Abstract
OBJECTIVE
To investigate the effects of both the Fc fragment in tumor necrosis factor (TNF) inhibitors and rheumatoid factor (RF) titers on treatment survival, disease activity, and laboratory parameters in patients with rheumatoid arthritis (RA).
METHODS
In this retrospective cohort study, patients with RA who had started any anti-TNF therapy between January 2017 and March 2020 and who had stayed on this treatment for at least six months were included. The data of the patients were compared separately according to continuation or discontinuation of treatment and the presence or absence of Fc portion in the structure of anti-TNFs. Patients who were taking certolizumab pegol (CZP) without the Fc fragment were placed in the “without Fc group” (wo/Fc), while patients who were taking other drugs (adalimumab, etanercept, golimumab, and infliximab) were placed in the “with Fc group” (w/Fc).
RESULTS
Among the 221 RA patients whose data were available, 52 patients met the inclusion criteria and were included in the study. There was a significant difference in the DAS28-CRP score between wo/Fc group and w/Fc group in the third month of treatment (p=0.012). However, this difference did not persist at the sixth month of treatment (p=0.384). According to the cox-regression results, RF titers were determined to have a significant impact on the drug survival of anti-TNF agents when adjustments were made for the effects of other candidate predictors (Hazard ratio: 1.007 (1.002–1.012), p=0.009).
CONCLUSION
Our results suggest that compared to the Fc fragment, RF titers were the more important risk factor in survival of anti-TNF drugs.
Keywords: Fc fragments, rheumatoid arthritis, rheumatoid factor, TNF inhibitors, treatment
Highlight key points
RF titers affect the survival of anti-TNF drugs.
RF antibody, which binds to the Fc portion of the immunoglobulin, may also bind to TNF inhibitors containing Fc fragment due to this feature and may change their activities.
We investigated the effects of both the Fc fragment and RF titers on treatment survival of anti-TNF biologic agents.
We showed that RF titers have significant effects on the survival of anti-TNFs, but we did not find the same result with the Fc fragment.
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that causes inflammation and destruction of the synovial joints. Irreversible structural damage due to the disease leads to a decrease in quality of life and increased disability and mortality [1, 2]. Although RA can occur at any age, the peak incidence is between the fourth and sixth decades. It is about two to three times more common in women [3]. In addition to clinical findings, inflammatory markers (e.g., erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) and antibodies such as anti-cyclic citrullinated peptide (anti-CCP) and rheumatoid factor (RF) are used for diagnosis. RF, which is an antibody that binds to the Fc part of immunoglobulin G (IgG), can also be detected positive in other rheumatic diseases, as well as in infections and malignancies and in healthy individuals [4, 5]. RF responses can be of different isotypes, such as IgG, IgA, or IgM [6]. Although RF and anti-CCP autoantibodies are not detectable in all patients with RA, they are thought to be indicators of impaired immune tolerance and worsening disease course [7]. In the literature, there are studies reporting that both RF subtypes and anti-CCP positivity can change biological therapy responses [8–10].
In the treatment of RA, besides tumor necrosis factor (TNF) inhibitors, non-TNF therapies, such as rituximab, abatacept, tocilizumab, and small oral molecules (JAK inhibitors), are frequently used in clinical practice. Five different forms of TNF inhibitors, which were often the first biological treatment options available for patients unresponsive to conventional synthetic disease-modifying antirheumatic drug (csDMARD) treatments, have been used in the treatment of RA, with different molecular structures, doses, application frequencies, and half-lives [11]. Of the three IgG1 antibodies, infliximab (IFX) is chimeric (mouse–human), while adalimumab (ADA) and golimumab (GOL) have a pure humanized structure. Another anti-TNF drug, etanercept (ETA), is a fusion protein of the Fc part of IgG1 and the human TNF receptor 2. Finally, certolizumab pegol (CZP) is formed by the combination of polyethylene glycol (PEG) and the Fab fragment of a humanized anti-TNF antibody. CZP has no Fc component, unlike the other four TNF inhibitors [12, 13]. Although anti-TNF therapies are used effectively in both RA and in other diseases (e.g., spondyloarthritis, uveitis, and inflammatory bowel disease), treatment is discontinued in some patients due to primary or secondary unresponsiveness or intolerance [11]. The literature has reported that the rate of those who do not tolerate or do not respond adequately to the treatment within one year after starting anti-TNF therapy varies from 21–58% [14]. In previous studies, predictors such as age, gender, initial level of disability, concurrent methotrexate (MTX) usage, RF and anti-CCP levels have been associated with the response to anti-TNF drugs [15, 16]. Recently, it has been suggested that RF antibody, which binds to the Fc portion of the immunoglobulin, may also bind to TNF inhibitors containing Fc fragment due to this feature and may change their activities. A retrospective cohort study reported that CZP without Fc fragment may be a better option than other anti-TNFs in RA patients with high RF titers [17].
In this study, considering the different molecular structures of CZP and the possible effect of RF on anti-TNF therapies in RA patients, we aimed to evaluate disease activity, laboratory parameters, and treatment survival of patients with RA using TNF inhibitors with and without Fc fragments. In addition, we also aimed to examine the effect of RF titers on anti-TNF therapies.
MATERIALS AND METHODS
In this retrospective cohort study, after Erciyes University Faculty of Medicine Ethics Committee approval (date: 08 June 2022, approval number: 2022/448), both the manual files and electronic hospital records of 221 RA patients diagnosed according to American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2010 classification criteria and followed by the Erciyes University Faculty of Medicine rheumatology outpatient clinic were retrospectively scanned. The study was conducted in accordance with the Declaration of Helsinki. Due to the retrospective nature of this study, the requirement for informed consent was waived by the Ethics Committee. Patients who were started on any anti-TNF biologic agent between January 2017 and March 2020 and stayed on this treatment for at least six months were included. This six-month period was determined in order to observe the DAS-28 CRP scores after six months of treatment and to rule out primary unresponsiveness [18], because routine outpatient clinic visits of our patients are made once every three months. The exclusion criteria included the following: 1) being diagnosed after March 2020, 2) starting anti-TNF treatment before January 2017, 3) having used anti-TNF for less than six months, 4) using a non-TNF biologic, and 5) using csDMARD treatment only. Demographic data including age, gender, smoking status, height and weight; and clinical parameters, such as duration of the disease, whether the patient’s first biological treatment was anti-TNF, the name of the anti-TNF drug started within the prescribed dates, the start date of the anti-TNF, whether the anti-TNF therapy was still ongoing, the end date if treatment had been discontinued, the duration of treatment, whether non-TNF biologics were used before anti-TNF was initiated, the presence of steroids or csDMARDs used in combination, numbers of DMARDs used in the past, concomitant nonsteroidal anti-inflammatory drug (NSAID) usage (daily or when needed) with anti-TNF therapy were recorded separately. In order to calculate the follow-up time, the start date and end date of anti-TNF drugs were used for the patients whose treatment ended, and the deadline for those who continued their treatment was March 31, 2022. Anti-TNF therapies were divided into two groups according to the presence of the Fc fragment. Patients taking drugs containing Fc components, such as ADA, ETA, GOL, and IFX, formed the with Fc (w/Fc) group, while those taking CZP formed the without Fc (wo/Fc) group. Consistent with previous studies on RF titers measured by the turbidimetric method, the normal reference range is 0–14 IU/mL, low titer is 14–42 IU/mL (<3 times the upper limit of normal [ULN] for laboratory and testing), and >42 IU/mL (>3 times the upper limit of normal [ULN] for laboratory and testing) indicates high titer [19]. The DAS28-CRP score, which is calculated by combining the number of tender joints, the number of swollen joints, CRP, and patient’s global health (0–100) values, is routinely used in our outpatient clinic to measure RA disease activity. A DAS28 score <2.6 indicates remission, a score of 2.6 to ≤3.2 indicates low disease activity, a score of 3.2 to ≤5.1 indicates moderate disease activity, and a score >5.1 indicates high disease activity [20]. ESR, CRP, and DAS28-CRP values were recorded separately at the beginning, third, and sixth months of anti-TNF therapy to identify changes in disease activity.
Statistical Analyses
The normality of the distribution of data was tested using the Shapiro–Wilk test. Descriptive statistics for numerical variables are expressed as mean±standard deviation or median (interquartile range [IQR]), while those for categorical variables are expressed as numbers and percentages. Between the two independent groups, the independent samples t-test was used to compare normally distributed data, and the Mann–Whitney U test was used to compare non-normally distributed data. The relationships between the groups were assessed using the chi-square test for categorical variables. The Friedman test was used to compare the numerical data of more than two dependent variables (post hoc test: Dunn’s). Cox regression analyses (the univariate, enter, and backward Wald elimination methods) were used to estimate the hazard ratio (HR) for factors associated with drug survival of anti-TNF therapy. IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. A p-value of <0.05 was considered statistically significant.
RESULTS
After the inclusion and exclusion criteria were applied, 52 of the 221 RA patients followed in our rheumatology department were included in the study (Fig. 1). Of the patients, 48 (92.3%) were female, four (7.7%) were male, and the mean age was 51.7±13.0 years. The most commonly used anti-TNF biologic agent was ETA, and 23.1% of patients were using CZP. The patients’ demographic, clinical, and laboratory data are provided in Table 1.
FIGURE 1.
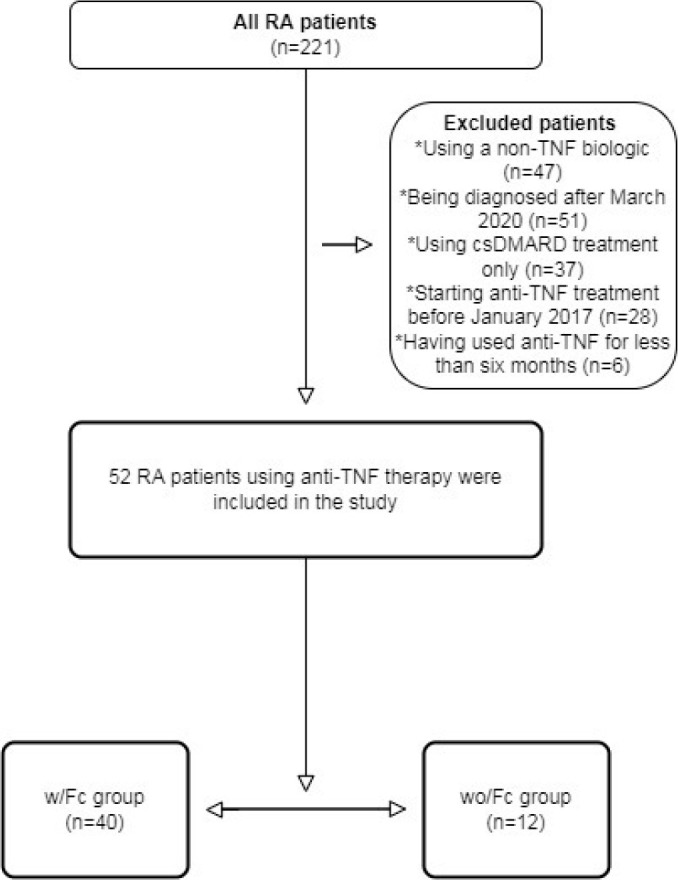
Flowchart of the study
TABLE 1.
Demographic, clinical, and laboratory findings of RA patients
| Number of the RA patients, n | 52 |
|---|---|
| Age, years | 51.7±13.0 |
| Female/male, n (%) | 48/4 (92.3/7.7) |
| BMI, kg/m2 | 28.9±5.0 |
| Disease duration, years | 13.8±7.2 |
| Follow-up time, months | 35.0±14.7 |
| Distributions of anti-TNF drugs, (%) | |
| Etanercept | 32.7 |
| Certolizumab | 23.1 |
| Adalimumab | 23.1 |
| Golimumab | 15.4 |
| Infliximab | 5.8 |
| Types of anti-TNF, (%) | |
| w/Fc | 76.9 |
| wo/Fc | 23.1 |
| Anti-TNF is first biologic drug, (%) | |
| Yes | 75.0 |
| No | 25.0 |
| Still on anti-TNF drug, (%) | |
| Yes | 55.8 |
| No | 44.2 |
| Duration of stay on the anti-TNF drugs, (%) | |
| ≤24 months | 30.8 |
| >24 months | 69.2 |
| CsDMARDs used with anti-TNF, (%) | |
| None | 23.0 |
| CsDMARD monotherapy | 63.5 |
| CsDMARD combination | 13.5 |
| MTX usage along with anti-TNF, (%) | |
| Yes | 48.1 |
| No | 51.9 |
| Steroid usage along with anti-TNF, n (%) | |
| Yes | 40.4 |
| No | 59.6 |
| RF titres, IU/mL | 58.1 (104.8) |
| RF, (%) | |
| ≤42 IU/mL | 46.2 |
| >42 IU/mL | 53.8 |
| Anti-CCP, (%) | |
| Positive | 69.2 |
| Negative | 30.8 |
| Comorbidities, (%) | |
| Yes | 50 |
| No | 50 |
| Number of comorbidity | 1 (2) |
| Smoking, n (%) | |
| Yes | 17.3 |
| No | 82.7 |
| Previous number of bDMARD | 1 (2) |
| Previous number of csDMARD | 3 (1) |
| Isoniazid prophylaxis, (%) | |
| Yes | 67.3 |
| No | 32.7 |
| NSAID usage, (%) | |
| Yes | 71.2 |
| No | 28.8 |
Continuous variables are presented as either mean±SD or median (IQR) according to normality. Categorical variables are given in percentage. BMI: Body mass index; bDMARD: Biological disease-modifying antirheumatic drug; csDMARD: Conventional synthetic disease-modifying antirheumatic drug; CCP: Cyclic citrullinated peptides; MTX: Methotrexate; NSAID: Nonsteroidal anti-inflammatory drug; RF: Rheumatoid factor; TNF: Tumor necrosis factor; w/Fc: With Fc; wo/Fc: Without Fc.
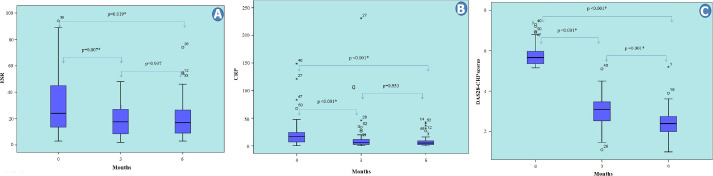
It was determined that the ESR, CRP, and DAS28-CRP values of all the RA patients significantly decreased at the third and sixth months of anti-TNF treatment compared to baseline (p=0.003, p<0.001 and p<0.001, respectively). While the median values of ESR and CRP were not different between the third and sixth months (p>0.05), the DAS28-CRP scores showed a statistically significant difference from each other at all three evaluations (p<0.05), and gradually decreased compared to the baseline (Fig. 2A–C).
FIGURE 2.
Changes in laboratory data and DAS28 scores in the first six months of anti-TNF therapy. *: P<0.05; CRP: C-reactive protein; DAS: Disease activity score; ESR: Erythrocyte sedimentation rate; RF: Rheumatoid factor; w/Fc: With Fc; wo/Fc: Without Fc; 0: Initiation of treatment; 3: Third month of treatment; 6: Sixth month of treatment.
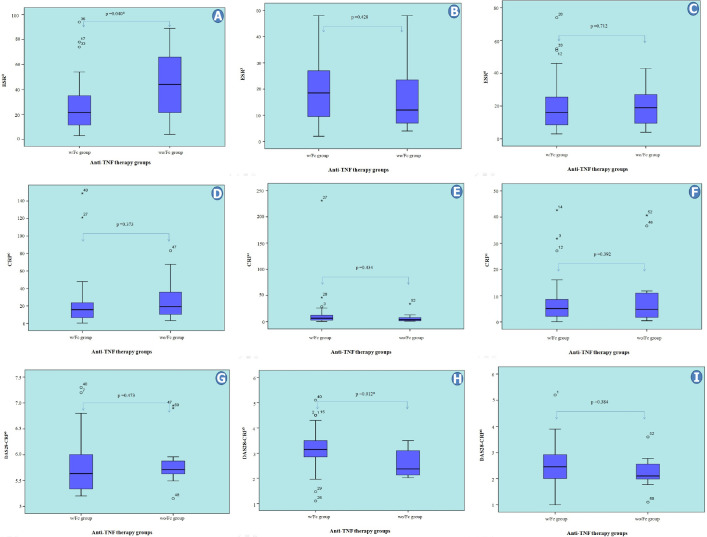
In two independent group comparisons based on whether the anti-TNF biological agents contain Fc fragments, the median ESR values were significantly higher in the wo/Fc group at the beginning of treatment (p=0.040). However, the ESR values did not differ in the third and sixth months after treatment (p=0.428 and p=0.712, respectively). We did not find any significant difference between the groups in terms of CRP values at all three-time points (p=0.373, p=0.434, and p=0.392, respectively). On the other hand, although there was no significant difference between the median DAS28-CRP scores of the two groups at the start of anti-TNF treatment (numerically higher in the wo/Fc group), the median DAS28-CRP score of the wo/Fc group was statistically significantly lower at the third month (p=0.012). This difference persisted numerically in the sixth month of treatment, but it was not statistically significant (p=0.384) (Fig. 3A–I). The mean age was lower in the wo/Fc group (p=0.006). The median RF titers did not differ between the groups (p=0.182). In addition, we did not find any significant difference between the two groups in terms of concomitant MTX or steroid usage (p=0.329 and p=0.587, respectively) (data not shown).
FIGURE 3.
Comparison of laboratory data and DAS28 scores according to anti-TNF therapy groups. *: P<0.05; CRP: C-reactive protein; DAS: Disease activity score; ESR: Erythrocyte sedimentation rate; RF: Rheumatoid factor; w/Fc: With Fc; wo/Fc: Without Fc; 0: Initiation of treatment; 3: Third month of treatment; 6: Sixth month of treatment.
At the time the study data were reviewed, 29 (55.8%) of the patients were still continuing the anti-TNF treatment that was started within the date range specified in the inclusion criteria, while 23 (44.2%) were not. The median values of RF titers and the median DAS28-CRP3 score were significantly higher in the group not continuing treatment (p=0.008 and p=0.004, respectively). In terms of other parameters, the two groups were similar (for all; p>0.05) (Table 2).
TABLE 2.
Comparison of demographic, clinical, laboratory, and disease activity parameters depending on whether or not to continue anti-TNF treatment at the last evaluated date
| Continuing with treatment (n=29) | Discontinuation (n=23) | p | |
|---|---|---|---|
| Age, years | 53.7±12.4 | 50.3±11.9 | 0.328 |
| Gender, (%) | 0.602 | ||
| Female | 56.3 | 43.8 | |
| Male | 50 | 50 | |
| BMI, kg/m2 | 29.2±5.7 | 28.6±4.0 | 0.674 |
| Disease duration, years | 13.9±6.1 | 14.0±8.4 | 0.969 |
| Types of anti-TNF, (%) | 0.299 | ||
| w/Fc | 52.5 | 47.5 | |
| wo/Fc | 66.7 | 33.3 | |
| Anti-TNF is first biologic drug, (%) | 0.629 | ||
| Yes | 59 | 41 | |
| No | 46.2 | 53.8 | |
| Non-TNF use before anti-TNF, (%) | 0.561 | ||
| Yes | 53.8 | 46.2 | |
| No | 56.4 | 43.6 | |
| CsDMARDs used with anti-TNF, (%) | 0.922 | ||
| None | 50 | 50 | |
| CsDMARD monotherapy | 60.6 | 39.4 | |
| CsDMARD combination | 42.9 | 57.1 | |
| MTX usage along with anti-TNF, (%) | 0.805 | ||
| Yes | 52 | 48 | |
| No | 59.3 | 40.7 | |
| Steroid usage along with anti-TNF, (%) | 0.491 | ||
| Yes | 47.6 | 52.4 | |
| No | 61.3 | 38.7 | |
| RF, (%) | 0.051 | ||
| Positive | 48.8 | 51.2 | |
| Negative | 81.8 | 18.2 | |
| RF titres, IU/mL | 28.4 (77.7) | 87.9 (126.8) | 0.008* |
| RF, (%) | 0.236 | ||
| <42 IU/mL | 66.7 | 33.3 | |
| >42 IU/mL | 46.4 | 53.6 | |
| Anti-CCP, (%) | 0.058 | ||
| Positive | 47.2 | 52.8 | |
| Negative | 75 | 25 | |
| Comorbidities, (%) | 0.782 | ||
| Yes | 57.7 | 42.3 | |
| No | 53.8 | 46.2 | |
| Number of comorbidity | 1 (2) | 0 (2) | 0.758 |
| Smoking, (%) | 0.366 | ||
| Yes | 66.7 | 33.3 | |
| No | 53.5 | 46.5 | |
| Previous number of bDMARD | 1 (2) | 1 (2) | 0.969 |
| Previous number of csDMARD | 3 (1) | 3 (1) | 0.335 |
| History of isoniazid prophylaxis, (%) | 0.991 | ||
| Yes | 54.3 | 45.7 | |
| No | 58.8 | 41.2 | |
| NSAID usage, (%) | 0.824 | ||
| Yes | 56.8 | 43.2 | |
| No | 53.3 | 46.7 | |
| ESR0 | 22 (26) | 27 (36) | 0.320 |
| ESR3 | 16 (18.5) | 19 (15) | 0.319 |
| ESR6 | 18 (24) | 16 (13) | 0.828 |
| CRP0 | 14.9 (17.2) | 20.5 (25.3) | 0.315 |
| CRP3 | 4.6 (8.9) | 6.7 (10.6) | 0.159 |
| CRP6 | 5.6 (6.6) | 4.6 (8.1) | 0.934 |
| DAS28-CRP0 | 5.6 (0.5) | 5.7 (1.1) | 0.173 |
| DAS28-CRP3 | 3 (0.9) | 3.4 (0.7) | 0.004* |
| DAS28-CRP6 | 2.4 (0.7) | 2.4 (1) | 0.531 |
: P<0.05. Continuous variables are presented as either mean±SD or median (IQR) according to normality. Categorical variables are given in percentage. BMI: Body mass index; bDMARD: Biological disease-modifying antirheumatic drug; csDMARD: Conventional synthetic disease-modifying antirheumatic drug; CCP: Cyclic citrul- linated peptides; CRP: C-reactive protein; DAS: Disease activity score; ESR: Erythrocyte sedimentation rate; MTX: Methotrexate; NSAID: Nonsteroidal anti-inflammatory drug; RF: Rheumatoid factor; TNF: Tumor necrosis factor; w/Fc: With Fc; wo/Fc: Without Fc; 0: Initiation of treatment; 3: Third month of treatment; 6: Sixth month of treatment.
In the Cox regression analyses, we initially evaluated the potential factors affecting anti-TNF survival separately by using a univariate model. In these analyses, RF titers and anti-CCP status variables were determined to have significant effects (HR: 1.005 (1.001–1.008), p=0.010 and HR: 2.963 (0.999–8.790), p=0.050, respectively). Then, candidate predictors were entered into the multiple model. After adjusting for the effects of predictors [anti-TNF subtypes, age, body mass index (BMI), number of comorbidities, history of treatment with csDMARDs/bDMARDs, concomitant use of steroids or methotrexate, and anti-CCP status] in the enter model, we found that RF titers were the most important independent risk factor for survival of anti-TNF therapy (HR: 1.007 (1.002–1.012), p=0.009) (Table 3). Finally, the backward Wald elimination method was applied, and RF titers were determined to be the most significant predictor (HR: 1.005 (1.001–1.008), p=0.010).
TABLE 3.
Evaluation of factors associated with the survival of anti-TNF therapies
| Univariate analysis | Enter model | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p | HR | 95% CI | p |
| Age | 0.989 | 0.956-1.023 | 0.507 | 0.961 | 0.916-1.008 | 0.100 |
| BMI | 0.987 | 0.912-1.069 | 0.755 | 0.980 | 0.875-1.097 | 0.721 |
| Disease duration | 1.006 | 0.944-1.073 | 0.847 | |||
| Number of comorbidity | 1.022 | 0.680-1.534 | 0.917 | 0.992 | 0.620-1.588 | 0.973 |
| Previous number of bDMARD | 0.940 | 0.688-1.286 | 0.701 | 0.816 | 0.566-1.178 | 0.278 |
| Previous number of csDMARD | 1.637 | 0.713-3.757 | 0.245 | 1.614 | 0.606-4.302 | 0.338 |
| Types of anti-TNF | ||||||
| Wo/Fc | ||||||
| W/Fc | 1.690 | 0.573-4.984 | 0.341 | 2.011 | 0.593-6.821 | 0.262 |
| RF status | ||||||
| Negative Positive |
3.961 | 0.924-16.976 | 0.064 | |||
| RF titers | 1.005 | 1.001-1.008 | 0.010* | 1.007 | 1.002-1012 | 0.009* |
| RF | ||||||
| ≤42 IU/mL >42 IU/mL |
1.954 | 0.827-4.619 | 0.127 | |||
| Anti-CCP status | ||||||
| Negative Positive |
2.963 | 0.999-8.790 | 0.050 | 1.837 | 0.563-5.992 | 0.313 |
| MTX | ||||||
| Yes | ||||||
| No | 0.777 | 0.343-1.764 | 0.547 | 0.645 | 0.266-1560 | 0.330 |
| Steroid usage | ||||||
| Yes | ||||||
| No | 0.587 | 0.256-1.347 | 0.209 | 0.392 | 0.153-1.004 | 0.051 |
: P<0.05; BMI: Body mass index; bDMARD: Biological disease-modifying antirheumatic drug; csDMARD: Conventional synthetic disease-modifying antirheumatic drug; CI: Confidence interval; RF: Rheumatoid factor; w/Fc: With Fc; wo/Fc: Without Fc.
DISCUSSION
In this study, we investigated the effects of both RF and TNF inhibitors (with and without Fc fragments) on treatment-related parameters. Our results demonstrated that not Fc fragment but RF titers might be effective on drug survival of anti-TNFs. Although there was no difference in DAS28-CRP scores between patients using CZP and those using other anti-TNF agents at the beginning of treatment, there was a significant difference between the two groups in the third month. However, this difference did not persist in the sixth month.
In the treatment of RA, it is recommended that patients start DMARD treatments as early as possible to achieve better clinical outcomes. EULAR recommends first MTX monotherapy or combination treatment with other csDMARDs after the diagnosis of RA, and in case of unresponsiveness, a switch to targeted therapies is recommended [21]. TNF-alpha is one of the cytokines found in the inflamed synovium of RA patients, and the treatments that block it (anti-TNF) are the drugs that were first used to treat millions of RA patients [22]. In comparisons made by taking into consideration the standard ACR treatment response criteria (ACR20, ACR50, and ACR70 response rates), it was revealed that the response rates of five different anti-TNF drugs were similar [23]. Likewise, in a study comparing head-to-head two anti-TNF drugs with and without an Fc fragment, Smolen et al. [24] reported similar outcomes in efficacy and safety data. With these similarities in efficacy and safety, anti-TNF drugs have different doses, dose optimization, half-lives, and drug administration routes. Of course, these pharmacokinetic and pharmacodynamic differences sometimes have the potential to affect the drug preferences of patients or physicians [23]. In the current study, the effects of anti-TNF drugs containing Fc fragment on the laboratory and disease activity parameters were similar to those of CZP, consistent with the literature. Only DAS28-CRP in the third month of treatment was lower in the CZP group. This result may be due to the four different drugs, doses and frequency of administration in the Fc-fragmented group, although there was only one drug and the same administration procedure in the CZP group. Indeed, this difference had disappeared and DAS28-CRP scores were <2.6 (remission) in both groups in the sixth month. These results are in line with studies [24] that found the short- and long-term efficacy of ADA (containing Fc fragments) and CZP to be similar.
The DAS28 score, which is calculated by adding the ESR or CRP to the physician’s and patient’s subjective evaluations, is frequently utilized for the evaluation of RA disease activity in clinical practice [25]. It is reported that DAS28-CRP is more commonly used than DAS28-ESR due to its ease of access and ability to show changes in inflammatory activity at an earlier stage. However, it should be kept in mind that some of the patients thought to be in remission based on both DAS28-CRP and DAS28-ESR show persistent synovitis on ultrasound (US) and magnetic resonance imaging (MRI) studies [26]. In our study, there was a significant improvement in the ESR, CRP, and DAS28-CRP values of all the RA patients in the third and sixth months compared to the onset of anti-TNF. However, we would like to point out that our patient records did not include any US or MRI information evaluating the presence of synovitis.
Studies conducted previously in different countries showed that anti-TNFs were started at a high rate as the first bDMARD in patients who did not respond to traditional DMARD treatments. This has been attributed to the fact that TNF inhibitors were historically the first biologics and targeted synthetic DMARD therapies were not available at that time. However, despite its widespread use with RA and non-RA indications (e.g., inflammatory spine diseases, inflammatory bowel diseases, uveitis, psoriasis, etc.), anti-TNF therapy cannot be continued in approximately 30–40% of patients due to primary failure, secondary loss of response, or intolerance [11]. Studies have reported that the main reason for discontinuing these drugs is loss of clinical efficacy [27]. One of the common mechanisms in loss of response is the formation of antibodies to TNF inhibitors [18]. The level of this immune response, called immunogenicity, is higher in IFX compared to ADA and ETA, especially when combined MTX is not used. Also, factors such as the molecular structures, doses, and treatment durations of drugs, together with the patient’s genetic background, affect the development of these antibodies [23, 27]. In RA, the effects of RF and anti-CCP autoantibodies, which are associated with a poor prognosis, on anti-TNF therapies are still unclear and previous studies have shown conflicting results [28, 29]. Julià et al. [28] reported that the simultaneous presence of RF and anti-CCP was associated with better outcomes than the presence of only one of the two autoantibodies. Conversely, negative effects of both RF positivity and high RF titers on remission rates and survival time of anti-TNF therapies have been reported in various studies [29–32]. Also, high titers of RF and ACPA are identified as poor prognostic factors in the 2019 EULAR recommendations for the treatment of rheumatoid arthritis [33]. In a recent retrospective study, Nakayama et al. [17] reported that CZP without Fc may be more effective than anti-TNFs with Fc in RA patients with high RF titers. Here, it was hypothesized that RF could bind to the Fc part of TNF inhibitors with its ability to bind to the Ig Fc part and change the clinical efficacy of these drugs. In our RA patient cohort, which had a mean follow-up time of 35 months, 44.2% of patients were unable to continue anti-TNF therapy. We did not find any difference in the use of combined csDMARDs or combined MTX between patients who continued the anti-TNF treatment and those who did not. For the third and sixth months, the clinical and laboratory data for each of the two treatment groups (the w/Fc and the wo/Fc groups) were substantially similar with the same median RF values. According to the cox-regression results, it was observed that RF titers significantly affected the survival of anti-TNF therapies while the Fc fragment did not. Although the effect of RF titers is consistent with the literature, whether the relationship between RF titers and the Fc fragment affects survival should continue to be investigated.
Our study has some limitations. This is a retrospective study that includes relatively few patients, and our results may have been influenced by other factors present in patients but not recorded at the time of the outpatient clinic visits. Although improvements in laboratory parameters and DAS28 scores were noted, structural damage in patients may have been different between the w/Fc and wo/Fc groups; however, the persistence of synovitis on radiological progression scores or MRI and US was not measured. Finally, the accuracy of our results may come into question for patients who meet the definition of difficult-to-treat RA [34], and if these patients had different numerical distributions in the different groups.
Conclusion
This study showed that RF titers have significant effects on the survival of anti-TNF therapies, but we did not find the same result with the Fc fragment. Disease activity in the third month of treatment was significantly lower in the wo/Fc group compared to the w/Fc group, but at later follow-up, treatment responses were similar. The possible effects of the relationship between RF titers and the Fc fragment on anti-TNF therapies should continue to be investigated in larger patient groups.
Footnotes
Cite this article as: Kaplan H, Cengiz G, Cuce I, Sas S, Senkoy E, Calis M, et al. Rheumatoid factor titers, but not Fc fragments, may be strongly associated with drug survival of anti-TNF agents in patients with rheumatoid arthritis. North Clin Istanb 2024;11(2):147–157.
Ethics Committee Approval
The Erciyes University Clinical Research Ethics Committee granted approval for this study (date: 08.06.2022, number: 2022/448).
Authorship Contributions
Concept – HK, GC, ES, SS, IC; Design – HK, GC, ES, SS, IC; Supervision – MC, HD, MK, OO; Data collection and/or processing – HK, ES, SS, GC, MC, OO; Analysis and/or interpretation – HK, GC, OO, IC; Literature review – HK, IC, ES, SS; Writing – HK, SS, ES, IC, GC, MC, HD, MK, OO; Critical review – GC, MC, MK, HD, OO.
Conflict of Interest
No conflict of interest was declared by the authors.
Use of AI for Writing Assistance
Not declared.
Financial Disclosure
The authors declared that this study has received no financial support.
Peer-review
Externally peer-reviewed.
References
- 1.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–74. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 2.Köhler BM, Günther J, Kaudewitz D, Lorenz HM. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8:938. doi: 10.3390/jcm8070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–88. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 4.Littlejohn EA, Monrad SU. Early diagnosis and treatment of rheumatoid arthritis. Prim Care. 2018;45:237–55. doi: 10.1016/j.pop.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers. 2013;35:727–34. doi: 10.1155/2013/726598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Delft MA, Huizinga TW. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun. 2020;110:102392. doi: 10.1016/j.jaut.2019.102392. [DOI] [PubMed] [Google Scholar]
- 7.Hecht C, Englbrecht M, Rech J, Schmidt S, Araujo E, Engelke K, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis. 2015;74:2151–6. doi: 10.1136/annrheumdis-2014-205428. [DOI] [PubMed] [Google Scholar]
- 8.Gualtierotti R, Ciavarella T, Meroni PL. Rheumatoid factors. In: Shoenfeld Y, Meroni PL, Gershwin E, editors. Autoantibodies. Amsterdam: Elsevierxs; 2014. pp. 751–60. [Google Scholar]
- 9.Can M, Najip A, Yılmaz N, Inanc N, Yavuz S. Immunoglobulin subtypes predict therapy response to the biologics in patients with rheumatoid arthritis. Rheumatol Int. 2013;33:1455–60. doi: 10.1007/s00296-012-2560-8. [DOI] [PubMed] [Google Scholar]
- 10.Maneiro R, Salgado E, Carmona L, Gomez-Reino JJ. Rheumatoid factor as predictor of response to abatacept, rituximab and tocilizumab in rheumatoid arthritis: Systematic review and meta-analysis. Semin Arthritis Rheum. 2013;43:9–17. doi: 10.1016/j.semarthrit.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Rubbert-Roth A, Szabó MZ, Kedves M, Nagy G, Atzeni F, Sarzi-Puttini P. Failure of anti-TNF treatment in patients with rheumatoid arthritis: the pros and cons of the early use of alternative biological agents. Autoimmun Rev. 2019;18:102398. doi: 10.1016/j.autrev.2019.102398. [DOI] [PubMed] [Google Scholar]
- 12.Van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2013;9:164–72. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 13.Curtis JR, Mariette X, Gaujoux-Viala C, Blauvelt A, Kvien TK, Sandborn WJ, et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open. 2019;5:e000942. doi: 10.1136/rmdopen-2019-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kekow J, Mueller-Ladner U, Schulze-Koops H. Rituximab is more effective than second anti-TNF therapy in rheumatoid arthritis patients and previous TNFα blocker failure. Biologics. 2012;6:191–9. doi: 10.2147/BTT.S32244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyrich K, Watson K, Silman A, Symmons DP. Predictors of response to anti-TNF-α therapy among patients with rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45:1558–65. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 16.Cuchacovich M, Bueno D, Carvajal R, Bravo N, Aguillón JC, Catalán D, et al. Clinical parameters and biomarkers for anti-TNF treatment prognosis in rheumatoid arthritis patients. Clin Rheumatol. 2014;33:1707–14. doi: 10.1007/s10067-014-2756-2. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama Y, Watanabe R, Murakami K, Murata K, Tanaka M, Ito H, et al. Differential efficacy of TNF inhibitors with or without the immunoglobulin fragment crystallizable (Fc) portion in rheumatoid arthritis: the answer cohort study. Rheumatol Int. 2022;42:1227–34. doi: 10.1007/s00296-021-05086-w. [DOI] [PubMed] [Google Scholar]
- 18.Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varache S, Cornec D, Morvan J, Devauchelle-Pensec V, Berthelot JM, Le Henaff-Bourhis C, et al. Diagnostic accuracy of ACR/EULAR 2010 criteria for rheumatoid arthritis in a 2-year cohort. J Rheumatol. 2011;38:1250–7. doi: 10.3899/jrheum.101227. [DOI] [PubMed] [Google Scholar]
- 20.Greenmyer JR, Stacy JM, Sahmoun AE, Beal JR, Diri E. DAS28-CRP cutoffs for high disease activity and remission are lower than DAS28-ESR in rheumatoid arthritis. ACR Open Rheumatol. 2020;2:507–11. doi: 10.1002/acr2.11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccarelli F, Massafra U, Perricone C, Idolazzi L, Giacomelli R, Tirri R, et al. Anti-TNF treatment response in rheumatoid arthritis patients with moderate disease activity: a prospective observational multicentre study (MODERATE) Clin Exp Rheumatol. 2017;35:24–32. [PubMed] [Google Scholar]
- 22.Caporali R, Pallavicini FB, Filippini M, Gorla R, Marchesoni A, Favalli EG, et al. Treatment of rheumatoid arthritis with anti-TNF-alpha agents: a reappraisal. Autoimmun Rev. 2009;8:274–80. doi: 10.1016/j.autrev.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Radner H, Aletaha D. Anti-TNF in rheumatoid arthritis: an overview. Wien Med Wochenschr. 2015;165:3–9. doi: 10.1007/s10354-015-0344-y. [DOI] [PubMed] [Google Scholar]
- 24.Smolen JS, Burmester GR, Combe B, Curtis JR, Hall S, Haraoui B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763–74. doi: 10.1016/S0140-6736(16)31651-8. [DOI] [PubMed] [Google Scholar]
- 25.Targońska-Stępniak B, Grzechnik K, Zwolak R. The relationship between platelet ındices and ultrasound, clinical, laboratory parameters of disease activity in patients with rheumatoid arthritis. J Clin Med. 2021;10:5259. doi: 10.3390/jcm10225259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orr CK, Najm A, Young F, McGarry T, Biniecka M, Fearon U, et al. The utility and limitations of CRP, ESR and DAS28-CRP in appraising disease activity in rheumatoid arthritis. Front Med (Lausanne) 2018;5:185. doi: 10.3389/fmed.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707–18. doi: 10.1038/nrrheum.2017.187. [DOI] [PubMed] [Google Scholar]
- 28.Julià A, López-Lasanta M, Blanco F, Gómez A, Haro I, Mas AJ, et al. Interactions between rheumatoid arthritis antibodies are associated with the response to anti-tumor necrosis factor therapy. BMC Musculoskelet Disord. 2021;22:372. doi: 10.1186/s12891-021-04248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos-Moreno P, Sánchez G, Castro C. Rheumatoid factor as predictor of response to treatment with anti-TNF alpha drugs in patients with rheumatoid arthritis: results of a cohort study. Medicine (Baltimore) 2019;98:e14181. doi: 10.1097/MD.0000000000014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Mulligen E, Ahmed S, Weel AE, Hazes JM, van der Helm-van Mil A, de Jong PH. Factors that influence biological survival in rheumatoid arthritis: Results of a real-world academic cohort from the Netherlands. Clin Rheumatol. 2021;40:2177–83. doi: 10.1007/s10067-020-05567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi T, Miyasaka N, Inui T, Yano T, Yoshinari T, Abe T, et al. High titers of both rheumatoid factor and anti-CCP antibodies at baseline in patients with rheumatoid arthritis are associated with increased circulating baseline TNF level, low drug levels, and reduced clinical responses: a post hoc analysis of the RISING study. Arthritis Res Ther. 2017;19:1–11. doi: 10.1186/s13075-017-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potter C, Hyrich K, Tracey A, Lunt M, Plant D, Symmons D, et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis. 2009;68:69–74. doi: 10.1136/ard.2007.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smolen JS, Landewé RB, Bijlsma JW, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–99. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 34.Nagy G, Roodenrijs NM, Welsing PM, Kedves M, Hamar A, Van Der Goes MC, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80:31–5. doi: 10.1136/annrheumdis-2020-217344. [DOI] [PMC free article] [PubMed] [Google Scholar]