Abstract
INTRODUCTION
Aducanumab selectively targets aggregated forms of amyloid beta (Aβ), a neuropathological hallmark of Alzheimer's disease (AD).
METHODS
PRIME was a Phase 1b, double‐blind, randomized clinical trial of aducanumab. During the 12‐month placebo‐controlled period, participants with prodromal AD or mild AD dementia were randomized to receive aducanumab or placebo. At week 56, participants could enroll in a long‐term extension (LTE), in which all participants received aducanumab. The primary endpoint was safety and tolerability.
RESULTS
Amyloid‐related imaging abnormalities–edema (ARIA‐E) were the most common adverse event. Dose titration was associated with a decrease in the incidence of ARIA‐E. Over 48 months, aducanumab decreased brain amyloid levels in a dose‐ and time‐dependent manner. Exploratory endpoints suggested a continued benefit in the reduction of clinical decline over 48 months.
DISCUSSION
The safety profile of aducanumab remained unchanged in the LTE of PRIME. Amyloid plaque levels continued to decrease in participants treated with aducanumab.
Highlights
PRIME was a Phase 1b, double‐blind, randomized clinical trial of aducanumab.
We report cumulative safety and 48‐month efficacy results from PRIME.
Amyloid‐related imaging abnormalities–edema (ARIA‐E) were the most common adverse event (AE); 61% of participants with ARIA‐E were asymptomatic.
Dose titration was associated with a decrease in the incidence of ARIA‐E.
Aducanumab decreased levels of amyloid beta (Aβ) in a dose‐ and time‐dependent manner.
Keywords: aducanumab, Alzheimer's disease, amyloid‐related imaging abnormalities, clinical trials, PRIME
1. BACKGROUND
Accumulation of aggregated amyloid beta (Aβ) peptides is hypothesized to drive neuropathological changes in Alzheimer's disease (AD), including neurodegeneration, that lead to cognitive symptoms. 1 , 2 , 3
Aducanumab is a human monoclonal antibody that selectively targets aggregated forms of Aβ, including soluble oligomers and insoluble fibrils. 4 Biomarker and clinical efficacy results from the fixed‐dose portion of the placebo‐controlled (PC) period of the PRIME study demonstrated that aducanumab reduced brain amyloid plaque levels in a dose‐ and time‐dependent manner while slowing clinical decline compared with placebo. 4 Amyloid‐related imaging abnormalities (ARIA) have been observed in participants treated with investigational anti‐Aβ monoclonal antibodies, including aducanumab. ARIA–edema (ARIA‐E) were the most common adverse event (AE) in aducanumab‐treated participants in the PC period of the PRIME study; the incidence of ARIA‐E was dose‐ and apolipoprotein E (APOE) ε4 carrier status‐dependent. 4
Following the PC period, eligible participants could enroll in the PRIME long‐term extension (LTE) to receive dose‐blinded aducanumab. Here, we report the cumulative safety data for PRIME and 48‐month (12‐month PC period and 36‐month LTE) efficacy data for all PRIME cohorts, including a titration to 10 mg/kg cohort. The reported cumulative safety data will help characterize the safety profile of aducanumab.
2. METHODS
2.1. Participants
2.1.1. PC period
The screening process and entry criteria for the PC period have been reported previously. 4 , 5 See the Methods section in the Supplementary Material for the key inclusion criteria. Randomization and first dose of treatment began on October 5, 2012, for the first cohort and ended on March 30, 2015, for the last cohort.
2.1.2. LTE
At week 56, participants who had completed the PC period, had received ≥11 doses of study treatment (without missing more than 2 consecutive doses), and had a Mini‐Mental State Examination (MMSE) score of >10 at month 12 could enroll in the LTE. The LTE period began on November 2, 2013. To be eligible to participate in the LTE, participants must have been ambulatory and should have had a reliable informant or caregiver. To be eligible to participate in the additional years of the LTE (i.e., years 3 and 4 of the study), participants would need to also have had an MMSE score of >10. The LTE was planned until the last participant who entered had completed 6 years (1‐year PC period, 5‐year LTE). Due to the staggered‐start design, the first enrolled participants had the potential to participate for up to 9 years. However, the study was terminated prematurely based on a preplanned futility analysis of the two aducanumab Phase 3 studies. The results of the futility analysis have been disclosed previously. 6 The maximum duration of treatment in PRIME was nearly 6.5 years.
RESEARCH IN CONTEXT
Systematic review: Following the publication of the initial results from the placebo‐controlled period of the PRIME study [1], this study reports cumulative safety data and 48‐month efficacy results from PRIME. This study was the first to evaluate titration dosing of aducanumab as a means to ameliorate the incidence of amyloid‐related imaging abnormalities (ARIA).
Interpretation: Safety results were consistent with previous findings during the PRIME placebo‐controlled period, indicating that ARIA–edema (ARIA‐E) is clinically manageable with dose titration and routine brain imaging. Long‐term efficacy results also indicate that aducanumab modifies one of the underlying pathological hallmarks of Alzheimer's disease.
Future directions: Future studies will continue to inform the safety and efficacy profile of aducanumab.
2.2. Study design
PRIME was a U.S.‐based, multicenter, double‐blind, multiple‐dose, staggered‐start, parallel‐group study in which participants were randomized 3:1 to intravenous aducanumab (1, 3, 6, or 10 mg/kg) or placebo every 4 weeks. Each fixed‐dose cohort was stratified by APOE ε4 status (carrier/noncarrier). Following fixed‐dose cohort enrollment, a titration cohort of APOE ε4 carriers only was added to evaluate the impact of up‐titration on ARIA incidence. Titration to 10 mg/kg occurred over 44 weeks: two doses of 1 mg/kg, four doses of 3 mg/kg, five doses of 6 mg/kg, and 10 mg/kg of aducanumab thereafter.
The protocol‐specified primary endpoint for the PC period was safety and tolerability, as measured by outcomes including incidence of AEs, serious AEs (SAEs), ARIA‐E, and ARIA–hemorrhage or –hemosiderosis (ARIA‐H). Secondary objectives were the effect on Aβ plaque levels, as measured by 18F‐florbetapir positron emission tomography (PET) at month 6, and serum pharmacokinetics (PK) of multiple aducanumab doses. 7 Exploratory objectives included the effect of aducanumab on Aβ plaque levels at month 12 and the clinical decline of AD.
2.2.1. LTE
Participants meeting the eligibility criteria at week 56 were enrolled in a dose‐blind, multiple‐dose LTE phase in which all participants received intravenous aducanumab every 4 weeks (Figure S1). Participants treated with fixed doses of 3, 6, or 10 mg/kg aducanumab in the PC period continued the same assigned dose in the LTE. Participants previously treated with 1 mg/kg in the PC period switched to 3 mg/kg aducanumab in the LTE. Participants in the titration group during the PC period received 10 mg/kg in the LTE. Placebo‐treated participants during the PC period switched to one of three regimens in the LTE: aducanumab 3 mg/kg, titration to 6 mg/kg (two doses of 3 mg/kg followed by 6 mg/kg thereafter), or titration to 10 mg/kg (following the same regimen from the PC period: 1 mg/kg [two doses], 3 mg/kg [four doses], 6 mg/kg [five doses], and 10 mg/kg thereafter).
The primary endpoint for the LTE was safety and tolerability. Other LTE endpoints, including change in amyloid PET and measures of clinical decline, were exploratory. Protocol‐defined rules were changed during the study such that patients who received a dose reduction in the PC period due to ARIA were eligible to titrate up to the original planned dose.
2.3. Endpoints and assessments
2.3.1. Safety assessments
Safety assessments were performed at regular intervals and included the incidence of treatment‐emergent AEs, including ARIA, SAEs, clinical laboratory tests, vital signs, physical and neurological examinations, and 12‐lead electrocardiography. Brain magnetic resonance imaging (MRI) for ARIA monitoring was performed at screening, at scheduled times throughout the study, and at unscheduled visits if ARIA were previously detected. 6 See the Methods in the Supplementary Material for the ARIA management protocol. Incidence was calculated by treatment group for each AE.
2.3.2. Amyloid PET imaging
Aβ plaque levels were measured by 18F‐florbetapir PET at screening at week 26 (primary pharmacodynamic [PD] endpoint), week 54 (exploratory PD endpoint during the PC period), weeks 110, 166, and 222 (exploratory PD endpoint during the LTE), and at study termination. A composite standardized uptake value ratio (SUVR; also expressed on the Centiloid [CL] scale 8 ) was computed as described previously. 5
2.3.3. Pharmacokinetics (PK)
See the Methods in Supplementary Material for the PK assessments.
2.3.4. Exploratory efficacy endpoints
Exploratory objectives included effect of aducanumab on the clinical decline of AD as assessed by the Clinical Dementia Rating–Sum of Boxes (CDR‐SB), MMSE, Neuropsychological Test Battery (NTB), and Free and Cued Selective Reminding Test (FCSRT). 6 The CDR‐SB and MMSE were conducted biannually through year 4, and then annually and on study termination. The NTB and Neuropsychiatric Inventory Questionnaire (NPI‐Q) were conducted biannually through year 2. The FCSRT was conducted annually through year 2. The Columbia Suicide Severity Rating Scale was administered biannually and on termination from the study. To maintain blinding to AEs, CDR‐SB raters were not permitted to conduct other clinical assessments and were blinded to other clinical and safety assessments.
2.4. Oversight
This study (NCT01677572) was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization and Good Clinical Practice guidelines and received ethics committee approval at each participating site. All participants provided written informed consent. An independent data monitoring committee oversaw study conduct and routinely reviewed safety data.
2.5. Statistical analyses
The safety population was defined as all randomized participants who received at least one dose of aducanumab. The safety MRI population was defined as all randomized participants who received at least one dose of aducanumab and had at least one postbaseline MRI. Safety analyses include data from the PC period 6 as well as cumulative safety data from the PC period and LTE after first exposure to active treatment and up to the final safety follow‐up visit. Safety data are presented according to active treatment dose, with placebo switchers who were assigned originally to placebo (who represented a mix of dose assignments in the LTE) separated according to LTE dose group. AEs were coded using the Medical Dictionary for Regulatory Activities, v22.0. AEs are reported as incidence (percentage based on number of participants in the safety population). ARIA are reported as incidence (percentage based on the number of treated participants with at least one MRI during the active treatment period). Each patient was counted only once within each category. All AEs were analyzed based on the principle of treatment emergence.
Because amyloid PET annual assessments were conducted only through year 4 of the study, the amyloid PET and clinical endpoint analyses presented here include data from the 12‐month PC and 36‐month LTE periods. Participants included in these analyses received either 48 months of continued aducanumab treatment (for participants originally randomized to active drug from study start) or 36 months of aducanumab treatment (for participants randomized to placebo at study start). For PK analyses, placebo switchers were separated into LTE dose groups.
Amyloid PET and exploratory clinical efficacy analyses through the month 48 visit used a mixed model for repeated measures (MMRM) for longitudinal change from baseline data, adjusting for baseline and APOE ε4 status (carrier/noncarrier). Visit and treatment group were treated as categorical variables in the model along with their interaction. An unstructured covariance matrix was assumed to model the within‐patient variability. For each time point, adjusted means for each treatment, pairwise adjusted differences with placebo switchers, 95% confidence intervals (CIs) for the pairwise differences, and associated nominal p values for the comparison were calculated. No adjustments were made for multiple comparisons or multiple interim analyses. Given the complex nature of the PRIME LTE study design and the exploratory nature of the clinical measures, caution should be used in the interpretation of statistical findings in the present study.
2.6. Availability of data and materials
Data deposition statement can be found in the Methods in the Supplementary Material.
3. RESULTS
3.1. Patient disposition and treatment exposure
Overall, 197 participants were randomized, and 196 received at least one dose during the PC period (Figure S2). At baseline, no apparent differences in demographics and clinical characteristics were observed, except for numerically lower mean composite PET SUVR levels in the titration group (Table S1).
The mean ± standard deviation (SD) number of active treatment infusions during the combined PC and LTE periods was 35 ± 25 for all aducanumab groups combined.
3.2. Safety
The incidence of AEs and SAEs was balanced across treatment groups during the PC period (Table 1). The incidence of ARIA‐E during the PC period was numerically higher among APOE ε4 carriers than noncarriers across aducanumab dose groups. Among APOE ε4 carriers, the incidence of ARIA‐E was numerically lower in participants receiving aducanumab titrated to 10 mg/kg (35%) than in those receiving aducanumab fixed doses of 6 mg/kg (43%) or 10 mg/kg (55%). The incidence of ARIA‐H microhemorrhage during the PC period was 22% in the fixed‐dose 10‐mg/kg group, whereas it was numerically lower in the titration to 10 mg/kg group (17%). However, the different treatment regimen in the 10‐mg/kg group did not affect the incidence of ARIA‐H superficial siderosis during the PC period, as it was 13% in both the fixed‐dose 10‐mg/kg group and the titration to 10 mg/kg group, respectively.
TABLE 1.
Summary of safety data during the PC period.
| Aducanumab | ||||||
|---|---|---|---|---|---|---|
| Safety population |
Placebo (n = 48) |
1 mg/kg (n = 31) |
3 mg/kg (n = 32) |
6 mg/kg (n = 30) |
10 mg/kg (n = 32) |
Titration to 10 mg/kg (n = 23) |
| Participants with an AE, n (%) | 47 (98) | 29 (94) | 27 (84) | 28 (93) | 29 (91) | 21 (91) |
| Participants with an SAE, n (%) | 16 (33) | 4 (13) | 4 (13) | 4 (13) | 12 (38) | 4 (17) |
| Safety MRI population | Placebo (n = 46) | 1 mg/kg (n = 31) | 3 mg/kg (n = 32) | 6 mg/kg (n = 30) | 10 mg/kg (n = 32) | Titration to 10 mg/kg (n = 23) |
|---|---|---|---|---|---|---|
| ARIA‐E, n (%) a | 0/46 (0) | 1/31 (3) | 2/32 (6) | 11/30 (37) | 13/32 (41) | 8/23 (35) |
| APOE ε4 carrier | 0/32 (0) | 1/19 (5) | 1/21 (5) | 9/21 (43) | 11/20 (55) | 8/23 (35) |
| APOE ε4 noncarrier | 0/14 (0) | 0/12 (0) | 1/11 (9) | 2/9 (22) | 2/12 (17) | — |
| Brain microhemorrhage, n (%) | 3/46 (7) | 1/31 (3) | 3/32 (9) | 5/30 (17) | 7/32 (22) | 4/23 (17) |
| Localized superficial siderosis, n (%) | 0/46 (0) | 2/31 (6) | 1/32 (3) | 2/30 (7) | 4/32 (13) | 3/23 (13) |
Notes: AEs for fixed‐dose cohorts during the PC period were reported previously in Sevigny et al. (2016) 4 . Titration to 10 mg/kg: two doses of 1 mg/kg, four doses of 3 mg/kg, five doses of 6 mg/kg, and 10 mg/kg of aducanumab thereafter.
Abbreviations: AE, adverse event; APOE ε4, apolipoprotein E ε4; ARIA, amyloid‐related imaging abnormalities; MRI, magnetic resonance imaging; PC, placebo controlled; SAE, serious adverse event.
Incidence of ARIA based on MRI.
Table 2 details safety data from the combined PC and LTE period after the first exposure to aducanumab, along with the mean ± SD treatment duration between each treatment arm. The most common AEs (≥15%) by preferred term were fall (29%), headache (29%), ARIA‐E (25%), urinary tract infection (22%), ARIA‐H (19%), diarrhea (16%), nasopharyngitis (16%), and upper respiratory tract infection (16%) in all aducanumab dose groups combined.
TABLE 2.
Cumulative aducanumab safety data: PC period and LTE after first aducanumab dose.
| Safety population | 1 to 3 mg/kg (n = 31) | 3 mg/kg (n = 42) | 6 mg/kg (n = 30) | 10 mg/kg (n = 32) | Titration, 3 to 6 mg/kg (n = 19) | Titration, 1to 3 to 6 to 10 mg/kg (n = 31) | All aducanumab dose groups combined (n = 185) |
|---|---|---|---|---|---|---|---|
| No. of weeks on study treatment, mean (±SD) | 123 (105) | 158 (120) | 165 (98) | 120 (113) | 146 (87) | 155 (72) | 145 (103) |
| AEs, n (%) | 29 (94) | 39 (93) | 30 (100) | 29 (91) | 19 (100) | 31 (100) | 177 (96) |
| SAEs, n (%) | 11 (35) | 19 (45) | 14 (47) | 16 (50) | 12 (63) | 12 (39) | 84 (45) |
| Fatal AEs, n (%) a | 1 (3) | 1 (2) | 1 (3) | 2 (6) | 1 (5) | 0 | 6 (3) |
| AEs leading to discontinuation of treatment, n (%) | 4 (13) | 9 (21) | 5 (17) | 16 (50) | 6 (32) | 3 (10) | 43 (23) |
| Safety MRI population | 1 to 3 mg/kg (n = 31) | 3 mg/kg (n = 42) | 6 mg/kg (n = 30) | 10 mg/kg (n = 32) | Titration, 3 to 6 mg/kg (n = 19) | Titration, 1to 3 to 6 to 10 mg/kg (n = 31) | All aducanumab dose groups combined (n = 185) |
|---|---|---|---|---|---|---|---|
| ARIA‐E, n/total (%) | 4/31 (13) | 5/42 (12) | 11/30 (37) | 13/32 (41) | 3/19 (16) | 10/31 (32) | 46/185 (25) |
| APOE ε4 carriers | 4/19 (21) | 3/25 (12) | 9/21 (43) | 11/20 (55) | 3/13 (23) | 10/31 (32) | 40/129 (31) |
| APOE ε4 homozygote | 0/1 | 1/5 (20) | 2/4 (50) | 3/6 (50) | 1/3 (33) | 2/4 (50) | 9/23 (39) |
| APOE ε4 heterozygote | 4/18 (22) | 2/20 (10) | 7/17 (41) | 8/14 (57) | 2/10 (20) | 8/27 (30) | 31/106 (29) |
| APOE ε4 noncarriers | 0/12 (0) | 2/17 (12) | 2/9 (22) | 2/12 (17) | 0/6 (0) | — | 6/56 (11) |
| In participants with ARIA‐E, n/total (%) | |||||||
| Asymptomatic | 2/4 (50) | 2/5 (40) | 7/11 (64) | 8/13 (62) | 1/3 (33) | 8/10 (80) | 28/46 (61) |
| Symptomatic | 2/4 (50) | 3/5 (60) | 4/11 (36) | 5/13 (38) | 2/3 (67) | 2/10 (20) | 18/46 (39) |
| ARIA‐E, discontinued treatment, n/total (%) | 1/31 (3) | 2/42 (5) | 3/30 (10) | 9/32 (28) | 3/19 (16) | 2/31 (6) | 20/185 (11) |
| Brain microhemorrhage | 2/31 (6) | 10/42 (24) | 8/30 (27) | 7/32 (22) | 4/19 (21) | 5/31 (16) | 36/185 (19) |
| Localized superficial siderosis | 2/31 (6) | 1/42 (2) | 3/30 (10) | 4/32 (13) | 0/19 | 4/31 (13) | 14/185 (8) |
Notes: Columns reflect patient aducanumab dose assignment while on active treatment. The treatment assignment of 1to 3 mg/kg represents an assignment to 1 mg/kg in the PC period and 3 mg/kg in the LTE.
Abbreviations: AE, adverse event; APOE ε4, apolipoprotein E ε4; ARIA, amyloid‐related imaging abnormalities; LTE, long‐term extension; MRI, magnetic resonance imaging; PC, placebo controlled; SAE, serious adverse event; SD, standard deviation.
Two additional deaths in participants who received placebo.
ARIA‐E and falls were the most common (≥5% in all aducanumab dose groups combined) SAEs reported. Seven participants (six receiving aducanumab, one receiving placebo) died during the study, and one placebo‐treated participant died after withdrawing from the study during the PC period. During the PC period, two participants died (one placebo‐treated participant with cardiac arrest and one patient receiving fixed‐dose aducanumab 10 mg/kg who died due to a cerebrovascular accident). Only one death (participant who received aducanumab at 1 mg/kg and then 3 mg/kg), which was due to cerebral hemorrhage, was considered treatment related by the investigator. Other reported causes of death were consistent with AD and comorbid conditions, encompassing AD, myocardial infarction, and cardiorespiratory arrest due to coronary atherosclerotic disease.
Across all aducanumab dose groups combined, the incidence of ARIA‐E was 46 of 185 (25%) in the safety MRI population, with 40 of 129 (31%) occurring in APOE ε4 carriers and 6 of 56 (11%) occurring in noncarriers (Table 2). Among APOE ε4 carriers, the incidence of ARIA‐E was 9 of 23 (39%) and 31 of 106 (29%) in homozygous and heterozygous carriers, respectively. The incidence of brain microhemorrhages and localized superficial siderosis was 36 of 185 (19%) and 14 of 185 (8%), respectively, across all aducanumab dose groups combined. APOE ε4 carriers titrating to 10 mg/kg had a numerically lower incidence of ARIA‐E compared with APOE ε4 carriers in the 10‐mg/kg fixed‐dose group (10 of 31, 32% vs 11 of 20, 55%, respectively). In the 10‐mg/kg titration group, the incidence of ARIA‐E was 50% (2 of 4) and 30% (8 of 27) in APOE ε4 homozygous and heterozygous carriers, respectively. In the 10‐mg/kg fixed‐dose group, ARIA‐E incidence was 50% (3 of 6) in homozygous carriers and 57% (8 of 14) in heterozygous carriers. For 35 of 46 participants (76%) with ARIA‐E events, ARIA‐E occurred within the first 6 months of treatment (Figure S3). ARIA‐E radiographically resolved in 44 of 46 participants within 4–12 weeks and was ongoing in two participants at the time of study withdrawal.
In some cases, ARIA were serious. Twenty participants had at least one SAE of ARIA during the active treatment period. The incidence of serious ARIA was as follows (note that participants may have experienced more than one type): ARIA‐E in 19 participants (10%); ARIA‐H superficial siderosis in six participants (3%); and ARIA‐H microhemorrhage in four participants (2%). Two cases of ARIA required hospitalization. In the safety MRI population, three of 185 participants (2%) experienced ARIA‐H macrohemorrhage, which were reported as AEs. In addition, macrohemorrhage was reported as MRI findings for two participants without being reported as an AE.
Three of 31 participants (10%) titrated to 10 mg/kg discontinued treatment due to an AE (2 of 31 [6%] were due to ARIA). The study protocol required permanent treatment discontinuation in participants with ARIA under certain circumstances (see Section 2). In eight participants (8 of 185 [4.3%]), there was more than one occurrence of ARIA‐E.
Among participants with ARIA‐E, 28 of 46 (61%) did not have accompanying clinical symptoms in the setting of the ARIA episode (Table 2). When symptoms were reported, 61% (11 of 18) were mild in clinical severity. The most common symptoms were headache, dizziness, and confusion. Severe symptoms were reported in 3 of 18 participants (17%) and included headache, confusion, and in one patient, seizures.
3.3. Aducanumab PK
PK parameters observed in this study were consistent with those of other immunoglobulin G1 monoclonal antibodies (Table S2). 9
3.4. Changes in amyloid plaque levels with aducanumab
During the PC period, a dose‐ and time‐dependent reduction in amyloid PET was observed in participants from the fixed‐dose and titration groups (Figure 1A). Placebo switchers showed a numerical decline in amyloid plaque levels following a period of minimal change during the first 12‐month PC period. During the first year of the LTE, amyloid plaque levels decreased in the 6‐mg/kg, 10‐mg/kg fixed‐dose, and 10‐mg/kg titration groups relative to the placebo switchers. Specifically, at month 24, composite SUVR adjusted mean ± standard error (SE) change from baseline ranged from −0.149 ± 0.032 (−32.8 ± 7.0; 1‐ to 3‐mg/kg group) to −0.322 ± 0.032 (−71.1 ± 7.0 CL; 10‐mg/kg fixed‐dose group). Similar to the 10‐mg/kg fixed‐dose group, the 10‐mg/kg titration group (average expected dose of 7.6 mg/kg) demonstrated an adjusted mean change of −0.275 ± 0.033 (−60.7 ± 7.3 CL) from baseline. Amyloid plaque levels generally continued to decline in a dose‐dependent manner from month 24 to 48. Specifically, at month 48, the adjusted mean changes from baseline in amyloid PET composite SUVR ranged from −0.227 ± 0.044 (−50.0 ± 9.6 CL; 1‐ to 3‐mg/kg group) to −0.335 ± 0.043 (−73.8 ± 9.6 CL; 10‐mg/kg fixed‐dose group) (Figure 1A). From month 24 to 48, both the 10‐mg/kg fixed‐dose and the titration groups experienced an apparent plateau in amyloid reduction.
FIGURE 1.
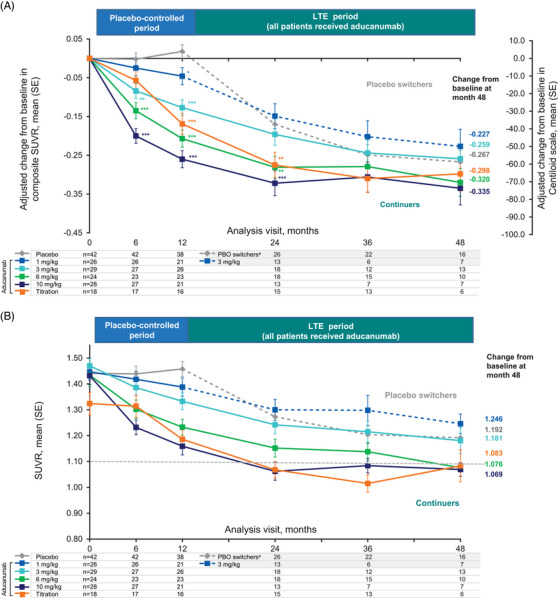
Effect of aducanumab on amyloid plaque levels through 48 months. APOE ε4, apolipoprotein E ε4; CL, Centiloid; LTE, long‐term extension; MMRM, mixed model for repeated measures; PBO, placebo; SE, standard error; SUVR, standardized uptake value ratio. (A) Effect of aducanumab on amyloid plaque levels (composite SUVR and CL units) over 48 months. Results based on MMRM fitted with change from baseline as a dependent variable and fixed effects for categorical treatment, categorical visit and treatment‐by‐visit interaction, continuous baseline value, and laboratory APOE ε4 status (carrier/noncarrier). (B) Descriptive summary of mean SUVR (SE). A mean SUVR value of 1.10 is a quantitative cut point reported to discriminate between an amyloid‐positive and amyloid‐negative scan. 10 aPlacebo switchers received aducanumab 3 mg/kg or titration (3–6 mg/kg or 1–10 mg/kg) in the LTE. Nominal *p < 0.05; **p < 0.01; ***p < 0.001 versus placebo in the placebo‐controlled period and versus placebo switchers in the LTE.
To better understand this observation, we analyzed the mean composite SUVR at each time point of the follow‐up period (Figure 1B). Participants in the titration and 10‐mg/kg fixed‐dose groups during the PC period who continued aducanumab treatment to month 24 reached a mean composite SUVR of 1.07 and 1.06, respectively. At month 48, the mean composite SUVR in the titration and 10‐mg/kg fixed‐dose groups remained similar, respectively. These composite SUVR means fell below 1.10, a quantitative cut point reported to discriminate between amyloid‐positive and amyloid‐negative scans. 10 At month 24, nine (69%) and 10 participants (67%) in the 10‐mg/kg fixed‐dose group and 10‐mg/kg titration group, respectively, had a composite SUVR of ≤1.10 (69%); at month 48, five (71%) and four participants (67%) in the 10‐mg/kg fixed‐dose group and 10‐mg/kg titration group, respectively, had a composite SUVR of ≤1.10. Placebo switchers also experienced a reduction in amyloid as measured by the decline in composite SUVR from month 12 to 48.
3.5. Clinical effects of aducanumab
Analyses of change from baseline in the exploratory endpoints, CDR‐SB (Figure 2A) and MMSE (Figure 2B) through month 48, suggested a continued slowing in clinical decline in aducanumab‐treated cohorts, measured as numerical differences from the change from baseline in placebo switchers. Effects were most apparent for participants in the 10‐mg/kg titration and fixed‐dose groups. Adjusted mean changes in NTB, FCSRT, or NPI‐Q scores at month 24 are reported in Table 3.
FIGURE 2.
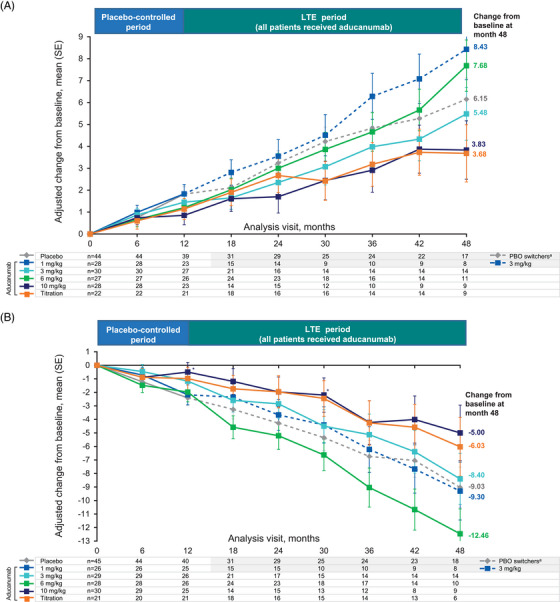
Effect of aducanumab on clinical endpoints through 48 months. APOE ε4, apolipoprotein E ε4; CDR‐SB, Clinical Dementia Rating–Sum of Boxes; LTE, long‐term extension; MMRM, mixed model for repeated measures; MMSE, Mini‐Mental State Examination; PBO, placebo; SE, standard error. (A) Effect of aducanumab on clinical decline as measured by the CDR‐SB (exploratory endpoint) over 48 months. (B) Effect of aducanumab on clinical decline as measured by the MMSE (exploratory endpoint) over 48 months. Results based on MMRM fitted with change from baseline as a dependent variable and fixed effects for categorical treatment, categorical visit and treatment‐by‐visit interaction, continuous baseline value, and laboratory APOE ε4 status (carrier/noncarrier). *Nominal p < 0.05. aPlacebo switchers received aducanumab 3 mg/kg or titration (3–6 mg/kg or 1–10 mg/kg) in the LTE.
TABLE 3.
Change from baseline in exploratory clinical endpoints at month 24.
| Continuersc | ||||||
|---|---|---|---|---|---|---|
| Adjusted change from baseline, mean (SE) | Placebo switchersa | 1 to 3 mg/kgb | 3 mg/kg | 6 mg/kg | 10 mg/kg | Titration |
| NTB overall z score | −0.40 (0.11) n = 28 | −0.67 (0.14) n = 13 | −0.29 (0.13) n = 16 | −0.38 (0.13) n = 19 | −0.24 (0.14) n = 15 | −0.18 (0.14) n = 16 |
| FCSRT sum of free recall score | −5.00 (1.36) n = 26 | −5.46 (1.80) n = 14 | −2.32 (1.78) n = 14 | −6.52 (1.59) n = 21 | 0.03 (1.74) n = 15 | −2.10 (1.86) n = 16 |
| NPI‐Q total distress score | 0.68 (0.93) n = 28 | 1.32 (1.24) n = 14 | 1.03 (1.15) n = 17 | 2.25 (1.11) n = 21 | 0.96 (1.22) n = 15 | −0.29 (1.30) n = 16 |
Notes: Adjusted mean and SE for each treatment group were based on the MMRM. The MMRM was fitted with change from baseline as a dependent variable and included fixed effects for categorical treatment, categorical visit and treatment‐by‐visit interaction, continuous baseline value, and laboratory APOE status (carrier and noncarrier). The NTB includes the Rey Auditory Verbal Learning Test Immediate and Delayed Recall, Wechsler Memory Scale Verbal Pair Associate Learning Test Immediate and Delayed Recall, Delis‐Kaplan Executive Function System Verbal Fluency Conditions 1 and 2, and Wechsler Adult Intelligence Scale Fourth Edition Symbol Search and Coding Subsets.
Abbreviations: APOE ε4; apolipoprotein E ε4; FCSRT, Free and Cued Selective Reminding Test; LTE, long‐term extension; MMRM, mixed model for repeated measures; NPI‐Q, Neuropsychiatric Inventory Questionnaire; NTB, Neuropsychological Test Battery.
Placebo switchers received aducanumab 3 mg/kg or titration (3–6 mg/kg or 1–10 mg/kg) in the LTE.
Participants in the 1‐ to 3‐mg/kg arm received aducanumab 3 mg/kg in the LTE.
Participants who were randomized to receive 3, 6, or 10 mg/kg or titration were scheduled to continue the same dose throughout the LTE.
4. DISCUSSION
In this report, combined data from the PC and LTE periods of the PRIME study are presented. ARIA‐E occurred primarily during the first 24 weeks of treatment and was transient and mostly asymptomatic in participants who received aducanumab during the PC period. The incidence of ARIA‐E in placebo switchers during the LTE was consistent with this observation. Specifically, the instances of ARIA‐E were more prevalent during the initial stages of treatment among the placebo switchers. This observation contributed to the count of participants who discontinued treatment in the LTE phase due to AEs among placebo switchers. Dose titration appeared to reduce the incidence of ARIA‐E in APOE ε4 carriers.
Amyloid plaque levels continued to a dose‐ and time‐dependent decrease in aducanumab‐treated participants who completed 48 months of treatment, with participants from the 10‐mg/kg and titration cohorts reaching a plateau at month 24. Analyses revealed that mean (net) amyloid plaque levels reached and remained at an SUVR level below 1.10, a quantitative cut point proposed to discriminate between a positive and negative amyloid PET scan (e.g., pathological vs nonpathological accumulation). 10 After 36 months of treatment, participants in the titration cohort reached a mean change from baseline in amyloid plaque levels comparable to that in the 10‐mg/kg group. This observation is consistent with the expected average aducanumab dose received in the titration group: 8.4 mg/kg by month 36 and 8.8 mg/kg by month 48. Overall, amyloid plaque levels appeared to plateau in the later stages of the trial (e.g., after 36 months), although caution should be used in this interpretation, as the later time points comprised very small patient numbers. As expected, a decline in amyloid plaque levels was not observed in participants randomized to placebo during the PC period. In contrast, placebo switchers experienced a decline in amyloid PET levels in the LTE period.
Exploratory analyses of the changes from baseline in CDR‐SB and MMSE showed a dose‐dependent reduction in clinical decline for aducanumab. During the LTE period, participants continuing aducanumab treatment were compared with placebo switchers, given that there was no placebo group for comparison after the 12‐month PC period. The trajectories for changes from baseline in CDR‐SB score for participants continuing aducanumab showed separation from placebo switchers, with adjusted mean ± SE changes from baseline at month 48 of 3.83 ± 1.33 and 3.68 ± 1.31 in the 10‐mg/kg fixed‐dose and 10‐mg/kg titration groups, respectively, compared with 6.15 ± 0.97 for placebo switchers. Although these exploratory results should be interpreted with caution, they suggest a continued benefit in the reduction of clinical decline over 48 months with the highest doses of aducanumab.
Limitations of this study include the small number of participants per cohort coupled with moderate attrition and the inherent selection bias associated with any LTE period. Placebo switchers were treated with a range of aducanumab doses when entering the LTE period, making analysis of such a group complex. In addition, heterogeneity existed in this study regarding ARIA management, as certain aspects of ARIA management were modified over time.
In conclusion, the PD effects seen in aducanumab‐treated participants indicated that aducanumab modifies the underlying pathology of AD in a dose‐dependent manner. Clinically, aducanumab‐treated participants with the highest doses (10 mg/kg fixed and titration to 10 mg/kg) experienced benefits over placebo in reduction of clinical decline as measured by changes from baseline in CDR‐SB and MMSE scores starting at 1 year; beyond 1 year, the clinical benefit appeared to continue with longer‐term treatment compared with participants who switched from placebo to aducanumab treatment. ARIA is monitorable via brain MRI, and aducanumab dose titration can reduce the incidence of ARIA. EMBARK (NCT04241068), an open‐label, longitudinal, single‐arm, global, Phase 3b study in participants from aducanumab studies that were halted in March 2019, is expected to provide further information regarding the long‐term safety of aducanumab.
CONFLICT OF INTEREST STATEMENT
Tianle Chen, John O'Gorman, Gioacchino G. Curiale, and Priya Singhal are employees and shareholders of Biogen Inc. Carmen Castrillo‐Viguera, Rajasimhan Rajagovindan, Ying Tian, Dakshaben Patel, Philipp von Rosenstiel, Christian von Hehn, Samantha Budd Haeberlein, and Alfred Sandrock were employees of Biogen at the time of this study and have since left the company. Christoph Hock and Roger M. Nitsch are employees and shareholders of Neurimmune. Stephen Salloway was a site investigator for the PRIME study and is a consultant to Biogen. He also receives research support and is a consultant to Eisai, Novartis, Genentech, Roche, Avid, and Lilly. Author disclosures are available in the Supporting Information.
CONSENT STATEMENT
All participants provided written informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the patients and their family members for participating in the aducanumab studies, as well as the investigators and the staff conducting these studies. We thank Guanfang Wang, Sarah Gheuens, Claudia Prada, Jeff Sevigny, Yan Ling, Brendon Boot, and Ahmed Enayetallah for their contributions to this study. This study (NCT01677572) was funded by Biogen Inc. (Cambridge, MA, USA). Medical writing and editorial support were provided by Nucleus Global Ltd., in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp‐2022), and funded by Biogen Inc.
Chen T, O'Gorman J, Castrillo‐Viguera C, et al. Results from the long‐term extension of PRIME: A randomized Phase 1b trial of aducanumab. Alzheimer's Dement. 2024;20:3406–3415. 10.1002/alz.13755
Carmen Castrillo‐Viguera, Rajasimhan Rajagovindan, Ying Tian, Dakshaben Patel, Philipp von Rosenstiel, Christian von Hehn, Samantha Budd Haeberlein, and Alfred Sandrock were employees of Biogen at the time of this study and have since left the company.
Trial Registration: ClinicalTrials.gov: NCT01677572
REFERENCES
- 1. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353‐356. [DOI] [PubMed] [Google Scholar]
- 2. Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184‐185. [DOI] [PubMed] [Google Scholar]
- 3. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50‐56. [DOI] [PubMed] [Google Scholar]
- 5. Sevigny J, Suhy J, Chiao P, et al. Amyloid PET screening for enrichment of early‐stage Alzheimer disease clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord. 2016;30:1‐7. [DOI] [PubMed] [Google Scholar]
- 6. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9:197‐210. [DOI] [PubMed] [Google Scholar]
- 7. Ostrowitzki S, Lasser RA, Dorflinger E, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. 2017;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1‐15.e1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633‐659. [DOI] [PubMed] [Google Scholar]
- 10. Joshi AD, Pontecorvo MJ, Lu M, Skovronsky DM, Mintun MA, Devous MD, Sr . A semiautomated method for quantification of F 18 Florbetapir PET images. J Nucl Med. 2015;56:1736‐1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
Data deposition statement can be found in the Methods in the Supplementary Material.


