Abstract
Introduction
Microvascular invasion (MVI) is one of the most important prognostic factors for hepatocellular carcinoma (HCC) recurrence, but its application in preoperative clinical decisions is limited. This study aimed to identify preoperative predictive factors for MVI in HCC and further evaluate oncologic outcomes of different types and extents of hepatectomy according to stratified risk of MVI.
Methods
Patients with surgically resected single HCC (≤5 cm) who underwent preoperative gadoxetic acid-enhanced magnetic resonance imaging (MRI) were included in a single-center retrospective study. Two radiologists reviewed the images with no clinical, pathological, or prognostic information. Significant predictive factors for MVI were identified using logistic regression analysis against pathologic MVI and used to stratify patients. In the subgroup analysis, long-term outcomes of the stratified patients were analyzed using the Kaplan-Meier method with log-rank test and compared between anatomical and nonanatomical or major and minor resection.
Results
A total of 408 patients, 318 men and 90 women, with a mean age of 56.7 years were included. Elevated levels of tumor markers (alpha-fetoprotein [α-FP] ≥25 ng/mL and PIVKA-II ≥40 mAU/mL) and three MRI features (tumor size ≥3 cm, non-smooth tumor margin, and arterial peritumoral enhancement) were independent predictive factors for MVI. As the MVI risk increased from low (no predictive factor) and intermediate (1–2 factors) to high-risk (3–4 factors), recurrence-free and overall survival of each group significantly decreased (p = 0.001). In the high MVI risk group, 5-year cumulative recurrence rate was significantly lower in patients who underwent major compared to minor hepatectomy (26.6 vs. 59.8%, p = 0.027).
Conclusion
Tumor markers and MRI features can predict the risk of MVI and prognosis after hepatectomy. Patients with high MVI risk had the worst prognosis among the three groups, and major hepatectomy improved long-term outcomes in these high-risk patients.
Keywords: Hepatocellular carcinoma, Microscopic vascular invasion, Magnetic resonance imaging, Hepatectomy, Recurrence
Introduction
Hepatectomy is the standard treatment for single hepatocellular carcinoma (HCC) with well-preserved liver function; however, high postoperative recurrence rate is the main obstacle to cure [1, 2]. HCC is a hypervascular tumor that invades the vessels around the tumor, even in the early stage, and spreads further into the liver and distant organs via the portal and systemic circulation [3]. Microscopic vascular invasion (MVI) is the main route of intrahepatic metastasis and is associated with a higher risk of tumor recurrence after hepatectomy [4–7].
Given the mechanisms of HCC spread, anatomical resection (AR), the complete removal of the tumor-bearing portal territory, has been recommended [8]. However, previous studies have reported conflicting results regarding the superiority of AR over nonanatomical resection (NAR) for HCC [9–12]. Therefore, instead of the uniform application of AR, surgical approaches such as type or extent of resection should be determined individually based on specific tumor biological features and patient’s liver function.
Although MVI is the main indicator of local spread and aggressive tumor behavior, it has limited application in preoperative clinical decisions, as it is an indicator confirmed by postoperative microscopic examination of the surgical specimens. Several studies have attempted to identify preoperative predictive factors for MVI using tumor markers [13–15] or preoperative imaging findings, including computed tomography (CT) [16], contrast-enhanced magnetic resonance imaging (MRI) [13, 17–19], and 18F-fluorodeoxyglucose positron emission tomography [20, 21]. MRI features have been widely validated, and several imaging features such as tumor margins, arterial-phase peritumoral enhancement, and low signal intensity (SI) on the hepatobiliary phase (HBP) have demonstrated significant correlation with MVI [13, 17, 22]. Preoperative prediction of MVI can guide the optimal selection of initial treatment in patients with small (≤3 cm) HCC who are candidates for hepatectomy or radiofrequency ablation [5]. However, no study has investigated the surgical extent of hepatectomy based on the preoperative risk of MVI in patients with resectable HCC.
We aimed to develop a predictive model for MVI using preoperative serum markers and imaging features of gadoxetic acid-enhanced MRI in patients who underwent hepatectomy for single HCC (≤5 cm) and to compare long-term outcomes between different types or extents of hepatectomy in patients stratified according to the risk of MVI.
Materials and Methods
Study Population
This retrospective study was reviewed and approved by the Yonsei University Institutional Review Board, which waived the requirement for informed consent (No. 4-2021-0173). A flow diagram of patient selection is shown in Figure 1. A total of 608 patients who underwent curative resection for suspected HCC between January 2008 and December 2018 were retrospectively recruited. The following inclusion criteria were used: (1) Child-Pugh class A patients with treatment-naïve suspected HCC; (2) solitary tumor ≤5 cm; (3) preoperative gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced MRI (EOB-MRI) performed within 1 month of the surgery date. As a result, 200 patients were excluded for the following reasons: (1) HCC size larger than 5 cm (n = 94); (2) HCC not feasible for minor resection (n = 41), such as located near the major portal vein, hepatic vein, or centrally (hilum), which was decided by a single surgeon with 17 years of experience; (3) recurrent tumor (n = 26); (4) multiple HCCs (n = 17); (5) inadequate pathologic report, missing important information on factors such as MVI (n = 7); (6) final pathologic diagnosis of cholangiocarcinoma (n = 6); (7) poor image quality, which means that the radiologist could not determine radiologic factors on multiple phases (n = 5); (8) preoperative MRI with extracellular contrast agent (n = 2); and (9) gross vascular thrombosis on MRI (n = 2). A total of 408 patients (318 men and 90 women; mean age ± standard deviation, 56.7 ± 9.3 years) were included in the final study population.
Fig. 1.
A flow diagram of patient selection.
Histopathological Diagnosis
The following pathologic information was acquired from the pathologic reports and evaluated: histologic tumor grade; presence of cirrhosis on background liver tissue, microscopic and macroscopic vascular invasion, bile duct invasion, and satellite nodules; and resection margins (cm). According to the Edmondson-Steiner criteria, histologic tumor grade was categorized into two groups: I–II and III–IV. MVI was defined as microvessel (a newly developed microvascular structure in the tumor capsule or compressed and fibrotic peritumoral nonneoplastic liver excluding the portal or hepatic veins, or the hepatic artery), portal vein, hepatic vein, or hepatic artery invasion visible only on microscopic examination [23]. Satellite nodules were defined as microscopic nodules within 2 cm of the main tumor [24].
Preoperative Clinical Factors and Surgical Procedures
Preoperative laboratory examinations and operative information were retrospectively obtained. From the electronic medical records, demographic and medical information including age, sex, etiology of liver disease (hepatitis B virus, hepatitis C virus, non-B, non-C hepatitis), serum levels of albumin, platelet count, serum total bilirubin, serum alanine transaminase, serum aspartate transaminase, alpha-fetoprotein (α-FP), and protein induced by vitamin K absence-II (PIVKA-II) were obtained.
Surgical type was divided into AR and NAR, or the extent of hepatectomy (minor or major). AR involved hepatectomy of at least one liver segment defined by Couinaud’s classification [25]. NAR was defined as hepatectomy regardless of Couinaud’s classification. Depending on the extent of the operation, minor hepatectomy included two or fewer segments and major hepatectomy included three or more segments [26, 27]. The types of surgical procedures are summarized in Table 1.
Table 1.
Type of surgical procedure
| Surgical type (N = 408) | n (%) |
|---|---|
| Anatomical resection | 306 (75.0) |
| Right hepatectomy | |
| Conventional (n = 50, 12.3%) | 72 (17.7) |
| Ventral segment-preserving (n = 22, 5.4%) | |
| Right anterior sectionectomy | 20 (4.9) |
| Right posterior sectionectomy | 35 (8.6) |
| Central bisectionectomy | 20 (4.9) |
| Left hepatectomy | 30 (7.4) |
| Left lateral sectionectomy | 36 (8.8) |
| Left medial sectionectomy | 2 (0.5) |
| Segmentectomy | 92 (22.5) |
| Nonanatomical resection | 102 (25.0) |
| Extent of hepatectomy | |
| Major hepatectomy (>2 segments) | 122 (29.9) |
| Minor hepatectomy (≤2 segments) | 286 (70.1) |
Image Acquisition
Liver dynamic MRI scans were obtained using 3.0-T MR scanners (Magnetom Trio Tim System, Siemens Healthcare; Ingenia or Intera Achieva, Philips Healthcare; Discovery MR 750, GE Medical Systems). Routinely performed MR sequences at our institution were as follows: dual gradient-echo in-phase and out-of-phase T1-weighted images; navigator-triggered single-shot and multishot T2-weighted images; diffusion-weighted imaging with b values of 0, 50, 400, and 800 s/mm2; ADC map; and dynamic fat-suppressed spoiled gradient-echo T1-weighted images performed before and after intravenous injection of 10 mL of gadoxetic acid (Primovist, Bayer Schering Pharma) at a fixed rate of 1 mL/s, followed by a 20 mL saline bolus injection. The test bolus or bolus-tracking technique was used to determine the scan timing of the arterial-phase images. Additionally, portal venous phase, late portal venous phase, transitional phase, and HBP images were acquired at 60, 90, and 150 s as well as 15 or 20 min after administration of the contrast agent, respectively. Detailed MRI parameters are specified in online supplementary Table 1 (for all online suppl. material, see https://doi.org/10.1159/000531786).
Image Analysis
Two board-certified abdominal radiologists with 4 and 23 years of experience in hepatic imaging independently reviewed the preoperative dynamic liver MRI of the included patients. For each HCC, both reviewers who were unaware of the pathologic results and follow-up outcomes evaluated the following imaging features for MVI [13, 17, 22, 28]: (1) tumor margins, classified as smooth (defined as nodular tumors with smooth contours) or non-smooth margins (defined as non-nodular tumors with irregular contours in all imaging sequences), (2) presence or absence of arterial-phase peritumoral enhancement (defined as detectable portion of parenchymal enhancement adjacent to the tumor margin in the arterial phase, which became isointense when compared with background liver parenchyma in the delayed phase), (3) HBP SI, categorized as low, iso/high, or heterogeneous SI compared with background liver parenchyma, (4) presence or absence of peritumoral low SI on HBP (defined as hypointense area of the liver parenchyma adjacent to the tumor margin on HBP images), (5) presence or absence of satellite nodules (defined as small nodules located less than 2 cm from the primary tumor), and (6) presence or absence of LR-M features defined by the Liver Imaging Reporting and Data System (LI-RADS) version 2018 [19]. The location (liver segment) and maximal diameter of the tumor measured on the axial HBP images were also recorded. In this study, for consistency, we selected the axial HBP images to measure tumor size. In case of disagreement between the two reviewers, a consensus was reached through discussion.
Postoperative Follow-Up
All patients were postoperatively evaluated for serum tumor markers (α-FP and PIVKA-II) and underwent dynamic CT scans. The postoperative follow-up was performed every 3 months for the first year, every 3–6 months for the second year, and every 6 months from the third year. When a possibility of recurrence was observed on CT scan, MRI was performed. If the HCC recurred, the treatment for the recurred lesion, such as transarterial chemoembolization, radiofrequency, repeated resection, or liver transplantation, was determined by a multidisciplinary team.
Statistical Analysis
Continuous variables are expressed as either mean ± standard deviation or median (range), as appropriate. Categorical variables are expressed as numbers and percentages. Parameters were compared between subgroups using a two-sided t test or analysis of variance test for parametric variables and Mann-Whitney U test or Welch’s test for nonparametric variables. Categorical data were evaluated using Fisher’s exact test or χ2 test. Logistic regression analysis was performed to assess the clinical and MRI features for predicting MVI. For tumor markers such as α-FP and PIVKA-II, the optimal cut-off value chosen to predict MVI was determined using the maximum Youden index from a receiver operating characteristic (ROC) curve analysis. A combination of α-FP and PIVKA-II was used for analysis as it was shown to be more closely associated with recurrence and MVI of HCC after hepatectomy than each tumor marker individually [29]. For multivariate logistic regression analysis, variables with p < 0.05 in the univariate logistic regression analysis were selected. In multivariate logistic regression analysis, variables with p < 0.05 were selected as predictive factors for MVI. ROC curve analysis was performed to determine the number of factors predicting MVI. The area under the ROC curve (AUC), sensitivity, and specificity were also calculated.
Overall survival (OS) and recurrence-free survival (RFS) were estimated by Kaplan-Meier method using stratified Cox proportional hazards regression analyses and compared using log-rank test for patients classified by the predicted risk of MVI. To investigate the effect of the surgical procedure on HCC recurrence according to MVI risk, cumulative recurrence rate was estimated and compared using Kaplan-Meier method and log-rank test according to surgical type: AR versus NAR and minor versus major hepatectomy, respectively.
The intraclass correlation coefficient (ICC) using the two-way random-effects model was used to analyze the interobserver reproducibility of tumor size: ICC ≤0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, almost perfect reliability [30]. The interobserver reproducibility of tumor margin, arterial-phase peritumoral enhancement, peritumoral low SI on HBP, and LR-M features was evaluated using Cohen’s κ coefficient: κ <0.01, poor; 0.01–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, almost perfect agreement [30].
Statistical analyses were performed using the R software (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria) and SPSS for Windows v.26.0 (IBM Corp.; Armonk, NY, USA). A p value <0.05 was considered statistically significant. The Bonferroni correction was applied to adjust p values for multiple comparisons.
Results
The baseline clinicopathological characteristics of the patients are summarized in Table 2. In the histopathologic report, macroscopic vascular invasion was confirmed in four (1.0%) patients and microscopic vascular invasion in 176 (43.1%) patients. The mean age of patients was 56.7 ± 9.3 years, and median follow-up duration was 69.3 (0.8–154.8) months.
Table 2.
Clinicopathologic and EOB-MR imaging characteristics of the study patients (n = 408)
| Variables | |
|---|---|
| Age, years | 56.7±9.3 |
| Gender (male/female) | 318 (77.9)/90 (22.1) |
| BMI, kg/m2 | 24.1±2.9 |
| Follow-up duration, median, months | 69.3 (0.8–154.83) |
| Etiology (HBV/HCV/NBNC) | 281 (68.9)/14 (3.4)/113 (27.7) |
| Liver cirrhosis (absent/present) | 216 (52.9)/192 (47.1) |
| Preoperative serum α-FP, ng/mL | 14.9 (1.0–38978.8) |
| Preoperative serum PIVKA-II, mAU/mL | 350.6±47.0 |
| Histologic features | |
| Edmondson-Steiner grade (I–II/III–IV) | 187 (45.8)/220 (53.9)/1 (N/A) |
| Macroscopic vascular invasion (absent/present) | 404 (99.0)/4 (1.0) |
| Microscopic vascular invasion (absent/present) | 232 (56.9)/176 (43.1) |
| Microscopic bile duct invasion (absent/present) | 404 (99.0)/4 (1.0) |
| Satellite nodule (absent/present) | 394 (96.6)/14 (3.4) |
| EOB-MR imaging features | |
| Tumor size, mean, cm | 2.77±0.95 (0.9–5.0) |
| Tumor margin (smooth/non-smooth) | 171 (41.9)/237 (58.1) |
| Arterial peritumoral enhancementa (absent/present) | 270 (66.0)/126 (30.9) |
| LR-M featuresb (absent/present) | 378 (92.6)/30 (7.4) |
| Peritumoral HBP low SI (absent/present) | 332 (81.4)/76 (18.6) |
| HBP SI (Low/Iso or high/heterogeneous) | 349 (85.5)/16 (3.9)/43 (10.5) |
| Satellite nodule (absent/present) | 380 (93.1)/28 (6.9) |
Values are mean±SD, median (range) or frequency (%). BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B, non-C hepatitis; α-FP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; HBP, hepatobiliary phase; SI, signal intensity.
aArterial peritumoral enhancement of 12 patients was not evaluable due to the poor quality of arterial-phase images.
bLR-M features were defined by the Liver Imaging Reporting and Data System version 2018.
According to the EOB-MRI image analysis, among 408 HCCs, tumor margin was smooth in 171 (41.9%) and non-smooth in 237 (58.1%) patients. Arterial peritumoral enhancement features were identified in 126 (30.9%), presence of any one of the LR-M features in 30 (7.4%), peritumoral HBP low SI in 76 (18.6%), and satellite nodules in 28 (6.9%). Low HBP SI was confirmed in 349 (85.5%), iso/high HBP SI in 16 (3.9%), and heterogeneous HBP SI in 43 (10.5%).
Measurement of tumor size showed almost perfect interobserver agreement (ICC, 0.963; 95% confidence interval [CI], 0.947–0.974). Interobserver agreement of imaging features for MVI ranged from moderate to substantial agreement between the two radiologists (κ for LR-M features, 0.474; tumor margin, 0.760; arterial-phase peritumoral enhancement, 0.782; peritumoral HBP low SI; 0.689).
Predictive Factors for Microscopic Vascular Invasion
The optimal cut-off values for α-FP and PIVKA-II determined by ROC analysis for predicting MVI were 25 ng/mL and 40 mAU/mL, respectively. In univariate logistic regression analysis, among preoperative clinical features, α-FP ≥25 ng/mL and PIVKA-II ≥40 mAU/mL, and among EOB-MR imaging features, tumor size ≥3 cm, non-smooth tumor margin, LR-M feature, arterial peritumoral enhancement, and peritumoral HBP low SI were statistically significant predictive factors for MVI. In the multivariate logistic regression, independent predictive factors for MVI included (1) α-FP ≥25 ng/mL and PIVKA-II ≥40 mAU/mL (odds ratio [OR]: 2.267; 95% CI: 1.384–3.712; p = 0.001); (2) tumor size ≥3 cm (OR: 2.314; 95% CI: 1.481–3.615; p < 0.001); (3) non-smooth tumor margin (OR: 2.430; 95% CI: 1.536–3.845; p < 0.001); (4) arterial peritumoral enhancement (OR: 2.134; 95% CI: 1.336–3.408; p = 0.002; Table 3).
Table 3.
Univariate and multivariate logistic regression analysis of preoperative clinical and EOB-MR imaging features for microvascular invasion in study patients
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age, years | 1.020 (0.998–1.042) | 0.069 | ||
| Gender [female, ref.] | 1.134 (0.708–1.816) | 0.600 | ||
| Etiology | ||||
| NBNC | 1 (reference) | |||
| HBV | 0.689 (0.444–1.069) | 0.096 | ||
| HCV | 2.636 (0.781–8.901) | 0.118 | ||
| Liver cirrhosis | 1.214 (0.819–1.799) | 0.334 | ||
| Serum albumin, g/dL | 0.764 (0.439–1.332) | 0.343 | ||
| Serum total bilirubin, mg/dL | 1.543 (0.749–3.179) | 0.239 | ||
| Serum platelets, 10^3/μL | 1.000 (1.000–1.000) | 0.588 | ||
| Serum ALT, IU/L | 0.996 (0.987–1.005) | 0.349 | ||
| Serum AST (IU/L) | 0.995 (0.995–1.007) | 0.380 | ||
| α-FP ≥25 ng/mL and PIVKA-II ≥40 ng/mL | 2.522 (1.601–3.974) | <0.001* | 2.267 (1.384–3.712) | 0.001* |
| EOB-MR imaging feature | ||||
| Tumor size ≥3 cm and ≤5 cm | 2.253 (1.496–3.391) | <0.001* | 2.314 (1.481–3.615) | <0.001* |
| Non-smooth tumor margin [smooth, ref.] | 3.009 (1.977–4.580) | <0.001* | 2.430 (1.536–3.845) | <0.001* |
| LR-M featuresa | 2.846 (1.297–6.248) | 0.009* | ||
| Arterial peritumoral enhancement | 2.621 (1.699–4.045) | <0.001* | 2.134 (1.336–3.408) | 0.002* |
| Peritumoral HBP low SI | 2.929 (1.743–4.924) | <0.001* | ||
| Iso/High HBP SI [low SI, ref.] | 0.408 (0.129–1.289) | 0.127 | ||
| Satellite nodule | 1.570 (0.727–3.390) | 0.251 | ||
OR, odds ratio; CI, confidence interval; NBNC, non-B, non-C hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; ALT, serum alanine transaminase; AST, serum aspartate transaminase; α-FP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; HBP, hepatobiliary phase; SI, signal intensity.
*Statistically significant results from logistic regression analysis. Variables with p < 0.05 in the univariate logistic regression analysis were subjected to multivariate logistic regression analysis.
aLR-M features were defined by the Liver Imaging Reporting and Data System version 2018.
The risk of MVI stratified by the number of predictive factors is shown in Figure 2a, b. As the number of predictive factors increased, the risk of MVI also increased; specifically, microvessels and microscopic portal veins increased (p < 0.001). The ROC analysis to predict the presence of MVI with the four predictive factors is presented in Figure 2c. The AUC, sensitivity, and specificity were 0.706 (95% CI: 0.655–0.757), 64.2%, and 66.4%, respectively.
Fig. 2.
a, b Predicted risk of microscopic vascular invasion and (c) ROC according to the number of predictive factors for MVI. As the number of predictive factors increased, the risk of MVI increased, specifically, microvessel and microscopic portal vein invasion increased (p < 0.001). The AUC, sensitivity, and specificity were 0.706 (95% confidence interval: 0.655–0.757), 64.2%, and 66.4%, respectively.
Long-Term Outcomes and Patient Characteristics according to MVI Risk
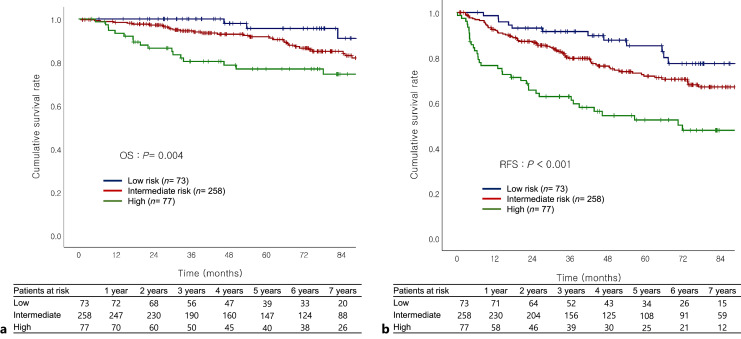
Patients were classified into three different risk groups: patients with zero predictive factors (n = 73, low-risk group), 1–2 factors (n = 258, intermediate-risk group), and 3 or more factors (n = 77, high-risk group). Clinical characteristics and surgical data of the low-, intermediate-, and high-risk groups of patients are summarized (see online suppl. Table 1). PIVKA-II was highest in high-risk patients. The surgical margin and preoperative laboratory results were similar among the three groups. AR was performed more frequently in high-risk patients, but no significant difference was observed in the rate of major hepatectomy between the groups. When the patients were stratified according to MVI risk, both OS and RFS gradually worsened as the MVI risk increased and was the worst in high-risk group (OS: p = 0.004; RFS: p < 0.001; Fig. 3a, b).
Fig. 3.
a, b Overall survival and recurrence-free survival depending on MVI risk OS and RFS gradually got worse as the MVI risk increased and were worst in the high-risk group (OS: p = 0.004; RFS: p < 0.001.
Recurrence and Long-Term Outcomes according to the Surgical Procedure for Each MVI Risk Group
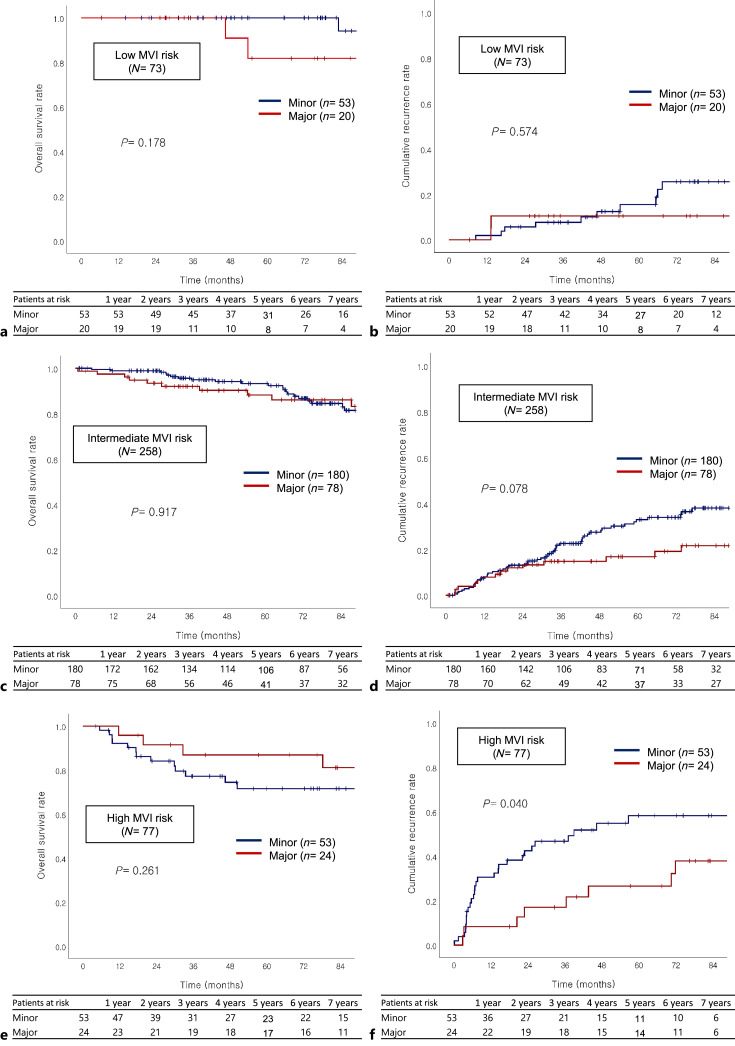
Subgroup analysis was performed to investigate recurrence according to major and minor hepatectomy in each risk group. Major versus minor hepatectomy was performed in 20 (27.4%) versus 53 (72.6%) patients in the low-risk group, 78 (30.2%) versus 180 (69.8%) in the intermediate-risk group, and 24 (31.2%) versus 53 (68.8%) in the high-risk group. In low-risk group, cumulative recurrence rates were not significantly different between major and minor hepatectomy (10.5% vs. 5.7% at 2 years, 10.5% vs. 15.7% at 5 years, p = 0.574; Fig. 4b). In intermediate risk group, cumulative recurrence rate was similar between major and minor hepatectomy, especially until 2 years (12.0% vs. 13.2% at 2 years, 16.9% vs. 33.1% at 5 years, p = 0.078; Fig. 4d). In contrast, in high-risk group, cumulative recurrence rate was significantly lower in patients with major hepatectomy than that in patients with minor hepatectomy (17.1% vs. 42.4% at 2 years, 26.6% vs. 58.3% at 5 years, p = 0.040; hazard ratio [HR]: 0.445; 95% CI: 0.201–0.983, p = 0.045; Fig. 4f).
Fig. 4.
Overall survival and cumulative recurrence rate according to extent of hepatectomy on low (a, b), intermediate (c, d), and high MVI risk group (e, f), respectively. Cumulative recurrence rate was similar between major versus minor hepatectomy in low (10.5% vs. 15.7% in 5-year, p = 0.574) and intermediate (16.9% vs. 33.1% in 5-year, p = 0.078) groups, and significantly worse in the high-risk group (26.6% vs. 59.8%, p = 0.040) by the Kaplan-Meier method and log-rank test.
In the low- and intermediate-risk groups, OS of patients who underwent minor and major hepatectomy did not show a statistically significant difference (low: p = 0.178; intermediate: p = 0.917; Fig. 4a, c). In the high MVI risk group, OS of patients with minor and major hepatectomy was not statistically different; however, OS of major hepatectomy was numerically higher than that of minor hepatectomy (91.5% vs. 84.1% at 2 years, 86.9% vs. 71.6% at 5 years, p = 0.261; Fig. 4e).
For the low-, intermediate-, and high-risk groups, AR was performed in 50 (68.5%), 180 (69.8%), and 68 (86.1%) patients, respectively. Regarding the type of surgical resection, OS and RFS of AR and NAR were not significantly different among the three risk groups (low, OS, p = 0.716; RFS, p = 0.450; intermediate, OS, p = 0.513; RFS, p = 0.261; high, OS, p = 0.705; RFS, p = 0.834).
Discussion
The present study demonstrated that elevated tumor marker levels (α-FP ≥25 ng/mL and PIVKA-II ≥40 mAU/mL) and three EOB-MRI features (tumor size ≥3 cm, non-smooth tumor margin, and arterial peritumoral enhancement) were independent predictive factors of MVI in patients with single HCC ≤5 cm. As the MVI risk increased from low (no predictive factor) and intermediate (1–2 factors) to high risk (3–4 factors), the RFS and OS of each group gradually decreased. The high-risk group had significantly poorer RFS and OS than the low- and intermediate-risk groups, respectively. Additionally, major hepatectomy resulted in a significantly lower cumulative recurrence rate than minor resection in the high-risk group.
Preoperative prediction of MVI has been an active research topic in recent years because it is one of the most reliable indicators of biological aggressiveness of HCC. Several studies have revealed that preoperative tumor markers and specific findings in preoperative imaging studies are associated with the presence of MVI in HCCs [13–17, 19–22]. In accordance with these studies, elevated tumor marker levels (α-FP ≥25 ng/mL and PIVKA-II ≥40 mAU/mL), tumor size ≥3 cm, non-smooth tumor margin, and arterial peritumoral enhancement were found to be independent predictive factors for the presence of MVI in our study. Tumor marker levels and tumor size are well-proven factors for tumor invasiveness and poor prognosis. α-FP and PIVKA-II are known to be complementary markers for HCC [31]. Furthermore, our previous study reported that combined assessment of α-FP and PIVKA-II was more strongly associated with MVI and prognosis after surgical resection than individual tumor markers [29]. Non-smooth tumor margins might represent non-boundary gross growth patterns, including single nodular with extranodular growth type, multinodular confluent type, and infiltrative type. Previous studies have reported that these gross patterns showed a higher risk of MVI, more stemness features, and poorer prognosis than single nodular growth type [32–34]. Arterial peritumoral enhancement on contrast-enhanced MRI has been reported to be a predictive factor for MVI in previous studies [17, 18, 35], which may be attributed to arterial hyperperfusion in compensation for decreased or absent portal flow in the area of microvascular invasion (MVI) [36].
Our MVI prediction model using four predictive factors for HCC less than 5 cm showed moderate accuracy with an AUC value of 0.706, sensitivity 64.2%, and specificity 66.4% [37]. In a previous study, the MVI prediction model using four predictive factors (α-FP and PIVKA-II, arterial peritumoral enhancement, and peritumoral HBP low SI) showed an AUC value for 0.87 for small single HCC of less than 3 cm [5]. In our model, these four predictive factors could stratify the predicted risk of MVI from 17.8% in patients with zero factors to 88.9% in those with four. As the MVI risk increased from low to intermediate to high, the RFS and OS of each group gradually decreased, in agreement with poor prognostic effect of long-term outcomes of MVI. In addition, high-risk group had a poorer prognosis than the intermediate group due to the higher predicted proportion of microscopic portal vein invasion, which had a worse prognosis than microvessel invasion in patients who underwent surgical resection or liver transplantation for HCC in our previous study [23].
Development of an appropriate method for preoperative prediction of MVI should be clinically incorporated into the proper selection of treatment modality and optimal selection of the type and extent of hepatectomy in patients with HCC. Imai et al. [15] found that α-FP ≥15 ng/mL, PIVKA-II ≥100 mAU/mL, and tumor size ≥2 cm were associated with the presence of MVI in patients with single small HCC (≤3 cm). In patients with 2 or 3 predictive factors, radiofrequency ablation resulted in poorer OS and higher local recurrence than in patients with zero or 1 predictive factor. Bai also reported that hepatectomy demonstrated decreased recurrence and improved OS compared to radiofrequency ablation in Milan criteria HCC patients with a predicted high risk of MVI [7, 38]. Lee et al. [5] developed an MVI risk score to accurately predict MVI using tumor markers and imaging features of EOB-MRI in patients with a single, small (≤3 cm) HCC, and demonstrated that surgical resection provided a significantly lower early recurrence rate than radiofrequency ablation in patients at high risk of MVI. These results may be because wider resection or AR can remove the main and microsatellite tumors more radically than radiofrequency ablation in HCC with MVI. Therefore, HCC with high MVI risk through a preoperative prediction system should receive surgical resection rather than radiofrequency ablation as the initial treatment, even if the tumor size is 3 cm or less.
Traditionally, AR has been recommended for HCC [8]. Two meta-analyses demonstrated that AR seems to provide better long-term outcomes than NAR in patients who underwent curative resection for HCC [9, 10]. However, several studies have not shown any superiority of AR in terms of long-term oncologic outcomes [11, 12]. These contradictory results may be mainly due to heterogeneity in the tumor stage. The reported detection rate of MVI ranged from 23.3 to 74.4% in the pathological examinations [4]. Several recent studies have demonstrated that AR is associated with reduced postoperative recurrence and prolonged OS in HCC with MVI [34, 39, 40]. Yamamoto et al. [41] demonstrated that sectionectomy or larger resection was suitable for patients with MVI, and Wong et al. [42] suggested that major hepatectomy is required to improve long-term survival in HCC (<5 cm) with MVI. Therefore, AR or major hepatectomy seems to have oncologic benefits only in patients with MVI.
To the best of our knowledge, this study is the first to demonstrate that different types and extents of hepatectomy should be selected according to the preoperatively predicted risk of MVI. In patients without predictive factors, AR or major hepatectomy did not provide any advantages over NAR or minor hepatectomy. However, major hepatectomy resulted in a significantly lower cumulative recurrence rate than minor hepatectomy in patients with high MVI risk. In our previous report, HCC vascular invasion progressed from peritumoral microvessel invasion to microscopic portal vein invasion located further away from the original tumor [23]. Due to a higher proportion (22.0–27.8%) of microscopic portal vein invasion in these patients, major hepatectomy rather than minor hepatectomy seemed to radically remove the main tumor and micrometastases confined to the same liver.
This study had several limitations. First, it only included patients with single and small HCC (≤5 cm). The poor prognosis of large and multiple HCCs after surgical treatment is related to tumor characteristics rather than the type and extent of surgical resection [12, 43]. Therefore, to investigate the necessity of a tailored surgical approach according to the risk of MVI, we confined the study population to a single HCC ≤5 cm in diameter. Second, the tumor location can also affect the extent of hepatectomy. In our study, despite the possibility of selection bias, 41 patients were excluded because minor hepatectomy was not feasible due to either the location in the deep parenchyma or proximity to the hilar vascular structure. Third, major hepatectomy is not recommended in patients with poor liver function or small future remnant volume. Our study only included Child-Pugh A patients, and ventral segment-preserving right hepatectomy (n = 22) and central bisectionectomy (n = 20) were performed in patients with small future remnant volumes instead of right hepatectomy and extended right hepatectomy, respectively [8, 44, 45]. Lastly, this is a single-center, retrospective study that lacks external validation. An internal validation done by splitting our cohort into training and validation datasets could be another option for a more reliable prediction system of MVI in HCC. However, several studies have demonstrated the correlation between specific MRI features and tumor markers with the presence of MVI [17, 22, 28], which were also validated in our study. Our primary emphasis was on investigating the effects of various types and extent of hepatectomy on oncologic outcomes, considering the preoperative risk of MVI. After consulting with a statistician, it was determined that internal validation would not be performed due to the difficulty in achieving significant validation with our data and the potential reduction in sample size. However, future studies should adopt a multicenter approach and validate our findings using external cohorts to ensure the generalizability and reliability of our results.
In conclusion, increased serum levels of α-FP and PIVKA-II, tumor size ≥3 cm, non-smooth tumor margins, and arterial peritumoral enhancement were significantly correlated with the risk of MVI in this study. As the MVI risk increased from low (0 predictive factors), intermediate (1–2 factors), to high (3–4 factors), RFS and OS of each group gradually decreased. In addition, major hepatectomy provided a significantly lower cumulative recurrence rate than minor resection in patients with 3 or more predictive factors for MVI.
Statement of Ethics
This study protocol was reviewed and approved by the Yonsei University Institutional Review Board, approval No. 4-2021-0173. The Yonsei University Institutional Review Board waived the requirement for informed consent (approval No. 4-2021-0173).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding resources to declare.
Author Contributions
Study design: N.R.K., B.J. H.S.H., D.H.H., and J.S.C. Data acquisition: N.R.K., H.S.H., B.J., D.H.H., K.S.K., J.S.C., G.H.C., and M.-S.P. Statistical analysis: N.R.K., B.J., and H.S.H. Interpreted the data: N.R.K., D.H.H., K.S.K., J.S.C., and G.H.C. Drafting and revising the manuscript: N.R.K., B.J., C.H.C., and M.S.P.
Funding Statement
The authors have no funding resources to declare.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
References
- 1. Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Korean Liver Cancer Association KLCA and National Cancer Center (NCC) Korea . 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28(4):583–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44(Suppl 19):112–8. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–39. [DOI] [PubMed] [Google Scholar]
- 5. Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2021;273(3):564–71. [DOI] [PubMed] [Google Scholar]
- 6. Zhong X-P, Zhang Y-F, Mei J, Li S-H, Kan A, Lu L-H, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma with microscope vascular invasion: a propensity score matching analysis. J Cancer. 2019;10(17):3950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bai S, Yang P, Xie Z, Li J, Lei Z, Xia Y, et al. Preoperative estimated risk of microvascular invasion is associated with prognostic differences following liver resection versus radiofrequency ablation for early hepatitis B virus-related hepatocellular carcinoma. Ann Surg Oncol. 2021;28(13):8174–85. [DOI] [PubMed] [Google Scholar]
- 8. Shindoh J, Makuuchi M, Matsuyama Y, Mise Y, Arita J, Sakamoto Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64(3):594–600. [DOI] [PubMed] [Google Scholar]
- 9. Jiao S, Li G, Zhang D, Xu Y, Liu J, Li G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surg. 2020;80:243–55. [DOI] [PubMed] [Google Scholar]
- 10. Moris D, Tsilimigras DI, Kostakis ID, Ntanasis-Stathopoulos I, Shah KN, Felekouras E, et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(7):927–38. [DOI] [PubMed] [Google Scholar]
- 11. Marubashi S, Gotoh K, Akita H, Takahashi H, Ito Y, Yano M, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015;102(7):776–84. [DOI] [PubMed] [Google Scholar]
- 12. Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143(4):469–75. [DOI] [PubMed] [Google Scholar]
- 13. Cho ES, Choi JY. MRI features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol. 2015;16(3):449–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawasaki Y, Yang SJ, Choi GH, Han DH, Lee JH, Iino S, et al. New scoring system for resectable hepatocellular carcinoma with a maximum tumor size of ≤5 cm based on preoperative tumor factors. HPB. 2019;21(10):1393–9. [DOI] [PubMed] [Google Scholar]
- 15. Imai K, Yamashita YI, Yusa T, Nakao Y, Itoyama R, Nakagawa S, et al. Microvascular invasion in small-sized hepatocellular carcinoma: significance for outcomes following hepatectomy and radiofrequency ablation. Anticancer Res. 2018;38(2):1053–60. [DOI] [PubMed] [Google Scholar]
- 16. Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62(3):792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67(3):526–34. [DOI] [PubMed] [Google Scholar]
- 18. Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, et al. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19(7):1744–51. [DOI] [PubMed] [Google Scholar]
- 19. Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, et al. Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, et al. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015;40(4):843–51. [DOI] [PubMed] [Google Scholar]
- 21. Kornberg A, Freesmeyer M, Barthel E, Jandt K, Katenkamp K, Steenbeck J, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transpl. 2009;9(3):592–600. [DOI] [PubMed] [Google Scholar]
- 22. Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B. Microvascular invasion in hepatocellular carcinoma: is it predictable with pretransplant MRI? AJR Am J Roentgenol. 2011;196(5):1083–9. [DOI] [PubMed] [Google Scholar]
- 23. Kang I, Jang M, Lee JG, Han DH, Joo DJ, Kim KS, et al. Subclassification of microscopic vascular invasion in hepatocellular carcinoma. Ann Surg. 2021;274(6):e1170–8. [DOI] [PubMed] [Google Scholar]
- 24. Jang JY, Lee JS, Kim H-J, Shim J-J, Kim JH, Kim BH, et al. The general rules for the study of primary liver cancer. J Liver Cancer. 2017;17(1):19–44. [Google Scholar]
- 25. Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB. 2013;15(1):31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kokudo T, Hasegawa K, Shirata C, Tanimoto M, Ishizawa T, Kaneko J, et al. Assessment of preoperative liver function for surgical decision making in patients with hepatocellular carcinoma. Liver Cancer. 2019;8(6):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giordano M, Lopez-Ben S, Codina-Barreras A, Pardina B, Falgueras L, Torres-Bahi S, et al. Extra-Glissonian approach in liver resection. HPB. 2010;12(2):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim KA, Kim M-J, Jeon HM, Kim KS, Choi J-S, Ahn SH, et al. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35(3):629–34. [DOI] [PubMed] [Google Scholar]
- 29. Chon YE, Choi GH, Lee MH, Kim SU, Kim DY, Ahn SH, et al. Combined measurement of preoperative α-fetoprotein and des-γ-carboxy prothrombin predicts recurrence after curative resection in patients with hepatitis-B-related hepatocellular carcinoma. Int J Cancer. 2012;131(10):2332–41. [DOI] [PubMed] [Google Scholar]
- 30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 31. Aoyagi Y, Oguro M, Yanagi M, Mita Y, Suda T, Suzuki Y, et al. Clinical significance of simultaneous determinations of alpha-fetoprotein and des-gamma-carboxy prothrombin in monitoring recurrence in patients with hepatocellular carcinoma. Cancer. 1996;77(9):1781–6. [DOI] [PubMed] [Google Scholar]
- 32. Rhee H, Chung T, Yoo JE, Nahm JH, Woo HY, Choi GH, et al. Gross type of hepatocellular carcinoma reflects the tumor hypoxia, fibrosis, and stemness-related marker expression. Hepatol Int. 2020;14(2):239–48. [DOI] [PubMed] [Google Scholar]
- 33. Murakata A, Tanaka S, Mogushi K, Yasen M, Noguchi N, Irie T, et al. Gene expression signature of the gross morphology in hepatocellular carcinoma. Ann Surg. 2011;253(1):94–100. [DOI] [PubMed] [Google Scholar]
- 34. Zhao H, Chen C, Gu S, Yan X, Jia W, Mao L, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: a propensity score matching analysis. J Gastroenterol Hepatol. 2017;32(4):870–8. [DOI] [PubMed] [Google Scholar]
- 35. Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, et al. Can current preoperative imaging Be used to detect microvascular invasion of hepatocellular carcinoma? Radiology. 2016;279(2):432–42. [DOI] [PubMed] [Google Scholar]
- 36. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273(1):30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–93. [DOI] [PubMed] [Google Scholar]
- 38. Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. 2016;151(4):356–63. [DOI] [PubMed] [Google Scholar]
- 39. Zhang XP, Wang K, Wei XB, Li LQ, Sun HC, Wen TF, et al. An eastern hepatobiliary surgery hospital microvascular invasion scoring system in predicting prognosis of patients with hepatocellular carcinoma and microvascular invasion after R0 liver resection: a large-scale, multicenter study. Oncologist. 2019;24(12):e1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsumoto T, Kubota K, Aoki T, Iso Y, Kato M, Shimoda M. Clinical impact of anatomical liver resection for hepatocellular carcinoma with pathologically proven portal vein invasion. World J Surg. 2016;40(2):402–11. [DOI] [PubMed] [Google Scholar]
- 41. Yamamoto M, Ariizumi S, Katagiri S, Kotera Y, Takahashi Y. The value of anatomical liver sectionectomy for patients with a solitary hepatocellular carcinoma from 2 to 5 cm in greatest diameter. J Surg Oncol. 2009;100(7):585–8. [DOI] [PubMed] [Google Scholar]
- 42. Wong TC, Cheung TT, Chok KS, Chan AC, Dai WC, Chan SC, et al. Treatment strategy to improve long-term survival for hepatocellular carcinoma smaller than 5 cm: major hepatectomy vs minor hepatectomy. World J Surg. 2014;38(9):2386–94. [DOI] [PubMed] [Google Scholar]
- 43. Han DH, Choi GH, Park JY, Ahn SH, Kim KS, Choi JS, et al. Lesson from 610 liver resections of hepatocellular carcinoma in a single center over 10 years. World J Surg Oncol. 2014;12:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xiao Y, Li W, Wan H, Tan Y, Wu H. Central hepatectomy versus major hepatectomy for patients with centrally located hepatocellular carcinoma: a meta-analysis. Int J Surg. 2018;52:297–302. [DOI] [PubMed] [Google Scholar]
- 45. Orimo T, Kamiyama T, Kakisaka T, Shimada S, Nagatsu A, Asahi Y, et al. Central hepatectomy versus major hepatectomy for centrally located hepatocellular carcinoma: a propensity score matching study. Ann Surg Oncol. 2021;28(11):6769–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.