Abstract
Introduction
Intratumoral administration of pexa-vec (pexastimogene devacirepvec), an oncolytic and immunotherapeutic vaccinia virus, given to patients with hepatocellular carcinoma (HCC), is associated with both local and distant tumor responses. We hypothesized subsequent treatment with sorafenib could demonstrate superior efficacy.
Methods
This random phase III open-label study evaluated the sequential treatment with pexa-vec followed by sorafenib compared to sorafenib in patients with advanced HCC and no prior systemic treatment. The primary endpoint is overall survival (OS). Key secondary endpoints included time to progression (TTP), progression-free survival, overall response rate (ORR), and disease control rate (DCR). Safety was assessed in all patients who received ≥1 dose of study treatment.
Results
The study was conducted at 142 sites in 16 countries. From December 30, 2015, to the interim analysis on August 2, 2019, 459 patients were randomly assigned (pexa-vec plus sorafenib: 234, sorafenib: 225). At the interim analysis, the median OS was 12.7 months (95% CI: 9.89, 14.95) in the pexa-vec plus sorafenib arm and 14.0 months (95% CI: 11.01, 18.00) in the sorafenib arm. This led to the early termination of the study. The median TTP was 2.0 months (95% CI: 1.77, 2.96) and 4.2 months (95% CI: 2.92, 4.63); ORR was 19.2% (45 patients) and 20.9% (47 patients); and DCR was 50.0% (117 patients) and 57.3% (129 patients) in the pexa-vec plus sorafenib and sorafenib arms, respectively. Serious adverse events were reported in 117 (53.7%) patients in the pexa-vec plus sorafenib and 77 (35.5%) patients in the sorafenib arm. Liver failure was the most frequently reported in both groups.
Conclusion
Sequential pexa-vec plus sorafenib treatment did not demonstrate increased clinical benefit in advanced HCC and fared worse compared to sorafenib alone. The advent of the added value of checkpoint inhibitors should direct any further development of oncolytic virus therapy strategies.
Keywords: Pexa-vec, JX-594, Sorafenib, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer – the third most frequent cause of cancer death worldwide in 2020 [1]. The incidence of HCC has been increasing steadily, attributed to the recent hepatitis C and current obesity epidemics [2]. In recent years, first-line therapies for HCC have evolved from only including the tyrosine kinase inhibitor (TKI) sorafenib [3], to lenvatinib [4], and more recently, checkpoint inhibitor combination therapies including atezolizumab plus bevacizumab [5], and recently, a dual checkpoint inhibitors therapy of single-dose tremelimumab plus durvalumab [6].
Oncolytic virotherapy and immunotherapeutic strategies have gained significant attraction in recent years. Pexa-vec (pexastimogene devacirepvec, JX-594) is a vaccinia virus engineered to express the immune-boosting human granulocyte-macrophage colony-stimulating factor (hGM-CSF), designed selectively to replicate within and destroy cancer cells. Pexa-vec mechanisms of action include tumor cell infection and lysis, necrosis, and acute vascular disruption followed by long-term anti-tumor immunity, mediated by expression of the transgene GM-CSF [7–12]. In early clinical studies of HCC, pexa-vec was well tolerated and demonstrated dose-dependent survival benefits [13, 14].
We hypothesized that the activities of pexa-vec followed by the anti-angiogenic effects of sorafenib might allow for additive anti-cancer activity in a combination treatment strategy. To promote tumor cell selectivity of pexa-vec, the thymidine kinase 1 (TK1) gene in the pexa-vec genome has been deactivated, rendering it dependent on an active cell cycle in its host cell for replication [15]. However, as sorafenib is designed to stop the cell cycle of constantly dividing cells, it may have the ability to inhibit pexa-vec replication. While this inhibitor effect has been confirmed in preclinical investigations, preliminary clinical studies employing a sequential treatment regime to allow pexa-vec wash-out prior to sorafenib administration showed promising results [16, 17]. Based on these observations, PHOCUS was developed as a phase 3, randomized, open-label study assessing sequential administration of pexa-vec and sorafenib, exclusively HCC, in patients without prior systemic treatment.
Methods
Study Design
The open-label, randomized, phase 3 study, PHOCUS, was conducted at 142 centers in Asia-Pacific, Europe, and North America. Patients were randomly assigned 1:1 by the Interactive Voice/Web Response System to pexa-vec plus sorafenib versus sorafenib. Within two main subgroups (Asian or non-Asian), patients were randomized using a dynamic stochastic minimization procedure [18] for the following minimization factors: study center, main etiology, presence of extrahepatic disease, presence of vascular invasion, Eastern Cooperative Oncology Group (ECOG) performance status [19], and alpha-fetoprotein (AFP) levels [20]. The dynamic minimization used a stochastic treatment allocation algorithm based on the variance method.
The primary objective of the study was to determine and compare the overall survival (OS) of patients with advanced HCC without prior systemic therapy treated with pexa-vec followed by sorafenib versus sorafenib. Secondary objectives included time to progression (TTP), progression-free survival (PFS), overall response rate (ORR), disease control rate (DCR), and determining and comparing the safety profiles of the two treatment arms. TTP was defined as the time from randomization to the date of the first documented radiographic tumor progression. If a patient did not experience tumor progression at the cut-off date for analysis, TTP was censored at the date of the last evaluable tumor assessment before the cut-off.
Sample Size
Sample size was originally determined by comparing the estimated OS in patients treated with pexa-vec plus sorafenib to that in those receiving sorafenib alone. It was estimated that a total of 474 events of death (in 570 evaluable patients) had to be observed to reject the null hypothesis of no pexa-vec effect, with a power of 86% using a stratified log-rank test (1-sided cumulative 2.5% level of significance), assuming that HR = 1 for the first 6 months and 0.6 thereafter. However, as the study terminated early, the final analysis was based on the actual number of patients and events observed by the study cut-off date for each individual patient.
Patients
Eligible patients were ≥18 years of age with a histologically or cytologically confirmed diagnosis of HCC eligible for and have not received prior systemic therapy, with Child-Pugh A [21], had an ECOG performance status of 0–1, Barcelona Clinic Liver Cancer (BCLC) Stage B or C [22], and at least one measurable and viable liver tumor (based on radiographic assessment) injectable under imaging guidance [20]. Only patients with a life expectancy of ≥3 months were eligible. A history of severe ascites, bleeding esophageal varices, hepatic encephalopathy, or pleural effusions related to liver insufficiency within 6 months of screening; significant immunodeficiency due to underlying illness; severe eczema; or cardiovascular disease were exclusionary. Patients were not eligible if they had tumors encompassing >50% of the liver volume and/or inferior vena cava invasion or tumor(s) invading other key anatomical structures. Ongoing anticoagulant or anti-platelet medication that could not be interrupted prior to pexa-vec injections and the inability to suspend treatment with anti-hypertensive medication for 48 h prior to and after pexa-vec injections were also exclusionary.
The manuscript complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. In the manuscript, all 154 authors state that subjects have given their written informed consent and that the study protocol was approved by each institute’s committee on human research. This study protocol was reviewed and approved by the US National Cancer Institute and posted on clinicaltrials.gov under approval number NCT02562755.
All patients received oral and written information about the study and signed a written informed consent before study inclusion. All study documentation was reviewed and approved by Local Institutional Ethics Committees/Institutional Review Boards, as applicable.
Treatment
Provided clinical and laboratory pre-evaluation criteria were met. Patients in the pexa-vec plus sorafenib arm received intratumoral pexa-vec injections on days 1, 15, and 29, with a treatment window of +1/+7 days. Each pexa-vec injection was administered as a dose of 1 × 109 plaque-forming units (pfu), suspended in sterile saline buffered with sodium bicarbonate.
All injections were performed using ultrasound and/or CT guidance by an interventional radiologist or by an investigator who had received training materials provided by the sponsor. All viable, safely injectable intrahepatic tumors ≥1 cm as longest diameter (LD) were treated, with a maximum of five tumors treated on a given day. The administered volume was up to 25% of the tumor volume, and the total volume was divided proportionally between the treated tumors.
Sorafenib was administered orally (400 mg BID). In the pexa-vec plus sorafenib arm, sorafenib treatment was initiated at the week 6 visit or ≥2 weeks after the latest pexa-vec injection. In the sorafenib arm, continuous treatment was initiated on day 1. Treatment continued for as long as patients benefitted clinically, at least until radiographic progression or unacceptable toxicity occurred.
Follow-Up
A safety follow-up visit took place at least 28 days (but not more than 2 months) after the last study treatment administration. Patients and/or their specified contacts were contacted approximately every 4 weeks for follow-up and collection of information on subsequent anti-cancer therapy received until death, loss to follow-up, or withdrawal of consent. Patients who discontinued treatment prior to documented progression were followed for PFS every 6 weeks.
Interim Analysis
The first interim analysis for futility was planned to be performed when 190 deaths (40% of required events for the final analysis) were documented in the intent-to-treat (ITT) population. The p value of the re-randomization test was to be calculated with a stopping boundary defined as HR = 1.1 and a p value defined as 0.744 for crossing the futility boundary. The analysis was conducted after 197 deaths were observed and concluded that the study did not meet its original primary objective of OS improvement.
This resulted in early termination, and enrollment was stopped on August 02, 2019, with no further administration of pexa-vec. Sorafenib treatment continued until individual patients no longer received benefit from the treatment or until October 31, 2019. The revised primary objective was to determine radiographic responses in pexa-vec plus sorafenib versus sorafenib based on central assessments using modified RECIST (mRECIST) for HCC [23] for TTP, ORR, DCR, and time to tumor marker elevation (TTME), with OS and PFS being key revised secondary endpoints (Data Supplement, Revised Statistical Analysis Plan dated January 15, 2020).
Endpoint Definitions
OS was defined as the time from randomization to death, resulting from any cause. TTP was defined as the time from randomization to the date of the first documented radiographic tumor progression. PFS was defined as the time from randomization to the date of the first documented radiographic tumor progression or death due to any cause. The ORR was defined as the proportion of patients whose best overall response during participation was either complete response (CR) or partial response (PR). Duration of response (DoR) was evaluated only for patients whose best overall response was CR or PR. The DoR was defined as the time (in months) from the first AFP decrease to tumor progression (per mRECIST/RECIST1.1 or AFP increase >400 ng/mL) or death due to underlying cancer. If a patient was alive or if the cancer had not progressed at the cut-off date for analysis, DoR was censored at the date of the last evaluable tumor assessment before the cut-off. DCR was defined as the proportion of patients whose best overall response was either CR, PR, or stable disease. TTME was defined as time from nadir AFP to AFP >400 ng/mL. If a patient had no AFP value of >400 ng/mL at their cut-off date for analysis, TTME was censored at the date of the last AFP recorded before their cut-off. Only patients who had a decrease in AFP from baseline and who also had nadir AFP ≤400 ng/mL were included in the TTME analyses.
Statistical Analysis
The ITT population included all patients randomly assigned to study treatment. Primary and secondary endpoints were analyzed using the per-protocol (PP) population. The PP population included all patients from the ITT population without any major protocol deviations who had completed the minimum exposure requirement. A re-randomization test was performed 1,000 times to obtain 1-sided p values [24]. Analyses of radiological endpoints were performed based on central assessments using modified mRECIST criteria for HCC and repeated using central RECIST 1.1 criteria [23, 25]. For the TTP endpoint, a re-randomization test using a stratified log-rank test was performed to compare the two treatment arms [24]. Estimates of the HRs (pexa-vec plus sorafenib over sorafenib) with 95% CI were obtained from a Cox proportional hazard (PH) model. For the ORR and DCR endpoints, Cochran-Mantel-Haenszel tests were performed to compare the two treatment arms at a 1-sided 2.5% significance level [26]. Re-randomization tests based on the Mantel-Haenszel χ2 statistic were performed to obtain 1-sided p values. For the TTME endpoint, a Kaplan-Meier curve was constructed for each treatment arm. Median TTME and 25% and 75% quartiles were presented, along with 95% CIs for each treatment arm. Re-randomization tests using stratified log-rank test was performed to compare OS and PFS in the two treatment arms [24]. Region was the only statistical stratification factor and was used in primary, secondary, and sensitivity analyses.
The DoR was summarized by the treatment arm. A Kaplan-Meier (KM) curve was constructed for each treatment arm. Median DoR and 25% and 75% quartiles were presented along with 95% CIs for each treatment arm. In addition, the KM estimates with 95% CIs at 3, 6, and 9 months were presented by the treatment arm.
TTME was compared between the 2 treatment groups using a stratified re-randomization test based on 1,000 re-randomizations. This test utilized the stratified log-rank statistic stratified only by region.
Safety analyses were performed on the safety population, which included all patients who received at least one dose of pexa-vec or sorafenib. Safety assessments collected from the first administration of any study treatment up to 28 days after the end date of any study treatment are provided. All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 22.0.
All statistical analyses were conducted using SAS® software version 9.2 or higher. An independent Data Monitoring Committee was employed to regularly assess the safety, progress of the study, and integrity of the data for benefit/risk evaluation.
Results
Demographics
From December 30, 2015, to August 2, 2019, a total of 459 patients at 142 sites from 16 countries were randomly assigned at a 1:1 ratio to the pexa-vec followed by sorafenib group (n = 234) or to the sorafenib alone group (n = 255; Fig. 1). Patient demographics and baseline characteristics for the ITT population were generally well balanced and are summarized in Table 1. There were more males (84.1%) than females (15.9%) in the study, and the mean age was 60.9 (±10.55) years. The majority of the patients (60.8%) were Asian, over 90% of whom were from Asian countries. Prior HCC therapies included surgical resection, locoregional therapy, and radiation therapy.
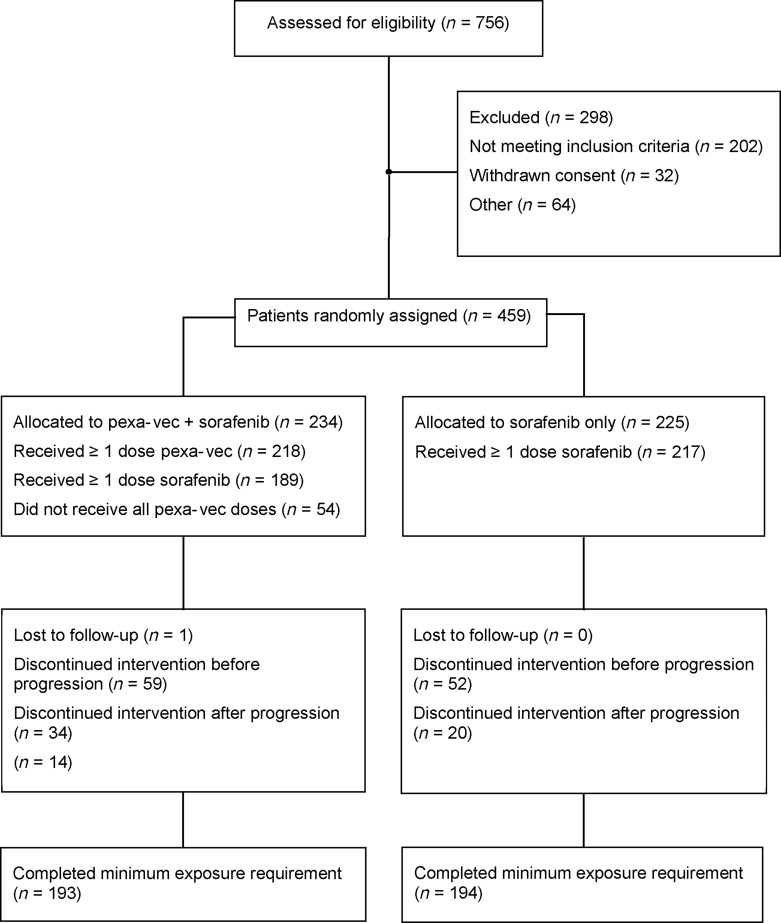
Fig. 1.
CONSORT diagram for the PHOCUS study. The intent-to-treat (ITT) population included all patients randomly assigned to study treatment. The per-protocol (PP) population included all patients from the ITT population without any major protocol deviations who had completed the minimum exposure requirement. Minimum exposure requirements were ≥1 pexa-vec injection in the pexa-vec plus sorafenib arm and ≥2 consecutive weeks of sorafenib treatment in the sorafenib arm.
Table 1.
Baseline patient demographics and clinical characteristics in the ITT population (n = 459)
| Characteristic | Pexa-vec + sorafenib (n = 234) | Sorafenib (n = 225) |
|---|---|---|
| Age, years | ||
| n | 234 | 225 |
| Median | 62 | 61 |
| Range | 35–84 | 28–84 |
| Sex, n (%) | ||
| Male | 204 (87.2) | 182 (80.9) |
| Female | 30 (12.8) | 43 (19.1) |
| Region**, % | ||
| Asian | 127 (54.3) | 129 (57.3) |
| Non-Asian | 107 (45.7) | 93 (42.7) |
| Race, n (%) | ||
| White | 71 (30.3) | 69 (30.7) |
| African American | 8 (3.4) | 6 (2.7) |
| Asian | 145 (62.0) | 134 (59.6 |
| Native Hawaiian/other Pacific Islander | 4 (1.7) | 5 (2.2) |
| Other | 6 (2.6) | 11 (4.9) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 5 (2.1) | 6 (2.7) |
| Not Hispanic or Latino | 229 (97.9) | 219 (97.3) |
| BCLC stage, n (%) | ||
| B – intermediate | 84 (35.9) | 73 (32.4) |
| C – advanced | 150 (64.1) | 151 (67.1) |
| Missing | 0 | 1 (0.4) |
| Body mass index, kg/m2 | ||
| n | 218 | 213 |
| Mean (SD) | 19.782 (28.5548) | 18.573 (28.8455) |
| Median | 24.279 | 24.355 |
| Range | 15.06–44.25 | 13.91–49.88 |
| Time since initial diagnosisa, months | ||
| n | 234 | 225 |
| Mean (SD) | 19.782 (28.5548) | 18.573 (28.8455) |
| Median | 7.627 | 6.312 |
| Range | 0.13–146.63 | 0.07–204.46 |
| Tumor size (SLD) of target tumors (RECIST 1.1) | ||
| n | 228 | 217 |
| Mean (SD) | 125.23 (71.339) | 120.74 (75.029) |
| Median | 114.50 | 111.00 |
| Range | 11.0–349.0 | 12.0–479.0 |
| Tumor size (SLD) of target tumors (mRECIST) | ||
| n | 218 | 206 |
| Mean (SD) | 100.82 (62.835) | 99.85 (65.356) |
| Median | 89.00 | 89.00 |
| Range | 10.0–337.0 | 12.0–322.0 |
| AFP levels, ng/mL | ||
| n | 234 | 225 |
| Mean | 19,594.53 (77,469.303) | 20,791.32 (63,783.454) |
| Median | 191.80 | 187.56 |
| Range | 1.2–882,865.4 | 1.1–453,514.0 |
| ECOG performance status, % | ||
| 0 | 146 (62.4) | 142 (63.1) |
| 1 | 88 (37.6) | 83 (36.9) |
| Extrahepatic disease**, % | ||
| Present | 96 (41.0) | 96 (42.7) |
| Not present | 88 (37.6) | 83 (36.9) |
| Vascular invasion**, % | ||
| Present | 81 (34.6) | 79 (35.1) |
| Not present | 153 (65.4) | 146 (64.9) |
| Etiology of the diseaseb, n (%) | ||
| Hepatitis B | 122 (52.1) | 114 (50.7) |
| Hepatitis C | 53 (22.6) | 57 (25.3) |
| ETOH | 47 (20.1) | 40 (17.8) |
| NASH | 17 (7.3) | 25 (11.1) |
| Other | 26 (11.1) | 18 (8.0) |
| Missing | 4 (1.7) | 4 (1.8) |
| Prior HCC therapies, n (%) | ||
| Surgical resection | 72 (30.8) | 75 (33.3) |
| Local-regional therapy | ||
| TACE | 102 (43.6) | 85 (37.8) |
| PEI | 4 (1.7) | 2 (0.9) |
| RFA | 38 (16.2) | 23 (10.2) |
| CA | 1 (0.4) | 0 |
| Radiation therapy | ||
| Stereotactic | 8 (3.4) | 3 (1.3) |
| Conformational | 5 (2.1) | 5 (2.2) |
| Brachytherapy | 0 | 1 (0.4) |
| Prior therapyc (≤7 days) | 132 (56.4) | 128 (56.9) |
SLD, sum of longest diameter; AFP, alpha-fetoprotein; BCLC, Barcelona Liver Clinic Liver Cancer; SD, standard deviation; ETOH, ethyl alcohol; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; PEI, percutaneous ethanol injection; CA, cryoablation.
**Based on IVRS. aDefined as the date of HCC diagnosis to date of randomization.
bPatients could have multiple etiologies; therefore, the total could be greater than 100%.
cDefined as any therapy such as sorafenib, HCC radiation therapy, HCC local-regional therapy of ≤7 days duration.
Among the 234 patients randomized to pexa-vec plus sorafenib, 218 received ≥1 dose of pexa-vec, and 189 received at least 1 dose of sorafenib. A majority (164 or 75.2% of patients) received all 3 doses of pexa-vec (27 patients [12.4%] received 1 dose, and 27 patients [12.4%] received 2 doses). The duration of sorafenib exposure (defined as the end date of sorafenib – the start date of sorafenib + 1) was mean 152.9 (SD: 207.91), median 81.0 (range: 0–1,000) days. The average daily dose of sorafenib was mean 553.97 (SD: 313.235), median 602.33 (range: 0–2,320.0) mg/day. The total duration of study treatment was mean of 194.17 (SD: 210.8) days and median of 124.50 (range: 14.0–1,044.0) days.
Among the 225 randomized to sorafenib, 217 received ≥1 dose of sorafenib. The duration of sorafenib exposure was mean 191.9 (SD: 235.5), median 106.0 (range: 4–1,291) days. The average daily dose of sorafenib was mean 647.96 (SD: 207.404), median 717.76 (range: 111.9–1,173.3) mg/day. The total duration of study treatment was mean 191.91 (SD: 235.5), median 106.00 (range: 4.0–1,291.0) days.
Primary Endpoint
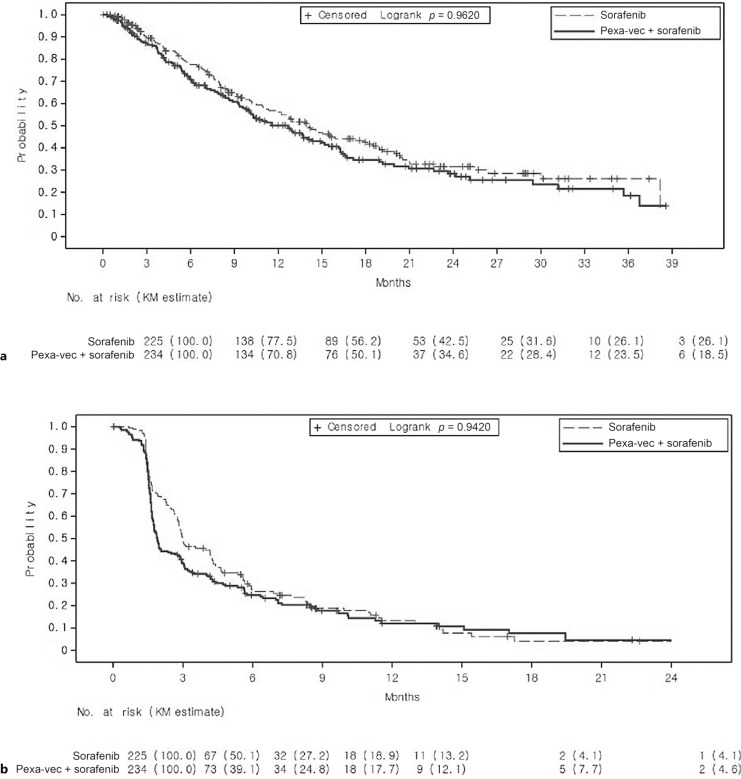
The median OS in the pexa-vec plus sorafenib was 12.7 months (95% CI: 9.89, 14.95) and in the sorafenib 14.0 months (95% CI: 11.01, 18.00), with a HR of 1.193 (95% CI: 0.928, 1.535; p = 0.9260). The median follow-up time was 8.0 months (range: 0.4–39.4) in the pexa-vec plus sorafenib arm and 8.3 months (range: 0.5–39.8) in the sorafenib arm. Kaplan-Meier estimates (Fig. 2a) indicated that the 6-month OS rate for pexa-vec plus sorafenib was 70.8% (95% CI: 64.1, 76.5) and for sorafenib 77.5% (95% CI: 71.0, 82.8). No statistically significant improvement in PFS was observed for pexa-vec plus sorafenib over sorafenib (p = 0.9420, online suppl. Table S1; for all online suppl. material, see https://doi.org/10.1159/000533650).
Fig. 2.
Kaplan-Meier estimates of OS (a) and PFS (b) in the intention-to-treat (ITT) population, displayed by treatment arm.
Supplementary analyses in the OS subgroup provided some insight into the patient population most likely to respond to pexa-vec treatment (online suppl. Table S2). Patients with more advanced disease (i.e., BCLC Stage C and baseline tumor size ≥75th percentile) experienced longer OS when treated with sorafenib alone, compared to the pexa-vec plus sorafenib combination. Subgroup analyses suggested a correlation between the number of doses administered and OS, though this finding is also consistent with the hypothesis that patients with indolent tumors were able to receive more doses. Patients on the pexa-vec plus sorafenib arm who received the full three doses of pexa-vec experienced a median OS of 16.3 months (95% CI: 13.04, 20.96), compared to only 1.8 months (95% CI: 1.31, 12.68) for patients who received one dose of pexa-vec (online suppl. Table S2).
Secondary Endpoints
The ORR was 19.2% (45 patients) in the pexa-vec followed by sorafenib treatment group and 20.9% (47 patients) in the sorafenib only treatment group for the ITT population. No statistically significant difference in ORR was observed for pexa-vec followed by sorafenib treatment compared with the sorafenib only treatment group (difference of −1.7%, p value = 0.6470). Stable disease was reported in 72 (30.8%) patients in the pexa-vec followed by sorafenib treatment group and 82 (36.4%) patients in the sorafenib only treatment group.
No statistically significant improvement in DoR was observed for pexa-vec followed by sorafenib treatment over sorafenib only treatment group (p value = 0.4800). The HR was 0.795 (95% CI: 0.412, 1.531). Median DoR was 2.8 months (95% CI: 0.36, 9.40) in the pexa-vec followed by sorafenib treatment group and 2.9 months (95% CI: 1.31, 8.34) in the sorafenib only treatment group.
In the pexa-vec plus sorafenib arm, 146 (62.4%) of the 234 enrolled patients experienced tumor progression, and 88 (37.6%) patients were censored due to having no event as of the cutoff date or being not evaluable for efficacy. In the sorafenib arm, 111 (49.3%) of the 225 enrolled patients experienced tumor progression, and 114 (50.7%) patients were censored for the reasons mentioned above.
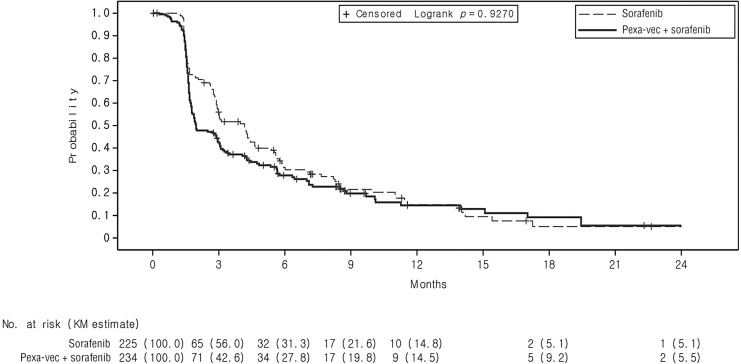
The median TTP was 2.0 months (95% CI: 1.77, 2.96) in the pexa-vec plus sorafenib arm and 4.2 months (95% CI: 2.92, 4.63) in the sorafenib arm. No statistically significant improvement in TTP was observed for pexa-vec plus sorafenib over sorafenib (p = 0.9270; HR: 1.196, 95% CI: 0.932, 1.533). Kaplan-Meier estimates of the TTP rate at 3 and 6 months in the pexa-vec plus sorafenib arm were 42.6% (95% CI: 35.3, 49.7) and 27.8% (95% CI: 21.2, 34.9), respectively, compared to 56.0% (95% CI: 47.4, 63.7) and 31.3% (95% CI: 23.2, 39.8), respectively, in the sorafenib arm (Fig. 3).
Fig. 3.
Kaplan-Meier estimates of the time to progression (TTP) in the intention-to-treat (ITT) population, displayed by treatment arm.
The median PFS was 1.9 months (95% CI: 1.74, 2.73) in the pexa-vec plus sorafenib arm and 3.0 months (95% CI: 2.79, 4.24) in the sorafenib arm, with a HR of 1.218 (95% CI: 0.967, 1.535). Kaplan-Meier estimates (Fig. 2a) showed that the PFS rates at 3 and 6 months in the pexa-vec plus sorafenib arm were 39.1% (95% CI: 32.3, 45.8) and 24.8% (95% CI: 18.7, 31.3), respectively, and 50.1% (95% CI: 42.1, 57.7) and 27.2% (95% CI: 20.0, 34.9), respectively, in the sorafenib arm (online suppl. Table S1).
The ORR per mRECIST criteria for the ITT population was 19.2% (45 patients) in the pexa-vec plus sorafenib arm and 20.9% (47 patients) in the sorafenib arm, with no statistically significant difference detected (difference of −1.7%, 95% CI: −9.13, 5.86; p = 0.6470; data not shown). Stable disease was reported in 72 (30.8%) patients in the pexa-vec plus sorafenib arm and 82 (36.4%) patients in the sorafenib arm. The DCR per mRECIST for the ITT population was 50.0% (117 patients) in the pexa-vec plus sorafenib arm and 57.3% (129 patients) in the sorafenib arm, with no statistically significant difference observed (difference of −7.3%, 95% CI: −16.45, 1.95; p = 0.0690; data not shown). The DCR per RECIST 1.1 was 45.7% (107 patients) in the pexa-vec plus sorafenib arm and 58.2% (131 patients) in the sorafenib arm for the ITT population. The DCR difference between the pexa-vec plus sorafenib arm over the sorafenib arm as per RECIST 1.1 was −12.5% (95% CI: −21.54, −2.11); the difference was statistically significant (p = 0.0040).
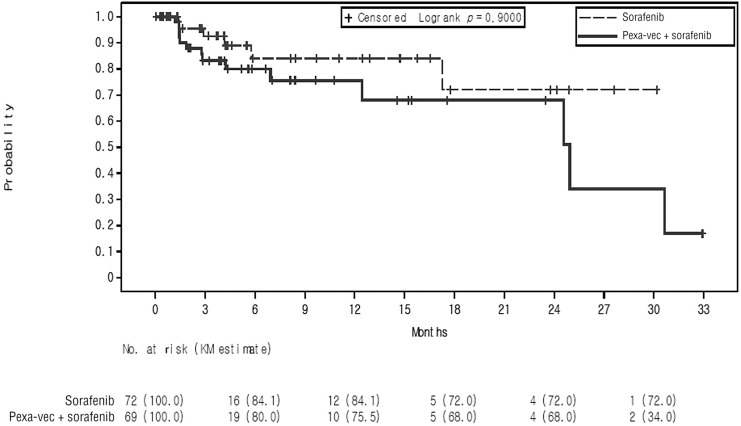
A total of 141 patients experienced a decrease in AFP from baseline with a nadir AFP of ≤400 ng/mL and were included in the TTME analyses (69 patients in the pexa-vec plus sorafenib arm and 72 in the sorafenib arm). TTME vs. time for the two study arms are shown graphically in Figure 4). In the pexa-vec plus sorafenib arm, the median TTME was 24.9 months (95% CI: 12.45, not estimable [NE]); the TTME could not be estimated in the sorafenib arm, as not enough data (events) were available to estimate the corresponding quartiles or confidence limits. Based on Kaplan-Meier estimates, the TTME rates at 3 and 6 months in the pexa-vec plus sorafenib arm were 83.2% (95% CI: 69.0, 91.3) and 80.0% (95% CI: 64.6, 89.2), respectively, and 92.6% (95% CI: 78.5, 97.6) and 84.1% (95% CI: 64.3, 93.4), respectively, in the sorafenib arm (online suppl. Table S1).
Fig. 4.
Kaplan-Meier estimates of TTME in the intention-to-treat (ITT) population, displayed by treatment arm.
Safety Analyses
Of the 459 randomized patients, 435 (94.8%) patients received at least one dose of study treatment and were included in the safety population (Fig. 1). Treatment-emergent adverse events (TEAEs) were reported by 218 patients (100%) in the pexa-vec plus sorafenib arm and 214 (98.6%) patients in the sorafenib arm (Table 2).
Table 2.
Treatment emergent adverse events in the safety population
| Pexa-vec + sorafenib (n = 218), n (%) | Sorafenib (n = 217), n (%) | |
|---|---|---|
| Any | 218 (100.00) | 214 (98.62) |
| Leading to treatment discontinuationa | 65 (29.8) | 41 (18.9) |
| Leading to study discontinuation | 45 (20.6) | 40 (18.4) |
| Leading to sorafenib dose modification | 66 (30.3) | 70 (32.3) |
| Pyrexia | 184 (84.40) | 28 (12.90) |
| Diarrhea | 107 (49.08) | 116 (53.46) |
| Decreased appetite | 85 (38.99) | 64 (29.49) |
| Nausea | 74 (33.94) | 63 (29.03) |
| Palmar-plantar erythrodysesthesia syndrome | 73 (33.49) | 99 (45.62) |
| Chills | 71 (32.57) | 4 (1.84) |
| Fatigue | 65 (29.82) | 64 (29.49) |
| Abdominal pain | 62 (28.44) | 60 (27.65) |
| Weight decrease | 12 (26.61) | 49 (22.58) |
| Vomiting | 56 (25.69) | 27 (16.59) |
| Constipation | 52 (23.85) | 51 (23.50) |
| Ascites | 46 (21.10) | 36 (16.59) |
| Hypertension | 44 (20.18) | 39 (17.97) |
| Abdominal pain, upper | 43 (19.72) | 30 (13.82) |
| Rash, pustular | 39 (17.89) | 2 (0.92) |
| Influenza-like illness | 37 (16.97) | 5 (2.30) |
| Hypotension | 35 (16.06) | 2 (0.92) |
| Anemia | 33 (15.14) | 23 (10.60) |
| Headache | 33 (15.14) | 23 (10.60) |
| Alopecia | 32 (14.68) | 46 (21.20) |
| Cough | 31 (14.22) | 25 (11.52) |
| Aspartate aminotransferase elevation | 30 (13.76) | 39 (17.97) |
| Back pain | 29 (13.30) | 17 (7.83) |
| Edema, peripheral | 27 (12.39) | 21 (9.68) |
| Injection site pain | 27 (12.39) | 0 (0.00) |
| Abdominal distension | 26 (11.93) | 26 (11.98) |
| Rash | 23 (10.55) | 28 (12.90) |
| Asthenia | 22 (10.09) | 21 (9.68) |
| Arthralgia | 20 (9.17) | 15 (6.91) |
| Dizziness | 20 (9.17) | 13 (5.99) |
| Pain in extremity | 19 (8.72) | 12 (5.53) |
| Hypokalemia | 18 (8.26) | 19 (8.76) |
| Dyspnea | 18 (8.26) | 11 (5.07) |
| Blood bilirubin elevation | 17 (7.80) | 29 (13.36) |
| Stomatitis | 12 (7.80) | 24 (11.06) |
| Upper respiratory tract infection | 17 (7.80) | 23 (10.60) |
| Hyponatremia | 16 (7.34) | 10 (4.61) |
| Insomnia | 16 (7.34) | 20 (9.22) |
| Oropharyngeal pain | 16 (7.34) | 10 (4.61) |
| Pruritus | 16 (7.34) | 17 (7.83) |
| Tachycardia | 16 (7.34) | 0 (0.00) |
| Dyspepsia | 15 (6.88) | 13 (5.99) |
| Hypoalbuminemia | 15 (6.88) | 11 (5.07) |
| Platelet count reduction | 15 (6.88) | 15 (6.91) |
| Hyperkalemia | 14 (6.42) | 4 (1.84) |
| Musculoskeletal pain | 14 (6.42) | 12 (5.53) |
| Epistaxis | 13 (5.96) | 9 (4.15) |
| Sinus tachycardia | 13 (5.96) | 3 (1.38) |
| Procedural pain | 12 (5.50) | 2 (0.92) |
| Myalgia | 11 (5.05) | 8 (3.69) |
| Dysphonia | 10 (4.59) | 12 (5.53) |
| Muscle spasms | 10 (4.59) | 12 (5.53) |
Data are sorted in decreasing frequency for the pexa-vec plus sorafenib treatment arm.
The frequency threshold used for this table was 5% in either treatment group. All events were collected by systematic assessment.
aEither or both study treatments in the pexa-vec + sorafenib treatment group or sorafenib in the sorafenib only treatment group.
The majority of TEAEs were mild in severity. In the pexa-vec plus sorafenib arm, 12 (5.5%) patients and 116 (53.2%) patients reported at least one Grade 4 and Grade 3 TEAE, respectively. In the sorafenib arm, 18 (8.3%) patients and 87 (40.1%) patients reported at least one Grade 4 and Grade 3 TEAE, respectively. TEAEs leading to treatment discontinuation were reported in 45 (20.6%) patients in the pexa-vec plus sorafenib arm and 40 (18.4%) patients in the sorafenib arm, with the most common being palmar-plantar erythrodysesthesia syndrome in 7 (3.2%) patients in the pexa-vec plus sorafenib arm and 4 (2.8%) patients in the sorafenib arm, and hepatic failure in 3 (1.4%) patients in the pexa-vec plus sorafenib arm and 4 (1.8%) patients in the sorafenib arm. TEAEs leading to death were reported in 57 (13.1%) patients, including 32 (14.7%) patients in the pexa-vec plus sorafenib arm and 25 (11.5%) patients in the sorafenib arm (Table 3). In the pexa-vec plus sorafenib arm, three deaths were considered by the investigator to be related to pexa-vec or sorafenib: hepatic rupture, post-procedural hemorrhage, and gastrointestinal hemorrhage. No sorafenib-related TEAEs leading to death were reported in the sorafenib arm.
Table 3.
Serious treatment emergent adverse events by treatment, system organ class and preferred term – safety population (in at least 2 patients in any treatment group)
| Event | Pexa-vec + sorafenib (n = 218), n (%) | Sorafenib (n = 217), n (%) |
|---|---|---|
| Any | 33 (15.14) | 25 (11.52) |
| Leading to death | 33 (15.14) | 25 (11.52) |
| Leading to death, attributed to treatment | 3 (1.38) | 0 (0.00) |
| Occurring in ≥0.5% (≥1) of patients in either group | ||
| Hepatic failure | 11 (5.05) | 8 (3.69) |
| Ascites | 8 (3.67) | 4 (1.84) |
| Pyrexia | 8 (3.67) | 1 (0.46) |
| Abdominal pain, upper | 7 (3.21) | 3 (1.38) |
| Hepatic encephalopathy | 5 (2.29) | 4 (1.84) |
| Abdominal pain | 4 (1.83) | 5 (2.30) |
| Anemia | 4 (1.83) | 1 (0.46) |
| Esophageal varices | 4 (1.83) | 3 (1.38) |
| Gastrointestinal hemorrhage | 4 (1.83) | 1 (0.46) |
| Neoplasm progression | 4 (1.83) | 4 (1.84) |
| Peritonitis bacterial | 4 (1.83) | 0 (0.00) |
| Upper gastrointestinal hemorrhage | 4 (1.83) | 1 (0.46) |
| Asthenia | 3 (1.38) | 5 (2.30) |
| Diarrhea | 3 (1.38) | 3 (1.38) |
| Hemoptysis | 3 (1.38) | 0 (0.00) |
| Hepatic function abnormal | 3 (1.38) | 0 (0.00) |
| Hepatic rupture | 3 (1.38) | 0 (0.00) |
| Liver carcinoma rupture | 3 (1.38) | 2 (0.92) |
| Musculoskeletal chest pain | 3 (1.38) | 0 (0.00) |
| Pneumonia | 3 (1.38) | 4 (1.84) |
| Portal vein thrombosis | 3 (1.38) | 0 (0.00) |
| Angina pectoris | 2 (0.92) | 0 (0.00) |
| Aspartate aminotransferase elevation | 2 (0.92) | 0 (0.00) |
| Bile duct stenosis | 2 (0.92) | 0 (0.00) |
| Blood bilirubin elevation | 1 (0.46) | 4 (1.84) |
| Cholecystitis | 2 (0.92) | 0 (0.00) |
| Constipation | 2 (0.92) | 2 (0.92) |
| Gastric hemorrhage | 2 (0.92) | 1 (0.46) |
| General physical health deterioration | 2 (0.92) | 1 (0.46) |
| Hematemesis | 2 (0.92) | 0 (0.00) |
| HCC | 2 (0.92) | 0 (0.00) |
| Hepatic hemorrhage | 2 (0.92) | 0 (0.00) |
| Hepatorenal syndrome | 2 (0.92) | 0 (0.00) |
| Hypotension | 2 (0.92) | 0 (0.00) |
| Ischemic stroke | 0 (0.00) | 2 (0.92) |
| Jaundice cholestatic | 2 (0.92) | 0 (0.00) |
| Liver abscess | 2 (0.92) | 0 (0.00) |
| Multiple organ dysfunction syndrome | 2 (0.92) | 1 (0.46) |
| Myocardial infarction | 2 (0.92) | 0 (0.00) |
| Pulmonary embolism | 2 (0.92) | 1 (0.46) |
| Rash, maculo-papular | 0 (0.00) | 2 (0.92) |
| Respiratory failure | 2 (0.92) | 1 (0.46) |
| Sepsis | 1 (0.46) | 4 (1.84) |
| Tumor pain | 2 (0.92) | 1 (0.46) |
| Tumor rupture | 2 (0.92) | 0 (0.00) |
HHC, hepatocellular carcinoma.
SAEs were coded using Medical Dictionary for Regulatory Activities (MedDRA), version 22.0.
The total number of SAEs counted all SAEs for patients. At each level of patient summarization, a patient was counted once for the most severe event if the patient reported 1 or more events. “n” represents the number of patients at each level of summarization.
Data are sorted in decreasing frequency for the pexa-vec plus sorafenib treatment arm.
As outlined in Table 2, the most frequently reported TEAEs in the pexa-vec plus sorafenib arm overall (in ≥25% of patients) were pyrexia, diarrhea, decreased appetite, nausea, palmar-plantar erythrodysesthesia syndrome, chills, fatigue, abdominal pain, weight decreased, and vomiting. In the sorafenib arm, the most frequently reported TEAEs (in ≥25% of patients) were diarrhea, palmar-plantar erythrodysesthesia syndrome, fatigue, decreased appetite, nausea, and abdominal pain.
TEAEs in which there was a ≥5% difference in frequency in patients treated with pexa-vec plus sorafenib compared to sorafenib included the following: pyrexia (84.4% vs. 12.9%), chills (32.6% vs. 1.84%), rash, pustular (17.9% vs. 0.9%), hypotension (16.1% vs. 0.9%), influenza-like illness (17.0% vs. 2.3%), injection-site pain (12.4% vs. 0%), decreased appetite (39.0% vs. 29.5%), vomiting (25.7% vs. 16.6%), tachycardia (7.3% vs. 0%), abdominal pain, upper (19.7% vs. 13.8%), and back pain (13.3% vs. 7.8%). TEAEs in which there was a ≥5% frequency in patients treated with sorafenib compared to pexa-vec plus sorafenib included the following: palmar-plantar erythrodysesthesia syndrome (45.6% vs. 33.5%), alopecia (21.2% vs. 14.7%), and blood bilirubin elevation (13.4% vs. 7.8%).
As outlined in Table 3, the incidence of serious TEAEs was higher in the pexa-vec plus sorafenib arm compared to the sorafenib arm (117 [53.7%] versus 77 [35.5%] patients, respectively). The most frequently reported (in ≥5 patients) serious TEAEs in the pexa-vec plus sorafenib arm were hepatic failure, pyrexia, abdominal pain upper, ascites, and hepatic encephalopathy. The most frequently reported serious TEAEs in the sorafenib arm were hepatic failure, abdominal pain, and asthenia. Overall, the occurrence of TEAEs and analyses of other safety assessments indicated that both study treatments were safe at the doses administered in this study. The most commonly reported TEAEs (in ≥2 patients) leading to death in the pexa-vec plus sorafenib arm were hepatic failure, HCC, neoplasm progression, tumor rupture, hepatic rupture, and esophageal varices hemorrhage. Within the sorafenib arm, the most common TEAEs leading to death were hepatic failure, neoplasm progression, ruptured liver carcinoma, asthenia, and sepsis.
Further anti-cancer therapies were administered to 83 (35.5%) patients in the pexa-vec plus sorafenib arm and 88 (39.1) patients in the sorafenib arm (online suppl. Table S3). More patients in the sorafenib arm received rescue therapy prior to disease progression compared to the pexa-vec plus sorafenib arm (68 [30.2%]) patients in the sorafenib arm and 34 (14.5%) patients in the pexa-vec plus sorafenib arm). The most commonly administered further anti-cancer therapies were regorafenib (24 [10.3%]) patients in the pexa-vec plus sorafenib arm, 39 (17.3%) patients in the sorafenib arm, and nivolumab (24 [10.3%]) patients in the pexa-vec plus sorafenib arm, 27 (2.0%) patients in the sorafenib arm.
Discussion
With sorafenib providing limited benefit for patients with advanced HCC, it was evident at the time this clinical trial commenced that there was a significant unmet need for agents with novel mechanisms of action [2]. PHOCUS was a phase 3 study conducted to assess the clinical benefit and safety profile of pexa-vec followed by sorafenib treatment, compared with sorafenib only treatment, in patients with advanced HCC without prior systemic therapy. Due to lack of OS benefit observed at the first interim analysis, the study was terminated early, and the originally planned protocol efficacy analyses were modified according to a revised regulatory strategy of Orphan Drug Designation for pexa-vec for the treatment of HCC.
Several factors related to the study design may have affected the survival results obtained at the interim analysis. As a live virus, pexa-vec can produce a number of progeny virions by replicating its own genetic material, eventually leading to lysis of the host cell. However, to promote tumor cell selectivity, the TK1 gene in pexa-vec has been deactivated. Thus, it cannot synthesize thymine nucleotides by itself and is dependent on an active cell cycle in its host cell for replication [15]. However, tyrosine kinase inhibitors, including sorafenib, are designed to stop the cell cycle of constantly dividing cells. While being an efficient mechanism of action, this effectively prevents the viral factory function required for the self-amplification of pexa-vec [17]. These antagonistic effects of agents in the cell cycle inhibitor family on pexa-vec replication have been confirmed in a number of studies [17, 27]. The PHOCUS study was thus designed to prevent concurrent active treatment with the two agents by allowing a 2-week wash-out period between the administration of pexa-vec and sorafenib. However, the impact of sorafenib on sustained pexa-vec replication and immune activation beyond the washout period may have deleteriously impacted pexa-vec activity. It is notable that prior studies of pexa-vec showed continued tumor shrinkage and clinical benefit beyond a third IT dose, while sorafenib, in addition to the above impact on virus replication, has also shown immunosuppressive effects [17]. In the combined pexa-vec plus sorafenib treatment arm, sorafenib was not started until the completion of the pexa-vec therapy based on previously reported pre-clinical hypothesis [17]. Hypothetically, this delay of sorafenib therapy may have permitted tumor growth, resulting in the shorter TTP in the combination treatment arm.
At the time of study initiation, no drugs had been approved for the treatment of HCC after progression with sorafenib. However, during its course, several new agents were approved and administered to study patients [28, 29]. In addition, more patients in the sorafenib arm were administered rescue medication prior to progression, and one of the new protein kinase inhibitors (regorafenib) was more frequently administered in the sorafenib arm, which may have skewed post-progression survival results (online suppl. Table S3).
Additionally, OS might be influenced by post-study medication [28, 30]. Use of immune checkpoint inhibitors or new TKIs as a second line or later was not very common before 2015, when this study started patient recruitment. The relatively shorter PFS of 3 months with a longer OS of 14.0 months in the sorafenib arm of this study compared with the sorafenib arm from the phase III SHARP study with a PFS of 4.3 months and an OS of 10.7 months (19.2 vs. 13.4 months), published in 2008, may provide an insight into the effect of downstream treatment following progression.
Elevated AFP in patients with HCC is associated with a worse prognosis compared with the general HCC population [31–35], and a decline in serum AFP levels during treatment has been associated with tumor response in HCC patients who received various other systemic therapies. Zhu et al. [36] recently reported that HCC patients treated with ramucirumab were more likely to experience an AFP response (decrease) post-baseline compared with patients treated with placebo (ramucirumab: 35.4% vs. placebo: 9.3%; p < 0.0001) and were less likely to experience AFP progression (increase) at any time post-baseline compared with those treated with placebo (ramucirumab: 62.0% vs. placebo: 76.1%; p = 0.0005). In the current study, we assessed the time to AFP >400 ng/mL; no statistically significant difference in TTME was observed between the two treatment arms.
As evident by recent findings across the oncolytic virus (OV) field, direct oncolysis of host cells is not the only, and perhaps not the most important, mechanism of action of OVs [37, 38]. The ability of OVs (particularly those armed with immune-boosting features) to induce long-term anti-tumor immune responses is emerging as an important factor in treatment success, and it is particularly interesting that efficient oncolysis may not always be required to initiate anti-tumor immunity [39, 40].
The occurrence of TEAEs and analyses of other safety assessments indicated that both study treatments (pexa-vec followed by sorafenib and sorafenib alone) were safe at the doses administered. TEAEs were observed in a high percentage of patients in both treatment arms, in 100% of patients in the pexa-vec plus sorafenib arm, and in 98.6% of patients in the sorafenib arm. However more TEAEs were observed in the pexa-vec plus sorafenib treatment arm than in the sorafenib arm (4,062 TEAEs in 218 patients treated with pexa-vec plus sorafenib vs. 2,890 TEAEs in 217 patients treated with sorafenib). The majority of TEAEs in both study arms were low grade, 89.2% in the pexa-vec plus sorafenib arm and 88.0% in the sorafenib arm. In the pexa-vec plus sorafenib arm versus sorafenib arm, TEAEs included pyrexia (84.4% vs. 12.9%), chills (32.6% vs. 1.84%), rash (17.9% vs. 0.9%), hypotension (16.1% vs. 0.9%), influenza-like illness (17.0% vs. 2.3%), and injection-site pain (12.4% vs. 0%), respectively. These TEAEs would be anticipated based on the toxicity profile of pexa-vec, which expressed GM-CSF. TEAEs leading to treatment discontinuation were higher in the pexa-vec plus sorafenib arm than in the sorafenib arm, 65 (29.8%) patients versus 41 (18.9%) patients, respectively. This could have been anticipated considering the increased toxicity with the combination regimen. Despite that, the frequency of TEAEs leading to study discontinuation were similar in the two treatment arms: 45 (20.6%) patients in the pexa-vec plus sorafenib arm and 40 (18.4%) patients in the sorafenib arm. The percentage of patients experiencing a serious adverse event (SAE) was moderately higher in patients treated with pexa-vec plus sorafenib compared to sorafenib, alone, 15.1% versus 11.5%, respectively. All SAEs were fatal in both treatment arms. However, the percentages of SAEs which led to death and which were attributed to treatment were similarly very low in both treatment arm, 1.4% and 0%, respectively. Thus, both treatment arms were reasonably well tolerated.
No new safety signal for pexa-vec was observed in this study. However, the full compliance rate of 3 IT injections in combination group was 75.2%, and 16 adverse events were reported as related to either IT injection or pexa-vec. Also, higher compliance showed the trend of longer OS. Therefore, another method of pexa-vec delivery may improve compliance, tolerability, and, thereby, perhaps the efficacy of treatment (online suppl. Table S2).
It is well known that the immune system plays a crucial role in hepatocarcinogenesis and HCC progression, and as such, immunotherapy and checkpoint inhibitor treatment, in particular, have been the main focus of recent research [40, 41]. The successful improvement of OS obtained with atezolizumab and bevacizumab in combination versus sorafenib with a hazard ratio of 0.66 (95% CI: 0.52, 0.85), leading to its approval for the treatment of advanced HCC, provides an indication of the importance of multi-pronged immune modulation in the HCC setting [5, 41].
Pexa-vec exerts its immune booster functions via expression of the hGM-CSF cassette and has been confirmed to induce both antibody-mediated complement-dependent cytotoxicity and adaptive responses with reactivity to tumor antigens [13]. This priming of anti-tumor immunity may complement the effect of immune checkpoint inhibitors, which are designed to enable an existing anti-tumor immune response, or other types of immunotherapies. As such, it is possible that potent immune modulation, aligning with the bevacizumab-atezolizumab strategy, could be achieved using a checkpoint inhibitor and immune booster (e.g., pexa-vec) combination. We hypothesize that multi-target treatments including tyrosine kinase inhibitors, angiogenesis inhibitors, and locoregional therapies together with immunotherapy may be the way forward for HCC treatment, with the aim of boosting response rates and minimizing resistance. Importantly, the cell cycle inhibitor family, e.g., tyrosine kinase inhibitors, antagonistic effects on pexa-vec replication, and immune stimulation, must be avoided by allowing a sufficient washout prior to pexa-vec treatment and combining with more rationale partner agents.
Despite the novel approach to therapy, the study had a few limitations. A key one was the lack of tissue collection for assessment of virus-tumor interactions. Baseline and on study tissue and blood collection including measurement of virus dissemination and replication, transgene expression, immune induction, or other tissue-based examinations, would have allowed the evaluation of the study population for patients who were more or less likely to benefit from pexa-vec. Along that line, the relatively limited number of patients with BCLC-C staged HCC is a reality reminder of the local therapeutic clinics approach of pexa-vec for BCLC-B patients, while BCLC-C patients could be provided a valuable clinical trial opportunity, and learns more with amply provided tissue. Such data may also have been of interest in order to evaluate any immune-based changes induced by pexa-vec and perhaps allowed for a more rationale selection of any future agents to pair with pexa-vec.
Future studies of pexa-vec in HCC and other potential cancer indications should uniformly attempt to address these shortcomings with appropriately conceived correlate assessments in order to more rationally apply this potential therapeutic and partner in a more scientifically based manner.
Acknowledgments
The authors thank all the patients and their loved ones for their contribution to the study. The authors also thank all the supporting staff for the help and dedication.
Statement of Ethics
The manuscript complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. In the manuscript, all 154 authors state that subjects have given their written informed consent and that the study protocol was approved by each institute’s committee on human research. This study protocol was reviewed and approved by the US National Cancer Institute and was posted on clinicaltrials.gov under approval number NCT02562755. This study protocol was reviewed and approved by ethics committees at each of the participating sites. This full list of participating site and ethics committees can be found at clinicaltrials.gov/study/NCT02562755 and online supplementary Table S4. All patients received oral and written information about the study and signed a written informed consent before study inclusion. All study documentation was reviewed and approved by Local Institutional Ethics Committees/Institutional Review Boards, as applicable.
Conflict of Interest Statement
Ghassan K. Abou-Alfa reports research support from Arcus, Astra Zeneca, BioNtech, BMS, Celgene, Flatiron, Genentech/Roche, Genoscience, Incyte, Polaris, Puma, QED, Silenseed, Yiviva, and consulting support from Adicet, Alnylam, Astra Zeneca, Autem, Beigene, Berry Genomics, Boehringer Ingelheim, Celgene, Cend, CytomX, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Genoscience, Helio, Helsinn, Incyte, Ipsen, Merck, Nerviano, Newbridge, Novartis, QED, Redhill, Rafael, Servier, Silenseed, Sobi, Vector, Yiviva, plus patent PCT/US2014/031545 filed on March 24, 2014, and priority application Serial No.: 61/804,907; filed: March 25, 2013. Peter R. Galle reports research support from Bayer and Roche and consulting support from Bayer, Boston Scientific, AstraZeneca, Adaptimmune, BMS, Eisai, MSD, Sirtex, Lilly, Roche, Guerbet, and Ipsen. Joseph Erinjeri reports consulting support from AztraZeneca. Jeong Heo received grants/research support from Roche, Yuhan, and Gilead and participates on trials steering committee for Sillajen, Oncolys, Gilead, and AstraZeneca. Mitesh J. Borad report research support from Senhwa Pharmaceuticals, Adaptimmune, Agios Pharmaceuticals, Halozyme Pharmaceuticals, Celgene Pharmaceuticals, EMD Merck Serono, Toray, Dicerna, Taiho Pharmaceuticals, Sun Biopharma, Isis Pharmaceuticals, Redhill Pharmaceuticals, Boston Biomed, Basilea, Incyte Pharmaceuticals, Mirna Pharmaceuticals, Medimmune, Bioline, Sillajen, ARIAD Pharmaceuticals, PUMA Pharmaceuticals, Novartis Pharmaceuticals, QED Pharmaceuticals, Pieris Pharmaceuticals, and consulting support from ADC Therapeutics, Exelixis Pharmaceuticals, Inspyr Therapeutics, G1 Therapeutics, Immunovative Therapies, OncBioMune Pharmaceuticals, Western Oncolytics, Lynx Group, Genentech, Merck, Huya. James Burke is employed by CG Oncology and is consultant for Oncomyx, Kalivir, Western Oncolytics, Sonata, and Amped. Adina Pelusio is employed by Kalivir Immunotherapeutics and reports previous employment by SillaJen Biotherapeutics and Turnstone Biologics, Corp. Delphine Agathon reports previous employment by Transgene. Caroline Breitbach is an inventor on SillaJen patents. Edward Gane is a member of the Scientific Advisory Boards as an advisor and/or speaker for AbbVie, Abbott Diagnostics, Aligos, Arbutus, Arrowhead, Assembly, Dicerna, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Novartis, Roche, Surrozen, The Liver Company, Vaccitech, Virion Therapeutics, and Vir Bio and is on Speakers’ Bureau for AbbVie and Abbott Diagnostics. Yee Chao, Angelo Luca, Monika Lusky, and Shukui Qin report no conflicts of interest.
Funding Sources
This study was fully funded by Sillajen Inc.
Author Contributions
All authors Ghassan K. Abou-Alfa, Peter R. Galle, Yee Chao, Joseph Erinjeri, Jeong Heo, Mitesh J. Borad, Angelo Luca, James Burke, Adina Pelusio, Delphine Agathon, Monika Lusky, Caroline Breitbach, Shukui Qin, and Edward Gane planned the study and contributed to the interpretation of the data and revisions, and gave input at all stages of the study. All the authors have approved the final version of the manuscript.
Funding Statement
This study was fully funded by Sillajen Inc.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. World Health Organization International Agency for Research on Cancer (IARC) . GLOBOCAN 2020: Liver. [cited 2021 Apr 27].
- 2. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016 May 20;34(15):1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med Overseas Ed. 2008 Jul 24;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S, Han K, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 5. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_Suppl):267. [Google Scholar]
- 6. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 7. Lauer UM, Beil J. Oncolytic viruses: challenges and considerations in an evolving clinical landscape. Future Oncol. 2022 Jul 12;18(24):2713–32. [DOI] [PubMed] [Google Scholar]
- 8. Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006 Sep;14(3):361–70. [DOI] [PubMed] [Google Scholar]
- 9. Kim MK, Breitbach CJ, Moon A, Heo J, Lee YK, Cho M, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med. 2013 May 15;5(185):185ra63. [DOI] [PubMed] [Google Scholar]
- 10. Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9(6):533–42. [DOI] [PubMed] [Google Scholar]
- 11. Parato KA, Breitbach CJ, le Boeuf F, Wang J, Storbeck C, Ilkow C, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20(4):749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Breitbach CJ, Arulanandam R, de Silva N, Thorne SH, Patt R, Daneshmand M, et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013 Jan 15;73(4):1265–75. [DOI] [PubMed] [Google Scholar]
- 13. Breitbach CJ, Parato K, Burke J, Hwang TH, Bell JC, Kirn DH. Pexa-Vec double agent engineered vaccinia: oncolytic and active immunotherapeutic. Curr Opin Virol. 2015;13:49–54. [DOI] [PubMed] [Google Scholar]
- 14. Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013 Mar;19(3):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hengstschlagers M, Knoflero M, Miillner EW, Ogrisn E, Wintersberger E, Wawrall E. Different regulation of thymidine kinase during the cell cycle of normal versus DNA tumor virus-transformed cells. J Biol Chem. 1994;269(19):13836–42. [PubMed] [Google Scholar]
- 16. Breitbach CJ, Moon A, Burke J, Hwang TH, Kirn DH. A phase 2, open-label, randomized study of Pexa-Vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol Biol. 2015;1317:343–57. [DOI] [PubMed] [Google Scholar]
- 17. Heo J, Breitbach C, Cho M, Hwang TH, Kim CW, Jeon UB, et al. Phase II trial of Pexa-Vec (pexastimogene devacirepvec; JX-594), an oncolytic and immunotherapeutic vaccinia virus, followed by sorafenib in patients with advanced hepatocellular carcinoma (HCC); 2013. p. 4122. [Google Scholar]
- 18. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 19.ECOG Performance Status - ECOG-ACRIN. [cited 2021 May 20]. Available from: https://ecog-acrin.org/resources/ecog-performance-status.
- 20. Abou-Alfa GK, Galle PR, Chao Y, Brown KT, Heo J, Borad MJ, et al. PHOCUS: a phase 3 randomized, open-label study comparing the oncolytic immunotherapy Pexa-Vec followed by sorafenib (SOR) vs SOR in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy. J Clin Oncol. 2016 May 20;34(15_Suppl):TPS4146. [Google Scholar]
- 21. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973 Aug;60(8):646–9. [DOI] [PubMed] [Google Scholar]
- 22. Llovet JM, Fuster J, Bruix J; Barcelona-Clínic Liver Cancer Group . The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl):S115–20. [DOI] [PubMed] [Google Scholar]
- 23. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgan KL, Rubin DB. Rerandomization to improve covariate balance in experiments. Ann Statist. 2012 Apr;40(2) 1263–82. [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 26. Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel methods: applications and recent developments. Annu Rev Public Health. 1988;9:123–60. [DOI] [PubMed] [Google Scholar]
- 27. Peng C, Zhou Y, Cao S, Pant A, Campos Guerrero ML, McDonald P, et al. Identification of vaccinia virus inhibitors and cellular functions necessary for efficient viral replication by screening bioactives and fda-approved drugs. Vaccines. 2020 Sep 1;8(3):401–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018 Jul 5;379(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 Jan 7;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 30. Saad ED, Buyse M. Statistical controversies in clinical research: end points other than overall survival are vital for regulatory approval of anticancer agents. Ann Oncol. 2016;27(3):373–8. [DOI] [PubMed] [Google Scholar]
- 31. Zhu AX, Finn RS, Kang YK, Yen CJ, Galle PR, Llovet JM, et al. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. Br J Cancer. 2021 Apr;124(8):1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tyson GL, Duan Z, Kramer JR, Davila JA, Richardson PA, El-Serag HB. Level of α-fetoprotein predicts mortality among patients with hepatitis C-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011 Nov 1;9(11):989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Chen Y, Ge N, Zhang L, Xie X, Zhang J, et al. Prognostic significance of alpha-fetoprotein status in the outcome of hepatocellular carcinoma after treatment of transarterial chemoembolization. Ann Surg Oncol. 2012 Oct;19(11):3540–6. [DOI] [PubMed] [Google Scholar]
- 34. Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993 Jun 24;328(25):1797–801. [DOI] [PubMed] [Google Scholar]
- 35. Kudo M, Izumi N, Sakamoto M, Matsuyama Y, Ichida T, Nakashima O, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer. 2016;5(3):190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015 Jul 1;16(7):859–70. [DOI] [PubMed] [Google Scholar]
- 37. Yoo SY, Badrinath N, Woo HY, Heo J. Oncolytic virus-based immunotherapies for hepatocellular carcinoma. Mediators Inflamm. 2017;2017:5198798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaurasiya S, Fong Y, Warner SG. Oncolytic virotherapy for cancer: clinical experience. Biomedicines. 2021 Apr 13;9(4):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres-Domínguez LE, McFadden G. Poxvirus oncolytic virotherapy. Expert Opin Biol Ther. 2019;19(6):561–73. [DOI] [PubMed] [Google Scholar]
- 40. Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer. 2019;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.