Abstract
Introduction
Lenvatinib is indicated for the forefront treatment of advanced hepatocellular carcinoma (aHCC), but its use may be limited by the risk of esophagogastric varices (EGV) bleeding. This study assessed the prevalence, predictors, and complications of EGV in aHCC patients treated with lenvatinib.
Methods
In this multicenter international retrospective study, cirrhotic patients treated with lenvatinib for aHCC, were enrolled if upper-gastrointestinal endoscopy was available within 6 months before treatment. Primary endpoint was the incidence of EGV bleeding during lenvatinib therapy; secondary endpoints were predictors for EGV bleeding, prevalence, and risk factors for the presence of EGV and high-risk EGV at baseline, as well as impact of EGV bleeding on patients’ survival.
Results
535 patients were enrolled in the study (median age: 72 years, 78% male, 63% viral etiology, 89% Child-Pugh A, 16% neoplastic portal vein thrombosis [nPVT], 56% Barcelona Clinic Liver Cancer-C): 234 had EGV (44%), 70 (30%) were at high risk and 59 were on primary prophylaxis. During lenvatinib treatment, 17 patients bled from EGV (3 grade 5), the 12-month cumulative incidence being 3%. The only baseline independent predictor of EGV bleeding was the presence of baseline high-risk EGV (hazard ratio: 6.94, 95% confidence interval [CI]: 2.23–21.57, p = 0.001). In these patients the 12-month risk was 17%. High-risk varices were independently associated with Child-Pugh B score (odds ratio [OR]: 2.12; 95% CI: 1.08–4.17, p = 0.03), nPVT (OR: 2.54; 95% CI: 1.40–4.61, p = 0.002), and platelets <150,000/μL (OR: 2.47; 95% CI: 1.35–4.50, p = 0.003).
Conclusion
In hepatocellular carcinoma patients treated with lenvatinib, the risk of EGV bleeding was mostly low but significant only in patients with high-risk EGV at baseline.
Keywords: Cirrhosis, Portal hypertension, Varices, Neoplastic portal vein thrombosis, Bevacizumab
Introduction
Systemic treatment for hepatocellular carcinoma (HCC) includes different drugs as first-line options, such as sorafenib, lenvatinib, atezolizumab combined with bevacizumab, durvalumab combined with tremelimumab, according to chronological order of approval [1–4]. Selection of treatment is based on both effectiveness and safety considerations. As far as effectiveness, both combinations of atezolizumab with bevacizumab and durvalumab with tremelimumab resulted in superiority to sorafenib, while no direct comparisons are available for lenvatinib, which resulted in non-inferiority to sorafenib, as well as durvalumab monotherapy. Moreover, safety aspects are generally taken into consideration when treatment options are evaluated, particularly in patients with HCC who generally have underling cirrhosis which represents a competitive risk factor for lower tolerability and death [5]. Cirrhosis might affect the treatment decision process not only for concerns about liver function but also for clinically significative portal hypertension, since it might limit survival, increase the risk of decompensation and bleeding from esophageal/gastric varices (EGV) [5]. According to phase 3 randomized control trials, grade 3 or 4 (G3/G4) EGV bleeding events occurred in 2% of patients treated with sorafenib, while EGV bleeding events occurred in 2.4% of patients treated with the combination atezolizumab plus bevacizumab (1.8% G3/G4) versus 1.9% (0.6%) in sorafenib-treated patients, while row data on lenvatinib reported a 1.47% in the registration trial [1–3, 6]. As far as real-life studies, EV bleeding occurred in 12 (8%) patients after 36 (18–260) days of treatment with sorafenib, and a 4.4% of G3/G4 EGV bleeding for atezolizumab plus bevacizumab in a multicenter retrospective study [7, 8].
Data on EGV prevalence, EGV bleeding episodes in patients with HCC treated with lenvatinib are lacking even in clinical practice studies. The little available clinical data on lenvatinib suggest that it could aggravate portal hypertension [9]. The absence of data, as well as considering that lenvatinib might be an alternative in patients excluded from the combination of atezolizumab plus bevacizumab and taking into consideration the risk of bleeding of the latter, led us to investigate the prevalence, risk factors, and clinical consequences of EGV and occurrence of EGV bleeding in patients undergoing lenvatinib treatment for HCC.
Patients and Methods
For the scope of this study, we only included patients with upper-gastrointestinal endoscopy (UGE) performed within 6 months prior to start of therapy among patients included in an ongoing multicenter, investigator-driven, retrospective study to assess the safety and effectiveness of first-line systemic treatment in patients with HCC; part of the results has been previously published [10, 11]. Patients treated with lenvatinib as first-line therapy for advanced-stage HCC (Barcelona Clinic Liver Cancer [BCLC]-C) or early/intermediate HCC (BCLC-A and -B) who were deemed ineligible for first or re-treatment with surgical or locoregional therapies were included in the study population. Between November 2014 and April 2022, the overall cohort included Western and Eastern populations from 12 centers in three countries (Italy, Korea, and Japan), with data for analysis collected retrospectively. In accordance with local regulations, a written informed consent was obtained from participants to participate in the study, before data collection. Eligible patients had to have their HCC diagnoses confirmed histologically or clinically according to international guidelines and had not previously received systemic therapy. Primary endpoint of the study was the assessment of the incidence rate of EGV bleeding during lenvatinib treatment; secondary endpoints were risk factors of EGV bleeding, prevalence and risk factors for the presence of EGV and high-risk EGV at baseline, impact of EGV bleeding, and liver decompensation on patients’ survival and systemic treatment management.
Treatments and Definitions
All patients were treated with lenvatinib as first-line treatment. Lenvatinib was administered as described in the REFLECT trial (12 mg if baseline bodyweight was ≥60 kg or 8 mg if baseline bodyweight was <60 kg, given once daily orally) [3].
HCC was staged according to BCLC staging system, and cirrhosis was graded according to the Child-Pugh classification [12, 13]. Neoplastic portal vein thrombosis (nPVT) was defined at baseline CT scan or MRI as a filling defect, partially or completely occluding the vessel in the portal venous phase, with clear evidence of enhancement during the arterial phase of dynamic imaging. Portal vein thrombosis without this clear-cut evidence was considered as nonneoplastic.
Treatment interruptions and dose reductions were managed according to local policies and physicians’ judgment. Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0. Patients were commonly controlled every 2–3 months with triphasic scanning technique. Tumor assessment was maintained until radiological disease progression even after treatment was discontinued. Progression was diagnosed according to either mRECIST or RECIST 1.1 criteria according to local policies. In patients who were still alive at the radiological assessment, the adoption of any subsequent anticancer medication depended on physician’s decision.
Esophago/Gastric Varices
All patients underwent UGE within 6 months prior to lenvatinib treatment. EGVs were classified according to the North Italian Endoscopic Club criteria: size and location (F, graded 0–3), and red wale marks [14]. According to Baveno VI, small varices with red wale marks, medium/large esophageal varices, and gastric varices are considered at increased risk of bleeding and were retrospectively classified accordingly [15]. The management of patients with clinically significant portal hypertension was in accordance with national and international guidelines but ultimately based on the judgment of treating physicians.
Bleeding from EGV was defined according to the Baveno International Consensus Criteria and treated accordingly [16]. Before and during lenvatinib treatment, primary prophylaxis was managed according to international guidelines and local policies. Additional UGE surveillance during treatment with lenvatinib was scheduled according to local policies, unless clinically required.
Statistical Analysis
Categorical variables were reported as the number of cases and percentage and compared using χ2 or Fisher’s exact test when appropriate. Continuous variables were expressed as median and interquartile range (IQR) and compared using Kruskall-Wallis one-way analysis of variance. Prevalence of EGV, high-risk EGV, and variceal bleeding were expressed as percentage. Estimation of variceal bleeding incidence among patients with EGV was performed by Kaplan-Meier estimator during a follow-up time considered as the interval between first administration of lenvatinib and bleeding, death, or last visit. Logistic regression was used to identify predictive factors for presence of any kind of EGV and for high-risk varices only. Cox’s proportional hazard model was applied to assess variables associated with variceal bleeding among patients with EGV. Baseline variables considered in the analysis were as follows: sex, age, body mass index, underlying liver disease etiology, Child-Pugh-Turcotte, BCLC stage, nPVT, presence of extrahepatic disease, Eastern Cooperative Oncology Group Performance Status, alpha-fetoprotein, albumin, international normalized ratio (INR), bilirubin, aspartate aminotransferase, alanine aminotransferase, LDH, ALP, platelet count, white blood cell, neutrophils, neutrophil/lymphocyte ratio, creatinine, and, for variceal bleeding only, presence of high-risk EGV. For platelets, a cut-off value of 150,000/μL was considered, according to Baveno VII criteria [16]. Variables that tested significant in univariable analysis were included in the multivariable model. p values <0.05 were considered significant. Results were expressed as odds ratios (ORs), or hazard ratios (HRs) and 95% confidence interval (CI). Overall survival was measured from the date of starting lenvatinib therapy until the date of death from any cause or the date of last visit. Deaths due to GI hemorrhage, defined according to the Baveno International Consensus Criteria [15], were reported separately. All data generated or analyzed during this study are included in this article. Data management and analysis were performed using the STATA/SE 12.0 STATA package (Stata Corp., 4905 Lakeway Dr, College Station, TX 77845, USA).
Results
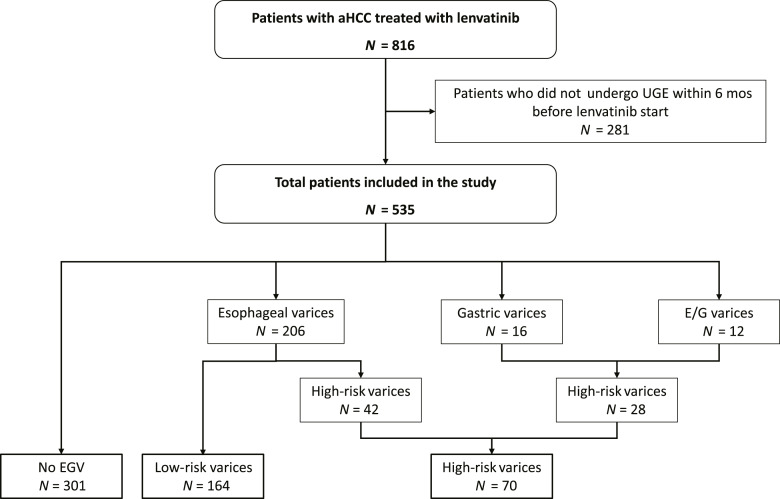
Between November 2014 and April 2022, 816 patients with confirmed diagnosis of advanced hepatocellular carcinoma (aHCC) started lenvatinib treatment. Among these patients, 535 (66%) were reported to have undergone UGE within 6 months before starting treatment and were evaluated in the present study (Fig. 1). Baseline characteristics of these patients are reported in Table 1. Briefly, median age was 72 years (IQR: 65–79), most patients were males with compensated cirrhosis, viral-induced liver disease, and preserved performance status. 297 (56%) patients were in BCLC stage C, 86 (16%) patients had nPVT, and 195 (36%) had extrahepatic disease. 435 (81%) were enrolled in Asia (406 in Japan and 29 in Korea), and 100 (19%) in Italy. Baseline characteristics according to geographical origin are reported in online supplementary Table 1 (for all online suppl. material, see https://doi.org/10.1159/000534127).
Fig. 1.
Patients’ disposition according to EGV at baseline.
Table 1.
Demographic and clinical characteristics of the 535 patients included in the study
| Baseline features | All patients (n = 535) |
|---|---|
| Age, years* | 72 (65–79) |
| Males, n (%) | 419 (78%) |
| Asian, n (%) | 435 (81%) |
| BMI, kg/m2* | 23.3 (21–26) |
| Etiology, n (%) | |
| Viral | 340 (63) |
| Alcohol | 88 (17) |
| MAFLD | 98 (18) |
| Others | 9 (2) |
| AST, IU/L* | 41 (28–63) |
| ALT, IU/L* | 30 (19–46) |
| ALP, IU/L* | 277 (145–426) |
| Bilirubin, mg/dL* | 0.8 (0.6–1.1) |
| Albumin, g/dL* | 3.7 (3.3–4.1) |
| INR* | 1.07 (1.01–1.15) |
| Creatinine, mg/dL* | 0.8 (0.7–1.0) |
| Neutrophil-to-lymphocyte ratio | 2.6 (1.8–3.8) |
| Platelets, mm3* | 130,000 (93,000–185,000) |
| >150,000/mm3, n (%) | 208 (39) |
| CPT B, n (%) | 59 (11) |
| ALBI score, n (%) | |
| 1 | 187 (35) |
| 2 | 334 (63) |
| 3 | 9 (2) |
| ECOG PS 0, n (%) | 417 (79) |
| nPVT, n (%) | 86 (16) |
| Extrahepatic disease, n (%) | 195 (36) |
| AFP, ng/mL* | 39.7 (5.6–617.2) |
| BCLC, n (%) | |
| A | 11 (2) |
| B | 227 (42) |
| C | 297 (56) |
| Previous treatments, n (%) | |
| Surgery | 54 (10) |
| Surgery+LRT | 91 (17) |
| Ablation | 110 (21) |
| TACE | 177 (33) |
| None | 103 (19) |
EGV, esophageal/gastric varices; BMI, body mass index; HCV, hepatitis C virus; HBV, hepatitis B virus; MAFLD, metabolic dysfunction-associated fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; INR, international normalized ratio; WBC, white blood cells; N/L, neutrophil/lymphocyte ratio; CPT, Child-Pugh-Turcotte; ALBI, albumin-bilirubin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; nPVT, neoplastic portal vein thrombosis; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; LRT, locoregional treatment; TACE, transarterial chemoembolization; IQR, interquartile range.
*Median (IQR).
Data are available for 301 patients.
At the time of the analysis (data lock May 2022), 189 (35%) patients were still alive, 329 (62%) had died, and 17 (3%) were lost to follow-up. Median overall survival was 15 months (IQR: 7–32 months). After patients’ stratification according to baseline liver function, the OS was 16 months (IQR: 8–32 months) in Child-Pugh A patients and 8.2 (IQR: 3.8–21) in Child-Pugh B patients (p < 0.001). Best response was evaluated in 514 of 535 patients: complete response in 27 (5%), partial response in 165 (32%), stable disease in 211 (41%), and progression in 111 (22%) patients, respectively. During study period, HCC progressed in 395/513 (77%) patients.
The most common AEs during treatment with lenvatinib were asthenia (235/432, 54%), decreased appetite (210/534, 39%), and arterial hypertension (204/534, 38%). Lenvatinib-related AEs, response treatment, and second-line drugs are reported in online supplementary Table 2. A second-line treatment was started in 237 (44%) patients after discontinuation of lenvatinib.
EGV Prevalence and Bleeding
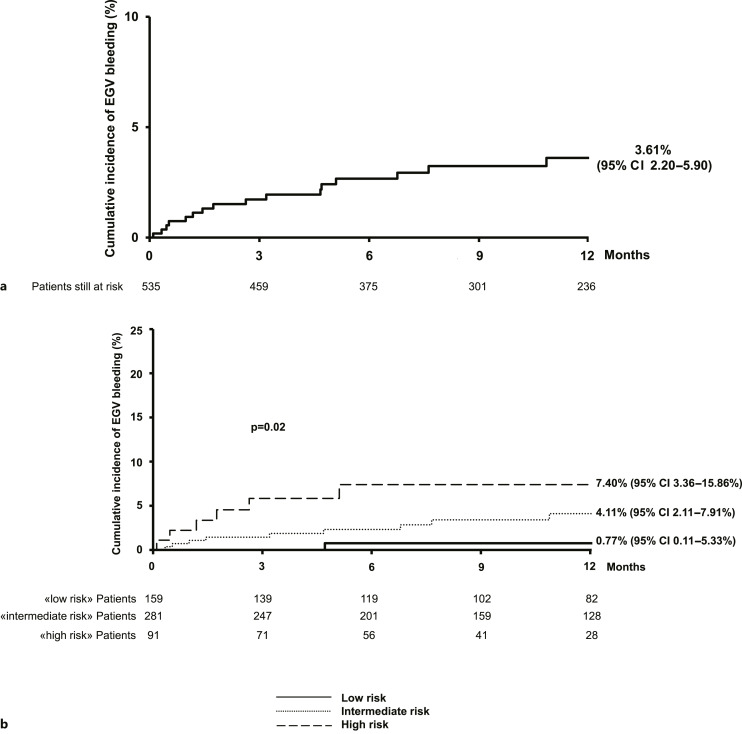
Patients recruited in the study performed UEG for 35 days (IQR: 12–92) before starting lenvatinib. EGV were detected in 234 (44%) patients: 206 (88%) esophageal varices, 16 (7%) gastric varices, and 12 (5%) both (Fig. 1). According to the primary endpoint of our study, during treatment (median duration: 10.5 months [IQR: 5–19 months]), EGV bleeding occurred in 17 (3%) patients, corresponding to a mean annual incidence of 2.82% and a cumulative incidence at 6 and 12 months of 2.67% (95% CI: 1.55–4.56) and 3.61% (95% CI: 2.20–5.90), respectively (Fig. 2a). Bleeding was moderate, not requiring intervention in 3 (18%, grade 2) and moderate-severe, requiring transfusion and urgent endoscopic band ligation (ESBL) or interventional radiology in 11 (64%, grade 3–4) patients, while 3 patients died due to EGV bleeding (18%, grade 5). Finally, among the patients with well-preserved liver function only (476 Child-Pugh A patients), the mean annual incidence of EGV bleeding was 6.35%, corresponding to a 6-month cumulative incidence of 5.33% (95% C.I.: 2.90–9.70).
Fig. 2.
One-year estimated cumulative incidence of EGV bleeding during treatment with lenvatinib in the 535 enrolled patients overall (a) and stratified per class of risk according to Child-Pugh, platelet count, and nPVT at baseline, by Kaplan-Meier (b).
High-risk varices were found in 70/234 (30%) patients (28 gastric varices, 42 esophageal varices). Among patients with high-risk EGV, 25/70 (36%) were on primary prophylaxis with nonselective beta-blockers (NSBB) at the time of lenvatinib start, 35 (50%) have been treated with ESBL (32 for primary and 3 for secondary prophylaxis), and 2 (3%) were treated with both ESBL and NSBB, while 8 patients received no prophylaxis (11%), mainly with gastric varices. The prevalence of both EGV and high-risk EGV was comparable between patients recruited in Asian and Italian centers (42% vs. 51%, p = 0.10 and 12% vs. 18%, p = 0.11).
Considering only the 234 patients with EGV at baseline, EGV bled in 16 (7%) patients during a median follow-up time of 9 months (IQR: 5–18 months), as often as 12 (17%) among those with high-risk varices. The corresponding mean annual incidence was 6.51%, and the cumulative incidences at 6 and 12 months were 5.54% (95% CI: 3.18–9.58) and 7.78% (95% CI: 4.68–12.80%), respectively. Among these 16 patients with baseline EGV who bled during treatment, 7 were in primary prophylaxis (4 NSBB and 3 ESBL), 1 in secondary, and 8 with no prophylaxis (4/8 with high-risk EGV).
Lenvatinib was discontinued in all patients after EGV bleeding and 8 (47%) underwent second-line treatment. In patients with EGV, bleeding median OS was shorter than patients without EGV bleeding (8 months, IQR: 2–15, vs. 15 months, IQR: 8–32, p = 0.007).
Risk Stratification
By Cox regression model, the presence of baseline EGV and of nPVT were independently associated with EGV bleeding in the whole cohort (HR: 17.70, 95% CI: 2.32–135.04, p value = 0.006 and HR: 2.79, 95% CI: 1.03–7.55, p value = 0.04) (Table 2). Limiting the analysis in patients with EGV, the only baseline variable independently related to EGV bleeding during lenvatinib treatment was the presence of high-risk EGV (HR: 6.94; 95% CI: 2.23–21.57, p = 0.001) (Table 3).
Table 2.
Univariable and multivariable Cox regression models to predict EGV bleeding during lenvatinib treatment in 535 patients included in the study
| Baseline variables | Type of variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age (years) | Continuous | 0.99 | 0.95–1.03 | 0.59 | |||
| Males | Yes versus no | 1.22 | 0.35–4.25 | 0.75 | |||
| Asian | Yes versus no | 0.91 | 0.14–1.17 | 0.10 | |||
| BMI (kg/m2) | Continuous | 1.05 | 0.93–1.83 | 0.43 | |||
| Viral etiology | Yes versus no | 0.85 | 0.32–2.23 | 0.73 | |||
| AST (IU/L) | Continuous | 1.00 | 1.00–1.01 | 0.13 | |||
| ALT (IU/L) | Continuous | 1.00 | 0.98–1.01 | 0.79 | |||
| ALP (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.83 | |||
| Bilirubin (mg/dL) | Continuous | 2.15 | 1.09–4.26 | 0.03 | 1.48 | 0.71–3.12 | 0.31 |
| Albumin (g/dL) | Continuous | 1.49 | 0.53–4.19 | 0.45 | |||
| INR | Continuous | 1.93 | 0.23–15.95 | 0.54 | |||
| Creatinine (mg/dL) | Continuous | 0.58 | 0.11–3.02 | 0.52 | |||
| Neutrophil/lymphocyte ratio | Continuous | 1.01 | 0.84–1.20 | 0.94 | |||
| Platelets (mm3) | Continuous | 1.00 | 0.99–1.00 | 0.61 | |||
| Platelets <150,000/mm3 | Yes versus no | 3.01 | 0.86–10.46 | 0.08 | |||
| CPT B | Yes versus no | 1.27 | 0.29–5.55 | 0.75 | |||
| ALBI score >1 | Yes versus no | 1.02 | 0.37–2.82 | 0.97 | |||
| ECOG PS 0 | Yes versus no | 0.97 | 0.28–3.41 | 0.97 | |||
| EGV | Yes versus no | 21.71 | 2.88–163.73 | 0.003 | 17.70 | 2.32–135.04 | 0.006 |
| nPVT | Yes versus no | 4.14 | 1.57–10.89 | 0.004 | 2.79 | 1.03–7.55 | 0.04 |
| Extrahepatic disease | Yes versus no | 0.84 | 0.29–2.38 | 0.74 | |||
| AFP (ng/mL) | Continuous | 1.00 | 0.99–1.00 | 0.40 | |||
| BCLC-C | Yes versus no | 1.09 | 0.42–2.84 | 0.86 | |||
EGV, esophageal-gastric varices; BMI, body mass index; HCV, hepatitis C virus; HBV, hepatitis B virus; MAFLD, metabolic dysfunction-associated fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; INR, international normalized ratio; WBC, white blood cells; N/L, neutrophil/lymphocyte ratio; CPT, Child-Pugh-Turcotte; ALBI, albumin-bilirubin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; nPVT, neoplastic portal vein thrombosis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; HR, hazard ratio; CI, confidence interval.
Table 3.
Univariable and multivariable Cox regression models to predict EGV bleeding during lenvatinib treatment in 234 patients with baseline EGV
| Baseline Variables | Type of variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age (years) | Continuous | 0.98 | 0.93–1.02 | 0.35 | |||
| Males | Yes versus no | 0.97 | 0.28–3.42 | 0.96 | |||
| Asian | Yes versus no | 0.46 | 0.16–1.35 | 0.16 | |||
| BMI (kg/m2) | Continuous | 1.04 | 0.92–1.19 | 0.51 | |||
| Viral etiology | Yes versus no | 0.86 | 0.31–2.36 | 0.77 | |||
| AST (IU/L) | Continuous | 1.01 | 1.00–1.02 | 0.04 | 1.01 | 0.99–1.02 | 0.21 |
| ALT (IU/L) | Continuous | 1.00 | 0.98–1.02 | 0.82 | |||
| ALP (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.96 | |||
| Bilirubin (mg/dL) | Continuous | 1.70 | 0.77–3.74 | 0.19 | |||
| Albumin (g/dL) | Continuous | 1.84 | 0.62–5.44 | 0.27 | |||
| INR | Continuous | 0.75 | 0.02–27.91 | 0.88 | |||
| Creatinine (mg/dL) | Continuous | 0.33 | 0.04–2.89 | 0.32 | |||
| N/L ratio | Continuous | 0.99 | 0.79–1.25 | 0.94 | |||
| Platelets (mm3) | Continuous | 1.00 | 0.99–1.00 | 0.83 | |||
| Platelets <150,000/mm3 | Yes versus no | 2.39 | 0.54–10.52 | 0.25 | |||
| CPT B | Yes versus no | 0.87 | 0.20–3.86 | 0.86 | |||
| ALBI score>1 | Yes versus no | 0.72 | 0.26–2.03 | 0.54 | |||
| ECOG PS 0 | Yes versus no | 0.90 | 0.25–3.18 | 0.87 | |||
| High-risk EGV | Yes versus no | 7.29 | 2.35–22.60 | 0.001 | 6.94 | 2.23–21.57 | 0.001 |
| nPVT | Yes versus no | 3.16 | 1.17–8.52 | 0.02 | 2.38 | 0.83–6.78 | 0.11 |
| Extrahepatic disease | Yes versus no | 0.83 | 0.27–2.57 | 0.74 | |||
| AFP (ng/mL) | Continuous | 1.00 | 0.99–1.00 | 0.48 | |||
| BCLC-C | Yes versus No | 1.07 | 0.40–2.87 | 0.89 | |||
EGV, esophageal-gastric varices; BMI, body mass index; HCV, hepatitis C virus; HBV, hepatitis B virus; MAFLD, metabolic dysfunction-associated fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; INR, international normalized ratio; WBC, white blood cells; N/L, neutrophil/lymphocyte ratio; CPT, Child-Pugh-Turcotte; ALBI, albumin-bilirubin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGV, esophagogastric varices; nPVT, neoplastic portal vein thrombosis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; HR, hazard ratio; CI, confidence interval.
Bilirubin, INR, platelet count <150,000/μL, Child-Pugh B, higher albumin-bilirubin score, and presence of nPVT were associated with presence of EGV (Table 4). INR, bilirubin, and albumin-bilirubin score were excluded from multivariable analysis as they are or include variables already part of the Child-Pugh score. By multiple logistic regression, independent predictors of presence of EGV at baseline were platelet count <150,000/μL (OR 3.19; 95% CI: 2.17–4.70, p < 0.001), Child-Pugh B (OR 2.11; 95% CI: 1.18–3.77, p = 0.01), and presence of nPVT (OR 2.44; 95% CI: 1.48–4.02, p < 0.001). The same variables independently predicted the presence of high-risk EGV (Table 5).
Table 4.
Univariable and multivariable logistic regression models to predict presence of EGV at baseline in 535 patients included in the study
| Baseline variables | Type of variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Age (years) | Continuous | 1.00 | 0.98–1.01 | 0.66 | |||
| Males | Yes versus no | 1.36 | 0.89–2.07 | 0.15 | |||
| Asian | Yes versus no | 0.70 | 0.45–1.08 | 0.11 | |||
| BMI (kg/m2) | Continuous | 1.04 | 0.99–1.10 | 0.12 | |||
| Viral etiology | Yes versus no | 1.32 | 0.92–1.88 | 0.13 | |||
| AST (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.80 | |||
| ALT (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.64 | |||
| ALP (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.99 | |||
| Bilirubin (mg/dL) | Continuous | 2.14 | 1.49–3.07 | <0.001 | Not included | Not included | Not included |
| Albumin (g/dL) | Continuous | 0.71 | 0.51–1.00 | 0.05 | |||
| INR | Continuous | 8.10 | 1.81–36.30 | 0.006 | Not included | Not included | Not included |
| Creatinine (mg/dL) | Continuous | 0.91 | 0.60–1.37 | 0.66 | |||
| Neutrophil/Lymphocyte ratio | Continuous | 1.07 | 0.95–1.05 | 0.93 | |||
| Platelets (mm3) | Continuous | 0.99 | 0.98–0.99 | <0.001 | Not included | Not included | Not included |
| Platelets <150,000/mm3 | Yes versus no | 3.02 | 2.08–4.38 | <0.001 | 3.19 | 2.17–4.70 | <0.001 |
| CPT B | Yes versus no | 2.38 | 1.36–4.16 | 0.002 | 2.11 | 1.18–3.77 | 0.01 |
| ALBI score>1 | Yes versus no | 1.50 | 1.04–2.15 | 0.03 | Not included | Not included | Not included |
| ECOG PS 0 | Yes versus no | 1.04 | 0.68–1.59 | 0.86 | |||
| nPVT | Yes versus no | 2.24 | 1.40–3.59 | 0.001 | 2.44 | 1.48–4.02 | <0.001 |
| Extrahepatic disease | Yes versus no | 0.71 | 0.50–1.02 | 0.06 | |||
| AFP (ng/mL) | Continuous | 1.00 | 0.99–1.00 | 0.51 | |||
| BCLC-C | Yes versus no | 0.91 | 0.65–1.29 | 0.61 | |||
EGV, esophageal-gastric varices; BMI, body mass index; HCV, hepatitis C virus; HBV, hepatitis B virus; MAFLD, metabolic dysfunction-associated fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; INR, international normalized ratio; WBC, white blood cells; N/L, neutrophil/lymphocyte ratio; CPT, Child-Pugh-Turcotte; ALBI, albumin-bilirubin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; nPVT, neoplastic portal vein thrombosis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; OR, odds ratio; CI, confidence interval.
Table 5.
Univariable and multivariable logistic regression models to predict presence of high-risk EGV at baseline in 535 patients included in the study
| Baseline variables | Type of variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Age (years) | Continuous | 1.00 | 0.97–1.02 | 0.81 | |||
| Males | Yes versus no | 0.92 | 0.51–1.68 | 0.80 | |||
| Asian | Yes versus no | 0.62 | 0.34–1.11 | 0.11 | |||
| BMI (kg/m2) | Continuous | 1.02 | 0.94–1.12 | 0.58 | |||
| Viral etiology | Yes versus no | 1.04 | 0.61–1.75 | 0.89 | |||
| AST (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.37 | |||
| ALT (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.63 | |||
| ALP (IU/L) | Continuous | 1.00 | 0.99–1.00 | 0.90 | |||
| Bilirubin (mg/dL) | Continuous | 1.90 | 1.25–2.89 | 0.003 | Not included | Not included | Not included |
| Albumin (g/dL) | Continuous | 0.83 | 0.51–1.37 | 0.47 | |||
| INR | Continuous | 1.63 | 0.37–7.10 | 0.52 | |||
| Creatinine (mg/dL) | Continuous | 0.69 | 0.30–1.56 | 0.37 | |||
| Neutrophil/lymphocyte ratio | Continuous | 1.00 | 0.93–1.08 | 0.95 | |||
| Platelets (mm3) | Continuous | 0.99 | 0.98–0.99 | 0.002 | Not included | Not included | Not included |
| Platelets <150,000/mm3 | Yes versus no | 2.33 | 1.30–4.21 | 0.005 | 2.47 | 1.35–4.50 | 0.003 |
| CPT B | Yes versus no | 2.33 | 1.20–4.52 | 0.01 | 2.12 | 1.08–4.17 | 0.03 |
| ALBI score>1 | Yes versus no | 1.42 | 0.82–2.47 | 0.21 | |||
| ECOG PS 0 | Yes versus no | 0.78 | 0.43–1.41 | 0.41 | |||
| nPVT | Yes versus no | 2.42 | 1.35–4.32 | 0.003 | 2.54 | 1.40–4.61 | 0.002 |
| Extrahepatic disease | Yes versus no | 0.61 | 0.35–1.07 | 0.08 | |||
| AFP (ng/mL) | Continuous | 1.00 | 0.99–1.00 | 0.36 | |||
| BCLC-C | Yes versus no | 0.77 | 0.47–1.28 | 0.32 | |||
EGV, esophageal-gastric varices; BMI, body mass index; HCV, hepatitis C virus; HBV, hepatitis B virus; MAFLD, metabolic dysfunction-associated fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; INR, international normalized ratio; WBC, white blood cells; N/L, neutrophil/lymphocyte ratio; CPT, Child-Pugh-Turcotte; ALBI, albumin-bilirubin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; nPVT, neoplastic portal vein thrombosis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; OR, odds ratio; CI, confidence interval.
Among the 476 Child-Pugh A patients only, the only baseline variable independently related to EGV bleeding in 197 Child-Pugh A patients with varices was the presence of high-risk EGV (HR: 6.65; 95% CI: 2.08–21.26, p = 0.001). Among these patients, independent predictors of presence of EGV at baseline were bilirubin levels (OR: 1.78, 95% CI: 1.05–3.01, p = 0.03), nPVT (OR: 2.78; 95% CI: 1.49–5.19, p = 0.001), and platelet count <150,000/μL (OR: 2.51; 95% CI: 1.48–4.25, p = 0.001). Independent predictors of the presence of high-risk EGV were nPVT (OR: 2.53; 95% CI: 1.24–5.16, p = 0.01) and platelet count <150,000/μL (OR: 2.56; 95% CI: 1.28–5.10, p = 0.008), while hepatitis B virus-infection resulted protective (OR: 0.28; 95% CI: 0.10–0.82, p = 0.02).
According to the three independent variables identified as predictors of high-risk EGV in the whole population (Child-Pugh, platelet count, and nPVT), we stratified the risk of bleeding in three groups: patients with no risk factors (low risk, 1/159 patient, 6-month cumulative incidence 0.77%, 95% CI: 0.11–5.33 and 12 months cumulative incidence 0.77%, 95% CI: 0.11–5.33), patients with one risk factor (intermediate risk, 10/281 patients, 6-month cumulative incidence 2.31%, 95% CI: 1.04–5.09 and 12 months cumulative incidence: 4.11%, 95% CI: 2.11–7.91), patients with two or 3 risk factors (high risk, 6/91 patients, 6-month cumulative incidence 7.40%, 95% CI: 3.36–15.86 and 12-month cumulative incidence: 7.40%, 95% CI: 3.36–15.86%) (Fig. 2b).
Discussion
This study demonstrated for the first time in a real-world practice that in HCC patients treated with lenvatinib the overall risk of EGV bleeding is low and limited to patients with baseline nPVT, platelets <150,000/μL, and less preserved liver function. A risk score to identify these patients was developed to identify a priori those patients in whom a UGE screening is warranted in the initial evaluation for the choice of the best systemic treatment for the patient with aHCC.
Currently, at least three different systemic treatments for aHCC have been approved, and other combinations will soon be available for first line. Selection of the most appropriate treatment should be considered through a multiparametric assessment, which includes several aspects of the tumor itself and underlying liver disease, among which the degree of portal hypertension and the risk of bleeding should also be considered. In our multicenter study, we detected 16 EGV bleeding episodes in 234 patients with EGV among 535 patients treated with lenvatinib in first line for aHCC. We identified low platelet count, less compensated cirrhosis, and presence of nPVT as independent predictors of presence of both EGV and high-risk EGV. Finally, among patients with EGV, the presence of high-risk EGV was the only independent predictor of bleeding. Thus, on the basis of these results, we could suggest stratifying the risk of bleeding and support the decision on forefront treatment for aHCC: Child-Pugh A patients with platelet count >150,000/μL without nPVT could avoid UGE evaluation before starting treatment and lenvatinib-based treatment can be considered; patients with either 1/3 or 2/3 risk factors (either Child-Pugh B or nPVT or platelet count<150,000/μL) should undergo UGE evaluation and those with high-risk EGV should be considered with caution for treatment with lenvatinib or other drugs with similar actions, while a pure immunotherapy regimen (either as single or combination agents) would be more suitable. Finally, in patients with all the three risk factors present, the cost/benefit ratio of initiating systemic treatment must be carefully considered, both for the risk of bleeding and for the reduced survival and the risk of death from worsening liver function in Child-Pugh B patients, as our study confirmed in line with previous data with sorafenib and with atezolizumab+bevacizumab treatments [8, 17]. Another aspect that our study helps reveal are the limits of the applicability of prophylaxis to reduce the risk of bleeding in aHCC: some of our patients have bled either without high-risk varices (4/16), or from gastric varices, in respect of which the approach has a lower level of agreement among experts. However, the relevance of a multidisciplinary approach to these patients with HCC, clinically significant portal hypertension, and nPVT is extremely relevant for survival [18].
These findings have currently – and near future – a clinical relevance for patients with aHCC, who can be offered different forefront therapeutic options and even more combinations might be available in the near future. In fact, while the degree of portal hypertension has well-demonstrated implications for the selection of candidates for surgery, little data exists for patients receiving systemic therapies. However, because the risk of EGV bleeding is a key concern in assessing, candidacy to both multikinase inhibitor (MKI) and bevacizumab, we sought to evaluate whether presence and severity of EGV together with other clinical features were associated with bleeding events. There is a proportion of patients who may suffer adverse outcomes, and this has been identified as patients with less preserved liver function, low platelet count, and nPVT. While the first two features are well-known predictors of EGV in cirrhosis, the presence of neoplastic portal invasion is a peculiarity of aHCC, whose weight has been highlighted not only as a predictor of bleeding in patients treated with MKI [7] but also as a factor limiting the effectiveness of systemic therapy in general, as recently shown even for atezolizumab+bevacizumab [19]. However, although limited by retrospective design, our study shows correlation between the presence of varices at the pretreatment EGD, particularly high-risk ones, and the development of bleeding events during treatment. The identification of the 3 categories of patients at different risk of bleeding may help tailor the management of patients in terms of EGV and systemic treatment. To this end, patients in higher risk group invariably are in Child-Pugh class B, a condition for which the benefit of lenvatinib has not been undoubtedly demonstrated, confirming the issues of whether MKI must be avoided in this subgroup. But even in Child-Pugh A patients, the presence of low platelet count and nPVT identifies patients with high-risk EGV. The microvascular changes induced by lenvatinib, as well as bevacizumab, can spark serious complications when used on the background of portal hypertension [20, 21]. Although we know that a direct comparison is not possible, the rate of EGV bleeding in our population is lower than that of the IMbrave-150 study, despite having recruited patients with more severe disease in terms of portal hypertension and reduced liver function. This figure is even more reassuring when compared with a historical case series from phase 2 trials, where the variceal prophylaxis was less standardized and bevacizumab-related bleeding events occurred in up to 10% of the patients [21]. If identified and adequately treated, the presence of varices is not associated with the risk of GI bleeding, thus making the delivery of lenvatinib-based treatment a safe option in this population.
Our study has some limitations. First, the retrospective nature of the database, cannot replace prospective studies, however the study should be regarded as mainly safety-oriented, without claiming to provide information on efficacy, as already highlighted in previous studies. Our findings are reinforced by the presence of patients enrolled in several Italian and Asian centers – both hepatological-oriented and oncological ones – with a wide spectrum of etiologies, degree of portal hypertension, and liver function status. Second, the real-life setting of our study implies a lack of standardization in clinical practice, including eligibility assessment, frequency of follow-up, management of AEs, and the presence of missing data. Lack of baseline UGE in 281 out of 816 patients included in the original population, for instance, might have led to selection bias. Reasons for incomplete adherence to pretreatment UGE screening are impossible to be fully retrieved in retrospective studies. However, the baseline characteristics of patients excluded from the study were slightly different from those of the included patients, however with similar treatment duration and OS. Finally, our study included 59 patients in Child-Pugh B class: the small number prevented a trustable evaluation of the use of lenvatinib in Child-Pugh B patients; however, the results of our study recommend a careful assessment of bleeding risk in lenvatinib-treated Child-Pugh-Turcotte B patients.
In conclusion, the overall risk of EGV bleeding in cirrhotic patients treated with lenvatinib is low but significant in a subgroup of patients. The identification of baseline risk factors both for the presence of high-risk EGV and for the bleeding itself during lenvatinib, provides clinically useful data to guide the decision-making process in clinical practice, suggesting an algorithm to stratify the risk of portal hypertensive bleeding according to the Baveno guidelines [15], that deserves validation in other forefront and second-line systemic treatments.
Statement of Ethics
This study protocol was reviewed and approved by the Ethical Committees of each center after first approval by Ethical Committee of San Raffaele Hospital, approval number DSAN854-A-OS/5. The current study complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws and fulfilled the Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016 on the protection of natural persons with regard to processing of personal data. In accordance with local regulations, a written informed consent was obtained from participants to participate in the study, before data collection.
Conflict of Interest Statement
M. Iavarone: speaking/teaching, consultant, and advisory board for Bayer, Gilead Sciences, BMS, Janssen, Ipsen, MSD, BTG-Boston Scientific, AbbVie, Guerbet, EISAI, Roche, and AstraZeneca; C. Soldà: consulting/advisory role for MSD and EISAI; speakers’ bureau for Roche and MSD; C. Yoo: received honoraria from Servier, Bayer, AstraZeneca, Merck Sharp & Dohme, Eisai, Celgene, Bristol-Myers Squibb, Debiopharm, Ipsen, Kyowa Kirin, Novartis, Boryung Pharmaceuticals, Merck Serono, Mundipharma, Roche, and Janssen; and received research grants from Servier, Bayer, AstraZeneca, Ono Pharmaceuticals, Celgene, Ipsen, Boryung Pharmaceuticals, Ildong Pharmaceutical, and Chong Kun Dang Pharm.; F. Piscaglia: AstraZeneca, Bayer, Bracco, EISAI, Esaote, Exact Sciences, IPSEN, MSD, Roche, Samsung, and Tiziana Life Sciences; P. Lampertico: advisory board/speaker bureau for BMS, Roche, Gilead Sciences, GSK, AbbVie, MSD, Arrowhead, Alnylam, Janssen, SPRING Bank, MYR, Eiger, Aligos, Antios, and Vir.
Funding Sources
This study was partially funded by the Italian Ministry of Health and current research IRCCS.
Author Contributions
Guarantor of the article: M. Iavarone and P. Lampertico; study design: M. Iavarone, E. Alimenti, A. Casadei-Gardini, and P. Lampertico; data collection: T. Tada, S. Shimose, G. Suda, C. Yoo, C. Soldà, F. Piscaglia, F. Marra, C. Vivaldi, F. Conti, M. Schirripa, H. Iwamoto, T. Sho, S.H. Lee, M.D. Rizzato, M. Tonnini, M. Rimini, C. Campani, G. Masi, F. Foschi, M. Bruccoleri, T. Kawaguchi, T. Kumada, A. Hiraoka, M. Atsukawa, S. Fukunishi, T. Ishikawa, K. Tajiri, H. Ochi, S. Yasuda, H. Toyoda, T. Hatanaka, S. Kakizaki, K. Kawata, F. Tada, H. Ohama, N. Itokawa, T. Okubo, T. Arai, M. Imai, and A. Naganuma; data analysis and interpretation: M. Iavarone, E. Alimenti, P. Lampertico, and G. Tosetti; manuscript preparation: M. Iavarone and E. Alimenti; and manuscript supervision: P. Lampertico, A. Casadei-Gardini, and F. Piscaglia. All authors have approved the final draft submitted.
Funding Statement
This study was partially funded by the Italian Ministry of Health and current research IRCCS.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author: anonymized dataset of the study is available to editors, reviewers, and readers upon request to the corresponding author.
Supplementary Material
References
- 1. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 3. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 4. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022 Jul 26;1(8):1. [DOI] [PubMed] [Google Scholar]
- 5. Cabibbo G, Aghemo A, Lai Q, Masarone M, Montagnese S, Ponziani FR, et al. Optimizing systemic therapy for advanced hepatocellular carcinoma: the key role of liver function. Dig Liver Dis. 2022;54(4):452–60. [DOI] [PubMed] [Google Scholar]
- 6. https://clinicaltrials.gov/ct2/show/results/NCT01761266.
- 7. Iavarone M, Primignani M, Vavassori S, Sangiovanni A, La Mura V, Romeo R, et al. Determinants of esophageal varices bleeding in patients with advanced hepatocellular carcinoma treated with sorafenib. United Eur Gastroenterol J. 2016;4(3):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D’Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76(4):1000–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hidaka H, Uojima H, Nakazawa T, Shao X, Hara Y, Iwasaki S, et al. Portal hemodynamic effects of lenvatinib in patients with advanced hepatocellular carcinoma: a prospective cohort study. Hepatol Res. 2020;50(9):1083–90. [DOI] [PubMed] [Google Scholar]
- 10. Rapposelli IG, Tada T, Shimose S, Burgio V, Kumada T, Iwamoto H, et al. Adverse events as potential predictive factors of activity in patients with advanced hepatocellular carcinoma treated with lenvatinib. Liver Int. 2021;41(12):2997–3008. [DOI] [PubMed] [Google Scholar]
- 11. Rimini M, Kudo M, Tada T, Shigeo S, Kang W, Suda G, et al. Nonalcoholic steatohepatitis in hepatocarcinoma: new insights about its prognostic role in patients treated with lenvatinib. ESMO Open. 2021;6:100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. [DOI] [PubMed] [Google Scholar]
- 14. North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices . Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983–9. [DOI] [PubMed] [Google Scholar]
- 15. de Franchis R; Baveno VI Faculty . Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–52. [DOI] [PubMed] [Google Scholar]
- 16. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty . Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–7. [DOI] [PubMed] [Google Scholar]
- 18. Vitale A, Cabibbo G, Iavarone M, Viganò L, Pinato DJ, Ponziani FR, et al. Personalised management of patients with hepatocellular carcinoma: a multiparametric therapeutic hierarchy concept. Lancet Oncol. 2023;24(7):e312–22. [DOI] [PubMed] [Google Scholar]
- 19. Breder VV, Vogel A, Merle P, Finn RS, Galle PR, Zhu AX, et al. IMbrave150: exploratory efficacy and safety results of hepatocellular carcinoma (HCC) patients (pts) with main trunk and/or contralateral portal vein invasion (Vp4) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in a global Ph III study. J Clin Oncol. 2021;39(15_Suppl l):4073.34724392 [Google Scholar]
- 20. Allaire M, Rudler M, Thabut D. Portal hypertension and hepatocellular carcinoma: des liaisons dangereuses. Liver Int. 2021;41(8):1734–43. [DOI] [PubMed] [Google Scholar]
- 21. Fang P, Hu JH, Cheng ZG, Liu ZF, Wang JL, Jiao SC. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: a systematic review of phase II trials. PLoS One. 2012;7(12):e49717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author: anonymized dataset of the study is available to editors, reviewers, and readers upon request to the corresponding author.