Abstract
Recent studies have demonstrated that metabolites produced by commensal bacteria causally influence health and disease. The sulfated metabolome is one class of molecules that has recently come to the forefront due to efforts to understand the role of these metabolites in host-microbiome interactions. Sulfated compounds have canonically been classified as waste products; however, studies have revealed a variety of physiological roles for these metabolites, including effects on host metabolism, immune response, and neurological function. Moreover, recent research has revealed that commensal bacteria either chemically modify or synthesize a variety of sulfated compounds. In this Review, we explore how host-microbiome collaborative metabolism transforms the sulfated metabolome. We describe bacterial and mammalian enzymes that sulfonate and desulfate biologically relevant carbohydrates, amino acid derivatives, and cholesterol-derived metabolites. We then discuss outstanding questions and future directions in the field, including potential roles of sulfated metabolites in disease detection, prevention, and treatment. We hope that this review inspires future research into sulfated compounds and their effects on physiology.
Introduction
The sulfated metabolome, or the collection of sulfate-containing small molecule metabolites, is an underappreciated source of unique, biologically active products. Historically, sulfated metabolites were studied almost exclusively as waste products produced by phase II metabolism1,2. The canonical reaction pathways of phase I metabolism (oxidation, reduction, or hydrolysis) and phase II metabolism (conjugation reactions including acylation, glucuronidation, and sulfonation) occur primarily in the liver and produce polar compounds that are more readily excreted1,2. The oxidized products of phase I metabolism were also once considered waste products, but research has shown that redox biology is essential for the control of key signaling pathways3,4. Likewise, recent research has revealed that sulfated metabolites possess signaling capabilities and affect disease-related phenotypes5–8. Moreover, we are now learning that host-microbiome interactions regulate the sulfated metabolome. Bacteria collaborate with the host to produce new molecules, either by metabolizing host-produced sulfated metabolites, producing novel metabolites that are then further modified, or by sulfonating metabolites directly (Fig. 1). These discoveries have placed us at the forefront of a renaissance in the study of the sulfated metabolome and its regulation by both the host and the microbiome.
Figure 1. The host and microbiome produce sulfated metabolites through collaborative metabolism.

The actions of bacterial and host enzymes modulate the pool of biologically active molecules that comprise the sulfated metabolome. Sulfotransferases (SULTs) from both the host and bacteria append a sulfo group (SO3−) to small molecules. Bacterial sulfatases (SULFs) cleave off this sulfo group, releasing inorganic sulfate and an organic alcohol. Finally, both the host and bacteria produce the substrates for these sulfur-modulating reactions, including carbohydrates, amino acid derivatives, and cholesterol-derived compounds. This collaborative sulfur metabolism alters the physiological effects of these compounds and influences their roles in host health and disease.
One of the key advances that has facilitated the study of the sulfated metabolome is the development of new liquid chromatography-mass spectrometry (LC-MS) technologies9,10. In the past, these compounds were analyzed by gas chromatography-mass spectrometry (GC-MS). However, due to the polar character of these metabolites, the sulfate group had to be chemically removed and the sample derivatized prior to analysis. While these methods provided valuable initial insights, their technical complexity increased the risk of error, sample loss, and loss of chemical information, ultimately limiting their widespread utility. Recent advancements in negative ion mode mass spectrometry as well as quadrupole time-of-flight (QTOF) and triple-quadrupole mass spectrometry technologies have allowed for the identification and assignment of hundreds of novel sulfated compounds in biological samples9,10. These studies have spurred efforts to elucidate the biological roles of these metabolites, and research has revealed that sulfated metabolites possess immunoregulatory5,6 and neuromodulatory functions8,11.
Two keystone chemical reactions regulate the composition of the sulfated metabolome: sulfonation and desulfation. Sulfonation is the enzymatically catalyzed transfer of a biologically activated sulfo group (SO3−) from a donor cofactor to the free hydroxyl of a substrate to form a sulfate ester. Sulfonation is catalyzed by sulfotransferases (SULTs). The products of these reactions are typically referred to as sulfates because the products contain an SO4 moiety. However, the transformation itself is referred to as sulfonation because it involves the catalytic transfer of a sulfo group (SO3−) rather than the transfer of a sulfate group (SO42−). These enzymes are classified based on their activities against different groups of compounds (e.g., bile acids, steroids, phenolic compounds) as well as specific substrates (e.g., dehydroepiandrosterone, 2-naphthol). The mammalian genome encodes 5 classes and over 15 isoforms of SULTs2. In bacteria, SULTs were known but had previously only been characterized in environmental and pathogenic microbes12. Interestingly, recent work has identified SULTs in human commensal bacteria with unique substrate profiles, thereby expanding the scope of host-microbiome sulfonation chemistry13,14.
Desulfation, in turn, is the removal of sulfate from sulfate-esters to produce free sulfate and an organic alcohol. Desulfation is catalyzed by sulfatases (SULFs). The mechanism of action of most SULFs requires a formyl glycine residue, which must be generated by a partner enzyme during protein biosynthesis15. Prior work had characterized the activity of microbiome derived SULFs against specific steroids and drugs16. Recently, new gut bacterial SULFs have been discovered possessing a wider range of activities against carbohydrates and steroid-derived compounds15.
In this review, we discuss sulfonation and desulfation mechanisms for three classes of biomolecules: carbohydrates, amino acids derivatives, and cholesterol derivatives (Fig. 1). We highlight microbiome- and host-driven biotransformations of biologically relevant metabolites in each class and summarize the key enzymes involved. Finally, we discuss the effects of the substrates and products of this collaborative sulfur metabolism on host health and disease.
Carbohydrates
Dextran sodium sulfate
Dextran sodium sulfate (DSS) is a chemical colitogen that induces gut inflammation in mice17. Treated animals exhibit weight loss, gut barrier disruption, shortening of colon/SI length, induction of pro-inflammatory cytokines, diarrhea, and colorectal cancer17. Because these phenotypes closely resemble human ulcerative colitis, DSS rodent models are widely used in study of inflammatory bowel diseases (IBD). DSS does not induce inflammation directly, but rather acts as a chemical toxin that targets gut epithelial cells, particularly in the colon. DSS induces cell cycle arrest and cell death, which in turn leads to disruption of gut barrier integrity and recruitment of immune cells, ultimately resulting in an onslaught of inflammatory responses in the gut18.
Even though DSS induces inflammation by acting directly on host epithelial cells, germ-free (GF) and antibiotic-treated mice display reduced colitis severity following DSS treatment19,20. These results suggest that a microbiome is required for DSS-induced colitis in rodent models. Several studies have reported links between the gut microbiome and phenotypic variations amongst DSS treated animals20–22. A recent study found that differences in microbiota composition alone could explain the DSS phenotypic variations. GF mice monocolonized with Duncaniella muricolitica and Alistipes okayasuensis, strains that were positively correlated with disease severity in conventional mice, exhibited worsened disease outcomes following DSS treatment compared to GF mice colonized with health-associated strains Anaerostipes faecis and Sangeribacter muris20. Interestingly, a different study found that a member of the genus Duncaniella conferred protection after DSS treatment23, indicating that bacterial genus-level identity alone is not sufficient to predict DSS susceptibility.
While DSS is highly toxic, dextran, its non-sulfated form, is non-toxic. Incubation of DSS with rat cecal contents in vitro under anaerobic conditions resulted in breakdown of DSS through subsequent desulfation and carbohydrate degradation24. This result suggests that the microbiome can degrade and potentially detoxify DSS. However, whether strains of bacteria that protect against DSS-induced colitis can desulfate DSS is currently unknown.
DSS can also modulate the microbiome. DSS treatment increases the abundance of sulfur-metabolizing bacteria, including A. muciniphila25. Release of mucin-associated sulfur by A. muciniphila glycosulfatases results in a bloom in sulfur-consuming bacteria, thus increasing the capacity of the microbiota to use sulfated carbohydrates as a carbon source25. In this context, increased abundance of sulfur-metabolizing bacteria affects the host by inducing desulfation of intestinal mucin. Mucin forms the first line of defense against gut bacteria and other gut luminal insults, preventing these factors from accessing the intestinal epithelium and immune cells26. Mucin degradation results in exposure of the gut epithelium to DSS and other inflammatory factors in luminal contents, which in turn leads to inflammation (Fig. 2a). Fecal microbiota transplants (FMTs) have been shown to reduce sulfated mucin degradation, thus reducing the abundance of sulfur-metabolizing bacteria and protecting against DSS colitis. However, in animals possessing a genetic mutation that prevents sulfonation of newly synthesized mucin, FMTs fail to protect against DSS-induced colitis because non-sulfated mucin is not sufficiently protective against inflammatory insults25.
Figure 2. Microbial desulfation of sulfated carbohydrates influences host biology.
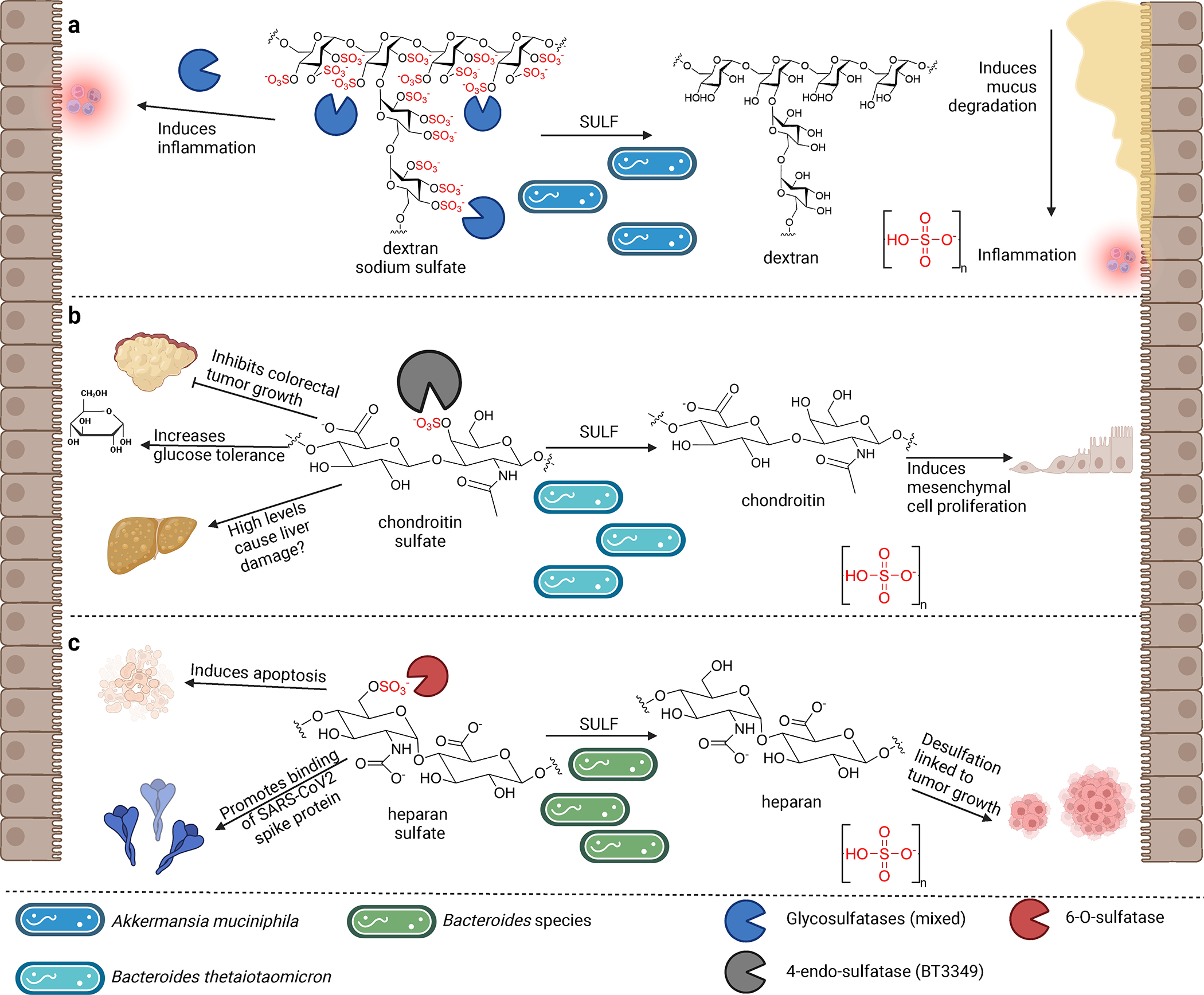
a, Dextran sodium sulfate (DSS) directly induces inflammation in the gut by acting as a chemical toxin. Desulfation of DSS by gut microbes produces non-toxic dextran but also releases sulfate. This free sulfate induces the growth of sulfur-metabolizing bacteria such as A. muciniphila, which in turn degrade host mucus, thereby increasing gut inflammation. b, Chondroitin sulfate (CS) inhibits growth of colorectal tumors, increases glucose tolerance, and can damage liver function at high levels. Desulfation of CS by commensal gut bacteria induces proliferation of mesenchymal cells. c, Heparan sulfate (HS) exhibits a plethora of activities in host cells, including induction of apoptosis, inhibition of senescence, and interaction with SARS-CoV-2 spike protein. Desulfation of HS by gut Bacteroides species may increase tumor growth.
From these collective results, we can conclude that sulfonation of DSS is critical to its effects as an inflammatory agent, either by directly acting as a chemical toxin or by indirectly stimulating growth of mucus-degrading bacteria. Moreover, these data suggest that the microbiome may be one reason for the lack of reproducibility in DSS colitis models27 and motivate further studies to identify DSS-degrading bacteria and the corresponding enzymes responsible. Preliminarily, these findings also suggest that inhibition of mucin desulfation by gut bacteria could protect against gut inflammatory conditions.
Chondroitin sulfate
Chondroitin sulfate (CS) is a sulfated glycosaminoglycan that acts as a building block of cartilage and plays an important role in maintaining tissue structural integrity28. CS is commonly used for treatment of osteoarthritis29. Many studies have documented both beneficial and detrimental effects of CS therapy beyond its effects on joint health. For example, some studies have found that CS inhibits colorectal cancer in mice, improves glucose tolerance, and promotes healthy aging30–32. CS treatment also increases levels of butyrate and beneficial amino acids in the gut28. In contrast, CS has been reported to induce liver damage, atherogenesis, and dementia-like pathogenesis in mice33. One study found that CS treatment can ameliorate inflammation by reducing systemic LPS levels, while other studies have found CS to induce IBD by increasing H2S production and inducing mucin degradation in the gut28,30,34, highlighting the complex physiological effects of this sulfated sugar.
Interestingly, the effects of CS on the host are largely dependent on its metabolism by the gut microbiome. The bioavailability of CS is estimated to be 0–13% due to gut microbiota-mediated transformation, particularly its desulfation. Patients are therefore prescribed 2–3 times higher doses of CS to achieve the desired effects on osteoarthritis remediation35. A recent study has postulated that the colonization levels of mucus-degrading bacteria such as Akkermansia muciniphila in the gut could determine the bioavailability and subsequent positive or negative effects of CS therapy30. Probiotics in human gut microbiota can degrade host glycosaminoglycans through their sulfatases36. In the case of CS, gut commensals such as Bacteroides thetaiotaomicron J1, Bacteroides thetaiotaomicron 82, Bacteroides ovatus E3, and Clostridium hathewayi R4 degrade CS by desulfation and hydrolysis35. Study of sulfatases in the B. thetaiotaomicron genome identified BT3349 as an active sulfatase for CS37. BT3349 encodes a 4-endo-O-desulfatase whose activity in the periplasm precipitates the breakdown of larger sugars into smaller saccharides that are then used by the bacterial cell for further metabolism37,38. Desulfation of CS can induce mesenchymal stem cell proliferation39. In addition, both breakdown of CS into disaccharide residues and a decrease in CS sulfonation have been shown to induce steatohepatitis and liver cirrhosis in mice30,34. On the other hand, in a liver cirrhosis rat model, an increase in CS oversulfation was observed in the fibrous lesion compared with the nonfibrous lesion. Oversulfation was characterized by an increase in GalNAc(4,6SO4) residues, the formation of which requires 4-sulfate-6-O-sulfotransferase (GalNAc4S-6ST) enzymes. GalNAc4S-6ST knock out animals are deficient in CS GalNAc(4,6SO4) residues, suggesting that CS oversulfation is likely performed by the host. The factors driving this oversulfation are currently unknown and warrant further study. Together, these results suggest that the degree of CS sulfonation and the action of gut microbiota on CS could modulate liver fibrosis33.
Importantly, CS treatment can modulate the gut microbiome. CS administration induces growth of sulfatase-secreting bacteria (SSB) in the colon such as Bacteroides thetaiotaomicron and can promote the growth of sulfate-reducing bacteria (SRB) in the gut, including Desulfovibrio piger and Akkermansia muciniphila40. Additionally, the presence of anSME (anaerobic sulfatase maturing enzyme), the enzyme responsible for activating sulfatases via the formation of a formyl glycine residue, was found to be essential for B. thetaiotaomicron to colonize the gut37. While an increase in SRB is associated with increased glucagon-like peptide-1 (GLP-1) and insulin secretion, as well as improved oral glucose tolerance in mice, SRB also induce gut inflammation and cancer by increasing colonic H2S levels and inducing mucin degradation40,41. Further investigation of both bacterial modification of CS and the effects of CS on gut microbiome community composition and function may lead to improved strategies for therapeutic use of this metabolite.
Heparan sulfate
Heparan sulfate (HS) is a sulfated glycosaminoglycan that can be found either as free, unconjugated chains or conjugated to proteins at the cell surface and in the extracellular matrix42. HS is synthesized by mammalian cells as a proteoglycan, assembled on core proteins by enzymes in the Golgi, and sulfonated in the cell43. HS chains are then presented on the cell surface as sulfated proteoglycans, which can further undergo proteolytic and glycosidic cleavage by the host42. HS deregulation can initiate and advance the progression of several malignancies44. HS induces apoptosis, thus inhibiting cancer initiation. Interestingly, increase in HS desulfation is liked to tumor growth, while inhibition of intracellular host HS sulfatases downregulates tumor progression44. HS is also a mediator of healthy aging and anti-senescence32,45. The degree of HS sulfonation is linked to inhibition of cellular senescence46, effects that that may be beneficial to the host in the context of healthy cells but detrimental in the context of cancer progression. To this point, reduction of HS sulfonation by depleting PAPSS2, an enzyme that synthesizes the sulfur donor PAPS, or via small molecule inhibitor-mediated repression of PAPSS2 led to premature cell senescence in cancer cells46. Furthermore, altered HS metabolism during development triggers dopamine-dependent autistic-behaviors in the MPS-IIIA mouse model of ASD47. MPS-IIIA mice showed an overall increase in charge density, with an increase in the degree of 2-O-sulfonation in MPS-IIIA. Finally, a recent study found that cellular HS interacts with the receptor-binding domain of the SARS-CoV-2 spike protein and is a necessary cofactor for SARS-CoV-2 infection48. Together, these results indicate that HS induces wide-ranging physiological effects on the host at the cellular level.
HS is present on the surface of small intestinal epithelial cells, suggesting that this compound is accessible to host microbes. The gut microbiome has a high capacity for HS desulfation49. Three Bacteroides species, B. xylanisolvens, B. thetaiotaomicron and B. vulgatus, are predicted to catabolize HS. HS-modifying bacteria reduce in abundance with age and are reduced in patients with COVID-19. Commensal host bacterial communities from the lung and nasal microbiome can modify HS and thereby modulate SARS-CoV-2 spike protein binding. Cell-surface HS on H1299 human lung cells was reduced by 60% upon exposure to cell-free supernatants from mid-log phase cultures of B. ovatus or B. thetaiotaomicron49–51. Together, these data suggest that desulfation of HS by the microbiome may affect host health in a context-dependent manner, including both inhibitory and stimulatory effects in the context of cancer cell growth and anti-infective effects in the context of SARS-CoV-2. Further studies are needed to causally connect bacterial desulfation of HS with specific host effects in vivo.
Conclusions
Human gut microbes modulate the degree of sulfonation of both exogenous and host-produced carbohydrates. It is apparent that microbial desulfation of carbohydrates can have substantial effects on host health, specifically in the context of gut inflammation. Bioinformatics and experimental studies have revealed that carbohydrate SULFs are frequently encoded as part of large polysaccharide utilization loci (PULs)52. These SULFs cleave 2-O, 4-O, 6-O, or 3-O linkages on the sugar activating the PUL37,52,53. In the gut microbiota, many members of the Bacteroides genus secrete SULFs that act against CS, HS, and mucin O-glycans53. Sequence similarity networks have also identified putative carbohydrate sulfatase genes in a diverse array of gut-colonizing microbes, including Alistepes, Hungatella, and Clostridia52. This research indicates that carbohydrate SULFs are more broadly distributed across the human gut microbiota than previously thought52. In vitro and in vivo experiments that pursue the hypotheses generated by bioinformatic and modeling studies will reveal the substrates of these enzymes, their biogeographic sites of operation in the GI tract, and the effects of the products on host health. Moreover, understanding the mechanisms by which bacterial sulfur metabolism mediates inflammation could lead to a deeper understanding of models like DSS and the ways in which microbes mediate host immune function.
Amino acid-derived metabolites
Indoxyl sulfate
Indoxyl sulfate is a pro-inflammatory uremic toxin. Gut bacteria, including members of the genus Bacteroides, convert tryptophan to indole via encoded tryptophanases (BT1492), which is then 3-hydroxylated to indoxyl by host cytochrome P450s, including CYP2E17,54. Indoxyl is then sulfonated in the host liver by SULT1A1, which has broad activity against phenolic compounds2,54. Indoxyl sulfate contributes to the progression of chronic kidney disease (CKD) and is a risk factor for thrombosis, atherosclerosis, and cardiovascular disease-related death amongst late-stage CKD patients undergoing dialysis55. This metabolite promotes vascular calcification and is associated with glucose intolerance in CKD patients56. Indoxyl sulfate acts as a ligand for the aryl hydrocarbon receptor (AHR). Importantly, sulfating indole greatly increases its potency as an AHR agonist57. Indoxyl sulfate induces TNFα expression and secretion by macrophages and NFkB activation via AHR58. Finally, indoxyl sulfate induced depression, anxiety, and cognitive impairment phenotypes in mice, demonstrating that this compound also exhibits neurotoxic effects59. Experiments in GF mice showed that while indole is beneficial to gut-barrier function, indoxyl sulfate lacks this effect60. However, while indoxyl sulfate induces detrimental phenotypes, indole and other indole metabolites can have positive and negative roles in the host as reviewed elsewhere61.
The amount of indoxyl sulfate in circulation is controlled by the interplay between host and microbial enzymatic transformations. Indoxyl sulfate concentration depends not only on bacterial production of indole, but also on bacterial conversion of tryptophan to other metabolites. Diet can also have a consequential impact on levels of indoxyl sulfate, as omnivores have significantly lower levels of indoxyl sulfate than carnivores. This effect is likely due to both a reduction of tryptophan substrate as well as a shift in microbial community composition caused by a high-fiber, low-protein diet62.
p-Cresol sulfate
p-Cresol sulfate (pCS) is a uremic toxin with carcinogenic and neuromodulatory properties that also influences AHR signaling (Fig. 3a–b). Bacteria in the distal colon synthesize p-cresol from tyrosine through a series of transamination, deamination, and decarboxylation reactions. Like indoxyl, p-cresol is then sulfonated in the host liver by SULT1A2,63. pCS is associated with chronic kidney disease and induces proliferation and migration of clear cell renal cell carcinoma. pCS induces epithelial-mesenchymal transition, migration, and cancer cell proliferation via HIF-1α and requires miR-21 for its activity64. Similar to IS, pCS promotes vascular calcification and is associated with glucose intolerance in CKD patients55. p-Cresol intestinal uptake and pCS urinary clearance is also associated with cardiovascular disease in human patients of CKD65. Finally, administration of the precursor p-cresol to mice induced anxiety- and depression-like behavior and caused social communication deficiencies66.
Figure 3. Amino acid-derived sulfated metabolites exhibit diverse biological effects.

a, Tryptophan and tyrosine are converted by bacterial enzymes into intermediates indole, p-cresol, and 4-ethylphenol (4EP) in the gut. These compounds are subsequently recirculated to the liver and sulfonated by host SULT1A1, producing indoxyl sulfate (IS), p-cresol sulfate (pCS), and 4-ethylphenyl sulfate (4EPS), respectively. b, IS, pCS, and 4EPS possess biological activities that are distinct from their non-sulfated precursors. These metabolites are associated with chronic kidney disease in patients. They also induce cardiovascular disease phenotypes and behavioral abnormalities in animal models and cause inflammation via activation of the aryl hydrocarbon receptor (AHR) in macrophages.
At least 55 bacterial strains present in the human microbiota have been identified that produce p-cresol. These strains are phylogenetically divergent and spread across the Bifidobacteriaceae, Enterobacteriaceae, Coriobacteriaceae, Bacteroidaceae, Fusobacteriaceae, Lactobacillaceae, and Clostridiaceae families66. The production of p-cresol by Clostridium difficile, a pathogen that causes severe diarrhea and colitis, provides a growth advantage to this bacterium67. Treatment of conventionally colonized animals with p-cresol changes the microbiota and enriches for p-cresol-producing commensals. Fecal microbiota transplantation from p-cresol-treated animals to naïve mice increases p-cresol production and also transfers behavioral deficits associated with p-cresol treatment68. Similar to IS, omnivores have significantly lower levels of pCS than carnivores62.
4-ethylphenyl sulfate
The uremic toxin 4-ethylphenyl sulfate (4EPS) is also implicated in AHR signaling (Fig. 3a–b). The toxin 4EPS is generated through host-commensal collaborative metabolism. Tyrosine is converted to p-coumaric acid by tyrosine ammonia lyases (AL) found in gut Bacteroides species. The p-coumaric acid that is secreted by Bacteroides species is imported by other bacteria, including Lactobacillus plantarum, where phenolic acid decarboxylase (PAD) and vinyl phenol reductase (VPR) enzymes convert this compound to 4-ethylphenol (4EP), which is converted to 4EPS by SULT1A1 in the liver11. This compound is not detected in germ free animals, further supporting the conclusion that the microbiome is required for the synthesis of 4EPS. This metabolite is toxic in high amounts and is linked to chronic kidney disease and autism69. 4EPS treatment impaired gut barrier integrity and induced anxiety-like behavior in maternal immune activation (MIA) mouse model of ASD. This sulfated metabolite reduces myelination of axons, impairs oligodendrocyte maturation and function, and decreases oligodendrocyte-neuron interactions in ex vivo brain cultures11. Production of 4EPS by host-bacteria collaborative metabolism in vivo increased ASD- and anxiety-like behaviors in mice11. In contrast, B. fragilis treatment reduced elevated 4EPS levels in MIA mice by over 8-fold. Whether B. fragilis or other gut commensals desulfate or otherwise metabolize 4EPS is unknown.
Conclusions
Production of sulfated amino acid derivatives is an example of how collaborative metabolism between bacteria and the host can produce bioactive metabolites. Future work may reveal that enzymatic amino acid transformations once thought to be exclusively performed by the host can also be catalyzed by gut bacterial enzymes. Many of these sulfated compounds are toxins and exhibit neuromodulatory activities in vitro and in vivo in rodent models. Levels of IS, pCS, and 4EPS are elevated in children with ASD70–75. Whether these compounds play a role in human neurological disease warrants further investigation. Finally, given the abundance of amino acids in the gut and the richness of microbial chemistry involving these substrates, future studies are likely to reveal new bioactive sulfated amino acid derivatives.
Cholesterol-derived metabolites
Cholesterol sulfate
Cholesterol sulfate (ChS) is a sulfated sterol molecule found in host tissues and gut contents (Fig. 4). ChS has a variety of known biological activities in host metabolism, including regulation of de novo cholesterol (Ch) biosynthesis via inhibition of HMG-CoA reductase76, alteration of membrane fluidity for immune77 and reproductive function78,79, and inhibition of T-cell migration in murine tissue5 (Fig. 5a). Additionally, ChS serves as a sex hormone precursor in liver metabolism79.
Figure 4. Host and microbial enzymes biosynthesize cholesterol-derived sulfated metabolites.

(1) Dietary consumption and endogenous metabolism both contribute to the pool of cholesterol in the host; (2) Host liver enzymes convert dietary and host-produced cholesterol into conjugated primary bile acids, which are stored in the gallbladder before being secreted into the gut. (3) Bacterial bile salt hydrolases then hydrolyze these compounds to produce unconjugated bile acids, (4) which are then converted into secondary bile acids such as lithocholic acid (LCA) by bacterial enzymes in the bile acid-inducible (bai) operon. (5) LCA agonism of VDR in the liver induces SULT2A, which catalyzes the sulfonation of bile acids to produce LCA-3-sulfate and cholic acid-7-sulfate. (6) Separately, cholesterol can be converted into the androgen dehydroepiandrosterone (DHEA) via host cytochrome P450 (cyp) enzymes. (7) Cholesterol and (8) DHEA can be sulfonated by host SULT 2B1 to produce DHEA-S and cholesterol sulfate (ChS). (9) The sulfated and desulfated forms of these metabolites can then be interconverted by bacterial SULTs and SULFs.
Figure 5. Cholesterol-derived sulfated metabolites are associated with host phenotypes.

a, Cholesterol-sulfate (ChS) influences host metabolism and immunity and is produced by both the host and the microbiome. ChS inhibits T cell migration and can alter membrane fluidity. ChS also inhibits host hepatic cholesterol production, thus affecting cholesterol metabolic pathways. b, Sulfonation of the microbial metabolite lithocholic acid (LCA) to lithocholic acid-3-sulfate (LCA3S) abolishes its ability to agonize TGR5. c, Host production of cholic acid-7-sulfate (CA7S) is induced by LCA. CA7S exhibits anti-diabetic properties and improves hyperglycemia via activation of TGR5 and induction of GLP-1 secretion. d, Dehydroepiandrosterone (DHEA) is an adrenal steroid that exacerbates polycystic ovary syndrome (PCOS) and cardiovascular disease phenotypes. Sulfonation of DHEA to DHEA-3-sulfate (DHEA-S) detoxifies this metabolite.
Recent studies have shown that the microbiome plays an important role in regulating cholesterol metabolism and homeostasis14,80. While ChS was once thought to be produced exclusively by host sulfotransferases (SULT2B1b)81, recent work has found that gut bacteria from the phylum Bacteroidetes produce ChS from cholesterol using SULT enzymes13,14. Homologs of these genes are widely distributed in Bacteroidetes and are also hypothesized to be present in other commensals13,14. In one study, bacterially synthesized ChS entered circulation and assisted in regulating global Ch levels14. In another study, an immunoregulatory function of bacterially produced ChS was identified, and SULT-dependent inhibition of T cell migration to mesenteric lymph nodes was observed in vivo in mice13.
Both the host78 and gut bacteria15,16 have also been shown to desulfate steroid sulfates. In the case of the host, the enzyme steroid sulfatase (hSTS) is broadly active against steroid sulfates in host tissues78. In bacteria, while previous gut microbe SULFs were not shown to act on ChS16, a recent study showed that bacterial SULFs hydrolyze ChS to cholesterol inside ChS-producing bacteria13. The authors hypothesized that the SULFs and SULTs of ChS-producing Bacteroidetes engage in a dynamic interplay that controls intracellular cholesterol ChS levels and that ChS is released and can interact with host targets upon bacterial cell lysis13. Together, these studies suggest that sulfonation of cholesterol likely acts as a method of communication between microbiome and host systems. Given the biological importance of sulfated and desulfated cholesterol, this interplay between bacterial and host production and metabolism of ChS warrants further exploration in vivo.
Lithocholic acid-3-sulfate
Lithocholic acid-3-sulfate (LCA-S) is a sulfated compound derived from the secondary bile acid lithocholic acid (LCA) (Fig. 4). Previous studies have identified conjugated salts of LCA-S (tauro-/glyco-N-acyl conjugates) in bile and intestinal contents82, but their functions have not yet been elucidated. While LCA is a potent agonist against the Takeda G-protein receptor 5 (TGR5) receptor, sulfonation of LCA abolishes the receptor-ligand affinity83. Recent work in murine cell culture has identified a potential immunoregulatory role for LCA-S as a ligand of retinoid-related orphan receptor γt (RORγt). This study found that LCA-S interacted with the ligand binding domain of RORyt with high affinity, suggesting a potential ability for LCA-S to modulate RORyt-expressing immune populations such as CD4+ Th17 cells84. This reported activity for LCA-S suggests a unique biological role for this compound beyond abolition of TGR5 agonism and should be further investigated in in vitro and in vivo studies. Moreover, this research motivates further work exploring the biological activities of LCA-S and other sulfated secondary bile acids.
LCA-S is produced through host-bacterial collaborative metabolism. First, a wide variety of gut bacteria convert host-produced conjugated chenodeoxycholic acid (tauro- or glyco-CDCA) into CDCA in the gut. Then Clostridium scindens and other closely related bacteria convert CDCA to LCA in the lower GI tract85. Bacterially synthesized LCA is transported from the gut lumen to the liver via the portal vein86, where it is then sulfonated by host enzyme SULT2A182. Whether gut bacteria can sulfonate LCA to produce LCA-S is currently unknown. Likewise, bacterial SULFs that can hydrolyze LCA-S have not yet been identified.
Cholic acid-7-sulfate
Cholic acid-7-sulfate (CA7S) is a sulfated bile acid metabolite produced by the host liver via the sulfonation of cholic acid, a host-produced bile acid, by host enzyme SULT2A187. CA7S levels were found to be increased in mice and humans following bariatric surgery88. Additionally, acute and chronic treatment of mice with CA7S increases glucose tolerance. While cholic acid is a weak TGR5 agonist, CA7S is a potent TGR5 agonist (half-maximum effective concentration (EC50) values of 12 μM and 170 nM, respectively). Agonism of TGR5 by CA7S stimulates secretion of the incretin hormone GLP-1 from intestinal L cells (Fig 5c). GLP-1 then travels to the pancreas, where it stimulates insulin secretion and improves glucose tolerance88. CA7S is restricted to the gut, and as a result, it agonizes TGR5 exclusively in the GI tract, thereby avoiding deleterious off-target effects of TGR5 activation outside the gut88.
Lack of a microbiome depletes host production of CA7S, suggesting that cholic acid sulfonation is microbiome-dependent86,89. Gut bacteria do not directly sulfonate cholic acid to produce CA7S. Instead, it was found that a shift in the microbiome post-bariatric surgery was sufficient to induce production of CA7S by the host. Surgery induces an increase in the transport of the microbially derived bile acid LCA from the gut to the liver via the portal vein. In the liver, LCA activates the vitamin D receptor (VDR), which induces sulfonation of cholic acid to produce CA7S (Fig. 4). This research provides evidence that cross-talk between gut microbiota and host metabolism can be mediated by a sulfated metabolite. Importantly, a cecal microbial transplant from mice post-sleeve gastrectomy to GF mice was sufficient to induce CA7S production, demonstrating that the gut microbiome influences production of this anti-diabetic sulfated metabolite86. Finally, consumption of vegetable lecithins, known to be beneficial for metabolic and nutritional health, modulates the gut microbiome and increases levels of CA7S in mice90, demonstrating that diet also affects CA7S biosynthesis.
Dehydroepiandrosterone-3-sulfate
DHEA-S is an abundant steroid derivative that is synthesized by the host through the sulfonation of dehydroepiandrosterone (DHEA) by SULT2A11. While DHEA is an adrenally produced steroid that possesses androgenic activity91, DHEA-S lacks hormonal activity but can still act as a neurosteroid and neurotrophin92 and is the most abundant steroid sulfate in human plasma91. DHEA has been advertised as a beneficial oral supplement despite conflicting reports of beneficial bioactivity79,91 (Fig. 5d).
It has been shown that DHEA induces a polycystic ovary syndrome (PCOS)-like phenotype in rat models93. In this study, DHEA created a permanently dysbiotic microbiome characterized by a loss of microbial diversity. Fecal microbiota transplants from the DHEA-fed, dysbiotic rats to untreated rats resulted in the development of disease phenotypes, suggesting that the shifted microbiome caused by DHEA treatment may influence disease initiation or progression93. In separate work, it was found that the gut commensal B. thetaiotaomicron sulfonates DHEA in bacterial culture to produce DHEA-S13 (Fig. 4). Considering the toxic effects of parent molecule DHEA and the canonical role of sulfonation in detoxifying molecules, it is worth exploring whether bacteria-mediated sulfonation could act as a protective mechanism against toxic small molecules such as DHEA. Finally, a recent study has also found that certain gut bacteria can desulfate DHEA-S15. Together, these data suggest that bacterial community composition could affect the ratio of DHEA to DHEA-S in vivo.
Conclusions
In the traditional view of sulfated metabolome dynamics, host sulfonation of steroids marks these compounds for excretion. The microbiome then desulfates a portion of these compounds, resulting in the reuptake of the parent steroids1,79,94. That model no longer encompasses the breadth and complexity of interactions of the host and microbiome with sulfated steroidal metabolites. New studies have revealed that sulfated steroids have important regulatory and signaling roles in the host. Moreover, recent studies have revealed the presence of steroid-targeting SULTs and SULFs in the microbiome, leading to the conclusion that levels of sulfated steroids are controlled by interactions between host and commensal metabolism. By coupling enzymatic discovery to biological testing, future studies will reveal how gut microbes shape the sulfated steroid metabolome and how these compounds affect the host.
Outlook
In this review, we have highlighted the rich biology and biochemistry of the sulfated metabolome. We discussed the desulfation of complex, host-produced carbohydrates, cooperative metabolism in the biosynthesis of sulfated amino acid derivatives, and the dynamic and expanding metabolism and biological role of cholesterol-derived sulfates1,79,94. In a broader context, each of these molecular classes highlights a different aspect of sulfur metabolism; however, there are further activities that have yet to be fully explored. Studies have found SULFs for cholesterol-derived molecules and carbohydrates, but the ability of bacteria to desulfate amino acid derivatives is an important but underexplored topic. Additionally, with new knowledge of the existence of SULTs in gut bacteria, the ability of the microbiome to engage in sulfonation needs to be further explored.
Increasing our understanding of the identities and activities of compounds in the sulfated metabolome has implications for the detection and treatment of human diseases. Modern LC-MS techniques have enabled detection and quantification of the sulfur metabolome. A recent study used a new analytical method to identify 206 novel sulfated metabolites modulated by the gut microbiome in human urine and fecal samples, which is a substantial increase compared with the number previously reported in the Human Metabolome Database95. Expansion of metabolomic analyses of patient samples to include sulfated compounds may reveal novel physiologically active molecules with causal roles in disease pathogenesis. The discovery of HS as a mediator of SARS-CoV-2 infection in lung cells has led to the suggestion that therapeutic agents that degrade HS could combat COVID-1948. Considering that the gut microbiota has the capacity to modify HS, whether the nasal or lung microbiome plays a role in susceptibility to COVID-19 warrants investigation. Detection of sulfated metabolites will also expand the plethora of markers for diseases in human patients. For example, pCS may serve as an important systemic biomarker for autism, especially in children68. Indoxyl sulfate is a potential biomarker for renal function decline and for predicting poor outcome after kidney transplants96.
Historically, sulfonation of metabolites is considered a detoxification mechanism that increases negative charge and polarity, thus restricting these compounds to the GI tract and ensuring excretion87. Recent studies have investigated sulfonation as a means of gut-selective therapeutic targeting. For example, activation of TGR5 in an intestine-specific manner by CA7S prevents off-target effects that arise from parenteral activation of this receptor88. Other TGR5 agonists that are systemically absorbed, while effective at improving hyperglycemia, have failed in clinical trials due to untargeted TGR5 agonism in blood vessels, heart, and the gallbladder97,98. Recently, a sulfated, gut microbiome-targeted covalent inhibitor protected against gut barrier dysfunction in an animal model of non-alcoholic steatohepatitis (NASH) without causing systemic off-target effects99,100. In both these cases, sulfonation of metabolites reduced off-target effects and thereby improved the selectivity of these metabolites in vivo.
Targeting sulfated metabolites for removal could also be explored as a strategy to treat certain diseases. In a pilot, single-cohort, open-label study, the oral adsorbent AB-2004 reduced levels of 4EPS, IS, pCS, and other bacterial metabolites in adolescents and improved some metrics of anxiety and irritability8. While this study is cause for optimism, caution is required, as the levels of many metabolites were altered by the adsorbent and no control arm was included to assess the potential of beneficial psychological effects from treatment. This work motivates the development of tools to selectively modulate the levels of sulfated compounds in vivo as well as further studies of the mechanisms by which these compounds are produced and their effects on host neurological development and function8. More broadly, investigation of the sulfur metabolome presents new opportunities for disease detection, prevention, and treatment.
In all, we have highlighted several classes of sulfated metabolites that are becoming increasingly prevalent in studies of microbiome-host interactions and may play an important role in human health and disease. We hope that our understanding of the sulfated metabolome continues to grow as studies reveal the physiological effects of sulfated molecules and the ability of gut microbes to modify and functionalize these compounds in unique ways.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants R35 GM128618, R01 DK126855, and R01DK110559, and an Alfred P. Sloan Fellowship (A.S.D). S.N.C. acknowledges an American Heart Association Postdoctoral Fellowship and an NIH K99/R00 Pathway to Independence Award (K99 DK128503). We thank Melissa Tran for helpful discussions, and we acknowledge BioRender for help with figure creation. Correspondence should be addressed to S.N.C. and A.S.D.
Footnotes
Competing interests
A. S. D. is an ad hoc consultant for Takeda Pharmaceuticals, Axial Therapeutics, and Ferring Pharmaceuticals. S. N. C. is an ad hoc consultant for Metis Therapeutics. G. D. D. declares no competing interests.
References
- 1.Jancova P, Anzenbacher P & Anzenbacherova E PHASE II DRUG METABOLIZING ENZYMES. Biomed Pap 154, 103–116 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Lindsay J, Wang L-L, Li Y & Zhou S-F Structure, function and polymorphism of human cytosolic sulfotransferases. Curr Drug Metab 9, 99–105 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Fleming I The factor in EDHF: Cytochrome P450 derived lipid mediators and vascular signaling. Vasc Pharmacol 86, 31–40 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Li T & Apte U Chapter Nine Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv Pharmacol 74, 263–302 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakurai T et al. Cholesterol sulfate is a DOCK2 inhibitor that mediates tissue-specific immune evasion in the eye. Sci Signal 11, eaao4874 (2018). [DOI] [PubMed] [Google Scholar]
- 6. Wang F, Beck-García K, Zorzin C, Schamel WWA & Davis MM Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat Immunol 17, 844–850 (2016). Cholesterol sulfate, historically considered a waste product, inhibits TCR signaling.
- 7.Lobel L, Cao YG, Fenn K, Glickman JN & Garrett WS Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 369, 1518–1524 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell AS et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial. Nat Med 28, 528–534 (2022). Oral administration of the sequestrant AB-2004 reduced levels of sulfonated aromatic amino acid metabolites and improved some ASD phenotypes in human patients. This is the first clinical trial resulting from the study of sulfonated microbial metabolites.
- 9.Correia MSP et al. Comparative dietary sulfated metabolome analysis reveals unknown metabolic interactions of the gut microbiome and the human host. Free Radical Bio Med 160, 745–754 (2020). [DOI] [PubMed] [Google Scholar]
- 10. Fitzgerald CCJ et al. Profiling Urinary Sulfate Metabolites With Mass Spectrometry. Frontiers Mol Biosci 9, 829511 (2022). This paper provides a novel analytical method for systematic, untargeted metabolic profiling of sulfated metabolites.
- 11. Needham BD et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 602, 647–653 (2022). Collaborative metabolism between host and the microbiome results in production of a sulfonated amino acid, 4-EPS, which modulates neurological activity.
- 12.Mougous JD, Green RE, Williams SJ, Brenner SE & Bertozzi CR Sulfotransferases and Sulfatases in Mycobacteria. Chem Biol 9, 767–776 (2002). [DOI] [PubMed] [Google Scholar]
- 13. Yao L et al. A biosynthetic pathway for the selective sulfonation of steroidal metabolites by human gut bacteria. Nat Microbiol 1–15 (2022) doi: 10.1038/s41564-022-01176-y. One of the first two papers to report cholesterol sulfonation by gut bacteria, this work also shows that ChS inhibits leukocyte migration.
- 14. Le HH, Lee M-T, Besler KR, Comrie JMC & Johnson EL Characterization of interactions of dietary cholesterol with the murine and human gut microbiome. Nat Microbiol 1–14 (2022) doi: 10.1038/s41564-022-01195-9. One of the first two papers to report cholesterol sulfonation by gut bacteria, this work also shows that ChS produced by gut bacteria enters systemic circulation.
- 15. Ervin SM et al. Structural Insights into Endobiotic Reactivation by Human Gut Microbiome-Encoded Sulfatases. Biochemistry-us 59, 3939–3950 (2020). This work structurally characterized representative sulfatases from across the gut microbiome, identified conserved motifs for active site activation, and identified key variations in gut microbial sulfatases that may give rise to substrate selectivity profiles.
- 16.Eldere JV, Parmentier G, Asselberghs S & Eyssen H Partial characterization of the steroidsulfatases in Peptococcus niger H4. Appl Environ Microb 57, 69–76 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chassaing B, Aitken JD, Malleshappa M & Vijay-Kumar M Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr Protoc Immunol 104, 15.25.1–15.25.14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ARAKI Y et al. Dextran sulfate sodium administered orally is depolymerized in the stomach and induces cell cycle arrest plus apoptosis in the colon in early mouse colitis. Oncol Rep 28, 1597–1605 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Chirlaque C et al. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J Crohn’s Colitis 10, 1324–1335 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Forster SC et al. Identification of gut microbial species linked with disease variability in a widely used mouse model of colitis. Nat Microbiol 7, 590–599 (2022). This paper reports that differences in susceptibility to DSS-induced colitis in mice are caused by gut bacteria.
- 21.Li M, Wu Y, Hu Y, Zhao L & Zhang C Initial gut microbiota structure affects sensitivity to DSS-induced colitis in a mouse model. Sci China Life Sci 61, 762–769 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Brinkman BM et al. Gut Microbiota Affects Sensitivity to Acute DSS-induced Colitis Independently of Host Genotype. Inflamm Bowel Dis 19, 2560–2567 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Chang C-S et al. Identification of a gut microbiota member that ameliorates DSS-induced colitis in intestinal barrier enhanced Dusp6-deficient mice. Cell Reports 37, 110016 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Akao T, Oyanagi Y, Shiotsuki S, Ishii Y & Sasahara M Metabolism of Dextran Sulfate Sodium by Intestinal Bacteria in Rat Cecum Is Related to Induction of Colitis. Biological Pharm Bulletin 38, 566–570 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Eichele DD & Kharbanda KK Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroentero 23, 6016–6029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhar P & McAuley J The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front Cell Infect Mi 9, 117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan I et al. Differential Susceptibility of the Gut Microbiota to DSS Treatment Interferes in the Conserved Microbiome Association in Mouse Models of Colitis and Is Related to the Initial Gut Microbiota Difference. Adv Gut Microbiome Res 2022, 1–20 (2022). [Google Scholar]
- 28.Liu F et al. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci Rep-uk 7, 6783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henrotin Y, Mathy M, Sanchez C & Lambert C Chondroitin sulfate in the treatment of osteoarthritis: from in vitro studies to clinical recommendations. Ther Adv Musculoskelet Dis 2, 335–348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Huang S-Q, Li C-Q, Xu Q & Zeng Q-P Akkermansia muciniphila May Determine Chondroitin Sulfate Ameliorating or Aggravating Osteoarthritis. Front Microbiol 8, 1955 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu R, Shen Q, Li P & Shang N Sturgeon Chondroitin Sulfate Restores the Balance of Gut Microbiota in Colorectal Cancer Bearing Mice. Int J Mol Sci 23, 3723 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewald CY Drug Screening Implicates Chondroitin Sulfate as a Potential Longevity Pill. Frontiers Aging 2, 741843 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habuchi H, Ushida T & Habuchi O Mice deficient in N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase exhibit enhanced liver fibrosis and delayed recovery from fibrosis in carbon tetrachloride-treated mice. Heliyon 2, e00138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao T et al. Chondroitin Sulfate Elicits Systemic Pathogenesis In Mice By Interfering With Gut Microbiota Homeostasis. Biorxiv 142588 (2017) doi: 10.1101/142588. [DOI] [Google Scholar]
- 35.Shang Q et al. Degradation of chondroitin sulfate by the gut microbiota of Chinese individuals. Int J Biol Macromol 86, 112–118 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Kawai K, Kamochi R, Oiki S, Murata K & Hashimoto W Probiotics in human gut microbiota can degrade host glycosaminoglycans. Sci Rep-uk 8, 10674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulmer JE et al. Characterization of glycosaminoglycan (GAG) sulfatases from the human gut symbiont Bacteroides thetaiotaomicron reveals the first GAG-specific bacterial endosulfatase. J Biological Chem 289, 24289–303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ndeh D et al. Metabolism of multiple glycosaminoglycans by Bacteroides thetaiotaomicron is orchestrated by a versatile core genetic locus. Nat. Commun. 11, 646 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim JJ & Temenoff JS The effect of desulfation of chondroitin sulfate on interactions with positively charged growth factors and upregulation of cartilaginous markers in encapsulated MSCs. Biomaterials 34, 5007–5018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shmagel A et al. The Effects of Glucosamine and Chondroitin Sulfate on Gut Microbial Composition: A Systematic Review of Evidence from Animal and Human Studies. Nutrients 11, 294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichette J, Fynn-Sackey N & Gagnon J Hydrogen Sulfide and Sulfate Prebiotic Stimulates the Secretion of GLP-1 and Improves Glycemia in Male Mice. Endocrinology 158, 3416–3425 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Davis DAS & Parish CR Heparan Sulfate: A Ubiquitous Glycosaminoglycan with Multiple Roles in Immunity. Front Immunol 4, 470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop JR, Schuksz M & Esko JD Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Nagarajan A, Malvi P & Wajapeyee N Heparan Sulfate and Heparan Sulfate Proteoglycans in Cancer Initiation and Progression. Front Endocrinol 9, 483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ST, Neo BH & Betts RJ Glycosaminoglycans: Sweet as Sugar Targets for Topical Skin Anti-Aging. Clin Cosmet Investigational Dermatology 14, 1227–1246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung SH et al. Heparan sulfation is essential for the prevention of cellular senescence. Cell Death Differ 23, 417–429 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Risi MD et al. Altered heparan sulfate metabolism during development triggers dopamine-dependent autistic-behaviours in models of lysosomal storage disorders. Nat Commun 12, 3495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clausen TM et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 183, 1043–1057.e15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martino C et al. Bacterial modification of the host glycosaminoglycan heparan sulfate modulates SARS-CoV-2 infectivity. Biorxiv 2020.08.17.238444 (2020) doi: 10.1101/2020.08.17.238444. [DOI] [Google Scholar]
- 50.Morris A et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Resp Crit Care 187, 1067–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Dwyer DN, Dickson RP & Moore BB The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J Immunol Baltim Md 1950 196, 4839–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luis AS et al. Sulfated glycan recognition by carbohydrate sulfatases of the human gut microbiota. Nat Chem Biol 18, 841–849 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luis AS et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature 598, 332–337 (2021). This paper is the first report of a bacterial gene and enzyme responsible for degrading host gut mucin via desulfation.
- 54.Devlin AS et al. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 20, 709–715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Opdebeeck B et al. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J Am Soc Nephrol 30, 751–766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung S, Kuo K, Wu C & Tarng D Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J Am Heart Assoc 6, e005022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroeder JC et al. The Uremic Toxin 3-Indoxyl Sulfate Is a Potent Endogenous Agonist for the Human Aryl Hydrocarbon Receptor. Biochemistry-us 49, 393–400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HY, Yoo T-H, Cho J-Y, Kim HC & Lee W-W Indoxyl sulfate–induced TNF-α is regulated by crosstalk between the aryl hydrocarbon receptor, NF-κB, and SOCS2 in human macrophages. Faseb J 33, 10844–10858 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Sun C-Y et al. Indoxyl sulfate caused behavioral abnormality and neurodegeneration in mice with unilateral nephrectomy. Aging Albany Ny 13, 6681–6701 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimada Y et al. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. Plos One 8, e80604 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Zhang B, Hu Y & Zhao Y New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front Pharmacol 12, 769501 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel KP, Luo FJ-G, Plummer NS, Hostetter TH & Meyer TW The Production of p-Cresol Sulfate and Indoxyl Sulfate in Vegetarians Versus Omnivores. Clin J Am Soc Nephro 7, 982–988 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gryp T, Vanholder R, Vaneechoutte M & Glorieux G p-Cresyl Sulfate. Toxins 9, 52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu T-K et al. The uremic toxin p-cresyl sulfate induces proliferation and migration of clear cell renal cell carcinoma via microRNA-21/ HIF-1α axis signals. Sci Rep-uk 9, 3207 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poesen R et al. Cardiovascular disease relates to intestinal uptake of p-cresol in patients with chronic kidney disease. Bmc Nephrol 15, 87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bermudez-Martin P et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome 9, 157 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrison MA et al. Clostridioides difficile para-Cresol Production Is Induced by the Precursor para-Hydroxyphenylacetate. J Bacteriol 202, e00282–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Y et al. The Role of Bacterial-Derived Aromatic Amino Acids Metabolites Relevant in Autism Spectrum Disorders: A Comprehensive Review. Front Neurosci-switz 15, 738220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsiao EY et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 155, 1451–1463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diémé B et al. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J Proteome Res 14, 5273–5282 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Altieri L et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 16, 252–260 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Gabriele S et al. Urinary p-cresol is elevated in young French children with autism spectrum disorder: a replication study. Biomarkers 19, 463–470 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Lussu M et al. The urinary 1H-NMR metabolomics profile of an italian autistic children population and their unaffected siblings. Autism Res 10, 1058–1066 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Ming X, Stein TP, Barnes V, Rhodes N & Guo L Metabolic Perturbance in Autism Spectrum Disorders: A Metabolomics Study. J Proteome Res 11, 5856–5862 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Needham BD et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. Biol Psychiat 89, 451–462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams ML, Hughes-Fulford M & Elias PM Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and sterol synthesis by cholesterol sulfate in cultured fibroblasts. Biochimica Et Biophysica Acta Bba - Mol Cell Res 845, 349–357 (1985). [DOI] [PubMed] [Google Scholar]
- 77.Cheetham JJ, Epand RM, Andrews M & Flanagan TD Cholesterol sulfate inhibits the fusion of Sendai virus to biological and model membranes. J Biol Chem 265, 12404–12409 (1990). [PubMed] [Google Scholar]
- 78.Strott CA & Higashi Y Cholesterol sulfate in human physiology what’s it all about? J Lipid Res 44, 1268–1278 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Sanchez LD et al. Cholesterol and oxysterol sulfates: Pathophysiological roles and analytical challenges. Brit J Pharmacol 178, 3327–3341 (2021). [DOI] [PubMed] [Google Scholar]
- 80.Kenny DJ et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 28, 245–257.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Javitt NB, Lee YC, Shimizu C, Fuda H & Strott CA Cholesterol and Hydroxycholesterol Sulfotransferases: Identification, Distinction from Dehydroepiandrosterone Sulfotransferase, and Differential Tissue Expression. Endocrinology 142, 2978–2984 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Dawson PA & Karpen SJ Intestinal transport and metabolism of bile acids. J Lipid Res 56, 1085–1099 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sato H et al. Novel Potent and Selective Bile Acid Derivatives as TGR5 Agonists: Biological Screening, Structure–Activity Relationships, and Molecular Modeling Studies. J Med Chem 51, 1831–1841 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Xiao R et al. Synthesis and identification of lithocholic acid 3-sulfate as RORγt ligand to inhibit Th17 cell differentiation. J Leukocyte Biol (2022) doi: 10.1002/jlb.1ma0122-513r. [DOI] [PubMed] [Google Scholar]
- 85.A B, Lipsky WRL, Fricke RJ & Hylemon PB Bile acid induction specificity of 7α-dehydroxylase activity in an intestinal Eubacterium species. Steroids 35, 103–109 (1980). [DOI] [PubMed] [Google Scholar]
- 86. Chaudhari SN et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 29, 408–424.e7 (2021). This paper shows that sulfonated cholic acid (CA7S) improves diabetes phenotypes. Subsequent work revealed that the microbial metabolite LCA signals in the liver to induce CA7S biosynthesis.
- 87.Alnouti Y Bile Acid Sulfation: A Pathway of Bile Acid Elimination and Detoxification. Toxicol Sci 108, 225–246 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Chaudhari SN et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat Chem Biol 17, 20–29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wahlström A, Sayin SI, Marschall H-U & Bäckhed F Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 24, 41–50 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Robert C et al. Impact of Rapeseed and Soy Lecithin on Postprandial Lipid Metabolism, Bile Acid Profile, and Gut Bacteria in Mice. Mol Nutr Food Res 65, 2001068 (2021). [DOI] [PubMed] [Google Scholar]
- 91.Rutkowski K, Sowa P, Rutkowska-Talipska J, Kuryliszyn-Moskal A & Rutkowski R Dehydroepiandrosterone (DHEA): Hypes and Hopes. Drugs 74, 1195–1207 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Prough RA, Clark BJ & Klinge CM Novel mechanisms for DHEA action. J Mol Endocrinol 56, R139–R155 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Han Q, Wang J, Li W, Chen Z-J & Du Y Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome 9, 101 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strott CA Sulfonation and Molecular Action. Endocr Rev 23, 703–732 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Ballet C et al. New enzymatic and mass spectrometric methodology for the selective investigation of gut microbiota-derived metabolites. Chem Sci 9, 6233–6239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng T-H et al. Indoxyl Sulfate, a Tubular Toxin, Contributes to the Development of Chronic Kidney Disease. Toxins 12, 684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhimanwar RS & Mittal A TGR5 agonists for diabetes treatment: a patent review and clinical advancements (2012-present). Expert Opin Ther Pat 32, 191–209 (2021). [DOI] [PubMed] [Google Scholar]
- 98.Cao H et al. Intestinally-targeted TGR5 agonists equipped with quaternary ammonium have an improved hypoglycemic effect and reduced gallbladder filling effect. Sci Rep-uk 6, 28676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adhikari AA et al. A Gut-Restricted Lithocholic Acid Analog as an Inhibitor of Gut Bacterial Bile Salt Hydrolases. Acs Chem Biol 16, 1401–1412 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li DK et al. Inhibition of microbial deconjugation of micellar bile acids protects against intestinal permeability and liver injury. Sci Adv 8, eabo2794 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]


