Abstract
Background
Persistent inflammation affects people with HIV (PWH) despite antiretroviral therapy (ART). Selective serotonin and serotonin-norepinephrine reuptake inhibitors (SSRIs, SNRIs), HMG-CoA reductase-inhibitors (statins), and angiotensin-converting enzyme inhibitors (ACEIs) have immunomodulant properties. We evaluated the potential impact of these drugs on inflammation and neurodegeneration in PWH.
Methods
Cross-sectional single-center (United States) analysis in 184 PWH on ART with plasma HIV RNA < 200 copies/mL. All participants had 10 biomarkers measured in blood and cerebrospinal fluid (CSF). To reduce dimensionality, hierarchical clustering and principal components (PCs) analysis were employed. The analyses were adjusted for duration of the drugs and clinical conditions.
Results
Participants were mostly middle-aged men, with median CD4+ T cells of 620/µL. In adjusted models, SSRI use was associated with 3 PCs: higher CSF and plasma Aβ42 and CSF CCL2 (aβ=.14, P = .040); lower CSF 8-oxo-dG, total tau, and sCD14 (aβ=−.12, P = .042); and higher plasma sCD14 with lower sCD40L (aβ=.15, P = .042). SNRI use was associated with higher values of CSF and plasma neopterin and CSF sTNFR-II (aβ=.22, P = .004). Statins and ACEIs showed no association.
Conclusions
SSRIs and SNRIs had distinct biomarker signatures. SSRIs were associated with reduced neurodegeneration, immune activation, and oxidative stress in CSF, suggesting a role of SSRIs as adjunctive therapy in PWH.
Keywords: chronic inflammation, immune modulation, comedications, antidepressants, HIV, SSRIs, SNRIs, statins, ACE inhibitors, neurodegeneration
Among virally suppressed PWH, SSRIs were associated with reduced neurodegeneration and lower immune activation, SNRIs with higher levels of myeloid and lymphoid activation, while ACEIs and statins had no distinct biomarker signatures. Specific comedications may affect HIV-related persistent immune activation.
Polypharmacy in aging people with human immunodeficiency virus (PWH) is common due to the increased risk of comorbidities, such as diabetes, hypertension, and depression [1]. Despite viral suppression, PWH have an increased risk of serious non-AIDS–defining conditions such as these comorbidities due to persistently heightened inflammation and immune activation [1]. This in turn leads to polypharmacy, and eventually to further adverse consequences such as frailty, drug-drug interactions, cognitive impairment, and impaired mental health. However, while comedications are not specifically targeted against inflammation and related neurodegeneration, some may have substantial “off-target” anti-inflammatory and neuroprotective effects.
For example, selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) may reduce neuroinflammation by regulating several immune signaling pathways that involve molecules such as peroxisome proliferator-activated receptor gamma (PPAR-γ), nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), Toll-like receptor 4 (TLR4), and inflammasomes [2, 3]. In ex vivo studies involving PWH, SSRIs enhanced natural killer and CD8+ T cell functioning [4], downregulated human immunodeficiency virus (HIV) receptor and coreceptors in peripheral blood mononuclear cells [5], and reduced HIV replication in macrophages and T lymphocytes [4].
Beyond their lipid-lowering activity, 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins) may downregulate several pathways of inflammation and immune activation [6, 7], and in vitro, they can induce resistance of CD4+ T cells to HIV-1 infection [8]. Statins may also reduce HIV-associated neuropathology by counteracting the proinflammatory activity of amyloid β and Tat in brain endothelial cells [9] and by shaping the phenotype and activation of monocytes [10]. In line with this, a lower likelihood of detectable cerebrospinal fluid (CSF) HIV RNA in participants on either statins or SSRIs has been observed [11]. However, as the interest in the anti-inflammatory properties of these drugs is growing, conflicting findings have also been reported [12–14]. Similarly, evidence of immunomodulatory properties of angiotensin-converting enzyme inhibitors (ACEIs) has been reported in the general population, but to date these effects have not been demonstrated in PWH [15, 16]. Because inflammation contributes to the pathogenesis of cardiovascular, mood, and cognitive disorders in PWH, whether comedications have beneficial immunomodulatory effects might lead to intervention trials using these readily available, regulatory agency-approved drugs. Such data could also inform prescribing and deprescribing practices in HIV clinic.
We evaluated the effects of concomitant nonantiretroviral medications on biomarkers of inflammation and neurodegeneration in PWH on effective antiretroviral therapy (ART), while taking into consideration the potential confounding activity of the comorbidities for which these medications were prescribed.
METHODS
Study Design
This retrospective cross-sectional study investigated in PWH on suppressive ART (plasma HIV RNA < 200 copies/mL) the associations between specific concomitant comedication classes prescribed for mood disorders (SSRIs, SNRIs), hypertension (ACEIs), or dyslipidemia (statins) and a panel of soluble biomarkers in blood and CSF. Comedications had been taken continuously for at least 3 months prior the evaluation.
Participants were assessed between 2016 and 2020 in an observational research project at the HIV Neurobehavioral Research Program at the University of California San Diego. All research protocols were approved by the Institutional Review Board, and all participants provided written informed consent.
Participants were not assessed if they were unable to perform the evaluations (eg, untreated systemic infection, acute intoxication), or if they had a severe, untreated neuropsychiatric disorder (eg, schizophrenia, untreated seizure disorder). People who had localized mild infections (eg, herpes simplex reactivation, mucosal candidiasis) or who used immunomodulating medications systemically administered (eg, corticosteroids) were not excluded, so analyses were adjusted for use of antimicrobials and immunomodulators.
Medical and Cognitive Assessment
Demographics (eg, age, sex, race), HIV parameters, blood and CSF biochemistry, clinical data, and data on comedications and comorbidities were collected. All participants completed a comprehensive neurocognitive test battery, from which the individual test scores were demographically corrected and combined to calculate the global deficit score (GDS), as previously detailed [17]. A GDS value of ≥0.5 indicated neurocognitive impairment [17]. Depressive symptoms were assessed by the Beck Depression Inventory II (BDI-II), and depressed mood defined for scores ≥14 [18]. The presence and severity of neuropathy was assessed by clinical examination (reflexes, vibration, and sharp discrimination) and self-reported dysesthesias.
Biomarkers were selected on the basis of their relevance to inflammation and neurodegeneration in the context of HIV. Table 1 summarizes the soluble biomarkers measured in blood and CSF biomarkers and the commercial immunoassays used to measure them. Blood-brain barrier function was assessed using the CSF-to-serum albumin ratio (CSAR), calculated by dividing CSF albumin mg/dL by serum albumin g/dL [19].
Table 1.
Blood and CSF Biomarkers Levels in the Study Population
| Biomarkers | Median (Q1–Q3) (n = 184) |
|---|---|
| Plasma biomarkers | |
| Oxidative stress | |
| 8-oxo-dG, ng/mL | 134.2 (95.9–199.7) |
| Amyloid metabolism | |
| Aβ42, pg/mL | 37.6 (15.3–74.4) |
| Inflammation and immune activation | |
| IL-6, pg/mL | 1.29 (0.87–1.82) |
| sTNFR-II, pg/mL | 6126 (4506–8429) |
| CCL2, pg/mL | 158.5 (131.4–193.4) |
| Neopterin, nmol/mL | 9.0 (7.0–12.2) |
| sCD14, ng/mL | 1092359 (478158–1522954) |
| sCD40L, pg/mL | 1113 (393–2343) |
| CSF biomarkers | |
| Oxidative stress | |
| 8-oxo-dG, ng/mL | 12.8 (10.1–22.5) |
| Amyloid metabolism | |
| Aβ42, pg/mL | 1270.4 (433.9–2027.0) |
| Inflammation and immune activation | |
| IL-6, pg/mL | 1.16 (0.91–1.56) |
| sTNFR-II, pg/mL | 515.5 (393.4–856.4) |
| CCL2, pg/mL | 423.5 (338.8–528.3) |
| Neopterin, nmol/mL | 8.2 (6.8–10.9) |
| sCD14, ng/mL | 84892 (49317–121334) |
| NFL, pg/mL | 1236.7 (881.1–1687.1) |
| Neuronal injury | |
| Tau, pg/mL | 193.2 (106.2–417.6) |
The immunoassays used were Meso Scale Discovery (IL-6, sTNFR-II, CCL2, Aβ42, Tau); R&D Systems (sCD14); ALPCO (neopterin); Millipore Sigma (sCD40L); Trevigen (8-oxo-dG); and UmanDiagnostics (NFL).
Abbreviations: 8-oxo-dG, 8-oxo-2′-deoxyguanosine; Aβ42, fragment 1–42 of β amyloid; CCL2, chemokine C-C motif ligand 2; CSF, cerebrospinal fluid; IL-6, interleukin 6; NFL, neurofilament light chain; Q, quartile; sCD14, soluble cluster of differentiation 14; sCD40L, soluble ligand of cluster of differentiation 40; sTNFR-II, soluble tumor necrosis factor receptor type II; tau, total tau protein.
Statistical Analysis
Data were reported as mean (standard deviation), median (first and third quartile, IQR), or absolute number (proportion), according to the type of variable and its distribution. Values of some variables (eg, HIV RNA) were log10-transformed to reduce skewness. Biomarkers were standardized into Z-scores to ease comparativeness and reduce batch bias.
Considering the number of biomarkers, dimensionality was reducted in 2 steps. First, hierarchical clustering was performed on biomarkers, after detecting evidence of significant correlations among them (as shown in Supplementary Figure 1). Clustering was based on between-groups linkage through squared Euclidean distance. Next, principal component analysis (PCA) was performed on the identified clusters to derive biomarkers’ principal component (PC) and for each participant the corresponding component score (regression method [20]).
Univariable analysis (linear regression) assessed the associations between PC coefficients and the following variables: the comedications, demographics (age, sex, race, past use of illicit drugs), HIV-related variables (CSF HIV RNA, CD4+ T-cell count and nadir, CD4/CD8 ratio, AIDS diagnosis, duration of HIV infection, duration of ART, and ART classes currently used), clinical parameters as listed in Table 2 (HCV serostatus, immunomodulators or antimicrobial therapy, active tobacco smoking, medical comorbidities, urine drug screening, body mass index, blood and CSF leukocytes, CSF total protein and glucose, CSAR), depressive symptoms (BDI-II), and cognitive performance (GDS).
Table 2.
Demographic, HIV-Related, and Clinical Characteristics of the Study Population
| Characteristic | Study Population (n = 184) |
|---|---|
| Age, y, mean (SD) | 56.1 (8.5) |
| Male sex | 147 (79.9) |
| Race | |
| White | 85 (46.2) |
| Black | 81 (44.0) |
| Other | 18 (9.8) |
| Education, y, mean (SD) | 13.0 (2.5) |
| HIV risk | |
| MSM | 120 (65.2) |
| Heterosexual | 40 (21.7) |
| pIDU | 24 (13.0) |
| Estimated duration of HIV infection, y, median (Q1–Q3) | 23 (16–27) |
| Duration of current ART regimen, mo, median (Q1–Q3) | 19 (9–66) |
| Duration of any ART, mo, median (Q1–Q3) | 190 (135–240) |
| ART regimen | |
| PI including | 50 (27.1) |
| NNRTI including | 57 (31.0) |
| INSTI including | 114 (62.0) |
| AIDS diagnosis | 139 (75.5) |
| CD4+ T-cell count, cells/µL, median (Q1–Q3) | 620 (410–836) |
| CD4/CD8 ratio, median (Q1–Q3) | 0.82 (0.50–1.23) |
| Nadir CD4+ T-cell count, cells/µL, median (Q1–Q3) | 139 (22–210) |
| Plasma HIV RNA <20 cp/mL | 134 (72.8) |
| Plasma HIV RNA, cp/mL, median (Q1–Q3)a | 41 (35–80) |
| CSF HIV RNA <20 cp/mL | 172 (93.5) |
| CSF HIV RNA, cp/mL, median (Q1–Q3)b | 41 (35–41) |
| HCV seropositive | 70 (38.0) |
| BMI, median (Q1–Q3) | 25.7 (21.1–29.8) |
| Serum albumin, g/dL, median (Q1–Q3) | 4.3 (4.1–4.6) |
| Serum creatinine, mg/dL, median (Q1–Q3) | 1.1 (0.9–1.3) |
| WBC, cells × 109/L, median (Q1–Q3) | 5.8 (4.7–7.0) |
| CSF WBC, cells/mL, median (Q1–Q3) | 2 (1–5) |
| CSF total protein, mg/dL, median (Q1–Q3) | 41 (33–54) |
| CSF glucose, mg/dL, median (Q1–Q3) | 64 (58–69) |
| CSAR, median (Q1–Q3) | 5.3 (4.8–5.8) |
| Comorbidities, No./patient, median (Q1–Q3) | 3 (1–4) |
| Diabetes | 36 (19.6) |
| Malignancy | 19 (10.3) |
| Hypertension | 98 (53.3) |
| Dyslipidemia | 74 (40.2) |
| Chronic pulmonary disease | 40 (21.7) |
| Chronic renal disease | 14 (7.6) |
| Chronic liver disease | 8 (4.3) |
| Dysesthesia | 67 (36.4) |
| Positive neuropathy signs | |
| Reflexes | 85 (46.2) |
| Vibration | 69 (37.5) |
| Sharp discrimination | 64 (34.8) |
| Positive urine screening | 27 (14.7) |
| Active tobacco smoker | 35 (19.0) |
| Drug of interest | |
| On SSRIs | 22 (12.0) |
| Duration of SSRIs use, mo, median (Q1–Q3) | 45 (8–115) |
| On SNRIs | 14 (7.6) |
| Duration of SNRIs use, mo, median (Q1–Q3) | 44 (10–124) |
| On statins | 36 (19.6) |
| Duration of statins use, mo, median (Q1–Q3) | 18 (8–46) |
| On ACEIs | 32 (17.4) |
| Duration of ACEIs use, mo, median (Q1–Q3) | 67 (20–94) |
| On antimicrobial therapy | 39 (21.1) |
| On immunomodulator | 23 (12.5) |
| Depressed mood | 68 (36.9) |
| BDI-II score, median (Q1–Q3) | 7 (3–15) |
| Neurocognitive impairment | 79 (42.9) |
| GDS, median (Q1–Q3) | 0.37 (0.12–0.79) |
Data are No. (%) except where indicated.
Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ART, antiretroviral therapy; BDI-II, Beck Depression Inventory II; BMI, body mass index; cp, copy; CSAR, CSF to serum albumin ratio; CSF, cerebrospinal fluid; GDS, global deficit score; INSTI, integrase strand transfer inhibitors; MSM, men who have sex with men; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitors; pIDUs, past intravenous drug users; Q, quartile; SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; WBC, white blood cell count.
aIn participants with plasma HIV, RNA > 20 cp/mL.
bIn participants with CSF HIV, RNA > 20 cp/mL.
Variables with P values <.10 in univariable analysis were included as covariates in multivariable analysis and the best-fitting final models were identified by backward selection. In case of significant association of a medication class in univariable analysis, the corresponding multivariable model was adjusted for the clinical condition commonly associated with the medication (BDI-II score for SSRIs and SNRIs, neuropathy for SNRIs, dyslipidemia for statins, and hypertension for ACEI) and for the duration of use of the medication. The adjustment was performed to take into account the potential effects on biomarkers played by the underlying clinical conditions rather than the drugs, and possible time-dependent effects of the drugs on the biomarker outcomes (eg, acute vs chronic exposure, adaptation, or lead and lag times in developing any effect). All the multivariable models were also adjusted for age, regardless of univariable significance. Standardized unadjusted and adjusted β coefficients (aβ) were reported.
As internal validation and to address potential bias due to outliers without reducing the sample size, we ran the same final multivariable linear models using bootstrapping (2000 samples with bias-corrected and accelerated 95% confidence interval). Analyses were performed with SPSS version 29 (IBM).
RESULTS
Participant Characteristics
Table 2 presents a summary of the demographics, disease, and laboratory data. Most participants were middle-aged (mean, 56.1 years) men (79.9%), of whom 46.2% were white and 44.0% were black. The median estimated duration of HIV disease was 23 (IQR 16–27) years, and 75.5% had been previously diagnosed with AIDS. Median CD4+ T cells were 620/µL. One hundred and thirty-four (72.8%) participants had undetectable HIV RNA in plasma and 172 (93.5%) in CSF. On average, participants had been on SSRIs (n = 22; 12.0%), SNRIs (n = 14; 7.6%), statins (n = 36; 19.6%), or ACEIs (n = 32; 17.4%) for approximately 3 (IQR 1–7) years. Supplementary Table 1 shows the specific drugs within the 4 categories.
Neurocognitive impairment and depressed mood were observed in 42.9% and 36.9% of the participants, respectively. Additionally, 21.1% of the participants were taking at least 1 antimicrobial agent (primarily trimethoprim/sulfamethoxazole, acyclovir/valacyclovir, or fluconazole), and 12.5% were on a systemic immunomodulator (primarily corticosteroids).
Biomarker Clusters and Associations With Drugs
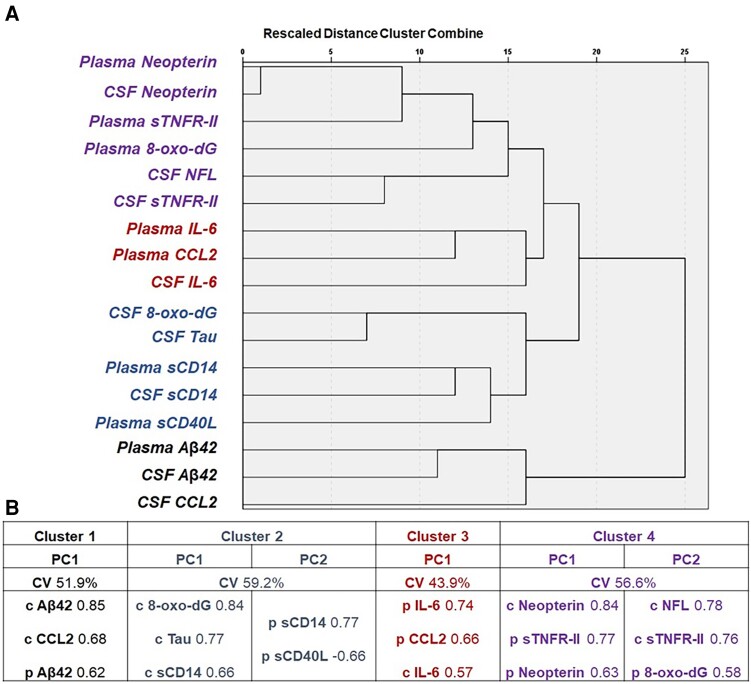
Hierarchical clustering identified 4 clusters (Figure 1A), and then PCA identified 1 unique PC in both cluster 1 (C1) and C3, and 2 PCs in both C2 and C4 (Figure 1B). Univariable analysis was run for each PC and significant associations are shown in Table 3. None of the comedications of interest were associated with PC2 of C4 (CSF neurofilament light chain [NFL], soluble tumor necrosis factor receptor type II [sTNFR-II], and plasma 8-oxo-2′-deoxyguanosine [8-oxo-dG]) nor with PC of C3 (CSF and plasma interleukin 6 [IL-6] and plasma chemokine C-C motif ligand 2 [CCL2]; data not shown); therefore, we did not proceed with further analyses for these.
Figure 1.
Results of hierarchical clustering and subsequent PC analysis of blood and CSF biomarkers. A, The 4 clusters identified at hierarchical clustering at a threshold of distance of 15–20: cluster 1 was composed of CSF and plasma BA42 and CSF CCL2; cluster 2 was composed of CSF and plasma sCD14, CSF tau, CSF 8-oxo-dG, and plasma sCD40L; cluster 3 was composed of plasma CCL2 and plasma and CSF IL-6; cluster 4 was composed of the remaining biomarkers. B, The component identified in each cluster, the cumulative proportion of variance explained within cluster, and the biomarkers factored into each PC with the respective loading factors. The sign of all loading factors goes in the same direction (positive), but for PC2 of cluster 2, where plasma sCD14 increases while plasma sCD40L decreases. Abbreviations: 8-oxo-dG, 8-oxo-2′-deoxyguanosine; Aβ42, fragment 1–42 of β amyloid; c, CSF, cerebrospinal fluid; CCL2, chemokine C-C motif ligand 2; CV, cumulative % variance; IL-6, interleukin 6; NFL, neurofilament light chain; p, plasma; PC, principal component; sCD14, soluble cluster of differentiation 14; sCD40L, soluble ligand of cluster of differentiation 40; sTNFR-II, soluble tumor necrosis factor receptor type II; tau, total tau protein.
Table 3.
Univariable and Multivariable Linear Regression Analysis for the Regression Coefficients of the Principal Components
| Standardized β (95% CI) | P | Standardized aβ (95% CI) | P | PC Direction | |
|---|---|---|---|---|---|
| CSF and plasma Aβ42 + CSF CCL2 (PC1 of C1 model P < .001, 39.2% explained variance) | |||||
| Age, y | .111 (−.035 to .256) | .134 | .048 (−.101 to .197) | .527 | … |
| CSF HIV RNA, cp/mL | .203 (.039 to .420) | .018 | .162 (−.017 to .340) | .075 | … |
| Male sex | −.193 (−.337 to −.050) | .009 | −.168 (−.314 to −.022) | .023 | Lower |
| NNRTI use | .143 (−.001 to .288) | .052 | .097 (−.050 to .244) | .093 | … |
| Current ART, mo | .093 (−.053 to .238) | .211 | .042 (−.105 to .188) | .574 | … |
| Positive urine screening | −.129 (−.274 to .016) | .081 | Excludeda | … | … |
| SSRI use | .172 (.028 to .316) | .020 | .144 (.001 to .287) | .040 | Higher |
| Duration of SSRIs, mo | .180 (.036 to .324) | .014 | .050 (−.385 to .485) | .227 | … |
| SNRI use | −.033 (−.672 to .427) | .661 | … | … | … |
| BDI-II, score | −.066 (−.212 to .080) | .460 | −.047 (−.190 to .096) | .507 | … |
| Statin use | .030 (−.292 to .443) | .684 | … | … | … |
| ACEI use | .040 (−.280 to .489) | .594 | … | … | … |
| Tobacco smoking | .153 (.009 to .298) | .038 | Excludeda | … | … |
| CSAR | .132 (−.013 to .277) | .074 | Excludeda | … | … |
| CSF 8-oxo-dG + CSF total Tau + CSF sCD14 (PC1 of C2 model P < .001, 61.8% explained variance) | |||||
| Age, y | .163 (.018 to .307) | .027 | .097 (−.148 to .342) | .264 | … |
| CSF HIV RNA, cp/mL | .307 (.143 to .471) | <.001 | .202 (.033 to .371) | <.001 | Higher |
| HIV infection, mo | .182 (.038 to .326) | .013 | Excludeda | … | … |
| SSRI use | −.171 (−.270 to −.072) | .021 | −.123 (−.231 to −.015) | .042 | Lower |
| Duration of SSRIs, mo | −.123 (−.268 to .022) | .096 | −.086 (−.298 to .126) | .579 | … |
| SNRI use | .072 (−.279 to .818) | .334 | … | … | … |
| BDI-II, score | .213 (.069 to .357) | .004 | .205 (.122 to .288) | .002 | Higher |
| Statin use | −.024 (−.427 to .308) | .751 | … | … | … |
| ACEI use | −.023 (−.445 to .324) | .758 | … | … | … |
| Tobacco smoking | −.162 (−.306 to −.017) | .028 | Excludeda | … | … |
| CSF total protein, mg/dL | .540 (.360 to .720) | <.001 | .547 (.377 to .717) | <.001 | Higher |
| CSAR | .183 (.040 to .327) | .013 | .149 (.043 to .252) | .016 | Higher |
| Plasma sCD14 + plasma sCD40L (PC2 of C2 model P = .006, 37.9% explained variance) | |||||
| Age, y | −.071 (−.216 to .075) | .341 | −.028 (−.173 to .116) | .698 | … |
| CD4+ T cell nadir, cells/µL | .151 (.006 to .295) | .041 | Excludeda | … | … |
| CD4+ T cell count, cells/µL | .130 (−.016 to .276) | .080 | Excludeda | … | … |
| Current ART, mo | −.127 (−.272 to .018) | .086 | Excludeda | … | … |
| BMI | .174 (.030 to .318) | .018 | .203 (.056 to .350) | .007 | Higher |
| SSRI use | .146 (.001 to .290) | .048 | .149 (.006 to .292) | .042 | Higher |
| Duration of SSRIs, mo | .101 (−.044 to .247) | .172 | −.009 (−.429 to .411) | .966 | … |
| SNRI use | −.073 (−.825 to .273) | .322 | … | … | … |
| BDI-II, score | .048 (−.100 to .195) | .524 | .075 (−.070 to .220) | .309 | … |
| Statin use | .023 (−.310 to .425) | .757 | … | … | … |
| ACEI use | −.056 (−.562 to .295) | .539 | … | … | … |
| Antimicrobial use | −.181 (−.325 to −.037) | .014 | −.216 (−.360 to −.072) | .004 | Lower |
| Immunomodulator use | −.132 (−.277 to .013) | .075 | Excludeda | … | … |
| CSF and plasma neopterin + CSF sTNFR-II (PC1 of C4 model P < .001, 46.5% explained variance) | |||||
| Age, y | .071 (−.047 to .189) | .235 | .091 (−.015 to .198) | .092 | … |
| PI use | .113 (−.004 to .230) | .058 | Excludeda | … | … |
| NNRTI use | −.124 (−.241 to −.007) | .038 | −.109 (−.221 to .004) | .058 | … |
| Current ART, mo | .045 (−.073 to .163) | .457 | .041 (−.067 to .0150) | .455 | … |
| BMI | .112 (−.006 to .229) | .063 | Excludeda | … | … |
| GDS | .126 (.017 to .235) | .024 | .137 (.030 to .243) | .012 | Higher |
| SSRI use | .033 (−.282 to .444) | .659 | … | … | … |
| SNRI use | .237 (.124 to .350) | <.001 | .223 (.072 to .374) | .004 | Higher |
| Duration of SNRIs, mo | .172 (.056 to .287) | .004 | .005 (−.145 to .156) | .943 | … |
| BDI-II, score | .111 (.001 to .221) | .049 | .025 (−.083 to .133) | .650 | … |
| Statin use | .045 (−.204 to .389) | .540 | … | … | … |
| ACEI use | −.069 (−.456 to .164) | .354 | … | … | … |
| Antimicrobial use | .114 (−.003 to .231) | .057 | Excludeda | … | … |
Linear regression models for PC regression coefficients. The table shows the results for the 4 medication classes, for significant (P value < .10) variables in univariable analysis, and for variables that were used to adjust the multivariable models regardless of univariable significance (univariable nonsignificant variables are not reported). Variables with P values <.10 on univariable analysis were included as candidate covariates in multivariable analysis and the best-fitting final models were identified through Akaike Information Criterion. Age, the clinical indication, and the duration of every drug that resulted associated on univariable analysis were retained to adjust the final model, regardless of univariable significance. P values <0.05 are highlighted in bold.
Abbreviations: 8-oxo-dG, 8-oxo-2′-deoxyguanosine; ACEI, angiotensin-converting enzyme inhibitors; ART, antiretroviral therapy; aβ, adjusted β; Aβ42, fragment 1–42 of β amyloid; BDI-II, Beck Depression Inventory II; BMI, body mass index; CCL2, chemokine C-C motif ligand 2; cp, copies; CSAR, CSF to serum albumin ratio; CSF, cerebrospinal fluid; GDS, global deficit score; NNRTI, nonnucleoside reverse transcriptase inhibitors; PC, principal component; PI, protease inhibitors; sCD14, soluble cluster of differentiation 14; sCD40L, soluble ligand of cluster of differentiation 40; SNRI, serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors; sTNFR-II, soluble tumor necrosis factor receptor type II.
aExcluded variables by backward selection are still shown in each model.
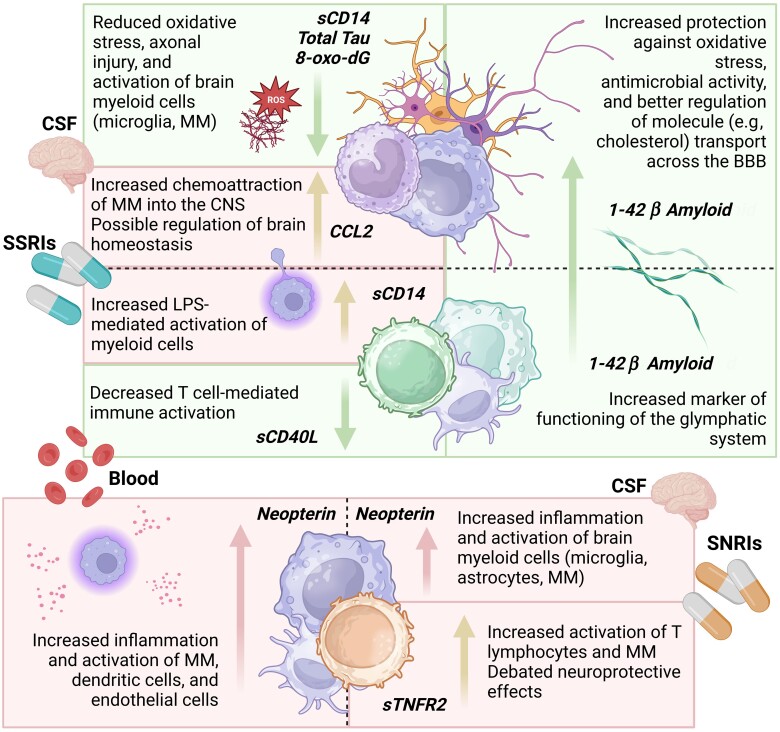
In a multivariable model adjusted for age, clinical indication for the comedication, duration of the comedication use, and univariable-significant covariates (Table 3), SSRI use was independently associated with higher levels of CSF and plasma fragment 1–42 of β amyloid (Aβ42) along with higher CSF CCL2 (PC of C1, aβ, .14; P = .040), with lower CSF levels of 8-oxo-dG, total tau, and soluble cluster of differentiation 14 (sCD14; PC1 of C2, aβ, −.12; P = .042), and with higher plasma sCD14 along with lower plasma sCD40L (PC2 of C2, aβ, .15; P = .042). In contrast, SNRI use was associated with higher levels of CSF and plasma neopterin and CSF sTNFR-II (PC1 of C4, aβ, .22; P = .004; Table 3). Of note, higher values of PC1 of C2 were also associated with higher BDI-II scores, while worse GDS was associated with higher PC1 of C4. Neither SSRI nor SNRI use was associated with neurocognitive impairment (data not shown). Figure 2 summarizes the significant associations between SSRIs, SNRIs, and biomarkers.
Figure 2.
Blood and CSF biomarker signatures associated with SSRIs and SNRIs use. Green boxes highlight favorable mechanisms that are associated with the increased or decreased levels of the respective biomarkers and the respective effects. The orange boxes summarize the associations between SSRI and SNRI use and biomarkers, for which the interpretation of the consequences of the observed association is uncertain (being either positive or negative). Abbreviations: 8-oxo-dG, 8-oxo-2′-deoxyguanosine; BBB; blood-brain barrier; CCL2, chemokine C-C motif ligand 2; CNS, central nervous system; CSF, cerebrospinal fluid; LPS, lipopolysaccharide; MM, monocytes/macrophages; sCD14, soluble cluster of differentiation 14; sCD40L, soluble ligand of cluster of differentiation 40; SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; sTNFR2, soluble tumor necrosis factor receptor type 2.
Sensitivity Analysis
After excluding participants with outlier level of biomarkers (n = 22) identified by z-score method (z-score value >3.0 or < −3.0), hierarchical clustering identified the same 4 clusters (composed by the same biomarkers) with partial differences in the distance/relationship between the biomarkers within clusters, suggesting that outliers did not affect significantly the validity of clustering procedure.
At bootstrapping, SSRI use and male sex remained associated with higher values of the PC of C1 (P = .044 and .031, respectively). The values of PC1 of C2 were associated with SSRI use (lower, P = .047), together with CSF HIV RNA, BDI-II score, CSAR, and CSF total protein (positive aβ for all; model P = .017). Higher values of PC2 of C2 remained associated with SSRI use (P = .040), together with higher BMI and no antimicrobial use (model P = .022). Lastly, bootstrapping confirmed the independent association of higher values of PC1 of C4 with the use of SNRIs (P = .012) and worse GDS (P = .034; data not shown).
DISCUSSION
Our study is unique in focusing on distinct associations of SSRIs and SNRIs with several blood and CSF biomarkers of immune activation, inflammation, oxidative stress, and neurodegeneration in virally suppressed PWH. Contrary to our hypotheses, SSRIs and SNRIs exhibited divergent biomarker signatures despite sharing similar clinical indications and having some pharmacological overlap. Specifically, the results suggest that SSRIs may have anti-inflammatory and neuroprotective effects, while SNRIs may not. Statins and ACEIs were not associated any with biomarker pattern.
The association of SSRIs with higher levels of CSF and plasma Aβ42 and lower total tau and 8-oxo-dG in CSF suggests reduced neurodegeneration and oxidative stress within the central nervous system (CNS). This is consistent with previous evidence. In vitro, fluoxetine blocks the degeneration of nigral dopaminergic neurons after lipopolysaccharide-induced microglial activation, and this was accompanied by reduced reactive oxygen species generation [21]. Vortioxetine reduced neurodegeneration and improved motor and cognitive dysfunction in the rotenone-induced murine Parkinson disease model [22]. In line with this, SSRIs, mainly fluoxetine, could benefit the cognitive performance of patients with Alzheimer and vascular dementia according to a large meta-analysis that included 14 randomized controlled trials [23].
The interpretation of the association of SSRIs with higher levels of sCD14 (marker of myeloid activation) in blood but lower in CSF is not straightforward. One possibility is that, despite adjustment for current depressive symptoms, alterations in gut microbiome linked with the mood disorders for which SSRIs were prescribed may drive the association. A second possibility is that, by reducing the expression and responsiveness of TLR4 (sCD14 receptor) [24], but not acting on sCD14 production, SSRIs could inhibit the pathway of response to circulating lipopolysaccharide downstream from the intervention of sCD14, which in turn may increase in the attempt to overcome such a block. The opposite direction of the association in blood and CSF may depend on the difference in the sensitivity to medications of the cell sources that produce sCD14 in the 2 compartments (mainly monocytes in blood and microglia in the CNS) [25]. Future tailored studies should assess these interpretations.
Multiple reports have supported a role of CCL2 in neuropathology, including in PWH [26]. However, the median CSF level of CCL2 in participants on SSRIs (444 pg/mL; IQR 361–594 pg/mL) was from 1- to 2-fold lower than the concentrations measured in untreated CNS HIV infection, HIV-associated dementia, or HIV CNS chronic infection with evidence of neuronal injury, while similar to those reported in people without HIV [26–28]. Of note, CCL2 has a physiological role in the maintenance of brain homeostasis, and regulates interactions, migration, differentiation, and proliferation of glial cells, astrocytes, and neurons [29]. Therefore, the higher levels of CSF CCL2 (within the normal range) along with higher Aβ42 among participants on SSRIs may fit the overall favorable immunological profile associated with these medications, as described by the other biomarkers, and may suggest a physiologic interplay between this chemokine and amyloid metabolism.
The inflammatory landscape found in association with SNRIs did not overlap with the one featuring SSRIs. Prior studies have shown pleiotropic anti-inflammatory effects of SNRIs [2, 3]. A potential explanation of the differences between our findings and prior reports is the nature of the samples: we studied PWH, whereas prior investigations were in different patient groups, or in vitro studies and animal models [2, 3]. Specifically, we found that SNRI use correlated with higher levels of neopterin in CSF and plasma and higher CSF sTNFR-II. Neopterin is a marker of myeloid activation with prognostic and predictive value for HIV progression and comorbidities, including mood disorders and cognitive impairment [30, 31]. Also in our study, higher CSF and plasma neopterin was associated with worse GDS. Neopterin increases during CNS inflammation but also in disorders characterized by peripheral neuropathies (eg, diabetic polyneuropathy, HIV-related neuropathy) [32, 33]; only 2 of the 36 participants with diabetes were on SNRIs and none had diabetes-related complications; furthermore, adjusting for either dysesthesias or objective signs of neuropathy did not modified the association between SNRIs and PC1 of C4 (shown in Supplementary Table 2). On the contrary, prescription practices may have preferred SSRIs as first-line antidepressants, and participants on SNRIs may represent a subgroup of patients suffering from treatment-resistant depression. While neopterin is less reliably associated with depressive symptoms, for which we adjusted our analysis, it consistently increases in individuals with 2 or more episodes of major depression compared to milder forms [34]. Therefore, rather than being a consequence of SNRI use, the higher levels of neopterin in participants on SNRIs may be due to the biological correlates of more severe or treatment-resistant depression.
Whether the association of SNRIs and higher CSF sTNFR-II has negative implications is also uncertain. TNFR-I antagonists and TNFR-II agonists blocked neuroinflammation and promoted neuronal survival in a mouse model of neurodegeneration related to Alzheimer disease, and the simultaneous blockade of TNFR-II activation nullified the neuroprotective effects of TNFR-I antagonists [35]. The activation of transmembrane TNFR-II represents an important mechanism of neuronal survival following different noxious stimuli and provides additional benefits, such as regulatory T-cell function and oligodendrocyte-precursor cell proliferation, maturation, and remyelination [36]. On the other hand, increased CSF levels of sTNFR-II have been proposed as a marker of mild cognitive impairment and Alzheimer disease in people without HIV [37]. Because sTNFR-II competes with the transmembrane TNF receptor by binding circulating TNF-α and thereby inhibiting its action [38], the elevated sTNFR-II in our population may also be a compensatory protective response to ongoing inflammation as reflected in the concomitant elevated neopterin that clustered together in the same PC.
Statins and ACEIs were not consistently associated with any of the biomarkers measured in this study. The potential immunomodulating properties of ACEIs are less well understood. Most of the immune effects are dependent on angiotensin-II signal transduction, although ACE has also been involved and both pro- and anti-inflammatory responses of angiotensin-II receptor type 1 and 2 activation have been reported [39]. This complexity may explain ours and previous negative findings in PWH [15, 16], in whom heterogeneity in the renin-angiotensin-aldosterone system may also affect the effects on inflammation, among others.
Several studies have described the immunoproperties of statins, which act mainly by the inhibition of isoprenoid synthesis and of protein geranylgeranylation/farnesylation [7]. However, proinflammatory effects have also been described in vitro [6, 7], and consistent with our findings, a previous single-arm pilot study in PWH found that atorvastatin did not influence CNS inflammation as indexed by CSF white blood cell count and neopterin [40]. Due to differences in dosing, pharmacokinetics, and pharmacodynamics, some authors have suggested that each statin possesses distinct pleiotropic immune effects [6]. In line with this, variable immunological outcomes of statin use have been reported in PWH that differed by baseline cardiovascular and inflammatory characteristics [13, 14, 41]. Our sample does not have sufficient power to compare the distinct statins used (atorvastatin, pravastatin, or rosuvastatin), and we are unable to account for differences in cardiovascular risk prior to starting the drugs. Further studies are required to evaluate possible immunoproperties of statins in PWH.
In addition to the limitations discussed above, our study was cross-sectional and unable to prove causality, the dimension reduction approach may have attenuated associations with individual biomarkers, and the sample size was also limited, with few participants on SSRIs or SNRIs, limiting generalizability and the strength of our hypotheses. Lastly, clustering procedures are particularly sensitive to outliers, and the identification of the best number of clusters in hierarchical approaches is not straightforward. Further studies on different and larger population are required to assess the replicability of our clusters and PCs, as well as of their eventual associations. However, this is also one of the few studies reporting real-life data on the associations of inflammation, immune activation, and neurodegeneration with commonly prescribed comedications in a modern cohort of PWH on suppressive ART. Furthermore, bootstrapping retained significance for every medication and biomarker component, supporting the reliability and strength of our findings.
In conclusion, the 2 most commonly used classes of antidepressants, SSRIs and SNRIs, showed divergent associations with the blood and CSF immune milieu of PWH on suppressive ART. SSRI use was favorably characterized by reduced CSF inflammation, neuronal injury, and activation of myeloid and lymphoid cells, despite higher plasma activation of monocytes/macrophages. In line with previous evidence, these associations support an effect of SSRIs in reducing inflammation in PWH. On the contrary, SNRI use was associated with increased blood and CSF lymphoid and myeloid activation, although this relationship may reflect prescribing practices rather than medication effects. Future longitudinal studies able to stratify by single drugs and distinct subcategories of comorbidities (eg, mild vs severe depression) are warranted to further clarify causality and the long-term consequences of antidepressants upon the immune system of PWH.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Mattia Trunfio, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA; Department of Medical Sciences, University of Turin, Turin, Italy.
Bin Tang, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Jennifer E Iudicello, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Qing Ma, Department of Pharmacy Practice, School of Pharmacy and Pharmaceutical Sciences, University of Buffalo, Buffalo, New York, USA.
Donald R Franklin, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Debra Cookson, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Patricia K Riggs, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Mariana Cherner, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
David J Moore, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Robert K Heaton, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Scott L Letendre, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Ronald J Ellis, HIV Neurobehavioral Research Program, Departments of Neurosciences and Psychiatry, University of California San Diego,San Diego, California, USA.
Notes
Author contributions. M. T., B. T., and R. J. E. contributed study conception and design. D. R. F, D. C., and P. K. R. performed data collection. M. T., B. T., J. E. I, Q. M., P. K. R., M. C., D. J. M., R. K. H, S. L. L., and R. J. E. analyzed and interpreted results. M. T. and R. J. E. drafted the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Data availability. The data that support the findings of this study are available from R. J. E. upon reasonable request.
Financial support. This work was supported by the National Institutes of Health (grant number P30 MH062512 to the HIV Neurobehavioral Research Center and grant number R01 AG063659 to Q.M., S.L.L, and R.J.E.).
References
- 1. Kettelhut A, Bowman E, Funderburg NT. Immunomodulatory and anti-inflammatory strategies to reduce comorbidity risk in people with HIV. Curr HIV/AIDS Rep 2020; 17:394–404. [DOI] [PubMed] [Google Scholar]
- 2. Dionisie V, Filip GA, Manea MC, Manea M, Riga S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: a review of human and rodent research studies. Inflammopharmacology 2021; 29:75–90. [DOI] [PubMed] [Google Scholar]
- 3. Gałecki P, Mossakowska-Wójcik J, Talarowska M. The anti-inflammatory mechanism of antidepressants—sSRIs, SNRIs. Prog Neuropsychopharmacol Biol Psychiatry 2018; 80:291–4. [DOI] [PubMed] [Google Scholar]
- 4. Benton T, Lynch K, Dubé B, et al. Selective serotonin reuptake inhibitor suppression of HIV infectivity and replication. Psychosom Med 2010; 72:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greeson JM, Gettes DR, Spitsin S, et al. The selective serotonin reuptake inhibitor citalopram decreases HIV receptor and coreceptor expression in immune cells. Biol Psychiatry 2016; 80:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheridan A, Wheeler-Jones CPD, Gage MC. The immunomodulatory effects of statins on macrophages. Immuno 2022; 2:317–43. [Google Scholar]
- 7. Zeiser R. Immune modulatory effects of statins. Immunology 2018; 154:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elahi S, Weiss RH, Merani S. Atorvastatin restricts HIV replication in CD4+ T cells by upregulation of p21. AIDS 2016; 30:171–83. [DOI] [PubMed] [Google Scholar]
- 9. András IE, Rha G, Huang W, et al. Simvastatin protects against amyloid beta and HIV-1 Tat-induced promoter activities of inflammatory genes in brain endothelial cells. Mol Pharmacol 2008; 73:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yadav A, Betts MR, Collman RG. Statin modulation of monocyte phenotype and function: implications for HIV-1-associated neurocognitive disorders. J Neurovirol 2016; 22:584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Letendre SL, Marquie-Beck J, Ellis RJ, et al. The role of cohort studies in drug development: clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. J Neuroimmune Pharmacol 2007; 2:120–7. [DOI] [PubMed] [Google Scholar]
- 12. Bedimo RJ, Mar H, Bosch RJ, et al. Brief report: no evidence for an association between statin use and lower biomarkers of HIV persistence or immune activation/inflammation during effective ART. J Acquir Immune Defic Syndr 2019; 82:e27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hearps AC, Angelovich TA, Trevillyan JM, et al. Effect of rosuvastatin therapy on biomarkers of inflammation and immune activation in people with human immunodeficiency virus at intermediate cardiovascular risk. J Infect Dis 2021; 224:667–72. [DOI] [PubMed] [Google Scholar]
- 14. Yadav A, Kossenkov AV, Showe LC, et al. Lack of atorvastatin effect on monocyte gene expression and inflammatory markers in HIV-1-infected ART-suppressed individuals at risk of non-AIDS comorbidities. Pathog Immun 2021; 6:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erlandson KM, Kitch D, Wester CW, et al. The impact of statin and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy on cognitive function in adults with human immunodeficiency virus infection. Clin Infect Dis 2017; 65:2042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cockerham LR, Yukl SA, Harvill K, et al. A randomized controlled trial of lisinopril to decrease lymphoid fibrosis in antiretroviral-treated, HIV-infected individuals. Pathog Immun 2017; 2:310–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol 2012; 26:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hobkirk AL, Starosta AJ, De Leo JA, Marra CM, Heaton RK, Earleywine M. Psychometric validation of the BDI-II among HIV-positive CHARTER study participants. Psychol Assess 2015; 27:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caligaris G, Trunfio M, Ghisetti V, et al. Blood-brain barrier impairment in patients living with HIV: predictors and associated biomarkers. Diagnostics (Basel) 2021; 11:867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harvey DT, Hanson BA; Learn PCA . Understanding scores and loadings. https://bryanhanson.github.io/LearnPCA/articles/Vig_04_Scores_Loadings.html. Accessed 3 November 2023.
- 21. Chung ES, Chung YC, Bok E, et al. Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic neurons by inhibiting microglia-mediated oxidative stress. Brain Res 2010; 1363:143–50. [DOI] [PubMed] [Google Scholar]
- 22. Nemutlu Samur D, Akçay G, Yıldırım S, et al. Vortioxetine ameliorates motor and cognitive impairments in the rotenone-induced Parkinson's disease via targeting TLR-2 mediated neuroinflammation. Neuropharmacology 2022; 208:108977. [DOI] [PubMed] [Google Scholar]
- 23. Xie Y, Liu P-P, Lian Y-J, Liu H-B, Kang J-S. The effect of selective serotonin reuptake inhibitors on cognitive function in patients with Alzheimer's disease and vascular dementia: focusing on fluoxetine with long follow-up periods. Signal Transduct Target Ther 2019; 4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sales MC, Kasahara TM, Sacramento PM, et al. Selective serotonin reuptake inhibitor attenuates the hyperresponsiveness of TLR2+ and TLR4+ Th17/Tc17-like cells in multiple sclerosis patients with major depression. Immunology 2021; 162:290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarassishin L, Suh H-S, Lee SC. LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia 2014; 62:999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monteiro de Almeida S, Letendre S, Zimmerman J, Lazzaretto D, McCutchan A, Ellis R. Dynamics of monocyte chemoattractant protein type one (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. J Neuroimmunol 2005; 169:144–52. [DOI] [PubMed] [Google Scholar]
- 27. Gisslen M, Keating SM, Spudich S, et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. PLoS One 2021; 16:e0250987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santaella A, Kuiperij HB, van Rumund A, et al. Cerebrospinal fluid monocyte chemoattractant protein 1 correlates with progression of Parkinson's disease. NPJ Parkinsons Dis 2020; 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwon MJ, Shin HY, Cui Y, et al. CCL2 mediates neuron–macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J Neurosci 2015; 35:15934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barco A, Orlando S, Stroffolini G, et al. Correlations between cerebrospinal fluid biomarkers, neurocognitive tests, and resting-state electroencephalography (rsEEG) in patients with HIV-associated neurocognitive disorders. J Neurovirol 2022; 28:226–35. [DOI] [PubMed] [Google Scholar]
- 31. Keegan MR, Chittiprol S, Letendre SL, et al. Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. Int J Tryptophan Res 2016; 9:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elbarbary NS, Ismail EAR, El-Hilaly RA, Ahmed FS. Role of neopterin as a biochemical marker for peripheral neuropathy in pediatric patients with type 1 diabetes: relation to nerve conduction studies. Int Immunopharmacol 2018; 59:68–75. [DOI] [PubMed] [Google Scholar]
- 33. Anderson AM, Jang JH, Easley KA, et al. Cognitive and neuronal link with inflammation: a longitudinal study in people with and without HIV infection. J Acquir Immune Defic Syndr 2020; 85:617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saloner R, Savini N, Letendre SL, Moore DJ, Montoya JL. Neopterin relates to lifetime depression in older adults with HIV on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr 2022; 89:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dong Y, Fischer R, Naudé PJW, et al. Essential protective role of tumor necrosis factor receptor 2 in neurodegeneration. Proc Natl Acad Sci U S A 2016; 113:12304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 2015; 302:2–22. [DOI] [PubMed] [Google Scholar]
- 37. Jiang H, Hampel H, Prvulovic D, et al. Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer's disease. Mol Neurodegener 2011; 6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov 2010; 9:482–93. [DOI] [PubMed] [Google Scholar]
- 39. Oosthuizen D, Sturrock ED. Exploring the impact of ACE inhibition in immunity and disease. J Renin Angiotensin Aldosterone Syst 2022; 2022:9028969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Probasco JC, Spudich SS, Critchfield J, et al. Failure of atorvastatin to modulate CSF HIV-1 infection. Neurology 2008; 71:521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nixon DE, Bosch RJ, Chan ES, et al. Effects of atorvastatin on biomarkers of immune activation, inflammation, and lipids in virologically suppressed, human immunodeficiency virus-1-infected individuals with low-density lipoprotein cholesterol <130 mg/dL (AIDS Clinical Trials Group Study A5275). J Clin Lipidol 2017; 11:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.