Abstract
Kaposi sarcoma (KS) continues to cause substantial morbidity and mortality in populations at risk in the southern United States. Utilizing biospecimens from the Houston site of the Young Men's Affiliate Project, 351 men who have sex with men had blood tested for KS-associated herpesvirus (KSHV) IgG. Seroprevalence, seroconversion between time points, and demographic and clinical correlates were measured. KSHV prevalence was 36.7% and incidence was 8.9 per 100 person-years. Furthermore, prevalence and incidence were higher among Black individuals, people living with HIV, and those with a history of syphilis. Further research on KSHV risk may improve health disparities in KS diagnosis and outcomes.
Keywords: HIV, human herpesvirus 8, Kaposi sarcoma, racial disparities, seroprevalence
Kaposi sarcoma continues to cause substantial morbidity and mortality in at-risk populations in the southern United States. Among young minority men who have sex with men in Houston, Texas, the prevalence of Kaposi sarcoma–associated herpesvirus was 36.7% and the incidence was 8.9 per 100 person-years.
Kaposi sarcoma (KS) remains a significant cause of morbidity and mortality in patients with HIV [1]. Since the introduction of antiretroviral therapy, KS incidence has decreased in the United States; however, this reduction has not been uniform. In the southern United States, KS incidence has instead increased among Black men [2], as a group that also experiences higher KS-related mortality than other groups [3]. KS-associated herpesvirus (KSHV) is the etiologic agent of KS, and infection with KSHV is uncommon in the general US population, around 3.5% among blood donors [4]. Among men who have sex with men (MSM) with HIV, the prevalence of KHSV infection is much higher, ranging from 30% to 70% [5, 6]. Little is known about the incidence or drivers of KSHV infection among young MSM in the southern United States, information that is critical to future prevention efforts.
Existing data from past and recent studies provide some clues to the geographic and demographic differences in KSHV seroprevalence. In a study of >5000 people with HIV who were enrolled in longitudinal linked randomized trials between 1997 and 2007, KSHV seroprevalence was 38%. Seroprevalence was higher among males, White individuals, those aged 30 to 49 years, residents of the West or Northeast, and those with a higher baseline CD4 count. KSHV incidence in this cohort was 4.07 per 100 person-years [7], which contrasts the aforementioned KS incidence trends (increasing among Black men in the South) and may be due to the study population of the selected clinical trials, the era of study, or both. A recent cross-sectional study based in Dallas, Texas, an area with high rates of KS, found an KSHV seroprevalence of 68% among MSM and transgender women with HIV but no significant differences in seroprevalence by race/ethnicity [6]. Taken together, these data suggest that subgroups within certain parts of the country are at higher risk for KSHV acquisition and that these trends have changed over time. To better understand which groups are most affected, current longitudinal data from racially diverse populations at risk are needed to estimate prevalence, incidence, and risk factors for KSHV.
In this study, we aim to measure KSHV seroprevalence, KSHV incidence, and demographic and clinical variables associated with KSHV infection, utilizing data and samples from an existing cohort with high potential for exposure to KSHV. The National Institutes of Health–funded Young Men's Affiliate Project (YMAP), which enrolled young urban MSM via a 2–time point study design, provides a unique opportunity to examine KSHV prevalence and incidence in a population at high risk for HIV and sexually transmitted infections.
METHODS
This study is a secondary analysis of an existing data set from the YMAP study [8]. The original YMAP participants were 755 MSM aged 16 to 29 years old living in Houston or Chicago who were enrolled between 2014 and 2016. Participants were recruited from the community by respondent-driven sampling (RDS) [9]. Inclusion criteria were male sex assigned at birth and current male identification, engagement in oral or anal sex with another man in the prior year, residence in and plans to remain in Houston or Chicago for the following year, and English-speaking ability. All participants signed informed consent, and all 3 participating institutions received approval from the institutional review boards (HSCSPH120830).
Surveys from Houston-based participants and blood samples for HIV and syphilis testing were collected from individuals at 2 time points (12–18 months apart). Survey data included sociodemographic characteristics, sexual behavior (number of sex partners), substance use (self-reported frequency of cannabis, methamphetamines, cocaine, inhalants, sedatives, hallucinogens, opioids, pain killers, and ecstasy), depression score based on the Brief Symptom Inventory–18, protective behaviors (preexposure prophylaxis [PrEP] use), social and sexual networks, and attendance at social and health-promoting venues.
Participants were tested with an antigen/antibody test (Alere Determine HIV-1/2 Combo), and those with reactive samples were referred to a clinic for care, with confirmation by HIV-1/HIV-2 multispot differentiation and HIV RNA (viral load) tests. Lifetime and active syphilis serology was assessed via a rapid plasma reagin test at both time points, followed by a confirmatory fluorescent treponemal antibody absorption test. Additional stored blood samples were tested for KSHV serology.
KSHV prevalence was defined as the proportion of individuals with a positive KSHV serology result in their baseline sample, defined as anti-KSHV IgG detection in plasma by enzyme-linked immunosorbent assay with K8.1 and ORF73 or by a bead-based multiplex antibody assay. This assay is sensitive and specific, and it can measure multiple KSHV antibodies in a single assay in a flexible format. This study included the same 2 KSHV antigens on which the enzyme-linked immunosorbent assay is based, and samples were considered positive if K8.1 or ORF73 was positive. KSHV incidence was defined as a positive result for KSHV IgG at time point 2 following a negative KSHV IgG result at time point 1.
KSHV prevalence was calculated for the cohort overall and per 100 persons and was stratified by race (non-Black vs Black), age group (16 to 22, 23–26, 27–29 years), baseline HIV status (negative or positive), and baseline syphilis status (negative or positive). KSHV incidence was calculated with 2 methods. First, incidence between time points 1 and 2 was calculated per 100 persons who were seronegative. Second, incidence rate was calculated per 100 person-years and stratified by race, age group, HIV status, and syphilis status, similar to prevalence. These estimates were RDS adjusted through Gile's sequential sampling estimators (with 95% CIs), which are weighted inversely by self-reported peer network size (degree) such that observations with lower reported degrees are weighted higher than observations with higher reported degrees [10]. We used the RDS program in the R version 4.2.0 statistical environment [11]. Univariate and multivariate logistic regression modeling was performed to identify independent risk factors for prevalent KSHV. Stepwise selection was used with a P value <.15 to enter and remain in the model.
RESULTS
The cohort consisted of 351 individuals who had blood tested for KSHV at baseline, from which 244 received follow-up blood work at their second visit. Overall, 206 (58.7%) identified as non-Hispanic Black, 69 (19.7%) as Hispanic, 55 (15.7%) as non-Hispanic White, and 21 (6.0%) as other. The average age was 24.7 years, with 95 (27.1%) younger than 23, 127 (36.2%) between 23 and 26, and 129 (36.8%) aged >26 to 29. At baseline, 221 (63%) were HIV negative and 130 (37%) were HIV positive. Approximately one-third (n = 112, 32%) tested positive for syphilis at baseline; 57 (16.2%) had ever taken PrEP; 203 (57.8%) used cannabis at least once in the past 3 months; and 12 (3.4%) had used methamphetamines in the past 3 months. Other demographic and baseline values for the cohort are shown in Supplementary Table 1.
KSHV prevalence was 132 (37.6%) and was higher among Black individuals, those with HIV at baseline, and those with positive syphilis test results at baseline, though prevalence did not differ by age group. In univariate analyses, Black race, older age (23–26 and >26–29 vs <23), HIV positivity, a positive syphilis test result, methamphetamine use, and having never taken PrEP were associated with KSHV prevalence. In adjusted multivariate analyses, the 23- to 26-year age group (odds ratio [OR], 1.91; 95% CI, 1.00–3.65), HIV-positive status (OR, 4.11; 95% CI, 2.37–7.13), and syphilis-positive status (OR, 2.80; 95% CI, 1.62–4.83) were independently associated with prevalent KSHV infection (Table 1).
Table 1.
Univariate and Multivariate-Adjusted Regression Analysis of KSHV Prevalence for Demographic, Clinical, and Social Variables
| Univariate/Unadjusted | Multivariate/Adjusted | ||||
|---|---|---|---|---|---|
| Variable | OR (95% CI) | P Value | P Value (LRT) | OR (95% CI) | P Value |
| Race: subgroups | <.001 | … | … | ||
| Hispanic | 1 [Reference] | … | … | ||
| Non-Hispanic White | 0.63 (.26–1.50) | .298 | … | … | |
| Non-Hispanic Black | 2.78 (1.52–5.08) | <.001 | … | … | |
| Non-Hispanic other | 0.30 (.06–1.41) | .127 | … | … | |
| Race: binary | <.001 | … | … | ||
| Non-Black | 1 [Reference] | 1 [Reference] | … | ||
| Black | 3.76 (2.31–6.11) | <.001 | 1.62 (.91–2.88) | .100 | |
| Age, y | .041 | … | … | ||
| 16 to 22 | 1 [Reference] | 1 [Reference] | … | ||
| 23–26 | 2.05 (1.16–3.61) | .013 | 1.91 (1.00–3.65) | .049 | |
| 27–29 | 1.49 (.84–2.64) | .169 | 1.43 (.74–2.75) | .289 | |
| Depression score | 1.00 (.96–1.04) | .871 | .871 | … | … |
| HIV status | <.001 | … | … | ||
| Negative | 1 [Reference] | 1 [Reference] | … | ||
| Positive | 6.99 (4.31–11.35) | <.001 | 4.11 (2.37–7.13) | <.001 | |
| FTA statusa | <.001 | … | … | ||
| Negative | 1 [Reference] | 1 [Reference] | … | ||
| Positive | 5.05 (3.12–8.18) | <.001 | 2.80 (1.62–4.83) | <.001 | |
| No. of sex partners (SQRT) | 0.96 (.82–1.14) | .658 | .655 | … | … |
| PrEP ever takenb | .003 | … | … | ||
| No | 1 [Reference] | … | … | ||
| Yes | 0.39 (.20–.76) | .006 | … | … | |
| Cannabis use: past 3 mo | .640 | … | … | ||
| Never | 1 [Reference] | … | … | ||
| Once or twice | 1.30 (.66–2.57) | .454 | … | … | |
| Monthly | 1.35 (.60–3.02) | .469 | … | … | |
| Weekly | 1.64 (.79–3.38) | .184 | … | … | |
| Daily | 1.37 (.80–2.37) | .254 | … | … | |
| Any cannabis use: past 3 mo | .136 | … | … | ||
| Never | 1 [Reference] | … | … | ||
| At least once | 1.40 (.90–2.18) | .138 | … | … | |
| Any methamphetamine use: past 3 moc | .038 | … | … | ||
| Never | 1 [Reference] | … | … | ||
| At least once | 3.47 (1.02–11.75) | .046 | … | … | |
| Noncannabis substance use: past 3 mod | .169 | … | … | ||
| No | 1 [Reference] | … | … | ||
| Yes | 0.73 (.47–1.14) | .169 | … | … | |
| No. of noncannabis substances used: past 3 mod | 0.96 (.86–1.08) | .505 | .503 | … | … |
Abbreviations: FTA, fluorescent treponemal antibody; KSHV, Kaposi sarcoma–associated herpesvirus; LRT, likelihood ratio test; OR, odds ratio; PrEP, preexposure prophylaxis; SQRT, square root.
aAbsorption test to determine syphilis status.
bMedication to reduce risk of HIV acquisition.
cMethamphetamine use has a limited sample size, with only 12 individuals indicating that they used it at least once in the past 3 months.
dCocaine, methamphetamine, inhalants, sedatives, hallucinogens, opioids, pain killers, ecstasy.
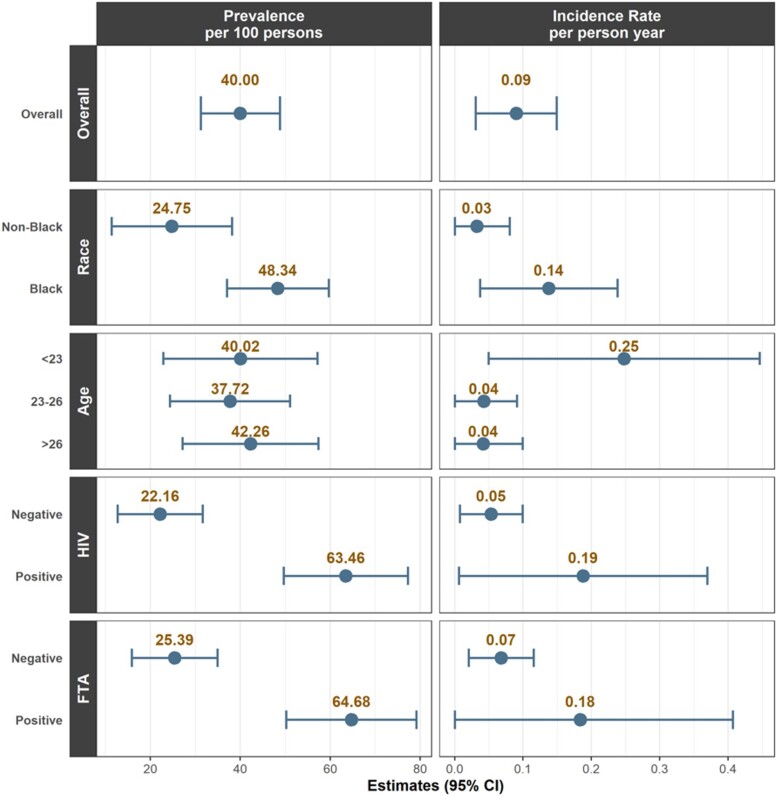
The incidence rate of KSHV was 8.86 per 100 person-years (16 seroconversions out of 155 participants), with measurements taken approximately 12 to 18 months apart (range, 10.0-28.5; median, 11.5). In the overall cohort, incidence rates were high among Black individuals, persons with HIV, and those with syphilis, though none of these differences were statistically significant. Incidence of KSHV was too low to conduct regression analyses. Population-level KSHV prevalence and incidence, overall and by subgroup, are summarized in Figure 1.
Figure 1.
Population estimates of Kaposi sarcoma–associated herpesvirus prevalence and incidence by overall cohort and subgroup (race, age, HIV status, syphilis). Prevalence estimates are calculated per 100 persons and incidence rate estimates by person-year. Error bars indicate 95% CIs. Syphilis was determined by fluorescent treponemal antibody (FTA) absorption test.
DISCUSSION
This is the first study to our knowledge to measure KSHV prevalence and incidence in a longitudinal cohort of young minority MSM in the southern United States. We identified high prevalence and incidence of KSHV, especially in specific subgroups. KSHV seroprevalence was twice as high in Black individuals as other groups (48.3% vs 24.8%) and nearly 3 times higher in MSM with HIV as compared with those without HIV (63.5% vs 22.2%). Furthermore, incident KSHV infection was 4 times higher among Black participants than non-Black participants (13.4 vs 3.2 per 100 person-years), which parallels the regional trends in KS incidence [2] and HIV, as the highest burden of new HIV diagnoses is among Black MSM in the South [12]. KSHV incidence in this study, 9 per 100 person-years, is more than twice that identified in a nationally representative study of people with HIV (4.07 per 100 person-years) [7] and a study of MSM in Seattle (3.8 per 100 person-years) [13], which may reflect geographic, sociodemographic, and temporal changes in KSHV epidemiology. Similar drivers of disparities in incident HIV infections may be involved in incident KSHV and likely involve a complex interplay of behavioral, microbiological, and socioeconomic factors, though more studies are needed.
A recent cross-sectional Dallas-based study among MSM and transgender women, all with HIV and within a broader age range, showed a similarly high prevalence of KSHV/HIV coinfection (68% vs our 63.5%), but KSHV seroprevalence did not differ significantly by racial/ethnic group [6]. The higher proportion of KSHV seropositivity in young Black MSM in the current study, which includes people with and without HIV, is more aligned with national trends in KS incidence.
Additional important findings include associations with KSHV prevalence and a positive syphilis test result, recent methamphetamine use (in preceding 3 months), and an inverse association with PrEP use. Syphilis infection could be associated with KSHV either as a biologic risk, given the mucosal ulceration caused by syphilis and the probable transmission of KSHV through oral fluids, or as a proxy for behavioral risk (ie, unprotected sex including oral fluids), which could predispose to KSHV transmission. Other studies have not found clear epidemiologic associations between syphilis and KSHV, though data are relatively limited [14]. The association between methamphetamine use and KSHV has been noted [6] and could relate to sexual disinhibition associated with methamphetamine use (chemsex) [15] and/or the impact of methamphetamines on oral homeostasis and KSHV shedding, though this has not been studied directly. The inverse association between PrEP use and KSHV seroprevalence could be confounded by HIV status in that those who were not undergoing PrEP acquired HIV and KSHV; no studies to date have explored the impact of PrEP on KSHV incidence or prevalence.
Our study has several limitations. First, the data are from a single study site and therefore may not pertain to other settings, though they may be applicable to parts of the country with populations at high risk of HIV and KSHV infections. Second, given the relatively short follow-up (12–18 months), there were few incident cases, limiting our ability to conduct multivariate analyses for this outcome. Also, we were unable to determine active viral shedding, as we did not have oral fluid samples and therefore could not determine transmissibility or KSHV molecular epidemiology in this cohort. Types of inhalants, such as poppers (which have been associated with KSHV), were not specifically assessed. Last, we were unable to assess KSHV-specific factors, such as whether participants were from KS-endemic areas or had preexisting or subsequent KSHV-related diseases, as this was not the focus of the parent study.
We have identified a high prevalence and incidence of KSHV infection in young MSM residing in Houston, the most populous metropolitan city in the US South, especially among Black individuals, people living with HIV, and those with a history of syphilis. Given the increasing rate of KS among Black men in the southern United States, despite a steady decline in other populations and locations, it is critical to better understand how the risk factors for KSHV acquisition affect health disparities in cancer diagnosis and outcomes. Future longitudinal studies should explore multilevel drivers of KSHV acquisition and transmission, including associations with individual-level risk behaviors, such as concurrent sexually transmitted infections, substance use, and factors affecting oral microbiome and immunity, as well as associations with community-level factors, including connections within social and sexual networks.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Maverick Salyards, Institute of Behavioral Research, Texas Christian University, Fort Worth.
Ank E Nijhawan, Division of Infectious Diseases and Geographic Medicine, Department of Internal Medicine, University of Texas Southwestern; Parkland Health, Dallas.
Jacky Kuo, Center for Health Promotion and Prevention Research, School of Public Health, The University of Texas Health Science Center at Houston.
Sheena M Knights, Division of Infectious Diseases and Geographic Medicine, Department of Internal Medicine, University of Texas Southwestern; Parkland Health, Dallas.
Susana Lazarte, Division of Infectious Diseases and Geographic Medicine, Department of Internal Medicine, University of Texas Southwestern; Parkland Health, Dallas.
Nazzarena Labo, Viral Oncology Section, AIDS and Cancer Virus Program, Leidos Biomedical Inc, Frederick National Laboratory for Cancer Research, Maryland.
Wendell Miley, Viral Oncology Section, AIDS and Cancer Virus Program, Leidos Biomedical Inc, Frederick National Laboratory for Cancer Research, Maryland.
Denise Whitby, Viral Oncology Section, AIDS and Cancer Virus Program, Leidos Biomedical Inc, Frederick National Laboratory for Cancer Research, Maryland.
Lu-Yu Hwang, Center for Infectious Diseases, School of Public Health, The University of Texas Health Science Center at Houston.
Anna-William Kornberg, Division of Cancer Prevention, Department of Epidemiology, University of Texas MD Anderson Cancer Center, Houston.
Kayo Fujimoto, Center for Health Promotion and Prevention Research, School of Public Health, The University of Texas Health Science Center at Houston.
Elizabeth Y Chiao, Division of Cancer Prevention, Department of Epidemiology, University of Texas MD Anderson Cancer Center, Houston.
Notes
Author contributions. M. S.: manuscript preparation. J. K., L.-Y. H., K. F., E. Y. C.: study design, recruitment, data analysis, manuscript preparation. A. E. N., S. M. K., S. L., N. L., D. W., A.-W. K.: data analysis, manuscript preparation. W. M.: laboratory procedures, manuscript preparation.
Financial support. This research extends from a parent study that was supported by the National Institute of Mental Health (1R01MH100021). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health. Additional funds came from the Frederick National Laboratory for Cancer Research, under contract HHSN261201500003I; National Cancer Institute contract 75N91019D00024 to D. W.; Sally W Vernon, PhD, Distinguished Professorship in Social Determinants of Health; UTHealth-MD Anderson Cancer Center Population Health Initiative Collaborative Project Award.
References
- 1. Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017; 4:e495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. White DL, Oluyomi A, Royse K, et al. Incidence of AIDS-related Kaposi sarcoma in all 50 United States from 2000 to 2014. J Acquir Immune Defic Syndr 2019; 81:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Royse KE, El Chaer F, Amirian ES, et al. Disparities in Kaposi sarcoma incidence and survival in the United States: 2000–2013. PLoS One 2017; 12:e0182750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pellett PE, Wright DJ, Engels EA, et al. Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion 2003; 43:1260–8. [DOI] [PubMed] [Google Scholar]
- 5. Liu Z, Fang Q, Zuo J, et al. Global epidemiology of human herpesvirus 8 in men who have sex with men: a systematic review and meta-analysis. J Med Virol 2018; 90:582–91. [DOI] [PubMed] [Google Scholar]
- 6. Knights S, Salyards M, Kendall N, et al. 827. High KSHV seroprevalence among MSM with HIV associated with oral intercourse and methamphetamine use in the southern United States. Open Forum Infect Dis 2021; 8(suppl 1):506. [Google Scholar]
- 7. Labo N, Miley W, Benson CA, Campbell TB, Whitby D. Epidemiology of Kaposi's sarcoma–associated herpesvirus in HIV-1–infected US persons in the era of combination antiretroviral therapy. AIDS 2015; 29:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujimoto K, Cao M, Kuhns LM, Li D, Schneider JA. Statistical adjustment of network degree in respondent-driven sampling estimators: venue attendance as a proxy for network size among young MSM. Soc Networks 2018; 54:118–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl 1997; 44:174–99. [Google Scholar]
- 10. Gile KJ. Improved inference for respondent-driven sampling data with application to HIV prevalence estimation. J Am Stat Assoc 2011; 106:135–46. [Google Scholar]
- 11. Handcock M, Fellows I, Gile K. RDS: respondent-driven sampling. R package version 0.7-6. 2016. https://CRAN.R-project.org/package=RDS. Accessed February 2023.
- 12. Centers for Disease Control and Prevention . HIV surveillance report, 2020; vol 33. May 2022. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed February 2023.
- 13. Casper C, Wald A, Pauk J, Tabet SR, Corey L, Celum CL. Correlates of prevalent and incident Kaposi's sarcoma–associated herpesvirus infection in men who have sex with men. J Infect Dis 2002; 185:990–3. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Liu S, Cao Y, et al. Prevalence of Kaposi's sarcoma associated herpesvirus among men attending sexually transmitted infections clinics in Anhui, China. J Med Virol 2016; 88:304–11. [DOI] [PubMed] [Google Scholar]
- 15. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy 2019; 63:74–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.