Abstract
OBJECTIVE
Long-term consequences of COVID-19 vary widely, representing a growing global health challenge. The aim of this report was to define the presence of symptoms in post-acute-COVID-19 syndrome (PCS) patients and to assess the frequency, associated factors, and the spectrum of persistent symptoms.
METHODS
In this longitudinal study, 487 adults with a previously diagnosed “Severe Acute Respiratory Syndrome Coronavirus 2” (SARS-CoV-2) who admitted to COVID-19 follow-up outpatient clinic between December 1, 2020 and November 31, 2021 were interviewed face-to-face three times. Data was collected on patient demographics, comorbidities, and symptoms. A questionnaire of 160 questions was asked and organized into the following: identification and consent, socio-demographic/epidemiological characteristics, previous medical history, diagnosis and clinical presentation of acute COVID-19, as well as systematic symptoms. Data were evaluated using univariate comparisons and multiple logistic regression.
RESULTS
The most prevalent symptoms among all PCS patients during their initial visit were dyspnea, weakness, forgetfulness, fatigue, and arthralgia respectively. The most common symptoms in patients with 6 months or more time from discharge to follow-up at the first and second visits, appear to be persistent. While incidence rates decreased by the third visit, the five most common symptoms remained the same. The possibility of weakness and arthralgia was found to be higher in non-hospitalized patients. Females were associated with the most common persistent symptoms and the strongest association was with arthralgia.
CONCLUSION
A large number of COVID-19 survivors had continuing symptoms at the first year of post-COVID-19-infection. Neither the presence of comorbidities of the patient nor smoking status were associated with the severity of PCS symptoms. A better understanding of the mechanisms, predisposing factors and evaluation require a multidisciplinary team approach.
Keywords: Long COVID, post-acute-COVID-19 syndrome, post-COVID syndrome, risk factors, symptoms
Highlight key points
While dyspnea was the most common symptom in all patients, forgetfulness, weakness, arthralgia and fatigue were seen in half of all Post-COVID-19-Syndrome (PCS) patients.
While incidence rates decreased at the third visit, the five most common symptoms remained the same.
The possibility of weakness and arthralgia was found to be higher in non-hospitalized patients.
Females were associated with the most common three persistent symptoms, with association being strongest for arthralgia.
The presence of comorbidities or smoking status of the patients were not associated with the presence of three major symptoms associated with PCS at 6 to 12 months following COVID-19.
As of June 2022, the COVID-19 pandemic resulted in greater than 500 million cases and mortality over 6 million [1]. Even though most are able to recover without complication, many patients have continued symptoms of post COVID-19 infection, while other have described new symptoms [2–4]. Despite, high mortality among those hospitalized during the initial infection course, studies of the long-term complications after COVID-19 pandemic are still limited due to its recent history [5–8].
As the number of infections rise, so does the population experiencing long-term effect, often referred to as “long haulers”. Thus, it is necessary for healthcare providers to identify, characterize, and gain a better understanding of long-term COVID-19. Currently, post-COVID symptoms have been documented as persistent or new onset complications that continue for 2 months following COVID-19 recovery with no other known cause. Symptoms may fluctuate or have a relapsing course that contributes to poorer overall health or quality of life [9]. Despite the increasing volume of publications, the available knowledge of post-acute-COVID-19 syndrome (PCS) is still controversial due to the heterogeneity of the studied populations and diagnostic follow-ups [2–8, 10–14].
Objectives
The goal of this report was to define the presence of symptoms in PCS patients who were mostly hospitalized and to assess the frequency, associated factors, and variety of persistent symptoms in long-term COVID-19 patients.
MATERIALS AND METHODS
Study Design and Participants
The current study was longitudinal and the target population of this study was 487 participants at the first visit, 365 at the second visit one month later, and 261 participants at the third visit three months later, who came to our outpatient clinic and agreeing to be followed up after COVID-19. All patients enrolled in the study was positive for SARS-Co V-2 virus (from a nasopharyngeal and throat swab) by reverse transcriptase-polymerase chain reaction (RT-PCR). Patients older than 18 and who were at least six weeks after RT-PCR positivity or discharge from the hospital were invited to voluntarily attend the post-COVID unit. Those who volunteered were given an informed consent form and their agreement was obtained. All patients were evaluated for the first time between December 1, 2020, and August 30, 2021. The controls of those who came for the third visit were also completed as of November 30, 2021.
Survey and Data Collection
Data was collected on patient demographics, comorbidities, and symptoms. A questionnaire of 160 questions was asked and organized into the following: identification and consent, socio-demographic/epidemiological characteristics, previous medical history, diagnosis and clinical presentation of acute COVID-19, and all systematic symptoms. We evaluated these symptoms according to a clinical case definition of post-COVID-19 condition by a Delphi consensus [9]. Consensus definition for adults:” Post-acute-COVID-19 condition occurs in individuals with a history of probable or confirmed COVID-19, usually three months from the onset, with symptoms lasting at least two months and cannot be explained by an alternative diagnosis. Symptoms might either be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms might also fluctuate or relapse over time.” The term “Long COVID” is used for symptoms lasting from week 12 to week 24, while the term “persistent post-COVID symptoms” is used for symptoms for lasting longer than 24 weeks. Volunteers were asked about new occurring or deteriorated symptoms since hospital discharge. Complaints that were present before COVID-19, and still continuing afterward were not included in the evaluation. These 160 questions were asked in the initial evaluation. In the subsequent control visits, 76 questions were asked only about the complaints of all systems.
Patients experiencing dyspnea were assessed using the Modified Medical Research Council scale, which stratifies dyspnea severity in respiratory diseases (i.e., COPD). The scale ranges from “0” (no breathlessness except with exercise) to “4” (breathlessness during general daily routines) levels of disability [15].
Ethics
This study was performed in accordance to the Act of the Ministry of Health and the Declaration of Helsinki. Written informed consent was obtained from all patients and the Diskapi Yildirim Beyazit Training and Research Hospital Ethics Committee approved the data collection and analyses (no: 110/12, date: 03.05.2021).
Statistics
All statistical analyses were performed using IBM SPSS Statistics software version 11.5 (Chicago, ILL, USA). Descriptive statistics including frequency, percentage, mean, and standard deviation (SD) was used for demographic data evaluation. Quantitative variables were represented as mean±SD and median (min–max), while categorical variables were written as numbers (n) and percentages (%). Uni- and multivariate logistic regression analysis were used to determine the risk factors affecting the occurrence of symptoms. A p-value of less than 0.05 was considered statistically significant.
RESULTS
General Characteristics
Of the 502 patients included in the evaluation, 15 patients with missing records were excluded. Basic demographic characteristics of the remaining 487 patients analyzed are presented in Table 1. Newly onset symptoms were reported in the initial evaluations. Ten percent of the patients had type II Diabetes Mellitus (DM), and 12% had interstitial lung disease after the active period of COVID-19.
TABLE 1.
Demographic characteristics of 487 post COVID-19 patients
| Characteristics | Total | Hospitalized | Outpatient | p |
|---|---|---|---|---|
| Sex, (%) | <0.001a | |||
| Male | 51.5 | 57.2 | 37.6 | |
| Female | 48.5 | 42.8 | 62.4 | |
| Age | <0.001c | |||
| Mean±SD | 53.77±13.88 | 57.13±12.14 | 45.54±14.48 | |
| Median | 55.00 | 57.00 | 47.00 | |
| (Min-Max) | (18.00-87.00) | (19.00-87.00) | (18.00-74.00) | |
| Marital status, (%) | 0.013a | |||
| Married | 81.3 | 84.1 | 74.5 | |
| Single | 18.7 | 15.9 | 25.5 | |
| People living with, (%) | 0.534a | |||
| Alone | 5.1 | 5.8 | 3.5 | |
| Parents and children | 83.4 | 83.2 | 83.7 | |
| Extended family | 11.5 | 11.0 | 12.8 | |
| Working status, (%) | <0.001a | |||
| Working | 34.5 | 27.7 | 51.1 | |
| Not working | 35.9 | 37.6 | 31.9 | |
| Retired | 29.6 | 34.7 | 17.0 | |
| Smoking, (%) | <0.001a | |||
| No | 92.6 | 96.5 | 83.0 | |
| Yes | 7.4 | 3.5 | 17.0 | |
| BMI, (%) | <0.001b | |||
| <18.5 | 2.5 | 1.4 | 5.0 | |
| 18.5-24.9 | 16.0 | 11.6 | 27.0 | |
| 25.0-29.9 | 35.7 | 24.7 | 38.2 | |
| 30.0-34.9 | 26.9 | 29.2 | 21.3 | |
| 35.0-44.9 | 17.5 | 21.1 | 8.5 | |
| >45.0 | 1.4 | 2.0 | 0.0 | |
| Post COVID-19 period, (%) | <0.001a | |||
| 6-11 weeks | 26.3 | 30.3 | 16.3 | |
| 12-23 weeks | 43.9 | 38.7 | 56.7 | |
| >24 weeks | 29.8 | 31.0 | 27.0 | |
| Intensive care unit, (%) | <0.001a | |||
| No | 72.1 | 60.7 | 100.0 | |
| Yes | 27.9 | 39.3 | 0.0 | |
| Intubation, (%) | 0.112b | |||
| No | 98.4 | 97.7 | 100.0 | |
| Yes | 1.6 | 2.3 | 0.0 | |
| Pneumonia, (%) | <0.001a | |||
| No | 28.1 | 3.2 | 89.4 | |
| Yes | 71.9 | 96.8 | 10.6 | |
| Other complications related to COVID-19, (%) | 0.364b | |||
| No | 97.3 | 96.8 | 98.6 | |
| Yes | 2.7 | 3.2 | 1.4 | |
| Types of other complications related to COVID, (%) | 0.302b | |||
| Pulmonary embolism | 1.2 | 1.8 | 0.0 | |
| Cerebrovascular accident | 0.2 | 0.4 | 0.0 | |
| Deep vein thrombosis | 0.5 | 0.7 | 0.0 | |
| Delirium | 0.5 | 0.7 | 0.0 | |
| Acute renal failure | 0.7 | 0.4 | 1.5 | |
| Previous comorbidities, (%)* | <0.001a | |||
| No | 24.2 | 19.9 | 34.8 | |
| One chronic disease | 24.6 | 23.4 | 27.7 | |
| Two chronic diseases | 19.1 | 18.8 | 19.9 | |
| Three or more chronic diseases | 32.3 | 37.9 | 17.6 | |
| Types of concomitant disease, (%) | ||||
| Hypertension | 37.4 | 42.8 | 24.1 | <0.001a |
| Tip II DM | 26.3 | 32.4 | 11.3 | <0.001a |
| Hyperlipidemia | 18. 1 | 21.1 | 10.6 | 0.007a |
| Coronary artery disease | 12.7 | 16.5 | 3.5 | <0.001a |
| Hypothyroidism | 12.5 | 11.3 | 15.6 | 0.190a |
| Asthma | 9.7 | 10.7 | 7.1 | 0.222a |
SD:Standard deviation; Min: Minimum; Max: Maximum; BMI: Body mass index; DM: Diabetes mellitus; a: Chi-square test; b: Fisher-exact test; c: Student-t test; *: 249 patients have had more than one disease.
Symptoms at the Time of Follow-up
Constitutional symptoms, respiratory, cardiovascular, musculoskeletal, gastrointestinal, neurological symptoms and sensory symptoms of the patients were questioned. In the first evaluation, although the duration of the patients after the COVID-19 varied between 6 weeks and 12 months, the most common five-six persistent symptoms were found to be interestingly similar. While dyspnea was the most common symptom in all patients, forgetfulness, weakness, arthralgia and fatigue were seen in half of all PCS patients. At this initial assessment, more than half of the patients past 6 months or even 9–12 months continue to have three or more continuous symptoms.
The most common symptoms at the first visit of all patients were dyspnea (61.4%), weakness (60.2%), forgetfulness (54.4%), fatigue (48.3%), and arthralgia (47.2%), respectively. Of the 365 patients who attended the second visit, 56.4% had dyspnea, 51.5% had forgetfulness, 50.1% had weakness, 44.7% had arthralgia, and 37.8% had fatigue. Of the 261 patients who attended the third visit, 49.4% had dyspnea, 43.3% had arthralgia, 42.1% had weakness, 41.8% had forgetfulness, and 33% had fatigue (Fig. 1, Table 2).
FIGURE 1.
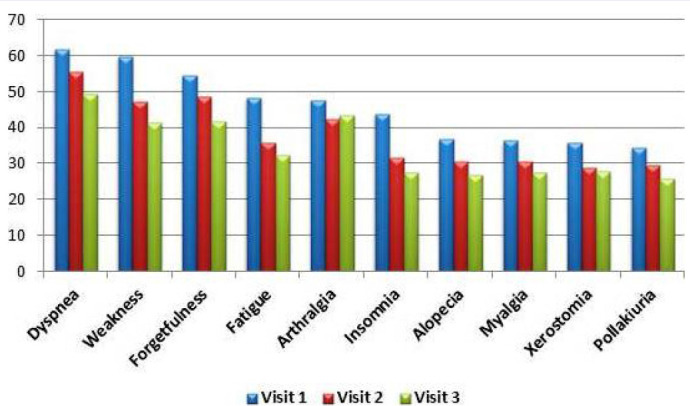
The most common symptoms of all (n=487) patients at three visits.
TABLE 2.
Most common symptoms at three visits for all patients and three visits for three post-COVID-19 periods
| Symptoms | All patients | 6-11 weeks | 12-24 weeks | 24-54 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. (%) |
2. (%) |
3. (%) |
1. (%) |
2. (%) |
3. (%) |
1. (%) |
2. (%) |
3. (%) |
1. (%) |
2. (%) |
3. (%) |
|
| Dyspnea | 61.4 | 56.4 | 49.4 | 64.8 | 54.4 | 47.4 | 58.9 | 54.0 | 48.7 | 62.1 | 62.4 | 52.9 |
| Weakness | 60.2 | 50.1 | 42.1 | 64.8 | 47.6 | 39.7 | 60.7 | 50.9 | 42.6 | 55.2 | 51.5 | 44.1 |
| Forgetfulness | 54.4 | 51.5 | 41.8 | 53.1 | 46.6 | 44.9 | 53.7 | 50.9 | 42.6 | 56.6 | 57.4 | 36.8 |
| Fatigue | 48.3 | 37.8 | 33.0 | 50.0 | 34.0 | 30.8 | 48.1 | 41.0 | 33.9 | 46.9 | 36.6 | 33.8 |
| Artralgia | 47.2 | 44.7 | 43.3 | 42.2 | 39.8 | 41.0 | 49.5 | 44.1 | 41.7 | 48.3 | 50.5 | 48.5 |
| Insomnia | 43.3 | 32.6 | 27.7 | 41.4 | 33.0 | 30.8 | 43.0 | 32.3 | 29.8 | 45.5 | 32.7 | 20.6 |
| Alopecia | 36.6 | 33.8 | 26.7 | 27.3 | 37.3 | 25.3 | 44.4 | 37.9 | 33.9 | 33.1 | 23.8 | 16.2 |
| Myalgia | 36.3 | 33.7 | 28.0 | 32.8 | 31.1 | 26.9 | 39.7 | 36.6 | 30.4 | 34.5 | 31.7 | 25.0 |
| Xerostomia | 35.7 | 31.0 | 28.4 | 35.9 | 28.2 | 25.6 | 37.9 | 29.2 | 25.2 | 32.4 | 36.6 | 36.8 |
| Pollakiuria | 34.2 | 30.5 | 25.8 | 35.9 | 34.0 | 21.8 | 35.2 | 31.9 | 24.6 | 31.0 | 24.8 | 32.4 |
The most common symptoms at the first visit of PCS patients who had passed the 12th week at the initial assessment were weakness (60.7%), dyspnea (58.9%), forgetfulness (53.7%), arthralgia (49.5%) and fatigue (48.1%). During the second visit one month later, the most common symptoms were dyspnea (54%), forgetfulness (50.9%), weakness (50.9%), arthralgia (44.1%), and fatigue (41.0%) from the same group. While incidence rates decreased by the third visit, the five most common symptoms remained the same. The most common symptoms at the first and second visits in patients with 6 months or more time from discharge (or recovery from disease) to follow-up appear to be persistent. At the third visit of this group of patients, dyspnea was still the most common symptom, while arthralgia was second and dry mouth was the fifth after weakness and forgetfulness (Fig. 2–4, Table 2).
FIGURE 2.
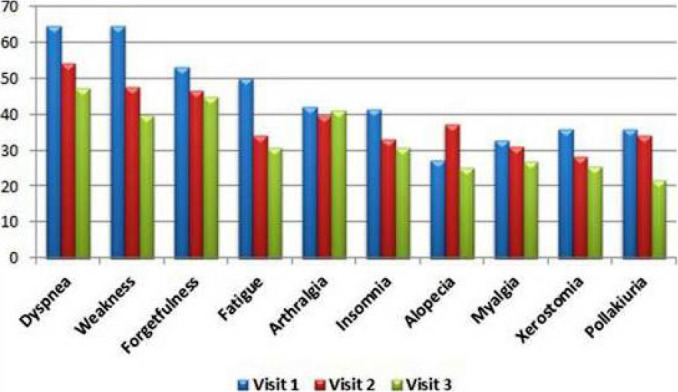
The most common symptoms at three separate visits of 128 patients with 6–11 weeks.
FIGURE 3.
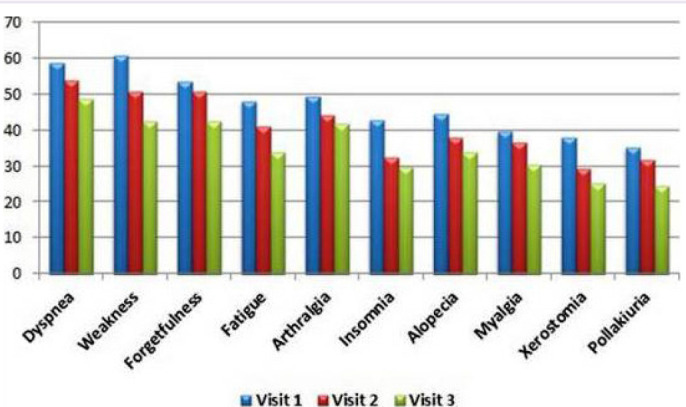
The most common symptoms at three visits of 214 patients with 12–23 weeks between discharge (or recovery from disease) and beginning of follow-up.
FIGURE 4.
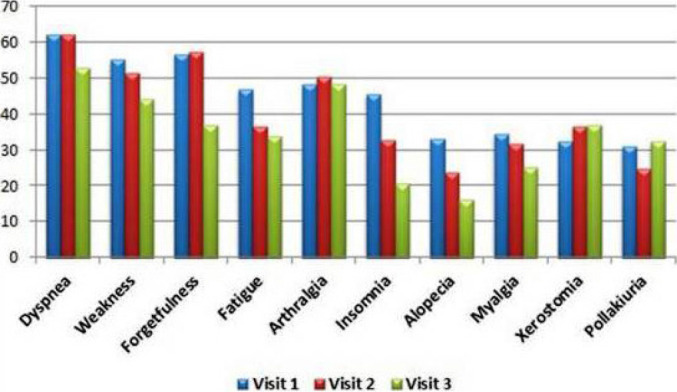
The most common symptoms at three visits of 145 patients with 6 months or more between discharge (or recovery from disease) and beginning of follow-up.
Evaluation of Risk Factors Affecting the Occurrence of Dyspnea, Weakness and Arthralgia
The risk factors affecting dyspnea were analyzed by univariate logistic regression analysis, and age, gender, and hospitalization in the intensive care unit were statistically significant (p=0.028, p=0.009, p=0.049 respectively). Multivariate logistic regression analysis was performed with these three variables. Age and gender were statistically significant risk factors together (p=0.013, p=0.004). In the presence of gender, the risk of dyspnea increases by 1.017 times as age increases by one unit. In the presence of age, being female increases the risk of dyspnea by 1.726 times compared to being male (Table 3).
TABLE 3.
Univariate logistic regression for factors affecting dyspnea
| Variables (reference) | β | SE | p | Odds ratio | 95% CI for odds ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.015 | 0.007 | 0.028 | 1.015 | 1.002 | 1.028 |
| Gender (male) | ||||||
| Female | 0.493 | 0.188 | 0.009 | 1.637 | 1.132 | 2.368 |
| BMI (<18.5) | ||||||
| 18.5-24.9 | 0.899 | 0.653 | 0.169 | 2.457 | 0.683 | 8.842 |
| 25.0-29.9 | 1.018 | 0.631 | 0.107 | 2.767 | 0.803 | 9.537 |
| 30.0-34.9 | 1.444 | 0.640 | 0.024 | 4.238 | 1.208 | 14.867 |
| 35.0-44.9 | 1.404 | 0.654 | 0.032 | 4.071 | 1.129 | 14.682 |
| >45 | 1.609 | 1.037 | 0.121 | 5.000 | 0.655 | 38.152 |
| Hospitalization (no) | ||||||
| Yes | 0.315 | 0.203 | 0.121 | 1.370 | 0.920 | 2.041 |
| Intensive care unit (no) | ||||||
| Yes | 0.420 | 0.214 | 0.049 | 1.522 | 1.001 | 2.315 |
| Comorbidity (no) | ||||||
| Yes | 0.346 | 0.214 | 0.106 | 1.413 | 0.929 | 2.151 |
| Smoking status (yes) | ||||||
| No | 0.261 | 0.349 | 0.455 | 1.298 | 0.655 | 2.572 |
SE: Standard error of mean; CI:Confidence interval; BMI:Body mass index.
The risk factors affecting weakness were examined with univariate logistic regression analysis. Gender and hospitalization were significant (p<0.001, p=0.023 respectively). If interpretation is made according to the results of univariate logistic regression analysis, the risk of weakness in the female sex increases 2.583 times compared to the male sex. In addition, the risk of weakness for those not hospitalized was 1.614 times greater than subjects who were hospitalized (Table 4).
TABLE 4.
Univariate logistic regression for factors affecting weakness
| Variables (reference) | β | SE | p | Odds ratio | 95% CI for odds ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | -0.002 | 0.007 | 0.775 | 0.998 | 0.985 | 1.011 |
| Gender (male) | ||||||
| Female | 0.949 | 0.192 | <0.001 | 2.583 | 1.774 | 3.762 |
| BMI (<18.5) | ||||||
| 18.5-24.9 | 0.134 | 0.630 | 0.832 | 1.143 | 0.332 | 3.929 |
| 25.0-29.9 | -0.035 | 0.605 | 0.953 | 0.965 | 0.295 | 3.161 |
| 30.0-34.9 | 0.050 | 0.612 | 0.935 | 1.051 | 0.317 | 3.488 |
| 35.0-44.9 | 0.374 | 0.629 | 0.552 | 1.454 | 0.424 | 4.992 |
| ≥45 | -0.624 | 0.962 | 0.517 | 0.536 | 0.081 | 3.533 |
| Hospitalization (yes) | ||||||
| No | 0.478 | 0.211 | 0.023 | 1.614 | 1.067 | 2.439 |
| Intensive care unit (yes) | ||||||
| No | 0.246 | 0.205 | 0.230 | 1.278 | 0.856 | 1.910 |
| Comorbidity (no) | ||||||
| Yes | 0.368 | 0.214 | 0.085 | 1.445 | 0.951 | 2.195 |
| Smoking status (no) | ||||||
| Yes | 0.043 | 0.355 | 0.904 | 1.044 | 0.520 | 2.094 |
SE: Standard error of mean; CI:Confidence interval; BMI: Body mass index.
The risk factors affecting arthralgia were analyzed by univariate logistic regression analysis. Gender and hospitalization were significant risk factors (p<0.001, p=0.008). Gender and hospitalization were not significant together (p<0.001, p=0.118). According to the univariate logistic regression results, female sex increases the risk of arthralgia 3.6 times. In addition, the risk of arthralgia for those not hospitalized was 1.7 times greater than those hospitalized (Table 5).
TABLE 5.
Univariate logistic regression for factors affecting arthalgia
| Variables (reference) | β | SE | p | Odds ratio | 95% CI for odds ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | -0.001 | 0.007 | 0.906 | 0.999 | 0.986 | 1.012 |
| Gender (male) | ||||||
| Female | 1.279 | 0.191 | <0.001 | 3.594 | 2.473 | 5.225 |
| BMI(≥45) | ||||||
| <18.5 | 0.624 | 0.962 | 0.517 | 1.867 | 0.283 | 12.310 |
| 18.5-24.9 | 0.442 | 0.797 | 0.579 | 1.556 | 0.2326 | 7.415 |
| 25.0-29.9 | -0.013 | 0.779 | 0.986 | 0.987 | 0.214 | 4.542 |
| 30.0-34.9 | 0.150 | 0.784 | 0.848 | 1.162 | 0.250 | 5.397 |
| 35.0-44.9 | 0.311 | 0.794 | 0.695 | 1.365 | 0.288 | 6.471 |
| Hospitalization (yes) | ||||||
| No | 0.539 | 0.202 | 0.008 | 1.714 | 1.154 | 2.544 |
| Intensive care unit (yes) | ||||||
| No | 0.298 | 0.204 | 0.144 | 1.347 | 0.903 | 2.009 |
| Comorbidity (no) | ||||||
| Yes | 0.259 | 0.213 | 0.225 | 1.295 | 0.852 | 1.968 |
| Smoking status (no) | ||||||
| Yes | 0.361 | 0.348 | 0.300 | 1.435 | 0.725 | 2.840 |
SE: Standard error of mean; CI:Confidence interval; BMI: Body mass index.
DISCUSSION
This study was performed on critically ill patients that had a rate of hospitalization at 71%, ICU unit rate of 27.9%, and prevalence of pneumonia of 71.9%. The mean age of the patients and the frequency of the most common comorbid diseases are similar to the results found in other studies. In this report, the most common comorbidities were hypertension (37.4%), DM (26.3%), hyperlipidemia (18.1), coronary artery disease (12.7%) and hypothyroidism (12.6). In a study in which 65 patients were evaluated at the 7th month after hospital discharge, these rates were as follows: hypertension (29.2%), DM (25%), dyslipidemia (20.8%) and hypothyroidism (8.3%) [3]. In another study, 101 patients were evaluated 10 months after discharge, and hypertension was found to be 41%, coronary artery disease 16%, and DM 12% [4]. In a Spanish study by Maestre-Muniz et al. [5], 67.4% of those hospitalized had hypertension, 24.7% had DM, 24% hyperlipidemia, and 8.1% hypothyroidism.
We observed that dyspnea, weakness, arthralgia, forgetfulness and fatigue were the most common complaints. At the initial assessment, more than half of the patients in the past six months or even 9–12 months continue to have three or more persistent symptoms. Staudt et al. [4] reported that dyspnea, weakness, and cognitive injury were the major symptoms (39 to 49%) 10 months after hospitalization. Our findings are similar to previous a long-term COVID-19 follow-up study, which was done in Spain [5]. Maestre-Muniz et al. [5] examined the presence of PCS one year following discharge from hospital. Patients had persistent symptoms (66.8%) and the most common was dyspnea (54.7%) followed by weakness (tiredness) (54.7%), ageusia (30.2%) and anosmia (26.3%), respectively. Huang et al. [6] showed that 76% of patients reported at least a single symptom at 6 months following the acute COVID-19 infection. Symptoms included weakness (63%), sleep distress (26%), mood swings (anxiety 23%, depression 22%), hair loss (11%), loss of smell (11%), and arthralgia (9%). The results of a meta-analysis study conducted by Fernandez-de-las-Penas et al. [7], which included 15244 hospitalized patients, showed that 63.2, 71.9, and 45.9% had greater than one post-COVID-19 symptom at 30, 60, and 90 days post-COVID-19 infection. Fatigue and dyspnea were most common with a range of 35 to 69% depending on follow-up. These results are very similar to the results of our study. The only difference is that the proportions of patients with three or more symptoms (93.9%, 87.5% and 89%) were higher in our study at all three visits. In a study conducted in Russia [16], it was found that some symptoms continued in half of the patients 6–8 months after COVID-19 infection, and these were fatigue, shortness of breath and forgetfulness. Lopez-Leon et al. [17] performed 21 meta-analyses in which 47,910 patients were included (age 17–87) and the frequency of long-term effects was determined. The studies classified long-COVID patients as those 14 to 110 days post-viral infection. Approximately 80 percent of the COVID-19 subjects had one or more long-term symptoms. This result is similar to the result found in our study. An observational study from 38 hospitals in Michigan examined 1,250 patients discharged [18]. The frequency of ongoing symptoms following COVID-19 ranged from 32.6% to 87% of hospitalized patients. Our results are consistent with previously published these reports. Females were associated with the most common three persistent symptoms, with association being strongest for arthralgia. Other studies of previously reported that females were at risk for long-term COVID symptoms such as dyspnea, weakness, fatigue, pain, hair loss and worse sleep quality [18–20]. In a study by Bai et al. [21], women were found to be three times more likely to be diagnosed with Long-COVID, and the reasons for this were discussed. Among these reasons are the role of hormones in maintaining the hyperinflammatory state of the acute phase even after recovery, and a stronger IgG antibody production in women in the early phase of the disease.
Our study shows that the presence of comorbidities or smoking status of the patient were not associated with the presence of three major symptoms associated with PCS at 6 to 12 months following COVID-19. In another study, an association with the other comorbidities and disease severity was shown. In addition, COVID-19 severity was associated with the level of post-COVID-19 manifestations [22]. In our study, conversely, the possibility of weakness and arthralgia was found to be higher in non-hospitalized patients. When previous studies are examined, it seems possible to encounter different or partially similar results. Moreno-Perez et al. [23] reported that following multivariate modification, there was no features that behaved as independent predictors of “PCS” including age, sex, comorbidity, acute COVID-19 infection severity, inflammatory markers, ICU-admission, hospital/ICU length of stay, or treatment. However, contradictory findings were also reported in two separate studies included in a meta-analysis by Lopez-Leon et al. [17]. In our opinion, the fact that the patients who were treated in the hospital have mostly taken steroids in the treatment may explain that the complaints of joint pain were less in this group and more in the outpatient group that did not receive steroids. In an article evaluating the rheumatologic complications of COVID-19, administration of corticosteroids to hospitalized patients was shown as a plausible reason for the lower incidence of musculoskeletal inflammation related manifestations [24].
Although the three-times, face to face evaluation of a significant number of patients between 6 weeks and 12 months post-COVID strengthens the study, it has some limitations. It was a single center study with that may have produced a potential selection bias. Patients also self-reported with no objective assessment, which may have introduced an information bias. For example; objective assessment methods such as spirometry and body plethysmography or the 6-minute walk test could not be routinely conducted for patients with dyspnea. The second, potentially eligible all participants were not enrolled. Although, all discharged patients from our tertiary hospital after recovering from the COVID-19 were called and invited, only those who came voluntarily were included and followed. The third, the variability of the patients’ post-COVID elapsed time created an obstacle to a standardized assessment.
Conclusions
A large number of COVID-19 survivors had continual symptoms in the first year following infection. Neither the presence of comorbidities of the patient nor smoking status were associated with major symptoms linked to PCS. Females were associated with higher risk of persistent symptoms. A better understanding of the mechanisms, predisposing factors and evaluation necessitates a multidisciplinary team approach.
Footnotes
Cite this article as: Emiroglu C, Dicle M, Demirelli Ozagar S, Gorpelioglu S, Aypak C. Evaluation of post-acute-COVID-19, and long-COVID symptoms with a questionnaire: Within one year, a longitudinal study. North Clin Istanb 2024;11(2):105–114.
Ethics Committee Approval
The Diskapi Yildirim Beyazit Training and Research Hospital Clinical Research Ethics Committee granted approval for this study (date: 03.05.2021, number: 110/12).
Authorship Contributions
Concept – CE, MD, SG, CA; Design – CE, CA, SG, SDO; Supervision – CE, CA, SG; Fundings – CE, MD, CA, SDO, SG; Materials – CA, CE, MD; Data collection and/or processing – CA, CE, SDO, MD; Analysis and/or interpretation – CA, SG, CE; Literature review – CE, MD, SDO, CA, SG; Writing – CE, CA; Critical review – CE, CA, SG, MD, SDO.
Conflict of Interest
No conflict of interest was declared by the authors.
Use of AI for Writing Assistance
Not declared.
Financial Disclosure
The authors declared that this study has received no financial support.
Peer-review
Externally peer-reviewed.
References
- 1.World Health Organization WHO COVID-19 dashboard. Available at: https://covid19.who.int Accessed Jun 30 2022.
- 2.Carfi A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–5. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alwan NA, Johnson L. Defining long-COVID: going back to the start. Med. 2021;2:501–4. doi: 10.1016/j.medj.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staudt A, Jörres RA, Hinterberger T, Lehnen N, Loew T, Budweiser S. Associations of post-acute COVID-19 syndrome with physiological and clinical measures 10 months after hospitalization in patients of the first wave. Eur J Intern Med. 2022;95:50–60. doi: 10.1016/j.ejim.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maestre-Muniz MM, Arias A, Mata-Vazquez E, Martin-Toledano M, Lopez-Larramona G, Ruiz-Chicote AM, et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10:2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–2. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-de-las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suvvari TK, Kutikuppala LVS, Tsagkaris C, Corriero AC, Kandi V. Post-COVID-19 complications: multisystemic approach. J Med Virol. 2021;93:6451–5. doi: 10.1002/jmv.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post-COVID-19 Condition A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–7. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-de-las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID Symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence COVID-19 rapid guideline: managing the long -term effects of COVID-19. Available at: www.nice.org.uk/guidance/ng188 Accessed Jan 19 2024. [PubMed]
- 12.Mumoli N, Conte G, Evangelista I, Cei M, Mazzone A, Colombo A. Post-COVID or long-COVID: two different conditions or the same? J Infect Public Health. 2021;14:1349–50. doi: 10.1016/j.jiph.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113–9. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of post-acute sequelae of SARS-Co-V-2 infection: a systematic review. JAMA Netw Open. 2021;4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021;51:1107–20. doi: 10.1111/cea.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021;27:601–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashif A, Chaudhry M, Fayyaz T, Abdullah M, Malik A, Anwer JMA, et al. Follow-up of COVID-19 recovered patients with mild disease. Sci Rep. 2021;11:13414. doi: 10.1038/s41598-021-92717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-de-las-Penas C, Martin-Guerrero JD, Pellicer-Valero OJ, Navarro-Pardo E, Gomez-Mayordomo V, Cuadrado ML, et al. Female sex is a risk factor associated with long-term post-COVID related symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM multicenter study. J Clin Med. 2022;11:413. doi: 10.3390/jcm11020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mule G, et al. Female gender is associated with long-COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2021;28:611. doi: 10.1016/j.cmi.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterization of post-COVID-19 manifestations. Int J Clin Prac. 2021;75:e13746. doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Perez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jimenez J, et al. COVID19-ALC research group Post-acute COVID-19 syndrome incidence and risk factors: a Mediterranean cohort study. J Infec. 2021;82:378–83. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacharias H, Dubey S, Koduri G, D’Cruz D. Rheumatological complications of Covid 19. Autoimmun Rev. 2021;20:102883. doi: 10.1016/j.autrev.2021.102883. [DOI] [PMC free article] [PubMed] [Google Scholar]


