Abstract
Background
Intrahepatic cholangiocarcinoma (iCCA) is often diagnosed at an advanced stage, leading to limited treatment options and a poor prognosis. So far, standard systemic therapy for advanced iCCA has been a combination of gemcitabine and cisplatin. However, recent advancements in the understanding of the molecular characteristics of iCCA have opened new possibilities for molecular-targeted therapies and immunotherapy.
Summary
Reportedly, 9–36% of iCCA cases have an inflamed tumor immune microenvironment (TME) based on the immune gene expression signature, which is characterized by the presence of immune cells involved in anti-tumor immune responses. The majority of iCCA cases have a non-inflamed TME with a lack of effector T cells, rendering immune checkpoint inhibitors (ICIs) ineffective in these cases. Interestingly, alterations in the fibroblast growth factor receptor (FGFR2) gene and IDH1/2 gene mutations are often observed in the non-inflamed TME in iCCA. Several mechanisms have been reported for the role of driver mutations on the establishment of TME unique for iCCA. For example, IDH1/2 mutations, which cause an increase in DNA methylation, are associated with the downregulation and hypermethylation of antigen processing and presentation machinery, which may contribute to the establishment of a non-inflamed TME. Therefore, inhibitors targeting IDH1/2 may restore the DNA methylation and expression status of molecules involved in antigen presentation, potentially improving the efficacy of ICIs. FGFR inhibitors may also have the potential to modulate immunosuppressive TME by inhibitingthe suppressor of cytokine signaling 1 and activating the interferon-γ signaling as a consequence of inhibition of the FGFR signal. From this perspective, understanding the molecular characteristics of iCCA, including the TME and driver mutations, is essential for the effective application of ICIs and molecular-targeted therapies.
Key Messages
Combination approaches that target both the tumor and immune system hold promise for improving the outcomes of patients with iCCA. Further research and clinical trials are needed to validate these approaches and optimize the treatment strategies for iCCA.
Keywords: Cholangiocarcinoma, Immune checkpoint inhibitors, Tumor immune microenvironment, Driver mutation, Molecular-targeted agents
Introduction
Intrahepatic cholangiocarcinoma (iCCA) accounts for almost 3–6% of primary liver cancers, and its proportion has increased gradually in recent years. Chronic hepatitis virus infections, such as chronic hepatitis B and C, are associated with the risk of iCCA and hepatocellular carcinoma (HCC). Therefore, caution should be exercised when diagnosing liver tumors that develop in the presence of chronic hepatitis and liver cirrhosis because the chemotherapy agents required for these two types of cancers are completely different. Generally, iCCA is asymptomatic in the early stages, and currently, there is no solid screening program for its detection. Therefore, although curative resection is the first choice of treatment, there are many inoperable patients who should be treated with systemic chemotherapy because of tumor progression, and the development of effective systemic therapy is required for improving the prognosis of patients with iCCA.
To date, the combination therapy using gemcitabine and cisplatin (GC) has been considered as the standard therapy for advanced cholangiocarcinoma, including iCCA [1]. A recent phase 3 trial also revealed the superiority of GC treatment in combination with tegafur, gimeracil, and oteracil potassium, commonly referred to as TS-1, over GC treatment alone in terms of overall survival (OS) and progression-free survival (PFS) in patients with unresectable or recurrent tumors [2].
In addition, molecular-targeted agents (MTAs) targeting driver mutations responsible for the tumor development have shown promising efficacy in advanced cholangiocarcinoma with disease progression on first-line chemotherapy. For example, according to the findings from the phase 2/3 clinical trials, anti-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitors (TKIs) and isocitrate dehydrogenase (IDH) inhibitors have exhibited effectiveness specifically in tumors carrying FGFR2 fusions or rearrangements and IDH1 mutations, respectively [3, 4]. Regarding first-line chemotherapy, an immune checkpoint inhibitors (ICIs) and GC combination significantly improves anti-tumor response and survival in patients with iCCA compared to GC treatment alone [5].
Since MTAs and ICIs can be used for the treatment of iCCAs, understanding the role of tumor immune microenvironment (TME) and driver mutations is essential because the TME affects the tumor response to ICIs, and the status of the driver mutation is associated with the efficacy of MTAs [6]. In this review, we focused on the TME and mutational status of iCCAs to determine the effectiveness of applyingMTAs and ICIs for advanced iCCAs. We especially focus on the impact of specific mutations for developing the unique TME because blocking the oncogenic signal with MTAs may reverse the immune-suppressive TME for the treatment using ICIs.
Molecular Classification of iCCA
To date, several studies have described the transcriptome-based classification of cholangiocarcinoma, including iCCA, and their clinical significance [7–9]. These molecular subclasses are useful in predicting treatment outcomes and responses. Comprehensive genomic and transcriptomic analyses of surgically resected iCCAs revealed two distinct tumor classes with varying clinical outcomes that were associated with KRAS or BRAF mutations [7]. Based on cluster analysis using the expression of 238 differentially expressed genes, tumors can be classified into two clusters, with differences in 5-year OS and time to recurrence [7]. All patients carrying KRAS/BRAF mutations are classified into groups characterized by a poor prognosis [7]. Generally, iCCA with KRAS/BRAF mutations and poor prognosis is associated with large ductal type histology, whereas tumors with IDH1 mutations, FGFR2 fusion/rearrangement, and relatively good prognosis are more common in the small ductal type of iCCA [10].
Molecular analyses have also been employed to identify different biological classes of cholangiocarcinoma with unique activation profiles for specific signaling pathways. Sia et al. [11] performed an integrated molecular analysis of iCCA and identified two tumor classes that had different outcomes: 38% of tumors were classified into the inflammation class and the remaining 62% were considered as a proliferation class. The inflammation class exhibited the activation of inflammatory signaling pathways and overexpression of cytokines with the activation of signal transducer and activator of transcription 3 (STAT3). In contrast, the proliferative class was characterized by KRAS/BRAF mutations, activation of oncogenic signaling pathways, and gene expression associated with unfavorable outcomes [11]. Patients with iCCAs in the proliferation class exhibited notably worse OS compared to those with tumors classified in inflammation class [11].
Development of TME in iCCA
In the early stages of cancer, tumor cells are eliminated by anti-tumor immunity caused by tumor-specific peptides and damage-associated molecular patterns derived from damaged tumor cells. In this phase, the innate and adaptive immune systems cooperate and recognize transformed cells for elimination before they develop into visible tumors [12]. In the next step, the growth of cancer cells and their destruction by the immune system reach equilibrium, where the progression of tumor volume is limited, and cellular immunogenicity is edited by the adaptive immune system [12]. Finally, the activation of immunosuppressive and immune-evasive pathways overcomes anti-tumor immunity, which is attributed to the alteration of immune cell components and cytokine profiles, accompanied by the expression of immune checkpoint molecules. Thus, cancers diagnosed in routine clinical practice carry a complex suppressive TME, in which cancer cells may escape anti-tumor immunity.
The growing focus on immunotherapeutic approaches in oncology has shed light on the clinical significance of the immune-based classifications of iCCA. The TME of iCCA is characterized by a lack of cytotoxic immune cells and an abundance of immunosuppressive components, including tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and cancer-associated fibroblasts (CAFs). Bao et al. [13] showed that iCCA can be classified into three subtypes that are associated with chromatin remodeling, metabolism, and chronic inflammation, respectively, using multi-omics analysis. The chronic inflammation subtype of iCCA, characterized by apolipoprotein E (APOE)+, complement C1q B chain (C1QB)+ TAMs, had poor outcomes. Another study performed single-cell transcriptomic analysis and identified two iCCA tumor subtypes based on the expression of S100 calcium-binding protein P (S100P) and secreted phosphoprotein 1 (SPP1, osteopontin), each with a distinct TME and prognosis. The S100P+SPP1− type represented the large duct type, and the S100P−SPP1+ type was associated with the small duct type iCCA characterized by less aggressive tumor behavior [14]. The TME affects the efficacy of ICIs in cancer treatment; TME with high levels of CD14+ monocytes was reportedly resistant to anti-programmed cell death-1 (PD-1) antibodies and had poor outcomes. CD14+ monocytes were characterized by the overexpression of immunosuppressive cytokines and chemokines, which induced suppression of CD4+ T-cell function and played a role in the immunosuppressive TME [15].
CAFs also play a pivotal role in the immune milieu of iCCA [16]. Cholangiocarcinoma is typically identified by a desmoplastic reaction marked by a prominent composition of CAF. Recent research has uncovered the distinct CAF subsets for modulating TME as well as promoting malignant tumors [17]. Myofibroblastic CAF and inflammatory CAF (iCAF) originate from hepatic stellate cells (HSCs). Mainly, myofibroblastic CAFs show high α-SMA expression and are located close to cancer cells, playing a role in stromal remodeling. In contrast, iCAFs are situated farther from cancer cells. While they have lower α-SMA levels, they produce more inflammatory molecules, including interleukin (IL)-6, IL-8, and IL-11; iCAFs may potentially promote immune suppression through the activation of STAT3 signaling. A rare subset known as mesothelial CAF originates from portal fibroblasts and expresses mesothelial markers [17]. Additionally, another analysis identified additional CAF subtypes in iCCA; antigen-presenting CAFs and vascular CAFs. Notably, the antigen-presenting CAF expresses a major histocompatibility complex class 2 family and B-cell marker (CD74), which is associated with the immunomodulatory effect through the interaction with CD4+ T cell [18]. The vascular CAFs are marked by CD146 expression and IL-6/STAT3 signaling, predominantly infiltrating the tumor core and microvascular regions [19]. This localization of CAF subsets within the TME may play a crucial role in establishing interactions with other cells. Besides paracrine signaling, CAFs secrete various growth factors and cytokines that maintain and differentiate CAF subsets as well as attract several immune-suppressive cells including regulatory T cell (Treg), MDSC, and TAM. Notably, CAFs also secrete WNT-2 that act as a suppressor for activation and differentiation of dendric cell (DC), which also result in the establishment of immune-suppressive TME [20]. In addition, members of the transforming growth factor (TGF) superfamily have been implicated in inducing a pro-tumorigenic phenotype in CAFs [16].
A separate study has linked the role of CAF to varying immune infiltration patterns in iCCA, offering the potential for patient stratification based on the TME features. Generally, immune “cold” tumors exhibit a deficiency in immune cell infiltration, wherein CAFs and the extracellular matrix (ECM) may impede the infiltration of T cells and their interaction with tumor cells. Moreover, several reports indicate the existence of a subset of iCCA cases characterized by the presence of immune-suppressive cells and a desmoplastic reaction. Essentially, these tumors exhibit enhanced TGF-β signaling, angiogenesis, and Notch signaling pathways, indicating altered and suppressive anti-tumor responses attributed to the functions of CAFs (Fig. 1). Therefore, the dynamic interplay among various cell types and the ECM within the TME significantly influences tumor progression and constrains the effectiveness of anti-tumor therapies.
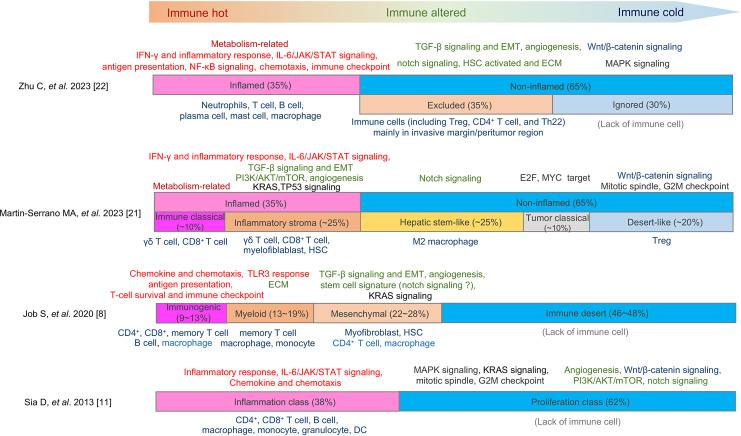
Fig. 1.
Comparison of TME classification in iCCA. Four different classifications of the tumor immune microenvironment (TME) in intrahepatic cholangiocarcinoma (iCCA) are presented. The length of each bar represents the reported frequency of each TME subclass. The primary pathways associated with each subclass are depicted at the top of the bar. Inflammation-related pathways are denoted in red, pathways related to mesenchymal response in green, and those linked to cell cycle and tumor growth in black. Unique to typical immune hot and immune cold tumors are the metabolism-related and Wnt/β-catenin signaling pathways, respectively, which are indicated in brown and blue. The predominant cell types infiltrating each subclass of tumors are shown at the bottom of the bar.
Activation of Oncogenic Signaling and Immune Subclass of iCCA
Conversely, transcriptome-based classifications of TME are also reported. Job et al. [8] identified four immune subclasses, including immune desert, immunogenomic, myeloid, and mesenchymal subclasses, which showed different immune evasion feature and patient outcomes, using transcriptomics from 198 iCCA specimens. The immune-desert subclass, characterized by downregulation of TME gene signatures, was the dominant class observed in approximately 46–48% of the cases. Martin-Serrano et al. [21] also reported a novel molecular classification of iCCA that integrated immune, stroma, and tumor characteristics and classified tumors into non-inflamed classes (65% of the cases) and inflamed class (35% of the cases). They also subclassified the non-inflamed classes into the “hepatic stem-like,” “tumor classical,” and “desert-like.” The “hepatic stem-like” subclass was characterized by the enrichment of stemness-related pathway and infiltration of M2 macrophages and occurrence of IDH and BRCA1 associated protein-1 (BAP1) mutations and alterations of FGFR2; the “tumor classical” subclass represented activation of cell cycle pathway and showed unfavorable patient outcomes. The “desert-like” subclass showed activation of Wnt/β-catenin signaling and lack of immune cells.
On the contrary, the inflamed class-exhibited activation of the IL-6/JAK/STAT and interferon-γ signaling pathways, accompanied by an increase in inflammatory responses. Notably, the inflamed class was further subcategorized into “immune classical” and “inflammatory stroma.” The former was characterized by an enhancement of metabolic-related pathways, while the latter displayed an increase in several oncogenic pathways, such as TGF-β, phosphatidylinositol-3 kinase (PI3K)/AKT, and KRAS signaling.
Zhu et al. [22] reported on the three spatial immunophenotypes of iCCA and explored the immune escape mechanisms associated with them. The inflamed class, which accounted for 35% of the cases, showed immune cell infiltration into the tumor, an increase in IFN-γ and inflammatory responses, and the activation of the IL-6/JAK/STAT signaling pathway. In contrast, the “excluded” and “ignored” phenotypes comprised the non-inflamed class, characterized by the upregulation of TGF-β and Wnt/β-catenin signaling pathways, as well as angiogenesis. The excluded class, where immune cells were primarily observed at the invasive margin and peritumor region, exhibited an increase in Notch signaling and the activation of HSCs. The ignored class, characterized by a lack of immune cells, showed an increase in mitogen-activated protein kinase signaling.
Based on these findings, generally, inflamed tumors displayed an activation of immune responses, including IFN-γ responses, antigen presentation, and IL-6/JAK/STAT signaling. Although immune checkpoint molecules could be expressed due to continuous immune stimulation and T-cell exhaustion, ICIs may be effective in this case. On the other hand, there are cases with altered immune phenotypes, showing an increase in TGF-β signaling, angiogenesis, Notch signaling, and the activation of HSCs. In such situations, several mesenchymal reactions may be enhanced, including the induction of abnormal ECM, neovascularization, and an increase in mesenchymal stem cells. Abnormal ECM limits the infiltration of T cells and activation of TGF-β, vascular endothelial growth factor (VEGF), and Notch signaling lead to the attraction of Tregs and M2 pro-tumoral macrophages. In cases with a lack of immune cells in the tumor and peritumoral region, anti-tumor immune reactions are expected to be absent, rendering them refractory to treatment with ICIs. The activation of the Wnt/β-catenin signaling pathway may play a role in this type of immune evasion (Fig. 1).
Unique Genetic/Epigenetic Alterations and Establishment of TME
The accumulation of genetic and epigenetic alterations occurs during carcinogenesis, and driver mutations that emerge during multistep carcinogenesis may affect the TME of cancer. Therefore, understanding the oncogenic alterations that are unique to each type of tumor is crucial. In many solid tumors, including liver cancer, various genetic and epigenetic alterations accumulate, leading to alterations in oncogenic signaling pathways. These changes play a major role in the establishment of the TME through altered expression of immune-related molecules [23]. Alterations of oncogenic signaling pathways in cancer cells facilitate resistance to ICIs by modulating cancer immune surveillance. The activation of oncogenic signaling, including receptor tyrosine kinases, MAPK, PI3K-AKT-mTOR, JAK-STAT, Hippo, and Wnt/β-catenin, modulates cancer immunity and induces ICI resistance [23]. For example, in melanoma, activating mutations in Wnt/β-catenin signaling suppresses the infiltration of DC and CD8+ T cells into tumor tissues by downregulating the CC chemokine ligand (CCL) 4. Wnt/β-catenin signaling also inhibits DC by upregulating IL-10 in melanoma [24]. Mutations in the Wnt/β-catenin signaling pathway act as drivers of hepatocarcinogenesis and are closely associated with a non-inflamed TME with the downregulation of CCL5, where the efficacy of ICIs should be attenuated [25]. The activation of yes-associated protein (YAP), which is regulated by the tumor suppressor Hippo signaling, also participates in the establishment of an immunosuppressive TME in a mouse HCC model. YAP induces CCL2 through direct transcriptional regulation, leading to the induction of tumor-associated M2 macrophages, which are known to confer resistance to ICIs [26]. Hence, oncogenic alterations in tumors that drive carcinogenesis may affect the establishment of a unique TME in cancer.
Classification of the TME status of iCCAs was performed using a transcriptome dataset of immune-related molecules from public databases [21]. Approximately 35% of iCCAs have an inflamed TME, and the remaining 65% are classified as non-inflamed. Tumors belonging to the non-inflamed class may not respond well to ICIs because of the lack or malfunction of effector T cells in anti-tumor immunity. Additionally, tumors carrying actionable mutations/alterations, such as FGF2R alterations and IDH1/2 mutations, are predominantly classified into the non-inflamed class [21].
Mutations commonly observed in iCCAs are associated with the TME status [8]. Mody et al. [27] conducted an integrative cluster analysis of tumors and found that FGFR2 fusion is inversely associated with the infiltration of immune cells in tumors. Another study revealed that many tumors with FGFR2 alterations represented decreased immune cell infiltrations [28]. Similarly, some studies have indicated that IDH1/2 mutations contribute to the establishment of a cold immune microenvironment [29]. IDH1/2 mutations are mainly observed in iCCA with a hepatic stem cell-like feature in the non-inflamed type [21]. However, another study reported that hepatitis B virus-related iCCAs carrying IDH1/2 mutations showed T-cell infiltration in tumor tissues [28].
Driver Mutations May Cause Non-Inflamed TME in Liver Cancer
The mutation profiles of genes involved in carcinogenesis differ between HCC and iCCA, and such alterations may trigger the establishment of a unique TME. In the case of HCC, Montironi et al. [30] reported that 63% of tumors were carrying a “non-inflamed” TME with enrichment of CTNNB1 mutations. Mutations in the Wnt/β-catenin pathway reportedly induced a downregulation of CCL5 and the exclusion of tumor-infiltrating lymphocytes (TILs) in a mouse model of HCC (Fig. 2a) [25]. In addition, the activation of β-catenin is associated with a poor response to ICI treatment in patients [31]. We also reported the association between mutations in genes involved in the Wnt/β-catenin pathway and the anti-tumor effects of anti-PD-1 monotherapy in HCC cases [32]. The presence of mutations in the Wnt/β-catenin pathway was related to a lack of TILs and shorter PFS on anti-PD-1 antibody. Activation of Wnt/β-catenin pathway, decrease in TILs, and expression of programmed cell death-ligand 1 (PD-L1) correlate well with the duration of PFS [32].
Fig. 2.
Role of driver mutation in non-inflamed tumor immune microenvironment (TME): A comparison of HCC and iCCA. a Driver mutation and establishment of non-inflamed TME in HCC. Mutations in the Wnt/β-catenin pathway, including the CTNNB1 mutation, are a well-known driver for hepatocarcinogenesis. The activation of this pathway, reportedly, induces a transcription repressor ATP3 and downregulates CCL4 in melanoma and CCL5 in HCC. Decrease of expression of CCL4/5 may inhibit the recruitment of CD103+ dendric cell (DC) and CD8+ cytotoxic T cell (CTL). b Driver mutation and establishment of non-inflamed TME in iCCA. Mutations in IDH1/2, and FGFR2 fusions/rearrangements are the characteristic driver mutations for iCCA. Mutant IDH1/2 induces a potent inhibitor of α-KG-dependent DNA demethylases and ten–eleven translocation (TET) enzymes, D-2-hydroxyglutarate (D-2-HG) that catalyze the iterative demethylation of 5-methylcytosine. Therefore, this type of mutation may cause hypermethylation and downregulation of genes including those involved in antigen processing and presentation, contributing to the establishment of a “non-inflamed” in iCCAs. Regarding the FGFR2 alterations, their fusions, and rearrangements induce the constitutive activation of the FGF signaling that lead to the inhibition of the IFN-γ signaling through the induction of SOCS1. Decrease of IFN-γ signaling may also result in the downregulation of antigen processing and presenting machineries. Therefore, it is possible that FGFR2 alterations also contribute to the establishment of a “non-inflamed” phenotype in iCCAs. The red arrows represent activation/induction events, and the gray arrows show inactivation/inhibition events, respectively.
In contrast, regarding the iCCA cases, mutations in IDH1/2, KRAS, BAP1, and FGFR2 fusions/rearrangements are the characteristic driver mutations. IDH1-mutated tumors may lead to increased production of D-2-hydroxyglutarate (D-2-HG), potentially exerting inhibitory effects on immune cells, including CD4+ and CD8+ T cells [33]. Importantly, mutant IDH1/2 also induces epigenetic changes, where the conversion of α-ketoglutarate (α-KG) to D-2-HG take place and D-2-HG acts as an inhibitor of KG-dependent DNA demethylases and ten–eleven translocation (TET) enzymes that catalyze the demethylation of 5-methylcytosine, further induces an increase in the methylation and downregulation of corresponding genes [34]. Recently, we reported that “non-inflamed” iCCAs were associated with the downregulation of genes involved in antigen processing and presentation [35]. Interestingly, these genes were hypermethylated and downregulated in tumors classified as non-inflamed class (Fig. 2b). We also found that the expressions of these antigen processing and presenting machineries were inversely correlated with their DNA methylation levels in iCCAs [35]. Interestingly, in mouse models, combining IDH1 inhibition with anti-PD-1 or anti- cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) resulted in improved outcomes compared to IDH1 inhibition alone [29, 33].
Alterations in genes related to antigen processing and presentation can, reportedly, cause an immune cold phenotype in many types of cancers. Colorectal cancers with microsatellite instability reportedly show a decrease in TILs, which is attributed to the downregulation of HLA-A, HLA-B, and HLA-C genes [36]. In addition, Job et al. [8] reported that decreased expressions of antigen-presenting machineries are the characteristics of immune desert subclass of iCCA. Therefore, methylation-induced downregulation of the molecules involved in antigen presentation may contribute to the formation of a “non-inflamed” TME in various cancers, including iCCAs. Because an increase in DNA methylation may be, at least partially, attributed to mutations in IDH1/2, we analyzed the association between these mutations and the expression/methylation patterns of antigen processing and presentation machineries. As expected, the majority of mutations in IDH1/2 were detected in tumors with hypermethylation and downregulation of antigen processing and presenting machinery in iCCA cases [35].
Aberrant FGFR signaling at the tumor level can also lead to downregulation of antigen-presenting MHC II molecules and upregulation of PD-L1 in some tumor types [37]. Furthermore, stimulation of FGFR can upregulate PD-1 on effector T cells, promote survival of Tregs through STAT5 phosphorylation, and skew macrophages toward the M2 phenotype, collectively generating an overall immunosuppressive effect [37]. On the other hand, Jusakul et al. [38] found that iCCAs enriched in FGFR alterations and IDH1/2 mutations represented hypermethylation at the CpG shore. Stimulation of FGF signaling induces activation of the suppressor of cytokine signaling 1 (SOCS1) and suppresses the interferon-γ (IFN-γ) signaling [39]. This inhibition leads to the downregulation of β2-microglobulin through the induction of DNA methylation [40]. We found that alteration of the FGFR2 gene was also associated with downregulation or hypermethylation of antigen processing and presenting machinery [35]. Therefore, FGFR2 alterations may also contribute to the establishment of a “non-inflamed” tumor (Fig. 2b).
Oncogenic KRAS upregulates the expression of toll-like receptor 4 (TLR4), inducing downstream IL-1 production, which promotes polarization of M2 macrophages and the accumulation of Th17 cells and MDSCs, ultimately suppressing the activation of CD8+ T cells [41]. In mouse models, KRAS inhibition reversed this phenotype, leading to an increased M1/M2 macrophage polarization ratio and an increase in CD4+ and CD8+ TILs [42]. Although amplification of HER2 is not common in iCCA, targeting amplified HER2 receptors can induce anti-tumor effects partly by eliciting adaptive and innate immune responses [43].
MTAs for iCCA
Significant progress has been made in terms of exploring and treating cholangiocarcinoma based on the identification of actionable genetic alterations. In recent years, molecular agents that target driver mutations frequently detected in iCCA have been developed. Distinct differences of mutational landscapes in iCCA, perihilar CCA (pCCA), and distal CCA (dCCA) are observed. Actionable genetic changes such as FGFR2 fusions or rearrangement and IDH1 mutations are more common in iCCA, whereas HER2 amplifications and KRAS mutations are more frequently found in pCCA and dCCA than in iCCA. These actionable genetic alterations, found in a significant percentage of cholangiocarcinoma cases, have transformed therapeutic approaches by enabling the use of systemic chemotherapy for advanced-stage diseases.
FGFR2 fusion and rearrangement are representative actionable alterations in iCCA. To activate FGFR signaling, receptor dimerization is required upon ligand binding. The fusion and rearrangement of FGFR2 induces dimerization without ligand binding, which leads to the constitutive activation of FGF signaling. Pemigatinib, a selective and potent inhibitor of FGFR1, 2, and 3, which inhibits the phosphorylation in the intracellular domain of the FGF receptor for FGF signaling, was approved by FDA; Abou-Alfa et al. [44] reported that efficacy of pemigatinib on locally advanced or metastatic cholangiocarcinoma with fusion and rearrangement of the FGFR2 gene. They found that 35.5% of iCCA patients carrying FGFR2 fusions or rearrangements showed an objective response (OR) with three cases of complete responses. These data indicate the therapeutic potential of pemigatinib for tumors with FGFR2 fusion or rearrangement. Another FGFR inhibitor, infigratinib, has demonstrated promising results in phase 2 trials, with an OR rate (ORR) of 23.1% [45]. A next-generation covalently binding FGFR1-4 inhibitor, futibatinib, has also shown efficacy in iCCA patients with FGFR2 alterations, with an ORR of 41.7% [4]. However, overcoming acquired resistance to FGFR inhibitors, generally associated with alterations in the kinase domain of FGFR2, remains challenging. Some studies have reported that futibatinib shows selective anti-tumor activity against FGFR-deregulated cholangiocarcinoma in cases where patients have experienced progression on reversible FGFR inhibitors [46].
Tumors carrying IDH1 mutations, which are predominantly found in iCCA, can be treated with IDH1 inhibitors. Mutations in IDH1 and IDH2 have been detected in 20.6–29.1% and 2.5–4.4% of iCCA cases, respectively [47]. In 2020, ivosidenib, an IDH1 inhibitor, reportedly showed superiority in PFS and OS compared to a placebo in patients with cholangiocarcinoma and IDH1 gene mutations refractory to conventional chemotherapy [3]. Other IDH1 inhibitors such as olutasidenib are also under investigation. In contrast, IDH1/2 mutation confers an oncogenic function that leads to the production of D-2-HG. Accumulation of D-2-HG inhibits the function of histone demethylases, which are critical for homologous recombination DNA repair, including the repair of DNA double-strand breaks. Poly ADP-ribose polymerase (PARP) is essential for single-stranded DNA break repair. In IDH mutant tumors, PARP inhibitors induce synthetic lethality by suppressing the repair of single-strand breaks, which eventually leads to their conversion into double-strand breaks [48]. Therefore, in cases of IDH1 mutations, PARP inhibitors have been explored in combination with IDH1 inhibitors.
BRAFV600E mutations, occurring in a small subset of iCCA cases [49], can be targeted using a combination of BRAF inhibitor (dabrafenib) and MEK inhibitor (trametinib). This therapeutic combination has received FDA approval for the treatment of previously treated non-colorectal solid tumors [50]. HER2 alterations are more common in pCCA and dCCA compared to iCCA; for patients with HER2-positive CCA, a treatment approach involving combination of anti-HER2 antibody, trastuzumab, and pertuzumab has shown promise. The antibody-drug conjugate trastuzumab deruxtecan has also shown efficacy in patients with biliary tract cancers (BTCs) with HER2 amplification or overexpression [51]. Other HER2-targeted therapies and novel antibody-drug conjugates have also been evaluated. NTRK, RET, and ROS1 fusions, although rare in cholangiocarcinoma, have demonstrated therapeutic potential as TRK, RET, and ROS1 inhibitors, respectively [16].
ICIs for iCCA
Immunotherapy using ICIs has undergone significant changes in its role in the treatment of cholangiocarcinomas, including iCCAs. The ICI, pembrolizumab, has shown efficacy in cholangiocarcinoma carrying mismatch repair deficient (dMMR)/microsatellite instability-high. Pembrolizumab showed an ORR of 40.8% and a CRR of 13.5% in patients with dMMR/microsatellite instability-high non-colorectal cancers [52]; it is approved for patients with tumors and a high tumor mutation burden. Although only a limited number of patients show a dMMR or high tumor mutation burden in iCCA cases, a combination of ICIs and cytotoxic chemotherapy has shown promising results. Cisplatin is a crucial component of the current first-line GC therapy and can induce immunomodulation through various mechanisms, including the upregulation of MHC class 1, recruitment and proliferation of CD8+ T cells and other immune effector cells, reduction of MDSCs and Tregs, and enhancement of the lytic activity of cytotoxic effectors [53]. The cytotoxic agents also induce the release of neoantigen from tumor. Studies have reported better outcomes in patients treated with the combination of anti-PD-1 antibodies and conventional chemotherapy than those with chemotherapy alone. Preliminary data suggest that this combined approach with ICIs and conventional chemotherapy improves PFS and OS in patients with advanced-stage BTCs [54]. In addition, the phase 3 study TOPAZ-1, which compared the efficacies of the PD-L1 antibody (durvalumab) combined with GC and placebo plus GC group, revealed the superiority of the PD-L1 antibody combined group in patients with previously untreated unresectable or metastatic BTC [5]. Combination therapy with durvalumab significantly improved OS, PFS, and OR rates compared to the placebo control. Another phase 3 trial, KEYNOTE-966, demonstrated the efficacy of combining gemcitabine, cisplatin, and ICIs as the first-line treatment of advanced-stage cholangiocarcinoma [55].
Trials for Overcoming the Immune-Suppressive Microenvironment in iCCA
Effective immunotherapy can drive the elimination of tumors, which is observed as a complete response. However, if the applied treatment fails to overcome the immunosuppressive TME, the cancer becomes resistant to immunotherapy. Importantly, driver mutations unique to each type of cancer play a role in establishing an immunosuppressive TME [23]. Generally, genetic alterations that emerge during tumor progression are irreversible; therefore, tumor-specific TME is occasionally difficult to overcome with ICI monotherapy because such genetic alterations may induce major components of anti-tumor immunity, including immunosuppressive cytokines, chemokines, metabolites, and immune checkpoint molecules [23]. From this point of view, combination therapy of ICIs with MTAs should theoretically be one of the promising approaches to enhance the efficacy of immunotherapy, including ICIs because MTAs may have the potential to suppress the effect of driver mutations that contribute to the establishment of an immunosuppressive TME and alter it to immune proficiency through the inhibition of the oncogenic pathway responsible for the activation of immunosuppressive cytokines and chemokines [6].
In a mouse model of cholangiocarcinoma, targeting MDSCs with an anti-Ly6G antibody or a liver X receptor agonist, combined with TAM targeting using an anti-CSF1R antibody, enhanced the efficacy of anti-PD-1 antibodies and improved survival [56]. Therefore, simultaneous targeting of TAMs and MDSCs is a promising approach because inhibition of TAM may induce the compensatory induction of MDSCs that are another immunosuppressive myeloid population. Neutralizing GM-CSF, which plays a role in myeloid cell programming, reduces and repolarizes TAMs and MDSCs and contributes to the induction of T-cell responses, leading to tumor reduction in mouse cholangiocarcinoma [57]. Activation of CD40 on antigen-presenting cells through an agonistic anti-CD40 antibody, in combination with anti-PD-1 antibodies, decreased the tumor burden and increased the activation of macrophages, DCs, CD8+, and CD4+ T cells, and NK cells in a mouse iCCA model [58]. The combination of agonistic anti-CD40 and anti-PD-1 antibodies improved the efficacy of GC combination therapy in this model.
The MEK inhibitor, trametinib, reportedly upregulates MHC I and PD-L1 expressions. Combining the MEK inhibitor with anti-PD-1 antibodies showed improved anti-tumor efficacy in mouse iCCA [59]. These preclinical studies support the idea that modulating the TME can enhance the anti-tumor response and effectiveness of ICIs in iCCA. Other ongoing clinical trials are exploring various combinations of ICIs with chemotherapy or TKIs (Table 1) [16]. For example, IDH1 mutations, predominantly found in iCCA, may synergize with ICIs due to their immunosuppressive effects; trials are exploring ivosidenib with nivolumab for BTCs. Blockade of FGFR and RGFR signaling, which are known for promoting an immunosuppressive environment, is also being studied in combination with ICIs in trials. HER2 amplification is another target for BTC, and HER inhibitors are being investigated for combination therapy. PARP inhibitors combined with ICIs are being studied, particularly in homologous repair-deficient BTCs; preliminary results from trials like olaparib plus pembrolizumab show promise. Although early results with ICI-TKI combinations have been modest, further investigation is underway.
Table 1.
Clinical trials of combination therapy including ICI for iCCA currently ongoing
| ID1 | Disease, condition | Phase | Line | ICI-based therapy2 | Combination3 | For comparison4 | First posted5 |
|---|---|---|---|---|---|---|---|
| NCT05247996 | iCCA | Real-world study | 1st | Optimal type of ICIs | TACE combined with MTAs | Intravenous chemotherapy with GEMOX | 2022 |
| NCT05823311 (GPLET) | CCA, advanced, not previously received immunotherapy | III | 1st/2nd | Tislelizumab | Lenvatinib + GC | GC | 2023 |
| NCT05342194 | iCCA, irresectable | III | 1st | Toripalimab | GC or GEMOX±Lenvatinib | GC or GEMOX | 2022 |
| NCT05820906 | BTC, advanced | II | 1st | Cadonilimab | Regorafenib + GC | N.A. | 2023 |
| NCT05775159 | Solid tumor, locally advanced or metastatic | II | Any | MEDI5752 or AZD2936 | Bevacizumab, lenvatinib, or GC | N.A. | 2023 |
| NCT05532059 (GPLET) | CCA, advanced, not previously received immunotherapy | II | 1st/2nd | Tislelizumab | Lenvatinib + GC | GC | 2022 |
| NCT05429697 | BTC, refractory disease after chemotherapy | IIb | 2nd | SMT-NK inj. + Pembro | N.A | Pembro. monotherapy | 2022 |
| NCT05557578 (GOT) | iCCA, resectable | II | Neoadjuvant | Tislelizumab | GEMOX | N.A. | 2022 |
| NCT05174650 | iCCA with FGFR2 fusions/rearrangement | II | 2nd | Atezo | Derazantinib | N.A. | 2022 |
| NCT05451043 (BLOCKED) | BTC, unresectable | II | 1st | Durva/Treme | GC + propranolol | N.A. | 2022 |
| NCT05254847 | BTC, received R0 resection | II | Adjuvant | Tislelizumab | Capecitabine + lenvatinib | N.A. | 2022 |
| NCT04941287 | BTC, unresectable | II | 2nd | Atezo | CDX-1127 (varlilumab)±cobimetinib | N.A. | 2021 |
| NCT05010681 | iCCA, previously treated with ICIs | II | 2nd | Sintilimab | Lenvatinib | N.A. | 2021 |
| NCT05007106 (KEYVIBE-005) | BTC, unresectable | II | 1st/2nd | MK-7684A | With or without other anticancer therapies | Pembro. monotherapy | 2021 |
| NCT04907851 | BTC, after progression on one prior systemic therapy | II | 2nd | RXC004 + Denosumab + Pembro | N.A. | N.A. | 2021 |
| NCT04506281 | iCCA, resectable, and high risk for recurrence | II | Neoadjuvant, adjuvant | Toripalimab | GEMOX + lenvatinib | No anti-tumor drug before surgery | 2020 |
| NCT04298008 | Refractory BTC, patients who failed immunotherapy | II | 2nd | Durva | AZD6738 | N.A. | 2020 |
| NCT04306367 | BCT, progressed on gemcitabine-based therapy | II | 2nd | Pembro | Olaparib | N.A. | 2020 |
| NCT04057365 | BTC, advanced, previously treated | II | 2nd | Nivo | DKN-01 | N.A. | 2019 |
| NCT03991832 | Solid Tumors, IDH-mutated | II | 2nd | Durva | Olaparib | N.A. | 2019 |
| NCT04056910 | Advanced solid tumor, curative treatment is not available, IDH1-mutated | II | 2nd | Nivo | Ivosidenib | N.A. | 2019 |
| NCT05742750 | BTC, inoperable or metastatic | I/II | 1st | Camrelizumab | Apatinib + GC | N.A. | 2023 |
| NCT05849480 | BTC, progressed on first-line systemic therapy | I/II | 2nd | CDX-1140 + Pembro | Oxaliplatin + Capecitabine | N.A. | 2023 |
| NCT05749900 (HERBOT) | BTC, advanced, HER2-positive | Ib/II | 1st | Nivo | Trastuzumab + GC | N.A. | 2023 |
| NCT05872867 | BTC, advanced or metastatic | I | Any | WM-A1-3389 + Pembro | N.A. | N.A. | 2023 |
| NCT05220722 | iCCA, previously received 1 line of standard therapy | Ib/II | 2nd | ICIs (Pembro, Nivo, Ipi) | SD-101 via HAI | N.A. | 2022 |
| NCT05311618 | CCA, advanced or metastatic | I/Ib | 2nd | NGM438 with/without Pembro | N.A. | N.A. | 2022 |
| NCT05253053 | BTC, advanced | Ib/II | Any | TT-00420 + Atezo | N.A. | N.A. | 2022 |
| NCT05510427 | CCA, FGFR2 fusion/amplification | Ib | 2nd | Atezo/Bev | Infigratinib | N.A. | 2022 |
| NCT05052099 | BTC, advanced | I/II | 2nd | Atezo/Bev | mFOLFOX6 | N.A. | 2021 |
| NCT04989218 | iCCA | I/II | 1st | Durva/Treme | GC | N.A. | 2021 |
| NCT05000294 | BTC, after prior systemic therapy | I/II | 2nd | Atezo | Tivozanib | N.A. | 2021 |
| NCT04913337 | CCA, advanced or metastatic | I/II | 2nd | NGM707 with/without Pembro | N.A. | N.A. | 2021 |
| NCT04708067 | iCCA, after first-line chemotherapy | I | 2nd | Bintrafusp Alfa | HFR | N.A. | 2021 |
| NCT04660929 | BTC, HER2 overexpressing | I | Any | CT-0508 + Pembro | N.A. | N.A. | 2020 |
| NCT05215574 | CCA, advanced or metastatic | I/Ib | Any | NGM831 with/without Pembro | N.A. | N.A. | 2019 |
| NCT04430738 | Solid tumor | I | Any | TRK-950 + Nivo. or Pembro | N.A. | N.A. | 2019 |
ICI, immune checkpoint inhibitor; iCCA, intrahepatic cholangiocarcinoma; CCA, cholangiocarcinoma; BTC, biliary tract cancer; FGFR, fibroblast growth factor receptor; HER2, human epidermal growth factor receptor 2; Pembro, pembrolizumab; Atezo, atezolizumab; Durva/Treme, durvalumab and tremelimumab combination; Atezo/Bev, atezolizumab and bevacizumab combination; Nivo, nivolumab; Ipi, Ipilimumab; TACE, transarterial chemoembolization; GEMOX, gemcitabine and oxaliplatin combination; MTAs, molecular-targeted agents; GC, gemcitabine and cisplatin combination; N.A., not applicable; HAI, hepatic artery infusion; HFR, hypofractionated radiation therapy; mFOLFOX6, modified fluorouracil, leucovorin, and oxaliplatin.
1ID: ClinicalTrials.gov Identifier.
2ICI-based therapy. Treatment using ICIs in the trial. Function of the specific agents are as follow: MEDI5752 (CTLA-4/Anti-PD-1 Bispecific Antibody), AZD2936 (anti-TIGIT/anti-PD-1 bispecific antibody), SMT-NK (Human natural killer [NK] cell therapy), MK-7684A (pembrolizumab/vibostolimab co-formulation), RXC004 (inhibitor targeting the WNT pathway), AZD6738 (ataxia telangiectasia and Rad3-related [ATR] kinase inhibitor), DKN-01 (humanized antibody targeting dickkopf-1 [DKK1]), CDX-1140 (CD40 agonist), WM-A1-3389 (anti-IGSF1 [immunoglobulin superfamily member 1]), NGM438 (leukocyte associated immunoglobulin-like receptor 1 [LAIR1] antagonist antibody), TT-00420 (tinengotinib, a spectrum-selective multi-kinase inhibitor that target Aurora A/B), fibroblast growth factor receptor [FGFR] 1/2/3, vascular endothelial growth factor receptors [VEGFRs], Janus kinase [JAK]1/2, and colony-stimulating factor 1 receptor [CSF1R], NGM707 (humanized monoclonal antibody that binds the immune inhibitory receptors [immunoglobulin-like receptor] ILT2 and ILT4 and blocks interactions with their HLA ligands), bintrafusp alfa (anti-PD-L1/TGF-β Trap), CT-0508 (human epidermal growth factor receptor 2 [HER2] targeted chimeric antigen receptor macrophage [CAR-Macrophage]), NGM831 (ILT3 antagonist antibody), TRK-950 (humanized antibody raised against CAPRIN-1).
3Combination: combination therapy with ICI-based therapy for clinical trial. Function of the specific agents is as follow: SD-101 (TLR [toll-like receptor] 9 agonist). Derazantinib (FGFR1/2/3 inhibitor), infigratinib (FGFR1/2/3 inhibitor), tivozanib (VEGFR inhibitor).
4For comparison: active comparator of the trials.
5First posted: the date on which the study was first available on ClinicalTrials.gov.
Especially, as mentioned above, mutations in IDH1/2 may induce DNA methylation and downregulation of genes, including those involved in antigen processing and presentation, and may contribute to the formation of the “non-inflamed” iCCAs (Fig. 2b) [35]. Therefore, IDH inhibitors cloud reverse the high methylation status of DNA and induce the expression of these machineries, thus improving the efficacy of ICIs. Interestingly, Wu et al. [29] reported that mutant IDH1 plays a role in tumor maintenance through immune evasion induced by D-2-HG, leading to the decrease of infiltration of CD8+ T cell and suppression of TET2 DNA demethylase in a mouse model. Inhibition of mutant IDH1 restored the recruitment of CD8+ T cell into tumor and IFN-γ expression, and promoted TET2-dependent induction of genes involved in IFN-γ response in tumor cells. In addition, immune checkpoint activation reduced the efficacy of mutant IDH1 inhibitors; combination with CTLA4 blockade provided therapeutic synergy for anti-tumor immunity [29]. Therefore, IDH1 inhibitors are promising agents for restoring the non-inflamed type of TME and enhancing the efficacy of ICIs. Similarly, FGFR inhibitor may also have a potential to alter the immunosuppressive TME in iCCAs. As mentioned above, inhibition of FGF signal can suppress the induction of PD-1, Tregs, and M2 polarization of macrophages [37]. It also downregulates SOCS1 and activate the IFN-γ signaling [39]; FGFR tyrosine kinase inhibitor may also play a role for restoring the non-inflamed TME. As IDH inhibitors and FGFR TKIs have already been approved for the treatment of patients with BTCs carrying these genetic alterations [6], a deeper understanding of iCCA drug therapy using molecular-targeted drugs and ICIs is required.
Conclusion
Based on a previous report, the majority of iCCA cases show a non-inflamed TME; therefore, it is necessary to consider how to manage cholangiocarcinoma using ICIs. Some driver mutations, such as alterations in IDH1/2 and FGFR2 are associated with a non-inflamed TME, and their inhibition may restore the anti-tumor immunity of iCCAs. Recent clinical trials have shown that the treatment of iCCAs with ICIs appears promising, and efforts to understand the mechanisms of resistance and identify predictive biomarkers will be crucial for maximizing the potential of ICIs in the treatment of this type of cancer.
Conflict of Interest Statement
M.K. has received grants from Taiho Pharmaceuticals, Chugai Pharmaceuticals, Otsuka, Takeda, Sumitomo Dainippon-Sumitomo, Daiichi Sankyo, AbbVie, Astellas Pharma, and Bristol-Myers Squibb. He has also received grants and personal lecture fees from Merck Sharpe and Dohme (MSD), Eisai, and Bayer and is an adviser for MSD, Eisai, Bayer, Bristol-Myers Squibb, Eli Lilly, Chugai, AstraZeneca, and ONO Pharmaceuticals. M.K. is an Editor-in-Chief of Liver Cancer, and N.N. is an Editorial Board member of Liver Cancer.
Funding Sources
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI: 21K07184, N.N.) and a grant from Smoking Research Foundation (N.N.).
Author Contributions
Conceptualization, investigation, data curation, writing – original draft preparation, writing – review and editing, visualization, funding acquisition, N.N.; supervision, N.N. and M.K.
Funding Statement
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI: 21K07184, N.N.) and a grant from Smoking Research Foundation (N.N.).
Data Availability Statement
As this is a review manuscript, all data used in this study can be available in the references.
References
- 1. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010 Apr 8;362(14):1273–81. [DOI] [PubMed] [Google Scholar]
- 2. Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023 Jan;30(1):102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021 Nov 1;7(11):1669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023 Jan 19;388(3):228–39. [DOI] [PubMed] [Google Scholar]
- 5. Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8). [DOI] [PubMed] [Google Scholar]
- 6. Harding JJ, Khalil DN, Fabris L, Abou-Alfa GK. Rational development of combination therapies for biliary tract cancers. J Hepatol. 2023 Jan;78(1):217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012 Apr;142(4):1021–31.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020 Sep;72(3):965–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabro R, Pinyol R, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020 Aug;73(2):315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung T, Park YN. Up-to-date pathologic classification and molecular characteristics of intrahepatic cholangiocarcinoma. Front Med. 2022;9:857140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013 Apr;144(4):829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019 Mar;16(3):151–67. [DOI] [PubMed] [Google Scholar]
- 13. Bao X, Li Q, Chen J, Chen D, Ye C, Dai X, et al. Molecular subgroups of intrahepatic cholangiocarcinoma discovered by single-cell RNA sequencing-assisted multiomics analysis. Cancer Immunol Res. 2022 Jul 1;10(7):811–28. [DOI] [PubMed] [Google Scholar]
- 14. Song G, Shi Y, Meng L, Ma J, Huang S, Zhang J, et al. Single-cell transcriptomic analysis suggests two molecularly subtypes of intrahepatic cholangiocarcinoma. Nat Commun. 2022 Mar 28;13(1):1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keenan BP, McCarthy EE, Ilano A, Yang H, Zhang L, Allaire K, et al. Circulating monocytes associated with anti-PD-1 resistance in human biliary cancer induce T cell paralysis. Cell Rep. 2022 Sep 20;40(12):111384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ilyas SI, Affo S, Goyal L, Lamarca A, Sapisochin G, Yang JD, et al. Cholangiocarcinoma: novel biological insights and therapeutic strategies. Nat Rev Clin Oncol. 2023 May 15;20(7):470–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minini M, Fouassier L. Cancer-associated fibroblasts and extracellular matrix: therapeutical strategies for modulating the cholangiocarcinoma microenvironment. Curr Oncol. 2023 Apr 14;30(4):4185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019 Aug;9(8):1102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge C, et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020 Nov;73(5):1118–30. [DOI] [PubMed] [Google Scholar]
- 20. Huang TX, Tan XY, Huang HS, Li YT, Liu BL, Liu KS, et al. Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut. 2022 Feb;71(2):333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin-Serrano MA, Kepecs B, Torres-Martin M, Bramel ER, Haber PK, Merritt E, et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut. 2023 Apr;72(4):736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu C, Ma J, Zhu K, Yu L, Zheng B, Rao D, et al. Spatial immunophenotypes predict clinical outcome in intrahepatic cholangiocarcinoma. JHEP Rep. 2023 Aug;5(8):100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishida N. Role of oncogenic pathways on the cancer immunosuppressive microenvironment and its clinical implications in hepatocellular carcinoma. Cancers. 2021 Jul 21;13(15):3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015 Jul 9;523(7559):231–5. [DOI] [PubMed] [Google Scholar]
- 25. Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, Lindblad KE, Maier B, Sia D, et al. β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019 Aug;9(8):1124–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cassetta L, Kitamura T. Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology. 2018 Nov;155(3):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mody K, Jain P, El-Refai SM, Azad NS, Zabransky DJ, Baretti M, et al. Clinical, genomic, and transcriptomic data profiling of biliary tract cancer reveals subtype-specific immune signatures. JCO Precis Oncol. 2022 Jun;6:e2100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin Y, Peng L, Dong L, Liu D, Ma J, Lin J, et al. Geospatial immune heterogeneity reflects the diverse tumor-immune interactions in intrahepatic cholangiocarcinoma. Cancer Discov. 2022 Oct 5;12(10):2350–71. [DOI] [PubMed] [Google Scholar]
- 29. Wu MJ, Shi L, Dubrot J, Merritt J, Vijay V, Wei TY, et al. Mutant IDH inhibits ifnγ-TET2 signaling to promote immunoevasion and tumor maintenance in cholangiocarcinoma. Cancer Discov. 2022 Mar 1;12(3):812–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montironi C, Castet F, Haber PK, Pinyol R, Torres-Martin M, Torrens L, et al. Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification. Gut. 2023 Jan;72(1):129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019 Apr 1;25(7):2116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morita M, Nishida N, Sakai K, Aoki T, Chishina H, Takita M, et al. Immunological microenvironment predicts the survival of the patients with hepatocellular carcinoma treated with anti-PD-1 antibody. Liver Cancer. 2021 Jul;10(4):380–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018 Aug;24(8):1192–203. [DOI] [PubMed] [Google Scholar]
- 34. Fortin J, Chiang MF, Meydan C, Foox J, Ramachandran P, Leca J, et al. Distinct and opposite effects of leukemogenic Idh and Tet2 mutations in hematopoietic stem and progenitor cells. Proc Natl Acad Sci U S A. 2023 Jan 24;120(4):e2208176120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishida N, Aoki T, Morita M, Chishina H, Takita M, Ida H, et al. Non-inflamed tumor microenvironment and methylation/downregulation of antigen-presenting machineries in cholangiocarcinoma. Cancers. 2023 Apr 20;15(8):2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawazu M, Ueno T, Saeki K, Sax N, Togashi Y, Kanaseki T, et al. HLA class I analysis provides insight into the genetic and epigenetic background of immune evasion in colorectal cancer with high microsatellite instability. Gastroenterology. 2022 Mar;162(3):799–812. [DOI] [PubMed] [Google Scholar]
- 37. Ruan R, Li L, Li X, Huang C, Zhang Z, Zhong H, et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol Cancer. 2023 Mar 25;22(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017 Oct;7(10):1116–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adachi Y, Kamiyama H, Ichikawa K, Fukushima S, Ozawa Y, Yamaguchi S, et al. Inhibition of FGFR reactivates IFNγ signaling in tumor cells to enhance the combined antitumor activity of lenvatinib with anti-PD-1 antibodies. Cancer Res. 2022 Jan 15;82(2):292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vlkova V, Štěpánek I, Hrušková V, Šenigl F, Mayerova V, Šrámek M, et al. Epigenetic regulations in the IFNγ signalling pathway: IFNγ-mediated MHC class I upregulation on tumour cells is associated with DNA demethylation of antigen-presenting machinery genes. Oncotarget. 2014 Aug 30;5(16):6923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor cell-derived IL1β promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 2020 Mar 1;80(5):1088–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kemp SB, Cheng N, Markosyan N, Sor R, Kim IK, Hallin J, et al. Efficacy of a small-molecule inhibitor of KrasG12D in immunocompetent models of pancreatic cancer. Cancer Discov. 2023 Feb 6;13(2):298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010 Aug 9;18(2):160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020 May;21(5):671–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021 Oct;6(10):803–15. [DOI] [PubMed] [Google Scholar]
- 46. Meric-Bernstam F, Bahleda R, Hierro C, Sanson M, Bridgewater J, Arkenau HT, et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2022 Feb;12(2):402–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pirozzi CJ, Yan H. The implications of IDH mutations for cancer development and therapy. Nat Rev Clin Oncol. 2021 Oct;18(10):645–61. [DOI] [PubMed] [Google Scholar]
- 48. Javle M, Curtin NJ. The role of PARP in DNA repair and its therapeutic exploitation. Br J Cancer. 2011 Oct 11;105(8):1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018 May;68(5):959–69. [DOI] [PubMed] [Google Scholar]
- 50. Subbiah V, Lassen U, Elez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020 Sep;21(9):1234–43. [DOI] [PubMed] [Google Scholar]
- 51. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021 Sep;22(9):1290–300. [DOI] [PubMed] [Google Scholar]
- 52. Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022 Sep;33(9):929–38. [DOI] [PubMed] [Google Scholar]
- 53. de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res. 2014 Nov 1;20(21):5384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022 Jun;7(6):522–32. [DOI] [PubMed] [Google Scholar]
- 55. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023 Jun 3;401(10391):1853–65. [DOI] [PubMed] [Google Scholar]
- 56. Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020 Oct 1;130(10):5380–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruffolo LI, Jackson KM, Kuhlers PC, Dale BS, Figueroa Guilliani NM, Ullman NA, et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut. 2022 Jul;71(7):1386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Diggs LP, Ruf B, Ma C, Heinrich B, Cui L, Zhang Q, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021 May;74(5):1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wabitsch S, Tandon M, Ruf B, Zhang Q, McCallen JD, McVey JC, et al. Anti-PD-1 in combination with trametinib suppresses tumor growth and improves survival of intrahepatic cholangiocarcinoma in mice. Cell Mol Gastroenterol Hepatol. 2021;12(3):1166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this is a review manuscript, all data used in this study can be available in the references.