Abstract
Background
Atezolizumab + bevacizumab represent the current standard of care for first-line treatment of advanced hepatocellular carcinoma (HCC). However, direct comparison with other combination treatments including immune checkpoint inhibitors (ICI) + tyrosine kinase inhibitors (TKIs) are lacking.
Objectives
This network meta-analysis (NMA) aims to indirectly compare the efficacy and the safety of first-line systemic therapies for unresectable advanced HCC.
Method
A literature search of MEDLINE, Embase, and SCOPUS databases was conducted up to October 31, 2022. Phase 3 randomized controlled trials (RCTs) testing TKIs, including sorafenib and lenvatinib, or ICIs reporting overall survival (OS) and progression-free survival (PFS) were included. Individual survival data were extracted from OS and PFS curves to calculate restricted mean survival time. A Bayesian NMA was performed to compare treatments in terms of efficacy (15- and 30-month OS, 6-month PFS) and safety, represented by grade ≥3 (severe) adverse events (SAEs). The incremental safety-effectiveness ratio as measure of net health benefit was calculated as the difference in SAE probability divided by survival difference between the 2 most effective treatments.
Results
Nine RCTs enrolling 6,600 patients were included. Atezolizumab plus bevacizumab showed the highest probability (88%) of achieving the 30-month OS landmark. Lenvatinib showed a probability of 86% of achieving best PFS outcomes. ICI monotherapies ranked as most tolerable. Atezolizumab plus bevacizumab showed the best net health benefit for OS, compared to durvalumab plus tremelimumab. When evaluating the net health benefit for PFS, at a willingness-to-risk threshold of 10% of SAEs for life-month gained, atezolizumab plus bevacizumab was favoured in 78% of cases, while at threshold of 30% of SAEs for life-month gained, lenvatinib was favoured in 76% of cases.
Conclusions
Atezolizumab plus bevacizumab is the best treatment in terms of net benefit and therefore it should be recommended as standard of care. Compared to atezolizumab plus bevacizumab, lenvatinib monotherapy had the best net benefit for PFS when physicians and patients are available to accept a higher risk of toxicity.
Keywords: Hepatocellular carcinoma, Systemic treatment, First-line treatment, Immunotherapy, Tyrosin-kinase inhibitors, Anti-vascular endothelial growth factor
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer death worldwide [1] and its prognosis is highly heterogeneous, being related not only to tumour burden but also to the severity of underlying chronic liver disease [2]. Despite the application of surveillance programs to diagnose HCC in earlier stages, more than half of patients are diagnosed at advanced stage and are suitable only for systemic therapy.
The oral tyrosine kinase inhibitor (TKI) sorafenib was the only approved drug for first-line treatment of advanced HCC [3] until 2018, when another TKI, lenvatinib, proved to be non-inferior in terms of overall survival (OS), providing significantly longer progression-free survival (PFS) compared to sorafenib [4]. Since then, the systemic treatment landscape has rapidly evolved with the advent of immune checkpoint inhibitors (ICIs), including programmed cell death-1 pathway inhibitors (PD-1). Although monotherapy with anti-PD-1 agents has shown promising clinical activity [5–7], leading to objective radiological response in 15–20% of patients, it failed to show superiority in OS in first-line [8], while pembrolizumab monotherapy recently showed to be significantly better than placebo in second-line [9]. Conversely, combination of ICIs and agents with different mechanisms of action provided to be effective in phase 3 randomized controlled trials (RCTs). Particularly, the combination of atezolizumab, an anti-PD-L1 agent, with bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, showed to be superior compared to sorafenib in terms of OS, PFS, and quality of life [10, 11], establishing itself as the current standard of care for advanced HCC. Similar results were obtained with the combination of sintilimab, an anti-PD-1 agent, plus IBI305, a bevacizumab biosimilar, in a phase 3 RCT enrolling mostly patients with HBV infection [12]. More recently, the HIMALAYA RCT demonstrated the superiority of the combination of two ICIs, including an anti-PD-L1 agent, durvalumab, plus an anti-CTLA4 agent, tremelimumab and the non-inferiority of durvalumab monotherapy in improving OS compared to sorafenib [13]. Finally, the combination of ICI plus TKI has been explored in phase 3 RCTs showing different results on PFS and OS: the combination of atezolizumab plus cabozantinib has showed to significantly improve PFS compared to sorafenib, but with no significant OS benefit [14]; camrelizumab plus apatinib showed to be superior compared to sorafenib in terms of both PFS and OS [15], while lenvatinib plus pembrolizumab did not significantly increase PFS and OS compared to lenvatinib monotherapy [16].
According to the results of the above-mentioned phase 3 RCTs, ICI-based combinations currently represent the most effective treatment options for first-line treatment. However, it is not clear which is the best class of drugs to associate with ICIs and whether it is more effective to combine them with anti-VEGF, with another ICI or with TKIs. The majority of combination treatments were tested versus a common comparator, sorafenib, but no direct randomized comparisons exist among them, and it is unlikely that RCTs will be performed in the future to test this hypothesis.
Therefore, the identification of the most effective first-line systemic treatment for HCC still remains an unmet medical need. Several network meta-analyses (NMAs) [17–21] attempted to address this issue, demonstrating atezolizumab plus bevacizumab as the most effective treatment option in prolonging both OS [17–21] and PFS [17–19]. However, all of them were performed including only aggregate data, without considering the proportionality of hazard between treatments [17–21]. This last point is particularly relevant from a clinical point of view, as ICI-based therapies are often associated with a late survival benefit in a proportion of patients. This could violate the assumption of proportional hazards (PHs), making the hazard ratio (HR) unsuitable for the evaluation of the clinical benefit when using this drug class [22–24] and underlining the need for innovative measures for pooling data in NMA. Moreover, the first published NMAs [17, 18] did not include both durvalumab plus tremelimumab and the recently evaluated combination therapies of ICIs plus TKIs [17–19]. We performed a NMA of individual participant survival data by using an innovative pooling method, with the aim of comparing the efficacy, safety, and the net benefit of first-line systemic treatment agents for patients with advanced HCC.
Methods
This NMA was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement [25].
Data Sources and Search Strategies
A systematic literature search of MEDLINE, Embase, and SCOPUS databases was performed for full-text articles published up through October 2022. To identify additional studies, the computer search was supplemented with manual searches of the reference lists of all review articles and primary studies retrieved. When the results of a single study were reported in more than one publication, only the most recent and complete data were included in the meta-analysis. We also searched abstracts presented in the American Society of Clinical Oncology (ASCO) meeting library and the European Society for Medical Oncology (ESMO) conference platform during the last 5 years. Abstracts published subsequently as full-text studies already included in our analysis were excluded. The search strategy included the terms “tyrosine kinase inhibitor” OR “multikinase inhibitors” OR “sorafenib” OR “lenvatinib” OR “cabozantinib” OR “regorafenib” AND “vascular endothelial growth factor inhibitor” OR “bevacizumab” AND “immune checkpoint inhibitor” OR “avelumab” OR “pembrolizumab” OR “durvalumab” OR “nivolumab” OR “atezolizumab” OR “tremelimumab” OR “tislelizumab” AND “hepatocellular carcinoma.”
Eligibility Criteria and Study Selection
Randomized phase 3 clinical trials were included if: (1) the control arm was represented by an active treatment; (2) they were performed in first-line setting; (3) they compared TKI monotherapy, including sorafenib and lenvatinib, with ICI monotherapy or combination therapies of ICIs with another agent; (4) they reported Kaplan-Meier curves of OS and PFS for all treatment arms; (5) they were published in English language. Studies were excluded if: (1) they evaluated systemic treatments in combination with locoregional treatments; (2) they were performed in second- or further lines setting. Three individual reviewers (Ci.C., G.E.M.R., and P.G.) identified and reviewed full-text articles and abstracts that were deemed relevant by screening the list of titles. Disagreements between the 3 reviewers were resolved with consensus.
Trial-Level Data Extraction
OS and PFS median times and HRs with corresponding 95% confidence intervals (95% CI) were extracted as measures of treatment efficacy. The rate of grade ≥3 treatment-related adverse events (TRAEs), defined as severe adverse events (SAEs), and the rate of TRAEs leading to treatment discontinuation were assessed as safety measures. We also obtained the following patient-level covariates: age, sex, geographical region, aetiology of liver disease (HBV, HCV, or non-viral), Child-Pugh class, albumin-bilirubin (ALBI) grade, Barcelona Clinic Liver Cancer (BCLC) stage, alpha-fetoprotein (AFP) levels, macrovascular invasion, extrahepatic spread, and use of post-progression systemic treatments.
Individual Patient Survival Data Extraction
Individual patient data (IPD) from OS and PFS Kaplan-Meier curves were extracted as previously described [26, 27], by using Engauge Digitizer software version 12 and by using Guyot algorithm [28] to reconstruct the data. This algorithm was applied to assembled patients with predicted survival times and a predicted event of interest (i.e., alive or dead; progression or progression-free) with digitized data on survival probabilities, time, and total numbers of patients and events. Each reconstructed survival curve was inspected for accuracy and compared with the originally published curves.
Risk of Bias
The Cochrane Collaboration’s tool for assessing the risk of bias in the trial was used [29] which includes the following domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. Two reviewers independently assessed trial quality (G.E.M.R. and P.G.) and disagreements were resolved by referring to a third reviewer (Ci.C.).
Statistical Analysis
We first checked if the PHs assumption between treatment arms was valid for OS and PFS in each trial by using Schoenfeld residual statistics. To take into account cases of non-PH among the studies, we used the restricted mean survival times (RMSTs) as effect measure which has the advantage of not assuming PHs, differently from the HR. RMST reflects average survival from time 0 to a specified time-point t, that was determined from Kaplan-Meier estimates of survival functions. RMST can be interpreted readily as the area under the survival curve within a specific time window. For each trial, we reanalysed the reconstructed IPD and then calculated RMSTs for OS and PFS at pre-specified time horizons. For PFS, a single early time-point was defined at 6 months. For OS, two time-points were defined at 15 and at 30 months. Because RMST could be sensitive to the survival curve estimation method used, as a comparison, we also estimated RMST by separated Royston-Parmar models [30].
NMA was performed according to a Bayesian approach. For OS and PFS, the mean differences between RMSTs and 95% CI were estimated. All treatments were compared with atezolizumab plus bevacizumab. We calculated the relative ranking of the interventions for OS and PFS as their Surface Under the Cumulative Ranking (SUCRA) [31]. SUCRA ranges between 0 (when a treatment is certainly the worst) and 1 (when a treatment is certainly the best). Univariate meta-regression analysis was used to examine associations between patient-level covariates and mean differences. A NMA of difference of proportions for SAEs and TRAEs leading to treatment discontinuation was estimated and 95% CI were calculated.
Efficacy and safety were combined to calculate the incremental safety-effectiveness ratio (ISER), an innovative measure of net health benefit [26, 27]. It was defined as the difference in the rate of SAEs between 2 treatments, divided by their difference in OS or in PFS. This unit of measure expresses the incremental percentage of SAEs for each month gained in OS or PFS. Two-way sensitivity analysis for ISER was performed by varying both the numerator (percentage difference in SAEs) and the denominator (life-month gained) within their CIs. Analogous to the willingness-to-pay threshold of a cost-effectiveness analysis, a willingness-to-risk threshold value must be established for ISER. Different willingness-to-risk thresholds for ISER, and their visual effect in terms of estimated areas, across the potential range of SAEs and life-month gained, have been calculated. All the analyses were performed using R (R core team, 2020).
Results
Literature Search and Study Characteristics
The flowchart of the study selection process is shown in online supplementary Figure S1 (for all online suppl. material, see https://doi.org/10.1159/000531744) . Our primary search identified 1,746 titles and abstracts. After review of the studies, 9 phase 3 RCTs (6 full-papers and 3 abstracts) [4, 8, 11–16, 32] enrolling 6,600 patients fulfilled the inclusion criteria and were selected for the NMA. All included RCTs had two treatment arms, except for HIMALAYA [13] and COSMIC [14] RCTs that had 3 arms. For COSMIC [14], the monotherapy cabozantinib arm was not included in the analysis since OS curve was not available. Sorafenib represented the control arm in 8 RCTs and it was compared with TKI monotherapy (lenvatinib) in one RCT [4], with ICI monotherapy in 3 RCTs (nivolumab, durvalumab, tislelizumab) [7, 13, 32], with ICI combined with anti-VEGF in 2 RCTs (atezolizumab plus bevacizumab, sintilimab plus IBI305) [11, 12], with ICI combined with TKI in 2 RCTs (atezolizumab plus cabozantinib, camrelizumab plus apatinib) [14, 15], and with ICI combined with another ICI in one RCT (durvalumab plus tremelimumab) [13]. In only one RCT, lenvatinib represented the control arm and it was compared with ICI combined with TKI (pembrolizumab plus lenvatinib) [16]. All RCTs were designed for testing superiority except from REFLECT, HIMALAYA (for the comparison between durvalumab monotherapy and sorafenib), and RATIONALE-301 RCTs [4, 13, 32].
The characteristics of patients included in RCTs are shown in online supplementary Table S1. The proportion of Asian patients ranged from 28% [14] to 100% [12]. Viral aetiology (HBV or HCV) was present in a proportion of patients ranging from 55% [8] to 98% [12]. The prevalence of macrovascular invasion ranged from 15% [32] to 43% [11] and extrahepatic spread ranged from 52% [13] to 75% [12]. Efficacy and safety outcomes of included RCTs are shown in online supplementary Table S2. The proportion of patients receiving post-progression systemic treatments ranged from 20% [11, 14] to 44% [15] for experimental arms and from 37% [14] to 60% [12] for control arms. The risk of bias assessment showed an overall low risk of bias (online suppl. Table S3). Non-proportionality of hazards was present in 1 [4] and in 4 [4, 8, 16, 32] out of 9 RCTs for OS and PFS, respectively (online suppl. Fig. S2, S3).
NMA of OS
Eleven treatments from 9 RCTs were evaluated for OS (Fig. 1). When evaluating 15-month OS, atezolizumab plus bevacizumab was significantly better than sorafenib, tislelizumab, and durvalumab, while it was not significantly better compared to other treatments (online suppl. Fig. S4). Combinations of ICI plus anti-VEGF (sintilimab plus IBI305 and atezolizumab plus bevacizumab) achieved the highest probability score of being the most effective (probabilities of 95% and 84%, respectively) (Table 1).
Fig. 1.
NMA of comparisons between first-line systemic treatments for advanced HCC.
Table 1.
Ranking of the first-line systemic treatments for advanced HCC according to their probability of being the best in OS and PFS
| Fifteen-month OS | Thirty-month OS | Six-month PFS | |||
|---|---|---|---|---|---|
| treatment | SUCRA | treatment | SUCRA | treatment | SUCRA |
| Sintilimab + IBI305 | 0.946 | Atezolizumab + bevacizumab | 0.883 | Pembrolizumab + lenvatinib | 0.926 |
| Atezolizumab + bevacizumab | 0.843 | Camrelizumab + apatinib | 0.865 | Lenvatinib | 0.858 |
| Camrelizumab + apatinib | 0.804 | Pembrolizumab + lenvatinib | 0.722 | Camrelizumab + apatinib | 0.786 |
| Pembrolizumab + lenvatinib | 0.586 | Durvalumab + tremelimumab | 0.626 | Sintilimab + IBI305 | 0.712 |
| Lenvatinib | 0.553 | Nivolumab | 0.506 | Atezolizumab + cabozantinib | 0.695 |
| Atezolizumab + cabozantinib | 0.467 | Lenvatinib | 0.466 | Atezolizumab + bevacizumab | 0.518 |
| Durvalumab + tremelimumab | 0.410 | Tislelizumab | 0.352 | Sorafenib | 0.347 |
| Nivolumab | 0.319 | Durvalumab | 0.342 | Durvalumab + tremelimumab | 0.324 |
| Durvalumab | 0.264 | Atezolizumab + cabozantinib | 0.120 | Nivolumab | 0.153 |
| Tislelizumab | 0.195 | Sorafenib | 0.114 | Durvalumab | 0.107 |
| Sorafenib | 0.111 | – | – | Tislelizumab | 0.070 |
SUCRA, Surface Under the Cumulative Ranking.
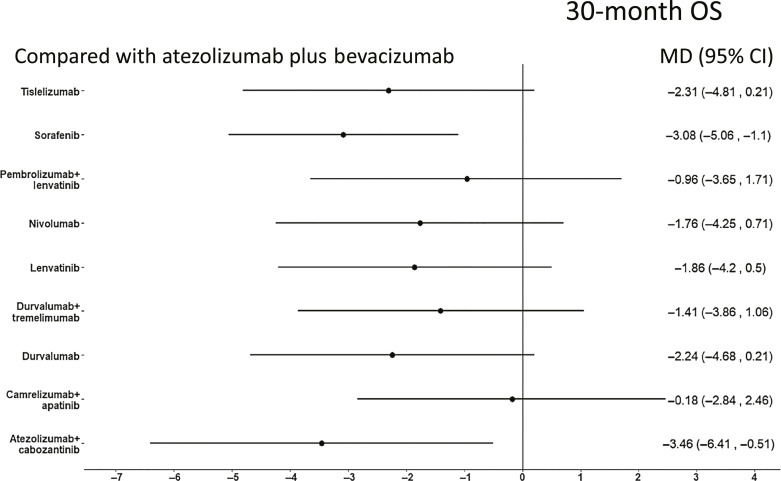
When evaluating 30-month OS, ORIENT-32 RCT was not included due to the shorter length of follow-up. Atezolizumab plus bevacizumab was significantly better than sorafenib and atezolizumab plus cabozantinib, while it was not significantly superior to other treatments (Fig. 2). Atezolizumab plus bevacizumab and camrelizumab plus apatinib achieved the highest probability score of being the most effective (probabilities of 88% and 86%, respectively) (Table 1).
Fig. 2.
Forest plot of NMA for OS of first-line systemic treatments for advanced HCC compared to atezolizumab plus bevacizumab. 30-month OS.
Similar results were obtained when comparing treatments by using Kaplan-Meier method (online supp. Fig. S5, S6; Table S4). Univariate meta-regression analysis showed that no patient- or study-level covariates were significantly associated with PFS and OS.
NMA of PFS
Compared to atezolizumab plus bevacizumab, pembrolizumab plus lenvatinib and lenvatinib were significantly better, while atezolizumab plus bevacizumab was significantly better than tislelizumab, sorafenib, nivolumab, durvalumab, and durvalumab plus tremelimumab (Fig. 3). According to SUCRAs, lenvatinib plus pembrolizumab and lenvatinib had the highest probability of being ranked the most effective intervention (93% and 86%, respectively) (Table 1). Similar results were obtained by using Kaplan-Meier method (online suppl. Fig. S6; Table S4).
Fig. 3.
Forest plot of NMA for 6-month PFS of first-line systemic treatments for advanced HCC compared to atezolizumab plus bevacizumab.
NMA of Safety
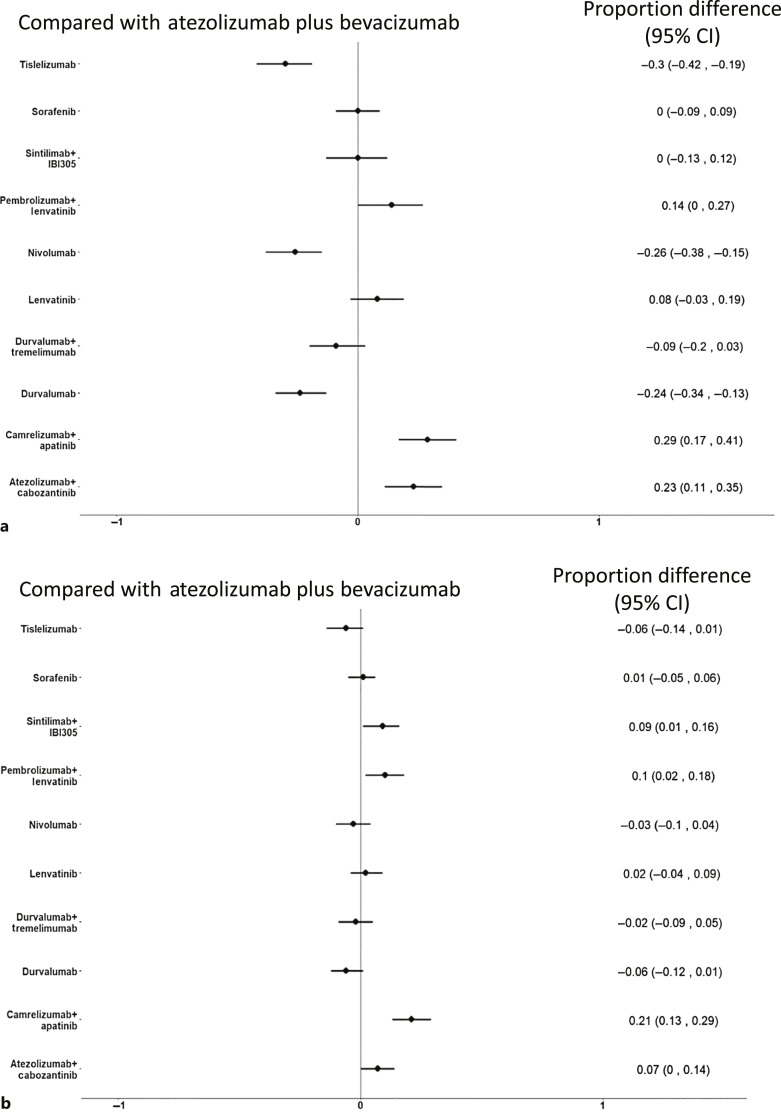
Compared to atezolizumab plus bevacizumab, camrelizumab plus apatinib and atezolizumab plus cabozantinib were associated with a higher risk to develop SAEs, while tislelizumab, nivolumab, and durvalumab were associated with a significantly lower risk (Fig. 4a). According to SUCRAs, tislelizumab and nivolumab had the highest probability of being ranked the most tolerable intervention (97% and 89%, respectively) (online suppl. Table S5).
Fig. 4.
Forest plot of NMA for adverse events (AEs) of first-line systemic treatments for advanced HCC compared to atezolizumab plus bevacizumab. a grade ≥3 (severe) AEs (SAEs). b AEs leading to treatment discontinuation.
When evaluating the risk of TRAEs leading to treatment discontinuation, sintilimab plus IBI305, pembrolizumab plus lenvatinib, camrelizumab plus apatinib, and atezolizumab plus cabozantinib were associated with a significantly higher risk compared with atezolizumab plus bevacizumab, while no treatment was associated with a significantly lower risk (Fig. 4b). According to SUCRAs, tislelizumab and durvalumab had the highest probability of being associated with the lowest risk of TRAEs leading to treatment discontinuation (93% and 91%, respectively) (online suppl. Table S5).
Net Health Benefit Analysis
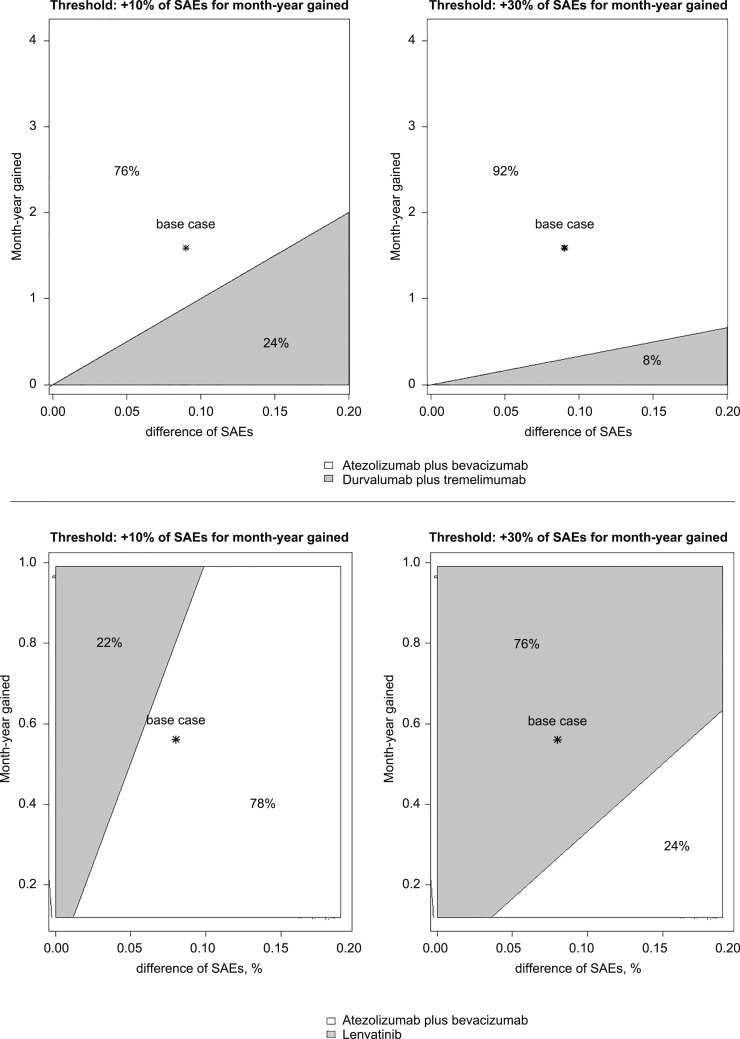
We compared the ISER of atezolizumab plus bevacizumab versus durvalumab plus tremelimumab for OS and atezolizumab plus bevacizumab versus lenvatinib monotherapy for PFS. Compared to atezolizumab plus bevacizumab, durvalumab plus tremelimumab was associated with a decrease of 6% of SAEs for life-month gained. A two-way sensitivity analysis, varying SAEs and life-month gained, evaluated which therapy would be preferred at different willingness-to-risk thresholds. At a willingness-to-risk threshold of 10% of SAEs for life-month gained, atezolizumab plus bevacizumab was favoured in 76% of cases; at a threshold of 30% of SAEs for life-month gained, atezolizumab plus bevacizumab was favoured in 92% of cases (Fig. 5, top panel).
Fig. 5.
Two-way sensitivity analysis of ISER (ISER, i.e., delta SAEs %/survival difference) to indicate which treatment is favoured according to different “willingness-to-risk” thresholds. Two different scenarios were reported with two different willingness-to-risk thresholds: 10% and 30% of SAEs for month-life gained. Top panel: comparison of ISER between atezolizumab plus bevacizumab and durvalumab plus tremelimumab for OS. Grey area favoured durvalumab plus tremelimumab, while white area favoured atezolizumab plus bevacizumab. Bottom panel: comparison of ISER between atezolizumab plus bevacizumab and lenvatinib monotherapy for PFS. Grey area favoured lenvatinib monotherapy, while white area favoured atezolizumab plus bevacizumab.
Compared to atezolizumab plus bevacizumab, lenvatinib monotherapy was associated with an increase of 14% of SAEs for progression-free month gained. At a willingness-to-risk threshold of 10% of SAEs for progression-free month gained, atezolizumab plus bevacizumab was favoured in 78% of cases; at a threshold of 30% of SAEs for progression-free month gained, lenvatinib monotherapy was favoured in 76% of cases (Fig. 5, bottom panel). The comparison of ISER between atezolizumab plus bevacizumab and pembrolizumab plus lenvatinib is reported in online supplementary Figure S8.
Discussion
This updated NMA, indirectly comparing first-line systemic treatments for advanced HCC, collecting individual survival data of 6,600 patients enrolled in 9 phase 3 RCTs and using innovative measures of benefit, showed that atezolizumab plus bevacizumab is the most effective regimen in improving OS, while lenvatinib is most effective in improving PFS. ICI monotherapy (tislelizumab, durvalumab, and nivolumab) provided the lowest probability of developing severe toxicity. Although ICI monotherapies resulted as the safest treatments, our NMA clearly demonstrated that they are significantly less effective than combination treatments including ICIs in terms of both OS and PFS. Finally, compared to durvalumab plus tremelimumab, the net health benefit favoured atezolizumab plus bevacizumab for OS while, compared to atezolizumab plus bevacizumab, the net health benefit favoured lenvatinib for PFS at the cost of an increased risk of toxicity.
Atezolizumab plus bevacizumab is currently considered as the standard of care for first-line treatment of advanced HCC, according to a significant improvement in OS, PFS, and quality of life, compared to sorafenib monotherapy [10, 11]. Moreover, preliminary data suggest that atezolizumab plus bevacizumab could significantly improve recurrence-free survival in early-stage HCC treated with surgical resection or ablation [33]. In the setting of advanced HCC, atezolizumab plus bevacizumab combination has never been directly compared with other first-line treatments, combining anti-PD-1/PD-L1 with TKIs or with anti-CTLA-4. This innovative NMA confirm atezolizumab plus bevacizumab as the preferred choice for first-line treatment with a probability of about 90% of being the best treatment in prolonging OS. However, it should be considered that OS difference between atezolizumab plus bevacizumab and durvalumab plus tremelimumab was small and just over a month. This small difference could be explained by clinical, methodological, or biological issues.
From a clinical point of view, baseline patient characteristics among RCTs are heterogeneous, especially in terms of ethnicity, aetiology of liver disease, and prevalence of macrovascular invasion and main portal vein invasion. Particularly, the prevalence of macrovascular invasion was higher in patients treated with atezolizumab plus bevacizumab, compared to durvalumab plus tremelimumab, that also excluded the subgroup of patients with main portal trunk invasion. However, it should be considered that durvalumab plus tremelimumab could be the preferred option when bevacizumab is contraindicated due to high risk of bleeding.
From a methodological point of view, although OS still represents the most robust outcome to assess treatment benefit [34], it could be biased by the use of post-progression treatments. Notably, the proportion of patients receiving systemic treatments after progression is about the double for durvalumab plus tremelimumab compared to atezolizumab plus bevacizumab. Finally, from a biological perspective, co-inhibition of VEGF or CTLA-4 in conjunction with PD-1 pathway blockade affect a biologically diverse spectrum of negative regulators of anti-cancer immunity, such as impaired vascular permeability, regulatory T-cell proliferation, dendritic cell maturation [35].
Although our results are in line with those of two recently published NMAs [20, 21], clearly demonstrating that atezolizumab plus bevacizumab is the most effective in prolonging OS, our study provided different results in PFS, showing that, among approved regimens, lenvatinib monotherapy was associated with the highest probability of being the most effective. PFS represents a widely used surrogate radiology-based endpoint in HCC trials and it was evaluated as co-primary endpoint with OS in the most recent phase 3 RCTs. However, benefit in PFS does not always translate into OS benefit, being the surrogacy of PFS with OS highly heterogeneous according to the class of drugs and the evaluation time-point [34, 36]. Although our NMA showed that lenvatinib monotherapy was most effective treatment in terms of 6-month PFS, this did not translate into a similar result in terms of OS, being lenvatinib ranked only as the fifth or the sixth most effective treatment in 15- and 30-month OS analyses, respectively. Similarly, the significant PFS benefit of Atezolizumab plus cabozantinib compared to sorafenib did not translate into a significant OS benefit in COSMIC-312 trial [14, 37] and, conversely, the significant OS benefit of durvalumab plus tremelimumab compared to sorafenib was not associated with significant differences in PFS in the HIMALAYA trial [13]. The reasons for the unsatisfactory surrogacy between PFS and OS could be related to several confounding factors, including the different mechanisms of action between ICIs and TKIs [36], differences in treatment toxicity and the competing risk of hepatic decompensation. It has been suggested that PFS could be overestimated when treatment toxicity is high because of informative censoring bias, that consists in the censorship of patients who drop-out early from the trial due to SAEs, before that radiological progression could be documented [38]. In this line, it should be noted that combination of ICI with TKI resulted from our analysis as the treatments associated with the higher risk of SAEs and treatment discontinuation for adverse events. Unfortunately, the rate of hepatic decompensation, that represents a competing driver of death in patients with advanced HCC, is not usually reported in phase 3 RCTs of systemic treatments and this hampered its evaluation in our analysis [39, 40].
Our NMA of safety data showed that ICI monotherapies (durvalumab, tislelizumab, and nivolumab) were associated with the lowest risk of developing SAEs among monotherapies and durvalumab plus tremelimumab was the safest among combination regimens. However, the best safety profile associated with ICI monotherapies is counterbalanced by a significantly lower efficacy, since ICI monotherapies never proved to be superior compared to sorafenib, with durvalumab and tislelizumab that achieved non-inferiority.
The American Society of Clinical Oncology (ASCO) statements [41] clearly recommend net health benefit, that is an innovative measure that combines efficacy and safety, and that should be used to assess the difference between 2 different therapeutic strategies [42]. According to ASCO and differently from all previously published NMAs [17–21], we calculated an innovative measure combining efficacy and safety, called ISER. Atezolizumab plus bevacizumab showed the best net health benefit for OS. Conversely, lenvatinib monotherapy showed the best net health benefit for PFS only in patients with higher willingness-to-risk threshold. These results strongly support the importance of collecting and reporting data on quality of life and patient-reported outcomes, both in RCTs and in real-world studies. For the first time in the setting of HCC to the best of our knowledge, we used an innovative measure of efficacy, i.e., RMST, to overcome the non-proportionality of HR for PFS assessment, that was present in several RCTs. Differently from all previous NMAs, that used HR aggregate data, the extraction of individual survival patient data allowed us the calculation of RMST.
Our NMA presents some limitations. Firstly, unfortunately, individual data regarding patient-level covariates were not available, hampering the identification of baseline features able to explain heterogeneity. So, it is not surprising that our meta-regression analysis based only on aggregate data failed to identify neither patient- nor study-level covariates able to explain the different observed outcomes. In this line, no firm conclusions about the role of patient ethnicity, aetiology of liver disease, and tumour burden on outcomes of patients treated with ICIs can be drawn by using aggregate data. Moreover, our NMA was not able to compare treatments in terms of risk for liver decompensation, which is a well-demonstrated driver of death in patients with HCC [40, 43, 44]. Therefore, time to liver decompensation and decompensation-free survival should be collected and reported in future trials [40]. Second, looking at the NMAs, number of studies per comparisons and the NMA configuration may affect estimates of ranking probabilities [45]. Finally, surrogate (PFS) and true (OS) outcomes of treatments were analysed separately in this and previously published NMAs. Further innovative approaches, including multivariate NMAs able to simultaneously compare RCTs with different treatment regimens and different true and surrogate endpoints should be explored in the setting of advanced HCC [46].
In conclusion, our NMA of first-line systemic treatments for advanced HCC conducted with innovative methods using individual patient survival data showed that: (1) atezolizumab plus bevacizumab is the best treatment in terms of net benefit, and therefore it should be recommended as standard of care; (2) compared to atezolizumab plus bevacizumab, lenvatinib monotherapy had the best net benefit for PFS when physicians and patients are available to accept a higher risk of toxicity. Waiting for future RCTs designed to directly compare first-line combination regimens, individual patient-data NMAs could be useful to improve the personalization of first-line systemic combination treatments for patients with HCC.
Statement of Ethics
The paper is exempt from Ethical Committee approval according to decision of Ethic Committee of Palermo, and written consent was not required as only data extracted from published randomized controlled trials were used. This study protocol was reviewed and the need for consent was waived by the University of Palermo.
Conflict of Interest Statement
Giuseppe Cabibbo participated in advisory board for Bayer, Eisai, Ipsen, and AstraZeneca. Ciro Celsa received speaker fees from Eisai, Ipsen, and MSD. David James Pinato received lecture fees from BMS, Roche, and EISAI; travel expenses from BMS and Bayer Healthcare; consulting fees from Mina Therapeutics, EISAI, Roche, Da Volterra, Ewopharma, and AstraZeneca; research funding (to the institution) from MSD, GSK, and BMS; grant funding from the Wellcome Trust Strategic Fund (PS3416) and from the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG Grant ID 25697); and support from the NIHR Imperial Biomedical Research Centre, the Imperial Experimental Cancer Medicine Centre (ECMC), and the Imperial College Tissue Bank. Calogero Cammà participated in the advisory board for Bayer, MSD/Merck, Ipsen, AstraZeneca, Roche, and Eisai. The other authors have no disclosure to declare.
Funding Sources
The research leading to these results has received funding from the European Union – NextGenerationEU through the Italian Ministry of University and Research under PNRR M4C2I1.3 project PE_00000019 “HEAL ITALIA” CUPB73C22001250006 to Prof. Calogero Cammà (section of gastroenterology & hepatology, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, PROMISE, University of Palermo, Palermo, Italy). Ciro Celsa is funded by the European Union-FESR or FSE, PON Research and Innovation 2014-2020-DM 1062/2021. Marco Enea is funded by “FFR2021.”
Author Contributions
Ciro Celsa: conception and design of the work, analysis, and interpretation of data; drafting, writing and revising critically the work for important intellectual content; and final approval of the version. Giuseppe Cabibbo: conception and design of the work, analysis, and interpretation of data; drafting, writing and revising critically the work for important intellectual content; and final approval of the version to be published. David James Pinato: critical revision of the work for important intellectual content and final approval of the version to be published. Gabriele Di Maria: statistical analysis; critical revision of the work for important intellectual content; and final approval of the version to be published. Marco Enea: statistical analysis; interpretation of data; critical revision of the work for important intellectual content; and final approval of the version to be published. Marco Vaccaro and Salvatore Battaglia: statistical analysis; critical revision of the work for important intellectual content; and final approval of the version to be published. Giacomo Emanuele Maria Rizzo, Carmelo Marco Giacchetto, Gabriele Rancatore, and Maria Vittoria Grassini: acquisition of the data and final approval of the version to be published. Calogero Cammà: conception and design of the work, analysis and interpretation of data; drafting, writing and revising critically the work for important intellectual content; and final approval of the version.
Funding Statement
The research leading to these results has received funding from the European Union – NextGenerationEU through the Italian Ministry of University and Research under PNRR M4C2I1.3 project PE_00000019 “HEAL ITALIA” CUPB73C22001250006 to Prof. Calogero Cammà (section of gastroenterology & hepatology, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, PROMISE, University of Palermo, Palermo, Italy). Ciro Celsa is funded by the European Union-FESR or FSE, PON Research and Innovation 2014-2020-DM 1062/2021. Marco Enea is funded by “FFR2021.”
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material files. Further enquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. Erratum in: CA Cancer J Clin. 2020 Jul;70(4):313. [DOI] [PubMed] [Google Scholar]
- 2. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010 Apr;51(4):1274–83. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 5. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017 Jun 24;389(10088):2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018 Jul;19(7):940–52. Erratum in: Lancet Oncol. 2018 Sep;19(9):e440. [DOI] [PubMed] [Google Scholar]
- 7. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020 Jan 20;38(3):193–202. [DOI] [PubMed] [Google Scholar]
- 8. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022 Jan;23(1):77–90. [DOI] [PubMed] [Google Scholar]
- 9. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J Clin Oncol. 2023;41(7):1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382(20):1894–905. [DOI] [PubMed] [Google Scholar]
- 11. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab versus sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022 Apr;76(4):862–73. [DOI] [PubMed] [Google Scholar]
- 12. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021 Jul;22(7):977–90. Erratum in: Lancet Oncol. 2021 Aug;22(8):e347. [DOI] [PubMed] [Google Scholar]
- 13. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 14. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022 Aug;23(8):995–1008. [DOI] [PubMed] [Google Scholar]
- 15. Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. LBA35 Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. 2022;33:S1401–2. [Google Scholar]
- 16. Finn RS, Kudo M, Merle P, et al. LBA34 - primary results from the phase III LEAP-002 study: lenvatinib+pembrolizumab versus lenvatinib as first-Line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33(Suppl l_7):S808–9. [Google Scholar]
- 17. Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 2020 Dec 1;6(12):e204930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vogel A, Rimassa L, Sun HC, Abou-Alfa GK, El-Khoueiry A, Pinato DJ, et al. Comparative efficacy of atezolizumab plus bevacizumab and other treatment options for patients with unresectable hepatocellular carcinoma: a network meta-analysis. Liver Cancer. 2021 Jun;10(3):240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fulgenzi CAM, D’Alessio A, Airoldi C, Scotti L, Demirtas CO, Gennari A, et al. Comparative efficacy of novel combination strategies for unresectable hepatocellular carcinoma: a network metanalysis of phase III trials. Eur J Cancer. 2022 Oct;174:57–67. [DOI] [PubMed] [Google Scholar]
- 20. Zhang B, Tao B, Li Y, Yi C, Lin Z, Ma Y, et al. Dual immune checkpoint inhibitors or combined with anti-VEGF agents in advanced, unresectable hepatocellular carcinoma [published online ahead of print, 2022 Dec 31]. Eur J Intern Med. 2023;111:37–46. [DOI] [PubMed] [Google Scholar]
- 21. Fulgenzi CAM, Scheiner B, Korolewicz J, Stikas CV, Gennari A, Vincenzi B, et al. Efficacy and safety of frontline systemic therapy for advanced HCC: a network meta-analysis of landmark phase III trials. JHEP Rep. 2023;5(5):100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–5. Erratum in: Epidemiol. 2011 Jan;22(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uno H, Claggett B, Tian L, Inoue E, Gallo P, Miyata T, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014 Aug 1;32(22):2380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pak K, Uno H, Kim DH, Tian L, Kane RC, Takeuchi M, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017 Dec 1;3(12):1692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 26. Cabibbo G, Reig M, Celsa C, Torres F, Battaglia S, Enea M, et al. First-line immune checkpoint inhibitor-based sequential therapies for advanced hepatocellular carcinoma: rationale for future trials. Liver Cancer. 2022;11(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cabibbo G, Celsa C, Enea M, Battaglia S, Rizzo GEM, Grimaudo S, et al. Optimizing sequential systemic therapies for advanced hepatocellular carcinoma: a decision analysis. Cancers. 2020 Jul 31;12(8):2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012 Feb 1;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JP, Altman DG, Gøtzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct 18;343(oct18 2):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freeman SC, Carpenter JR. Bayesian one-step IPD network meta-analysis of time-to-event data using Royston-Parmar models. Res Synth Methods. 2017 Dec;8(4):451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment metaanalysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. [DOI] [PubMed] [Google Scholar]
- 32. Qin S, Kudo M, Meyer T, Finn R, Vogel A, Bai Y, et al. LBA36 - final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol. 2022;33(Suppl l_7):S1402–3. [Google Scholar]
- 33. Chow P, Chen M, Cheng A-L, et al. Abstract CT003: IMbrave050: phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma at high risk of disease recurrence following resection or ablation. Presented at: 2023 AACR annual meeting; April 14-19, 2023; Orlando, Florida.
- 34. Cabibbo G, Bruix J. Radiological endpoints as surrogates for survival benefit in hepatocellular carcinoma trials: all that glitters is not gold. J Hepatol. 2023 Jan;78(1):8–11. [DOI] [PubMed] [Google Scholar]
- 35. Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, et al. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020 Apr;39(18):3620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cabibbo G, Celsa C, Enea M, Battaglia S, Rizzo GEM, Busacca A, et al. Progression-free survival early assessment is a robust surrogate endpoint of overall survival in immunotherapy trials of hepatocellular carcinoma. Cancers. 2020 Dec 30;13(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cabibbo G, Celsa C, D'Alessio A, Fulgenzi CAM, Pinato DJ. COSMIC-312: mounting immunotherapy enigmas for hepatocellular carcinoma. Lancet Oncol. 2022;23(10):e441. [DOI] [PubMed] [Google Scholar]
- 38. Tannock IF, Pond GR, Booth CM. Biased evaluation in cancer drug trials-how use of progression-free survival as the primary end point can mislead. JAMA Oncol. 2022 May 1;8(5):679–80. [DOI] [PubMed] [Google Scholar]
- 39. Cabibbo G, Aghemo A, Lai Q, Masarone M, Montagnese S, Ponziani FR, et al. Optimizing systemic therapy for advanced hepatocellular carcinoma: the key role of liver function. Dig Liver Dis. 2022 Apr;54(4):452–60. [DOI] [PubMed] [Google Scholar]
- 40. Reig M, Cabibbo G. Antiviral therapy in the palliative setting of HCC (BCLC-B and -C). J Hepatol. 2021 May;74(5):1225–33. [DOI] [PubMed] [Google Scholar]
- 41. Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American society of clinical oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015 Aug 10;33(23):2563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998 Apr-Jun;18(2 Suppl l):S68–80. [DOI] [PubMed] [Google Scholar]
- 43. Cabibbo G, Petta S, Barbara M, Attardo S, Bucci L, Farinati F, et al. Hepatic decompensation is the major driver of death in HCV-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J Hepatol. 2017 Jul;67(1):65–71. [DOI] [PubMed] [Google Scholar]
- 44. Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019 Aug;71(2):265–73. [DOI] [PubMed] [Google Scholar]
- 45. Kibret T, Richer D, Beyene J. Bias in identification of the best treatment in a Bayesian network meta-analysis for binary outcome: a simulation study. Clin Epidemiol. 2014 Dec 3;6:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Owen RK, Bujkiewicz S, Tincello DG, Abrams KR. Multivariate network meta-analysis incorporating class effects. BMC Med Res Methodol. 2020 Jul 8;20(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material files. Further enquiries can be directed to the corresponding author.