Abstract
In the absence of viral envelope gene expression, cells expressing human immunodeficiency virus type 1 (HIV-1) gag and pol, accessory HIV functions, and a vector genome RNA produce and secrete large amount of noninfectious virus-like particles (VLPs) into the conditioned medium. After partial purification, such HIV-1 VLPs can be made infectious in cell-free conditions in vitro by complex formation with lipofection reagents or with the G protein of vesicular stomatitis virus (VSV-G). The resulting in vitro-modified HIV-1 particles are able to infect nondividing cells. Infectivity of envelope-free HIV VLPs can also be induced by prior modification of target cells through exposure to partially purified VSV-G vesicles. Similarly, infection can be carried out by attachment of envelope-free noninfectious VLPs to unmodified cells followed by subsequent treatment of cells with VSV-G. We interpret these findings to indicate that interaction between a viral envelope and a cell surface receptor is not necessary for the initial virus binding to the cells but is required for subsequent cell entry and infection.
Lentivirus vectors such as those based on human immunodeficiency virus (HIV) are attractive reagents for gene therapy studies because they combine advantageous features of conventional Moloney murine leukemia virus (MLV)-based retrovirus vectors with an ability to infect nondividing cells (4, 18–20, 22, 26). Nevertheless, clinical application of this vector system is complicated by safety considerations, some of which are being addressed by the development of improved vector production methods with more complete elimination of HIV genes (10, 16, 29). We have been interested in the in vitro assembly of gene transfer vectors that contain some viral elements for cell recognition and entry and have previously published a partially cell-free method to produce infectious MLV-based particles by addition of fusion functions in vitro to noninfectious virus-like particles. We now report extension of these earlier results to the HIV-based vectors and also to include a characterization of the mechanisms of retrovirus infection.
HIV type 1 (HIV-1) particles contain a central core consisting largely of the product of the viral gag gene, the viral RNA, the reverse transcriptase and integrase products of the polymerase gene, and additional accessory gene products (11). The Gag protein of a number of mammalian retroviruses, including MLV, HIV-1, and others, has the ability to oligomerize and assemble into virus-like particles, not only in packaging cell lines but also in vitro (8, 13, 23, 28, 31, 32). In the absence of viral envelope protein synthesis in retrovirus packaging cells, the viral Gag protein alone assembles efficiently into virus-like particles that bud from the cell and are released in large numbers into the conditioned medium of the cells. When the packaging cell line also contains and expresses an integrated retroviral genome, the assembled particles also associate with the packageable viral RNA. However, such envelope-free Gag-Pol-RNA (GPR) particles are noninfectious, apparently because they lack mechanisms for recognizing and binding to specific virus receptors on the surface of cells and thereby cannot initiate the membrane fusion events required for entry of the particles into the cell. We have recently reported that noninfectious envelope-free MLV GPR particles produced by packaging cell lines can be converted into infectious particles in cell-free conditions in vitro by the addition of one of several agents intended to provide a fusion function to the particles, including a variety of lipofection reagents and the G protein of vesicular stomatitis virus (VSV-G) (2, 25). These agents function by interacting with the immature, noninfectious particles to form complexes with physicochemical properties very similar to those of mature virus. Unlike the envelope-free GPR particles, such in vitro-assembled particles are able to enter and infect cells because of the presence of the fusiogenic function provided by the lipofectin reagents or VSV-G (2, 25).
It is particularly important to understand the mechanisms of assembly, maturation, and cell entry of HIV because identification of agents able to interfere with these steps in the HIV life cycle may provide important therapeutic opportunities to prevent spreading HIV infection and possibly facilitate the production of HIV-based lentivirus vectors for gene transfer and gene therapy studies. We have therefore examined the ability of HIV-1 GPR particles to form infectious complexes with pelleted VSV-G vesicles. In this report, we demonstrate that the conditioned medium from human 293 cells doubly transfected with plasmid vectors expressing HIV gag-pol and a neomycin phosphotransferase vector contain large amounts of immature, noninfectious GPR particles and that addition of Lipofectin or VSV-G vesicles in cell-free conditions in vitro makes the particles infectious when assayed on either growing or growth-arrested HT1080 cells. We also demonstrate that addition of VSV-G vesicles to monolayers of HT1080 cells followed by addition of noninfectious GPR particles results in infection of cells with an efficiency directly related to the amount of VSV-G bound to the cells. Similarly, addition of noninfectious GPR particles to HT1080 cells followed by addition of VSV-G also results in infection of cells with an efficiency similar to that of virus produced by conventional in vivo methods, demonstrating that initial binding of virus particles to the cells does not require specific interaction between an envelope protein and a cell surface receptor.
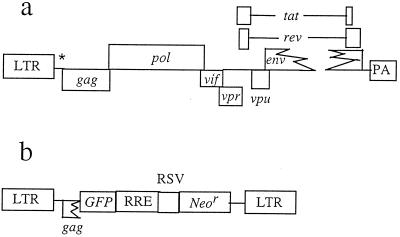
The HIV-1-derived GPR particles were produced by cotransfection of human 293 cells with plasmids shown in Fig. 1 by methods similar to those previously described for MLV (2, 25). Plasmid p107ΔΨΔ3′LTR is a pNL4-3-based HIV-1 plasmid that expresses gag, pol, vif, vpr, vpu, tat, and rev genes (3). It is a modification of plasmid GB107 with a 426-bp in-frame deletion in the envelope gene (gift from G. Buchschacher) and was further modified by deleting a portion of the packaging signal and the 3′ long terminal repeat (LTR) and by replacing a portion of the nef gene and the 3′ LTR with the rabbit β-globin gene poly(A) signal (33). The vector pGFPRNL-HIV is also derived from pNL4-3 and provides a packageable viral RNA. The plasmid construct has intact HIV-1 5′ and 3′ LTRs, an intact packaging signal, green fluorescent protein (GFP) cDNA expressed from the 5′ LTR, and a Rous sarcoma virus (RSV) promoter driving the neomycin phosphotransferase gene. The VSV-G expression plasmid pCMV-G has been described previously (33). The cell lines 293 and HT1080 were obtained from the American Type Culture Collection and maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum. In studies of infection of growth-arrested HT1080 cells, aphidicolin was added to culture medium to a concentration of 10 μg/ml 24 h before infection and for 24 h after infection (18).
FIG. 1.
Genetic organization of plasmids used to produce replication-defective HIV-1-based retroviral vector particles. (a) Plasmid p107ΔΨΔ3′LTR, which expresses the HIV gag, pol, vif, vpr, vpu, tat, and rev genes. nef and the 3′ LTR are replaced by the rabbit β-globin polyadenylation signal (PA). (b) Vector plasmid pGFPRNL-HIV, which provides the packageable viral RNA and expresses GFP from the 5′ LTR and the neomycin phosphotransferase gene (Neor) from an internal RSV LTR promoter. RRE, rev-responsive element.
To produce authentic VSV-G-pseudotyped HIV-1 vectors, 293 cells growing in a 10-cm-diameter dish were transfected with 10 μg of p107ΔΨΔ3′LTR, 10 μg of pGFPRNL-HIV, and 5 μg of pCMV-G, using the established calcium phosphate transfection method (20, 34). The conditioned medium containing virus particles was collected 48 and 72 h after transfection. To obtain HIV-1 GPR particles, 293 cells were transfected with 10 μg of p107ΔΨΔ3′LTR and 10 μg of pGFPRNL-HIV, and conditioned medium was collected at 48 and 72 h. Both preparations were concentrated by centrifugation at 25,000 rpm for 2 h at 4°C in a Beckman SW28 rotor. The pellets were suspended in DMEM in a volume to produce a 500-fold concentration. The gag gene product p24 was measured by enzyme-linked immunosorbent assay (ELISA) (Coulter Diagnostics, Miami, Fla.).
For the preparation of VSV-G vesicles, 293 cells were transfected with pCMV-G by the calcium phosphate method; the conditioned medium was collected 48 and 72 h later, filtered through a 0.45-μm-pore-size filter, and centrifuged at 25,000 rpm and 4°C for 2 h. The pellet was suspended in DMEM to achieve a 500-fold concentration. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis with Coomassie blue staining of purified VSV-G and known amounts of marker proteins (Sigma Biochemicals) was used to quantitate VSV-G and to determine its purity (1). A major band corresponding to authentic VSV-G at approximately 60 kDa was found (data not shown).
Methods for the preparation of GPR complexes with Lipofectin (Gibco-BRL) or VSV-G were identical to those previously reported for the MLV-based GPR assembly (2, 25).
Infection of HT1080 cells was carried out under standard transduction conditions in the presence of Polybrene (8 μg/ml) (34). Table 1 summarizes results of two experiments with the HIV-derived GPR particles in the absence and presence of Lipofectin or VSV-G. In all cases, infection efficiency with the GPR complexes represents the number of G418-resistant colonies at 14 days compared with transduction with native mature HIV vector and normalized to p24 content. In all experiments, exposure of cells to unmodified GPR particles produced no G418-resistant colonies. In contrast, the addition of Lipofectin to the particles produced virus particles with an infectivity titer of approximately 103 CFU/ml, similar to the results reported for MLV-based particles (25). Equal amounts of GPR particles complexed with VSV-G vesicles produced virus preparations with titers more than 2 orders of magnitude higher, (>105 CFU/ml). Normalization of these titers to p24 content showed a corresponding increase in infectivity of between 100- and 1,000-fold. In parallel experiments and under identical assay conditions, native vector produced by the conventional cotransfection method demonstrated a titer of >106 CFU/ml and a p24-normalized titer 10- and 100-fold higher than that of the GPR–VSV-G complex. In all cases, titers determined on HT1080 cells growth arrested with aphidicolin were indistinguishable from those determined on control HT1080 cells.
TABLE 1.
Infection of HT1080 cells with GPR particles from vector GFPRNL-HIV-1a
| Particles | Titer (CFU/ml of neomycin-resistant colonies)
|
Titer normalized to μg of p24 | |
|---|---|---|---|
| Replicating HT1080 | Growth-arrested HT1080 | ||
| GPR alone | <10 | <10 | <10 |
| GPR + Lipofectinb | 1.7 × 103 | ND | 35 |
| 2.8 × 103 | 2.3 × 103 | 60 | |
| GPR + VSV-Gc | 5.1 × 105 | ND | 1.0 × 104 |
| 2.4 × 105 | 2.1 × 105 | 6.3 × 103 | |
| GFPRNL-HIV-1d | 4.2 × 106 | ND | 7.5 × 105 |
| 2.1 × 106 | 2.0 × 106 | 4.2 × 105 | |
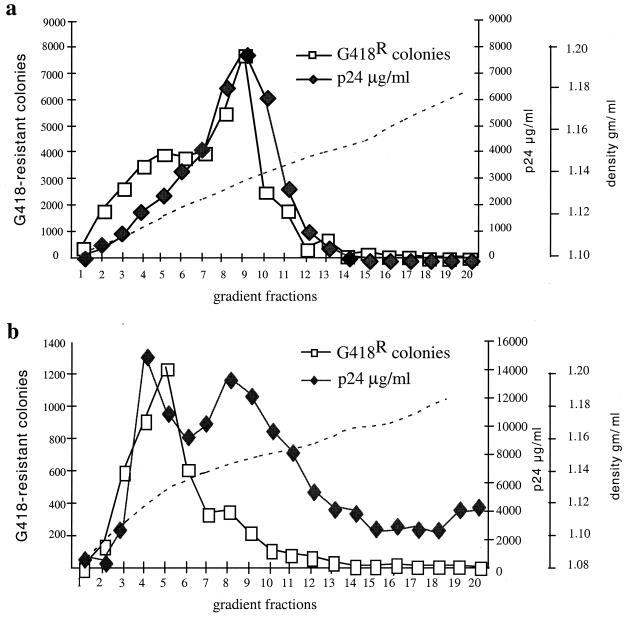
We used sucrose density gradient centrifugation to characterize the physicochemical properties of the complexes formed by the HIV-1 GPR particles and VSV-G vesicles and to compare the in vitro-assembled particles with native HIV-1 vector (Fig. 2). The GPR particles were prepared from the conditioned medium of cells cotransfected as described above. Samples of native HIV-1 vector and the in vitro-assembled, VSV-G-complexed particles were loaded onto preformed sucrose gradients spanning densities of 1.12 to 1.20 or 1.08 to 1.18 g/ml and centrifuged for 24 h at 25,000 rpm at 4°C in an SW28 rotor. Fractions of 500 μl were collected and assayed for infectivity and for p24 content by ELISA as described above. In the case of native vector produced by triple transfection (Fig. 2a), p24-containing material exhibited a broad peak at a density of approximately 1.15 to 1.16 g/ml. Most of the infectivity cosedimented with the major p24 peak, although a significant broad and lighter shoulder of infectious material was detected at a density of 1.13 to 1.15 g/ml. This finding of a significant amount of infectivity in particles on the light side of the majority of particles is consistent with the results from our previous characterization of MLV particles in which infectivity of MLV derived from traditional producer cells was found to be maximal on the light side of the major reverse transcriptase peak in similar sucrose gradients (2).
FIG. 2.
Physicochemical properties of HIV-1 particles. (a) Sucrose density gradient analysis of native VSV-G-pseudotyped HIV-1 vector particles produced by triple transfection of 293 cells; (b) sucrose density gradient characterization of in vitro-assembled HIV-1 GPR particles with VSV-G protein. The left ordinate shows the number of G418-resistant (G418R) colonies; the right ordinate shows levels of p24 and sucrose densities. Fractions were collected and analyzed for p24 by ELISA; infectious virus particles were assayed by infection of HT1080 cells.
In the case of GPR–VSV-G complexes (Fig. 2b), infectivity was detected in a major peak of p24-containing material at a density of approximately 1.11 to 1.14 g/ml, lighter than the majority of unmodified GPR particles and also lighter than most infectious particles in traditional virus preparations derived from producer cells (Fig. 2a). The in vitro technique therefore seems to generate particles whose physicochemical properties generally resemble native VSV-G-pseudotyped HIV-1 with the highest infectivity/p24 ratio.
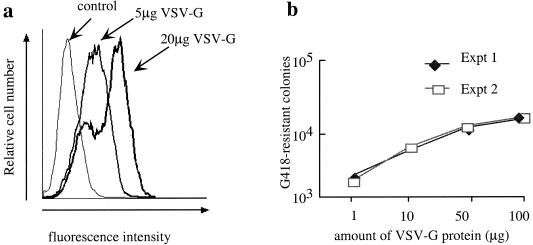
Since the lipid bilayers of the GPR particles and the target cell should be very similar, and if VSV-G functions by providing a bridging fusiogenic function to the GPR particles, it may be possible to induce the same degree of infectivity onto GPR particles by exposing the target cells to VSV-G prior to exposure to unmodified GPR particles. We examined the initial attachment and infectivity of uncomplexed GPR particles on HT1080 cells exposed to VSV-G vesicles. To examine the binding of VSV-G to target cells, we exposed monolayers of HT1080 cells in six-well plates at a density of approximately 8 × 105 cells per well to VSV-G at concentrations of 5 or 20 μg/ml of full culture medium for 30 min, after which the cells were washed extensively and further incubated with I1 antibody to VSV-G (5). The presence of cell-bound VSV-G was detected by fluorescence-activated cell sorting analysis using an fluorescein isothiocyanate-labeled anti-mouse secondary antibody (Southern Biotechnologies) (Fig. 3a). At both concentrations of VSV-G examined, virtually all cells contained VSV-G on their surface, as indicated by the complete shift of virtually all cells to positions of increased fluorescence. At the higher concentration (20 μg), most cells displayed an increased amount of VSV-G, although a subpopulation of cells seemed to be saturated with the lower level of bound VSV-G.
FIG. 3.
Binding and infectivity of HT1080 cells pretreated with VSV-G. (a) Fluorescence-activated cell sorting analysis of VSV-G vesicles bound to HT1080 cells. HT1080 cells were incubated with two different amounts of VSV-G vesicle, and binding of VSV-G to the cell surface was detected by reaction with antibody I1 to VSV-G followed by labeling with fluorescein isothiocyanate-coupled anti-mouse secondary antibody. In all cases, a total of 10,000 events were visualized. (b) Infectivity of HT1080 cells expressed as the number of G418-resistant colonies following GPR infection of HT1080 cells preexposed to 1 to 100 μg of VSV-G protein. The concentration of VSV-G is in the range of 0.5 to 50 μg/ml.
Parallel cultures of VSV-G-exposed HT1080 cells growing as described above were used to determine the infectivity of unmodified GPR particles. Cells were treated with 1, 10, 50, or 100 μg of VSV-G for 30 min, washed, exposed to unmodified GPR particles in the presence of Polybrene (8 μg/ml), and maintained under G418 selection conditions, as described above, to determine infectivity titer. Infectivity was evident even at the lowest dose of VSV-G (1 μg) (Fig. 3b) and increased to a plateau at approximately 50 to 100 μg. Interestingly, this priming effect on the infectivity of GPR particles remained detectable for several days after a single 30-min exposure of HT1080 to VSV-G, with apparent titers of GPR particles remaining at 25% of starting titers at 24 h and approximately 10% of starting titers as late as 60 h (data not shown). Even though there were no apparent morphological changes in the cells or evidence for syncytium formation, the persistence of the priming effect indicates the retention in the plasma membrane of VSV-G in amounts and in a distribution that permits fusion with the membranes of the GPR particles for effective binding and uptake.
To compare the efficiency of infection by VSV-G-complexed particles in HT1080 cells with that of unmodified particles in VSV-G-treated cells, we added amounts of uncomplexed HIV GPR particles identical to those used to prepare the VSV-G complexes described above to cultured HT1080 cells that had been previously exposed for 30 min to VSV-G at a concentration of 50 μg/ml. In a parallel experiment, we exposed cells to unmodified, envelope-free GPR particles first for 30 min in the presence of Polybrene (8 μg/ml) followed by extensive washing to remove free unbound particles and finally by treatment with VSV-G for 30 min at a concentration of 50 μg/ml.
Another set of infection experiments was performed with MLV-based GPR particles prepared from conditioned medium of 293GP cells containing LZRNL provirus as previously described (2, 25). Table 2 summarizes the infectivity of identical amounts of envelope-free GPR particles rendered infectious by three different methods: prior addition of VSV-G to the GPR particles, addition of VSV-G to target cells followed by addition of unmodified GPR particles, or addition of envelope-free GPR particles to uncomplexed cells followed by subsequent addition of VSV-G. Results in Table 2 indicate that the three different methods of inducing infectivity produce indistinguishable titers. The fact that pretreatment of cells with envelope-free GPR particles followed by exposure to VSV-G produces an efficiency indistinguishable from that of the other two methods of infection demonstrates that the envelope protein-free GPR particles bind to the cells with an efficiency indistinguishable from that of mature envelope-containing virus particles. While VSV-G binding to HT1080 cells did not require Polybrene as shown in Fig. 3a, infection with GPR particles and VSV-G in the absence of Polybrene did not produce any evidence of infection, suggesting that induction of cell entry process and not virus particle binding requires the presence of a polycation such as Polybrene (data not shown). We obtained similar results with MLV-GPR particles in infectivity studies in rat 208F rat fibroblasts (data not shown).
TABLE 2.
Infection of HT1080 cells with HIV- and MLV-based GPR particlesa
| Infection procedure | Titer (CFU/ml)
|
|
|---|---|---|
| HIV-GPR (LGFPRNL) | MLV-GPR (LZRNL) | |
| GPR-VSV complex onto HT1080 cells | 1.2 × 105 | 6.9 × 105 |
| 1.4 × 105 | 4.0 × 105 | |
| GPR particles onto VSV-G-treated HT1080 cells | 7.8 × 104 | 3.8 × 105 |
| 9.0 × 104 | 9.8 × 104 | |
| GPR particles onto untreated cells followed by VSV-G addition | 1.1 × 105 | 4.0 × 105 |
| 8.2 × 104 | 2.4 × 105 | |
All infections were carried out in duplicate in the presence of Polybrene (8 μg/ml), using identical amounts of GPR particle preparations. Complexes of GPR particles with VSV-G and treatment of cells with VSV-G were performed as described in the text.
A full understanding of the mechanisms by which retroviral particles recognize, attach to, and infect cells would be very helpful for the development of antiviral therapies and for the design of improved retrovirus vectors for gene transfer, vector targeting, and gene therapy. The mechanisms of HIV and MLV attachment and cell uptake have been studied extensively, and families of cell surface receptors and coreceptors for these retroviruses have been identified and characterized. While an initial step of specific interaction of a virus envelope with its receptor(s) has generally been thought to represent the initial step in most kinds of the virus infection, our observations demonstrate that such an interaction is not essential for the initial binding of HIV- and MLV-based retrovirus particles to target cells but rather is central to the subsequent process of membrane fusion, cell entry, and subsequent infection. We interpret this requirement to indicate that the retrovirus envelope protein plays a less important role than previously thought in initial attachment to cells and that its principal function is either to induce the fusion between the viral and cellular membranes that allow entry of the particles into the cell or to allow transfer of the bound particles to another receptor for subsequent cell uptake. These studies do not approach questions related to the function of such retrovirus or lentivirus coreceptors, such as are known to be important for infection with HIV (7, 9, 12).
Polycations such as Polybrene have long been known to be required for efficient infection with retroviruses. Our present studies confirm that cell-bound GPR particles, like mature virus particles, cannot be induced by subsequent treatment with VSV-G to become infectious in the absence of Polybrene, suggesting that electrostatic interactions may play a role in postattachment mechanisms of virus infection or that attachment of virus particles in the absence or presence of polycations may be different.
These results extend several recent studies describing features of retrovirus infection. Pizzato et al. demonstrated that ecotropic and envelope protein-free MLV particles bind to human cells with similar efficiency as those of amphotropic viruses (21). In those previous studies, the role of polycations in the attachment process was not studied, leaving open questions of the relationship between initial binding and subsequent membrane fusion and cell uptake. More recently, Lavillette et al. have reported that the addition of soluble forms of type C mammalian retrovirus receptor binding domain to target cells is sufficient to allow efficient infection with cell entry-defective retroviruses carrying mutant envelope glycoproteins (17). While the latter results also do not examine the relationship between the attachment and fusion functions of the retrovirus envelopes by analyzing the Env-free particles, they provide clear evidence for a common entry pathway activated by conserved features of their envelope glycoproteins.
The results of our present studies of attachment, cell uptake, and infectivity of envelope-free virus-like particles in both the HIV and MLV systems underscore the need to understand the relative roles of envelope proteins in defining virus attachment and uptake toward the goals of designing improved antiviral agents in general and improving the design of vectors targeted to specific tissues for tissue-specific in vivo gene delivery. Many studies aimed at cell-specific targeting of retroviruses through addition of peptide, single-chain antibodies, or other ligands to the envelope proteins of retroviruses have been carried out (6, 14, 15, 24, 27, 30). In most studies with MLV-based vectors, the efficiency of cell-specific infection has not been great, and it has generally been assumed that poor infection resulted from structural changes in the chimeric envelope proteins that do not allow the conformational changes required for membrane fusion and virus particle uptake into exposed cells. On the other hand, reproducible functional targeting has been reported with spleen necrosis virus vectors modified to contain cell-specific single-chain antibody ligands in the envelope protein (6). It has been assumed that this targeting resulted from a cell-specific attachment event followed by uptake and infection after membrane fusion. The relative failure of studies in the MLV system may reflect differences in mechanisms of infection with MLV and spleen necrosis virus and a misleading concept of the early events in retrovirus infection.
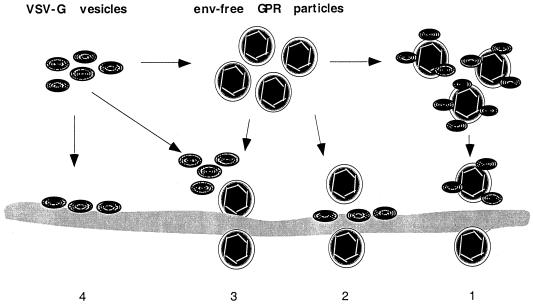
Our working model of the interactions of GPR particles with VSV-G and with target cells is presented in Fig. 4. Although the precise mechanisms by which VSV-G vesicles permit gene transfer by the GPR particles remain uncertain, it seems likely that VSV-G plays no significant role in initial particle binding to cells but rather acts as a fusion-inducing bridge between the cell membrane and the envelope of the GPR particles. Because the attachment of virus particles is very efficient even in the absence of envelope protein, we think that strategies aimed largely at influencing initial binding of the retroviral envelope protein to its receptor(s) will continue to be difficult to carry out and to interpret. We expect that further investigation of the in vitro cell-free conditions of retrovirus assembly will be useful to elucidate the infection mechanisms and will also lead to production of infectious particles of even higher titers.
FIG. 4.
A proposed model for interaction of envelope protein-free retroviral GPR particles with VSV-G vesicles and with target cells. In mechanism 1, VSV-G is complexed with GPR particles to produce infectious particles that interact with unmodified regions of the cellular membrane and allow cell uptake and infection. Alternatively, in mechanism 2, GPR particles devoid of envelope protein interact with regions of the cell membrane to which VSV-G had previously been complexed. Mechanism 3 indicates that envelope-free GPR particles also are able to bind effectively to regions of the cellular membrane not containing retroviral envelope proteins but are then able to infect ells upon subsequent addition of VSV-G. Effective binding of VSV-G alone to the cellular lipid membrane is indicated in mechanism 4.
Acknowledgments
This study was supported by grants DK49023 and HL53680 from the National Institutes of Health and from the UCSD Center for AIDS Research (NIAID 2 P30 AI 36214). We also thank J. Corbeil of UCSD Center for AIDS Research and the Veterans Medical Research Foundation Genomics Core for the HIV-1 gag p24 ELISA.
REFERENCES
- 1.Abe A, Miyanohara A, Friedmann T. Enhanced gene transfer with fusogenic liposomes containing vesicular stomatitis virus G glycoprotein. J Virol. 1998;72:6159–6163. doi: 10.1128/jvi.72.7.6159-6163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe A, Chen S-T, Miyanohara A, Friedmann T. In vitro cell-free conversion of noninfectious Moloney retrovirus particles to an infectious form by the addition of the vesicular stomatitis virus surrogate envelope G protein. J Virol. 1998;72:6356–6361. doi: 10.1128/jvi.72.8.6356-6361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchschacher G L, Jr, Panganiban A T. Human immunodeficiency virus vectors for inducible expression of foreign genes. J Virol. 1992;66:2731–2739. doi: 10.1128/jvi.66.5.2731-2739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu T-H T, Dornburg R. Toward highly efficient cell-type-specific gene transfer with retroviral vectors displaying single-chain antibodies. J Virol. 1997;71:720–725. doi: 10.1128/jvi.71.1.720-725.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolate of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 8.Dickson C, Eisenman R, Fan H, Hunter E, Teich N. Protein biosynthesis and assembly. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. pp. 513–648. [Google Scholar]
- 9.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanam C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.Dull T, Zufferey R, Kelly M, Mandel R J, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 13.Garnler L, Wills J W, Verderame M E, Sudol M. WW domains and retrovirus budding. Nature. 1996;381:744–745. doi: 10.1038/381744a0. [DOI] [PubMed] [Google Scholar]
- 14.Hall F L, Gordon E M, Wu L, Zhu N L, Skotzko M J, Starnes V A, Anderson W F. Targeting retroviral vectors to vascular lesions by genetic engineering of the MoMLV gp70 envelope protein. Hum Gene Ther. 1997;8:2183–2192. doi: 10.1089/hum.1997.8.18-2183. [DOI] [PubMed] [Google Scholar]
- 15.Kasahara N, Dozy A, Kan Y W. Targeting retroviral vectors to specific cells. Science. 1995;269:417. doi: 10.1126/science.7618110. [DOI] [PubMed] [Google Scholar]
- 16.Kim V N, Mitrophanous K, Kingsman S M, Kingsman A J. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavillette D, Ruggieri A, Russell S J, Cosset F-L. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J Virol. 2000;74:295–304. doi: 10.1128/jvi.74.1.295-304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis P, Henselle M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 21.Pizzato M, Marlow A A, Blair E D, Takeuchi Y. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J Virol. 1999;73:8599–8611. doi: 10.1128/jvi.73.10.8599-8611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poeschla E, Corbeau P, Wong-Staal F. Development of HIV vectors for anti-HIV gene therapy. Proc Natl Acad Sci USA. 1996;93:11395–11399. doi: 10.1073/pnas.93.21.11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakalian M, Parker S D, Weldon R A, Jr, Hunter E. Synthesis and assembly of retrovirus Gag precursors into immature capsids in vitro. J Virol. 1996;70:3706–3715. doi: 10.1128/jvi.70.6.3706-3715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnierle B S, Moritz D, Jeschke M, Groner B. Expression of chimeric envelope proteins in helper cell lines and integration into Moloney murine leukemia virus particles. Gene Ther. 1996;3:334–342. [PubMed] [Google Scholar]
- 25.Sharma S, Murai F, Miyanohara A, Friedmann T. Noninfectious virus-like particles produced by Moloney murine leukemia virus-based retrovirus packaging cells deficient in viral envelope become infectious in the presence of lipofectin reagents. Proc Natl Acad Sci USA. 1997;94:10803–10808. doi: 10.1073/pnas.94.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada T, Fujii H, Mitsuya H, Nienhuis A W. Targeted and highly efficient gene transfer into CD4+ cells by a recombinant human immunodeficiency virus retrovirus vector. J Clin Investig. 1991;88:1043–1047. doi: 10.1172/JCI115365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somia N V, Zoppe M, Verma I M. Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene delivery. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasakumar N, Hammarskjeold M L, Rekosh D. Characterization of deletion mutations in the capsid region of human immunodeficiency virus type 1 that affect particle formation and Gag-Pol precursor incorporation. J Virol. 1995;69:6106–6114. doi: 10.1128/jvi.69.10.6106-6114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasakumar N, Chazal N, Helga-Maria C, Prasad S, Hammarskjold M-L, Rekosh D. The effect of viral regulatory protein expression on gene delivery by human immunodeficiency virus type 1 vectors produced in stable packaging cell lines. J Virol. 1997;71:5841–5848. doi: 10.1128/jvi.71.8.5841-5848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valsesia-Wittmann S, Morling F J, Hatziioannou T, Russell S J, Cosset F-L. Receptor co-operation in retrovirus entry: recruitment of an auxiliary entry mechanism after retargeted binding. EMBO J. 1997;16:1214–1223. doi: 10.1093/emboj/16.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogt V M. Proteolytic processing and particle maturation. Curr Top Microbiol Immunol. 1996;214:95–132. doi: 10.1007/978-3-642-80145-7_4. [DOI] [PubMed] [Google Scholar]
- 32.Yamshchikov G V, Ritter G D, Vey M, Compans R W. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology. 1995;214:50–58. doi: 10.1006/viro.1995.9955. [DOI] [PubMed] [Google Scholar]
- 33.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee J K, Friedmann T, Burns J C. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43:99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]