth ventricle (Benton, 1988). Modern concepts of functional localization, highlighting where select cognitive processes (e.g., language, memory, visuospatial abilities) are localized and organized in the brain, began in earnest in the 19th century with the observations, experiments, and writings of physicians including Franz Joseph Gall, Johann Spurzheim, Hughlings Jackson, David Ferrier, Paul Broca, Carl Wernicke, and Eduard Hitzig.
Historically, brain–behavior relations were inferred through individual case studies or aggregates of cases with a common lesion locus, where a localized brain lesion was associated with a specific functional deficit. Gall, in the early 1800s, was the first to confidently hypothesize that the brain consisted of different parts or regions, and that these different regions subserved disociable cognitive functions (Benton, 1988). Gall’s theory was strengthened when, in the mid-1800s, Paul Broca observed a series of patients with speech production impairment, who on autopsy were observed to have left frontal lobe damage (Berker et al., 1986). From these observations, Broca hypothesized that the left frontal lobe was responsible for productive speech (Lazar, 2011; Lazar and Mohr, 2011). Broca’s discovery of the relationship between non-fluent aphasia and anterior left hemisphere disease was critical to the inception of hemispheric specialization and localization of function, identifying the neural mechanisms underlying behavioral processes (Benton, 1988). In a similar vein, Carl Wernicke observed a series of patients who had difficulty comprehending spoken language (Wilkins and Brody, 1970). Unlike Broca’s patients who were unable to produce words, many of Wernicke’s patients could produce speech; however, the speech produced was generally unintelligible. Autopsy revealed lesions in the posterior temporal lobe which led Wernicke to postulate that the posterior temporal lobe subserved the ability to understand and produce intelligible language.
Although reports of these brain–behavior relationships were groundbreaking, Jackson (1878) wisely cautioned his fellow scientists that “to locate the lesion which destroys speech and to locate speech are two different things.” Kurt Goldstein reiterated Jackson’s warning about correlation and specificity in the mid-1900s (Goldstein, 1946):
We are by no means justified to infer directly from a correlation between a localized defect and a defect in performance a relationship between the concerned area and a definite performance. The facts allow only localisation of defects, but not a localisation of performances. The latter remains a theoretical interpretation which can be tried only after a careful analysis of the functions corresponding to the performance and defect. Such an analysis induces a concept about brain function and its relation to performance which, as unsatisfactory as it may have been till now, differs in principle from the concept of circumscribed localisation
(pp. 25–26).
A simple correlation or relation between anatomical structure and behavioral function provides evidence for an association and can be useful in exploratory analysis, but it is inadequate for demonstrating specificity between a neuroanatomical structure and behavioral function.
SINGLE DISSOCIATION MODEL: LESION STUDIES
Demonstrating a single dissociation between structures and functions extends a simple association by showing that a lesion is related to a specific cognitive, motor, or behavioral function and is not related to a different cognitive, motor, or behavioral function. As Figure 11.1 shows, a dissociation is demonstrated when lesion “1” is associated with impairment in function “1” and lesion “1” is not associated with impairment in function “2.” Alternatively, one can demonstrate that function ‘1’ is associated with lesion “1” and not associated with lesion “2.” This dissociation model provides stronger evidence of the specificity between structure and function compared with simply demonstrating an association between a single structure and a single function.
Fig. 11.1.
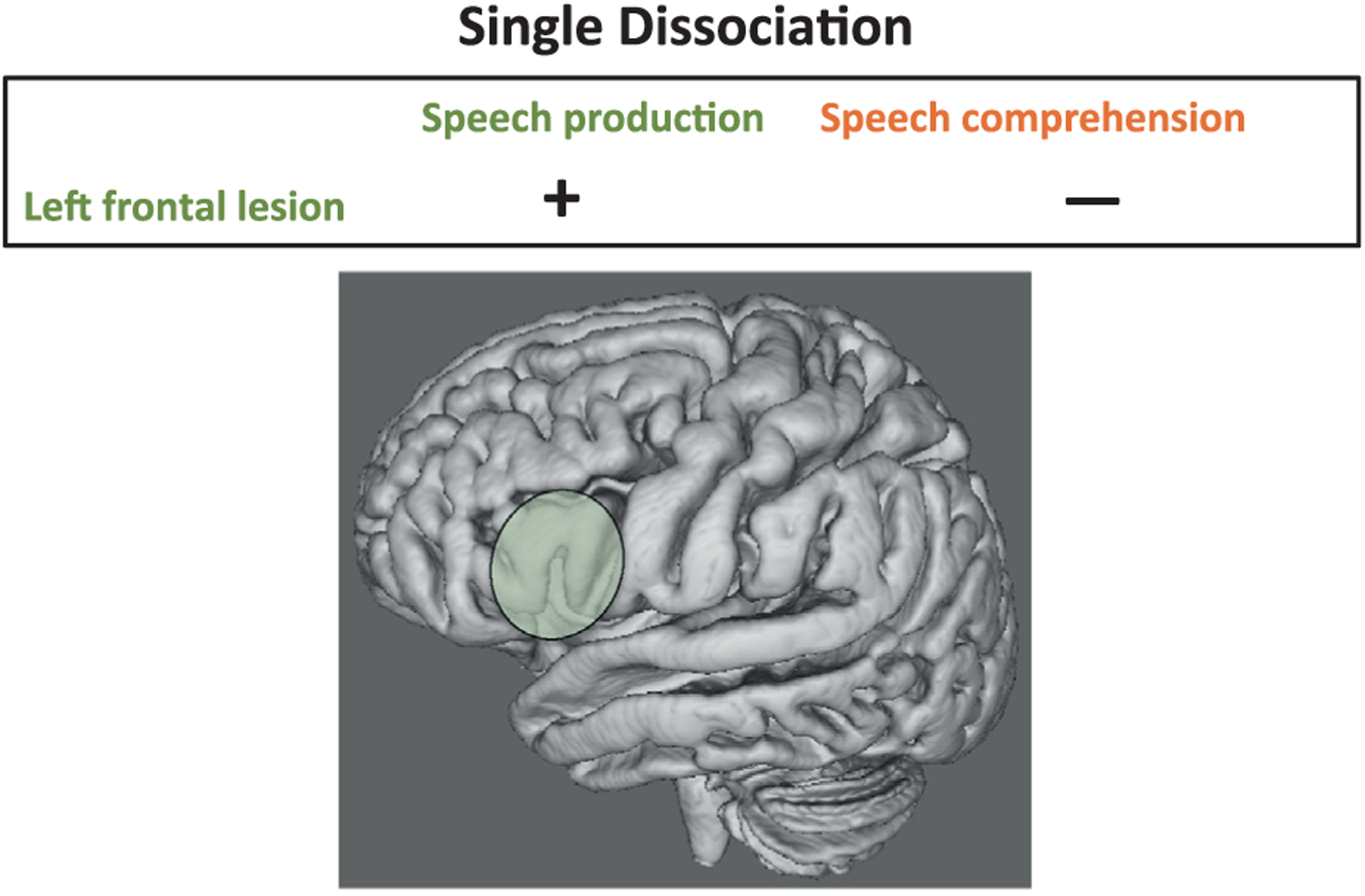
This figure demonstrates the single dissociation model. Individuals with lesions in the left frontal lobe (green-shaded area) often demonstrate speech production but not speech comprehension impairments.
The single dissociation model is inadequate for drawing conclusions about the specificity between brain structure and function. Lashley (1952) and Teuber (1955) highlighted a number of these problems, which they cautioned could lead to incorrect conclusions about functional localization. For example, deficits observed in function “1” and not function “2” could be due to differences between the tests used to assess these functions (one test being more or less demanding or sensitive to brain impairment or requiring greater cognitive resources than the other) as opposed to demonstrating brain–behavior specificity (Jones, 1983; Young et al., 2000). Thus, differences in structure–function associations could be due to test demand differences and not to selectivity between brain structure and function.
DOUBLE DISSOCIATION MODEL: LESION STUDIES
Ability to parse component processes of complex behaviors is necessary for understanding structure–function relationships and drawing conclusions about functional localization. Deficits in memory, for example, can result from impairment in a number of different cognitive component processes affecting encoding, storage, or retrieval of learned information. Frontal lobe lesions are often associated with impairments in the ability to organize and plan, and these impairments can contribute to problems with memory; this is not to say, however, that the frontal lobe is the “seat of memory functions.” Thus, even when a brain region is associated with a particular cognitive or motor ability and that brain region is also shown to not be associated with a different function, it does not necessarily reflect a selective relationship between brain structure and function. Rather, it could demonstrate that this brain region serves a supportive role in the mediation of that function.
To address the limitations of the single dissociation model, Teuber (1955) championed the model of double dissociation (for historical reviews, see Milner and Teuber, 1968; Bates et al., 2003; Bigler, 2009). In the double dissociation model, structure–function relationships can be inferred between two or more individuals or clinical diagnoses when two functions are disrupted independently from one another: Lesion “1” is associated with impairment in function “1” but not in function “2,” and lesion “2” (a lesion in a different area of the brain) is associated with impairment in function “2” but not in function “1.” This model lends stronger evidence than does the single dissociation model that a particular brain region is specifically associated with a particular function. Figure 11.2 demonstrates a double dissociation based on the example of language production and comprehension. In this example, a left frontal cortical lesion is associated with speech production but not language comprehension, whereas a left temporoparietal cortical lesion is associated with language comprehension but not speech production. This double dissociation supports the hypothesis that the left frontal lobe is selectively related to speech production, whereas the left temporoparietal lobe is selectively related to language comprehension.
Fig. 11.2.
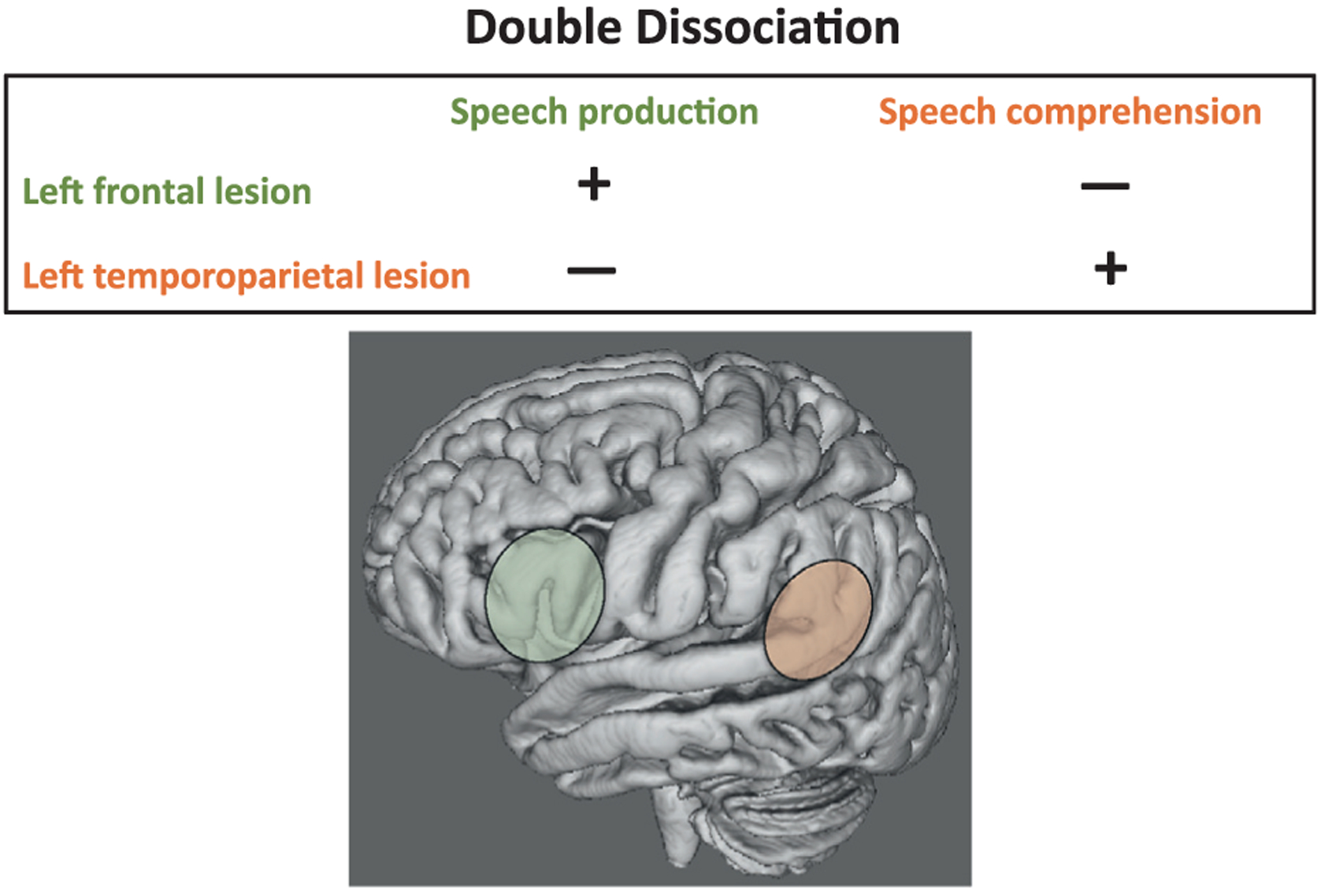
This figure demonstrates the classical double dissociation model. Left frontal lobe lesions (green-shaded area) are associated with impairments in speech production and not speech comprehension, whereas left temporoparietal lobe lesions (orange-shaded area) are associated with the opposite pattern, specifically, impairment in speech comprehension with relative sparing of speech production. This double dissociation lends confidence to the selectivity of brain structure–function relations, where a specific brain region is more relevant to a selective process than to another.
DOUBLE DISSOCIATION MODEL IN CONDITIONS AFFECTING MULTIPLE NEURAL SYSTEMS
Modifications of the classic double dissociation model used to establish specificity between brain structure and function in lesion studies have been successfully used in neurologic conditions that affect multiple brain structures, regions, and systems. Models include studies that compare clinical groups with a control group, clinical groups with one another, and heterogeneity within a single clinical group. These modifications to the classic double dissociation model are presented next.
Between-groups model
Many neurodegenerative conditions (e.g., Alzheimer’s disease, Parkinson’s disease, Huntington’s disease (HD)) affect multiple neural systems and cannot be characterized by a single, discrete lesion. In these neurologic diseases, a double dissociation can be demonstrated and brain–behavior relations inferred by showing that one group of patients with a specific disease (group “1”) demonstrates one pattern of spared and impaired functions (impaired function “1” and spared function “2”), whereas another group of patients with a different disease (group “2”) demonstrates the opposite pattern of spared and impaired functions (impaired function “2” and spared function “1”) (Jones, 1983). This pattern of observations suggests that brain abnormalities in these two conditions result in different functional impairments and that these functional impairments, in turn, are related to specific and dissociable neural networks selectively disrupted by the disease.
Dissociability of memory processes (e.g., explicit versus implicit memory processes) has been demonstrated using this model. Individuals, for example, with Korsakoff’s syndrome (KS), an amnesia associated with chronic heavy drinking and concomitant thiamine deficiency, show severe anterograde explicit memory impairment with relatively intact implicit, procedural memory compared with control subjects (Cermak et al., 1985; Fama et al., 2006). By contrast, individuals with HD demonstrate the opposite pattern, with relatively intact explicit memory but impaired implicit, procedural memory (Heindel et al., 1988; Gabrieli et al., 1997). These patterns of sparing and impairment lend support to the hypothesis that explicit memory and implicit memory are dissociable processes and that the different neuropathology associated with KS and HD underlies the dissociation: thalamic regions and associated neural networks in KS and striatal regions and associated neural networks in HD. This type of double dissociation can be demonstrated by comparing two neurologic groups to a normal control group (Fig. 11.3, left) or to each other (Fig. 11.3, right) (Jones, 1983). A control group can be helpful in establishing whether clinical groups show impairment relative to controls as well as a difference in cognitive performance pattern. For example, one group demonstrates impairment compared with control subjects while the other group performs comparably to controls, or both clinical groups demonstrate impairment that simply differs in level of severity between the groups.
Fig. 11.3.
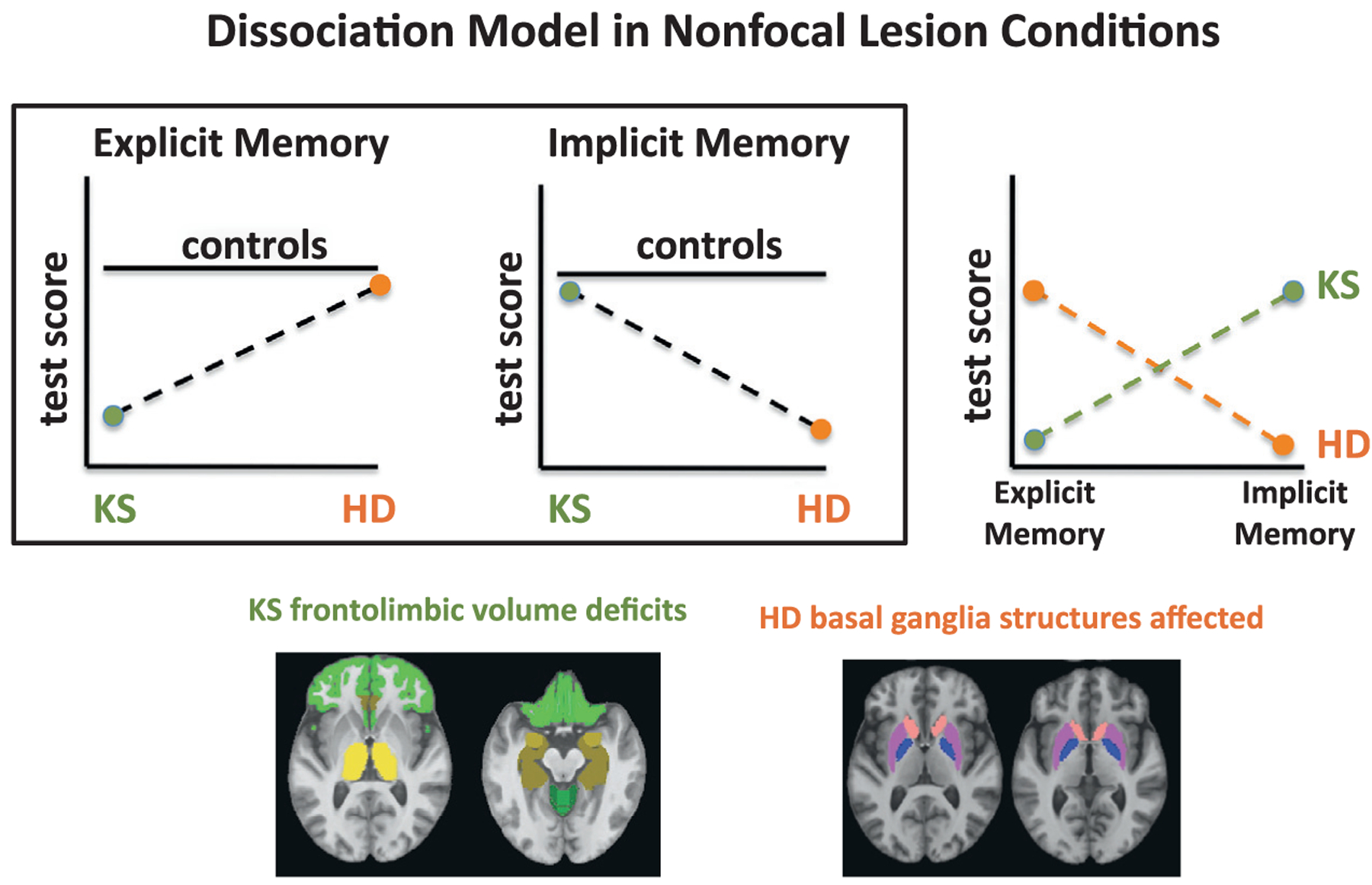
Dissociations can be demonstrated in non-focal lesion conditions. Here, explicit and implicit memory scores in individuals with Korsakoff’s syndrome (KS) and others with Huntington’s disease (HD) are compared with a control group and between themselves. Studies have generally demonstrated that explicit memory processes are more affected than implicit memory processes in KS, whereas the opposite pattern, greater impairment in implicit than explicit memory processes, has been demonstrated in conditions with primary subcortical dysfunction such as HD. This pattern of deficits can be visually compared with a group of controls (left and middle graphs) or can be shown as a cross-over interaction between the two neurologic groups (right graph). The differences in mnemonic processes observed in these conditions are presumably caused by the different neuroanatomic regions affected in these disorders: frontolimbic deficits in KS and basal ganglia dysfunction in HD.
Within-group model
Another modification of the classic double dissociation model can be used within a single neurologic condition when heterogeneity and variance in brain damage and behavioral impairment are adequate for correlational analyses. Alcohol use disorders modify brain structures and, although not a result of a frank lesion, neurologic dysfunction in alcoholism includes widespread neuronal damage and cognitive, motor, and behavioral deficits. These deficits show a specific pattern of selective damage, with particular brain structures (e.g., frontal lobes, corpus callosum, and cerebellum) and selective neural networks more affected than others (Fein et al., 1995; Pfefferbaum et al., 1997, 2006, 2009; Meyerhoff, 2005; Makris et al., 2008). Understanding how alcohol-related brain abnormalities affect cognition, motor, and emotional processes is clinically and theoretically relevant. Identifying patterns of impairment and sparing lends evidence as to the specificity of different neural systems and cognitive processes (Caramazza, 1986) and to the functional and neural separation of abilities (Bates et al., 2003).
Unlike neurodegenerative conditions, brain impairments and behavioral deficits associated with alcoholism can improve or worsen depending on the time assessed during its typical dynamic course. Abstinence, for example, can be associated with increased volumes of gray and white matter and improvement in selective cognitive and motor functioning (Cardenas et al., 2007; Rosenbloom and Pfefferbaum, 2008; Sullivan et al., 2010). Heterogeneity in both brain structure and cognitive processes measured with quantitative measures of brain structure (magnetic resonance imaging (MRI): regional tissue volume) and quantitative measures of behavioral processes (e.g., neuropsychologic tests of cognition, motor, and emotional abilities) allows for brain—behavior associations to be demonstrated with correlational analysis on a continuum. Single and double dissociations in brain structure and function have been observed in alcoholism; examples of how this model can be used in alcoholism are shown below.
A single dissociation involving cerebellar volume and postural balance (Sullivan et al., 2006) was demonstrated when longer sway paths measured on a force platform, indicating imbalance during quiet standing, correlated with smaller volumes of the anterior cerebellar vermis (Fig. 11.4). In addition to this simple association, no relationship between lateral cerebellar volumes and postural balance was observed. This pattern of findings demonstrates the selectivity of the relationship between vermian (but not lateral) cerebellar volumes and postural stability using the single dissociation model.
Fig. 11.4.
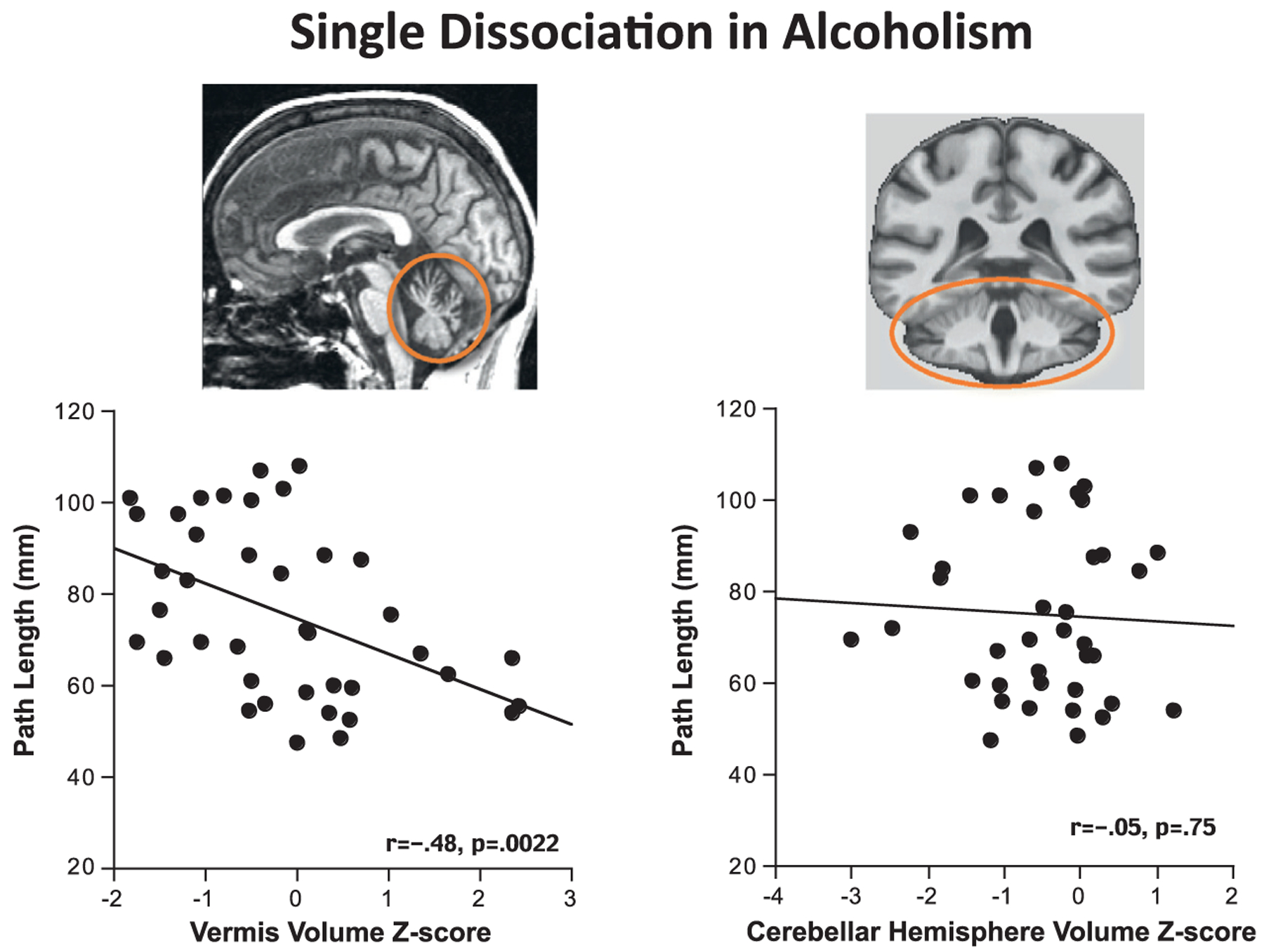
These scatterplots depict a single dissociation within a group of alcoholics. Here it was demonstrated that volumes of the anterior vermis, but not lateral cerebellar hemisphere volumes, were associated with balance stability as assessed by path length sway.
A double dissociation in alcoholism was identified by Pfefferbaum and colleagues (2006) (Fig. 11.5) using the mean diffusivity metric of MR diffusion tensor imaging measuring local microstructural integrity of the corpus callosum and two cognitive tests. In this study, lower working memory scores were associated with greater diffusivity in the genu but not the splenium, whereas poorer matrix reasoning scores correlated with higher diffusivity in the splenium but not the genu. This double dissociation provides evidence for the specificity of fiber tract integrity of the anterior versus posterior corpus callosum to information processing and reasoning. These examples demonstrate that selective brain regions within chronic alcoholism can differentially affect behavioral processes.
Fig. 11.5.
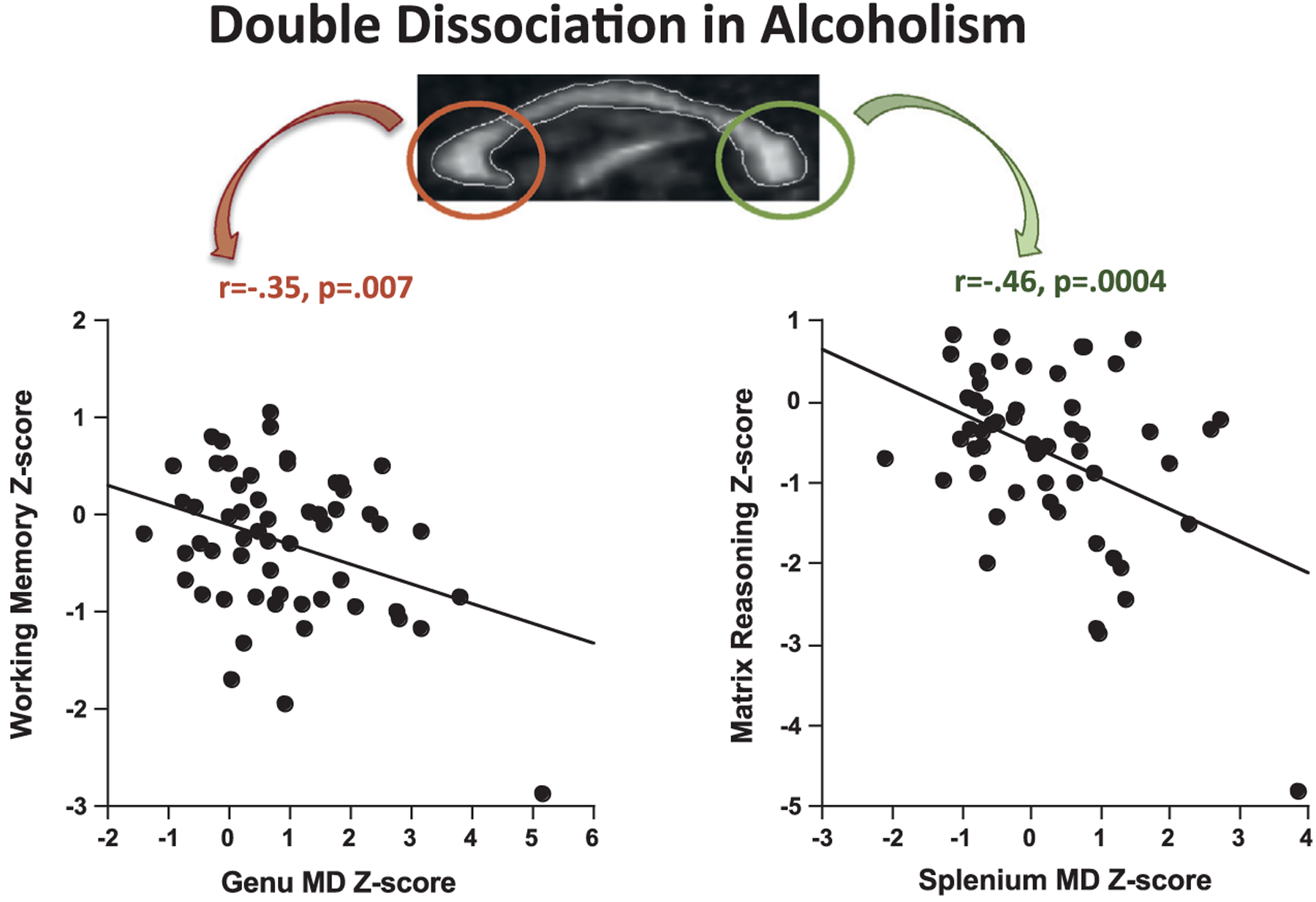
In a group of 57 alcoholics, lower working memory scores were selectively correlated with high mean diffusivity (MD) in the genu but not the splenium, whereas lower visuospatial test scores were selectively correlated with high MD in the splenium but not the genu. Selectivity of these relations was established with multiple regression analysis. As reported in Pfefferbaum et al. (2006, p. 1004), “Genu and splenium MD values of the focal regions were entered as simultaneous predictors of working memory and accounted for 13% of the variance. Genu MD made a significant independent contribution to the variance (partial F = 4.10, p < 0.05), whereas splenium MD did not (partial F = 1.18, p > 0.70). For matrix reasoning, both genu MD (partial F = 3.55, p < 0.07) and splenium MD (partial F = 4.92, p = 0.03) were independent contributors to the total variance (26%). When all four regional DTI [diffusion tensor imaging] measures (i.e., genu fractional anisotropy (FA) and MD and splenium FA and MD) were entered as predictors of matrix reasoning, 28% of the variance was accounted for but only splenium MD made a significant independent contribution (partial F = 4.28, p < 0.05) to the overall variance. (For a detailed description of DTI methods, see Chapter 17 in this volume.)
Multiple dissociations and relevance to establishing network selectivity
Although the classic double dissociation model and its modifications provide strong evidence for selective brain structure–function relations, there are theoretic limitations to this model (Van Orden et al., 2001; Barr and Goldberg, 2003). A fundamental limitation is the assumption of modularity of function, which assumes that each structure and function can be separate and dissociable (Shallice, 1988), and that there are at most two components to differentiate from one another (Baddeley, 2003). That limitation, however, might be considered a “straw man” argument and be readily supplanted by models involving multiple dissociations. Neural networks and systems underlie complex neuropsychologic functions; a functional and structural network model has supplanted the simple localization model. The network approach posits that neuropsychologic functions (e.g., memory, attention, visuospatial processes) comprise multiple component processes, any of which, if impaired, affects that function.
The fundamental principles of double dissociation can be applied to intrinsic functional networks, which are identified with resting-state functional MRI, are replicable across study sites, are dissociable, and appear to be a network substrate of complex constellations of behavior. For example, nodes of the default mode network (DMN), comprising the medial prefrontal cortex, posterior cingulate cortex, precuneus, and medial temporal lobe (Raichle et al., 2001; Greicius et al., 2003, 2009), are maximally active during rest and considered to serve functions of self-reference. The DMN is distinct from other intrinsic networks, such as the executive control network, which links dorsolateral frontal and parietal structures and is relevant to salience processing functions (Seeley et al., 2007; Tomasi et al., 2009). These networks are dissociable and are differentially vulnerable to disease (Seeley et al., 2009). The distinction of these networks indicates their dissociability and therefore the need for rigorous application of selectivity models to establish their specificity constellation of functions.
Regardless of the model, brain–behavior relationships demonstrate associations (correlations) and do not speak to causation or directionality of effect. Despite these limitations, the double (or multiple) dissociation model has been and continues to be a powerful heuristic and statistical tool to pursue objective and quantitative understanding of complex cognitive systems (Baddeley, 2003). Knowledge of the current limitations of the double dissociation model and understanding of the strength of the inferences obtained from the results of these studies can guide future hypotheses concerning the relationships of brain structure and function in chronic alcoholism and other conditions affecting the brain and nervous system whether characterized by discrete or widespread damage.
ACKNOWLEDGMENT
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism AA017347, AA010723, AA017168, and AA021697. We thank Adolf Pfefferbaum, M.D. for his helpful comments on the text and supplying the MR images for the figures throughout the chapter.
REFERENCES
- Baddeley A (2003). Double dissociations: not magic, but still useful. Cortex 39: 129–131. [DOI] [PubMed] [Google Scholar]
- Barr WB, Goldberg E (2003). Pitfalls in the method of double dissociation: delineating the cognitive functions of the hippocampus. Cortex 39: 153–157. [DOI] [PubMed] [Google Scholar]
- Bates E, Appelbaum M, Salcedo J et al. (2003). Quantifying dissociations in neuropsychological research. J Clin Exp Neuropsychol 25: 1128–1153. [DOI] [PubMed] [Google Scholar]
- Benton AL (1988). Neuropsychology: past, present, and future. In: Boller F, Grafman J (Eds.), In: Handbook of Neuropsychology, Vol. 1. Elsevier Science, Amsterdam, pp. 1–29. [Google Scholar]
- Berker EA, Berker AH, Smith A (1986). Translation of Broca’s 1865 report. Localization of speech in the third left frontal convolution. Arch Neurol 43: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Bigler ED (2009). Hans-Lukas Teuber and ‘The riddle of frontal lobe frontal in man’ as published in The frontal granular cortex and behavior (1964). Neuropsychol Rev 19: 9–24. [DOI] [PubMed] [Google Scholar]
- Caramazza A (1986). On drawing inferences about the structure of normal cognitive systems from the analysis of patterns of impaired performance: the case for single-patient studies. Brain Cogn 5: 41–66. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S et al. (2007). Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak LS, Talbot N, Chandler K et al. (1985). The perceptual priming phenomenon in amnesia. Neuropsychologia 23: 615–622. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV (2006). Visuoperceptual priming in alcoholic Korsakoff syndrome. Alcohol Clin Exp Res 30: 680–687. [DOI] [PubMed] [Google Scholar]
- Fein G, Meyerhoff DJ, Weiner MW (1995). Magnetic resonance spectroscopy of the brain in alcohol abuse. Alcohol Health Res World 19: 3056–3314. [PMC free article] [PubMed] [Google Scholar]
- Finger S (1994). Origins of Neuroscience: a history of explorations into brain function. Oxford University Press, New York. [Google Scholar]
- Gabrieli JD, Stebbins GT, Singh J et al. (1997). Intact mirror-tracing and impaired rotary-pursuit skill learning in patients with Huntington’s disease: evidence for dissociable memory systems in skill learning. Neuropsychology 11: 272–281. [DOI] [PubMed] [Google Scholar]
- Goldstein K (1946). Remarks on localisation. Confin Neurol 7: 25–34. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL et al. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V et al. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel WC, Butters M, Salmon DP (1988). Impaired learning of a motor skill in patients with Huntington’s disease. Behav Neurosci 102: 141–147. [DOI] [PubMed] [Google Scholar]
- Jackson HJ (1878). On affections of speech from disease of the brain. Brain 1: 304–330. [Google Scholar]
- Jones GV (1983). On double dissociation of function. Neuropsychologia 21: 397–400. [DOI] [PubMed] [Google Scholar]
- Lashley KS (1952). Functional interpretation of anatomic patterns. Res Publ Assoc Res Nerv Ment Dis 30: 529–547. [PubMed] [Google Scholar]
- Lazar RM (2011). Broca and the biology of language. Neuropsychol Rev 21: 225–226. [DOI] [PubMed] [Google Scholar]
- Lazar RM, Mohr JP (2011). Revisiting the contributions of Paul Broca to the study of aphasia. Neuropsychol Rev 21: 236–239. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK et al. (2008). Decreased volume of the brain reward system in alcoholism. Biol Psychiatry 64: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ (2005). Brain spectroscopic imaging, morphometry, and cognition in social drinkers and recovering alcoholics. Alcohol Clin Exp Res 29: 153–154. [Google Scholar]
- Milner B, Teuber H-L (1968). Alteration of perception and memory in man: reflections: reflections on methods. In: Weiskrantz L (Ed.), Analysis of Behavioral Change. Harper & Row, New York, pp. 268–375. [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH et al. (1997). Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 21: 521–529. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV (2006). Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol Aging 27: 994–1009. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T et al. (2009). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 65: 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M, MacLeod A, Snyder A et al. (2001). A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A (2008). Magnetic resonance imaging of the living brain: evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Res Health 31: 362–376. [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J et al. (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T (1988). From Neuropsychology to Mental Structure. Cambridge University Press, Cambridge. [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A (2006). Effect of vision, touch, and stance on cerebellar vermian-related sway and tremor: a quantitative MRI and physiological study. Cereb Cortex 16: 1077–1086. [DOI] [PubMed] [Google Scholar]
- Sullivan EV,Harris RA,Pfefferbaum A(2010).Alcohol’s effects on brain and behavior. Alcohol Res Health 33: 127–143. [PMC free article] [PubMed] [Google Scholar]
- Teuber H-L (1955). Physiological psychology. Annu Rev Psychol 6: 267–296. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R et al. (2009). Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One 4: e6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Orden GC, Pennington BF, Stone GO (2001). What do double dissociations prove? Cognit Sci 25: 111–172. [Google Scholar]
- Wilkins RH, Brody IA (1970). Wernicke’s sensory aphasia. Arch Neurol 22: 279–282, English translation of C. Wernicke (1874) Der aphasische Symptomencomplex: Eine psychologische Studie auf anatomischer Basis. Breslau: Max Cohn & Weigert. [DOI] [PubMed] [Google Scholar]
- Young MP, Hilgetag CC, Scannell JW (2000). On imputing function to structure from the behavioural effects of brain lesions. Philos Trans R Soc Lond B Biol Sci 355: 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S (1995). Localization of brain function: the legacy of Franz Joseph Gall (1758–1828). Annu Rev Neurosci 18: 359–383. [DOI] [PubMed] [Google Scholar]


